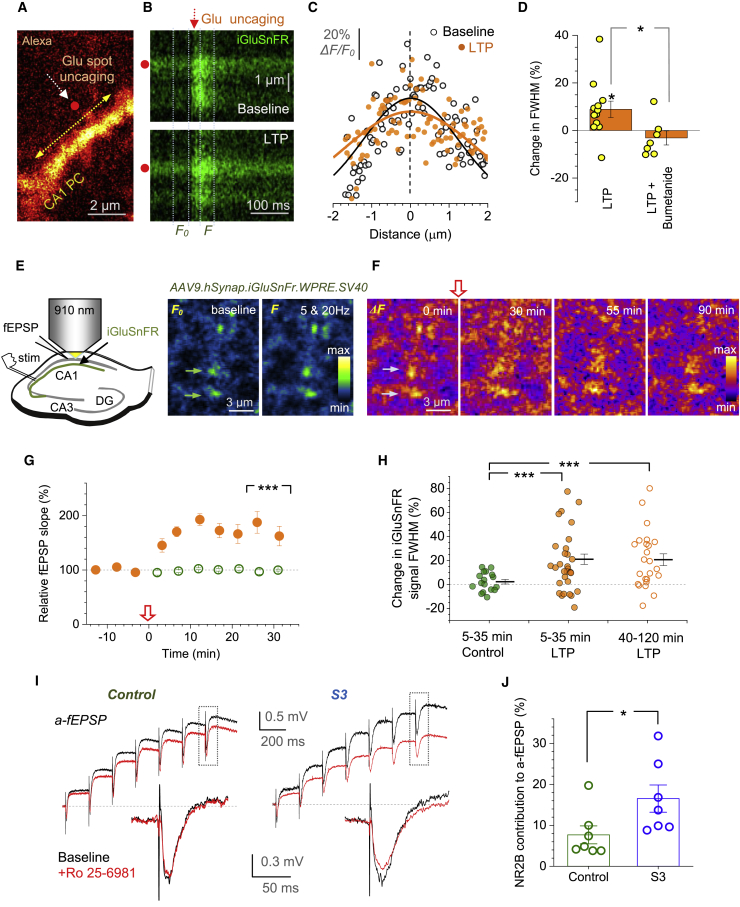
Figure 6.
LTP Induction Broadens Evoked Extracellular Glutamate Transients
(A) Dendritic fragment, CA1 pyramidal cell (AF 594 channel); red dot, glutamate uncaging spot; yellow arrow, line scan position for iGluSnFR monitoring (Figures S6A–S6C).
(B) Line scans (as in A; iGluSnFR channel) showing fluorescence transients in response to a 1 ms uncaging pulse (arrow, onset; red dot, position), before (top) and 20–25 min after the spot-uncaging LTP induction (bottom); dotted lines, time windows to sample baseline (F0) and evoked (F) fluorescence profiles, giving signal profile ΔF = F − F0 (STAR Methods).
(C) iGluSnFR fluorescence profiles (dots, pixel values) from test in (B); zero, uncaging spot position; black and orange lines, best-fit Gaussian.
(D) Summary of tests shown in (A)–(C): relative change (%, mean ± SEM) in ΔF/F0 signal full-width-at-half-magnitude (FWHM) ~25 min after LTP induction (LTP, 9.0% ± 3.4%; n = 12; ∗p < 0.03), and with 20 μM bumetanide inside astroglia (LTP+Bumetanide; −3.1% ± 3.0%; n = 7; ∗p < 0.02, df = 15; Figures S6D–S6G); dots, individual tests.
(E) Diagram, monitoring evoked glutamate release from Schaffer collateral boutons with iGluSnFR, acute slices. Images: iGluSnFR fluorescence landscape s. radiatum in resting conditions (F0) and during five stimuli at 20 Hz (F); arrows, two tentative axonal boutons, false colors.
(F) Evoked iGluSnFR signal landscapes (ΔF = F − F0; ROI as in E) just before (0 min, as in E) and 30, 55, and 90 min after LTP induction (red arrow; Figure S6H; STAR Methods); false colors.
(G) Relative fEPSP slope (%, mean ± SEM, n = 8 slices), protocol as in (E) and (F); arrow, LTP induction; ∗∗∗p < 0.001 (relative to no-HFS control, n = 4; over 25–35 min post-induction; df = 10).
(H) The FWHM of evoked iGluSnFR ΔF signals relative to baseline, over 5–35 min in control conditions (control, n = 17 boutons), 5–35 min (n = 31), and 40–120 min (n = 21) after LTP induction, as shown; dots, individual boutons; bars, mean ± SEM; ∗∗∗p < 0.005 (df = 46; 4 slices).
(I) Upper traces, examples of CA1 astrocyte-recorded fEPSPs (a-fEPSP, current clamp, isolated NMDAR component; 3–5 trial average) evoked by 7 stimuli at 5 Hz, in baseline conditions (black) and after blocking GluN2B-containing NMDARs (1 μM Ro 25-6981, red); control cell and one dialyzed with 200 μM peptide S3 shown, as indicated; lower traces, fragments (rectangles) showing the 7th a-fEPSPs (pre-pulse baseline adjusted; see Figure S6I for extended traces).
(J) Summary of tests shown in I; ordinate, reduction of the a-fEPSP amplitude by Ro 25-698; dots, individual cells; bars, mean ± SEM; ∗p < 0.05 (n = 7 in control and S3; df = 12).