Abstract
Background
Fetal Alcohol Spectrum Disorders (FASD) are a global health concern. Early intervention mitigates deficits, yet early diagnosis remains challenging. We examine whether children can be screened and meet diagnoses for FASD at 1.5 years compared to 5 years post-birth.
Methods
A population cohort of pregnant women in 24 neighborhoods (N=1258) was recruited and 84.5%−96% were reassessed at two weeks post-birth, 0.5 years, 1.5 years, 3 years, and 5 years later. A two-step process was followed to diagnose FASD; first, a paraprofessional screened the children and then a physician evaluated the child. Logistic regression models examined differences in children receiving a positive FASD screening (n=160) at 1.5 years vs. 5 years and maternal predictors of the FASD positive screening and diagnosis.
Results
Screening positive for FASD more than doubled from 1.5 years to 5 years (from 6.8% to 14.8%). About one quarter of children who screened positive and were evaluated by a physician, were diagnosed as having a FASD. However, half did not complete second stage screening. Mothers without Stage 2 screening were more likely to live in informal housing than those who did (81.3% vs. 62.5%, p=0.014).
Conclusions
We found that children can be screened and diagnosed for FASD at 1.5 and 5 years. As FASD characteristics develop over time, repeated screenings are necessary to identify all affected children and launch preventive interventions. Referrals for children to see a physician to confirm diagnosis and link children to care remains a challenge. Integration with the primary healthcare system might mitigate some of those difficulties.
Keywords: Fetal Alcohol Spectrum Disorders, Alcohol Misuse, Community Health Workers, Prenatal Alcohol Exposure, Child Development
INTRODUCTION
Alcohol use during pregnancy and fetal alcohol spectrum disorders (FASD) are global health concerns (Popova, Lange, Probst, Gmel, & Rehm, 2017) and a recent meta-analysis found the global prevalence of FASD among children and youth to be 7.7 per 1000 population (95% CI, 4.9–11.7) (Lange, Probst, et al., 2017). FASD causes deficits in height and weight, immune system development, executive functioning, problem solving ability, short- and long-term memory, communication skills, and attention (Brown et al., 2018; Gauthier, 2015; Lange, Rovet, Rehm, & Popova, 2017). FASD can result in reduced quality of life and significant economic impacts by reducing productivity due to morbidity and mortality and by increasing burdens on the health care, special education, residential care, and criminal justice systems (Greenmyer, Klug, Kambeitz, Popova, & Burd, 2018).
There is substantial and unequivocal evidence that prenatal alcohol exposure is associated with the serious and long term effects of FASD (Lange, Rovet, et al., 2017; Philip A May et al., 2016). The quantity, frequency, and timing of maternal drinking determines the manifestation of FASD symptoms, with mothers who drink more, more often, and earlier during pregnancy giving birth to children with the most severe outcomes (Philip A May et al., 2013; Rehm et al., 2017). However, even light to moderate levels of alcohol exposure in utero can result in increased risks, including diminished cognitive capacity in children (Willford, Leech, & Day, 2006).
The highest rates of prenatal alcohol use and FASDs globally have been documented in South Africa. South Africa has one of the highest rates of alcohol consumption in the world, and alcohol consumption per capita has increased in the last decade (World Health Organization, 2014). Although about 40% of the population does not drink alcohol, national surveys show that those who drink are likely to binge drink (Peltzer, Davids, & Njuho, 2011; Ramsoomar & Morojele, 2012;. World Health Organization, 2016). When reporting anonymously, about 25% of mothers report alcohol consumption while pregnant (Davis, Rotheram-Borus, Weichle, Rezai, & Tomlinson, 2017; O’Connor et al., 2011). In selected peri-urban communities in South Africa, about 11% of children entering first grade have been diagnosed with FAS (Roozen et al., 2016).
Fortunately, early interventions that address cognitive functioning in children with FASD have been successful in reducing developmental deficits (Matzopoulos, Truen, Bowman, & Corrigall, 2014; Parry, Rehm, Poznyak, & Room, 2009; Reid et al., 2015). However, it can be difficult to diagnose FASD early and to link children to the care and the early intervention they need (Burd, Cotsonas-Hassler, Martsolf, & Kerbeshian, 2003). Only a small portion of children with a FASD are receiving appropriate early diagnosis (Chamberlain, Reid, Warner, Shelton, & Dawe, 2017). Because early intervention is so important for children with FASD, we evaluated FASD symptoms at 1.5 and 5 years to examine the earliest age in this sample at which a reliable diagnosis of FASD could be made. We also examined the characteristics of mothers who followed-up to complete a second diagnostic interview and assessment, which required visiting a physician.
METHODS
The study was approved by the Institutional Review Boards of the University of California, Los Angeles, and Stellenbosch University (IRB#10–000386). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study is registered with ClinicalTrials.gov (NCT00996528).
Participants
Cape Town has a network of townships with both formal homes and informal shacks. Most families (70%) earn 0–800 ZAR per month (about 60 USD). We identified 24 neighborhoods of similar size (450–600 households) that were within five kilometers of health clinics, were non-contiguous and/or separated by natural barriers (e.g., a highway), with similar patterns of migration among residents, and had five to seven alcohol bars (shebeens). Shebeens are informal public drinking venues typically that serve both home brew, beer, and wine and whiskey, and are important community-based gathering places in South Africa.
Recruiters (local women from nearby neighborhoods) were trained to conduct house-to-house visits from May 2009 to September 2010 to invite all pregnant women ages 18 years or older to participate in the study (Rotheram-Borus et al., 2011). Pregnant women were recruited at an average 26 weeks of pregnancy (range, 3–40 weeks); only 2% of women (n=25/1283) refused participation. Recruiters identified pregnant women, obtained consent to contact, and referred to the assessment team. A driver took potential participants to a centralized assessment center located at the intersection of three townships. Among all participants, 91.9% were reassessed at two weeks post-birth, 85.6% at six months, 91.7% at 1.5 years, 84.7% at 3 years and 84.5% at 5 years post-birth. During the study period, a total of 105 children died, 7 of which were screened for FASD at either time point. Children with FASD were no more likely to die compared to those without FASD. Multiple births were removed (n=13), reducing the total sample (n=1225). With the remaining singleton children, 977 children (79.8%); (n=809) were screened for FASD at 1.5 years and/or 5 years (n=858).
Measures
Mothers
All women in the sample were of Black African (e.g. Xhosa) ethnicity. Mothers reported their age, education, income, and housing characteristics. Food insecurity was measured with the US Household Food Security Survey Module (Carlson, Andrews, & Bickel, 1999): an 8-item food insecurity scale, which measured the frequency of running out of food, being unable to afford balanced meals, and skipping meals because of lack of food over six months (Carlson et al., 1999; Tsai, Hung, & Weiser, 2012; Tsai, Tomlinson, Comulada, & Rotheram-Borus, 2016). This module has been used in a variety of populations and countries, including in South Africa (Abrahams, Lund, Field, & Honikman, 2018; Tsai et al., 2012; Ukegbu, Nwofia, Ndudiri, Uwakwe, & Uwaegbute, 2019). To screen for depression, we administered the Edinburgh Postnatal Depression Scale (EPDS) with a cut-off of ≥13 to indicate depressed mood (Lawrie, Hofmeyr, de Jager, & Berk, 1998). Interpersonal partner violence was captured by asking whether the woman had been slapped, pushed or shoved, and/or threatened with a weapon by a current partner in the past 12 months (Straus, 1979).
Maternal alcohol consumption was self-reported at all assessments using the 3-item AUDIT-C, which ranges from 0 points (non-drinking) to 12 (very high-risk drinking). The three questions on the screen include: 1) days of any alcohol use; 2) usual number of drinks per day; and 3) binge episodes of five or more drinks in a single day. Previous research has found high concurrence between AUDIT-C (≥ 3/4) and the full AUDIT. Previous research has found high concurrence between the AUDIT-C (≥ 3/4) and the full AUDIT (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993) cut-off score of ≥ 7/8 (Morojele et al., 2017). For this study, question 3 was modified to define a binge episode as heavy episodic drinking of four or more drinks in a single day to conform to the National Institute on Alcohol Abuse and Alcoholism definition of hazardous drinking in women (NIAAA, 2005). Five additional questions were from the TWEAK, a measure recommended to assess alcohol misuse in pregnant women (CDC, 2009). An AUDIT-C score of four or more drinks in a single day at least once a month and at least one positive symptom from the TWEAK (e.g., needing alcohol to get up each morning) was used to indicate high risk drinking (Bush, Kivlahan, McDonell, Fihn, & Bradley, 1998). This study focused on maternal alcohol use and binge drinking at two time points during pregnancy; drinking prior to the mother recognizing that she was pregnant and following pregnancy recognition.
Mother’s HIV status was obtained by self-reports and examination of their children’s health record summarized on the Road-to-Health Card. At an 8-year follow-up assessment, rapid HIV tests were administered to mothers. Comparing maternal self-reports of HIV status, there were 40 potential discrepancies, including both false positives and false negatives, among 1041 mothers (a 3.8% discrepancy rate).
Children
The primary outcome for this paper was whether a child of a participant received a positive FASD screening and diagnosis at 1.5 years and/or 5 years. At the time of this study, FASD diagnostic criteria required that mothers acknowledged drinking alcohol during pregnancy and that a physician assessed and validated the child’s status.
A senior U.S.-based senior alcohol researcher personally trained paraprofessional screeners to identify select FASD characteristics based on the U.S. Institute of Medicine (IOM) criteria (Hoyme et al., 2005; P. A. May et al., 2009). Training methods have been previously described (O’Connor et al., 2014). In brief, screening components consisted of 1) evidence of growth retardation (height or weight ≤ 10th percentile); 2) evidence of deficient brain growth (occipital frontal circumference (OFC) ≤ 10th percentile); and 3) evidence of a pattern of facial anomalies characteristic of FAS (flat upper vermillion border (lip) or flat philtrum). The lip and philtrum were assessed using the Lip-Philtrum Guide (Astley, 2004), which is used to objectively measure philtrum smoothness and the flatness of the upper vermillion border of the lip (O’Connor et al., 2014). A child with a rank of 1 has a well-formed upper lip or deeply grooved philtrum, and a rank of 5 reflects an upper lip or philtrum that is smooth (O’Connor et al., 2014). Scores of 4 or greater indicate a positive facial feature. The formal training period included the use of realistic dolls that served as models of black infants with features consistent with FASD. Reliability was assessed between the screeners and a trained clinical psychologist with expertise in FASD, which resulted in 95% agreement on lip and 100% agreement on philtrum. Continued supervision and reliability of screening outcome was overseen by an on-sight alcohol researcher on a weekly basis. Any child meeting criteria of height, weight, or OFC ≤ 10th percentile; or receiving a philtrum or lip score ≥ 4 received a positive FASD screening and referral for further evaluation.
Stage 2, clinical FASD evaluations were performed by a pediatrician employed by the study who was trained to reliability to assess FASD by the senior alcohol researcher. All children were photographed and photos were evaluated by the senior alcohol researcher who was blind to physician diagnoses. FASD diagnoses were based on the IOM criteria (which included growth retardation, facial features of flat philtrum, flat upper lip, and short palpebral fissures) and included classifications of FAS, partial FAS (pFAS), or alcohol related neurodevelopment disorder (ARND) (Hoyme et al., 2005; O’Connor et al., 2014). For the purpose of this study, we grouped the FASD diagnoses into a dichotomous variable measuring any FASD diagnosis versus none.
All mothers were contacted repeatedly and offered transportation and a 20 ZAR (about 8 USD at the time) incentive to complete each evaluation. We were not able to conduct the second stage screening to the 24% of children who had relocated to the Eastern Cape. The Eastern Cape is a deeply rural area with significant distances between households and clinic sites. While mothers and children in the Eastern Cape were reassessed by interviewer teams, there were no physicians available to assess children referred for FASD.
Data Analysis
We estimated the prevalence of positive FASD screening and FASD diagnosis at 1.5 years, 5 years, and the consistency between the two assessments. We compared the characteristics of mothers of children who screened positive with mothers whose children screened negative using t-tests for continuous variables and chi-square tests for discrete variables.
We compared mothers of children with a positive FASD screening who were referred and followed up by visiting the study physician to mothers who did not follow-up with the referral to the physician using t-tests for continuous variables and chi-square tests for discrete variables. We used SAS 9.4 and Stata® SE software version 14.2.
RESULTS
Table 1 summarizes the maternal characteristics of the mothers based on children’s FASD diagnostic status: screening negative, screening positive, and diagnosed with a FASD. Overall, the mean age of mothers in our sample was 26.5 ± 5.6 years, a quarter had less than a high school level of education, just over half had a monthly household income <R 2,001 (about $150 USD in 2010), and all were black. Most were informally housed (68.1%), and almost half did not have water and/or a flush toilet on their premises. However, most had electricity. Half of the mothers reported being hungry in the last week. The HIV prevalence in our sample was high at 28.3% in pregnancy. About a third of mothers experienced depressive symptoms (35.3%) or interpersonal partner violence (36.3%) in the last year.
Table 1.
Maternal characteristics organized on the basis of the results of Children’s fetal alcohol spectrum disorder (FASD) screening for at 1.5 years and/or 5 years
| Total (N=977) | Negative FASD Screen (N=817) | Positive FASD Screen (N=160) | FASD Diagnosis†(N=18) | P-value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | N | % | N | % | N | % | N | % | |
| Age, mean (SD) | 26.5 | (5.6) | 26.4 | (5.58) | 27.1 | (5.86) | 29.7 | (6.97) | 0.190 |
| >35 years | 74 | 7.6 | 58 | 7.1 | 16 | 10.0 | 6 | 33.3 | 0.205 |
| Education level < high school | 249 | 25.5 | 198 | 24.2 | 51 | 31.9 | 7 | 38.9 | 0.043* |
| Monthly household income ≤ R2,000 | 499 | 52.8 | 400 | 50.6 | 99 | 63.9 | 13 | 72.2 | 0.002** |
| Housing | |||||||||
| Informal housing | 665 | 68.1 | 550 | 67.3 | 115 | 71.9 | 11 | 61.1 | 0.258 |
| No water on site | 460 | 47.1 | 380 | 46.5 | 80 | 50.0 | 8 | 44.4 | 0.419 |
| No flush toilet | 445 | 45.6 | 361 | 44.2 | 84 | 52.5 | 8 | 44.4 | 0.053 |
| No electricity | 105 | 10.8 | 82 | 10.0 | 23 | 14.4 | 1 | 5.6 | 0.105 |
| Food Insecurity | 486 | 49.7 | 401 | 49.1 | 85 | 53.1 | 12 | 66.7 | 0.350 |
| EPDS score, mean (SD) | 10.8 | (7.0) | 10.5 | (6.90) | 12.6 | (7.00) | 14.2 | (7.10) | <0.001*** |
| EPDS score ≥ 13 | 345 | 35.3 | 271 | 33.2 | 74 | 46.3 | 10 | 55.6 | 0.002** |
| Any intimate partner violence | 354 | 36.3 | 282 | 34.5 | 72 | 45.3 | 12 | 66.7 | 0.010** |
| Mother living with HIV | 276 | 28.3 | 227 | 29.6 | 49 | 32.7 | 9 | 50.0 | 0.048* |
| Alcohol Use | |||||||||
| Drank alcohol during pregnancy (baseline) | 258 | 26.4 | 205 | 25.1 | 53 | 33.1 | 16 | 88.9 | 0.035* |
| Binge ≥ once per month during pregnancy (baseline) | 131 | 14.7 | 100 | 13.5 | 31 | 20.5 | 12 | 75.0 | 0.025* |
| Drank alcohol during pregnancy (60 month recall) 185 | 185 | 20.7 | 142 | 19.1 | 43 | 28.5 | 15 | 83.3 | 0.010** |
| Binge ≥once per month during pregnancy (60 month recall) | 152 | 17.0 | 120 | 16.2 | 32 | 21.2 | 12 | 66.7 | 0.133 |
p<0.05
p<0.01
p<0.001 p-values are from chi-squared, Fisher’s exact, and t-tests comparing positive and negative FASD screening outcomes. SD: standard deviation; EPDS: Edinburgh Postnatal Depression Scale; HIV: human immunodeficiency virus.
FASD diagnosis is a subsample of children who received positive FASD screening.
In the bivariate comparisons, mothers of children who received a positive FASD screening were less likely to have a high school education (31.9% vs. 24.2%, p=0.043); more likely to have a monthly income <R2,000 (63.9% vs. 50.6%, p=0.002), a depressed mood (46.3% vs. 33.2%, p=0.002); and to have experienced interpersonal partner violence (45.3% vs. 34.5%, p=0.010) compared to mothers whose children were not referred. As would be expected, mothers whose children screened FASD positive were significantly more likely to use alcohol and binge drink during pregnancy compared to mothers whose children screened negative. These general trends held for mothers of children that received an FASD diagnosis at either time point.
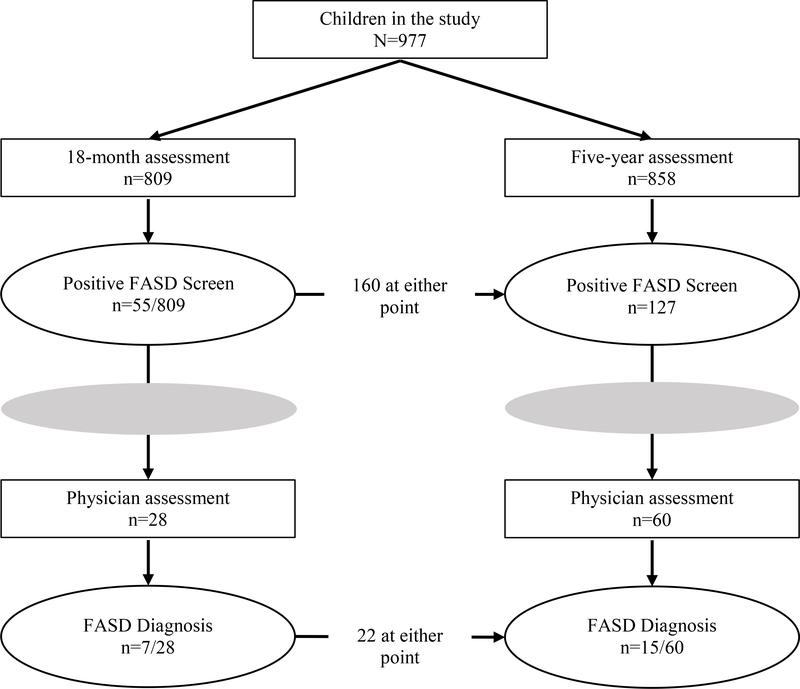
Figure 1 shows the diagnostic status of children over time for FASD, including the prevalence of positive FASD screenings and the prevalence of positive FASD diagnoses at both 1.5 years and 5 years of age. Among our sample of 977 children, there were 160 (16.4%) who screened positive for a FASD at the 1.5 year and/or 5 year assessments. Among those who screened positive, 55% (88/160) followed up with the physician, and 25% (22/88) received a FASD diagnosis. Looking across the assessment time points, at 1.5 years, 55 (6.8%) children screened positive, and at 5 years, 127 (14.8%) children screened positive. Among the 45 children referred at 1.5 years and not missing at 5 years, 22 (48.9%) were referred again at 5 years. Furthermore, among the five children diagnosed with a FASD at 1.5 years and not missing at 5 years, four were again diagnosed with a FASD at 5 years. One child, who screened positive, but did not follow up with the physician at 1.5 years, was re-screened and diagnosed with a FASD at 5 years.
Figure 1:
Flow of study children from FASD assessment to referral and diagnosis.
In our follow-up comparison of the mothers with children who screened positive for a FASD (80 children who followed up with the study physician and 80 children who did not), we found that mothers who did not see the physician were significantly more likely to live in informal housing (81.3% vs. 62.5%, p=0.014). No other covariates were significant.
DISCUSSION
This study conducted FASD paraprofessional screenings and physician diagnoses among children at 1.5 and 5-years post-birth in Cape Town, South Africa. Screenings at 5 years identified more than twice as many children as those at 1.5 years. About one quarter of children who screened positive and were evaluated by a physician, were diagnosed with a FASD. However, half were not evaluated by a physician. Mothers of children who received a positive FASD screening had lower levels of education and income and were more likely to experience the syndemics of interpersonal partner violence and binge drinking during pregnancy compared to mothers whose children screened negative. Children with positive FASD screenings were shorter and weighed less than those who screened negative.
Early identification and intervention for children with a FASD can minimize associated negative outcomes (Reid et al., 2015). The paraprofessional screeners in this study were able to identify children at-risk children for an FASD diagnosis at both 1.5 years and 5 years after birth. Further, among children who screened positive for FASD characteristics and went on to see a physician for diagnosis, 25% were found to have a FASD at both time points. This number is likely a low estimate for the positive predictive value of their screening, as about half of children screened positive at each assessment did not follow-up with a physician for diagnosis.
Referral to a physician was a major barrier in the diagnosis of FASD. Only half of mothers followed up the referral with a specialist physician, even when transportation was provided. Parental follow-up may be even more difficult outside the context of a research study (O’Connor et al., 2014). Mothers face multiple challenges, potentially feeling guilty for drinking alcohol during pregnancy or fear of FASD diagnosis (O’Connor et al., 2014; Olson, Jirikowic, Kartin, & Astley, 2007). A major issue was family migration to the Eastern Cape of South Africa. We were not able to transport children in rural South Africa to a physician and 25% of households had migrated by the time their children were 5 years of age. In our sample, we looked for differences between the 80 mothers who followed up with the physician and the 80 mothers who did not. We found living in informal housing was significantly associated with failure to follow-up the positive FASD screening; however, other factors (such as low income or education, depression, IPV, or HIV) were not statistically associated with follow-up screening.
Screenings at 5 years identified more than twice as many children with characteristics of a FASD than screenings at 1.5 years. This intuitively makes sense. As children age, facial characteristics – including the philtrum and vermillion border of the lips – become more defined. As they grow, deficits in height, weight, or head circumference become more pronounced. The rate for identifying FASD was low for both paraprofessionals and physicians at 1.5 years; however, the consistent positive predictive value of their screenings over time indicates it is more likely that some children with mild characteristics of a FASD cannot be identified early in childhood. As a result, identification of every child with a FASD may require repeated screenings over time. Yet, waiting for children to enter school before screening for a FASD may miss the opportunity to intervene early in a child’s life.
Ultimately, only about 2% of children in this study were diagnosed with a FASD. This is much lower than previous studies in areas with high levels of alcohol consumption. However, if we take into consideration the 80 children that screened positive for FASD characteristics, but did not follow-up with a physician for diagnosis, and assume that about a quarter of those children also would be diagnosed, the rate is closer to 4% of the child populations -a number is on the low end of the 2–11% reported in other South African studies (P. A. May et al., 2009; Roozen et al., 2016; Urban et al., 2008).
Limitations
At both the 1.5 and 5-year time points we were not able to link all children who screened positive for a FASD to a physician for diagnosis. Those missed physician evaluations decreased the effectiveness of screening, reduced our ability to assess the accuracy of the screen, and restricted the sample size, thus, limiting avenues for analysis and the generalizability of results related to the FASD diagnoses. In addition, although our screeners were trained to be non-stigmatizing, it is likely that mothers under-reported their alcohol use. At the time of this study, diagnosis required maternal report or documentation of alcohol use; however, this is no longer the case. This change in criteria, is useful since screenings that rely on maternal reports of alcohol use alone may miss 10–20% of children with a FASD. Further, we used two dichotomous variables for alcohol use and were not able to determine associations between more nuanced levels of alcohol use and FASD positive screenings.
Next Steps
Our study identified several challenges in early diagnosis and intervention for children with a FASD. First, self-reported alcohol use during pregnancy may underestimate true behaviors. There is currently no gold standard for an alcohol biomarker (Asiimwe et al., 2015), but a combination of self-report and biomarkers, such as the PEth test (Helander, Péter, & Zheng, 2012; Hoyme et al., 2005), could provide a valuable additional tool for assessing children’s risk of a FASD. While the PEth test is relatively simple, the current cost of more than $100 may not be feasible for use broadly in a low- and middle-income country. Second, as we lack widely accepted standardized criteria for diagnosing FASD, more research is needed to identify valid and reliable biomarkers for detecting fetal effects from different levels of alcohol use during pregnancy. Thus, more research is needed to investigate the pathways and neurochemistry involved in FASD development, which can inform both diagnostic methods and potential treatments (Ehrhart et al., 2019). Finally, despite free transportation and incentives, it was difficult to ensure follow-up of physician referrals over time. For paraprofessional screenings to ensure children receive early diagnosis and linkage to beneficial interventions, better integration with the healthcare system is required. However, South Africa lacks national surveillance, specialist diagnostic and support services, and training on FASD for health professionals. Therefore, we suggest a coordinated and comprehensive plan for this public health problem (Adebiyi, Mukumbang, & Beytell, 2019). Paraprofessionals can offer repeated referrals, information, and support to mothers, but this is not enough, without a healthcare system that is prepared to diagnose and intervene in the lives of children with FASD.
Conclusion
We conducted FASD paraprofessional screenings and physician diagnoses among children at 1.5 and 5 years in South Africa. Screenings at 5 years identified more than twice as many children than those at 1.5 years. One quarter of children who screened positive and were evaluated by a physician, were diagnosed with a FASD. However, referrals for children to see a physician to confirm diagnosis remains a challenge. Integration with the primary healthcare system might mitigate some of those difficulties. As FASD characteristics develop over time, repeated screenings are necessary to identify all affected children and launch preventive interventions.
Highlights.
Screening positive for FASD more than doubled from 1.5 years to 5 years of age (from 6.8% to 14.8%).
With free transportation and repeated scheduling, mothers fail to get second stage screening for FASD with a physician.
Better family integration with primary healthcare is required for early diagnosis and intervention for FASD.
Acknowledgments
Funding: This study was funded by NIAAA grant # 1R01AA017104 and supported by NIH grants MH58107, 5P30AI028697, 1R24AA022919 and UL1TR000124, the DJ Murray Trust, The Elma Foundation, and Ilyfa Labantwana Foundation.
Footnotes
Conflict of Interest: There are no conflicts of interest.
Declaration of Interest: There are no conflicts of interest; see author affiliations and funding sources below.
Trial Registration. ClinicalTrials.gov registration # NCT00996528; https://clinicaltrials.gov/ct2/show/NCT00996528
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrahams Z, Lund C, Field S, & Honikman S (2018). Factors associated with household food insecurity and depression in pregnant South African women from a low socio-economic setting: a cross-sectional study. Soc Psychiatry Psychiatr Epidemiol, 53(4), 363–372. doi: 10.1007/s00127-018-1497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi BO, Mukumbang FC, & Beytell AM (2019). To what extent is Fetal Alcohol Spectrum Disorder considered in policy-related documents in South Africa? A document review. Health Res Policy Syst, 17(1), 46. doi: 10.1186/s12961-019-0447-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiimwe SB, Fatch R, Emenyonu NI, Muyindike WR, Kekibiina A, Santos GM, … Hahn JA (2015). Comparison of Traditional and Novel Self-Report Measures to an Alcohol Biomarker for Quantifying Alcohol Consumption Among HIV-Infected Adults in Sub-Saharan Africa. Alcohol Clin Exp Res, 39(8), 1518–1527. doi: 10.1111/acer.12781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astley S (2004). Diagnostic guide for fetal alcohol spectrum disorders: The 4-Digit Diagnostic Code. Seattle: University of Washington Publication Services; 2004. In. [Google Scholar]
- Brown J, Trnka A, Harr D, Dodson K, Wartnik H, & Donaldson K (2018). Fetal alcohol spectrum disorder (FASD): A beginner’s guide for mental health professionals. Journal of Neurology and Clinical Neuroscience, 2(1). [Google Scholar]
- Burd L, Cotsonas-Hassler TM, Martsolf JT, & Kerbeshian J (2003). Recognition and management of fetal alcohol syndrome. Neurotoxicol Teratol, 25(6), 681–688. doi: 10.1016/j.ntt.2003.07.020 [DOI] [PubMed] [Google Scholar]
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, & Bradley KA (1998). The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med, 158(16), 1789–1795. doi: 10.1001/archinte.158.16.1789 [DOI] [PubMed] [Google Scholar]
- Carlson SJ, Andrews MS, & Bickel GW (1999). Measuring food insecurity and hunger in the United States: development of a national benchmark measure and prevalence estimates. J Nutr, 129(2S Suppl), 510s–516s. doi: 10.1093/jn/129.2.510S [DOI] [PubMed] [Google Scholar]
- CDC. (2009). Fetal alcohol spectrum disorders; competency-based curriculum development guide for medical and allied health education and practice.
- Chamberlain K, Reid N, Warner J, Shelton D, & Dawe S (2017). A qualitative evaluation of caregivers’ experiences, understanding and outcomes following diagnosis of FASD. Res Dev Disabil, 63, 99–106. doi: 10.1016/j.ridd.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Davis EC, Rotheram-Borus MJ, Weichle TW, Rezai R, & Tomlinson M (2017). Patterns of Alcohol Abuse, Depression, and Intimate Partner Violence Among Township Mothers in South Africa Over 5 Years. AIDS Behav, 21(Suppl 2), 174–182. doi: 10.1007/s10461-017-1927-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhart F, Roozen S, Verbeek J, Koek G, Kok G, van Kranen H, … Curfs LMG. (2019). Review and gap analysis: molecular pathways leading to fetal alcohol spectrum disorders. Mol Psychiatry, 24(1), 10–17. doi: 10.1038/s41380-018-0095-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier TW (2015). Prenatal Alcohol Exposure and the Developing Immune System. Alcohol Res, 37(2), 279–285. [PMC free article] [PubMed] [Google Scholar]
- Greenmyer JR, Klug MG, Kambeitz C, Popova S, & Burd L (2018). A Multicountry Updated Assessment of the Economic Impact of Fetal Alcohol Spectrum Disorder: Costs for Children and Adults. J Addict Med, 12(6), 466–473. doi: 10.1097/adm.0000000000000438 [DOI] [PubMed] [Google Scholar]
- Helander A, Péter O, & Zheng Y (2012). Monitoring of the alcohol biomarkers PEth, CDT and EtG/EtS in an outpatient treatment setting. Alcohol Alcohol, 47(5), 552–557. doi: 10.1093/alcalc/ags065 [DOI] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, … Khaole N (2005). A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics, 115(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Probst C, Gmel G, Rehm J, Burd L, & Popova S (2017). Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth: A Systematic Review and Meta-analysis. JAMA Pediatr, 171(10), 948–956. doi: 10.1001/jamapediatrics.2017.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange S, Rovet J, Rehm J, & Popova S (2017). Neurodevelopmental profile of Fetal Alcohol Spectrum Disorder: A systematic review. BMC Psychol, 5(1), 22. doi: 10.1186/s40359-017-0191-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie TA, Hofmeyr GJ, de Jager M, & Berk M (1998). Validation of the Edinburgh Postnatal Depression Scale on a cohort of South African women. S Afr Med J, 88(10), 1340–1344. [PubMed] [Google Scholar]
- Matzopoulos RG, Truen S, Bowman B, & Corrigall J (2014). The cost of harmful alcohol use in South Africa.. South African Medical Journal, 104(2), 127–132. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, & Hoyme HE (2009). Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev, 15(3), 176–192. doi: 10.1002/ddrr.68 [DOI] [PubMed] [Google Scholar]
- May PA, Hasken JM, Blankenship J, Marais A-S, Joubert B, Cloete M, … Roux S (2016). Breastfeeding and maternal alcohol use: Prevalence and effects on child outcomes and fetal alcohol spectrum disorders. Reproductive Toxicology, 63, 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PA, Tabachnick BG, Gossage JP, Kalberg WO, Marais A-S, Robinson LK, … Hoyme HE (2013). Maternal factors predicting cognitive and behavioral characteristics of children with fetal alcohol spectrum disorders. Journal of developmental and behavioral pediatrics: JDBP, 34(5), 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morojele NK, Nkosi S, Kekwaletswe CT, Shuper PA, Manda SO, Myers B, & Parry CD (2017). Utility of Brief Versions of the Alcohol Use Disorders Identification Test (AUDIT) to Identify Excessive Drinking Among Patients in HIV Care in South Africa. J Stud Alcohol Drugs, 78(1), 88–96. [DOI] [PubMed] [Google Scholar]
- NIAAA. (2005). Helping Patients Who Drink Too Much: A Clinician’s Guide. Retrieved from
- O’Connor MJ, Rotheram-Borus MJ, Tomlinson M, Bill C, LeRoux IM, & Stewart J (2014). Screening for fetal alcohol spectrum disorders by nonmedical community workers. J Popul Ther Clin Pharmacol, 21(3), e442–452. [PMC free article] [PubMed] [Google Scholar]
- O’Connor MJ, Tomlinson M, Leroux IM, Stewart J, Greco E, & Rotheram-Borus MJ (2011). Predictors of alcohol use prior to pregnancy recognition among township women in Cape Town, South Africa. Soc Sci Med, 72(1), 83–90. doi: 10.1016/j.socscimed.2010.09.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson HC, Jirikowic T, Kartin D, & Astley S (2007). Responding to the challenge of early intervention for fetal alcohol spectrum disorders. Infants & Young Children, 20(2), 172–189. [Google Scholar]
- Parry C, Rehm J, Poznyak V, & Room R (2009). Alcohol and infectious diseases: an overlooked causal linkage? Addiction, 104(3), 331–332. doi: 10.1111/j.1360-0443.2008.02500.x [DOI] [PubMed] [Google Scholar]
- Peltzer K, Davids A, & Njuho P (2011). Alcohol use and problem drinking in South Africa: findings from a national population-based survey. Afr J Psychiatry (Johannesbg), 14(1), 30–37. doi: 10.4314/ajpsy.v14i1.65466 [DOI] [PubMed] [Google Scholar]
- Popova S, Lange S, Probst C, Gmel G, & Rehm J (2017). Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: a systematic review and meta-analysis. Lancet Glob Health, 5(3), e290–e299. doi: 10.1016/s2214-109x(17)30021-9 [DOI] [PubMed] [Google Scholar]
- Ramsoomar L, & Morojele NK (2012). Trends in alcohol prevalence, age of initiation and association with alcohol-related harm among South African youth: implications for policy. S Afr Med J, 102(7), 609–612. doi: 10.7196/samj.5766 [DOI] [PubMed] [Google Scholar]
- Rehm J, Gmel GE Sr., Gmel G, Hasan OSM, Imtiaz S, Popova S, … Shuper PA (2017). The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction, 112(6), 968–1001. doi: 10.1111/add.13757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid N, Dawe S, Shelton D, Harnett P, Warner J, Armstrong E, … O’Callaghan F (2015). Systematic Review of Fetal Alcohol Spectrum Disorder Interventions Across the Life Span. Alcohol Clin Exp Res, 39(12), 2283–2295. doi: 10.1111/acer.12903 [DOI] [PubMed] [Google Scholar]
- Roozen S, Peters GJ, Kok G, Townend D, Nijhuis J, & Curfs L (2016). Worldwide Prevalence of Fetal Alcohol Spectrum Disorders: A Systematic Literature Review Including Meta-Analysis. Alcohol Clin Exp Res, 40(1), 18–32. doi: 10.1111/acer.12939 [DOI] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, le Roux IM, Tomlinson M, Mbewu N, Comulada WS, le Roux K, … Swendeman D (2011). Philani Plus (+): a Mentor Mother community health worker home visiting program to improve maternal and infants’ outcomes. Prev Sci, 12(4), 372–388. doi: 10.1007/s11121-011-0238-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption--II. Addiction, 88(6), 791–804. [DOI] [PubMed] [Google Scholar]
- Straus M (1979). Measuring Intrafamily conflict and violence: the conflict tactics (CT) scales.. J Marriage Fam, 41(1), 75–88. [Google Scholar]
- Tsai AC, Hung KJ, & Weiser SD (2012). Is food insecurity associated with HIV risk? Cross-sectional evidence from sexually active women in Brazil. PLoS Med, 9(4), e1001203. doi: 10.1371/journal.pmed.1001203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Tomlinson M, Comulada WS, & Rotheram-Borus MJ (2016). Food insufficiency, depression, and the modifying role of social support: Evidence from a population-based, prospective cohort of pregnant women in peri-urban South Africa. Soc Sci Med, 151, 69–77. doi: 10.1016/j.socscimed.2015.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukegbu P, Nwofia B, Ndudiri U, Uwakwe N, & Uwaegbute A (2019). Food Insecurity and Associated Factors Among University Students. Food Nutr Bull, 40(2), 271–281. doi: 10.1177/0379572119826464 [DOI] [PubMed] [Google Scholar]
- Urban M, Chersich MF, Fourie LA, Chetty C, Olivier L, & Viljoen D (2008). Fetal alcohol syndrome among grade 1 schoolchildren in Northern Cape Province: prevalence and risk factors. S Afr Med J, 98(11), 877–882. [PubMed] [Google Scholar]
- Willford J, Leech S, & Day N (2006). Moderate prenatal alcohol exposure and cognitive status of children at age 10. Alcohol Clin Exp Res, 30(6), 1051–1059. doi: 10.1111/j.1530-0277.2006.00119.x [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2014). Global status report on alcohol and health 2014. Management of Substance Abuse; Retrieved from http://www.who.int/substance_abuse/publications/global_alcohol_report/en/ [Google Scholar]
- World Health Organization. (2016). World Health Statistics data visualizations dashboard: harmful use of alcohol.. Retrieved from http://apps.who.int/gho/data/node.sdg.3-5-data?lang=en.