Intravascular polarimetry (IVP) with polarization-sensitive optical frequency domain imaging (OFDI) measures polarization properties of the coronary arterial wall in parallel with the intensity images of conventional OFDI (1,2). Tissues rich in collagen and smooth muscle cells (SMCs) appear birefringent, while the presence of lipid and macrophages causes depolarization (1,2). Because drug-eluting stents (DES) are designed to prevent SMC proliferation and collagen deposition, we hypothesized that neointimal tissue would exhibit low birefringence.
This study included 19 drug-eluting stents (DES) imaged with IVP (Figure-1A) in 13 patients at a median of 1.5 years post stenting. The ethics committee at the Erasmus University Medical Center approved the study and all patients gave written informed consent. Stented coronary segments were analyzed every 1 mm, resulting in a total of 455 frames with neointima. An additional 97 frames of native atherosclerosis were also examined, located at least 5 mm from the edges of the implanted stents. Neointima (lumen to inner boundary of the stent excluding guidewire shadow) was manually segmented in intensity images using MATLAB. The median depolarization was computed together with the median birefringence in areas with a depolarization of ≤ 0.2 across the segmented neointima in each frame (2). Frames presenting intensity features of macrophages, lipid or calcifications extending to at least one adjacent frame were classified as neoatherosclerosis (n = 112), and otherwise as normal neointima (n = 343). Polarization properties of native atherosclerosis (n = 97) were measured as previously described (2). We also categorized all frames of a stented segment according to the presence of in-stent restenosis (ISR) and/or stent thrombosis (ST) within that segment (204 frames from 6 stents in 5 patients). Statistical analysis of birefringence and the logarithm of depolarization used a univariate generalized linear model with a generalized estimating equation or one-way ANOVA.
Figure 1.
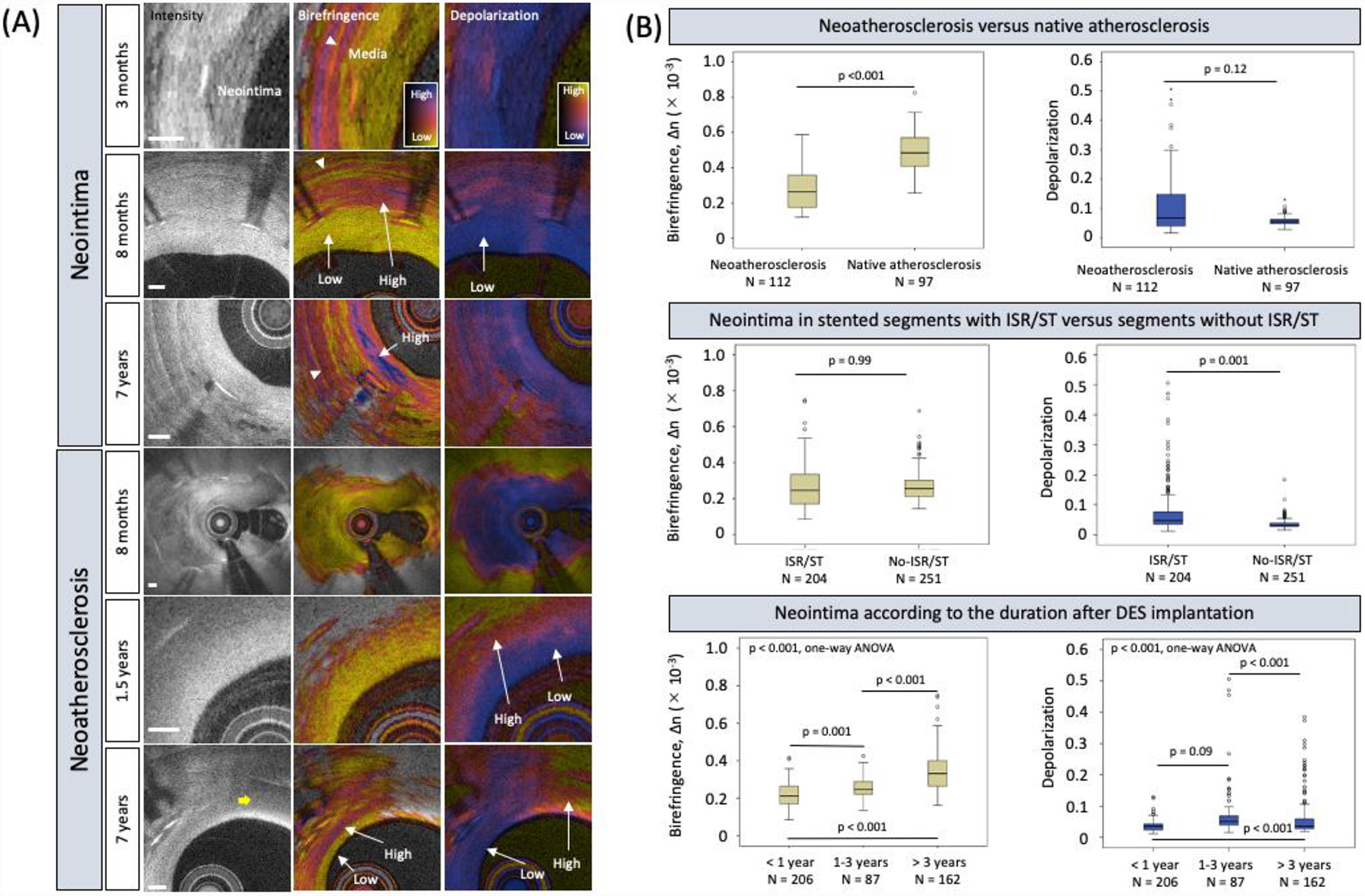
(A) Intensity and polarimetric images of neointima and neoatherosclerosis at various durations after stent implantation. Early neointimal tissues (3 months and 8 months) exhibit low birefringence and depolarization. In-stent restenosis in neoatherosclerosis (8 months) exhibits low birefringence and depolarization. Neoatherosclerosis (1.5 years) depicts spotty appearance of higher birefringence and increased depolarization. Neoatherosclerosis and neointimal tissues at later stage (7 years) feature increased birefringence. Neoatherosclerosis and macrophage accumulation (yellow arrow) feature pronounced depolarization. White arrowheads point to the tunica media. Scale bars are 200 μm. (B) Neoatherosclerosis exhibited lower birefringence and tended to have higher depolarization than native atherosclerosis (top panel). Neointima in stents with ISR/ST displayed higher depolarization than in stents without ISR/ST but comparable birefringence (middle panel). Birefringence and depolarization increased with the duration after stent implantation. ISR = in-stent restenosis and ST = stent thrombosis.
The major findings of the present study are: 1) neoatherosclerosis exhibited lower birefringence than native atherosclerosis (p < 0.001, Figure-1B top panel); 2) depolarization was positively associated with neoatherosclerosis (p < 0.001) and ISR/ST (p = 0.002), while birefringence was not (Figure-1B middle panel); 3) birefringence was positively correlated with the duration after DES implantation (p < 0.001, Figure-1B bottom panel).
The extracellular matrix (ECM) of the neointima within DES has been shown to contain less fibrous collagen than in bare-metal stents (4). Hence, it is comprehensible that we observed very low birefringence in early neointima. After completion of the drug elution with its antiproliferative effect the birefringence increases, likely in hand with neointimal SMC and collagen content. These observations suggest that birefringence can serve to measure the age of neointimal tissue.
Depolarization may quantitatively describe the early stages of neoatherosclerosis leading to ISR/ST. Neoatherosclerosis corresponds to the infiltration of neointimal tissue by lipid-laden macrophages, while the first phase of native atherosclerosis presents focal accumulation of lipid, but possibly without macrophage involvement (3). This difference in the role of macrophages may contribute to the clear association of depolarization with neoatherosclerosis and the tendency of neoatherosclerosis to depolarize more strongly than native atherosclerosis (Figure-1B top panel).
This study has limitations. 1) It consisted of a limited number of patients from two heterogeneous cohorts; 2) the limited imaging depth of IVP may bias depolarization towards increased plaque burden; and 3) histopathology would be required to validate the origin of the observed polarization features of neointima and neoatherosclerosis. In conclusion, IVP provides quantitative assessment of vascular healing after DES implantation and may help clinical decision making in patients at high risk of stent failure.
Acknowledgements
Dr. Otsuka acknowledges partial support from the Japan Heart Foundation / Bayer Yakuhin Research Grant Abroad, the Uehara Memorial Foundation Postdoctoral Fellowship, and the Japan Society for the Promotion of Science Overseas Research Fellowship.
Funding: This work was supported by the National Institutes of Health (grants P41EB-015903 and R01HL-119065) and by Terumo Corporation. Dr. Bouma was supported in part by the Professor Andries Querido visiting professorship of the Erasmus University Medical Center in Rotterdam.
Footnotes
Disclosures: Massachusetts General Hospital and the Erasmus University Medical Center have patent licensing arrangements with Terumo Corporation. Drs. Bouma, van Soest, and Villiger have the right to receive royalties as part of the licensing arrangements. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.Villiger M, Otsuka K, Karanasos A, et al. Coronary Plaque Microstructure and Composition Modify Optical Polarization. A New Endogenous Contrast Mechanism for Optical Frequency Domain Imaging. J Am Coll Cardiol Img 2018;11(11):1666–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Otsuka K, Villiger M, Karanasos A, et al. Intravascular Polarimetry in Patients With Coronary Artery Disease. J Am Coll Cardiol Img 2020:13(3)790–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahagi K, Kolodgie FD, Otsuka F, et al. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol 2016;13(2):79–98. [DOI] [PubMed] [Google Scholar]
- 4.Suna G, Wojakowski W, Lynch M, et al. Extracellular matrix proteomics reveals interplay of aggrecan and aggrecanases in vascular remodeling of stented coronary arteries. Circulation 2018;137(2):166–83. [DOI] [PMC free article] [PubMed] [Google Scholar]


