1. Introduction
High myopia is a significant risk factor for several blinding eye diseases including glaucoma, retinal detachment and macular degeneration, and therefore represents a leading cause of blindness worldwide (Buch et al., 2001). The prevalence of myopia is continuing to increase and is expected to affect nearly half of the global population by 2050 (Holden et al., 2016). Although clinical and experimental studies indicate that normal eye growth (emmetropization) is controlled by visual input (Wallman and Winawer, 2004), the cause of myopia in humans is not understood. However, sustained close work such as reading or working at computers might interrupt the normal vision-dependent mechanisms (Huang et al., 2015) that coordinate the regular growth of the cornea, lens and sclera. Interestingly, the most common structural abnormality associated with myopia is excessive lengthening of the posterior segment of the ocular globe which leads to negative refractive error due to a mismatch between the axial length and the focal length of the eye.
Animal models have provided valuable insights into the role of the visual environment on ocular growth control. In chicks, one of the best investigated animal models so far (Troilo et al., 2019), deprivation of form vision, through the use of visual “occluders” or “goggles” results in accelerated ocular growth and the development of myopia within a matter of days (Wallman et al., 1978, Hodos and Kuenzel, 1984). Again, an interruption of the normal visual stimuli is assumed that otherwise maintains normal ocular growth. By removing the occluders, normal visual input is restored resulting in a rapid deceleration in ocular elongation and eventual return to emmetropia (“recovery”) (Wallman and Adams, 1987).
It is well-established that visually induced changes in ocular length are the result of a “retina-to-choroid-to-scleral signaling cascade” that ultimately results in altered extracellular matrix (ECM) remodeling of the scleral shell (Rada et al., 1991; Norton and Rada, 1995;l Rada et al., 2000). While the signals in these processes are not understood, the vitamin A derivative, all-trans-retinoic acid (atRA) may be an important component for the control of postnatal ocular growth (Seko et al., 1998; Mertz and Wallman, 2000; McFadden et al., 2004; Troilo et al., 2006). Molecular biological approaches revealed that ocular atRA synthesis is regulated in response to visual stimuli exclusively via choroidal expression of the atRA synthesizing enzyme, retinaldehyde dehydrogenase 2 (RALDH2) (Rada et al., 2012; Harper et al., 2016). While the source of atRA production was unknown, it could be demonstrated in chicks and humans that RALDH2 is synthesized by a population of stromal cells, some of which are closely associated with blood vessels (Rada et al., 2012; Harper et al., 2015; 2016). In chicks, RALDH2 positive cells increase in number markedly over 1 – 7 days of recovery and accumulated to a greater extent in the direction of the choriocapillaris (Harper et al., 2016). Despite our documentation of the specific expression of RALDH2, a more precise identification of these cells has been elusive. Moreover, it is unclear as to whether the increase in RALDH2 positive cells is due to increased RALDH2 protein expression by a population of resident choroidal cells, increased cellular proliferation of choroidal RALDH2-expressing cells, or migration of RALDH2-positive cells into the choroid from neighboring tissues or from the vasculature. Therefore, this study was undertaken to better characterize the cells involved in the modulation of atRA biosynthesis in the chick and human choroid.
2. MATERIALS AND METHODS
2.1. Ethics and Animals.
Animals were managed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, with the Animal Welfare Act, and with the National Institutes of Health Guidelines. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center. White Leghorn male chicks (Gallus gallus) were obtained as 2-day-old hatchlings from Ideal Breeding Poultry Farms (Cameron, TX). Chicks were housed in temperature-controlled brooders with a 12-hour light/dark cycle and were given food and water ad libitum. At the end of experiments, chicks were euthanized by overdose of isoflurane inhalant anesthetic (IsoThesia; Vetus Animal Health, Rockville Center, NY), followed by decapitation.
2.2. Induction of Myopia and Recovery from Myopia.
Form deprivation myopia (FDM) was induced in 3 to 4 day-old chicks by applying translucent plastic goggles to one eye, as previously described (Rada et al., 1991). The contralateral eyes (left eyes) of all chicks remained untreated and served as controls. Chicks were checked daily for the condition of the goggles. Goggles remained in place for 10 days, after which time the goggles were removed and chicks were allowed to experience unrestricted vision (recover) for up to 15 days.
2.3. Tissue Preparation.
To prepare ocular tissues for analysis, chicks were euthanized by an overdose of isoflurane inhalant anesthetic (IsoThesia; Vetus Animal Health, Rockville Center, NY) following 10 days of form deprivation (day 0 recovery), and following 1, 4, 7, and 15 days of recovery. Eyes were enucleated and cut along the equator to separate the anterior segment and posterior eye cup. Anterior tissues were discarded, and the vitreous body was removed from the posterior eye cups. An 8 mm punch was taken from the posterior pole of the chick eye using a dermal biopsy punch (Miltex Inc., York, PA). Punches were located nasal to the exit of the optic nerve, with care to exclude the optic nerve and pecten oculi. With the aid of a dissecting microscope, the retina and majority of RPE were removed from the underlying choroid and sclera with a drop of phosphate buffered saline (PBS; 3 mM dibasic sodium phosphate, 1.5 mM monobasic sodium phosphate, 150 mM NaCl, pH 7.2) and gentle brushing. For western blots and retinaldehyde dehydrogenase activity assays, choroids were separated from the sclera using a small spatula, placed in 2 ml screw cap tubes, and snap frozen in liquid nitrogen and stored at −80°C. For immunolabelling experiments, choroids with sclera still attached were placed into a 48-well flat bottom plate (Corning Inc., Corning, NY). A small amount of RPE was left on the choroids to discriminate between the RPE and scleral side of the tissue. The tissues were then fixed with 4% paraformaldehyde (stock solution freshly prepared) in PBS O/N at 4°C.
All of our studies were carried out on choroidal punches from the posterior pole of the chick eyes with the same cross sectional area (8 mm diameter punch = 50.24 mm2). However, Wallman et al., (1995) and others have demonstrated that in response to myopic defocus, as a result of recovery from induced myopia or due to positive lens wear, the chick choroid undergoes substanstial thickening. Therefore, the total tissue volumes of recovering eyes is expected to increase during the recovery period (although not directly determined in the present study).
2.4. Retinaldehyde Dehydrogenase Activity Assays.
Retinaldehyde dehydrogenase activity in choroidal homogenates was determined by measuring the production of atRA in vitro as described previously with minor modifications (McCaffery et al., 1992). To prepare choroid homogenates, individual snap-frozen and stored tissue was homogenized in 200 μL RALDH homogenization buffer (20 mM triethanolamine-HCl pH 7.4, 1 mM dithiothreitol, 0.1 mM EDTA, [Sigma-Aldrich]) using an Omni Tip™ homogenizer (Omni International, Inc., Kennesaw, GA). Homogenates were transferred to thick-walled microfuge tubes (polyallomer tubes; Beckman Coulter, Brea, CA, USA) and ultracentrifuged (100,000g for 1 hour; Optimum MAX Ultracentrifuge, Beckman Coulter) at 4°C to isolate cytosol fractions (supernatant). All procedures with all-trans-retinaldehyde or atRA were performed under yellow lights (Feit Electric A19/BUG/LED; Lincolnwood, IL) in order to prevent light-induced isomerization and degradation (Kane and Napoli,. 2010). 200 μL of synthesis buffer (2.5% DMSO, 4 mM NAD, 32 mM tetrasodium pyrophosphate pH 8.2, 0.1 mM pyrazole, 5 mM glutathione, 1 mM EDTA, [Sigma-Aldrich]) was added to 200 μL of cytosol from homogenized ocular tissues (in homogenization buffer). The reaction was initiated by the addition of 50 μL of 250 μM all-trans-retinaldehyde to the reaction mixture (25 μM final concentration). Negative controls included: (1) 200 μL of RALDH homogenization buffer in place of cytosol, (2) homogenization buffer in place of all-trans-retinaldehyde, and (3) absence of NAD. The reactions were mixed and placed in a water bath at 37°C for 0 to 60 minutes, after which time, the reactions were stopped by immersion in ice water and addition of 3/2 volumes (675 μL) of methanol. The samples were processed by HPLC, as described previously (Farjo et al., 2011). Each activity measurement represented the average of two duplicate samples.
2.5. SDS-PAGE and Western Blot
To determine RALDH2 protein expression in chick ocular tissues, 10 μL of choroidal cytosol (prepared as described above) was prepared for SDS-PAGE and Western blot analysis. NuPAGE LDS Sample Buffer and NuPAGE Sample Reducing Agent were added to 10 μL of each sample so that the sample buffer and reducing agent came to a final concentration of 1X (Life Technologies). Samples then were placed in a 70°C water bath for 10 minutes and electrophoresed under reducing conditions on 10% Bis-Tris Gel NuPAGE SDS-PAGE gels (Life Technologies), according to standard protocols for the NuPAGE gel system. For Western blots, gels were electroblotted onto a nitrocellulose membrane (BioRad) using an electrotransfer unit (XCELL Sureback Electrophoresis Cell; Invitrogen) according to manufacturer’s instructions. Blots were incubated in blocking buffer (0.2% I-Block; Tropix, Bedford, MA, USA and 0.1% Tween-20 in PBS) overnight at 4°C with gentle rocking. Blots then were incubated with rabbit anti-chick RALDH2 (1:500 in blocking buffer) overnight at 4°C. Immunoblots were washed three times with PBS containing 0.05% Tween-20 followed by three washes with PBS. Immunoblots then were incubated with goat anti-rabbit IgG conjugated to alkaline phosphatase as a secondary labeling antibody (1:1,000 in PBS; BioRad). After incubation with the secondary antibody, blots were washed (as above), incubated in CDP Star Ready-to-Use with Nitro-BlockII (Tropix) for 5 minutes, and images were captured with a Chemigenius imager (Syngene, Frederick, MD, USA).
Quantification of band intensity was performed using the Manual Band Quantification feature of the Syngene GeneTools (Syngene) program. Automatic background correction was applied. To control for differences in labelling across the three different blots, all values for each time point (day 0, 1 and 4) were normalized to the average of the control values for each time point. In this way, we were able to assess relative differences between control and treated choroids at each time point.
2.6. Donor Eyes.
Human choroids were obtained from Cornea Bank of the Department of Ophthalmology, or the body donor program of the Department of Anatomy and Cell Biology, both Paracelsus Medical University Salzburg Austria in full accordance with the Declaration of Helsinki and also approved by the local ethics committee (415-EP/73/775–2018 and EK1012/2019). Human donors with a history of systemic disease or chemotherapy were excluded from the study. Choroids from human donors (n= 5, of both sexes, 59 to 78 years of age, post mortem time 8 to 15 hrs.) were dissected, fixed by immersion in PBS containing 4% PFA for 1 hour at room temperature (RT), rinsed in PBS overnight at 4°C, and transferred into PBS containing 15% sucrose (overnight at 4°C). Tissue was mounted in a cryostat (HM 550, Microm, Walldorf, Germany) in tissue embedding medium (OCT; Thermo Scientific, Vienna, Austria) and serial sections of 12–20 μm were collected on adhesion slides (Superfrost Plus; Thermo Scientific) and air-dried for at least 1hr at RT and stored at −20°C for further processing.
2.7. Immunolabelling of Chick Choroids.
After fixation, chick choroids were gently removed from the scleral tissue, as described above, and placed into a new 48-well flat bottom plate (Corning Inc., Corning, NY). Choroids were washed in PBS for 10 min (3X) on a shaking device at RT. Choroids were then blocked in PBS containing 2% bovine serum albumin (BSA), 0.2% Triton X-100, 0.004% sodium azide (pH 7.4) for 1 hr at RT with rocking and subsequently incubated in BSA-PBS containing primary antibodies (see Table 1) for 72 hrs at 4°C. Choroids were then washed in PBS for 10 min (6X) at RT with rocking, incubated with goat anti-rabbit IgG conjugated to AlexaFluor 488 (1:1000 in BSA-PBS; Life Technologies Grand Island, NY) for 24 hours at 4°C, and washed with PBS for 10 min (6X). For double-immunolabelling experiments, choroids were simultaneously incubated with primary antibodies for 72 hrs at 4°C, washed in PBS as described above, simultaneously incubated with goat anti-rabbit IgG conjugated to AlexaFluor 488 and goat anti-Mouse IgG conjugated to AlexaFluor 568 (1:1000 in BSA-PBS; Life Technologies) followed by PBS washes for 10 min (6X). For multiphoton imaging, choroids were placed into shallow wells created by wrapping glass microscope slides with parafilm (Bemis Company, Inc., Oshkosh, WI; approximately 5 times/slide) and cutting a 10 – 15 mm2 rectangle out of the center. Fluorescence mounting media (20 mM Tris, pH 8.0; 0.5% N-propyl gallate [Sigma-Aldrich]; 90% glycerol) was added to the wells containing choroids and wells were covered with coverslips. For confocal imaging, following the PBS washes, choroids were additionally stained with 4′,6-Diamidino-2 phenylindol dihydrochloride (DAPI; diluted 1:1000 in PBS from 5mg/ml stock in dH20, Life Technologies), mounted on glass slides and coverslipped using a fluorescence mounting media (Prolong Gold with DAPI, Thermo Fisher Scientific). All slides were stored at 4°C until imaging.
Table 1.
List of Primary Antibodies used for Immunolabeling Chick Tissues
| Antigen (species) | Recognized Cells | Manufacturer (catalog #) | Host | Dilution/ concentration | Reference |
|---|---|---|---|---|---|
| Ia antigen (chicken) | thymic macrophages/ dendritic cells | DSHB* (TAP1) | mouse monoclonal | 1:2.5 (4 μg/ml) | Guillemot et al., 1984 |
| Bromodeoxyuridine | proliferating cells | Millipore, Billerica, MA (MAB4072) | mouse monoclonal | 1:500 (2 μg/ml) | Chang et al., 2000 |
| CD5 (chicken) | T and B cells | Southern Biotech Birmingham, AL (8360-01) | mouse monoclonal | 1:100 (5 μg/ml) | Koskinen et al., 1998 |
| Collagen type I pro-peptide (sheep) | mesenchymal cells | DSHB* (SPI.D8) | mouse monoclonal | 1:6.75 (4 μg/ml) | Foellmer et al., 1983 |
| Desmin (chicken) | pericytes | DSHB* (D3) | mouse monoclonal | 1:5.5 (4.4 μg/ml) | Danto and Fischman, 1984 |
| FSP1/S100A4 | fibroblasts | LSBio (LS-B2381/76464 | goat polyclonal | 1:100 (5 μg/ml) | |
| IgY (chicken) | macrophages | Aves Labs (U-1010) | goat polyclonal | 1:200 (5 μg/ml) | |
| L-CAM/E-Cadherin Cadherin -1 (chicken) | epithelial cells | DSHB* (7D6) | mouse monoclonal | 1:5 (4 μg/ml) | Gallin et al., 1983 |
| leukocyte, activated, cell surface glycoprotein (chicken) | granulocytes | DSHB* (GRL-2) | mouse monoclonal | 1:5 (10 μg/ml) | Thomas et al., 1993 |
| MHC-II (chicken) | macrophages | DSHB* (2D5) | mouse monoclonal | 1:5 (10 μg/ml) | Uni et al., 1994 |
| Monocyte/ macrophage (chicken) | moncytes\macrophages activated microglia | Bio-Rad, Hercules, CA (KUL01/ MCA5770) | mouse monoclonal | 1:200 (2.5 μg/ml) | Mast et al., 1998 |
| Nestin (rat) | glial cells/schwann cells | DSHB* (rat-401) | mouse monoclonal | 1:5 (3.6 μg/ml) | Hockfield and McKay, 1985 |
| RALDH2 (chicken) | different cell types | Dr. Jody Summers, OUHSC, Oklahoma City, OK | rabbit polyclonal | 1:50 | Harper et al., 2016 |
| SSEA-1 antigen (mouse) | embryonic and germ cells | DSHB* (MC-480) | mouse monoclonal | 1:4.25 (4 μg/ml) | Solter and Knowles, 1978 |
| Tcr-ɣɗ-AF488 (chicken) | ɣδ T cells | Southern Biotech Birmingham, AL (8230-30) | mouse monoclonal | 1:100 (5 μg/ml) | Sowder et al., 1988 |
| Vimentin (chicken) | mesenchymal cells | DSHB* (AMF-17b) | mouse monoclonal | 1:10.5 (4 μg/ml) | Isaacs et al., 1989 |
Developmental Studies Hybridoma Bank, U Iowa, Iowa City, IA
2.8. Immunolabelling of Human Choroids.
After a 5 min rinse in TRIS-buffered saline (TBS; Roth, Karlsruhe, Germany) slides were incubated for 1 hr at RT in TBS containing 5% donkey serum (Sigma-Aldrich, Vienna, Austria), 1% BSA; (Sigma-Aldrich, Vienna, Austria), and 0.5% Triton X-100 (Merck, Darmstadt, Germany). Following a 5 min rinse, slides were incubated for single and double immunolabelling experiments with antisera generated against human epitopes as listed in Table 2 (all diluted in TBS, containing 1% BSA and 0.5% Triton X-100, 12 h at RT). After a rinse in TBS (three times 5 min) binding sites of primary antibodies were visualized by Alexa555- and/or Alexa488 tagged antisera (both 1:1000; raised in donkey; Invitrogen, Karlsruhe, Germany) in TBS, containing 1% BSA and 0.5% Triton X-100 (1 h at RT) followed by another rinse in TBS (three times 5 min). Whenever necessary, slides received an additional nuclear staining using DAPI. For that, slides were incubated 10 min (1:4000, stock 1 mg/ml, VWR, Vienna, Austria) followed by a rinse in TBS (three times 5 min) and slides were embedded in TBS-glycerol (1:1 at pH 8.6). Negative controls were performed by omission of the primary antibodies during incubation and revealed absence of immunoreactivity.
TABLE 2.
List of Primary Antibodies used for Immunolabeling Human Tissue
| Antigen (protein abbreviation) | Recognized Cells | Manufacturer (catalog #) | Host | Dilution |
|---|---|---|---|---|
| α-smooth muscle actin (ASMA) | smooth muscle cells | Sigma-Aldrich, Vienna, Austria | mouse monoclonal | 1:400 |
| Cluster of differentiation 31 (CD31) | endothelial cells | Invitrogen/Thermo, Fisher Vienna, Austria | mouse monoclonal | 1:50 |
| Cluster of differentiation 34 (CD34) | endothelial cells | Reliatech, WolfenbÜttel, Germay | mouse monoclonal | 1:200 |
| Cluster of differentiation 68 (CD68) | macrophages | Dako, Vienna, Austria | mouse monoclonal | 1:50 |
| Cluster of differentiation 146 (CD146) | endothelial cells | Invitrogen/Thermo Fisher, Vienna, Austria | mouse monoclonal | 1:500 |
| Desmin (DES) | pericytes | Santa Cruz, Heidelberg, Germany | goat polyclonal | 1:200 |
| Ionized calcium-binding adapter molecule 1 (IBA1) | microglia | Abcam, Cambridge, UK | goat polyclonal | 1:500 |
| Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) | lymphatic endothelial cells | Reliatech, WolfenbÜttel, Germay | mouse monoclonal | 1:800 |
| Protein-gene product 9.5 (PGP9.5) | pan neurons | Sigma-Aldrich, Vienna, Austria | guinea pig polyclonal | 1:400 |
| Retinaldehyde dehydrogenase 2 (RALDH2) | different cell types | Sigma-Aldrich, Vienna, Austria | rabbit polyclonal | 1:750 |
| Vimentin (VIM) | mesenchymal cells | Dianova, Hamburg, Germany | mouse monoclonal | 1:500 |
2.9. RALDH2/Bromodeoxyuridine Labeling.
Bromodeoxyuridine (BrdU; Sigma-Aldrich) was dissolved in PBS at a concentration of 10 mg/ml. Following 10 days of form deprivation, 6 chicks were injected intraperitoneally with BrdU (300 mg/kg) and occluders were removed. Three chicks were sacrificed 24 hrs later (1 day of recovery), and choroids were harvested and fixed in 4% PFA as described above. The remaining chicks were administered BrdU on day 2 and 3 of recovery, sacrificed on recovery day 4, and choroids isolated and fixed in 4% PFA. Double- immunolabelling for RALDH2 and BrdU in choroid whole mounts was carried out using rabbit anti- chick RALDH2 and anti-BrdU mouse monoclonal antibodies (described above) following prior digestion of choroids with proteinase K (5 μg/mL in PBS + 0.1% Tween 20, Amresco/VWR product number VWRV0706) and DNase I (10 U/μL Roche Diagnostics/Millipore Sigma) as previously described (Tkatchenko, 2006).
2.10. Microscopic Imaging of Chick Tissue.
For multiphoton microscopic analyses, choroids immunolabelled with anti-chick RALDH2 were viewed using a multiphoton microscope (Fluoview 1000MPE, Olympus America, Inc., Center Valley, PA, USA), and z-stack images were collected through the entire thickness of the choroid (36–144 μm) with each slice representing 3 μm. It has been previously shown that the chick choroid expands considerably in response to myopic defocus (Wallman, Wildsoet et al. 1995). Therefore the number of slices in z-stacks required to image the entire thickness of recovering choroids became substantially greater than that of control choroids after 1 day of recovery. RALDH2-positive cells were counted manually in each of two 512 × 512 pixel areas (corresponding to 510 × 510 μm) within each slice through the entire z-stack of each choroid from control and recovering eyes at each time point in a total of 12 – 48 slices/z-stack to determine the number and distribution of RALDH2 positive cells per choroidal region (0.0094 – 0.0374 mm3). RALDH2-positive cells were evaluated in 2 – 4 separate control and recovering choroids at each time point.
Due to overlapping fluorescence emission spectra of Alexa 488, Alexa 568, and DAPI, confocal microscopy was used for quantification of RALDH2 and BrdU immunolabelling in chick choroids. Confocal images of chick choroids were acquired using an Olympus Fluorview 1000 confocal microscope (Olympus America, Inc., Center Valley, PA, USA), by sequential scanning using a z-separation of 1 μm. RALDH2 and BrdU-positive cells were counted manually in 2 – 4 separate pixel areas (corresponding to 212 × 212 μm) to a depth of 14 – 33 μm in 3 separate choroids from control and treated eyes at each time point. The average number of labelled cells was calculated from all pixel areas counted in control and treated eyes at each time point.
2.11. Microscopic Imaging of Human Tissue.
In order to document single and double label immunohistochemistry in human tissue, a confocal laser-scanning unit (Axio ObserverZ1 attached to LSM710, Zeiss, Göttingen, Germany; ×20 dry or ×40 and ×60 oil immersion objective lenses, with numeric apertures 0.8, 1.30, and 1.4, respectively; Zeiss) was used. Sections were imaged using the appropriate filter settings for Alexa555 (555 nm excitation, coded red), Alexa488 (488 nm exicitation, coded green) and DAPI (405 nm excitation, coded blue), and additionally, when no Alexa488 tagged second antibody or DAPI was applied, background illumination was used whenever appropriate (488 nm excitation, coded green). Additionally, in some experiments the transmitted light detector of the confocal microscope was used. In all human specimens, the single optical section mode of the confocal microscope was chosen and representative micrographs were selected.
2.12. Statistics
Analyses between groups were made using a one-way ANOVA followed by a Bonferroni correction for multiple comparisons; analyses between pairs within a group were made using a paired or unpaired t-test (GraphPad Prism 5, La Jolla, CA). Results were considered significant with p-value ≤ 0.05.
3. RESULTS
3.1. RALDH2 Cells in Chick Choroidal Tissue
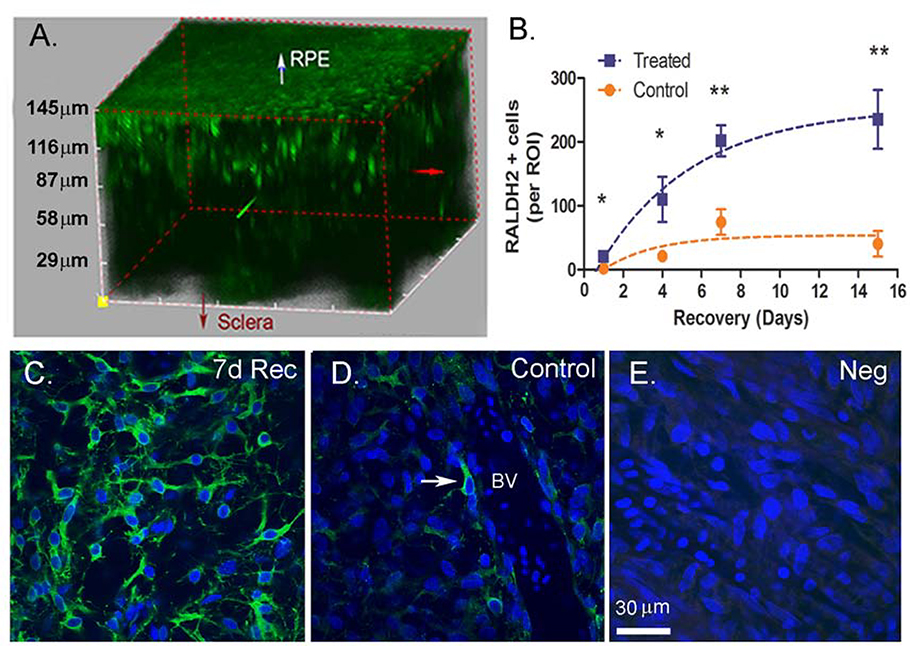
The animal model of myopia, monocular form deprivation myopia in the chick, was employed to induce accelerated axial elongation and myopia in treated eyes. Following 10 days of form deprivation, the occluders were removed to induce myopic defocus and subsequent recovery from the induced myopia. The number of RALDH2-immunopositive cells (RALDH+ cells) in chick choroids was evaluated throughout the entire thickness of the choroid by multiphoton microscopy following immunolabelling with anti-chick RALDH2 antibodies (Fig. 1A). RALDH2+ cells were more concentrated in the direction of the choriocapillaris, as described previously (Fig 1A) (Harper et al., 2016). This became most noticeable in recovering choroids as the choroids thickened substantially following four days of recovery (supplemental Video 1). In recovering choroids (Fig. 1B), the number of RALDH2+ cells per region of interest (ROI) increased significantly and almost linearly between 1 and 7 days of recovery and plateaued between 7 and 15 days compared to corresponding control eyes (d1: 20.75 ± 5.72 vs. 2.25 ± 1.44; d4: 110.00 ± 35.50 vs. 21.00 ± 3.85; d7: 201.75 ± 24.31 vs. 74.50 ± 19.93; d15:235.25 ± 46.14 vs. 40.50 ± 19.96; paired t-test p <0.05 at d1 and 4; p<0.01 at d7 and 15. Interestingly, a paralleled increase, although much smaller in magnitude, was observed in the number of RALDH2+ cells in contralateral control eyes which reached statistical significance on day 7 (day 1 vs day 7; p < 0.05, ANOVA with Bonferroni correction).
Figure 1.
Multiphoton images and quantification of RALDH2 expressing cells (green) in control and recovering choroids. (A) RALDH2 immunopositive cells in chick choroids following 7 days of recovery from induced myopia. (B) Average total RALDH2 cell number ( ± SEM) in control and treated choroids following 1 – 15 days of recovery. Results were calculated as total number of RALDH2-positive cells per each 510 μm x 510 μm x 3 μm (x, y, z, respectively) slice within each ROI [n = 2 regions of interest (ROI’s) for each of two animals in each group (4 total ROI’s / condition). (C, D) RALDH2-labeled cells were present as variably shaped cells throughout the extravascular choroidal stroma with some in close association with blood vessels (D, arrow). (E) Negative control (incubation in preimmune rabbit serum instead of primary antibody). 7d Rec, 7-day recovery; BV, blood vessel; Scale bar: 30 μm in (C–E). *p < 0.05; **p < 0.01 (paired t-test).
RALDH2+ cells were highly variable in morphology; some displayed round or spindle shaped cell bodies with cell diameters of 10 to15 μm and scarce cytoplasm, while others impressed with long, thin processes emerging from opposite poles of the cell (Fig. 1C). The majority of RALDH2+ cells were located in the choroidal stroma, a subpopulation of RALDH2+ cells were observed in close association with blood vessels and displayed a longitudinal orientation to the axis of the vascular wall (Fig. 1D, arrow).
3.2. RALDH2 Protein Expression and Activity during Recovery from Induced Myopia
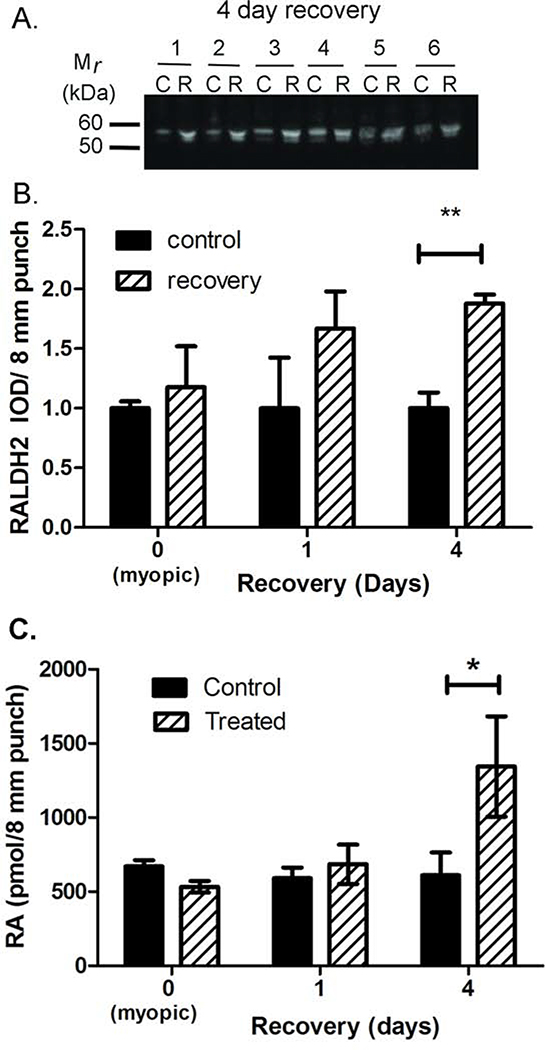
We have previously shown that protein levels of RALDH2 are significantly increased in the choroid during recovery from induced myopia, resulting in increased retinoic acid synthesis (Rada et al., 2012; Harper et al., 2016). In the present study, RALDH2 protein expression and activity were compared in choroids isolated from treated and control eyes following 10 days of form deprivation (= recovery day 0), 10 days of form deprivation followed by 1 day of recovery (= recovery day 1), and 10 days of form deprivation followed by 4 days of recovery (= recovery day 4) (Fig. 2). Following western blotting with anti-chick RALDH2 antibodies, RALDH2 was detected in control and recovering choroids as a ~55 kDa band. Additionally, an immunopositive band migrating at ~53 kDa was observed in choroid samples from control and recovering eyes (Fig. 2A). We suspect that this lower molecular weight band represents a partially degraded or alternatively spliced product of chick RALDH2, as we do not see this variant when chick RALDH2 is over-expressed in mammalian cell lines (data not shown). Quantification of the band intensities indicated that RALDH2 protein levels were significantly increased by 79.61 ± 11.46 %, (p < 0.01; paired t-test) in choroids following 4 days of recovery (n = 6) relative to controls [relative integrated optical densities (IOD) = 1.88 ± 0.075 in recovering eyes vs. 1.00 ± 0.132 in control eyes]. No statistically significant differences were detected in choroidal RALDH2 protein concentrations between control and treated eyes on day 0 or following 1 day of recovery, although RALDH2 protein concentrations exhibited an upward trend in recovering eyes following 1 day of recovery (Fig. 2B).
Figure 2.
Quantification of RALDH2 and retinoic acid in choroids. (A) RALDH2 was detected in tissue lysates from control (“C”) and four day recovering choroids (“R”) using anti-chick RALDH2 antibodies. RALDH2 was present as a ~55 kDa immuno-positive band together with a minor band migrating at ~53 kDa in control and recovering choroids at all time points examined. (B) Average RALDH2 protein concentration (± SEM) in choroids from myopic, recovering and control eyes was measured as the integrated optical density (IOD) of the 55 kDa immunopositive band in all samples (n = 4 – 6 choroids for each time point). (C) RALDH enzymatic activity was measured as the amount of NAD dependent retinoic acid synthesis using an HPLC/ spectrophotometric assay. Average RALDH enzymatic activity (±SEM) in choroids from myopic, recovering and control eyes (n = 4 – 6 choroids for each time point). **P <0.01, *P < 0.05 (paired t-test).
RALDH activity was examined in choroidal cytosol fractions using an atRA synthesis assay. To examine the kinetics of the assay, cytosol fractions from untreated choroids were incubated at 37°C with all-trans-retinaldehyde (25 μM) for 30 min as described previously (Harper et al., 2016). RALDH activity was measured in the choroids of control and treated eyes, following 10 days of form deprivation (0 days recovery), or after 1 day and 4 days of recovery (Fig. 2C). RALDH activity was significantly higher in choroids following four days of recovery (1344 ± 338.5 pmol/8 mm punch) compared to controls (611.1 ± 153.9 pmol/8 mm punch) (p < 0.05, paired t-test). No significant differences were detected in choroidal RALDH activity following 10 days of form deprivation or following 1 day of recovery.
3.3. Proliferation of RALDH2+ Cells
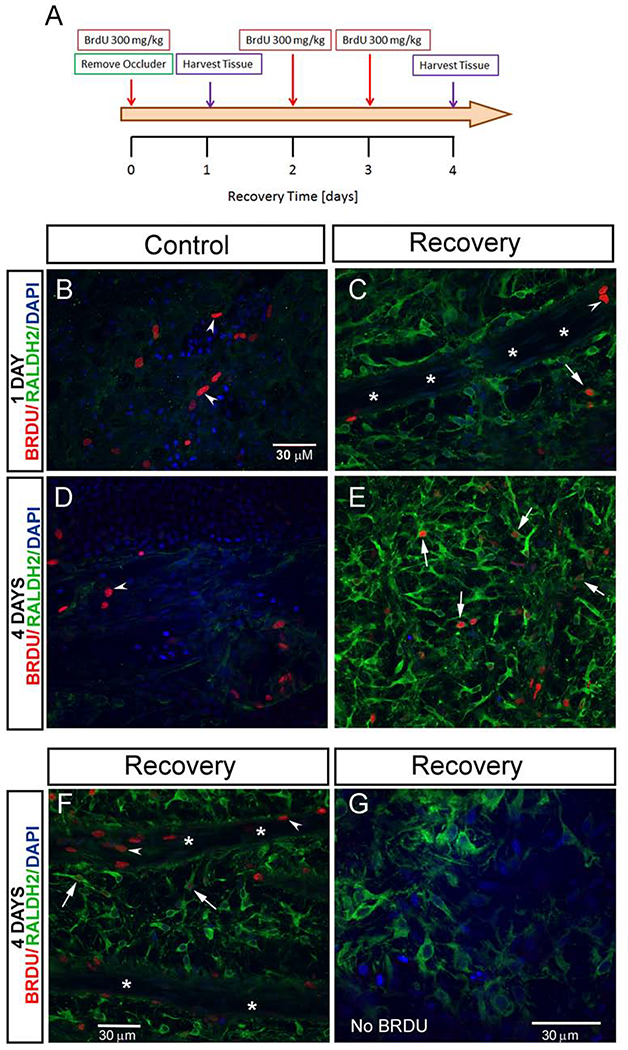
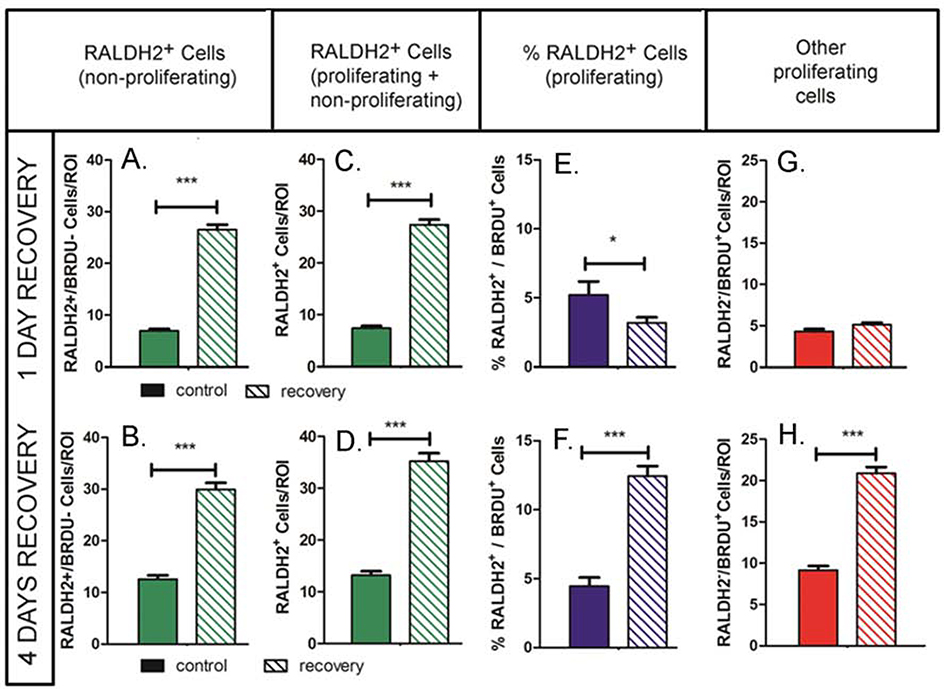
As we demonstrated here (Fig. 1) and in earlier reports (Harper et al., 2016), the number of RALDH2 immunopositive cells in the chick choroid increases during recovery from form deprivation myopia. In order to determine whether the increase in RALDH2+ cells observed during recovery is the result of cell proliferation, bromodeoxyuridine (BrdU) was administered to chicks on day 0 (following 10 days of form deprivation) and following 2 and 3 days of recovery to label cells in the S-phase of the cell cycle. During DNA synthesis, BrdU is incorporated into newly synthesized DNA, substituting for thymidine in DNA replication. Proliferating cells were then quantified using confocal microscopy using specific anti-BrdU antibodies. Following 1 day of recovery (and 1 day after BrdU administration) relatively few RALDH2+ cells in recovering eyes [n = 24.20 ± 0.69 cells ( ± SEM)] had incorporated BrdU (3.18± 0.40% of the RALDH2+ cell population) (Fig. 3C arrow, and Fig. 4D). Interestingly, the percentage of RALDH2+/BrdU+ cells in treated eyes following 1 day of recovery was significantly lower than that in control eyes (3.18 ± 0.40% in recovering eyes as compared with 5.20 ± 0.98% in control eyes, p < 0.05, Student’s t-test) (Fig. 4D), suggesting that the increase in choroidal RALDH2+ cells following 1 day of recovery was not due to cell proliferation. In contrast, a significant increase in the number of RALDH2+ /BrdU+ cells was observed in treated eyes following 4 days of recovery [n = 150.20 ± 10.60 cells ( ± SEM)] compared to controls [n = 13.71 ± 0.58 cells ( ± SEM)] which corresponded to 12.43 ± 0.73% of all RALDH2+ cells in recovering eyes as compared with 4.46 ± 0.63% in control eyes, p < 0.001, Student’s t-test) (Figs. 3E, F, arrows and Fig. 4D). These results indicate that cellular proliferation does contribute to the increase in RALDH2+ cells in recovering eyes between 1 and 4 days of recovery. We also observed a significant increase in proliferating cells that were RALDH2 negative in treated eyes following 4 days of recovery (20.89 ± 0.73 BrdU+ cells/ROI in recovering eyes as compared with 9.16 ± 0.50 BRDU+ cells/ROI in control eyes, p < 0.001, Student’s t-test) (Fig. 4C). BrdU-labelled nuclei of RALDH2-negative cells appeared as elongated nuclei located in the walls of blood vessels as well as round or indented nuclei within blood vessels and in the extravascular choroidal stroma (Fig. 3B–F, arrowheads).
Figure 3.
Proliferation of choroidal cells during recovery from myopia. (A) Scheme of the BrdU labeling experiment. BrdU was injected intraperitoneally into chicks following 10 days of form deprivation (day 0 recovery), followed by immediate removal of occluders to induce recovery. Additional BrdU injections were administered following 2 and 3 days of recovery. Tissue was harvested following 1 and 4 days of recovery (n = 3 chicks/ time point). After isolation of choroids, RALDH2 and BrdU immunolabeling was performed simultaneously as described in Materials and Methods and imaged using confocal microscopy. (B- F) Representative merged confocal images demonstrating RALDH2 and BrdU immunopositive cells. Intensely labelled RALDH2 positive cells (Alexa 488-labelled, green) were detected in choroids following 1 and 4 days of recovery (C,E,F,G). Proliferating RALDH2 positive cells were identified by the presence of BrdU labeling (Alexa 568-labelled, red) in the nucleus, some of which varied in intensity (C,E,F, arrows). Additional proliferating choroidal cells (RALDH2-negative) were identified by nuclear BrdU labelling (B,C,D, F, arrowheads). (G) No alexa-568 fluorescence was detected in tissues from chicks that were not administered BrdU.
Figure 4.
Quantitation of cell proliferation during recovery from myopia. (A, B) The average number of nonproliferating RALDH2 positive cells (RALDH2+/BRDU−) was significantly higher in recovering eyes following 1 and 4 days of recovery. (C, D) The total number of proliferating and non-proliferating RALDH2 positive cells (RALDH2+/BRDU− and RALDH2+/BRDU+) was significantly higher in recovering eyes following 1 and 4 days of recovery. (E, F) The percentage of choroidal RALDH2 positive cells that were proliferating (%RALDH2+/BRDU+) was significantly decreased following 1 day of recovery compared to contralateral controls, but was significantly increased in recovering eyes after 4 days of recovery. (G, H) The number of proliferating choroidal cells that were RALDH2 negative (RALDH2-/BrdU+) was significantly increased following 4 days of recovery. Results were calculated as the average of the total number of RALDH2-positive cells per each 212 μm x 212 μm x 1 μm (x, y, z, respectively) slice within each region of interest (ROI) (n = 2 – 4 ROI’s for n = 3 separate choroids/condition). Solid bars represent control choroids; hashed bars represent recovering choroids in A – H. 2 – 4 separate pixel areas were evaluated in 3 separate choroids from control and treated eyes at each time point. ***p < 0.001; *p < 0.05 (Student’s t-test).
3.4. Classification of Chick Choroidal RALDH2+ Cells
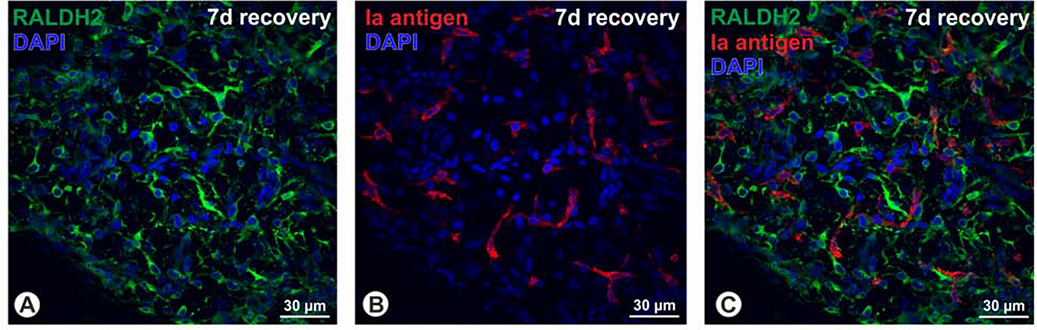
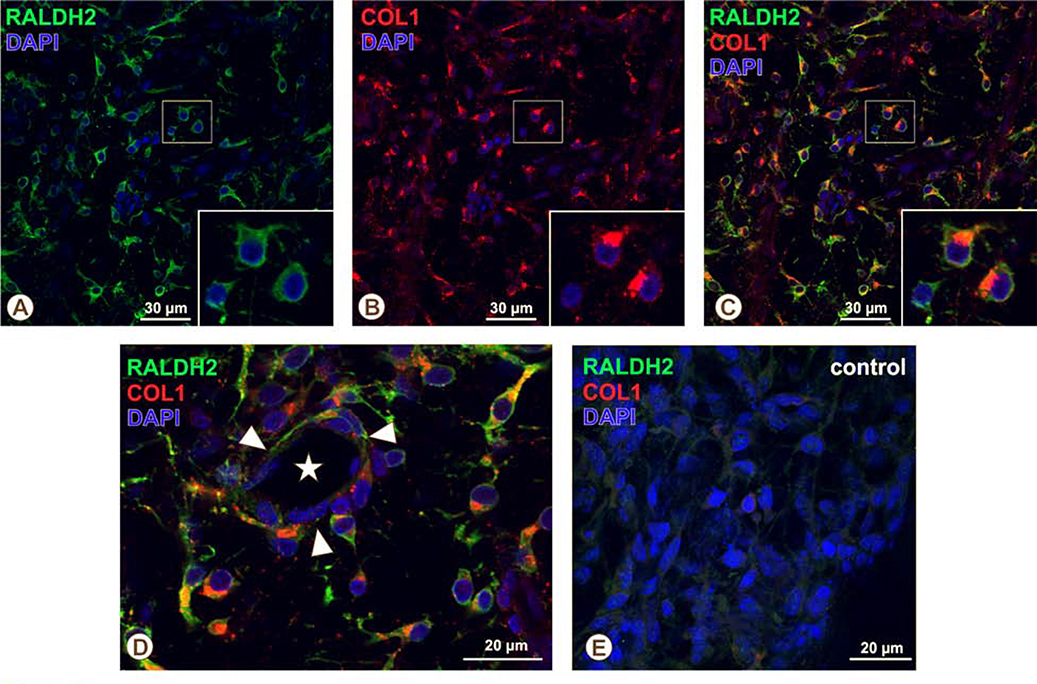
In an attempt to identify the cell type responsible for choroidal atRA synthesis, chick choroidal RALDH2+ cells were evaluated for their co-expression of a number of antigens (Table 1). The antigens listed in Table 1 were selected because: 1) they have been demonstrated on cell types known to be present in the choroid, 2) they have been previously demonstrated on RALDH2+ cells in other tissues, and 3) specific antibodies were available that have been confirmed by our lab or by other laboratories to react with chicken antigens. RALDH2+ cells were negative for the macrophage/dendritic cell markers KuL01, MHC-II, and IgY (Supplemental Figure 1). RALDH2+ cells were also negative for the MHC-II “Ia” antigen despite an abundant number of Ia-positive cells throughout the choroidal stroma that resembled RALDH2+ cells in size, shape and distribution (Fig. 5). Additionally, RALDH2+ cells did not co-localize with TCRγδ, CD5, or GRL-2 indicating they were not of hematopoietic origin (Supplemental Figure 1). RALDH2+ cells also did not co-localize with the pericyte markers desmin and nestin, E-cadherin (an epithelial marker), the SSEA antigen (germ/stem cell antigen), or the mesenchymal/fibroblast cell markers, vimentin and FSP1 (Supplmental Figure 2). Our further immunohistochemical analyses indicated that nearly all RALDH2+ choroidal cells contained intracellular vesicles immunopositive for pro-collagen Type I (Col1) (Fig. 6 A – D and supplemental Video 2). These RALDH2+ / Col1+ cells were distributed within extravascular spaces throughout the choroidal stroma as well as in close association with blood vessels (Fig. 6D). RALDH2+ cells lacking Col1-containing vesicles (RALDH2+/Col1−) were not detected.
Figure 5.
Immunohistochemical localization of choroidal macrophages and RALDH2 positive cells in recovery. Chick choroids were isolated after 7 days of recovery from induced myopia. The distribution of choroidal macrophages and RALDH2 positive cells was evaluated in choroidal whole mounts from recovering eyes by double immunolabeling with mouse anti-Ia antigen antibodies and rabbit anti-RALDH2 antibodies, respectively, as described in Materials and Methods and imaged using confocal microscopy. (A) Numerous, variably shaped RALDH2 positive cells (Alexa 488-labelled, green) are present throughout the choroid. (B) Ia-antigen positive macrophages/dendritic cells (Alexa 568-labelled, red) are distributed throughout the choroidal stroma and are similar in size and shape to RALDH2 positive cells, but are distinct from RALDH2 positive cells, as there is no co-localization of green and red labelling (C). Images are representative of results from four separate immunolabelling experiments using recovering eyes from four separate chicks. Bar = 30 μm in A – C.
Figure 6.
Immunohistochemical localization of pro-collagen type I and RALDH2 in recovering choroidal cells. Chick choroids were isolated after 7 days of recovery from induced myopia. The distribution of pro-collagen type I and RALDH2 expressing cells was evaluated in choroidal whole mounts from 7 day recovering eyes by double immunolabeling with mouse anti-procollagen type I antibodies and rabbit anti-RALDH2 antibodies, respectively, as described in Materials and Methods and imaged using confocal microscopy. (A-C) The majority of RALDH2-positive cells (Alexa 488-labelled, green) also have clusters of pro-collagen type I-containing intracellular vesicles (Alexa 568-labelled, red). Insets (A-C): Magnified region of choroid (boxed area) demonstrating two of three RALDH2-positive cells that also contain pro-collagen type 1-positive intracellular vesicles. (D) Higher magnification of an immunolabeled choroidal whole mount demonstrating close association of double-labelled cells with the outer wall of a choroidal blood vessel (arrowhead; asterisk depicts the lumen of the vessel) as well as their presence in the extravascular choroidal stroma. (E) Negative control of a choroidal whole mount from a 7 day recovering eye incubated with mouse IgG and pre-immune rabbit serum instead of primary antibodies (NEG). Images are representative of results from four separate immunolabelling experiments using recovering eyes from four separate chicks.
3.5. Classification of Human Choroidal RALDH2+ Cells.
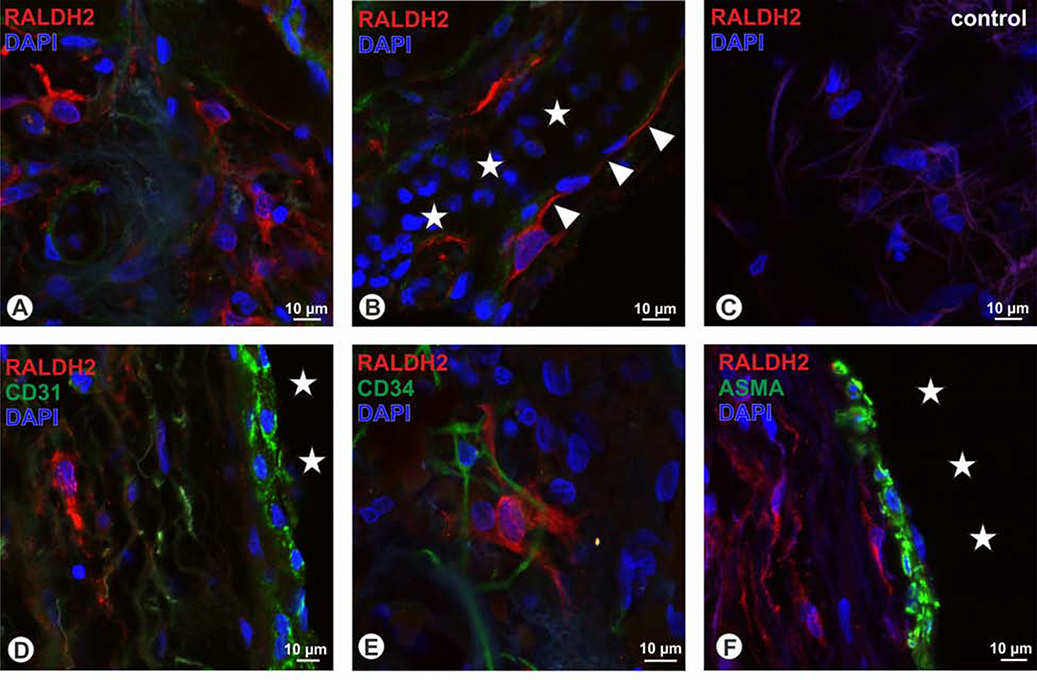
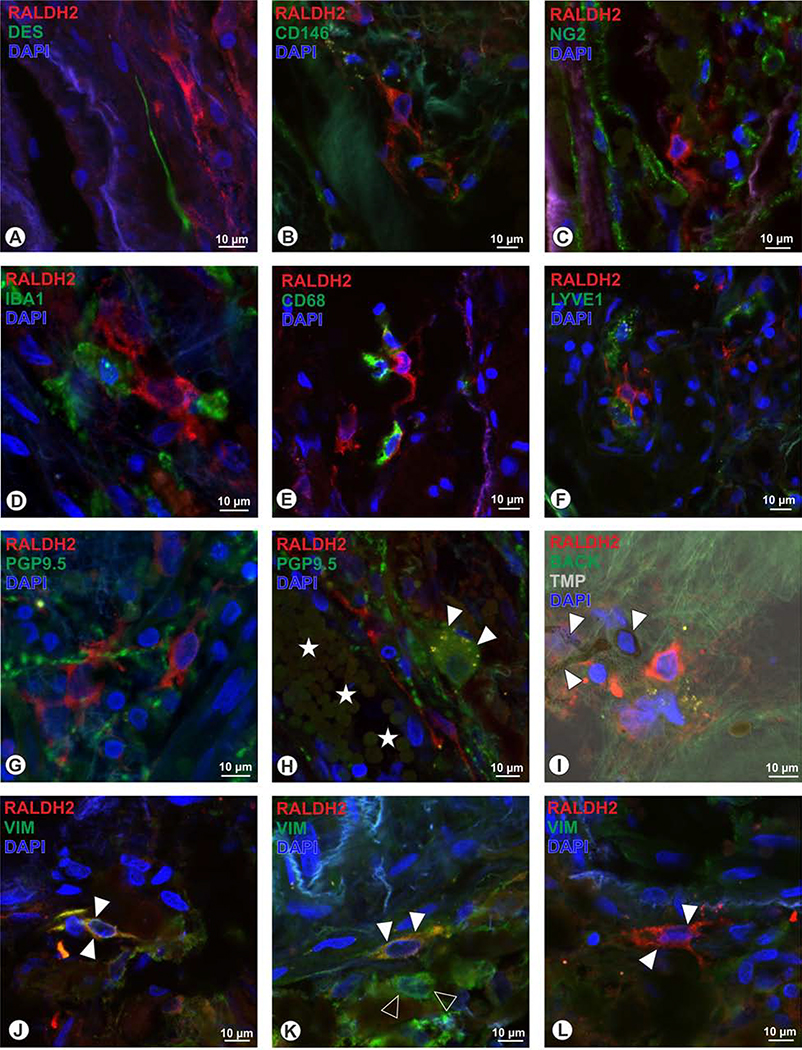
Within the human choroid, two types of RALDH2+ cells were discernible: cells located in the stroma that were rather small with cell diameters of around 10 μm and displayed short and more circumferential arranged processes (Fig. 7A). Others were located adjacent to choroidal blood vessels, these were larger (around 20 μm) and displayed scarce cytoplasm, with long processes that could be followed over up to 70μm (Fig. 7B). To determine whether these cells represent aberrant vascular structures, we used the endothelial cells markers CD31, CD34, and ASMA, but saw no co-localization (Figs. 7 D–F). While the vascular association of the RALDH2+ could also indicate pericytes, we tested for desmin, NG2 and CD146, but again an overlap with RALDH2 was not detectable (Figs. 8 A–C). Stromal RALDH+ cells by size and shape were reminiscent of microglia or macrophages, however, the microglia marker IBA1 (Fig. 8D), as well as the macrophage markers CD-68 and LYVE1 (Schroedl et al., 2008) (Figs. 8E–F) were not expressed in RALDH2+ cells. Further, also the pan-neuronal marker PGP9.5 was absent in RALDH2-immunoreactive cells (Fig. 8G) and RALDH2-immunoreactivity was also not present in intrinsic choroidal neurons (Fig. 8H). Combining fluorescence microscopy and the trans-illumination mode of the confocal microscope revealed that the RALDH2+ cells do not represent melanocytes, since melanin granules were absent in RALDH2-immunoreactive cells (Fig. 8I). When applying antiserum against the intermediate filament vimentin, a co-localization with stromal RALDH2+ cells was detected (Fig. 8J). However, not all cells co-localized for vimentin (Fig. 8K), and vimentin+ cells were observed that were lacking RALDH2 (Fig. 8L).
Figure 7.
In human, RALDH2+ positive cells (red) were detected in the choroidal stroma (A) as well as adjacent to choroidal blood vesses (asterisks) displaying long and thin processes (arrowheads). RALDH2 immunoreactivity was absent in corresponding negative controls (C). RALDH2+ cells were not co-localized with the vascular endothelial markers CD31 (D), CD34 (green E), and were also lacking ASMA (F). Asterisks in D and F display choroidal blood vessels; blue in A to F = DAPI
Figure 8.
Human RALDH2+ cells (red) could not be identified as pericytes, as the pericyte markers desmin (A, green), CD146 (B), and NG2 (C), revealed no co-localization. Further, the microglia marker IBA1 (D), and the macrophage markers CD68 (E) and LYVE1 (F) were absent in RALDH2+ cells. RALDH2+ cells do not belong to neuronal cell populations since PGP9.5 (G) revelaed no overlap, and further intrinsic choroidal neurons were lacking RALDH2 (H, arrowheads; yellow dots within the neuron represent lipofuscin granules; asterisks: choroidal blood vessel). When applying the trans-ilumination mode in the confocal microscope (TMP, I), melanocytes (arrowheads) could be identified by the presence of melanin granules, but were not co-localized for RALDH2. J to L: the intermediate filament vimentin (VIM) was co-localized in some RALDH2+ cells (arrowheads in J, K), while also other cells were detected, that displayed VIM only (open arrowheads, K) and further a subpopulation of RALDH2+ cells were lacking VIM immunoreactivity (arrowheads in L).
4. Discussion
All trans retinoic acid (atRA) is an attractive candidate as the final molecular signal in the retina-choroid-scleral signaling cascade since choroidal synthesis and concentrations of atRA are modulated in response to visual stimuli and atRA is a potent inhibitor of scleral proteoglycan synthesis at endogenous concentrations present in recovering choroids (4 × 10−9 M to 3 × 10−8 M) (Rada et al., 2012). Tissue concentrations of atRA are tightly controlled by the activities of synthesizing enzymes, retinol dehydrogenase (RDH), the retinaldehyde dehydrogenases RALDH1, RALDH2, and RALDH3 (also known as Aldh1A1, Aldh1A2, and Aldh1A3, respectively), and the atRA metabolizing enzyme CYP26, a member of the cytochrome P450 family (Napoli 2011). Additionally CYP1B1 may also contribute to atRA synthesis in the chick embryo (Chambers et al., 2007). Of all the atRA metabolizing enzymes examined, RALDH2 was the only enzyme that was altered during the recovery from experimentally induced myopia. RALDH2 mRNA, protein and catalytic activity have been shown to be increased in the choroids of chick eyes during the recovery from myopia, a condition characterized by decreased scleral proteoglycan synthesis and a deceleration in ocular elongation rates (Wallman and Adams, 1987; Rada et al., 1992; Rada et al., 2012; Harper et al., 2016). It is therefore of much interest to understand the regulation of choroidal RALDH2 protein expression as this information may provide insight into the regulation of choroidal atRA concentrations which is linked to scleral remodeling, as well as provide the basis of new strategies for the treatment of myopia.
In agreement with our previous reports, this study demonstrates that the number of RALDH2 positive cells increases substantially in the choroid within 1 – 15 days of recovery from induced myopia (↑9 – 55 fold, compared with the number of RALDH2 positive cells in choroids of contralateral control eyes). This increase in the number of RALDH2 positive cells coincides with significant increases in choroidal RALDH2 protein concentration and atRA synthesis by four days of recovery.
Quantification of BrdU incorporation into RALDH2-positive cells indicated that cellular proliferation was partially responsible for the increase in RALDH2 positive cells in recovering choroids observed following four days of recovery. However, the percentage of proliferating RALDH2 positive cells was significantly decreased in recovering eyes as compared with contralateral controls following only one day of recovery, indicating that the initial increase in the number of RALDH2 positive cells observed after one day of recovery is not due to cellular proliferation, but rather from increased protein expression in resident choroidal cells or possibly by migration of RALDH2 positive cells from adjacent tissues or the vasculature. This latter possibility is unlikely based on the rather uniform distribution of RALDH2+ cells within the choroid and that no RALDH2+ cells were ever detected within blood vessels. In contrast, the percentage of BRDU labeled RALDH2 positive choroidal cells was ≈ 3X higher in 4 day recovering choroids, suggesting that choroidal concentrations of RALDH2 are partially controlled by proliferation of RALDH2 positive cells with longer periods of recovery. However, the population of proliferating RALDH2 positive cells at 4 days of recovery represented only ≈ 12% of the total number of RALDH2 positive cells, indicating that mechanisms other than cell proliferation contribute to the increase in RALDH2 positive cells observed following 4 days of recovery. Moreover, RALDH2 positive cells were always observed scattered individually throughout the choroidal stroma - not distributed as clusters, as would be expected if they proliferated from a parent cell.
We also observed a significant increase in proliferating choroidal cells that did not express RALDH2 following 4 days of recovery. Based on their location and morphology, these BrdU-positive, RALDH2-negative cells are most likely endothelial cells and hematopoietic cells. These results suggest that recovery from induced myopia is associated with endothelial cell and blood cell proliferation within the choroid. Studies by Wallman et al., (Wallman et al., 1995) and Liang et al., (2004) have shown that the chick choroid undergoes a rapid and dramatic increase in thickness in response to myopic defocus. This visually driven thickening of the chick choroid results, at least in part, from swelling of the choroidal lacunae (Junghans et al., 1999; Rada et al., 2001), accumulation of hyaluronan in the choroidal stroma (Rada et al., 2010) and from extravascular tissue edema as a result of increased vascular permeability (Pendrak et al., 2000; Rada and Palmer, 2007). It should be noted that choroidal thickening also coincides with our observed increases in choroidal RALDH2 positive cells, RALDH2 protein concentration and atRA synthesis (Harper et al., 2016). Whether proliferation of RALDH2+ cells, and/or retinoic acid synthesis is directly responsible for the choroidal thickness is not clear that this point. Based on our studies on the location of RALDH2+ cells during recovery (Harper et al., 2016), we do not think that the increase in RALDH2 cell number is the direct cause of increased choroidal thickness, since the location of the majority of RALDH2+ cells is located in the proximal choroid, whereas the choroidal expansion is occurring more distally (near the scleral side). It is possible, however, that increased vascular permeability and increased hyaluronan synthesis (associated with choroidal thickening as described above) may be regulated, directly or indirectly, by retinoic acid, which in turn is regulated by RALDH2+ cell proliferation.
Interestingly, a large, transient increase in choroidal blood flow has been shown to precede changes in choroidal thickness following 7 – 30 hrs of recovery from form deprivation myopia (Fitzgerald et al., 2002). Therefore, it is plausible that the increased number of proliferating endothelial cells and blood cells observed in the present study might represent a manifestation of the choroidal vascular changes reported to occur early during the recovery process.
We carried out a series of immunohistochemical experiments to characterize RALDH2-positive cells in recovering and control chick choroids. RALDH2 positive cells were negative for monocyte, macrophage and dendritic cell- associated antigens including KULO 1, MHC class II, IgY, and Ia antigens. Interestingly, monoclonal antibodies specific for avian Ia antigen labeled a large population of choroidal cells that resembled macrophages and resident dendritic cells previously described in the mouse and human choroid (Kumar et al., 2014; McLeod et al; 2016). The Ia antigen-specific monoclonal antibody used in this study was raised against immunogens present on dendritic cells and macrophages in thymic cell suspensions. The presence of this large population of Ia antigen positive macrophages in the choroid is suggestive of similarities between the avian choroid and thymus gland. RALDH2-expressing cells in the chick choroid were also negative for T and B lymphocyte markers (CD5 and Tcr-γδ), a stem cell antigen (SSEA), and did not contain intermediate filaments associated with pericytes (desmin), neurons (nestin), and mesenchymal cells (vimentin and FSP1). RALDH2-positive cells were also negative for an epithelial cell marker (cadherin-1).
Virtually all RALDH2-positive cells in the chick choroid also contained procollagen type-1 (Col1), located within clusters of intracellular vesicles. The combination of negative immunolabeling results and positive labeling for Col1 indicates that choroidal RALDH2+ cells are most likely a subpopulation of connective tissue fibroblasts.
The presence of RALDH2+ cells in the human choroid of similar morphology and phenotype as observed in the chick suggests that these cells may also modulate their expression of RALDH2 or their proliferative state in response to visual stimuli to modulate choroidal atRA synthesis and scleral remodeling. As this is the first screening in human donor tissue, sources currently available did not yet allow for the investigation of adolescent tissue in order to compare the situation of the growing eye in the animal model; nevertheless, the presence of RALDH2+ cells in human choroid could be confirmed, and further also allowed for a pursuing classification of these cells. Similar to that observed in the chick, RALDH2+ cells in the human choroid were non-neuronal, non-muscular, non-pericyte, non-vascular, and non-macrophage and additionally were not melanocytes. While a specific fibroblast marker is hitherto not available, and moreover different tissue specific phenotypes of fibroblasts are also known (Nagalingam et al., 2019) the presence of vimentin in some (but not all) human RALDH2+ cells suggested mesenchymal origin (Barishak, 1992).
In a variety of tissues and disease processes, resident tissue fibroblasts or stromal cells have been shown to increase their synthesis of RALDH2 and atRA in response to inflammatory and injury-induced signals. Following ischemic stroke, the number of perivascular stromal cells (PSCs) expressing RALDH1, RALDH2, and collagen type 1 increase in ischemic areas of the brain (Kelly et al., 2016). Similarly, spinal cord injury results in the active migration and proliferation of RALDH2 positive cells from the arachnoid into the damaged area (Mey et al., 2005; Kern et al., 2007). Organ wide RALDH2 expression has also shown to be rapidly induced in the endocardium and epicardium of the zebrafish heart following ventricular injury or inflammatory stress (Kikuchi et al., 2011). Most recently, RALDH2 was upregulated in renal fibroblasts surrounding proximal tubules during their transition to myofibroblasts in several models of kidney injury and may stimulate tubular regeneration (Nakamura et al., 2019). Together, these studies demonstrate the dynamic nature of tissue fibroblasts and that RALDH2 expression is one aspect of their phenotypic variability in response to changes in their microenvironment.
The molecular mechanisms which stimulate RALDH2+ cell proliferation, migration or RALDH2 protein expression in the current and aforementioned studies remain unknown. Clues may come from the fact that RALDH2 was also induced in the zebrafish endocardium by lipopolysaccharide (LPS) injection (Kikuchi et al., 2011), suggesting that inflammatory cytokines or other bioactive molecules are released in response to the injury/stimulus which directly or indirectly result in local synthesis of retinoic acid, either through increased RALDH2 protein expression, RALDH2+ cell proliferation and/or RALDH2+ cell migration. In the choroid, we predict that these bioactive molecules are emanating from the RPE or choriocapillaris since: 1) the RPE and choriocapillaris are adjacent to the neural retina, where the visual stimuli are initially detected and transduced into chemical signals, and 2) RALDH2+ choroidal fibroblasts are located in highest concentration in the proximal choroid, near the RPE.
We speculate that myopic defocus, as a result of prior form deprivation, stimulates the synthesis and/or release of chemical mediators in the proximal choroid that directly or indirectly, stimulate RALDH2 protein expression and cellular proliferation of RALDH2+ choroidal stromal fibroblasts as well as the proliferation of other cell types in the choroid. Considering the multidimensional nature of the choroidal response during recovery, we predict that these chemical mediators act on multiple cell types in the choroid to initiate changes in choroidal thickness, blood flow, and permeability, associated with the choroidal response to myopic defocus. It is therefore of much interest to identify the upstream signals that mediate choroidal RALDH2 fibroblast proliferation and RALDH2 expression during visually guided eye growth.
4.1. Study Limitations –
The use of young chicks for experimental studies has the advantages of rapid induction of myopia, a robust recovery response, and low costs of animals and animal care. One drawback, however, is the relative lack of chicken specific antibodies for immunological characterization of cellular antigens. Therefore, it is highly probable that additional cellular markers for RALDH2+ choroidal fibroblasts will be identified if new chicken-specific antibodies become available, or are developed in our laboratory. We attempted to circumvent this limitation with the inclusion of studies on RALDH2+ cells in the human choroid using additional antibodies, which agree with and compliment the results obtained in chicks. Additionally, all immunohistochemistry on chick choroids was carried out using choroid whole mounts, in order to preserve tissue morphology and assess immunolabelling throughout the posterior pole, where the major changes in ocular growth have been demonstrated to occur (Rada et al., 1994). However, due to the limited depth of focus using confocal microscopy with the Olympus Fluoview1000 microscope, we could only assess the distribution of double labelled cells at a depth of 33 microns or less from the RPE. For control choroids, this depth was more than adequate for surveying the entire thickness of the choroid, but was not deep enough to assess the entire thickness of recovering choroids. Since the majority of RALDH2+ choroidal cells are located in the proximal choroid, the use of confocal microscopy was appropriate to clearly identify double and triple labelled RALDH2+ choroidal cells.
For studies assessing choroidal cell proliferation, BrdU was administered 1 – 3 times to chicks via intraperitoneal injection. Since choroidal cells were in various stages of the cell cycle at the time of BrdU administration, BrdU may not have been incorporated to all proliferating cells prior to being metabolized, thereby underestimating the number of proliferating cells at each time point.
Functional experiments specifically targeting choroidal RALDH2 will elucidate the role of the RALDH2+ cells in the myopia signalling cascade and the process of visually guided ocular growth.
Supplementary Material
Highlights.
Choroidal retinoic acid synthesis plays a role in postnatal ocular growth control.
Retinoic acid synthesis is regulated by retinaldehyde dehydrogenase 2.
Retinaldehyde dehydrogenase 2 is expressed by a novel choroidal cell type.
Proliferation of this novel cell type regulates choroidal retinoic acid synthesis.
Acknowledgements
The authors would like to acknowledge the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, for providing many of the monoclonal antibodies used in this study. Research reported in this publication was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P 30 GM122744 (JAS), NIH R01 grant EY09391 (JAS), Austrian National Bank Funds under grant number17617 (FS), and Research Support Funds of Paracelsus Medical University under grant number R-17/03/092-SCK (AKE)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barishak YR (1992). Embryology of the eye and its adnexae. Dev. Ophthalmol. 24, 1–142. [PubMed] [Google Scholar]
- Buch H, Vinding T and Nielsen NV (2001). Prevalence and causes of visual impairment according to World Health Organization and United States criteria in an aged, urban Scandinavian population: the Copenhagen City Eye Study. Ophthalmology 108, 2347–2357. [DOI] [PubMed] [Google Scholar]
- Chambers D, Wilson L, Maden M and Lumsden A (2007). RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development 134, 1369–1383. [DOI] [PubMed] [Google Scholar]
- Chang WY, Winegarden NA, Paraiso JP, Stevens ML and Westwood JT (2000) Visualization of nascent transcripts on Drosophila polytene chromosomes using BrUTP incorporation. BioTechniques 29, 934–936. [DOI] [PubMed] [Google Scholar]
- Danto SI and Fischman DA (1984) Immunocytochemical analysis of intermediate filaments in embryonic heart cells with monoclonal antibodies to desmin. J. Cell Biol. 98, 2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo KM, Moiseyev G, Nikolaeva O, Sandell LL, Trainor PA and Ma JX (2011). RDH10 is the primary enzyme responsible for the first step of embryonic Vitamin A metabolism and retinoic acid synthesis. Dev. Biol. 357, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald ME, Wildsoet CF and Reiner A (2002). Temporal relationship of choroidal blood flow and thickness changes during recovery from form deprivation myopia in chicks. Exp. Eye Res. 74, 561–570. [DOI] [PubMed] [Google Scholar]
- Foellmer HG, Kawahara K, Madri JA, Furthmayr H, Timpl R and Tuderman L (1983) A monoclonal antibody specific for the amino terminal cleavage site of procollagen type I. Eur. J. Biochem. 1341, 183–189. [DOI] [PubMed] [Google Scholar]
- Gallin WJ, Edelman GM, and Cunningham BA (1983) Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc. Natl. Acad. Sci. U.S.A. 80, 1038–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillemot FP, Oliver PD, Peault BM, and Le Douarin NM (1984) Cells expressing Ia antigens in the avian thymus. J. Exp. Med. 160,1803–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AR, Wang X, Moiseyev G, Ma JX and Summers JA (2016). Postnatal chick choroids exhibit increased retinaldehyde dehydrogenase activity during recovery from form deprivation induced myopia. Invest.Ophthalmol. Vis. Sci. 57, 4886–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper AR, Wiechmann AF, Moiseyev G, Ma JX and Summers JA (2015). Identification of active retinaldehyde dehydrogenase isoforms in the postnatal human eye. PLoS One 10, e0122008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockfield S, and McKay RD (1985) Identification of major cell classes in the developing mammalian nervous system. J. Neurosci. 5, 3310–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W and Kuenzel WJ (1984). Retinal-image degradation produces ocular enlargement in chicks.Invest. Ophthalmol. Vis. Sci. 25, 652–659. [PubMed] [Google Scholar]
- Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, Wong TY, Naduvilath TJ and Resnikoff S (2016). Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050.Ophthalmology 123, 1036–1042. [DOI] [PubMed] [Google Scholar]
- Huang HM, Chang DS and Wu PC (2015). The association between near work activities and myopia in children-a systematic review and meta-analysis. PLoS One 10, e0140419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs WB, Cook RK, Van Atta JC, Redmond CM, and Fulton AB (1989). Assembly of vimentin in cultured cells varies with cell type. J. Biol. Chem. 264, 17953–17960. [PubMed] [Google Scholar]
- Junghans BM, Crewther SG, Liang H and Crewther DP (1999). A role for choroidal lymphatics during recovery from form deprivation myopia? Optom. Vis. Sci. 76, 796–803. [DOI] [PubMed] [Google Scholar]
- Kane MA and Napoli JL (2010). Quantification of endogenous retinoids. Methods Mol. Biol. 652,1–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KK, MacPherson AM, Grewal H, Strnad F, Jones JW, Yu J, Pierzchalski K, Kane MA, Herson PS and Siegenthaler JA (2016). Col1a1+ perivascular cells in the brain are a source of retinoic acid following stroke. BMC Neurosci. 17, 49 https://bmcneurosci.biomedcentral.com/articles/10.1186/s12868-016-0284-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern J, Schrage K, Koopmans GC, Joosten EA, McCaffery P and Mey J (2007). Characterization of retinaldehyde dehydrogenase-2 induction in NG2-positive glia after spinal cord contusion injury. Int. J. Dev. Neurosci. 25, 7–16. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G and Poss KD (2011). Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Dev. Cell 20, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskinen R, Gobel TW, Tregaskes CA, Young JR, and Vainio O (1998). The structure of avian CD5 implies a conserved function. J. Immunol. 160, 4943–4950. [PubMed] [Google Scholar]
- Kumar A, Zhao L, Fariss RN, McMenamin PG, and Wong WT (2014). Vascular associations and dynamic process motility in perivascular myeloid cells of the mouse choroid: implications for function and senescent change. Invest. Ophthalmol. Vis. Sci. 55, 1787–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang H, Crewther SG, Crewther DP, and Junghans BM (2004). Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest. Ophthalmol. Vis. Sci. 45, 2463–2474. [DOI] [PubMed] [Google Scholar]
- Mast J, Goddeeris BM, Peeters K, Vandesande F, and Berghman LR (1998) Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet Immunol Immunopathol 61, 343–357. [DOI] [PubMed] [Google Scholar]
- McCaffery P, Lee MO Wagner MA Sladek NE and Drager UC (1992). Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development 115, 371–382. [DOI] [PubMed] [Google Scholar]
- McFadden SA, Howlett MH and Mertz JR (2004). Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res 44, 643–653. [DOI] [PubMed] [Google Scholar]
- McLeod DS, Bhutto I, Edwards MM, Silver RE, Seddon JM and Lutty GA (2016). Distribution and Quantification of Choroidal Macrophages in Human Eyes With Age-Related Macular Degeneration. Invest. Ophthalmol. Vis. Sci.57, 5843–5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertz JR and Wallman J (2000). Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp. Eye Res. 70, 519–527. [DOI] [PubMed] [Google Scholar]
- Mey J, Morassutti J, D., Brook G, Liu RH, Zhang YP, Koopmans G and McCaffery P (2005). Retinoic acid synthesis by a population of NG2-positive cells in the injured spinal cord. Eur. J. Neurosci 21, 1555–1568. [DOI] [PubMed] [Google Scholar]
- Nagalingam RS, Al-Hattab DS and Czubryt MP (2019). What’s in a name? On fibroblast phenotype and nomenclature 1. Can. J. Physiol. Pharmacol. 97, 493–497. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Sato Y, Kitai Y, Wajima S, Yamamoto S, Oguchi A, Yamada R, Kaneko K, Kondo M, Uchino E, Tsuchida J, Hirano K, Sharma K, Kohno K and Yanagita M (2019). Myofibroblasts acquire retinoic acid-producing ability during fibroblast-to-myofibroblast transition following kidney injury. Kidney Int 95, 526–539. [DOI] [PubMed] [Google Scholar]
- Napoli JL (2012). Physiological insights into all-trans-retinoic acid biosynthesis. Biochim. Biophys. Acta.1821,152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton TT and Rada JA (1995). Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res 35, 1271–1281. [DOI] [PubMed] [Google Scholar]
- Pendrak K, G., Papastergiou I, Lin T, Laties AM, and Stone RA (2000). Choroidal vascular permeability in visually regulated eye growth. Exp. Eye Res. 70, 629–637. [DOI] [PubMed] [Google Scholar]
- Rada JA, Hollaway LY, Li N and Napoli J (2012). Identification of RALDH2 as a Visually Regulated Retinoic Acid Synthesizing Enzyme in the Chick Choroid. Invest. Ophthalmol. Vis. Sci. 53, 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada JA, Huang Yand Rada KG (2001). Identification of choroidal ovotransferrin as a potential ocular growth regulator. Curr. Eye Res. 22, 121–132. [DOI] [PubMed] [Google Scholar]
- Rada JA, Matthews AL and Brenza H (1994). Regional proteoglycan synthesis in the sclera of experimentally myopic chicks. Exp. Eye Res. 59, 747–760. [DOI] [PubMed] [Google Scholar]
- Rada JA, McFarland AL, Cornuet PK and Hassell JR (1992). Proteoglycan synthesis by scleral chondrocytes is modulated by a vision dependent mechanism. Curr. Eye Res. 11, 767–782. [DOI] [PubMed] [Google Scholar]
- Rada JA, Nickla DL and Troilo D (2000). Decreased proteoglycan synthesis associated with form deprivation myopia in mature primate eyes. Invest. Ophthalmol. Vis. Sci. 41, 2050–2058. [PubMed] [Google Scholar]
- Rada JA and Palmer L (2007). Choroidal regulation of scleral glycosaminoglycan synthesis during recovery from induced myopia. Invest. Ophthalmol. Vis. Sci. 48, 2957–2966. [DOI] [PubMed] [Google Scholar]
- Rada JA, Thoft RA and Hassell JR (1991). Increased aggrecan (cartilage proteoglycan) production in the sclera of myopic chicks. Dev. Biol. 147, 303–312. [DOI] [PubMed] [Google Scholar]
- Rada JA, Wiechmann AF, Hollaway LR, Baggenstoss BA and Weigel PH (2010). Increased hyaluronan synthase-2 mRNA expression and hyaluronan accumulation with choroidal thickening: response during recovery from induced myopia. Invest. Ophthalmol. Vis. Sci. 51, 6172–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroedl F, Brehmer A, Neuhuber WL, Kruse FE, May CA and Cursiefen C (2008) The normal human choroid is endowed with a significant number of lymphatic vessel endothelial hyaluronate receptor 1 (LYVE-1)-positive macrophages. Invest. Ophthalmol. Vis. Sci.49, 5222–5229. [DOI] [PubMed] [Google Scholar]
- Seko Y, Shimizu M and Tokoro T (1998). Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic. Res. 30, 361–367. [DOI] [PubMed] [Google Scholar]
- Solter D, Knowles BB (1978). Monoclonal antibody defining a stage-specific mouse embryonic antigen (SSEA-1). Proc. Natl. Acad. Sci. U.S.A. 75, 5565–5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowder JT, Chen C, Ager LL, Chan MM, Cooper MD (1988) A large subpopulation of avian T cells express a homologue of the mammalian Tγ/δ receptor. J Exp Med 167, 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas JL, Pourquie O, Coltey M, Vaigot P, and Le Douarin NM (1993) Identification in the chicken of grl1 and grl2: two granule proteins expressed on the surface of activated leukocytes. Exp. Cell Res. 204, 156–166. [DOI] [PubMed] [Google Scholar]
- Tkatchenko AV (2006). Whole-mount BrdU staining of proliferating cells by DNase treatment: application to postnatal mammalian retina. Biotechniques 40, 29–30, 32. [DOI] [PubMed] [Google Scholar]
- Troilo D, Nickla DL, Mertz JR and Summers Rada JA (2006). Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest. Ophthalmol. Vis. Sci. 47, 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troilo D, Smith EL 3rd, Nickla DL, Ashby R, Tkatchenko AV, Ostrin LA, Gawne TJ, Pardue MT, Summers JA, Kee CS, Schroedl F, Wahl S and Jones L (2019). IMI - Report on Experimental Models of Emmetropization and Myopia. Invest. Ophthalmol. Vis. Sci. 60, M31–M88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uni Z, Pratt WD, Miller MM, O’Connell PH, and Schat KA (1994) Syngeneic lysis of reticuloendotheliosis virus-transformed cell lines transfected with Marek’s disease virus genes by virus-specific cytotoxic T cells. Vet. Immunol. Immunopathol. 441, 57–69. [DOI] [PubMed] [Google Scholar]
- Wallman J and Adams JI (1987). Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res 27, 1139–1163. [DOI] [PubMed] [Google Scholar]
- Wallman J, Turkel J and Trachtman J (1978). Extreme myopia produced by modest change in early visual experience. Science 201, 1249–1251. [DOI] [PubMed] [Google Scholar]
- Wallman J, Wildsoet C, Xu A, Gottlieb MD, Nickla D, Marran LL, Krebs W and Christensen AM (1995). Moving the retina: choroidal modulation of refractive state. Vision Res 35, 37–50. [DOI] [PubMed] [Google Scholar]
- Wallman J and Winawer J (2004). Homeostasis of eye growth and the question of myopia. Neuron 43, 447–468. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.