Abstract
Background and aims
We quantified the effects of smoking and smoking cessation on carotid artery atherosclerosis and wall thickness in two unique cohorts of smokers making a quit attempt.
Methods
Our primary analysis included 726 smokers making a quit attempt in a randomized clinical trial with long-term follow-up. Our secondary analysis included 889 smokers making a quit attempt in a subsequent trial. Participants underwent carotid artery ultrasonography at baseline and up to 3 subsequent visits. Primary outcomes were changes in carotid plaque score and intima media-thickness (IMT). We calculated a smoking burden score (SBS) that reflected the number of visits in which participants reported smoking after the quit attempt. Multivariable regression examined relations between SBS and carotid artery outcomes with adjustments for cardiovascular disease risk factors.
Results
In the primary analysis, participants were mean (standard deviation) 46.1 (10.3) years old (57.9% female) and smoked 21.1 (8.9) cigarettes per day (CPD). After a median of 7 years, lower SBS predicted less increase in carotid plaque score (Χ2=13.0, p=0.012). SBS independently predicted change in carotid plaque score (p=0.007; SBS 0 vs 4) as did baseline CPD (p=0.024) and age (p<0.0001). SBS did not affect carotid IMT change. In the secondary analysis, increasing SBS was associated with increased likelihood of new plaques over 3 years among participants that smoked ≥15 CPD, (Χ2=6.51, p=0.011).
Conclusions
Smoking cessation is associated with less progression of carotid plaque, but not IMT. Salutary associations of smoking cessation with carotid plaque progression are related to degree of abstinence.
Keywords: Carotid arteries, Cerebrovascular disease, Smoking, Atherosclerosis
Introduction
Cigarette smoking is a powerful risk factor for atherosclerosis and atherosclerotic cardiovascular disease (ASCVD) events.1–4 Smoking cessation at any age is one of the most important health interventions for reducing risks of ASCVD, cancer, and mortality.3–5,6–8 Most of the data regarding the benefits of smoking cessation on ASCVD risk have come from observational cohorts that did not include detailed information on smoking heaviness and time since quitting, so those studies were not able to quantify the timing and magnitude of the effects of smoking cessation on atherosclerosis burden.5–8 Furthermore, today’s smokers smoke fewer cigarettes per day (CPD) and have different comorbidities: they weigh more than smokers from three or more decades ago, use more lipid-lowering and antihypertensive medications, and may gain more weight after a successful quit attempt, which may mask or reduce the effects of smoking and smoking cessation on atherosclerosis.9,10,11
The purpose of this study was to quantify the effects of continued smoking and smoking cessation on carotid artery atherosclerosis and intima-media thickness (IMT), independent of ASCVD risk factors and therapies, in two unique cohorts of contemporary smokers making a quit attempt.
Materials and methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.12,13 These studies were approved the University of Wisconsin Health Sciences Institutional Review Board; all participants provided written informed consent.
Design and participants
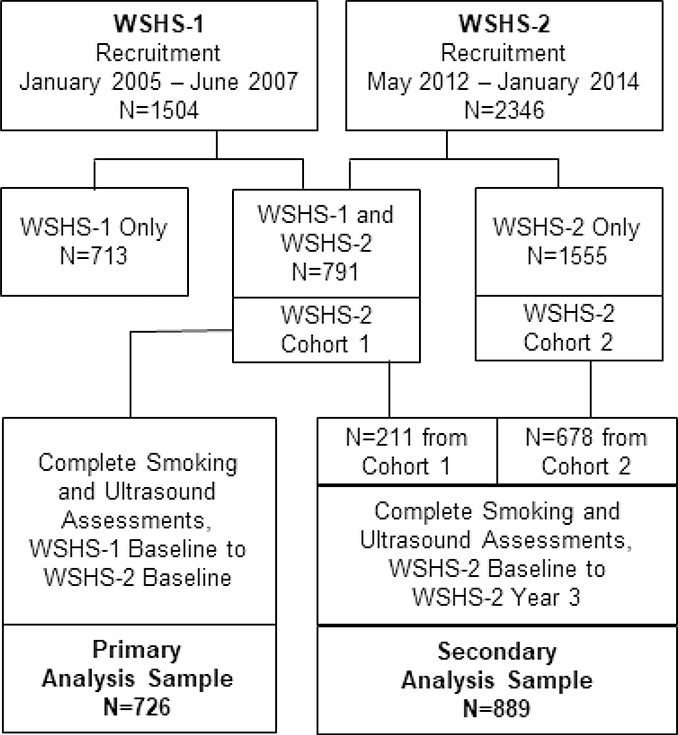
The Wisconsin Smokers Health Study (WSHS-1, clinicaltrials.gov registration NCT00332644) was a randomized, double-blind, placebo-controlled trial that evaluated the efficacy of five smoking cessation pharmacotherapies and the natural history of continued smoking and smoking cessation on ASCVD risk and other health outcomes; its methods have been reported previously.12,14–17 The Wisconsin Smokers Health Study (WSHS-2, clinicaltrials.gov registration number NCT01553084) was a subsequent randomized trial that evaluated the efficacy of three smoking cessation pharmacotherapies and also evaluated the natural history of continued smoking and smoking cessation on ASCVD risk and other health outcomes; its methods also have been reported previously.13,18 WSHS-1 recruited 1504 current smokers, of which 791 also participated in WSHS-2. WSHS-2 comprised 2346 smokers, the 791 from WSHS-1 and 1555 new recruits.
Our primary analysis was performed on participants in WSHS-1 from their baseline visit (January 2005–June 2007) through the baseline visit of WSHS-2 (May 2012–January 2014). This included 726 participants with complete smoking status and carotid ultrasound data over a median of 7 years (range 5–9 years). Our secondary analysis was performed on the 889 participants in WSHS-2 with complete smoking status and carotid ultrasound data from the baseline visit through study completion (May 2012–October 2016; 3 years of follow-up each).
Study procedures
In both studies, the baseline visit included assessment of sociodemographic variables, smoking status/history, medication use, physical and laboratory measurements, and carotid ultrasonography to measure intima-media thickness (a measure of arterial injury) and carotid plaques score (a measure of atherosclerosis burden). Participants also received smoking cessation counseling. Each participant was characterized as abstinent at year 1, 2, and 3 visits after their target quit day if they reported no smoking in the preceding year and had biochemical verification of abstinence (expired carbon monoxide <6 ppm).19 Repeat tests were performed at year 3. Study details for WSHS-1 and WSHS-2 are in prior reports.12,13 Enrollment and participant flow are shown in Figure 1.
Figure 1.
Participant flow diagram.
WSHS=Wisconsin Smokers Health Study
Carotid ultrasonography
Digital images of the right and left common carotid, bifurcation, and internal carotid artery segments were obtained from 3 angles by sonographers certified to perform the imaging protocol using one of two identical ultrasound systems with a high-resolution linear array transducer (CV70, L10–5 transducer, Siemens Medical Solutions, Mountain View, WA) (Supplemental Figure 1).16,20,21 All studies used the same imaging preset (dynamic range 70 dB, grayscale map L, depth 4.0 cm). Longitudinal and cross-sectional images of all segments were evaluated for the presence or absence of plaques defined as focal wall thickness ≥1.5 mm or 50% thicker than neighboring wall thickness. The carotid plaque score (0–12) was defined as the number of carotid artery segments that contained plaque (i.e., near and far walls of the right and left common, bifurcation, and internal segments). For carotid plaque score, intra-reader reproducibility was excellent (kappa=0.83, 95% confidence interval [CI] 0.70–0.96).20 The mean far wall IMT of the distal 1 cm of each common carotid artery was measured in triplicate from 3 complementary angles at the time of the ECG R-wave by a single reader, blinded to smoking status and other participant characteristics, using a semi-automated border detection program (Arterial Health Package software, Siemens Medical Solutions, Malvern, PA). For IMT measurement reproducibility, the intra-class correlation coefficient was excellent at 0.99.20
Statistical analysis
Analyses were conducted using SAS/STAT statistical software (version 13.2, SAS Institute, Cary, NC). For each participant, a smoking burden score (SBS) was computed based on yearly exhaled carbon monoxide-confirmed abstinence as well as daily (year 1) or monthly (years 2 and 3) reports of smoking assessed at multiple visits and phone follow-ups via timeline follow-back methods.22 When a smokers’ smoking status changed within year 1, we counted them as abstinent (smoking status=0) if self-reported smoking was <120 days and they had carbon monoxide-confirmed abstinence at year 1, otherwise year 1 smoking status=1 (smoking). In years 2 and 3, if a participant smoked <4 months and carbon monoxide-confirmed abstinence at the year’s end indicated abstinence, their smoking status=0 for that year; otherwise smoking status=1. Each participant’s SBS was computed as the sum of 0 or 1 scores for each year. For the primary analyses, the SBS ranged from 0 to 4; for the secondary WSHS-2 analyses, it ranged from 0 to 3.
For the primary analyses, the outcome variables were changes in carotid plaque score and IMT defined as final year minus baseline year. The distribution of carotid plaque change scores was right-skewed and ranged from −1 to 7. Carotid plaque change scores were evaluated in four ordered categories: 0=no increase in plaque number; 1=increase of 1; 2=increase of 2; and 3=increase of ≥3 plaques. The carotid plaque change score outcome was analyzed in a series of ordinal logistic regression models (SAS Proc Logistic; cumulative logit model). For carotid IMT, the change score was distributed normally with a range from −0.460 to 0.809 mm and was analyzed using linear regression (SAS Proc GLM).
Categorical variables included SBS, sex (0=female; 1=male), race (0=non-White; 1=White), study site (0=Milwaukee; 1=Madison), diabetes diagnosis, and three variables for use of antihypertensive, lipid-lowering, and diabetes medications. Each medication use variable was coded as 0=no use at any visit (reference category); 1=use at one visit, 2=use at two visits; and 3=use at all three visits. Continuous variables included age, baseline CPD, and changes (final year minus baseline year) for the following variables: high-density lipoprotein cholesterol (HDL-C), triglycerides, low-density lipoprotein cholesterol (LDL-C), systolic blood pressure, hemoglobin A1C, and weight. In addition, the interaction of SBS and baseline CPD was tested.
Our sequential modeling strategy started with a univariable model that tested the influence of SBS on changes in each carotid plaque score and IMT (model 0). Next (model 1), we added age, sex, race, and site to model 0. Site was dropped from further modeling since its estimate was not statistically significant (p <0.05). Model 2 added HDL-C, triglycerides, LDL-C, and systolic blood pressure to model 1. Model 3 added antihypertensive and lipid-lowering medication use; model 4 added a diagnosis of diabetes mellitus, hemoglobin A1C, and use of antiglycemic medication; model 5 added weight gain from the baseline visit to the final year; and model 6 added baseline CPD and the interaction of CPD*SBS. We then created a distilled multivariable model using the Hosmer and Lemeshow best-fitting model strategy. For the secondary analyses, we also performed an a posteriori analysis that evaluated the joint effect of SBS and baseline CPD (median split, <15 vs ≥ 15) on the binary change in carotid plaque score using a Chi-Square test.
Results
Participant characteristics (Table 1)
Table 1.
Participant characteristics
| Total WSHS-1 sample (N=1504) | Primary analysis sample (N=726) | Primary analysis sample at final visit (N=726) | |
|---|---|---|---|
| Mean (standard deviation) or N (%) | Mean (standard deviation) or N (%) | Mean (standard deviation) or N (%) | |
| Age, years | 44.7 (11.1) | 46.1 (10.3) | 53.6 (10.3) |
| Female sex, N (%) | 876 (58.2%) | 422 (58.1%) | 422 (58.1%) |
| Race | |||
| White, N (%) | 1258 (83.9%) | 608 (83.8%) | 607 (83.6%) |
| African-American, N (%) | 204 (13.6%) | 100 (13.8%) | 105 (14.5%) |
| Other, N (%) | 42 (2.7%) | 18 (2.4%) | 14 (1.9%) |
| Study site | |||
| Madison, N (%) | 883 (58.7%) | 430 (58.6%) | 430 (58.6%) |
| Milwaukee, N (%) | 621 (41.3%) | 304 (41.4%) | 304 (41.4%) |
| Cigarettes/day | 21.4 (8.9) | 20.9 (8.7) | 9.3 (10.0) |
| Smoking heaviness, pack-years | 29.4 (20.4) | 29.7 (19.2) | 15.9 (19.2) |
| Weight, kg | 83.7 (20.5) | 84.0 (20.0) | 87.6 (22.0) |
| Body-mass index, kg/m2 | 29.0 (6.5) | 29.1 (6.3) | 30.3 (6.8) |
| Systolic blood pressure, mmHg | 119.4 (14.4) | 119.3 (14.0) | 127.0 (16.3) |
| Antihypertensive medication, N (%) | 73 (4.9%) | 43 (6.0%) | 242 (34.7%) |
| Total cholesterol, mg/dL | 184.1 (35.5) | 185.7 (34.9) | 200.9 (40.9) |
| High-density lipoprotein cholesterol, mg/dL | 42.0 (13.6) | 42.6 (14.0) | 52.5 (17.3) |
| *Triglycerides, mg/dL | 120.9 (1.7) | 123.4 (1.7) | 128.1 (1.7) |
| Low-density lipoprotein cholesterol, mg/dL | 119.0 (30.6) | 119.4 (29.7) | 118.4 (35.8) |
| Lipid-lowering medication, N (%) | 60 (4.0%) | 36 (5.0%) | 170 (24.4%) |
| Diagnosis of diabetes mellitus, N (%) | 91 (6.1%) | 40 (5.6%) | 94 (13.8%) |
| Hemoglobin A1C, % | 5.57 (0.6) | 5.59 (0.6) | 5.9 (0.8) |
| Antiglycemic medication, N (%) | 35 (2.3%) | 17 (2.3%) | 58 (8.3%) |
| Carotid intima-media thickness, mm | 0.67 (0.15) | 0.64 (0.16) | 0.73 (0.18) |
| Carotid plaque presence, N (%) | 805 (55.1%) | 399 (55.0%) | 516 (71.1%) |
| Carotid plaque score, plaques | 1.6 (2.1) | 1.6 (2.0) | 2.6 (2.6) |
Geometric mean (standard deviation, log10 triglycerides)
Key characteristics of participants in the primary analysis sample were very similar to the full WSHS-1 sample with the exception of age (46.1 vs. 44.7 years) and use of antihypertensive medications (6.0% vs. 4.9%). Participants in the primary analysis were 57.9% female, 83.7% White, and weighed a mean (standard deviation) of 84.1 (20.1) kg. They smoked about 21.1 (8.9) CPD for 30.0 (19.7) pack-years. Distributions of carotid plaque scores and their changes are showed in Supplemental Figure 2.
Baseline associates of carotid plaque score and IMT in the primary analysis
Baseline associations of carotid plaque score are showed in Supplemental Table 1. The strongest associations for plaque scores were with age and pack-years. Baseline associations of carotid IMT are in showed Supplemental Table 2. The strongest associations for carotid IMT also were with age and pack-years.
Longitudinal smoking burden scores and weight changes in the primary analysis
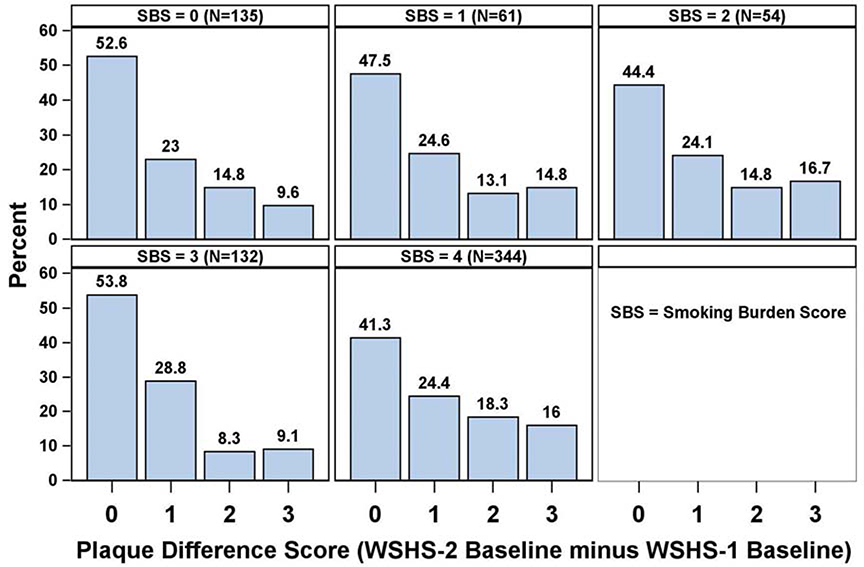
Among the 726 participants, 135 (18.6%) had an SBS=0 (i.e., abstinent at each visit after baseline); 61 (8.4%) had an SBS=1; 54 (7.4%) had an SBS=2; 132 (18.2%) had an SBS=3; and 344 (47.4%) had an SBS=4 (i.e., smoked at each visit). Weight gain decreased by increasing levels of SBS: 8.7 (8.7) kg for SBS=0; 7.7 (10.2) kg for SBS=1; 2.6 (9.3) kg for SBS=2; 4.9 (10.8) kg for SBS=3; and 0.62 (10.6) kg for SBS=4.
Longitudinal changes in carotid plaque score in the primary analysis
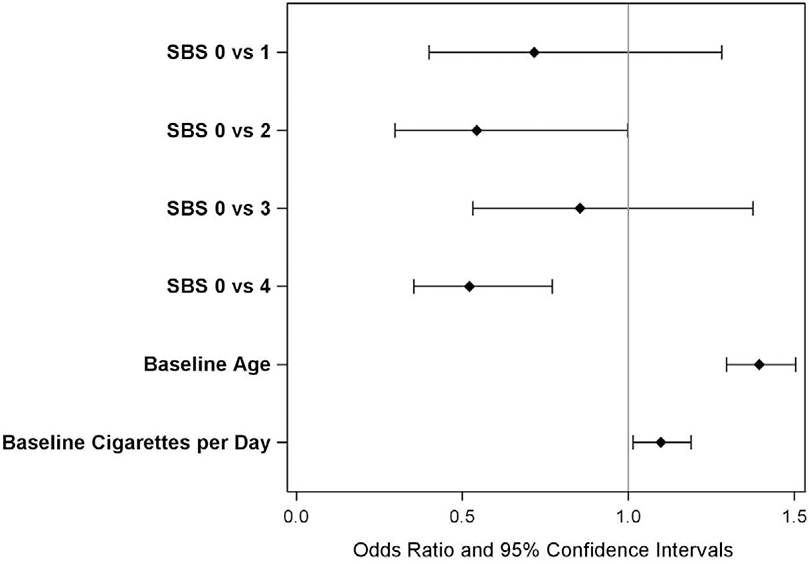
The mean change in carotid plaque score was 1.03 (1.25). Distributions of changes in carotid plaque scores by SBS are shown in Figure 2. The SBS predicted change in carotid plaque score (Χ2=13.0, p=0.011). Additional univariate predictors of changes in carotid plaque score were age, use of blood pressure medication, and change in weight (p <0.01 for each) as well as sex, use of lipid-lowering medication, and use of antiglycemic medication (p <0.05) but not race, study site, or changes in lipids, blood pressures, or hemoglobin A1C. In the best-fitting multivariable model, SBS was a statistically significant, independent predictor of change in carotid plaque score (p=0.007). Comparisons of individual SBS scores revealed significant differences between SBS score 0 vs. 2 (OR=0.544, 95%CI=0.297–0.998) and between SBS score 0 vs. 4 (OR=0.522, 95%CI=0.354–0.770), but not for SBS score 3 vs. 0 (OR=0.854, 95%CI=0.531–1.375). Other significant multivariable predictors were baseline CPD (per 5 CPD; OR=1.098, 95%CI=1.014–1.188, p=0.024) and age (per 5 years; OR=1.394, 95%CI=1.295–1.504, p <0.001) (Figure 3).
Figure 2.
Changes in carotid plaque scores by smoking burden score in the primary analysis.
SBS=smoking burden score; WSHS=Wisconsin Smokers Health Study
Figure 3.
Odds Ratios and 95% confidence intervals for predictors of changes in carotid plaque score in the best-fitting multivariable model (primary analysis).
Age per 5 years; cigarettes per day is in 5 per day. SBS=smoking burden score.
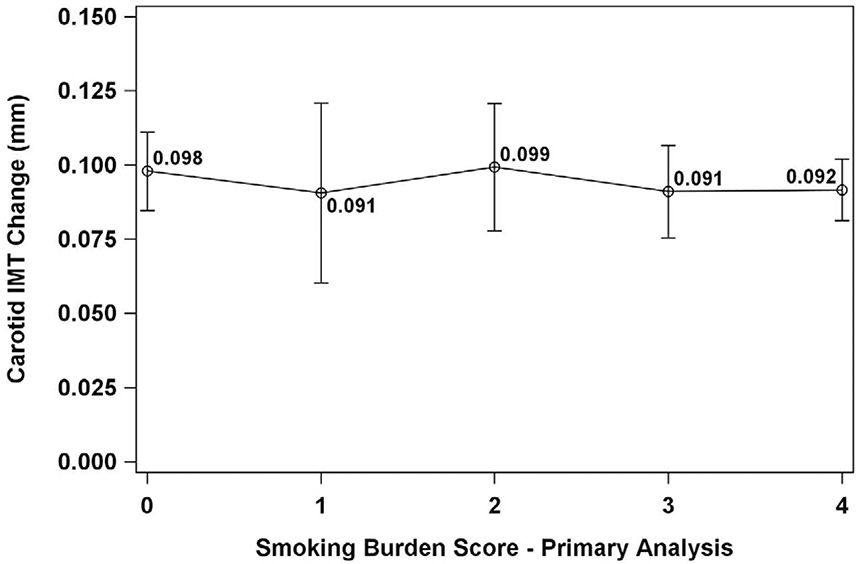
Longitudinal changes in carotid IMT in the primary analysis
The mean change in carotid IMT was 0.093 (0.094) mm. SBS did not predict change in carotid IMT (p=0.940) in any models. No significant pairwise differences were identified between changes in carotid IMT and any SBS score (all p>0.96) (Figure 4).
Figure 4.
Changes in carotid intima-media thickness by smoking burden score (primary analysis).
IMT=intima-media thickness
Secondary analysis
Participants in the secondary analysis smoked fewer cigarettes than those in the primary analysis (16.6 [8.3] vs. 21.1 [8.9] CPD, respectively) for fewer pack-years (25.6 [17.7] vs. 30.0 [19.7]). They were older (50.2 [11.4] vs. 46.1 [10.3] years), had higher HDL-C levels (50.1 [17.4] vs. 42.3 [14.0]), and were much more likely to use lipid-lowering medications (20.6% vs. 5.1%). 143 (16.1%) had SBS=0; 52 (5.9%) had SBS=1; 89 (10.0%) had BS=2; and 605 (68.1%) had SBS=3. Participants who were abstinent for the three years (SBS=0) gained an average of 6.3 (9.6) kg compared to participants with SBS score >0: 4.9 (9.4) kg for SBS=1; 3.0 (7.3) kg for SBS=2; and 0.6 kg (7.6) for SBS=3. Consistent with the primary analysis, the strongest associations of carotid plaque scores and IMT were with age and pack-years (Supplemental Tables 2 and 3).
The mean change in carotid plaque score in the secondary analysis sample was 0.64 (0.99), which was much lower than in the primary analysis. In the overall sample, SBS did not predict change in carotid plaque score (p=0.251). However, because participants in the secondary analysis sample had much less smoking exposure than those in the primary sample, we conducted additional analyses to determine whether change in plaque score differed as a function of smoking heaviness using a median split analysis. Indeed, among heavier smokers (≥ the median of 15 CPD), a linear increase in SBS was associated with an increased likelihood of new plaques across scores of 0 (34.1%) to 3 (47.2%) (Χ2 6.51, p=0.011), but not among those who smoked <15 CPD at baseline.
The mean change in carotid IMT in the secondary analysis was 0.063 (0.080) mm, which also was much lower than in the primary analysis. SBS did not predict change in carotid IMT (p=0.242); baseline smoking heaviness did not modify this relation.
Discussion
We studied the longitudinal relations of smoking burden with carotid artery plaque and IMT progression in two prospective studies of the effects of smoking and smoking cessation in daily smokers. Strengths of our analysis include its prospective longitudinal design, rigorous methods for serially quantifying cigarette smoking exposure over several years, contemporary smoker cohorts, and rigorous ultrasound methods that included using the same ultrasound instrumentation and scanning protocol at both sites and the same reader for all studies. Our major finding was that smoking cessation was associated with less progression of carotid plaque, but not IMT. The effects of smoking cessation on carotid atherosclerosis were related to the degree of abstinence and persisted after adjusting for baseline smoking heaviness and ASCVD risk factors. This association was not affected significantly by potential confounders such as weight gain or changes in medications.
We had intended for the primary analysis to cover the time period from the first visit of WSHS-1 through the final visit of WSHS-2, but because of attrition, only a very small number of participants were continuously abstinent from the first to last assessable study visits. Therefore, we chose the next longest observation period for our primary analysis (from the first visit in WSHS-1 through the first visit in WSHS-2). Because the WSHS-1 and WSHS-2 study populations differed substantially on important patient characteristics such as CPD (higher in WSHS-1), smoking pack-years (higher in WSHS-1), and age (higher in WSHS-2) and because duration of follow-up was shorter in WSHS-2, we performed a secondary analysis limited to participants in that study.
In the primary analysis, greater burden of continued smoking was associated with increased progression of carotid plaque, indicating atherosclerosis progression and increased ASCVD risk.20,22,23 The best-fitting multivariable model showed that smoking burden predicted carotid plaque progression, as did baseline smoking heaviness and increasing age. Smoking burden was not related to carotid IMT progression. These findings demonstrate that smoking cessation reduces progression of atherosclerosis and ASCVD risk in contemporary smokers, despite weight gain.
That carotid IMT progression was unrelated to smoking cessation may reflect the stronger influence of weight on wall thickness than on plaque formation.15,16,24,27 Indeed, other investigators have demonstrated that body mass is a key determinant of arterial and left ventricular wall thickness, but not atherosclerosis, consistent with the observation that carotid IMT in plaque-free arterial segments like the common carotid artery represents a multifactorial arterial injury rather than atherosclerosis.26,27 In an earlier report from WSHS-1 with shorter follow-up, we showed that early changes in carotid IMT after a quit attempt were related to weight gain.16 Also, in a previous report from WSHS-2, we demonstrated that reductions in common carotid artery echogenicity among successful quitters also was related to weight gain.18 These findings emphasize that carotid artery IMT and carotid plaque cannot be used interchangeably or referred to as “atherosclerosis”.25
Participants in the secondary analysis smoked fewer CPD for fewer pack-years and had a shorter follow-up period, which likely is why we did not detect a significant association between SBS and change in carotid plaque score in that analysis. However, we identified a significant relation between CPD and likelihood of new plaque formation in participants who smoked ≥ 15 CPD at baseline. This finding suggests that smoking cessation can affect plaque formation in a relatively short 3-year timeframe among heavier smokers. Although several cross-sectional studies have demonstrated that current and former smoking is associated with increased carotid IMT and plaque burden,15,20,28,29 those studies typically analyzed relations of smoking status variables that spanned lifetime exposure at a single time point. The current study suggests that the effects of cessation on new plaque formation can occur fairly rapidly.
Our study is novel because it prospectively followed two cohorts of contemporary cigarette smokers with detailed assessments of smoking habits, comorbidities, and use of medications from the time they made a quit attempt and we characterized the influences of smoking cessation versus continued smoking on two arterial measures. Baseline smoking heaviness was associated with carotid plaque score at baseline and carotid plaque progression over a median of seven years. Our longitudinal index of smoking burden independently predicted greater progression of carotid atherosclerosis, demonstrating the harm of continued smoking and the benefit of smoking cessation on atherosclerosis and ASCVD risk. It is especially important that in our primary analysis, the benefit of smoking cessation on progression of atherosclerosis was not mitigated by weight gain during follow-up, which was greater among those who smoked less.
Limitations
The major limitation of our study was drop out, as it is common among participants in smoking cessation studies, presumably due to those who did not quit smoking.30,31 Our conclusions are based on smokers with repeated follow-up and may not be generalizable to those who did not attend subsequent visits, however, participants in this analyses were very representative of the total WSHS-1 and WSHS-2 populations. Because this study focused on current smokers, it did not have a non-smoking control arm and we cannot exclude the effects of confounding variables. Smokers in our analyses all made a quit attempt, so our findings may not be generalizable to smokers unwilling to try to quit, although smokers wanting to quit tend to smoke more and cessation might yield even greater benefits on atherosclerosis burden. The WSHS-2 study had a relatively short follow-up period of 3 years and high prevalence of lipid-lowering therapy; it is possible that stronger relations between SBS and the carotid outcomes would have been found with longer follow-up and that use of lipid-lowering therapy may have reduced our sensitivity to identify changes. “Ever” of other nicotine-containing products and combustibles at the baseline visits of WSHS-1 and WSHS-2 was low (Supplemental Table 4), however, subsequent use of e-cigarettes and other nicotine-containing products in WSHS-2 was not ascertained. Comparisons of individual SBS scores revealed significant differences for plaque change scores for SBS 0 vs. 2 and SBS 0 vs. 4, but not for score 0 vs. 3. We cannot fully explain this observation. Those with an SBS of 3 had somewhat lower plaque scores at the WSHS-1 baseline visit than did the other SBS groups; this difference carried over to WSHS-2 baseline visit, such that those with SBS=3 also had lower plaque scores at that visit. We speculate that fortuitous baseline differences in vulnerability to plaque formation caused the SBS=3 group to behave differently than the other SBS groups.
Conclusion
Smoking cessation is associated with less progression of carotid plaque, but not IMT. The salutary associations of smoking cessation with carotid plaque progression are related to the degree of abstinence and persist after adjusting for baseline smoking heaviness and ASCVD risk factors. The association between smoking cessation and reduced atherosclerosis progression is not affected significantly by potential confounders such as weight gain, changes in other ASCVD risk factors, and medication use. Longitudinal changes in carotid plaque burden better reflect the influence of smoking cessation on arterial protection than do changes in carotid IMT.
Supplementary Material
Highlights.
We studied the longitudinal relations of smoking burden with carotid artery plaque and IMT progression in two prospective studies of the effects of smoking and smoking cessation in daily smokers.
Strengths of our analysis include its prospective longitudinal design, rigorous methods for serially quantifying cigarette smoking exposure over several years, contemporary smoker cohorts, and rigorous ultrasound methods that included using the same ultrasound instrumentation and scanning protocol at both sites and the same reader for all studies.
Our major finding was that smoking cessation was associated with less progression of carotid plaque, but not IMT.
The effects of smoking cessation on carotid atherosclerosis are related to degree of abstinence and persist after adjusting for baseline smoking heaviness and ASCVD risk factors.
This association was not affected significantly by potential confounders such as weight gain or changes in medications
Financial support
Supported in part by grants R01HL109031 from the NHLBI, K05CA139871 from the NCI, and P5019706 from the NIDA.
Footnotes
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke: A meta-analysis. Medicine (Baltimore). 2019;98(12):e14872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Larsson SC, Burgess S, Michaëlsson K. Smoking and stroke: A Mendelian randomization study. Ann Neurol 2019;86(3):468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Center for Chronic Disease Prevention Health Promotion Office on Smoking Health. The health consequences of smoking-50 years of progress: A report of the Surgeon General. Centers for Disease Control and Prevention (US). 2014. [Google Scholar]
- 4.Thun MJ, Carter BD, Feskanich D, Freedman ND, Prentice R, Lopez AD, Hartge P, Gapstur SM. 50-year trends in smoking-related mortality in the United States. N Engl J Med 2013;368:351–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH. Smoking cessation and decreased risk of stroke in women. JAMA. 1993;269:232–236. [PubMed] [Google Scholar]
- 6.Gordon T, Kannel WB, McGee D, Dawber TR. Death and coronary attacks in men after giving up cigarette smoking: A report from The Framingham Study. Lancet. 1974;2:1345–1348. [DOI] [PubMed] [Google Scholar]
- 7.Johansson S, Bergstrand R, Pennert K, Ulvenstam G, Vedin A, Wedel H, Wilhelmsson C, Wilhelmsen L, Aberg A. Cessation of smoking after myocardial infarction in women: Effects on mortality and reinfarctions. Am J Epidemiol 1985;121:823–831. [DOI] [PubMed] [Google Scholar]
- 8.Sato I, Nishida M, Okita K, Nishijima H, Kojima S, Matsumura N, Yasuda H. Beneficial effect of stopping smoking on future cardiac events in male smokers with previous myocardial infarction. Jpn Circ J 1992;56:217–222. [DOI] [PubMed] [Google Scholar]
- 9.Aubin HJ, Farley A, Lycett D, Lahmek P, Aveyard P. Weight gain in smokers after quitting cigarettes: Meta-analysis. BMJ. 2012;345:e4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeh HC, Duncan BB, Schmidt MI, Wang NY, Brancati FL. Smoking, smoking cessation, and risk for type 2 diabetes mellitus: A cohort study. Ann Intern Med 2010;152:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clair C, Rigotti NA, Porneala B, Fox CS, D’Agostino RB, Pencina MJ, Meigs JB. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, Baker TB. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry 2009;66:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker TB, Piper ME, Stein JH, Smith SS, Bolt DM, Fraser DL, Fiore MC. Effects of nicotine patch vs varenicline vs combination nicotine replacement therapy on smoking cessation at 26 weeks: A randomized clinical trial. JAMA. 2016;315:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson HM, Gossett LK, Piper ME, Aeschlimann SE, Korcarz CE, Baker TB, Fiore MC, Stein JH. Effects of smoking and smoking cessation on endothelial function: 1-year outcomes from a randomized clinical trial. J Am Coll Cardiol 2010;55:1988–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson HM, Piper ME, Jorenby DE, Fiore MC, Baker TB, Stein JH. Risk factors for subclinical carotid atherosclerosis among current smokers. Prev Cardiol 2010;13:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson HM, Piper ME, Baker TB, Fiore MC, Stein JH. Effects of smoking and cessation on subclinical arterial disease: A substudy of a randomized controlled trial. PLoS One. 2012;7:e35332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stein JH, Asthana A, Smith SS, Piper ME, Loh WY, Fiore MC, Baker TB. Smoking cessation and the risk of diabetes mellitus and impaired fasting glucose: Three-year outcomes after a quit attempt. PLoS One. 2014;9:e98278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell C, Piper ME, Smith SS, Korcarz CE, Fiore MC, Baker TB, Stein JH. Changes in carotid artery structure with smoking cessation. Vasc Med 2019;24:493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benowitz NL, Bernert JT, Foulds J, Hecht SS, Jacob P, Jarvis MJ, Joseph A, Oncken C, Piper ME. Biochemical verification of tobacco use and abstinence: 2019 update [published online ahead of print, 2019 Oct 1]. Nicotine Tob Res 2019;ntz132. doi: 10.1093/ntr/ntz132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tattersall MC, Gassett A, Korcarz CE, Gepner AD, Kaufman JD, Liu KJ, Astor BC, Sheppard L, Kronmal RA, Stein JH. Predictors of carotid thickness and plaque progression during a decade: The Multi-Ethnic Study of Atherosclerosis. Stroke. 2014;45:3257–3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gepner AD, Young R, Delaney JA, Budoff MJ, Polak JF, Blaha MJ, Post WS, Michos ED, Kaufman J, Stein JH. Comparison of carotid plaque score and coronary artery calcium score for predicting cardiovascular disease events: The Multi-Ethnic Study of Atherosclerosis. J Am Heart Assoc 2017;6:e005179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collins SE, Eck S, Torchalla I, Schroter M, Batra A. Validity of the timeline followback among treatment-seeking smokers in Germany. Drug Alcohol Depend. 2009;105:164–167. [DOI] [PubMed] [Google Scholar]
- 23.Hollander M, Bots ML, Del Sol AI, Koudstaal PJ, Witteman JC, Grobbee DE, Hofman A, Breteler MMB. Carotid plaques increase the risk of stroke and subtypes of cerebral infarction in asymptomatic elderly: the Rotterdam study. Circulation. 2002;105:2872–2877. [DOI] [PubMed] [Google Scholar]
- 24.Moreno M, Puig J, Moreno-Navarrete JM, Xifra G, Ortega F, Ricart W, Fernandez-Real JM. Lean mass, and not fat mass, is an independent determinant of carotid intima-media thickness in obese subjects. Atherosclerosis 2015;243:493–498. [DOI] [PubMed] [Google Scholar]
- 25.Raggi P, Stein JH. Carotid intima-media thickness should not be referred to as subclinical atherosclerosis: A recommended update to the editorial policy at atherosclerosis. Atherosclerosis 2020;312:119–120 [DOI] [PubMed] [Google Scholar]
- 26.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: A consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr 2008;21:93–111. [DOI] [PubMed] [Google Scholar]
- 27.Arnold M, Linden A, Clarke R, Guo Y, Du H, Bian Z, Wan E, Yang M, Wang L, Chen Y, et al. Carotid intima-media thickness but not carotid artery plaque in healthy individuals is linked to lean body mass. J Am Heart Assoc 2019;8:e011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Howard G, Burke GL, Szklo M, Tell GS, Eckfeldt J, Evans G, Heiss G. Active and passive smoking are associated with increased carotid wall thickness: The Atherosclerosis Risk in Communities Study. Arch Intern Med 1994;154:1277–1282. [PubMed] [Google Scholar]
- 29.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS, Stein JH. Distribution and predictors of carotid intima-media thickness in young adults. Prev Cardiol 2007;10:181–189. [DOI] [PubMed] [Google Scholar]
- 30.Hall SM, Delucchi KL, Velicer WF, Kahler CW, Ranger-Moore J, Hedeker D, Tsoh JY, Niaura R. Statistical analysis of randomized trials in tobacco treatment: Longitudinal designs with dichotomous outcome. Nicotine Tob Res 2001;3:193–202. [DOI] [PubMed] [Google Scholar]
- 31.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. Efficacy of Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release Bupropion for smoking cessation: A randomized controlled trial. JAMA. 2006;296:56–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.