Abstract
Niemann-Pick type C1 (NPC1) is a rare neurodegenerative disease. In NPC1 mouse cerebella, the antibacterial enzyme, lysozyme (Lyz2), is significantly increased in multiple cell types. Due to its possible role in toxic fibril deposition, we confirmed Lyz2 overexpression in culture in different control and NPC1 cell types including human NPC1 fibroblasts. Lyz2 expression is induced by Toll-like receptors potentially in response to lipid storage but does not play a functional role in NPC disease pathology.
1. Introduction
Niemann-Pick type C (NPC) disease is caused by reduced or loss of function of NPC1 or NPC2 proteins leading to the endolysosomal accumulation of sphingolipids and unesterified cholesterol [1]. In both human and mouse models of NPC, neuroinflammation is a component of the neuropathology, contributing to loss of cerebellar Purkinje neurons and a shorter lifespan [1, 2]. As part of the NPC cerebellar inflammation signature, lysozyme, a highly conserved antibacterial hydrolase, is overexpressed [2–5]. Using single-cell RNAseq we found that Lyz2 was not only upregulated in microglia, as expected, but expression was also significantly increased in Npc1−/− glial cells and neurons [6]. We proposed that elevated expression of lysozyme protein may functionally contribute to Purkinje neurons loss. This hypothesis was supported by a prior observation in a Sanfilippo syndrome type B mouse model. Although Lyz2 is ubiquitously expressed, lysozyme protein accumulates in disease sensitive neurons which also accumulate hyperphosphorylated TAU [7]. Aggregation of soluble lysozyme to form oligomers can mimic Aβ amyloid and induce the hyperphosphorylation of TAU [8]. NPC1 is considered a tauopathy and altered tau function influences NPC1 pathology [9, 10], thus we investigated the regulation of lysozyme expression and sought to determine if dysregulated lysozyme expression functionally contributes to NPC1 neuropathology.
2. Materials and Methods
2.1. Mouse models
Mouse experiments were approved by the NICHD ACUC. BALB/c-Npc1+/− [11] and C57BL/6-Lyz−/− mice [12] were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and crossed to obtain Npc1+/−:Lvz2+/− mice. These in turn were intercrossed to obtain control and Npc1−/−:Lvz2−/− mice. Genotype and phenotype characterization Npc1−/− mice have previously been described [5, 13].
2.2. Cell culture
Cell lines, culture conditions, TaqMan probes, RNA guides, plasmid and primer sequences are listed in Table S1. Immortalized myeloid (iMM) cells, cerebellar microglia and astrocytes were isolated and used as described previously [13–15]. Cells were treated with 2 μM U18666A (Sigma-Aldrich-Millipore, St-Louis, MO, USA) for 24 hours to inhibit NPC1 function and inhibition was confirmed by increased Srebf2 expression [16]. Hydroxypropyl-β-cyclodextrin (HPβCD, Kleptose HPB) and miglustat were used as previously described [13, 17]. Toll-like receptor (Tlr) 2 or 4 mutations were introduced into Neuro2a (N2a) cells using CRISPR/Cas9 genomic editing (PX458, https://www.addgene.org/crispr/zhang/) and guide RNAs designed with CHOPCHOP [18]. Genomic DNA from individual clones was screened using PCR (Table S1).
2.3. RNA extraction and qPCR
Total RNA was purified with Qiagen RNeasy Mini Columns (Qiagen, Hilden, Germany) and reverse-transcribed (1 μg) to obtain cDNA using a High-Capacity cDNA Archive Kit (ThermoFisher Scientific, Waltham, MA, USA). Probes used for gene expression analysis are listed in Table S1.
2.4. Statistical analysis
Statistical comparisons were performed with GraphPad Prism 5 software (San Diego, CA, USA). Mann-Whitney tests were used to compare 2 groups and two-way ANOVA testing was used when comparing two factors. Box and Whiskers plots display the Tukey post-hoc comparisons, n > 6 unless specified. Kaplan-Meier curves were compared using the log-rank test.
3. Results
3.1. Lysozyme 2 expression in NPC1 is dependent on lipid storage and Toll-like receptor activation
Microglia and astrocytes isolated from Npc1+/+ and Npc1−/− mice cerebella, N2a neurons, human fibroblasts and iMM cells all show increased expression of lysozyme when NPC1 function is decreased or absent (Fig. 1A–E). We and others have previously shown increased lysozyme protein expression in Npc1−/− cerebellar tissue [6, 19]. Increased lysozyme expression was prevented by treating microglia with HPβCD and reduced with miglustat (Fig. 1A). Miglustat inhibits glycosphingolipid synthesis and HPβCD reduces unesterified cholesterol storage in NPC1 cells. These data suggest that increased lysozyme expression is a cellular response to abnormal lipid storage. Lysozyme expression increases in response to Toll-like Receptor (TLR) activation [20], notably activation of TLR2 and 4. In the absence of Tlr4, U18666A induced expression of Lyz2 was abolished and in the absence of Tlr1 and Tlr2, induction of Lyz2 expression was attenuated (Fig. 1D). In contrast, disruption of Tlr6 had no effect on Lyz2 expression (Fig. 1D). Involvement of Tlr2 and Tlr4 was confirmed in N2a cells (Fig. 1C). TLR receptor signaling through MYD88 but not TRIF was confirmed using Mvd88 and Trif mutant cell lines (Fig. 1D).
Figure 1. Lysozyme in NPC1 pathology.
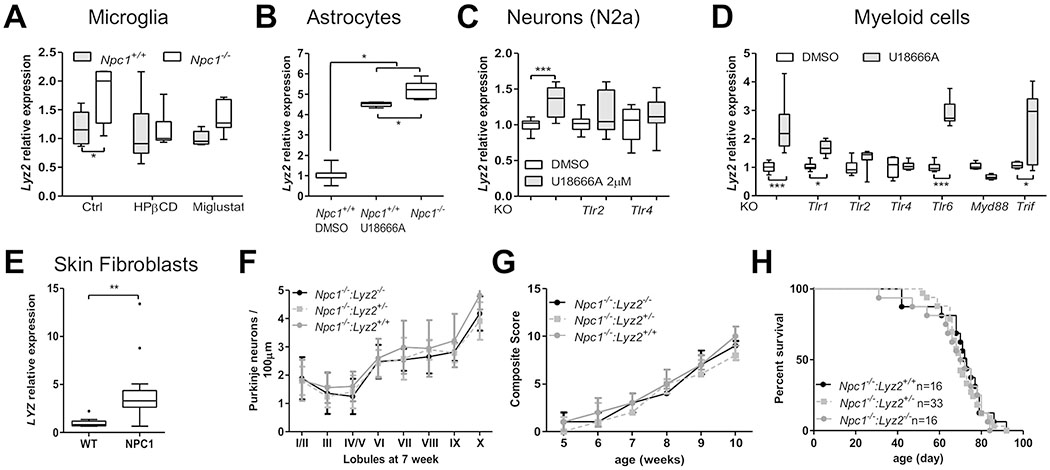
A) Relative expression by qPCR analysis of Lyz2 in Npc1+/+ and Npc1−/− microglia. Treating Npc1−/− microglia for 24 hours with either 100 μM miglustat or 100 μM HPβCD significantly decreased Lyz2 expression toward normal levels. B) Increased expression of Lyz2 in Npc1−/− astrocytes from 7-week old mice. Pharmacological inhibition of NPC1 function with 2 μM U18666A increased expression of Lyz2 in Npc1+/+ astrocytes. DMSO is a vehicle control C) Increased expression of Lyz2 in a neuronal cell line (N2a) when treated with U18666A to inhibit NPC1 function. Increased expression of Lyz2 is abrogated in Tlr2 and Tlr4 knockout (KO) lines. D) Increased Lyz2 expression in myeloid cell lines (control, Tlr1−/−, Tlr2−/−, Tlr4−/−, Myd88−/− or Trif−/−) treated with DMSO (vehicle control) or U18666A to inhibit NPC1 function. E) Increased LYZ expression in NPC1 patient skin fibroblasts. Genotypes are provided in table S1. F) Purkinje neuron density is not altered in cerebella from 7-week Npc1 mutant mice heterozygous or mutant for Lyz2 (n≥6). G) Phenotypic severity is similar in Npc1 mutant mice irrespective of the Lyz2 genotype (n≥6). H) Kaplan-Meier survival curves. Lyz2 genotype does not alter survival of Npc1−/− mice. Median survival was 71 and 72 days for Npc1−/−:Lyz2+/+ and Npc1−/−:Lyz2−/−mice, respectively (p=0.59). Statistical analysis used a log-rank test. Two-way ANOVA was used to analyze data in panels A, D , F and G. Mann-Whitney test was used to analyze data in panels B, C and E, and a log-rank test was used for data in panel H. For all panels: *p< 0.05, **p<0.01 and ***p<0.001.
3.2. Increased lysozyme expression does not functionally contribute to NPC1 neuropathology
Characteristic NPC1 histopathological findings related to both neuroinflammation and neurodegeneration were indistinguishable between the tested mouse genotypes: Npc1−/−:Lyz2+/+, Npc1−/−:Lyz2+/− and Npc1−/−:Lyz2−/− (Fig. 1F, S1). Lyz2 gene loss did not appear to play a role in the mice phenotype as it did not alter NPC disease progression (Fig. 1G) or survival (Fig. 1H).
4. Discussion
Neuroinflammation in NPC disease has been extensively characterized with Lyz2 identified as a gene commonly found to be overexpressed [4, 13, 19]. The present results suggest LYZ2, an antibacterial glycosyl-hydrolase, is overexpressed in several cell types of the cerebellum of NPC mice, including neurons and astrocytes. Our results show that Lyz2 is upregulated following TLR1/2 and TLR4 activation through MYD88. Based on substrate reduction using HPβCD and miglustat we propose that increased Lyz2 expression may be secondary to host-generated lipids accumulating in the endolysosomal compartment. As these TLRs have overlapping target genes, the inhibition of both TLR2 and TLR4 signaling may be needed for a protective effect on NPC1 disease progression [21]. Given that the NPC1 mouse phenotype is not appreciably altered in Npc1−/−:Lyz2−/− double mutant mice, increased lysozyme expression does not appear to play a direct role in NPC1 pathology.
However, lysozyme may serve as a useful biomarker that could provide insight into pathological mechanisms or a pharmacodynamic marker to support efficacy of therapeutic interventions. Our current work indicates that increased lysozyme expression is dependent on lipid accumulation, thus it may serve as a tool in future studies to identify specific sterols, lipids or glycolipids contributing to the neuroinflammatory state. Increased lysozyme has previously been identified as a biomarker of neuroinflammation [19]. Our current data extends this observation and shows that lysozyme is an indicator of abnormal TLR activation. Prior work by Suzuki et al. implicated endosomal accumulation and activation of TLR4 as a potential mechanism contributing to cell-autonomous microglial activation in NPC1 [21]. This would be consistent with observations that implicate microglial activation as an early pathological process preceding neuronal loss in NPC1 [6, 13], and underscores the potential of lysozyme to be used as a biomarker in assessing early disease progression.
Supplementary Material
Figure S1. Histological evaluation of Npc1−/−:Lyz2+/+, Npc1−/−:Lyz2+/− and Npc1−/−:Lyz2−/− mice cerebella. See below.
Supplementary Material: Table S1. List of reagents, primers and software used. See attached Excel file.
Acknowledgments:
We thank the NHLBI flow cytometry core and the NICHD animal facilities for technical assistance and animal husbandry. We thank Drs. Michael Dorrington and Iain Fraser (NIAID) for the iMM cell lines. The anti-mouse LYZ2 antibody was a gift from Dr. Edith Porter (California State University).
Funding: Intramural Research Programs of the Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Human Genome Institute. The Ara Parseghian Medical Research Fund at the University of Notre Dame.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interest
References
- 1.Vanier MT, Niemann-Pick disease type C. Orphanet J Rare Dis, 2010. 5: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cologna SM, Cluzeau CV, Yanjanin NM, Blank PS, Dail MK, Siebel S, Toth CL, Wassif CA, Lieberman AP, and Porter FD, Human and mouse neuroinflammation markers in Niemann-Pick disease, type C1. J Inherit Metab Dis, 2014. 37(1): p. 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao G, Wen Z, Irizarry K, Huang Y, Mitsouras K, Darmani M, Leon T, Shi L, and Bi X, Abnormal gene expression in cerebellum of Npc1−/− mice during postnatal development. Brain Res, 2010. 1325: p. 128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez ME, Klein AD, and Scott MP, Complement is dispensable for neurodegeneration in Niemann-Pick disease type C. J Neuroinflammation, 2012. 9: p. 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin KB, Williams IM, Cluzeau CV, Cougnoux A, Dale RK, Iben JR, Cawley NX, Wassif CA, and Porter FD, Identification of Novel Pathways Associated with Patterned Cerebellar Purkinje Neuron Degeneration in Niemann-Pick Disease, Type C1. Int J Mol Sci, 2019. 21(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cougnoux A, Yerger JC, Fellmeth M, Serra-Vinardell J, Martin K, Navid F, Iben J, Wassif CA, Cawley NX, and Porter FD, Single Cell Transcriptome Analysis of Niemann-Pick Disease, Type C1 Cerebella. Int J Mol Sci, 2020. 21(15). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohmi K, Kudo LC, Ryazantsev S, Zhao HZ, Karsten SL, and Neufeld EF, Sanfilippo syndrome type B, a lysosomal storage disease, is also a tauopathy. Proc Natl Acad Sci U S A, 2009. 106(20): p. 8332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vieira MN, Forny-Germano L, Saraiva LM, Sebollela A, Martinez AM, Houzel JC, De Felice FG, and Ferreira ST, Soluble oligomers from a non-disease related protein mimic Abeta-induced tau hyperphosphorylation and neurodegeneration. J Neurochem, 2007. 103(2): p. 736–48. [DOI] [PubMed] [Google Scholar]
- 9.Distl R, Treiber-Held S, Albert F, Meske V, Harzer K, and Ohm TG, Cholesterol storage and tau pathology in Niemann-Pick type C disease in the brain. J Pathol, 2003. 200(1): p. 104–11. [DOI] [PubMed] [Google Scholar]
- 10.Pacheco CD, Elrick MJ, and Lieberman AP, Tau deletion exacerbates the phenotype of Niemann-Pick type C mice and implicates autophagy in pathogenesis. Hum Mol Genet, 2009. 18(5): p. 956–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loftus SK, Morris JA, Carstea ED, Gu JZ, Cummings C, Brown A, Ellison J, Ohno K, Rosenfeld MA, Tagle DA, Pentchev PG, and Pavan WJ, Murine model of Niemann-Pick C disease: mutation in a cholesterol homeostasis gene. Science, 1997. 277(5323): p. 232–5. [DOI] [PubMed] [Google Scholar]
- 12.Orthgiess J, Gericke M, Immig K, Schulz A, Hirrlinger J, Bechmann I, and Eilers J, Neurons exhibit Lyz2 promoter activity in vivo: Implications for using LysM-Cre mice in myeloid cell research. European Journal of Immunology, 2016. 46(6): p. 1529–1532. [DOI] [PubMed] [Google Scholar]
- 13.Cougnoux A, Drummond RA, Collar AL, Iben JR, Salman A, Westgarth H, Wassif CA, Cawley NX, Farhat NY, Ozato K, Lionakis MS, and Porter FD, Microglia Activation in Niemann-Pick Disease, type C1 is Amendableto Therapeutic Intervention. Hum Mol Genet, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schildge S, Bohrer C, Beck K, and Schachtrup C, Isolation and culture of mouse cortical astrocytes. J Vis Exp, 2013(71). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J, Li N, Oh KS, Dutta B, Vayttaden SJ, Lin B, Ebert TS, De Nardo D, Davis J, Bagirzadeh R, Lounsbury NW, Pasare C, Latz E, Hornung V, and Fraser IDC, Comprehensive RNAi-based screening of human and mouse TLR pathways identifies species-specific preferences in signaling protein use. Science Signaling, 2016. 9(409). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu F, Liang Q, Abi-Mosleh L, Das A, De Brabander JK, Goldstein JL, and Brown MS, Identification of NPC1 as the target of U18666A, an inhibitor of lysosomal cholesterol export and Ebola infection. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams IM, Wallom K-L, Smith DA, Al Eisa N, Smith C, and Platt FM, Improved neuroprotection using miglustat, curcumin and ibuprofen as a triple combination therapy in Niemann–Pick disease type C1 mice. Neurobiology of disease, 2014. 67: p. 9–17. [DOI] [PubMed] [Google Scholar]
- 18.Labun K, Montague TG, Krause M, Torres Cleuren YN, Tjeldnes H, and Valen E, CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res, 2019. 47(W1): p. W171–W174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alam MS, Getz M, Yi S, Kurkewich J, Safeukui I, and Haldar K, Plasma signature of neurological disease in the monogenetic disorder Niemann-Pick Type C. J Biol Chem, 2014. 289(12): p. 8051–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klüter T, Fitschen-Oestern S, Lippross S, Weuster M, Mentlein R, Steubesand N, Neunaber C, Hildebrand F, Pufe T, and Tohidnezhad M, The antimicrobial peptide lysozyme is induced after multiple trauma. Mediators of inflammation, 2014 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki M, Sugimoto Y, Ohsaki Y, Ueno M, Kato S, Kitamura Y, Hosokawa H, Davies JP, Ioannou YA, Vanier MT, Ohno K, and Ninomiya H, Endosomal accumulation of Toll-like receptor 4 causes constitutive secretion of cytokines and activation of signal transducers and activators of transcription in Niemann-Pick disease type C (NPC) fibroblasts: a potential basis for glial cell activation in the NPC brain. J Neurosci, 2007. 27(8): p. 1879–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Histological evaluation of Npc1−/−:Lyz2+/+, Npc1−/−:Lyz2+/− and Npc1−/−:Lyz2−/− mice cerebella. See below.
Supplementary Material: Table S1. List of reagents, primers and software used. See attached Excel file.


