Abstract
Ischaemic stroke is an acute interruption of the blood supply to the brain, which leads to rapid irreversible damage to nerve tissue. Ischaemic stroke is accompanied by the development of neuroinflammation and neurodegeneration observed around the affected brain area. Heat shock protein 70 (Hsp70) facilitates cell survival under a variety of different stress conditions. Hsp70 may be secreted from cells and exhibits cytoprotective activity. This activity most likely occurs by decreasing the levels of several proinflammatory cytokines through interaction with a few receptors specific to the innate immune system. Herein, we demonstrated that intranasal administration of recombinant human Hsp70 shows a significant twofold decrease in the volume of local ischaemia induced by photothrombosis in the mouse prefrontal brain cortex. Our results revealed that intranasal injections of recombinant Hsp70 decreased the apoptosis level in the ischaemic penumbra, stimulated axonogenesis and increased the number of neurons producing synaptophysin. Similarly, in the isolated crayfish stretch receptor, consisting of a single sensory neuron surrounded by the glial envelope, exogenous Hsp70 significantly decreased photoinduced apoptosis and necrosis of glial cells. The obtained data enable one to consider human recombinant Hsp70 as a promising compound that could be translated from the bench into clinical therapies.
Keywords: Photothrombotic stroke, Hsp70, Neuroprotection, GAP43, Synaptophysin
Introduction
Stroke is the second leading cause of human disability and death worldwide (James et al. 2020; Powers et al. 2018). Ischaemic stroke (approximately 80% of all strokes) occurs due to a sudden rapid drop in cerebral blood flow that lasts for minutes, reduces the oxygen and glucose supply of a tissue, and leads to a decrease in ATP production and necrosis. The toxic factors (glutamate, K+-mediated depolarization, reactive oxygen species (ROS), oedema, etc.) spread slowly, over the course of several hours, from the infarction core to the surrounding tissue and expand the injured area. It is assumed that cells in the transition zone (penumbra) may be saved during the next 2–6 h (“therapeutic window”), or even during the first 24 h after stroke (Moskowitz et al. 2010; Khoshnam et al. 2017).
There are two main approaches for stroke therapy—thrombolysis and neuroprotection. Tissue plasminogen activator (tPA) is the only thrombolytic drug approved by the FDA (Venkat et al. 2018). However, it is effective only within the first 3–4.5 h after a stroke, and even in the USA, no more than 5% of patients are cured with this drug. Moreover, unfortunately, tPA has a number of adverse effects, including the risk of haemorrhage, overproduction of reactive oxygen and nitrogen species leading to oxidative damage to the nervous tissue, oedema and the activation of signalling cascades that may induce apoptosis (Radak et al. 2017; Uzdensky 2019).
Another potential approach in antistroke therapy is neuroprotection. Diverse proteins may be involved in the neuroprotection of penumbra cells from ischaemia/reperfusion (I/R) (Ferrer and Planas 2003; Puyal et al. 2013; Demyanenko et al. 2015; Uzdensky 2019). One promising protective protein is the molecular chaperone Hsp70, which belongs to the stress-induced protein group (Evgen’ev et al. 2014; Kim et al. 2018). As an intracellular house-keeping protein, Hsp70 promotes refolding or utilization of proteins damaged by various stress factors (Wegele et al. 2004). Up to 10% of synthesized Hsp70 is excreted out of the cells by a nonstandard mechanism that does not require the presence of an N-terminal signal sequence (similar to the secretion mechanism of several cytokines) (Robinson et al. 2005; Calderwood et al. 2007; Zhan et al. 2009). Overexpression of Hsp70 was shown to protect brain cells from ischaemic damage (Weinstein et al. 2004, Sharp et al. 2013: Kim et al. 2018). In contrast, inhibition of Hsp70 synthesis was shown to increase brain infarction (Lee et al. 2001). As shown in neuronal cultures and in vivo rodent models, secreted and recombinant Hsp70 exhibited neuroprotective properties (Lu et al. 2014; Bobkova et al. 2014; Evgen’ev et al. 2017). Upon intranasal administration, full-size Hsp70 effectively penetrates the blood-brain barrier. This may solve the problem of its delivery to the lesioned brain (Bobkova et al. 2014; Yurinskaya et al. 2015; Tytell et al. 2018).
Here, we described the effect of intranasal administration of recombinant human Hsp70 into the brain of mice subjected to photothrombotic stroke (PTS), a widely used model of ischaemic stroke (Uzdensky 2018). We showed that exogenous Hsp70 (eHsp70) decreased the level of apoptotic cell death in the PTS-induced penumbra and decreased the infarct volume after PTS. eHsp70 also stimulated axon regeneration and increased the number of neurons expressing synaptophysin that were reduced by PTS. Therefore, the recombinant Hsp70 may be a promising neuroprotective agent that stimulates reparative processes in the nervous tissue after ischaemic stroke in mammalian species, including humans.
Methods
Animal procedures
Male CD-1 mice (20–25 g) between the ages of 14 and15 weeks were obtained from the vivarium of the Rostov Scientific Research Institute of Microbiology and Parasitology, Russia. The animals were kept in groups of 4 to 5 mice with free access to food and water in 12 light/12dark cycles. All the experimental procedures were carried out in accordance with the European Union guidelines 86/609/EEC for the use of experimental animals and local legislation for the ethics of experiments on animals. The animal protocols used in this study were evaluated and approved by the Animal Care and Use Committee of the Southern Federal University (Approval No. 08/2016).
Local photothrombotic infarction of the cerebral cortex of mice
A local unilateral photothrombotic stroke (PTS) of the mouse cerebral cortex was used as a model of ischaemic stroke (Demyanenko et al. 2019; Uzdensky 2018). To induce a stroke, a solution of Bengal Rose photosensitizer (R4507, Sigma) at a concentration of 15 mg/ml was administered intraperitoneally at a dose of 10 μl/g weight. The hydrophilic photosensitizer Bengal Rose does not cross the blood-brain barrier and does not penetrate the cells but accumulates in the brain vessels. Subsequent local laser irradiation causes photoexcitation of the dye, intensive generation of highly toxic singlet oxygen, oxidative damage to the endothelium and basement membrane, and platelet aggregation and occlusion of microvessels; these processes lead to ischaemic damage and death of nearby neurons and glial cells (Lee et al., 2007). To induce these processes, the area of the mouse skull freed from the periosteum in the sensorimotor cortex area (2 mm lateral to Bregma) (300 mg/kg) (Franklin and Paxinos 2008), was irradiated with a diode laser under chloral hydrate anaesthesia 5 min after the administration of Bengal Rose. The irradiation parameters were as follows: wavelength 532 nm, intensity 0.2 W/cm2, beam diameter 1 mm and exposure 15 min. The control group included sham-operated animals that underwent the same operations but without the introduction of a photosensitizer. After 3, 7, 14 or 21 days, the animals were killed by an overdose of chloral hydrate (600 mg/kg), and the brain was extracted.
Expression and purification of recombinant Hsp70
The recombinant human Hsp70 used includes five substitutions at potential glycosylation sites (N35D, S153A, S362A, T419A, T489A) that reduce protein aggregation and were experimentally generated to allow the protein to be purified from the milk of transgenic animals (Gurskiy et al. 2016).The Hsp70 coding sequence was cloned into a pET-14b-derived plasmid to provide the N-terminal polyhistidine tag (MGSSHHHHHHSSGLVPRGSH). Recombinant Hsp70 protein was produced in the E. coli BL21 strain. Briefly, cells were transformed with an ampicillin-resistant pET-derived Hsp70-coding plasmid, and a single colony of the producer strain was resuspended in M9/0.1% peptone medium with ampicillin 150 μg/ml. Induction was achieved by the addition of IPTG, and after growth for 3 h, cells were collected by centrifugation at 4000×g for 10 min, washed with 100 mMNaCl and stored at − 70 °C. Then, cells were resuspended in water with egg lysozyme to 0.2 mg/ml and frozen once again at – 70 °С before lysis. The protein was purified in the native state on ice. Cells were lysed by sonication in 20 mL of buffer A (20 mM NaH2PO4, 500 mMNaCl, 25 mM imidazole, 0.01% NP-40, рН 7.0), the lysate was then centrifuged at 8000×g for 30 min at 4 °C, and the supernatant was incubated with Ni-NTA agarose (Qiagen) according to the manufacturer’s protocol. Subsequently, the suspension was washed with a 3× volume of buffer B (25 mM NaH2PO4, 500 mMNaCl, 40 mM imidazole, рН 7.0). Protein bound to the resin was eluted with buffer C (50 mM NaH2PO4, 300 mMNaCl, 350 mM imidazole, рН 6.0). The recombinant protein was desalted by dialysis at 4 °C against PBS, changing the buffer twice with 1 mMEDTA in the first change to remove traces of Ni+2. After the dialysis, the remaining LPS was removed on polymyxin-agarose (Sigma-Aldrich). Protein yield was assessed using Bradford reagent; purity of the resultant protein was determined using SDS-PAGE protein electrophoresis.
Administration of Hsp70
The concentration of Hsp70 was adjusted to 0.5 μg in 1 μl of sterile PBS and administered intranasally at 2 μg in 4 μl every 12 h for at least 7 days. The injections were performed 1 h after the PTS. Control animals were injected with PBS in the same volume.
Infarct volume evaluation
To determine the volume of myocardial infarction at different periods after the PTS, brain sections were stained with 2,3,5-triphenyltetrazolium chloride (TTC, T8877, Sigma). For this, the mice were anaesthetized and decapitated, and the brains were quickly removed and placed into a precooled adult mouse brain matrix (J&K Seiko Electronic Co., Ltd). A matrix with brain tissue was transferred to a freezer (− 80 °C) for 3–5 min, and 2-mm thick tissue slices were cut. Sections were stained in 1% TTC for 30 min at 37 °C in the dark. Using ImageJ software (http://rsb.info.nih.gov/ij/), the area of the infarction zones on each slice were measured, summed, and multiplied by the slice thickness (2 mm).
Imaging of apoptosis
Apoptotic cells were visualized using the TUNEL method (TdT-mediated dUTP-X nick end labelling) using the “In Situ Cell Death Detection Kit, TMR red” (12,156,792,910, Roche) (red fluorescence) or immunofluorescence analysis of active caspase 3. For TUNEL method, sections were treated with reagents from the kit according to the manufacturer’s recommendations and incubated for 1 h in the dark at 37 °C with a Hoechst 33342 cell nucleus marker (10 μg/ml, blue fluorescence). Detection of active caspase 3 was performed as described in the section on “Immunofluorescence microscopy”. The apoptotic coefficient (AI) was calculated as:
AI = (number of TUNEL-positive cells or caspase 3-positive cells/total number of cells stained by Hoechst 33342)*100%. The analysis was performed on 3 images for each of 8 animals in the group.
Estimation of necrosis and apoptosis in nerve cells of the isolated crayfish stretch receptor
To study the neuroprotective effect of Hsp70 on glia cells, we used an in vitro model of the isolated abdominal stretch receptor from the river crayfish Astacus leptodactilus. The model consisted of isolated mechanoreceptor neurons (MRNs) mounted on receptor muscles and surrounded by satellite glial cells. The advantage of this model is the ability to study identified neurons in a strictly controlled functional state and to simultaneously study the glial cells that surround this isolated neuron, which is difficult to perform when studying the brain using in vitro models (Rodkin et al. 2020).
An isolated MRN is able to generate action potentials (AP) for 6–8 h with a frequency depending on the degree of stretching of the receptor muscle (Rodkin et al. 2020). The isolated stretch receptors were placed in a plexiglass cuvette with 2 ml of physiological saline solution of van Harreveld for cold-blooded animals (mM: NaCl–205; KCl–5.4; NaHCO2–0.24; MgCl2–5.4; CaCl2–13.5; pH 7.2–7.4). APs were extracted extracellularly from axons using glass electrodes, amplified and digitized using an L-761 analogue-to-digital converter (L-Card, Moscow, Russia). Their frequency was continuously recorded. Oxidative stress in the neuron-glia system was achieved using photodynamic effects with the photosensitizer Photosens (NIOPIK, Moscow), and the semiconductor laser (670 nm, 0.4 W/cm2) served as the light source. The experiments were carried out at a temperature of 25 ± 4 °C. Each series of experiments included 4 groups: (Aigner and Caroni 1995) control, (Aneja et al. 2006) exposure to Hsp70, (Bobkova et al. 2015) photodynamic exposure (PDT) and (Bobkova et al. 2014) PDT in the presence of Hsp70. An Hsp70 solution (600 nM) was added to the cuvette with a crayfish stretch receptor 30 min before the start of irradiation, while continuing to record the impulse activity of the neurons. Visualization of necrosis and apoptosis of MRNs and glial cells in the stretch receptor was carried out 8 h after the termination of irradiation (the time required for apoptosis to develop). Visualization was performed by double dyeing the MRN preparation with propidium iodide (PI) (20 μM) (Sigma-Aldrich-Rus), penetrating only necrotic cells with a damaged membrane, and with a Hoechst-33,342 fluorochrome (10 μM) (Sigma-Aldrich), visualizing nuclear chromatin morphology and detecting apoptotic cells with fragmented nuclei. The preparations were photographed using a NIKON Eclipse FN1 fluorescence microscope (Japan).
Immunofluorescence microscopy
For immunofluorescence microscopy, mice were transcardially perfused with 10% formalin. The brain was fixed in formalin overnight and incubated for 48 h in a 20% solution of sucrose in phosphate buffer (PBS) at 4 °C. Frontal sections of the brain with a thickness of 20 μm (+ 2 mm from Bregma to − 4 mm) (Franklin and Paxinos 2008) were obtained on the Leica VT 1000 S vibratome (Leica, FRG). Sections were frozen in 2-methylbutane and stored at − 80 °C. After thawing, the sections were washed with PBS. Nonspecific antibody binding was blocked by 5% BSA with 0.3% Triton X-100 (1 h, 20–25 °C). Then, the sections were incubated overnight at 4 °C in the same solution with primary rabbit antibodies (all from Sigma-Aldrich): antiCaspase 3, active (C8487, 1:500), antiNeuN (MAB377; 1:1000), antiSynaptophysin (S5768; 1:500) or antiGAP43 (SAB4300525, 1:500). After washing in PBS, the sections were incubated for 1 h with fluorescence-labelled secondary antibodies: antirabbit CF488A (SAB4600045, 1:1000), antimouse CF488A (SAB4600043) or antimouse CF555 (SAB4600302, 1:1000). The sections were mounted on slides in 60% glycerol/PBS. The negative control consisted of similar sections without primary antibodies. The sections were analysed with the fluorescence microscope Eclipse FN1 (Nikon, Japan). Quantification of fluorescence was carried out on 10–15 images of experimental and control preparations that were obtained with the same settings of the digital camera. The average fluorescence intensity was determined using the ImageJ software. Threshold values were selected once and remained constant when processing all photos. Relative fluorescence changes ΔI:ΔI = (Iex–Iso or IPTS)/Iso or IPTS, where Iex is the average fluorescence intensity in the studied cortex regions. Iso is the mean fluorescence intensity in control samples; IPTS is the mean fluorescence intensity in animals subjected to photothrombotic stroke. Immunofluorescence data are presented as the mean ΔI ± S.E.M.
Statistical analysis
The distribution normality was evaluated by the Shapiro-Wilk criterion. Significant differences were assessed using a one-way analysis of variance (ANOVA) with the Tukey test. All data are presented as M ± SEM. Differences were considered significant at p < 0.05.
Results
Exogenous Hsp70 reduced PTS-induced apoptosis in the mouse cerebral cortex
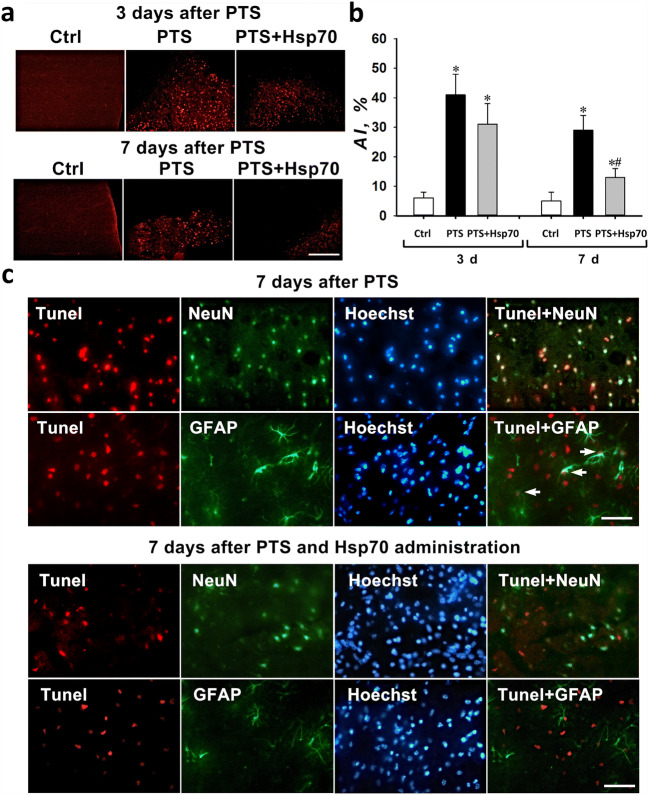
The number of Tunel-positive penumbra cells and the apoptotic coefficient increased at 3 and 7 days after PTS by approximately 7 and 6 times, respectively (Fig. 1a, b). Three days after administration of the Hsp70 preparation, the number of cells stained with Tunel (31%) did not significantly change compared with those from mice subjected to PTS (41%). However, after 7 days, Hsp70 administration reduced the apoptosis level of penumbra cells almost twofold (Fig. 1a, b).
Fig. 1.
Apoptosis of penumbra cells in the cortex of mice after photothrombotic stroke. a Typical fluorescence images of the Tunel-stained (red) cortex of mice at 3 and 7 days after a photothrombotic stroke and the administration of eHsp70. Scale bar 100 μm. b The changes of the apoptotic coefficient (AI, %) in different mice groups: sham-operated control mice (Ctrl) that were intranasally injected with a physiological solution; and 3 or 7 days after photothrombotic stroke in animals that were administered intranasally with a physiological solution or 3 and 7 days after PTS and on the background of the eHsp70 introduction 3 and 7 days after PTS (after injection of Hsp70 dissolved in physiological saline). n = 24 (three fields of view were analyzed for each mouse in groups consisting from eight animals). M ± SEM. One-way ANOVA. *P < 0.05 relative to the group of sham-operated animals, #P < 0.05 relative to the group with PTS. c Colocalization of Tunel-positive nuclei (red) and neuron marker NeuN (green) or astrocyte marker GFAP (green) in penumbra 7 days after a photothrombotic stroke. Arrows indicate astrocytes with apoptotic nuclei. Scale bar 100 μm
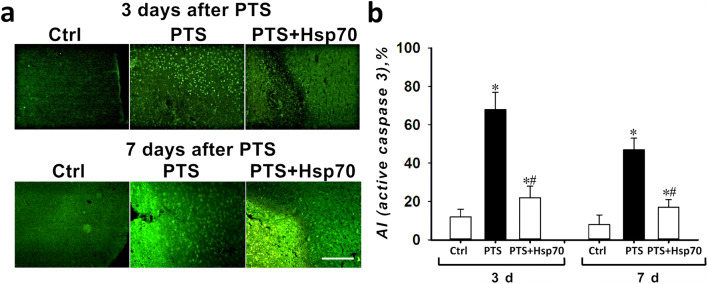
The dynamics of changes in the number of caspase 3-positive cells was similar (Fig. 2a, b). The percentage of caspase 3-positive cells in penumbra was increased in 3 and 7 days after PTS when compared with the sham-operated animals. Treatment with Hsp70 reduced the percentage of caspase 3-positive cells in the penumbra when compared with the PTS-group (Fig. 2a, b).
Fig. 2.
Immunofluorescence analysis of the active caspase 3 levels in the mice cerebral cortex in 3 and 7 days after PTS and the administration of Hsp70. a Typical fluorescence images of the caspase 3-stained (green) cortex of mice in 3 and 7 days after a photothrombotic stroke and the administration of eHsp70. Scale bar 100 μm. b The changes of the apoptotic coefficient (AI, %) in different mice groups: sham-operated control mice (Ctrl) that were intranasally injected with a physiological solution; and 3 or 7 days after photothrombotic stroke in animals that were administered intranasally with a physiological solution or 3 and 7 days after PTS and on the background of the eHsp70 introduction 3 and 7 days after PTS (after injection of Hsp70 dissolved in physiological saline). n = 24 (three fields of view were analyzed for each mouse in groups consisting of eight animals). M ± SEM. One-way ANOVA. *P < 0.05 relative to the group of sham-operated animals, #P < 0.05 relative to the group with PTS
Double immunofluorescence staining with Tunel and a NeuN neuron marker or GFAP astrocyte marker showed that Tunel-positive cells colocalized mainly with neurons and some astrocytes (Fig. 1c).
eHsp70 administration reduced the PTS-induced infarction volume
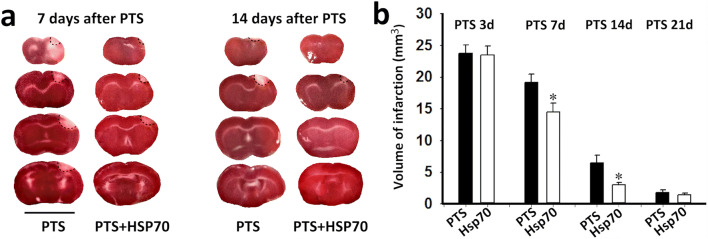
The PTS-induced infarction volume was approximately 24 mm3 at 3 days after the PTS, which corresponded to previously obtained data (Demyanenko et al. 2019). The administration of Hsp70 resulted in a 25% reduction in the infarction volume during the 7 days after the PTS and reduced the infarction volume more than twofold during the 14 days after the PTS. Apparently, this drop in volume was associated with a decrease in apoptosis of penumbra cells (Fig. 3a, b). After 21 days, the infarct volume decreased significantly (1.5–2 mm3) and did not differ between the PTS and PTS/Hsp70 groups.
Fig. 3.
The changes in the infarct volume in the mouse brain (mm3) at 3, 7, 14 and 21 days after PTS in the administration or absence of eHsp70. a Frontal brain slices stained with triphenyltetrazolium chloride at 7 and 14 days after PTS and Hsp70 administration (during the first 7 days). Scale bar 1 cm. b One-way ANOVA. n = 10. M ± SEM. * P < 0.05
eHsp70 restored the expression of synapse-associated proteins that were decreased after PTS
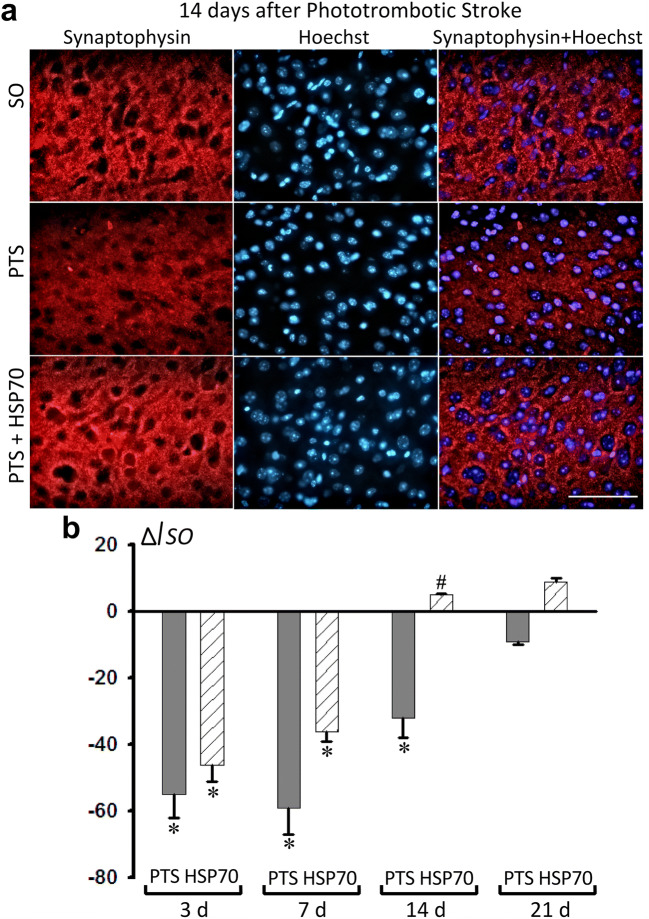
Synaptophysin (SYP) and growth-associated protein 43 (GAP43) are two critical synapse-associated proteins that play key roles in neuronal function. PTS decreased the level of SYP in penumbra cells by 55–59% at 3 or 7 days after the infarct relative to control group (Fig. 4). The reduced level of SYP (− 32%) was maintained up to 14 days after PTS (Fig. 4a, b). The administration of Hsp70 increased the expression of SYP by 37% compared with the PTS group, which did not receive Hsp70 treatment (Fig. 4a, b).
Fig. 4.
Immunofluorescence analysis of the synaptophysin levels in the mice cerebral cortex at different intervals after PTS (3, 7, 14 and 21 days) and the administration of Hsp70. a Representative images of the synaptophysin immunofluorescence (red) and cell nucleus marker Hoechst 33342 (blue) in sham-operated animals (SO), or in mice at 14 days after PTS and Hsp70 administration. Scale bar 50 μm. b The changes in the intensity of synaptophysin immunofluorescence relative to sham-operated mice (ΔISO). n = 24 (3 fields of view were analyzed for each of 8 animals in the group). M ± S.E.M. ANOVA. *P < 0.05 relative to sham-operated mice; # relative to PTS group
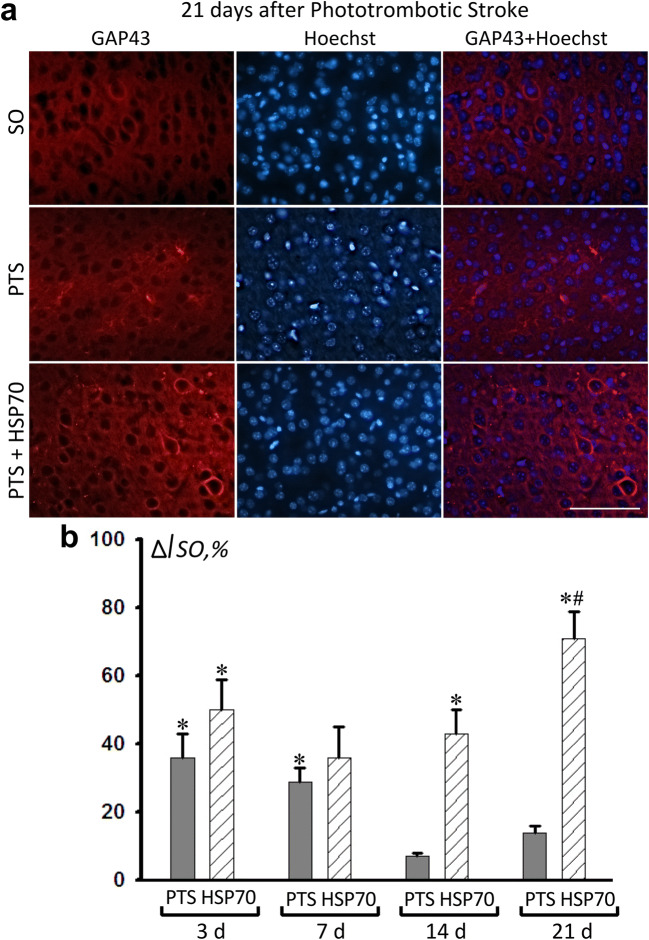
GAP43 is associated with the growth of neurites during the development and regeneration of the nervous system. PTS increased the GAP43 immunofluorescence in the penumbra after 3 and 7 days by 36% and 29%, respectively (Fig. 5). A PTS-induced increase in the cerebral level of GAP43 could be a result of two opposite processes: axon damage and regeneration (Li et al. 1998; Gorup et al. 2015). Notably, on the 14th day after the PTS, the level of GAP43 did not significantly differ from that in the sham-operated animals (Fig. 5b). The administration of Hsp70 increased the protein expression 14 days after PTS compared with that in the sham-operated animals by 43%, and after 21 days, GAP43 expression increased relative both to the control group (+44%) and to parameters for PTS (+64%) (Fig. 5a, b).
Fig. 5.
Immunofluorescence analysis of GAP43 expression in the cerebral cortex of mice at different periods after the PTS (3, 7, 14 and 21 days) and the administration of Hsp70. a Representative images of GAP43 immunofluorescence (red) and Hoechst (nucleus marker, blue) in sham-operated animals (SO), 21 days after the PTS and after administration of Hsp70. Scale: 50 μm. b Change in GAP43 fluorescence intensity relative to the level of the group of sham-operated mice (ΔISO) in %. N = 24 (3 fields of view were analyzed for each of 8 animals in the group). M ± S.E.M. ANOVA.*P < 0.05 relative to sham-operated animals; # relative to the PTS group
eHsp70 exhibits a significant cytoprotective effect in an in vitro model
In addition to our above studies on the role of Hsp70 in a stroke model of photothrombotic infarction (PTI) in the mouse cerebral cortex, we studied the effect of eHsp70 on photoinduced necrosis and apoptosis of neurons and glial cells in an isolated crayfish stretch receptor. The recombinant Hsp70 applied at a concentration of 600 nM in this model did not affect necrosis or timing of impulse activity of the neurons but did significantly reduce apoptosis and necrosis of glial cells after photodynamic damage (Fig. 6).
Fig. 6.
Effect of Hsp70 and photodynamic treatment (PDT) on the death of neurons and glial cells in crayfish stretch receptor. a Crayfish stretch receptor after PDT stained with propidium iodide (red) and Hoechst 33342 (blue). Arrow—nucleus of necrotic neuron. b Crayfish stretch receptor after PDT in the presence of Hsp70 stained with propidium iodide and Hoechst 33342. Arrow—nucleus of living neuron. c and d Fragmented apoptotic nuclei of satellite glial cells surrounding the proximal axon regions photosensitized in the absence and in the presence of Hps70, respectively. Necrotic nuclei of glial cells are orange-stained. Arrows on (c) and (d) indicate fragmented nuclei of apoptotic glial cells. e Apoptosis of glial cells in crayfish stretch receptor after PDT and in the dark with and without Hsp70 (600 nM) that was assessed by the number of fragmented glial nuclei in a 2-mm region along the axon. f necrosis of glial cells of crayfish stretch receptor after PDT and in the dark with and without Hsp70 (600 nM) that was calculated as percentage of necrotic glial cells surrounding neuronal body. g Timing of impulse activity of the receptor neuron in the dark and during PDT with and without Hsp70 (600 nM). h Neuron necrosis that was calculated as the percentage of necrotic receptor neurons after PDT and in the dark with and without Hsp70 (600 nM). N = 6. M ± S.E.M. One-way ANOVA; *p < 0.05 when compared to PDT; **p < 0.01 when compared with control; ***p < 0.001 when compared with PDT
Discussion
The pathogenesis of cerebral ischaemia has several features in common with neurodegenerative diseases. In both cases, an inflammatory reaction in the brain tissue plays an important role in the development of neurodegeneration after ischaemic stroke (Jayaraj et al. 2019). In this regard, the development of a new noninvasive strategy to treat ischaemia is aimed at finding drugs that combine antiinflammatory and neuroprotective properties. However, even drugs that showed promising results in vitro, or in animal experiments, were often found unsuitable for humans because of inefficiency or unacceptable adverse effects (Karsy et al. 2017; Rajah and Ding 2017; Zhou et al. 2018; Luo et al. 2019). Therefore, new strategies to treat ischaemic stroke are needed.
The cytoprotective effect of exogenous Hsp70 has been demonstrated in various in vitro and in vivo studies. Recombinant Hsp70, when added to the culture medium, has been shown to increase the survival of the primary motoneuron culture under growth factor deprivation. It increased the resistance of neuroblastoma cells to heat shock and other apoptosis inducers (Guzhova et al. 2001; Robinson et al. 2005). When administered intravenously, recombinant Hsp70 exhibited a neuroprotective effect on the focal cerebral ischaemia model and reduced the infarction volume and the activity of the NF-κB cascade (Zhan et al. 2010; Doeppner et al. 2013; Shevtsov et al. 2014).
However, the antiischaemic effect of intranasal administration of Hsp70 has not been studied. It was previously shown that human recombinant Hsp70 effectively penetrates the blood-brain barrier after intranasal administration and exerts a positive effect in a mouse model of Alzheimer’s disease. It reduced apoptosis of neurons in the hippocampus and frontal cortex, reduced the number and size of amyloid plaques, and improved various cognitive functions (Bobkova et al. 2014; Yurinskaya et al. 2015; Evgen’ev et al. 2017). Furthermore, it was shown that in aged mice, intranasal administration of eHsp70 increased the neurogenesis in hippocampal and cortical tissues and increased the synaptophysin level (Bobkova et al. 2015).
The photothrombotic stroke (PTS) model used here has a number of methodological advantages including consistent location and volume of infarct, good reproducibility, low mortality of experimental animals, and simple experimental procedures (Uzdensky 2018). In the present work, we used intranasal administration of recombinant human Hsp70, which is preferred over intravenous administration, to study the neuroprotective effect of exogenous Hsp70 on the PTS model. We showed a decrease in the apoptosis level and an upregulation of synaptophysin and GAP43 levels at the stage of the postPTS recovery that indicates an intensification of the neurorepair processes. SYP is a synaptic vesicle protein localized in the presynaptic membrane that is involved in the regulation of synaptic transmission efficiency (Wheeler et al. 2002). SYP is an important marker of synaptic plasticity (Smith et al. 2000). Ischaemic brain damage causes a decrease in SYP expression, which leads to memory deficit (Fonteles et al. 2016; Liu et al. 2018). Restoration of SYP expression level indicates recovery from the stroke (Zhang et al. 2017; Fang et al. 2010). An increase in synaptophysin levels during recovery from ischaemic stroke under the influence of eHsp70 correlates with the results obtained earlier when using recombinant Hsp70 in ageing mice (Bobkova et al. 2015).
GAP43 stimulates the accumulation of f-actin in neurites and thereby contributes to the organization of the cytoskeleton in the nerve endings (Aigner and Caroni 1995). After the onset of a stroke, one of the most noticeable regenerative events is the axon germination in penumbra, which is accompanied by high expression of GAP43 (Stroemer et al. 1993; Carmichael et al. 2005). In the transient middle cerebral artery occlusion (tMCAO) model, at an early stage, GAP43 is present in the ischaemic lesion site followed by its expression in the penumbra (Li et al. 1996). In the MCAO constant model, where the tissue is more severely and irreversibly damaged, GAP43 is absent in the lesion, but its expression increases in the penumbra, reaching a maximum on day 7 (Miyake et al. 2002). The discovery of the postsynaptic location of this protein and its colocalization with caspase 3 suggests that GAP43 not only promotes the growth of neurites but also participates in a more complex setup of intercellular networks necessary for proper tissue regeneration after ischaemia (Gorup et al. 2015). The increase in GAP43 after intranasal treatment with recombinant Hsp70 in model PTS has been shown for the first time.
Previous data indicate that eHsp70-mediated neuroprotection is associated with reduced neuroinflammation (Evgen’ev et al. 2017; Zheng et al. 2008). Secreted Hsp70 was shown to interact with a number of receptors such as TLR2/4, CD40, CD91 and several others (Calderwood et al. 2007). It was previously shown that exogenous Hsp70 inhibits the production of ROS, NO and TNFα by neutrophils and the expression of CD11b/CD18 receptors, and it restores the normal level of neutrophil apoptosis mediated by both lipopolysaccharide and lipoteichoic acid (the main endotoxin of gram-positive bacteria) (Rozhkova et al. 2010; Vinokurov et al. 2012). It was shown in vitro and in vivo that eHsp70 inhibited the intranuclear translocation of p65, the subunit of the transcription factor NF-κB. Hsp70 also suppressed the JNK stress cascade and the production of reactive oxygen species (Borges et al. 2013; Doeppner et al. 2013; Hsu et al. 2014; Ghosh et al. 2015; Troyanova et al. 2015).
We speculate that well-documented antiinflammatory activity of recombinant Hsp70 may account for part of the observed protective effects, but that other effects of Hsp70, such as refolding of and prevention of aggregation of denatured proteins may also play important role in ameliorating the consequences of the experimental stroke.
In cell cultures, e.g., our model of an isolated crayfish abdominal stretch receptor in which eHsp70 reduces the level of photo-induced apoptosis and necrosis of neuroglia, the protective effect of eHsp70 is obviously not associated with the suppression of inflammation and is probably based on other molecular mechanisms. Thus, it was demonstrated that eHsp70 is able to induce the synthesis of endogenous Hsp70 in target cells by means of activation of the TLR4 receptor (Lee et al. 2013). This interaction with eHsp70 leads to the activation of Akt and inhibition of the activity of glycogen synthase kinase (GSK-3β), one of the negative regulators of heat shock transcription factor (HSF1) (Lee et al. 2013). It was previously shown that mice with high endogenous Hsp70 levels usually have a lower infarct volume after cerebral ischaemia (Rajdev et al. 2000; Tsuchiya et al. 2003). The neuroprotective effect of endogenous Hsp70 is also associated with its ability to refold cellular proteins damaged by stress and to decrease protein aggregation (Tsuchiya et al. 2003; Giffard et al. 2004; Sun et al. 2006). In addition, intracellular Hsp70 has a high antiapoptotic activity mediated by inhibition of SAPK/JNK cascade and cytochrome c release, preventing Apaf-1/cytochrome c complex formation and decreasing activity of the proapoptotic factor Bax (Gabai et al. 1998; Mosser et al. 2000; Matsumori et al. 2005; Stankiewicz et al. 2005; Sun et al. 2006; Yenari et al. 2005). High levels of Hsp70 in the cells reduced apoptosis and necrosis in neurons and glial cells (Giffard et al. 2004; Yenari et al. 2005; Zhan et al. 2008).
To this end, the photothrombotic stroke model described herein showed a twofold decrease in endogenous Hsp70 in the penumbra on the first day after ischaemia, which may contribute to the death of neurons by apoptosis (Demyanenko and Uzdensky 2017). Thus, induction of endogenous Hsp70 in the nervous tissue may be another hypothetical mechanism explaining the observed neuroprotective effect of eHsp70.
The data presented here allow us to conclude that recombinant Hsp70 is a universal neuroprotector that promotes neurorepair and has prospects following: its administration as an intranasal solution to treat various neuropathologies in the clinic.
Abbreviations
- PTS
Photothrombotic stroke
- Hsp70
Heat shock protein 70
- SYP
Synaptophysin
Funding
Expression and isolation of human recombinant Hsp70 was performed using funding provided by Russian Science Foundation grant #19-14-00167 (D.G). All studies with two models of experimental stroke were funded by the Ministry of Science and Higher Education of Russian Federation grant #0852-2020-0028.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aigner L, Caroni P. Absence of persistent spreading, branching, and adhesion in GAP-43-depleted growth cones. J Cell Biol. 1995;128(4):647–660. doi: 10.1083/jcb.128.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aneja R, Odoms K, Dunsmore K, Shanley TP, Wong HR. Extracellular heat shock protein-70 induces endotoxin tolerance in THP-1 cells. J Immunol. 2006;177:7184–7192. doi: 10.4049/jimmunol.177.10.7184. [DOI] [PubMed] [Google Scholar]
- Bobkova NV, Garbuz DG, Nesterova I, Medvinskaya N, Samokhin A, Alexandrova I, Yashin V, Karpov V, Kukharsky MS, Ninkina NN, Smirnov AA, Nudler E, Evgen’ev M. Therapeutic effect of exogenous Hsp70 in mouse models of Alzheimer’s disease. J Alzheimers Dis. 2014;38(2):425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- Bobkova NV, Evgen’ev ME, Garbuz DG, Kulikov AM, Morozov A, Samokhin A, Velmeshev D, Medvinskaya N, Nesterova I, Pollock A, Nudler E. Exogenous Hsp70 delays senescence and improves cognitive function in aging mice. PNAS USA. 2015;112(52):16006–16011. doi: 10.1073/pnas.1516131112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges TJ, Lopes RL, Pinho NG, Machado FD, Souza AP, Bonorino C. Extracellular Hsp70 inhibits pro-inflammatory cytokine production by IL-10 driven down-regulation of C/EBPβ and C/EBPδ. Int J Hyperth. 2013;29:455–463. doi: 10.3109/02656736.2013.798037. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ, Jr, Theriault JR. Extracellular heat shock proteins in cell signaling. FEBS Lett. 2007;581:3689–3694. doi: 10.1016/j.febslet.2007.04.044. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S. Growth-associated gene expression after stroke: evidence for a growth-promoting region in peri-infarct cortex. Exp Neurol. 2005;193(2):291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Demyanenko S, Uzdensky A. Profiling of signaling proteins in penumbra after focal photothrombotic infarct in the rat brain cortex. MolNeurobiol. 2017;54(9):6839–6856. doi: 10.1007/s12035-016-0191-x. [DOI] [PubMed] [Google Scholar]
- Demyanenko SV, Panchenko SN, Uzdensky AB. Expression of neuronal and signaling proteins in penumbra around a photothrombotic infarction core in rat cerebral cortex. Biochemistry (Mosc) 2015;80(6):790–799. doi: 10.1134/S0006297915060152. [DOI] [PubMed] [Google Scholar]
- Demyanenko S, Berezhnaya E, Neginskaya M, Rodkin S, Dzreyan V, Pitinova M. Сlass II histone deacetylases in the post-stroke recovery period-expression, cellular, and subcellular localization-promising targets for neuroprotection. J Cell Biochem. 2019;120(12):19590–19609. doi: 10.1002/jcb.29266. [DOI] [PubMed] [Google Scholar]
- Doeppner TR, Kaltwasser B, Fengyan J, Hermann DM, Bähr M. TAT-Hsp70 induces neuroprotection against stroke via anti-inflammatory actions providing appropriate cellular microenvironment for transplantation of neural precursor cells. J Cereb Blood Flow Metab. 2013;33(11):1778–1788. doi: 10.1038/jcbfm.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evgen’ev MB, Garbuz DG, Zatsepina OG. (2014) Heat Shock Proteins and whole body adaptation to extreme environments. Springer. XVII: 218
- Evgen’ev MB, Krasnov GS, Nesterova IV, Garbuz DG, Karpov VL, Morozov AV, Snezhkina A, Samokhin A, SergeevA BNV. MolecularmechanismsunderlyingneuroprotectiveeffectofeHsp70 intransgenic 5XFADmice. J Alzheimer’s Desease. 2017;59(4):1415–1426. doi: 10.3233/JAD-170398. [DOI] [PubMed] [Google Scholar]
- Fang S, Yan B, Wang D, Bi X, Zhang Y, He J, Xu H, Yang Y, Kong J, Wu J, Li XM. Chronic effects of venlafaxine on synaptophysin and neuronal cell adhesion molecule in the hippocampus of cerebral ischemic mice. Biochem Cell Biol. 2010;88(4):655–663. doi: 10.1139/O10-015. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM. Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol. 2003;62(4):329–339. doi: 10.1093/jnen/62.4.329. [DOI] [PubMed] [Google Scholar]
- Fonteles AA, de Souza CM, de Sousa Neves JC, Menezes AP, Santos do Carmo MR, Fernandes FD, de Araújo PR, de Andrade GM. Rosmarinic acid prevents against memory deficits in ischemic mice. Behav Brain Res. 2016;297:91–103. doi: 10.1016/j.bbr.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G (2008) The mouse brain in stereotaxic coordinates, 1, 3th edn. Elsevier Academic Press, Amsterdam, p 325
- Gabai VL, Meriin AB, Yaglom JA, Volloch V, Sherman MY. Role of HSP70 in regulation of stress-kinase JNK: implications in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Sinha D, Mukherjee S, Biswas R, Biswas T. LPS stimulates and Hsp70 down-regulates TLR4 to orchestrate differential cytokine response of culture-differentiated innate memory CD8(+) T cells. Cytokine. 2015;73:44–52. doi: 10.1016/j.cyto.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, Sapolsky R, Steinberg G, Hu B, Yenari MA. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207(Pt 18):3213–3220. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- Gorup D, Bohaček I, Miličević T, Pochet R, Mitrečić D, Križ J, Gajović S. Increased expression and colocalization of GAP43 and CASP3 after brain ischemic lesion in mouse. Neurosci Lett. 2015;597:176–182. doi: 10.1016/j.neulet.2015.04.042. [DOI] [PubMed] [Google Scholar]
- Gurskiy YG, Garbuz DG, Soshnikova NV, Krasnov AN, Deikin A, Lazarev VF, Sverchinskyi D, Margulis BA, Zatsepina OG, Karpov VL, Belzhelarskaya SN, Feoktistova E, Georgieva SG, Evgen’ev MB. The development of modified human Hsp70 (HSPA1A) and its production in the milk of transgenic mice. Cell Stress Chaperones. 2016;21(6):1055–1064. doi: 10.1007/s12192-016-0729-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Yang RC, Lin SJ, Liou SF, Dai ZK, Yeh JL, Wu JR. Exogenous heat shock cognate protein 70 pretreatment attenuates cardiac and hepatic dysfunction with associated anti-inflammatory responses in experimental septic shock. Shock. 2014;42:540–547. doi: 10.1097/SHK.0000000000000254. [DOI] [PubMed] [Google Scholar]
- James SL, Castle CD, Dingels ZV, et al. (2020) Global injury morbidity and mortality from 1990 to 2017: results from the Global Burden of Disease Study 2017. Inj Prev injuryprev-2019-043494. doi:10.1136/injuryprev-2019-043494 [DOI] [PMC free article] [PubMed]
- Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16(1):142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsy M, Brock A, Guan J, Taussky P, Kalani MY, Park MS. Neuroprotective strategies and the underlying molecular basis of cerebrovascular stroke. Neurosurg Focus. 2017;42:E3. doi: 10.3171/2017.1.FOCUS16522. [DOI] [PubMed] [Google Scholar]
- Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF. Pathogenic mechanisms following ischemic stroke. Neurol Sci. 2017;38(7):1167–1186. doi: 10.1007/s10072-017-2938-1. [DOI] [PubMed] [Google Scholar]
- Kim JY, Han Y, Lee JE, Yenari MA. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin Ther Targets. 2018;22(3):191–199. doi: 10.1080/14728222.2018.1439477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Kim M, Yoon BW, Kim YJ, Ma SJ, Roh JK, Lee JS, Seo JS. Targeted hsp70.1 disruption increases infarction volume after focal cerebral ischemia in mice. Stroke. 2001;32(12):2905–2912. doi: 10.1161/hs1201.099604. [DOI] [PubMed] [Google Scholar]
- Lee JK, Park MS, Kim YS, Moon KS, Joo SP, Kim TS, Kim JH, Kim SH. (2007) Photochemically induced cerebral ischemia in a mouse model. Surg Neurol 67(6):620–625. 10.1016/j.surneu.2006.08.077 [DOI] [PubMed]
- Lee K, Jeong J, Yoo C. Positive feedback regulation of heat shock protein 70 (Hsp70) is mediated through toll-like receptor 4-PI3K/Akt-glycogen synthase kinase-3β pathway. Exp Cell Res. 2013;319(1):88–95. doi: 10.1016/j.yexcr.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Li GL, Farooque M, Holtz A, Olsson Y. Increased expression of growth-associated protein 43 immunoreactivity in axons following compression trauma to rat spinal cord. ActaNeuropathol. 1996;92(1):19–26. doi: 10.1007/s004010050484. [DOI] [PubMed] [Google Scholar]
- Li Y, Jiang N, Powers C, Chopp M. Neuronal damage and plasticity identified by microtubule-associated protein 2, growth-associated protein 43, and cyclin D1 immunoreactivity after focal cerebral ischemia in rats. Stroke. 1998;29(9):1972–1980. doi: 10.1161/01.str.29.9.1972. [DOI] [PubMed] [Google Scholar]
- Liu W, Xue X, Xia J, Liu J, Qi Z. Swimming exercise reverses CUMS-induced changes in depression-like behaviors and hippocampal plasticity-related proteins. J Affect Disord. 2018;227:126–135. doi: 10.1016/j.jad.2017.10.019. [DOI] [PubMed] [Google Scholar]
- Lu R, Tan M, Wang H, Xie A, Yu J, Tan L. Heat shock protein 70 in Alzheimer’s disease. Biomed Res Int. 2014;2014:435203. doi: 10.1155/2014/435203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Y, Tang H, Li H, Zhao R, Huang Q, Liu J. Recent advances in the development of neuroprotective agents and therapeutic targets in the treatment of cerebral ischemia. Eur J Med Chem. 2019;162:132–146. doi: 10.1016/j.ejmech.2018.11.014. [DOI] [PubMed] [Google Scholar]
- Matsumori Y, Hong SM, Aoyama K, Fan Y, Kayama T, Sheldon RA, Vexler ZS, Ferriero DM, Weinstein PR, Liu J. Hsp70 overexpression sequesters AIF and reduces neonatal hypoxic/ischemic brain injury. J Cereb Blood Flow Metab. 2005;25(7):899–910. doi: 10.1038/sj.jcbfm.9600080. [DOI] [PubMed] [Google Scholar]
- Miyake K, Yamamoto W, Tadokoro M, Takagi N, Sasakawa K, Nitta A, Furukawa S, Takeo S. Alterations in hippocampal GAP-43, BDNF, and L1 following sustained cerebral ischemia. Brain Res. 2002;935(1–2):24–31. doi: 10.1016/s0006-8993(02)02420-4. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The science of stroke: mechanisms in search of treatments. Neuron. 2010;67(2):181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DD, Caron AW, Bourget L, Meriin AB, Sherman MY, Morimoto RI, Massie B. The chaperone function of hsp70 is required for protection against stress-induced apoptosis. Mol Cell Biol. 2000;20:7146–7159. doi: 10.1128/mcb.20.19.7146-7159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL, American Heart Association Stroke Council Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46–e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- Puyal J, Ginet V, Clarke PG. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: a challenge for neuroprotection. ProgNeurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Radak D, Katsiki N, Resanovic I, Jovanovic A, Sudar-Milovanovic E, Zafirovic S, Mousad SA, Isenovic ER. Apoptosis and acute brain ischemia in ischemic stroke. CurrVascPharmacol. 2017;15(2):115–122. doi: 10.2174/1570161115666161104095522. [DOI] [PubMed] [Google Scholar]
- Rajah GB, Ding Y. Experimental neuroprotection in ischemic stroke: a concise review. Neurosurg Focus. 2017;42(4):E2. doi: 10.3171/2017.1.FOCUS16497. [DOI] [PubMed] [Google Scholar]
- Rajdev S, Hara K, Kokubo Y, Mestril R, Dillmann W, Weinstein PR, Sharp FR. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47(6):782–791. [PubMed] [Google Scholar]
- Robinson MB, Tidwell JL, Gould T, Taylor AR, Newbern JM, Graves J, Tytell M, Milligan CE. Extracellular heat shock protein 70: critical component for motoneuron survival. J Neurosci. 2005;25:9735–9745. doi: 10.1523/JNEUROSCI.1912-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodkin S, Khaitin A, Pitinova M, Dzreyan V, Guzenko V, Rudkovskii M, Sharifulina S, Uzdensky A. The localization of p53 in the crayfish mechanoreceptor neurons and its role in axotomy-induced death of satellite glial cells remote from the axon transection site. J Mol Neurosci. 2020;70(4):532–541. doi: 10.1007/s12031-019-01453-2. [DOI] [PubMed] [Google Scholar]
- Rozhkova E, Yurinskaya M, Zatsepina O, Garbuz D, Murashev A, Ostrov V, Boris M, Evgenev M, Vinokurov M. Exogenous mammalian extracellular HSP70 reduces endotoxin manifestations at the cellular and organism levels. Ann New-York Acad Sci. 2010;1197:94–107. doi: 10.1111/j.1749-6632.2009.05375.x. [DOI] [PubMed] [Google Scholar]
- Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp 27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4(6):685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevtsov MA, Nikolaev BP, Yakovleva LY, Dobrodumov AV, Dayneko AS, Shmonin AA, Vlasov TD, Melnikova EV, Vilisov AD, Guzhova IV, Ischenko AM, Mikhrina AL, Galibin OV, Yakovenko IV, Margulis BA. Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats. Drug Des DevelTher. 2014;8:639–650. doi: 10.2147/DDDT.S62024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20(17):6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE. Acute increase in expression of growth associated protein GAP-43 following cortical ischemia in rat. Neurosci Lett. 1993;162(1–2):51–54. doi: 10.1016/0304-3940(93)90557-2. [DOI] [PubMed] [Google Scholar]
- Sun Y, Ouyang YB, Xu L, Chow AM, Anderson R, Hecker JG, Giffard RG. The carboxyl-terminal domain of inducible Hsp70 protects from ischemic injury in vivo and in vitro. J Cereb Blood Flow Metab. 2006;26(7):937–950. doi: 10.1038/sj.jcbfm.9600246. [DOI] [PubMed] [Google Scholar]
- Troyanova NI, Shevchenko MA, Boyko AA, Mirzoyev RR, Pertseva MA, Kovalenko EI, Sapozhnikov AM. Modulating effect of extracellular HSP70 on generation of reactive oxygen species in populations of phagocytes. Bioorg.Khim. 2015;41(3):305–315. doi: 10.1134/s1068162015030097. [DOI] [PubMed] [Google Scholar]
- Tsuchiya D, Hong S, Matsumori Y, Kayama T, Swanson RA, Dillman WH, Liu J, Panter SS, Weinstein PR. Overexpression of rat heat shock protein 70 reduces neuronal injury after transient focal ischemia, transient global ischemia, or kainic acid-induced seizures. Neurosurgery. 2003;53(5):1179–1187. doi: 10.1227/01.neu.0000090341.38659.cf. [DOI] [PubMed] [Google Scholar]
- Tytell M, Davis A, Giles J, Snider L, Xiao R, Dozier S, Presley T, Kavanagh K. Alfalfa-derived HSP70 administered intranasally improves insulin sensitivity in mice. Cell Stress Chaperones. 2018;23(2):189–194. doi: 10.1007/s12192-017-0835-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzdensky AB. Photothrombotic stroke as a model of ischemic stroke. Transl Stroke Res. 2018;9(5):437–451. doi: 10.1007/s12975-017-0593-8. [DOI] [PubMed] [Google Scholar]
- Uzdensky AB. Apoptosis regulation in the penumbra after ischemic stroke:expression of pro- and antiapoptotic proteins. Apoptosis. 2019;24(9–10):687–702. doi: 10.1007/s10495-019-01556-6. [DOI] [PubMed] [Google Scholar]
- Venkat P, Shen Y, Chopp M, Chen J. Cell-based and pharmacological neurorestorative therapies for ischemic stroke. Neuropharmacology. 2018;134(Pt B):310–322. doi: 10.1016/j.neuropharm.2017.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinokurov M, Ostrov V, Yurinskaya M, Garbuz D, Murashev A, Antonova O, Evgen’ev M. Recombinant human Hsp70 protects against lipoteichoic acid-induced inflammation manifestations at the cellular and organismal levels. Cell Stress Chaperones. 2012;17:89–101. doi: 10.1007/s12192-011-0288-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegele H, Müller L, Buchner J. Hsp70 and Hsp90 – a relay team for protein folding. Rev Physiol Biochem Pharmacol Rev Physiol Biochem Pharmacol. 2004;151:1–44. doi: 10.1007/s10254-003-0021-1. [DOI] [PubMed] [Google Scholar]
- Weinstein PR, Hong S, Sharp FR. Molecular identification of the ischemic penumbra. Stroke. 2004;35(11 Suppl 1):2666–2670. doi: 10.1161/01.STR.0000144052.10644.ed. [DOI] [PubMed] [Google Scholar]
- Wheeler TC, Chin LS, Li Y, Roudabush FL, Li L. Regulation of synaptophysin degradation by mammalian homologues of seven in absentia. J Biol Chem. 2002;277(12):10273–10282. doi: 10.1074/jbc.M107857200. [DOI] [PubMed] [Google Scholar]
- Yenari MA, Liu J, Zheng Z, Vexler ZS, Lee JE, Giffard RG. Antiapoptotic and anti-inflammatory mechanisms of heat-shock protein protection. Ann N Y Acad Sci. 2005;1053:74–83. doi: 10.1196/annals.1344.007. [DOI] [PubMed] [Google Scholar]
- Yurinskaya M, Zatsepina OG, Vinokurov MG, Bobkova NV, Garbuz DG, Morozov AV, Kulikova DA, Mitkevich VA, Makarov AA, Funikov SY, Evgen’ev MB. The fate of exogenous human HSP70 introduced into animal cells by different means. Curr Drug Deliv. 2015;12(5):524–532. doi: 10.2174/1567201812666150724094207. [DOI] [PubMed] [Google Scholar]
- Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan R, Leng X, Liu X, Wang X, Gong J, Yan L, Wang L, Wang Y, Wang X, Qian LJ. Heat shock protein 70 is secreted from endothelial cells by a non-classical pathway involving exosomes. Biochem Biophys Res Commun. 2009;387:229–233. doi: 10.1016/j.bbrc.2009.06.095. [DOI] [PubMed] [Google Scholar]
- Zhan X, Ander BP, Liao IH, Hansen JE, Kim C, Clements D, Weisbart RH, Nishimura RN, Sharp FR. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41(3):538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Qiu B, Wang J, Yao Y, Wang C, Liu J. Effects of BDNF-transfected BMSCs on neural functional recovery and synaptophysin expression in rats with cerebral infarction. MolNeurobiol. 2017;54(5):3813–3824. doi: 10.1007/s12035-016-9946-7. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28(1):53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- Zhou Z, Lu J, Liu WW, Manaenko A, Hou X, Mei Q, Huang JL, Tang J, Zhang JH, Yao H, Hu Q. Advances in stroke pharmacology. PharmacolTher. 2018;191:23–42. doi: 10.1016/j.pharmthera.2018.05.012. [DOI] [PubMed] [Google Scholar]