Abstract
Collagen has been widely shown to promote osteogenesis of bone marrow mesenchymal stromal cells (BM-MSCs). Due to the invasive procedure of obtaining BM-MSCs, MSCs from other tissues have emerged as a promising alternative for regenerative therapy. MSCs originated from different sources, exhibiting different differentiation potentials. Therefore, the applicability of collagen type I (COL), combining with amniotic membrane (AM)-MSCs was examined through proliferation and differentiation assays together with the expression of surface markers and genes associated with stemness and differentiation under basal or induction conditions. No increase in cell growth was observed because AM-MSCs might be directed toward spontaneous osteogenesis. This was evidenced by the calcium deposition and elevated expression of osteogenic genes when AM-MSCs were cultured in collagen plate with basal media. Under the osteogenic condition, reciprocal expression of OCN and CEBPA suggested a shift toward adipogenesis. Surprisingly, adipogenic genes were not elevated upon adipogenic induction, although oil droplets deposition was observed. In conclusion, our findings demonstrated that collagen causes spontaneous osteogenesis in AM-MSCs. However, the presence of exogenous inductors could shift the direction of adipo-osteogenic gene regulatory network modulated by collagen.
Keywords: adipogenesis, Collagen, mesenchymal stromal cell, osteogenesis, stemness, surface markers
Introduction
Mesenchymal stromal cells (MSCs) have been traditionally isolated from the bone marrow. However, due to its invasive and painful procedure for the donor, MSCs derived from adult tissues such as adipose, amniotic membrane (AM), umbilical cord (UC) have been used as alternative sources for tissue engineering and regenerative medicine. Besides that, they have less ethical constraints, more accessible and low immunogenicity [1]. In addition to the prominent roles of MSCs for regenerative medicine, their profound functions as medicinal signalling cells have recently attracted great interest.
Nonetheless, MSCs need to be manipulated in vitro to obtain enough cell number for therapeutic purposes. To achieve this, natural or synthetic biomaterials have been extensively investigated for their potentials in maintaining stemness and directing the differentiation of MSCs [2–4]. Collagen type 1, a protein abundantly found in the extracellular matrix has been broadly shown to promote proliferation, survival, adhesion and osteogenesis in bone marrow MSCs [5,6]. It has also been demonstrated to promote endoderm differentiation of human embryonic stem cells [7]. However, the effects of collagen on other MSCs such as AM-MSCs are not well studied.
AM-MSCs as a promising source have been shown to be capable of self-renewal and multilineage differentiation. Emerging evidence has revealed that MSCs from different origins or culture conditions exhibiting discrepancy in term of their biological potentials such as proliferation, differentiation capability as well as gene expression profile [8–10]. It has been reported that AM-MSCs and UC-MSCs exhibited differences in cell growth and gene expression patterns [11]. For instance, AM-MSCs were shown to have the highest osteogenic potential compared with other neonatal MSCs [10]. Conversely, Araújo et al. demonstrated that neonatal MSCs were more efficient toward osteogenesis than adipogenesis [12]. Therefore, there is a necessity to evaluate how biomaterials modulate different types of MSCs before utilizing them for therapeutic purposes. In the present study, we examined the modulating effects of collagen type I (COL) on AM-MSCs covering the aspects of morphology, proliferation, differentiation, as well as the expression profiles of genes governing immunophenotyping, stemness and adipo-osteogenic commitment.
Materials and methods
Sample collection
The study was approved by the Ethics and Research Committee of Universiti Malaysia Sabah with approval code: JKEtika 1/16 (1). The informed consent was also obtained from patients. Full-term placenta from healthy newborn delivered by cesarean section was collected from Damai Specialist Hospital and KPJ Sabah Specialist Hospital, Kota Kinabalu. The samples were transferred immediately to the laboratory for cell isolation.
Isolation of AM-MSCs
The isolation process of AM-MSCs was done according to the isolation method published by Fatimah et al. [13] with some modifications. AM was peeled off aseptically from the placenta and washed with 1× Dulbecco’s phosphate-buffered saline (DPBS) (Gibco-Invitrogen, Carlsbad, CA, U.S.A.) several times to remove red blood cells. Then the membrane was cut into small pieces with a size of 2 × 2 cm2. The tissues (20 pieces) were proceeded to serial digestions with 0.05% trypsin-EDTA (Gibco-Invitrogen, Carlsbad, CA, U.S.A.) for 10 min initially followed by another three-times digestion for 30 min each. After that, the denuded membranes were washed with 1× DPBS followed by digestion using 0.1% collagenase type I (Worthington, Minnesota, U.S.A.) for 40 min at 37°C with constant agitation at 180 rpm. The cell suspension was collected and centrifuged at 5000 rpm for 5 min at room temperature. The resulting cell pellet was resuspended with culture medium and transferred to a 25-cm2 tissue culture flask.
Cell culture, cell count and morphological observation
Isolated cells were cultured using Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 (DMEM/F12) (Gibco-Invitrogen, Carlsbad, CA, U.S.A.) containing 10% fetal bovine serum (Gibco-Invitrogen, Carlsbad, CA, U.S.A.), 1% GlutaMAX (Gibco-Invitrogen, Carlsbad, CA, U.S.A.), 1% ascorbic acid (Merck, Darmstadt, Germany) and 1% antibiotic–antimycotic (Gibco-Invitrogen, Carlsbad, CA, U.S.A.). Medium was changed every 3 days until cells reached 80–90% confluency. This was followed by subculturing using 0.125% trypsin-EDTA. Cells were also cultured on microplates coated with COL (#152034: 6-well plates, #152036: 96-well plates) purchased from Thermo Fisher Scientific (Waltham, MA, U.S.A.). Cell viability assay was determined using 0.4% Trypan Blue solution (Gibco-Invitrogen, Carlsbad, CA, U.S.A.). Cells at specific passages were trypsinized at day 7 to obtain total cell number and their morphology at early, and late passage (P) was observed using an inverted microscope.
Cell proliferation assay
Cell proliferation assay was performed using PrestoBlue Cell Viability Reagent (Thermo Fisher Scientific, Waltham, MA, U.S.A.) according to the manufacturer’s procedure. Cells at a density of 5000 cells/well were seeded in 96-well microplates coated with or without collagen. After overnight incubation, the PrestoBlue solution was added to the cells and incubated for 1 h at 37°C in the dark. The fluorescence signal was measured at the excitation wavelength of 560 nm and an emission wavelength of 590 nm. The measurement was obtained every 2 days. Data were obtained from at least three independent experiments with each of them being performed in triplicates.
Differentiation assays
A total of 5.0 × 103 cells were first grown on 96-well plate, then induced at day 3 using StemPro osteogenesis and StemPro adipogenesis differentiation kit (Gibco-Invitrogen, Carlsbad, CA, U.S.A.). Media were changed every 3 days, and the cells were cultured for 2–3 weeks. After induction, cells were washed with 1× DPBS and fixed with 10% formalin (Merck, Darmstadt, Germany) for 20 min at room temperature. Calcium deposits and lipid droplets were stained using Alizarin Red S (ScienCell Research Laboratories, Carlsbad, CA, U.S.A.) and Oil Red O (Merck, Darmstadt, Germany), respectively, according to the manufacturers protocols. After washing, cells were observed under an inverted microscope.
RNA extraction and RT-PCR
Cells with a density of 3 × 104 cells were grown in each well of six-well microplate. Total RNA was extracted using TransZol Up Plus RNA Kit (Transgen Biotech, Beijing, China). RT-PCR and RT-qPCR were carried out using TransScript One-Step RT-PCR SuperMix (Transgen Biotech, Beijing, China) and OneStep iTaq Universal SYBR Green (Bio-Rad Laboratories, Irvine, CA, U.S.A.), respectively. Procedures were performed following the manufacturer’s instructions, except the annealing step that was performed at 55–58°C. Primers targeting stemness and differentiation genes were listed in Table 1. Surface markers were synthesized based on sequences published by Ali et al. [14]. PCR products were observed through 2% agarose gel electrophoresis. For semi-quantitative PCR, the gene expression levels in each sample were normalized to their respective β-actin expression levels and compared with control. For RT-qPCR, the relative expression was obtained by calculating fold change using the 2−ΔΔCt method for each pairwise analysis. Fold change ≥ 1.5 is considered a significant increase or decrease in expression.
Table 1. List of sequences of forward (F) and reverse (R) primers used in PCR.
| Genes | Primer sequences |
|---|---|
| OCT4 | F 5′-TGAGTAGTCCCTTCGCAAGC-3′ |
| R 5′-TTAGCCAGGTCCGAGGATCA-3′ | |
| NANOG | F 5′-ACCAGAACTGTGTTCTCTTCCACC-3′ |
| R 5′-CCATTGCTATTCTTCGGCCAGTTG-3′ | |
| RUNX2 | F 5′-GACCAGTCTTACCCCTCCTACC-3′ |
| R 5′-CTGCCTGGCTCTTCTTACTGAG-3′ | |
| OCN | F 5′-GTGCAGAGTCCAGCAAAGGT-3′ |
| R 5′-TCAGCCAACTCGTCACAGTC-3′ | |
| C/EBPB | F 5′-TTTGTCCAAACCAACCGCAC-3′ |
| R 5′-GCATCAACTTCGAAACCGGC-3′ | |
| C/EBPA | F 5′-GCAAACTCACCGCTCCAATG-3′ |
| R 5′-CTTCTCTCATGGGGGTCTGC-3′ | |
| ACTB | F 5′- GTCATTCCAAATATGAGATGCGT-3′ |
| R 5′- GCTATCACCTCCCCTGTGTG-3′ |
Statistical analysis
All experiments were performed using three independent biological samples and expressed as mean ± standard deviation (SD). Statistical analysis was done using paired t test, and P-value <0.05 was considered significant.
Results
Morphology and proliferation of AM-MSCs
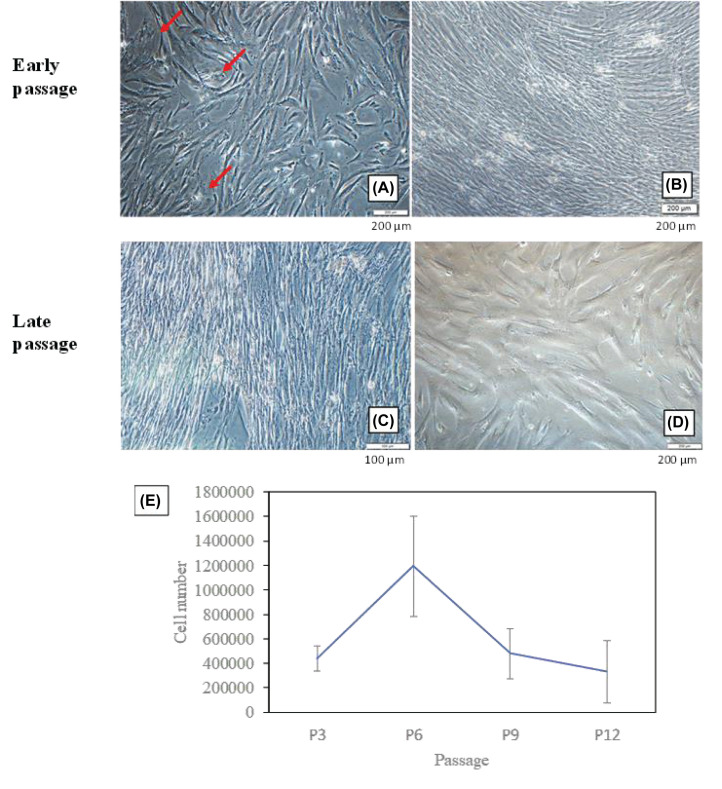
Due to the heterogeneity of freshly isolated MSCs, we observed cell morphology from early to late passages. At P3 (Figure 1A), spindle-shaped cells and a few round-shaped cells (red arrows) were seen in the culture. However, starting from P6 onward, we noticed the disappearance of round-shaped cells (Figure 1B–D). From our observation, if the majority of the isolated cells were appeared in round-shaped, the chances for cells to survive for longer passages was low (data not shown). Besides, cells at P12 became larger and longer in shape (Figure 1D). From the proliferation assay (Figure 1E), cells at P6 had the highest proliferation compared with other passages. A sharp decrease in cell count was found at late passages (P9 and P12). As the success rate of isolated cells proliferated beyond P10 was low, we had chosen P6 and P9 to represent early and late passages for further analysis.
Figure 1. The morphology and proliferation of AM-MSCs at early and late passages.
(A) P3, (B) P6, (C) P9 and (D) P12. (E) Cell numbers for each passage were calculated from three independent samples with triplicates. Red arrows indicate round-shaped cells.
Surface marker expression and differentiation potentials of AM-MSCs
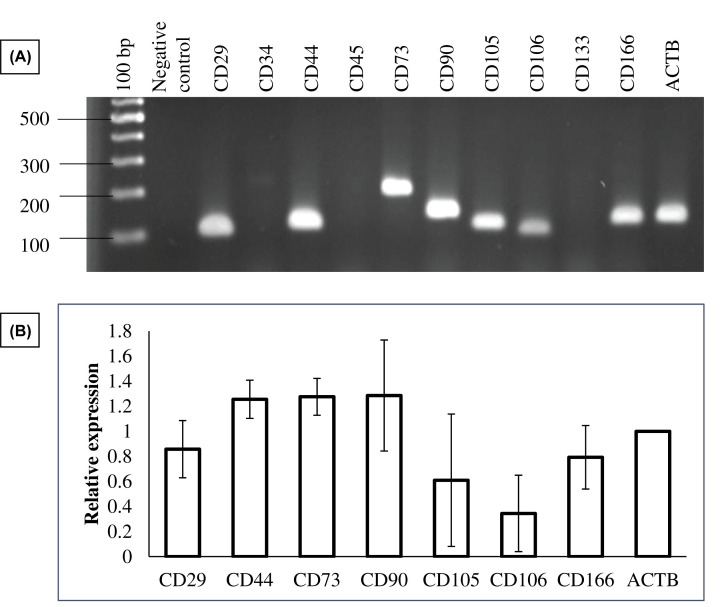
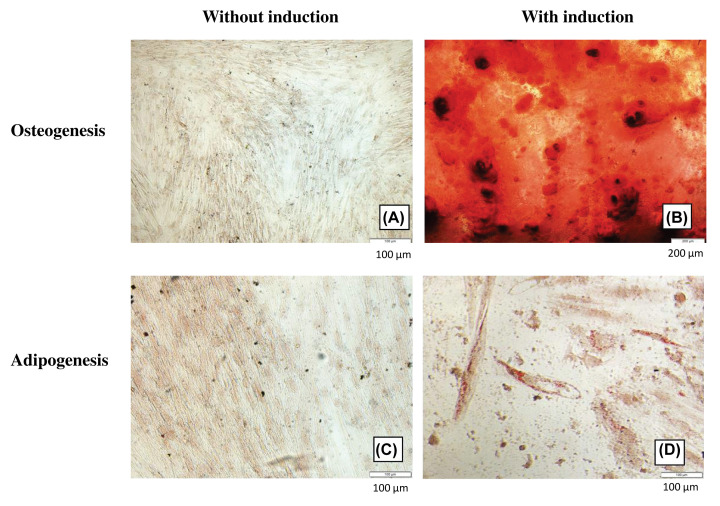
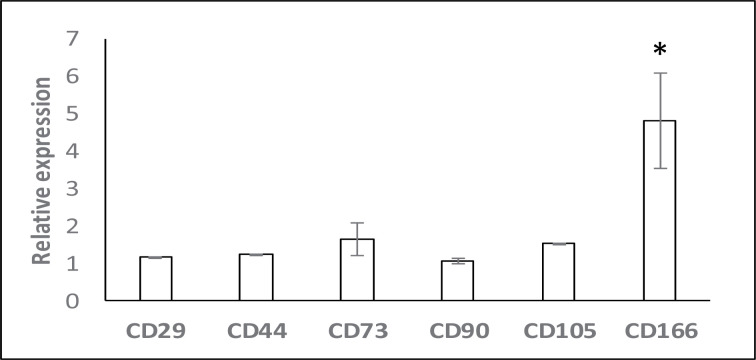
To confirm the identity of isolated cells, differentiation potentials and the mRNA expression of surface markers specific to MSCs were assessed (Figure 2). As expected, these cells displayed negative expression of both hematopoietic markers (CD34, CD45) and endothelial markers (CD133). Consistent with previous studies [10,15], isolated cells expressed all positive MSC markers: CD29, CD44, CD73, CD90, CD105, and CD166 which are frequently used for confirming the status of MSCs. Although the expression of CD106 is controversial, low expression of this surface marker has been reported in AM-MSCs [16]. CD271 which expresses in AM-MSCs but not epithelial stem cells [17] was confirmed through immunofluorescence staining (unpublished data). The presence of calcium deposition in osteocytes (Figure 3B) and lipid accumulation in adipocytes (Figure 3D) suggested that the isolated cells could differentiate into osteogenic and adipogenic lineages upon induction compared to the controls (Figure 3A,B).
Figure 2. The mRNA expression of surface markers in AM-MSCs.
(A) A representative agarose gel photo obtained by RT-PCR. (B) Relative expression of positive CD markers.
Figure 3. Differentiation potentials of AM-MSCs.
Cells cultured in the basal and induction media were stained Alizarin Red S (A,B) and Oil Red O (C,D), respectively.
Effects of collagen on cell morphology, proliferation and surface markers
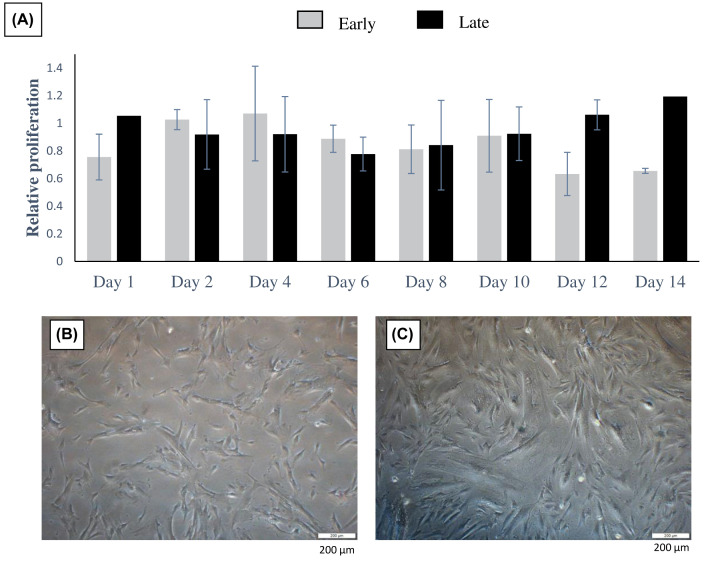
To ascertain the possible effect of collagen (COL) on the proliferation of AM-MSCs, cells were cultured on the collagen-coated plate (AM-MSCs-COL). As shown in Figure 4A, AM-MSCs-COL at the early passage showed comparable cell growth with the control as no obvious changes were observed up to day 10. However, drastic decreases in cell proliferation were shown on days 12–14. On the other hand, the growth pattern at late passage was steadily maintained throughout the culturing periods. We also found that some samples could survive up to 20 days while cells at the early passage were mostly dead and detached (data not shown). This indicated that collagen potentially prolongs cell survival, especially at the later passage, but more sample number is required to confirm this. No apparent difference between the early and late passages in term of cell morphology except cells looked less dense compared with those in collagen plate after 10 days (Figure 4). Among the positive surface markers, only the expression of CD166 was significantly increased to five-fold when compared with control (Figure 5). The gene expression of other surface markers was mostly unaltered in the presence of collagen.
Figure 4. The effects of collagen on cell proliferation and morphology.
(A) Relative cell proliferation of AM-MSCs cultured on collagen to control. The morphology of cells cultured on collagen at early (B) and late (C) passage.
Figure 5. The effects of collagen on positive surface markers.
Semi-quantitative analysis of CD markers expression. Asterisk (*) indicates statistical significance.
Collagen promotes osteogenic differentiation
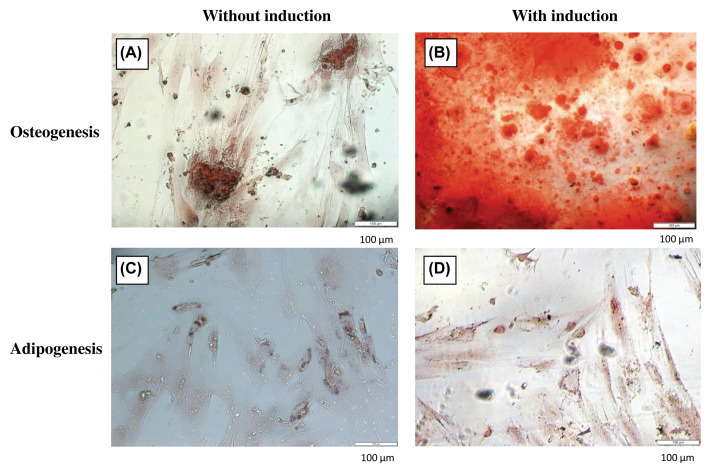
We further investigated whether collagen influences lineage commitment of AM-MSCs in the presence of exogenous inductors or without. From our results, we noticed that collagen was capable of inducing osteogenesis spontaneously in basal media (Figure 6A,C). AM-MSCs retained their osteogenic and adipogenic differentiation potentials when cultured in collagen plates (Figure 6B,D). The increase in calcium deposition was observed in AM-MSCs-COL (Figure 6B). Besides that, collagen had also caused more oil droplets accumulation upon adipogenic induction (Figure 6D). These results indicated a potential synergistic effect of collagen and exogenous inductors.
Figure 6. The effects of collagen on osteogenic and adipogenic differentiation of AM-MSC.
Cells cultured in the basal and induction media were stained with Alizarin Red S (A,B) and Oil Red O (C,D), respectively.
Effects of collagen on the expression of stemness and differentiation genes
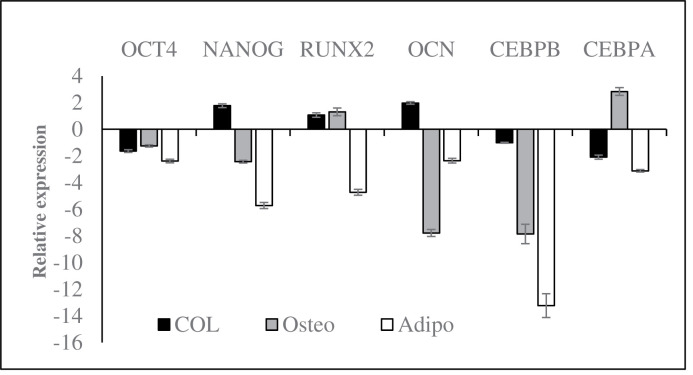
To confirm the observation shown in differentiation caused by collagen, we determined the expression levels of genes associated with stemness and differentiation. In basal media (Figure 7, dark), collagen was found to suppress the expression of OCT4 while increasing the expression of osteogenic genes (RUNX2 and OCN). This was in line with the spontaneous osteogenesis we observed above. We noticed the down-regulation of OCT4, NANOG, OCN and CEBPB in AM-MSCs-COL, while the expression of RUNX2 and CEBPA was up-regulated when cells were cultured in osteogenic media (Figure 7, grey). Surprisingly, the expression of CEBPA and CEBPB was down-regulated even in the presence of adipogenic media (Figure 7, white). This finding was negatively correlated with the oil deposition observed in adipogenesis assay. Nevertheless, this is insufficient to conclude that the inhibition of adipogenesis in AM-MSCs-COL as the gene regulatory may be activated via unknown mechanisms.
Figure 7. Collagen altered the expression of genes associated with stemness and differentiation in basal and induction media.
Discussion
Biomaterials with the ability to enhance proliferation and direct desired lineage differentiation are promising in overcoming inherent bottlenecks of MSCs. Comparative analysis of MSCs obtained from various sources has uncovered their distinct physiological properties that might restrict or favor their functional roles in a specific application. Therefore, combining biomaterials with different MSCs may have divergent implications on gene regulatory network. AM is an appealing source of MSCs owing to its high accessibility and high recovery rate and multilineage potentials [18,19]. Collagen has been widely used as a scaffold and shown in directing osteogenic commitment in bone marrow mesenchymal stromal cells (BM-MSCs). In the present study, freshly isolated AM-MSCs at early and late passages were subjected to the examination of cell morphology, expression of MSC surface markers and differentiation potentials to confirm their stemness properties. Based on the morphological and proliferation studies, AM-MSCs at early passage was used for further investigation to avoid inconsistent results
After ensuring the identity of isolated cells, they were applied on the collagen surface. Our results showed not many differences in term of proliferation when compared with cells cultured on tissue culture plate (control). However, this matrix has been shown to promote the proliferation of other MSCs [6,20]. From Alizarin staining, we noticed calcium deposition in AM-MSCs-COL without exogenous induction, suggesting the occurrence of spontaneous differentiation. Both collagen alone or collagen with other biomaterials have also been shown to induce osteogenesis in the 2D culture of human MSCs [21–24]. Besides, spontaneous differentiation was also observed in 3D microcarriers [24]. Similarly, this is in line with previous studies showing the reciprocal relationship between proliferation and differentiation in the basal medium [5,25].
On another note, our results showed that collagen elevated CD166 expression in AM-MSCs, but there was no significant alteration in the expression of other positive markers. CD166 (activated leucocyte cell adhesion molecule, ALCAM) is a transmembrane glycoprotein that belongs to the immunoglobulin superfamily [26]. They are cell adhesion molecules, thus increase in its expression could be due to AM-MSCs attached more tightly to collagen matrix as demonstrated by more closely packed morphology in Figure 5. CD166 has also been identified as cancer or cancer stem cell marker and involved in metastasis of various tumors [27–29]. However, only a few studies indicated the link between CD166 and other surface markers. For instance, reduction in CD166 and CD44 expression was observed in shRNA CD90 MSCs, leading to osteogenic differentiation in amniotic fluid, dental pulp and adipose MSCs [30]. However, collagen did not alter the expression levels of CD44 and CD90 in our study and also MSC markers mentioned by Parolini et al. [31]. High expression of CD166 surface marker was only reported in osteoarthritis (OA) MSCs showing increased expression of cartilage markers RUNX2 and COL10A1. It was hypothesized that OA-MSCs drive the OA progression [32]. To date, the role of CD166 in MSCs is still unclear.
Furthermore, the occurrence of spontaneous osteogenesis was supported by the up-regulation of osteogenic genes (RUNX2 and OCN). In contrast, the adipogenic genes (CEBPB and CEBPA) and OCT4 were simultaneously down-regulated. Although NANOG is also one of the master regulators of pluripotency, suppression of NANOG expression was not observed in AM-MSCs-COL cultured in basal media. It is well known that OCT4, SOX2 and NANOG are prominent in maintaining pluripotency of ESCs and MSCs despite conflicting findings [33–35]. According to Pierantozzi et al., NANOG was only detected in MSCs after in vitro expansion, and its expression was not associated with proliferative or differentiation potentials of MSCs [34]. The overexpression of NANOG was found to promote the proliferation and osteogenesis of human MSCs but impeded its commitment toward adipogenesis [36]. In contrast, the decrease in proliferation and differentiation of human dental pulp stem cells were observed after depletion of OCT4 and NANOG [37]. These findings demonstrated that NANOG might play a role in regulating lineage differentiation.
When examining the implication of osteogenic media on AM-MSCs-COL, we noticed the decreased NANOG and OCN expression levels were accompanied by an increase in the expression of CEBPA. This demonstrates a potential shift from differentiated osteocytes to adipocyte [38], as less calcium deposition was seen in Alizarin Red stained AM-MSCs. A parallel event of adipo-osteogenesis upon BMP2 induction has been reported by Ponce et al. [39], suggesting inverse expression of osteogenic and adipogenic genes was not exclusive.
Surprisingly, the down-regulation of adipogenic genes (CEBPA and CEBPB) was observed when AM-MSCs-COL was induced with adipogenic media despite seeing oil droplets in Oil Red stained cells (Figure 7D). Dexamethasone (Dex) has been shown to inhibit osteogenesis by suppressing the expression of OCN through PI3K/Akt signalling pathway [40–42]. Taken together, we suspect that the presence of exogenous factors such as Dex in induction media triggers a directional switch in differentiated AM-MSCs-COL, causing imbalance and dysregulation of adipo-osteogenic gene regulatory. The roles of Dex in trans-differentiation of MSCs have been shown in many studies [43,44]. It has been reported that Dex shifted osteoblastogenesis of BM-MSCs to adipogenesis by up-regulating the expression of CEBPA through DNA hypomethylation [45]. Besides that, collagen is known as the extracellular matrix protein, providing RGD binding sites for different types of integrin receptor depending on cell type [46]. Besides, a synergistic effect between collagen and inductors could not be excluded. Thus, this may also create an extracellular landscape different from the typical scenario leading to distinct gene regulatory network. Nonetheless, the possibility of collagen in directing lineage commitments through its topography should not be neglected as revealed by many studies [47,48].
Conclusion
We have demonstrated that collagen is capable of inducing spontaneous osteogenesis of AM-MSCs in exogenous inductors free condition without affecting their intrinsic properties in adipo-osteogenic differentiation by modulating associated gene pathways. This makes it a promising biomaterial for clinical application.
Acknowledgements
The authors thank Damai Specialist Hospital and KPJ Sabah Specialist Hospital, Kota Kinabalu for providing placental samples, and Ms Warda for isolating MSCs.
Abbreviations
- AM
amniotic membrane
- BM-MSC
bone marrow mesenchymal stromal cell
- BMP2
bone morphogenetic protein 2
- COL
collagen type I
- Dex
Dexamethasone
- DPBS
Dulbecco’s phosphate-buffered saline
- MSC
mesenchymal stromal cell
- OA
osteoarthritis
- RT-qPCR
quantitative reverse transcription polymerase chain reaction
- UC
umbilical cord
Data Availability
The data that support the findings of the present study are available upon request.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by the Ministry of Higher Education [grant number TRGS0003-SG-2/2014].
Author Contribution
H.M.A. performed the experiments, analyzed and interpreted the data and wrote the manuscript. P.L.T. designed and supervised the experiments, helped in interpreting the data, revised and approved the manuscripts.
References
- 1.Han Y., Tao R., Sun T., Chai J., Xu G. and Liu J. (2013) Optimization of human umbilical cord mesenchymal stem cell isolation and culture methods. Cytotechnology 65, 819–827 10.1007/s10616-012-9528-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha C., Liechty W.B., Khademhosseini A. and Peppas N.A. (2012) Designing biomaterials to direct stem cell fate. ACS Nano 6, 9353–9358 10.1021/nn304773b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoon J.K., Kang M.L., Park J.H., Lee K.M., Shin Y.M., Lee J.W. et al. (2018) Direct control of stem cell behavior using biomaterials and genetic factors. Stem Cells Int. 2018, 8642989 10.1155/2018/8642989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hiew V.V., Simat S.F. and Teoh P.L. (2018) The advancement of biomaterials in regulating stem cell fate. Stem Cell Rev. Rep. 14, 43–57 10.1007/s12015-017-9764-y [DOI] [PubMed] [Google Scholar]
- 5.Linsley C., Wu B. and Tawil B. (2013) The effect of fibrinogen, collagen type I and fibronectin on mesenchymal stem cells growth and differentiation into osteoblasts. Tissue Eng. Part A 19, 1416–1423 10.1089/ten.tea.2012.0523 [DOI] [PubMed] [Google Scholar]
- 6.Somaiah C., Kumar A., Mawrie D., Sharma A., Patil S.D., Bhattacharyya J. et al. (2015) Collagen promotes higher adhesion, survival and proliferation of mesenchymal stem cells. PLoS ONE 10, e0145068 10.1371/journal.pone.0145068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen C.H., Petersen D.R., Moeller J.B., Hansson M. and Dufva M. (2015) Collagen type I improves the differentiation of human embryonic stem cells towards definitive endoderm. PLoS ONE 10, e0145389 10.1371/journal.pone.0145389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okolicsanyi R.K., Carmilleri E.T., Oikari C.E., Yu C., Cool S.M., Wijnen A.J.V. et al. (2015) Human mesenchymal stem cells retain multilineage differentiation capacity including neural marker expression after extended in vitro expansion. PLoS ONE 10, e0137255 10.1371/journal.pone.0137255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teng S-W., Lo Y-S., Liu W-T., Hsuan Y. and Lin W. (2017) A genome-wide comparison of mesenchymal stem cells derived from human placenta and umbilical cord. Taiwanese J. Obstet. Gynecol. 56, 664–671 10.1016/j.tjog.2017.08.016 [DOI] [PubMed] [Google Scholar]
- 10.Ma J., Wu J., Han L., Jiang X., Yan L., Hao J. et al. (2019) Comparative analysis of mesenchymal stem cells derived from amniotic membrane, umbilical cord, and chorionic plate under serum-free condition. Stem Cell Res. Ther. 10, 19 10.1186/s13287-018-1104-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegmeyer H., Broske A.M., Leddin M., Kuentzer K., Nisslbeck A.K., Hupfeld J. et al. (2013) Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 22, 2606–2618 10.1089/scd.2013.0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araujo A.B., Salton G.D., Furlan J.M., Schneider N., Angeli M.H., Laureano A.M. et al. (2017) Comparison of human mesenchymal stromal cells from four neonatal tissues: amniotic membrane, chorionic membrane, placental decidua and umbilical cord. Cytotherapy 19, 577–585 10.1016/j.jcyt.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 13.Fatimah S.S., Tan G.C., Chua K., Fariha M.M., Tan A.E. and Hayati A.R. (2013) Stemness and angiogenic gene expression changes of serial-passage human amnion mesenchymal cells. Microvasc. Res. 86, 21–29 10.1016/j.mvr.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 14.Ali H., Al-Yatama M.K., Abu-Farha M., Behbehani K. and Al Madhoun A. (2015) Multi-lineage differentiation of human umbilical cord Wharton’s jelly mesenchymal stromal cells mediates changes in the expression profile of stemness markers. PLoS ONE 10, e0122465 10.1371/journal.pone.0122465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi Y.S., Park Y-B., Ha C-W., Kim J.A., Heo J-C., Han W-J. et al. (2017) Different characteristics of mesenchymal stem cells isolated from different layers of full term placenta. PLoS ONE 12, e0172642 10.1371/journal.pone.0172642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pogozhykh O., Pogozhykh D., Neehus A-L., Hoffmann A., Blasczyk R. and Müller T. (2015) Molecular and cellular characteristics of human and non-human primate multipotent stromal cells from the amnion and bone marrow during long term culture. Stem Cell Res. Ther. 6, 150 10.1186/s13287-015-0146-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soncini M., Vertua E., Gibelli L., Zorzi F., Denegri M., Albertini A. et al. (2007) Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 1, 296–305 10.1002/term.40 [DOI] [PubMed] [Google Scholar]
- 18.Díaz-Prado S., Muiños-López E., Hermida-Gómez T., Cicione C., Rendal-Vázquez M.E., Fuentes-Boquete I. et al. (2011) Human amniotic membrane as an alternative source of stem cells for regenerative medicine. Differentiation 81, 162–171 10.1016/j.diff.2011.01.005 [DOI] [PubMed] [Google Scholar]
- 19.Koike C., Zhou K., Takeda Y., Fathy M., Okabe M., Yoshida T. et al. (2014) Characterization of amniotic stem cells. Cell. Reprogram. 16, 298–305 10.1089/cell.2013.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clements L.E., Garvican E.R., Dudhia J. and Smith R.K. (2016) Modulation of mesenchymal stem cell genotype and phenotype by extracellular matrix proteins. Connect. Tissue Res. 57, 443–453 10.1080/03008207.2016.1215442 [DOI] [PubMed] [Google Scholar]
- 21.Salasznyk R.M., Williams W.A., Boskey A., Batorsky A. and Plopper G.E. (2004) Adhesion to vitronectin and collagen I promotes osteogenic differentiation of human mesenchymal stem cells. J. Biomed. Biotechnol. 2004, 24–34 10.1155/S1110724304306017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W., Sun H., Mou Y., Jiang X., Wei L., Wang Q. et al. (2015) Graphene oxide-collagen matrix accelerated osteogenic differentiation of mesenchymal stem cells. J. Biomater. Tissue Eng. 5, 257–266 10.1166/jbt.2015.1314 [DOI] [Google Scholar]
- 23.Kang B.J., Kim Y., Lee S.H., Kim W.H., Woo H.M. and Kweon O.K. (2013) Collagen I gel promotes homogenous osteogenic differentiation of adipose tissue-derived mesenchymal stem cells in serum-derived albumin scaffold. J. Biomater. Sci. Polym. Ed. 24, 1233–1243 10.1080/09205063.2012.745717 [DOI] [PubMed] [Google Scholar]
- 24.Bernhardt A., Lode A., Boxberger S., Pompe W. and Gelinsky M. (2008) Mineralised collagen–an artificial, extracellular bone matrix-improves osteogenic differentiation of bone marrow stromal cells. J. Mater. Sci. Mater. Med. 19, 269–275 10.1007/s10856-006-0059-0 [DOI] [PubMed] [Google Scholar]
- 25.Tseng P.C., Young T.H., Wang T.M., Peng H.W., Hou S.M. and Yen M.L. (2012) Spontaneous osteogenesis of MSCs cultured on 3D microcarriers through alteration of cytoskeletal tension. Biomaterials 33, 556–564 10.1016/j.biomaterials.2011.09.090 [DOI] [PubMed] [Google Scholar]
- 26.Bowen M.A., Patel D.D., Li X., Modrell B., Malacko A.R., Wang W. et al. (1995) Cloning, mapping and characterization of activated leukocyte-cell adhesion molecule (ALCAM) a CD6 ligand. J. Exp. Med. 181, 2213–2220 10.1084/jem.181.6.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidle U.H., Eggle D., Klostermann S. and Swart G.W. (2010) ALCAM/CD166: Cancer-related issues. Cancer Genomics Proteomics 7, 231–243 [PubMed] [Google Scholar]
- 28.Fujiwara K., Ohuchida K., Sada M., Horioka K., Iii C.D.U., Shindo K. et al. (2014) CD166/ALCAM expression is characteristic of tumorigenicity and invasive and migratory activities of pancreatic cancer cells. PLoS ONE 9, e107247 10.1371/journal.pone.0107247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao M., Wang X., Yan M. and Chen W. (2016) A systematic evaluation for the potential translation of CD166-related expression as a cancer biomarker. Expert Rev. Mol. Diagn. 16, 925–932 10.1080/14737159.2016.1211932 [DOI] [PubMed] [Google Scholar]
- 30.Moraes D.A., Sibov T.T., Pavon L.F., Alvim P.Q., Bonadio R.S., Da Silva J.R. et al. (2016) A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. 7, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parolini O., Alviano F., Bagnara G.P., Bilic G., Bühring H-J., Evangelista M. et al. (2008) Concise review: isolation and characterization of cells from human term placenta: outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 26, 300–311 10.1634/stemcells.2007-0594 [DOI] [PubMed] [Google Scholar]
- 32.Jayasuriya C.T., Hu N., Li J., Lemme N., Terek R., Ehrlich M.G. et al. (2018) Molecular characterization of mesenchymal stem cells in human osteoarthritis cartilage reveals contribution to the OA phenotype. Sci. Rep. 8, 7044 10.1038/s41598-018-25395-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babaie Y., Herwig R., Greber B., Brink T.C., Wruck W., Groth D. et al. (2007) Analysis of Oct4‐dependent transcriptional networks regulating self‐renewal and pluripotency in human embryonic stem cells. Stem Cells 25, 500–510 10.1634/stemcells.2006-0426 [DOI] [PubMed] [Google Scholar]
- 34.Pierantozzi E., Gava B., Manini I., Roviello F., Marotta G., Chiavarelli M. et al. (2011) Pluripotency regulators in human mesenchymal stem cells: expression of NANOG but not of OCT-4 and SOX-2. Stem Cells Dev. 20, 915–923 10.1089/scd.2010.0353 [DOI] [PubMed] [Google Scholar]
- 35.Rodda D.J., Chew J.L., Lim L.H., Loh Y.H., Wang B., Ng H.H. et al. (2005) Transcriptional regulation of NANOG by OCT4 and SOX2. J. Biol. Chem. 280, 24731–24737 10.1074/jbc.M502573200 [DOI] [PubMed] [Google Scholar]
- 36.Liu T.M., Wu Y.N., Guo X.M., Hui J.H., Lee E.H. and Lim B. (2009) Effects of ectopic Nanog and Oct4 overexpression on mesenchymal stem cells. Stem Cells Dev. 18, 1013–1022 10.1089/scd.2008.0335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C-E., Hu F-W., Yu C-H., Tsai L-L., Lee T-H., Chou M-Y. et al. (2014) Concurrent expression of Oct4 and Nanog maintains mesenchymal stem-like property of human dental pulp cells. Int. J. Mol. Sci. 15, 18623–18639 10.3390/ijms151018623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas D. and Kansara M. (2016) Epigenetic modifications in osteogenic differentiation and trans formation. J. Cell Biochem. 98, 757–769 10.1002/jcb.20850 [DOI] [PubMed] [Google Scholar]
- 39.Ponce M.L., Koelling S., Kluever A., Heinemann D.E.H., Miosge N., Wulf G. et al. (2008) Coexpression of osteogenic and adipogenic differentiation markers in selected subpopulations of primary human mesenchymal progenitor cells. J. Cell. Biochem. 104, 1342–1355 10.1002/jcb.21711 [DOI] [PubMed] [Google Scholar]
- 40.Mikami Y., Lee M., Irie S. and Honda M.J. (2011) Dexamethasone modulates osteogenesis and adipogenesis with regulation of osterix expression in rat calvaria-derived cells. J. Cell. Physiol. 226, 739–748 10.1002/jcp.22392 [DOI] [PubMed] [Google Scholar]
- 41.Hempel U., Möller S., Noack C., Hintze V., Scharnweber D., Schnabelrauch M. et al. (2012) Sulfated hyaluronan/collagen I matrices enhance the osteogenic differentiation of human mesenchymal stromal cells in vitro even in the absence of dexamethasone. Acta Biomater. 8, 4064–4072 10.1016/j.actbio.2012.06.039 [DOI] [PubMed] [Google Scholar]
- 42.Pan J.M., Wu L.G., Cai J.W., Wu L.T. and Liang M. (2019) Dexamethasone suppresses osteogenesis of osteoblast via the PI3K/Akt signaling pathway in vitro and in vivo. J. Recept. Signal Transduct. Res. 39, 80–86 10.1080/10799893.2019.1625061 [DOI] [PubMed] [Google Scholar]
- 43.Zhang Y., Khan D., Delling J. and Tobiasch E. (2012) Mechanisms underlying the osteo- and adipo-differentiation of human mesenchymal stem cells. Scientific World J. 2012, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taipaleenmäki H., Abdallah B.M., AlDahmash A., Säämänen A.M. and Kassem M. (2011) Wnt signalling mediates the cross-talk between bone marrow derived pre-adipocytic and pre-osteoblastic cell populations. Exp. Cell Res. 317, 745–756 10.1016/j.yexcr.2010.12.015 [DOI] [PubMed] [Google Scholar]
- 45.Li J., Zhang N., Huang X., Xu J., Fernandes J.C., Dai K. et al. (2013) Dexamethasone shifts bone marrow stromal cells from osteoblasts to adipocytes by C/EBP alpha promoter methylation. Cell Death Dis. 4, e832 10.1038/cddis.2013.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeltz C., Orgel J. and Gullberg D. (2014) Molecular composition and function of integrin-based collagen glues-introducing COLINBRIs. Biochim. Biophys. Acta 1840, 2533–2548 10.1016/j.bbagen.2013.12.022 [DOI] [PubMed] [Google Scholar]
- 47.Qian W., Gong L., Cui X., Zhang Z., Bajpai A., Liu C. et al. (2017) Nanotopographic regulation of human mesenchymal stem cell osteogenesis. ACS Appl. Mater. Interfaces 9, 41794–41806 10.1021/acsami.7b16314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiao F., Zhao Y., Sun Q. and Huo B. (2020) Spreading area and shape regulate the apoptosis and osteogenesis of mesenchymal stem cells on circular and branched micropatterned islands. J. Biomed. Mater. Res. A 108, 2080–2089 10.1002/jbm.a.36967 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of the present study are available upon request.