Introduction
Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma, with stage-dependent treatment and prognosis. Early-stage disease may be managed by skin directive therapies, whereas the treatment strategy for advanced stages ranges from vitamin A derivatives, immunomodulatory agents, radiation therapy, and chemotherapy to allogeneic hematopoietic stem cell transplantation (AHSCT). However, the treatment options for refractory advanced-stage MF remain limited, especially after failure of stem cell transplantation. Recurrence occurs in 45% of cases after AHSCT, resulting in a dismal prognosis.1 For these patients, the development of newer therapies is crucial. As immunotherapeutic oncologic agents are emerging, their role in the treatment of MF is yet to be defined.2 A recent publication described a positive outcome in patients with advanced MF and Sezary syndrome after treatment with the programmed cell death (PD)-1 antibody pembrolizumab.3 Additionally, radiation therapy is a pillar in the treatment of MF. Recent literature on the positive effects of immunotherapy in combination with radiation therapy in nonsmall cell lung cancer and malignant melanoma prompted us to explore this treatment option for advanced MF.4, 5, 6 Herein, we describe 2 patients with advanced MF who relapsed after AHSCT and were treated with subsequent pembrolizumab and radiation therapy, with positive outcomes.
Case report
Case 1
A 38-year-old African American woman diagnosed with MF stage IIB (T3N0M0B0) 8 years ago had been heavily pretreated with chemotherapy, focal radiation, narrow-band ultraviolet B, and carfilzomib until she underwent umbilical cord AHSCT, with complete remission. She had no evidence of chronic graft-versus-host disease (GvHD), except for an episode of gastrointestinal symptoms possibly due to GvHD, which completely resolved after a short course of systemic steroids. The patient relapsed a year later, with extensive tumors showing large-cell transformation. Subsequent treatment with nivolumab, lenalidomide, bexarotene, interferon alfa, and narrow-band ultraviolet B did not improve her condition, and the disease continued to progress. She presented in August 2019 with a body surface area of 90% with disseminated erythematous patches, plaques, and tumors and generalized lymphadenopathy. A positron emission tomography/computed tomography scan and blood work results were negative for systemic involvement. She was started on pembrolizumab (200 mg) infusions every 3 weeks in September 2019 (2112 days post AHSCT), and after cycle 2, she received low-dose total skin electron irradiation (1200 cGy/200 cGy in 6 fractions), with a boost to underdosed sites (palmar 700 cGy [1 fraction], plantar 200 cGy [1 fraction], scalp 800 cGy [200 cGy in 4 fractions], and perineum 800 cGy [1 fraction]). The pembrolizumab infusions were withheld during the 4 weeks of radiation therapy. Within 2 months after the treatment, the lesions throughout her entire body cleared or greatly improved, and her lymphadenopathy resolved (Fig 1). After 3 months, the patient received additional localized radiation treatment to her feet. However, she did not present with any other sites of active lesions. Ten months after treatment initiation (July 2020), the patient continued to respond to the treatment, with a body surface area of 2%. She is still receiving pembrolizumab (cycle 12), and she underwent additional single-fraction radiation treatment to 2 lesions on her feet. The patient experienced hypothyroidism as a side effect of pembrolizumab.
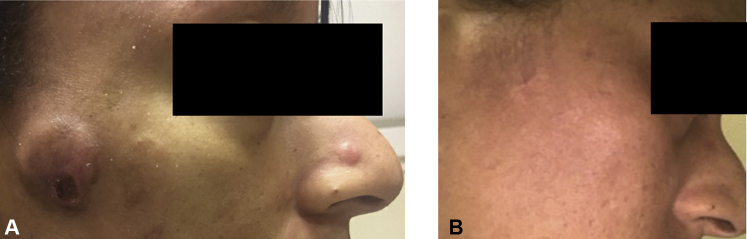
Fig 1.
A, Tumor lesion on the right cheek in a 38-year-old woman (case 1) with MF before pembrolizumab and TSEB (September 2019). B, Complete resolution of the tumor lesion on the right cheek after treatment (July 2020). Informed consent was obtained from the patient prior to publication. MF, Mycosis fungoides; TSEB, total skin electron beam radiation.
Case 2
A 58-year-old Caucasian man with MF IVB (T3N3M1(CNS)B0) was diagnosed in 2013 with folliculotropic MF with large-cell transformation and nodal involvement. His past medical history was significant for cutaneous squamous cell carcinoma and melanoma (IA in complete remission since 2013). His previous treatments included bexarotene, narrow-band ultraviolet B, interferon alfa, topical imiquimod, total skin electron beam radiation, pralatrexate, chemotherapy (Cyclophoshamid, Hydroxydaunorubicin, Vincristin, Etoposid, Prednisolon), and local radiation. After a clinical presentation of cauda equina syndrome, imaging and cerebrospinal fluid evaluation revealed progression to the central nervous system, and the patient received an HLA-matched unrelated AHSCT.
Following initial improvement post the transplantation, the patient was found to have a biopsy-proven recurrence of the disease on the right leg on day 60. In addition, he had recurrent cerebrospinal fluid involvement, requiring additional intrathecal chemotherapy, which subsequently resolved in May 2019. There were no signs of GvHD. Given the insufficiency of a durable response with the prior therapies and increased skin lesions, the patient was started on pembrolizumab (200 mg) every 3 weeks in July 2019 (264 days post AHSCT). At treatment initiation, the total body surface area involvement was 5%, with erythematous plaques on his face and extremities and a penile mass, which received localized radiation treatment (700 cGy/1 fraction) 1 month prior. All blood work results were negative for systemic involvement. The penile mass and a plaque on his hip received local radiation therapy during the pembrolizumab treatment (each lesion 700 cGy/1 fraction). A new tumoral lesion on the lower portion of his leg resolved after topical imiquimod treatment. His facial lesions resolved without localized radiotherapy. Throughout the entire treatment period of 12 months (July 2019-July 2020), all the cutaneous lesions resolved, and the patient currently has no active lesions or cerebrospinal fluid involvement (Fig 2). He continues to receive pembrolizumab (cycle 17) and does not report any side effects.
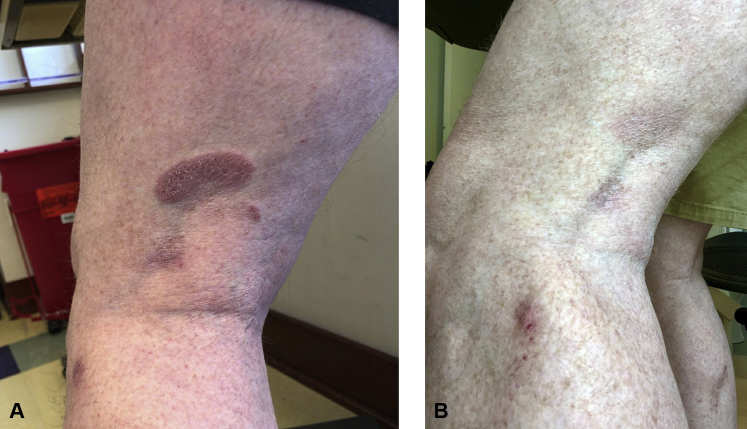
Fig 2.
A, Erythematous plaques on the posterior aspect of the left thigh and lateral aspect of the left knee of a 58-year-old MF patient (case 2) during pembrolizumab treatment (April 2020). B, Near complete resolution of the lesion on the posterior aspect of the left knee and improvement of the lesion on the lateral aspect of the left knee during pembrolizumab and localized imiquimod treatment without local radiation treatment to these lesions (July 2020). MF, Mycosis fungoides.
Discussion
AHSCT can provide prolonged remission for patients with advanced MF, mostly explained by graft-versus-lymphoma effects.7 Progression after AHSCT in MF is a common complication, mostly due to the lack of graft-versus-lymphoma, and these patients are frequently characterized by the absence of GvHD.8
In this case series, we demonstrated the positive outcomes in 2 patients with refractory advanced MF after failed AHSCT with no or mild GvHD. Patients 1 (MF IIB) and 2 (MF IVB) both displayed durable responses (10 and 12 months), with near complete remission with pembrolizumab and radiation therapy.
The combination of immunotherapy and radiation therapy resulting in a synergistic effect on cancer cells has been shown to produce positive outcomes in patients with nonsmall cell lung cancer and malignant melanoma.4,5 The use of immunotherapy for advanced MF is still an emerging field.2 However, the monoclonal PD-1 antibody pembrolizumab has been recently demonstrated to be effective in the treatment of advanced refractory MF.3 Pembrolizumab inhibits the PD-1/PD-L1 axis, which prevents immune cells from recognizing neoplastic cells.9 PD-1 is expressed by exhausted T cells, and the T-cell response is blunted by malignant cells expressing PD-L1. The potential risks of PD-1 inhibitors in T-cell malignancies have been the topics of many scientific papers as malignant T cells in MF and Sezary syndrome have also been shown to express PD-1.3,10
Moreover, the positive effect of combining immunotherapy with radiation therapy is hypothesized to result from a “vaccine-like” effect resulting from an increased antigen release from irradiated tumor cells and subsequent enhanced antitumor immunity via immunotherapeutic agents.11 To our knowledge, this application approach has not been published as a treatment strategy for advanced MF before, and this small case series might be the basis for additional studies. Interestingly, patient 1 did not respond to nivolumab in the past but responded to pembrolizumab and total skin electron beam radiation. This might be due to the higher response rates associated with pembrolizumab compared to that with nivolumab in refractory MF.3,12 Furthermore, the advantage of PD-1 inhibitors plus radiotherapy versus that of radiotherapy alone has been demonstrated in the past but has yet to be studied in MF, in particular with pembrolizumab and total skin electron beam radiation.13
Additionally, further questions regarding the duration of the treatment and long-term toxicities must be considered.3 To date, our described patients have not exhibited any late toxicities, except for hypothyroidism, and have tolerated the treatment very well.
In conclusion, pembrolizumab in combination with radiation therapy led to durable remission in 2 cases of refractory advanced-stage MF that had relapsed after AHSCT. Pembrolizumab in combination with radiation therapy might present a valuable treatment option for patients with refractory MF who fail to benefit from the graft-versus-lymphoma effect.
Footnotes
Funding sources: None.
Conflicts of interest: None disclosed.
IRB approval status: Not applicable.
References
- 1.Duarte R.F., Boumendil A., Onida F. Long-term outcome of allogeneic hematopoietic cell transplantation for patients with mycosis fungoides and Sezary syndrome: a European society for blood and marrow transplantation lymphoma working party extended analysis. J Clin Oncol. 2014;32(29):3347–3348. doi: 10.1200/JCO.2014.57.5597. [DOI] [PubMed] [Google Scholar]
- 2.Sivanand A., Surmanowicz P., Alhusayen R. Immunotherapy for cutaneous T-cell lymphoma: current landscape and future developments. J Cutan Med Surg. 2019;23(5):537–544. doi: 10.1177/1203475419867610. [DOI] [PubMed] [Google Scholar]
- 3.Khodadoust M.S., Rook A.H., Porcu P. Pembrolizumab in relapsed and refractory mycosis fungoides and Sézary syndrome: a multicenter phase II study. J Clin Oncol. 2020;38(1):20–28. doi: 10.1200/JCO.19.01056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh J.W., Tang C., De Groot P. Phase II trial of ipilimumab with stereotactic radiation therapy for metastatic disease: outcomes, toxicities, and low-dose radiation–related abscopal responses. Cancer Immunol Res. 2019;7(12):1903. doi: 10.1158/2326-6066.CIR-18-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luke J.J., Lemons J.M., Karrison T.G. Safety and clinical activity of pembrolizumab and multisite stereotactic body radiotherapy in patients with advanced solid tumors. J Clin Oncol. 2018;36(16):1611–1618. doi: 10.1200/JCO.2017.76.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formenti S.C., Rudqvist N.P., Golden E. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24(12):1845–1851. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu P.A., Kim Y.H., Lavori P.W., Hoppe R.T., Stockerl-Goldstein K.E. A meta-analysis of patients receiving allogeneic or autologous hematopoietic stem cell transplant in mycosis fungoides and Sezary syndrome. Biol Blood Marrow Transplant. 2009;15(8):982–990. doi: 10.1016/j.bbmt.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mori T., Shiratori S., Suzumiya J. Outcome of allogeneic hematopoietic stem cell transplantation for mycosis fungoides and Sezary syndrome. Hematol Oncol. 2020;38(3):266–271. doi: 10.1002/hon.2719. [DOI] [PubMed] [Google Scholar]
- 9.Raedler L.A. Keytruda (pembrolizumab): first PD-1 inhibitor approved for previously treated unresectable or metastatic melanoma. Am Health Drug Benefits. 2015;8(Spec Feature):96–100. [PMC free article] [PubMed] [Google Scholar]
- 10.Querfeld C., Leung S., Myskowski P.L. Primary T cells from cutaneous T-cell lymphoma skin explants display an exhausted immune checkpoint profile. Cancer Immunol Res. 2018;6(8):900–909. doi: 10.1158/2326-6066.CIR-17-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodge J.W., Guha C., Neefjes J., Gulley J.L. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology (Williston Park) 2008;22(9):1064–1070. 1064. [PMC free article] [PubMed] [Google Scholar]
- 12.Lesokhin A.M., Ansell S.M., Armand P. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34(23):2698–2704. doi: 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park S.S., Dong H., Liu X. PD-1 restrains radiotherapy-induced abscopal effect. Cancer Immunol Res. 2015;3(6):610–619. doi: 10.1158/2326-6066.CIR-14-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]