Highlights
-
•
PD-L1 expression in HGSOC requires further study with modern antibody reagents and platforms.
-
•
The association of PD-L1 positive cases with CD8+ immune cells is more prominent.
-
•
The checkpoint value in HGSOC is not yet clear, but there is an importance meaningful when the threshold is ≥5%.
-
•
PD-L1 expression shows two immunohistochemical staining patterns, patchy/diffuse and patchy/focal.
Keywords: High grade serous ovarian cancer, Programmed cell death 1, Programmed cell death ligand 1, Tumor infiltrating lymphocytes, Survival
Abstract
We investigated programmed cell death 1 (PD-1) / programmed cell death ligand 1 (PD-L1) expression in high grade serous ovarian cancer (HGSOC) and its relationship to tumor infiltrating lymphocytes (TIL) and prognosis. Formalin fixed paraffin embedded (FFPE) samples of 94 HGSOC cases were included in the study. Immunohistochemical analysis (CD3, CD4, CD8, PD-1 and PD-L1) was performed. Samples were analyzed for expression of immune proteins in the peritumoral stromal and intratumoral areas, scored, and expression was correlated with overall survival, stage, and age. PD-L1 staining ratio with a score greater than 0 was found to have lower survival. There were two positive staining patterns, patchy/diffuse and patchy/focal patterns, in 24 (25.5%) cases. Considering the threshold value ≥5%, we demonstrated that the PD-L1 positive cancer cell membrane immunoreactivity rate and patchy/diffuse PD-L1 expression were 9.6% (n = 9). There was statistically significant relationship between high PD-1 scores and PD-L1 cases of ≥ 5%. A statistically significant difference was found between PD-L1 staining and survival in patients with a threshold ≥ 5%. However an appropriate rate for treatment was determined in 9.6% cases. There was a statistically significant correlation between PD-1 positive TIL score and intratumoral CD3, peritumoral stromal CD3, intratumoral CD4 and intratumoral CD8 positive cells. Survival was lower in cases with higher PD-L1 positive stromal TIL score.
Introduction
In the last few decades, tumor-infiltrating lymphocytes (TILs), unlike ordinary inflammation cells, have been discovered in peritumoral stromal spaces and intratumoral areas in high grade serous ovarian cancer (HGSOC) [1], [2], [3], [4]. In some clinical studies, the presence of TILs, especially CD8+ T cells, has been associated with good prognosis and good survival in different cancers [[1], [2], [3],[5], [6], [7]]. It has also been found that programmed cell death 1 (PD-1) protein, a surface receptor of activated CD4+ and CD8+ T lymphocytes, could be a powerful regulatory mechanism in cancers [8,9]. Programmed cell death ligand 1 (PD-L1, B7-Homolog 1, B7-H1, CD274), is a ligand for PD-1, is expressed on the surface of cancer cells, tumor associated macrophages, myeloid derived suppressor cells, dendritic cells, T and B cells. T cells detect PD-L1 signal via PD-1 receptor [10]. Overexpression of PD-L1 has been found to be associated with resistance to immunotherapies and poor prognosis [11]. PD-1/PD-L1 inhibitors have been approved by The Food and Drug Administration for treatment of melanoma, non-small cell lung cancer, head and neck squamous cell carcinoma, colorectal cancer, renal cell carcinoma, hepatocellular carcinoma, urothelial carcinoma, Merkel cell carcinoma, Hodgkin lymphoma and cervical carcinoma [12]. However, in some cancers, the effects of PD-L1 in the response to these monoclonal antibodies are uncertain and controversial. It requires more detailed and deeper understanding, via more research [1]. It appears to have a highly favorable immune environment for studying PD-1/PD-L1 biology with TILs. HGSOC is one of these cancers that requires more studies [10].
HGSOC cells, by expressing high amounts of PD-L1 to avoid cytolysis by activated T cells, can form a immunosuppressor tumour microenvironment. Investigation of the immunological relationship in the microenvironment may contribute to the understanding of the relationship between high expression of PD-L1 and poor prognosis of ovarian cancer [1]. Additionally, in HGSOC, the scoring and evaluation criteria of peritumoral stromal and intratumoral PD-L1 in terms of mathematical aspect and antibody density, which is unconfirmed in pathology practice, continues to be an important problem.
We evaluated the relative expression levels of PD1/PD-L1 check point molecules by investigating the staining intensities and by scoring mathematically. Corelation between clinical and morphological findings were investigated in the present study.
Materials and methods
Patients
One hundred cases diagnosed as HGSOC were retrospectively reviewed at Zeynep Kamil Maternity and Pediatric Research and Training Hospital, Department of Pathology. Clinical findings were obtained from pathology archive and hospital registries. Ethics committee approval was received from Hitit University Faculty of Medicine Clinical Research Ethics Committee (Ethical approval code 2017-156).
HGSOC grading and staging was performed according to literature of Malpica et al. [13] and International Federation of Gynecology and Obstetrics (FIGO) staging system [14].
FFPE tissue blocks of 100 cases were selected with the widest tumor. These tissue sections, stained with Hematoxylin & Eosin (HE), were examined by two experienced pathologists (YB and NK). Immunohistochemical (IHC) P53, CK7, WT1, Ki-67 and Pax-8 were performed in 5 cases, where consensus cannot be reached on HE stained sections. FFPE samples of 94 cases that was considered to be reached a consensus, treated any chemotherapy previously, and included approximately more than 500 tumor cells, covered the study. From these blocks, the most dense tumor areas on HE-stained slides were marked and punch biopsies were collected in a diameter of 6 mm (28260000 µm2) and new FFPE blocks were prepared (Fig. 1). One of these new sections was re-stained with HE (Fig. 2).
Fig. 1.
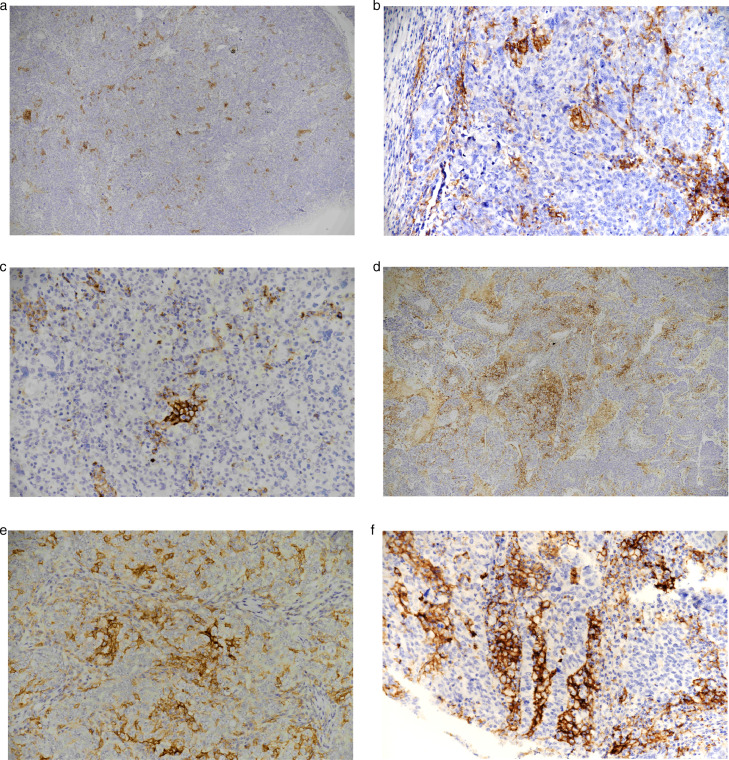
Representative IHC microscope slides containing four different cancer tissues, stained for CD8 and PD-L1 with four different cancer tissue. According to the slide, in the lower left corner there is a control placenta sample and in the lower right corner there is a control tonsil sample.
Fig. 2.
HE stained tumor sample in a diameter of 6 mm (28260000 µm 2), x2.
Age, stage, treatment after diagnosis, median overall survival, mortality after diagnosis and number of deaths were recorded in these 94 cases.
Tissue microarray construction and immunohistochemistry
Immunohistochemical staining was performed using the DAKO Omnis automated system according to the manufacturer's instructions. A mouse monoclonal antibody (Mouse Monoclonal PD1 Antibody, Clone NAT105) was used for PD-1. For determination of PD-L1, a “22C3 PharmDx (Mouse Monoclonal Anti- PD-L1) Clone” that binds to PD-L1 on the cell membrane of tumor cells, immune cells and cells of epithelial origin and does not cross-react with human PD-L2 protein, was studied. “Polyclonal Rabbit Anti-Human CD3 Ready-to-Use”, “Monoclonal Mouse Anti-Human CD4 (Clone 4B12)” and “Monoclonal Mouse Anti-Human CD8 (Clone C8/144B) were used, respectively for CD3, CD4 and CD8.
Four different cancer tissues in a diameter of 6 mm were placed on IHC microscope slide with positive tissue control from plasenta and tonsil (Fig. 3A-B). For the negative control, the internal control areas, that reacted negatively for cancer cells and that reacted positively for non-cancer cells, were investigated. Immunostained slides were evaluated and photographed under a microscope (Nikon Eclipse Ni). Numerical ratios per mm2 of the studied antibodies were calculted with a computer system (NIS-Elements D on Nikon).
Fig. 3.
(a) PD-L1 positive placenta sample was selected for positive control, x40. (b) PD-L1 positive tonsilla palatina sample was selected for positive control, x40.
Artefactive staining, diffuse cytoplasmic staining, staining of normal cells and necrotic areas were not included in the scoring. PD-1 displayed a membrane-accentuated expression, which was often accompanied by a cytoplasmic expression, though, was considered positive when surrounding membranous staining [2,11].
Evaluation and scoring of PD-L1 immunohistochemical staining in intratumoral and peritumoral stromal areas
Intratumoral PD-L1 and peritumoral stromal tissue PD-L1 staining in cancer cells and tumor-associated lymphocytes were examined separately. In the cell membrane and/or cytoplasm of cancer cells immunostaining for PD-L1 was observed. In cancer cells, focal weak, complete or partial membrane ≥1+ stained focus and staining areas in the peritumoral stromal tissue / perivascular space were marked.
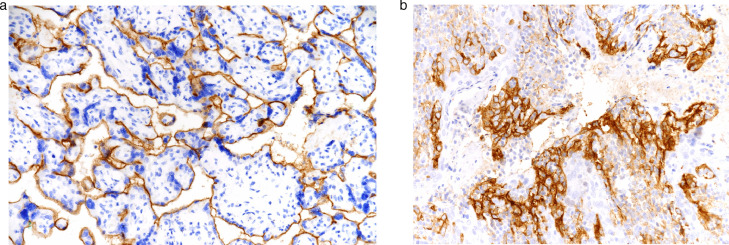
The scoring was performed based on previous studies [1,2,11,15] PD-L1 staining intensity in cancer cells was divided into 4 categories as follows: Negative (0) (score = 0) if no staining, weak expression (1+) (score = 1) if much weaker than positive control, moderate expressin (2+) (score = 2) if light staining and strong expression (3+) (score = 3) if there is expression equivalent to or stronger than the positive control (Fig. 4A-B).
Fig. 4.
(a) Weak expression (1+) (score = 1) if much weaker than the positive control, moderate expression (2+) (score = 2) if it is close to the positive control but slightly weak, x20. (b) Positive expression equivalent to or greater than (3+) (score = 3) was accepted, x20.
Algorithm was used to obtain a numerical score representing antigen frequency. In case of more than one area with low density, at least five high power field (HPF) were randomly selected and the expression intensity was determined [16]. A random area was selected in the sections where the staining seemed consistent and intense. The ratio of cancer cells showing high and low staining intensity in each selected area was determined by counting each cancer cell in HPF. Cases were considered positive for PD-L1 membranous expression if ≥5% of cancer cells, with moderate (score 2) and strong (score 3) density, exhibited complete circumferential or partial cell membrane staining. The 5% threshold is based on previous phase I trials in which PD-L1 positivity was associated with clinical response [17,18].
Tumor tissue in the whole slide was considered positive if ≥5% and at least moderately membranous PD-L1 expression detected in TILs [1].
Evaluation of immunohistochemical staining of PD-1 and TILs in Peritumoral stromal and intratumoral areas
The immune-positive intratumoral lymphocytes touching the cancer cells were separated from the peritumoral immune cells located in the stroma and/or perivascular spaces but not in direct contact with the cancer cells. TILs were separately counted as intratumoral and stromal at magnification HPF. When calculating the average number, five areas with the most infiltration were selected.
Statistical analysis
Statistical analysis was performed using SPSS 22 package program. Normality analyzes of variables were analyzed using Kolmogrov Smirnov test. Therefore, when the dependent variable was quantitative, Mann-Whitney U test was used to compare two independent groups and Kruskal Walliz test was used to compare more than two independent groups. Since the variables were not normally distributed, Kaplan Meier survival analyzes were performed on Median values. Descriptive statistics were obtained as a result of Kaplan Meier analyzes and Log-Rank tests were performed. The chi-square test of independence was used to investigate whether two qualitative variables affected each other.
Results
Clinical aspects
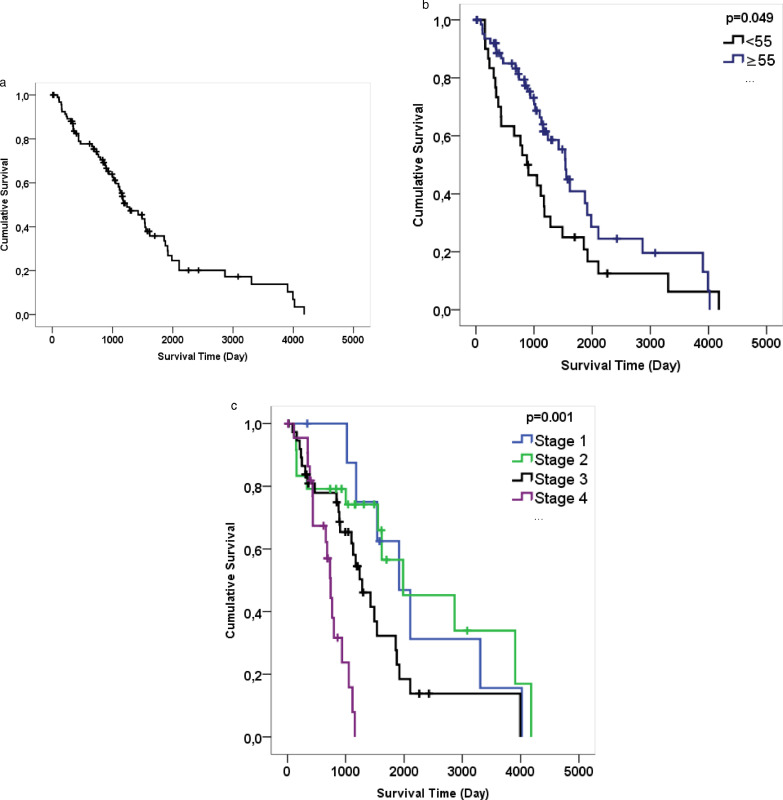
Ninety four cases with HGSOC diagnosis were included in the study. Clinical findings are summarized in Table 1. Cases were between the ages of 29-86 and the average age was 58.65 ±10.77. 40.4% of the cases were stage III and 24.5% were stage IV. All patients had received standard adjuvant platinium-based chemotherapy. 35.1% (n = 33) of the patients died. While the median of the survivors was 57.00, it was 62.00 in the patients who died (p = 0.02 <α 0.01).
Table 1.
Statistical analysis demonstrating the independent risk factors for the cancer-specific death of the patients with ovarian cancer (n = 94).
| Factors | Graphs | Groups | Median (Year) | Minimum (Day) | Maximum (Year) | Log Rank Test (Mantel-Cox) |
|---|---|---|---|---|---|---|
| All observations (n=94) | Fig. 3a | - | 3.39 | 87 with p = 0.99 | 11.61 with p = 0.00 | - |
| Age | Fig. 3b | <55 | 2.40 | 156 with p = 0.93 | 11.45 with p = 0.00 | x2 = 3.88 (p = 0.049*) |
| ≥55 | 4.22 | 87 with p = 0.98 | 11.01 with p = 0.00 | |||
| Stage (FIGO) | Fig. 3c | Stage 1 | 5.24 | 1021 with p = 88 | 11.01 with p = 0.00 | x2 = 25.38 (p = 0.000**) |
| Stage 2 | 5.44 | 114 with p = 0.96 | 11.45 with p = 0.00 | |||
| Stage 3 | 3.52 | 87 with p = 0.97 | 10.94 with p = 0.00 | |||
| Stage 4 | 2.03 | 112 with p = 0.96 | 3.17 with p = 0.00 | |||
| The ration of PD-L1 number of the positive square | Fig. 4a | <0.05 | 4.21 | 87 with p = 0.99 | 11.45 with p = 0.00 | x2 = 3.08 (p = 0.079) |
| ≥0.05 | 3.06 | 114 with p = 0.95 | 9.06 with p = 0.00 | |||
| The number of TPS | Fig. 4b | =0 | 3.90 | 112 with p = 0.99 | 11.45 with p = 0.00 | x2 = 3.29 (p = 0.070) |
| >0 | 3.17 | 87 with p = 0.96 | 9.06 with p = 0.00 | |||
| PDL1 stromal TIL score | Fig. 4c | 0 | 4.21 | 112 with p = 0.98 | 11.45 with p = 0.00 | x2 = 8.62 (p = 0.035*) |
| 1 | 3.39 | 114 with p = 0.95 | 10.94 with p = 0.00 | |||
| 2 | 0.97 | 307 with p = 0.83 | 3.06 with p = 0.17 | |||
| 3 | 1.28 | 87 with p = 0.88 | 9.06 with p = 0.00 | |||
| PD-L1 intratumoral TIL score | Fig. 4d | 0 | 4.21 | 112 with p = 0.98 | 11.45 with p = 0.00 | x2 =8.62 (p = 0.058) |
| 1 | 3.39 | 114 with p = 0.94 | 10.94 with p = 0.00 | |||
| 2 | 0.96 | 87 with p = 0.83 | 5.44 with p = 0.00 | |||
| 3 | 1.19 | 248 with p = 0.75 | 9.06 with p = 0.00 |
*p<0.05, **p<0.01.
The median survival of 94 patients was 3.39 years and the probability of survival of any patient for 87 days was 99% and for 11.61 years was 0% (Fig. 5A). When age variable 55 was selected, there was a significant difference on life expectancy (p = 0.049, <α 0.05) (Fig. 5B). According to the results, the median value of the age variables of the patients older than 55 years was 4.22 years, while the median value of survival time of those younger than 55 years was 2.40 years. The disease stage had a significant effect on survival (p = 0.000 <α 0.01). (Fig. 5C) While the life expectancy of FIGO stage I and II was approximately the same (5.24 and 5.44 years), it decreased in stage III (3.52 years) and stage IV (2.03 years). In fact, 100% probability of mortality in stage I was 11.01 years and 100% probability of mortality in stage IV was 3.7 years.
Fig. 5.
Survival time was analysed as day. (a) Graphic A show Kaplan Meier for the duration of estimated survival time on all data. (b) Graphic B show Kaplan Meier for age (≥55, <55). When age variable 55 was selected, there was a significant difference on life expectancy. (c) Graphic C show Kaplan Meier for FIGO Stage I, II, III and IV.
There was no statistically significant correlation between PD-L1 positive or negative and age (Mann-Whitney U; p = 0.322).
PD-L1 expression in tumor cells and TILs
When the cases (n = 94) were evaluated according to whether they were stained or not, we observed two positive staining patterns, wide patchy/diffuse and patchy/focal, in 24 cases (25.5%) (Fig. 6A-D). Wide patchy/diffuse staining was observed in only 5 (5.3%) cases. Most of them showed patchy/focal PD-L1 expression pattern, predominantly containing cancer cells at the invasive border. PD-L1 expression was statistically analyzed according to whether they were stained in cancer cells and TILs, whether the stained area was ≥5% and their relationship to peritumoral stromal/intratumoral TILs. The PD-L1 positive cancer cell membrane immunoreactivity rate was 9.6% (n = 9), considering the threshold value ≥5%. Seventy (74.5%) cases were characterized by complete absence of cancer cell membrane immunoreactivity or very weak staining.
Fig. 6.
Immunohistochemically samples PD-L1 (a) patchy/focal, x4, (b) patchy/focal, x20, (c) patchy/focal, x20, (d) patchy/diffuse staining, x4, (e) patchy/diffuse staining, x20, (f) patchy/diffuse staining, x20.
TILs were observed both in cancer stromal and intraepithelial area. Most TILs were in the peritumoral stromal area. PD-L1 positive cancer cells were associated with intratumoral CD3+ and CD8+ immune cells. There was a statistically significant relationship between them (Chi-Square; p = 0.002 and p = 0.02). PD-L1 staining increased the intratumoral CD3 and CD8 scores.
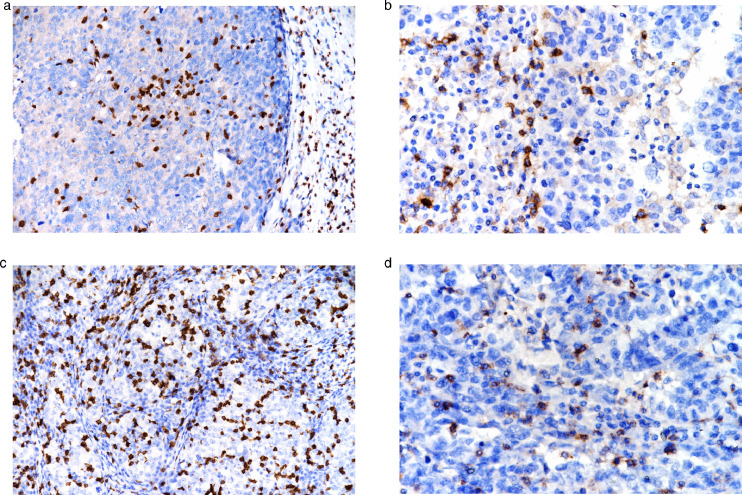
There was a statistically significant relationship between PD-L1 positive cases and stromal CD8+ immune cells (Chi-Square; p = 0.002). PD-L1 staining increased stromal CD8 score. There was a statistically significant relationship between PD-L1 positive cases and the cells count of CD3+, CD4+ and CD8+ in 5 different 20x HPFs (Mann-Whitney U; p = 0.001, p = 0.011, p = 0.000, respectively). The maximum cells count of CD3+, CD4+ and CD8+ in 5 different 20 HPFs was higher in the stained area than in the unstained area (Fig. 7A-D).
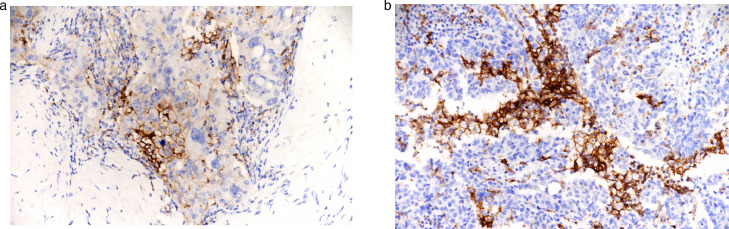
Fig. 7.
(a) CD3+ intratumoral TILs, x20. (b) CD4+ intratumoral TILs, x4. (c) CD8+ intratumoral TILs, x20. (d) PD-1+ intratumoral TILs x40.
There was statistically significant relationship between PD-L1 intratumoral TILs score in 5 different 20x HPFs and CD8+ cell count in 5 different HPF (Kruskal Wallis; p = 0.000). As the PD-L1 intratumoral TILs score increased in 5 HPFs, the count of CD8+ cells increased in the 5 most abundant positive 20x HPFs.
PD-L1 positive expression in tumor cells was more frequent in patients with advanced FIGO stage but there was no statistically significant relationship between being PD-L1 negative or positive and FIGO stages (Chi-Square; p = 0.904).
Correlation between survival and PD-L1
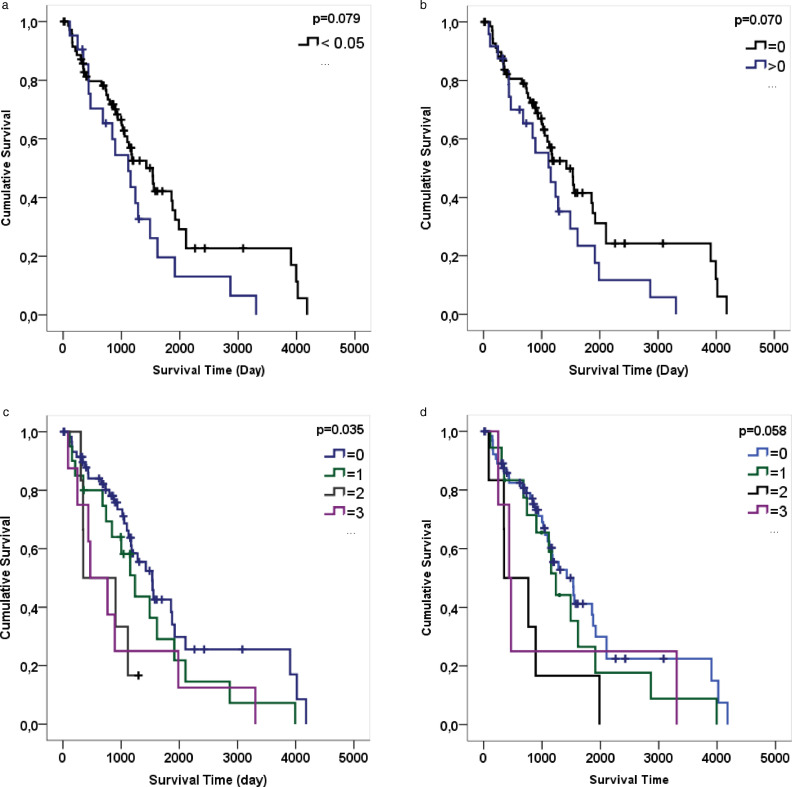
According to Kaplan-Meier analysis results, statistically significant difference was found between PD-L1 staining and life expectancy when threshold ≥5% was accepted (p = 0.079 > α 0.05) (Fig. 8A). Although no statistically significant association was found on PD-L1 stained area according to Kaplan-Meier analysis results (p = 0.070 > α 0.05), PD-L1 staining ratio having a score greater than 0 decreases the survival (Fig. 8B). There was a significant relationship between PD-L1 positive peritumoral stromal TIL score and survival (Kaplan Meier; p = 0.035 > α 0.05). In other words, survival was lower in cases with higher PD-L1 positive stromal TIL score (Fig. 8C). Although PD-L1 positive intratumoral TILs score does not have a statistically significant effect on survival, it could be said that an increase in PD-L1 positive intratumoral TIL score cause a decrease in survival (Kaplan Meier; p = 0.058 > α 0.05) (Fig. 8D).
Fig. 8.
Survival time was analysed as day (a) Graphic A show Kaplan Meier for the ratios of PD-L1 number of the positive square (groups: <0.05 and ≥0.05). There was a statistically significant difference between PD-L1 staining and life expectancy when the threshold was ≥ 5%. (b) Although there is no statistically significant difference according to the results of the analysis, the PD-L1 staining ratio has a score greater than 0 which lowers the life expectancy. (Groups: = 0 and > 0). (c) Graphic C show Kaplan Meier for PD-L1 stromal TILs score (Groups: 0, 1, 2, 3). In patients with high PD-L1 stromal TILs score, life expectancy was shortened. (d) Graphic D show Kaplan Meier for PD-L1 intratumoral TILs score (Groups: 0, 1, 2, 3). Increased PD-L1 intratumoral TILs score may lower survival time.
There was no statistically significant relationship between PD-L1 positive intratumoral TILs score and FIGO stage (Mann-Whitney U; p = 0.758 and Chi-Square; p = 0.904).
Correlation between tumor PD-1 expression and PD-L1 and TILs
The median count of PD-1 cells in 5 different 20x HPFs for PD-1 was 17.00 and the mean was 57.36 ±94.131. The majority of patients had a low (≤1) PD-1 TIL score of 80.90% and a low (≤1) PD-1 stromal score of 80.30%. There was a statistically significant relationship between high PD-1 scores and PD-L1 cases of ≥ 5% (Chi-squared test, p = 0.043). In PD-L1 stained cases, there was a statistically significant relationship with the count of PD-1 positive cells in the 5 most common positive areas in 20x HPFs (Mann-Whitney U; p = 0.000). In PD-L1 stained cases, PD-1 positive cell counts were higher in 5 different 20x HPFs than in non-stained areas. In cases with PD-L1 staining, a positive PD-1 peritumoral stromal immune cell score of >25 was 41.7%, whereas for negative cases it was 8.6%. There was a statistically significant relationship between PD-L1 staining and PD-1 peritumoral stromal immune cell score (CC = 0.358, CV = 0.357).
There was a statistically significant correlation between PD-1 positive TIL score and intratumoral CD3+, peritumoral stromal CD3+, intratumoral CD4+ and intratumoral CD8+ cells (Chi-Square; p = 0.003, p = 0.036, p = 0.003, p = 0.010, p = 0.008, respectively).
Discussion
The questions remain largely unanswered, such as which patients would benefit from immune checkpoint inhibitors and when to include immune checkpoint inhibitors in the treatment of gynecological malignancies [19]. In our study, disease stage was an independent prognostic factor. Statistically 100% probability of mortality in stage I was 11.01 years and 100% probability of mortality in stage IV was 3.7 years. These results are compatible with the results of Drakes et al. [15] and Deng et al. [20]. In our study, PD-L1 staining ratio with a score greater than 0 was found to have lower life expectancy (Fig. 4A). Statistically significant difference was found between PD-L1 staining and life expectancy in patients with a threshold ≥ 5%. The most abundant 5 different positive x20 HPFs for PD-1 were 57.36 ±94.131 on average, and the majority of patients had a low (≤1) PD-1 TIL score of 80.90% and a low (≤1) PD-1 stromal score of 80.3%. In the study of Drakes et al. [15], this molecule was expressed in 87% of tumors, but no significant relationship was found between survival and PD-1 or PD-L1 expression patterns in HGSOC studies. There are conflicting reports regarding a positive or negative relationship with the expression patterns of PD-1 in various cancers and with survival [[21], [22]–23].
In our study, PD-L1 expression in cancer cells exhibited two positive staining patterns, patchy/diffuse and patchy/focal. Patchy/diffuse staining was rare (Fig. 6). Most of the positive cases had patchy and focal PD-L1 expression patterns. In 24/94 (25.5%) cases, PD-L1 positive membrane immunoreactivity was observed in cancer cells. The remaining 70/94 (74.5%) cases had complete absence, partial/weak PD-L1 staining or <5% cancer cell membrane immunoreactivity. These results are similar with the results of Wang et al. [1] and Drakes et al. [15]. In the study of Wang et al. [1], PD-L1 membrane immunoreactivity was positive in 26/107 (24.30%) patients. In the remaining 81/107 (75.70%) patients, <5% were characterized by tumor membrane immunoreactivity or complete absence or very poor PD-L1 staining. In the study of Drakes et al. [15], PD-L1 expression was present in 33% of tumors.
A 5% PD-L1 IHC expression threshold used in other cancer types was found to be highly predictive for different drugs such as nivolumab, pembrolizumab, atesolimumab, durvalumab and avelumab [18]. However, in ovarian cancer, in many studies (Table 2), cut-off values differ in TILs and PD-L1 expression. In our study, we selected 5% membranous staining as a threshold value for PD-L1 positive expression. A statistically significant difference was found between PD-L1 staining and life expectancy in patients with a threshold ≥5%.
Table 2.
In the last few decades, with cut-off values varying in TILs and PD-L1 expression, different studies on HGSOC.
| First Author of Study | Country | Number of cases | Tumor Stage | Grade | Histologic Subtype | Specimen processing | TILs Phenotype | Location | Cut-off value |
|---|---|---|---|---|---|---|---|---|---|
| Raspollini 2005 (24) | Italy | 95 | III | High | Serous | FFPE | CD3 | Intratumoral | ≥5 cells/HPF |
| Callahan, 2008 (25) | USA | 184 | III, IV | High | Serous | FFPE | CD8 | Intraepithelial | Top quartile |
| Stumpf 2009 (26) | Germany | 100 | III | Mixed (G2- G3, 97%) | Serous | Tissue microarrays | CD8 | Intraepithelial | Any cells/HPF |
| Adams 2009 (27) | USA | 134 | III, IV | Mixed (G2- G3, 100%) | Mixed (Serous, 94%) | Cryosections | CD3, CD8, FoxP3 | Intraepithelial | CD3 (Any cells/HPF) CD8, FoxP3 (10 cells/HPF) |
| Bachmayr Heyda, 2013 (28) | Austria | 203 | Mixed (III-IV, 95.6%) | Mixed (G2-G3, 96.1%) | Mixed (Serous, 88.2%) | Tissue microarrays | CD8 | Intraepithelial | >median |
| DeLeeuw 2015 (29) | Canada | 187 | Mixed (III, 34%) | Mixed (G2- G3, 100%) | Serous | Tissue microarrays | CD4, CD8, CD25, FoxP3 | Intraepithelial | Any cells/HPF |
| Knutson 2015 (30) | USA | 348 | Mixed (III-IV, 86.2%) | Mixed (high, 97%) | Mixed (Serous, 81.4%) | Cryosections | CD4, CD8, CD25, FoxP3 | Intraepithelial | Any cells/HPF |
| Bösmüller 2016 (31) | Germany | 138 | Mixed (III-IV, 85%) | High | Serous | Tissue microarrays | CD3, CD8, CD103 | Intraepithelial | CD3: ≥7 cells/HPF CD8: ≥0.5 cells/HPF CD103: ≥1 cells/HPF |
| Strickland 2016 (32) | USA | 53 | NR | High | Serous | FFPE | CD3 | Intraepithelial | ≥35 cells/HPF |
| Darb Esfahani 2016 (2) | Germany | 215 | Mixed (III-IV, 86.6%) | High | Serous | Tissue microarrays | CD3, PD- 1, PD-L1 | Intratumoral | CD3: >65 cells/mm2 PD-1: >11cells/mm2 PD-L1: >20 cell/mm2 |
| Webb 2016 (8) | Canada | 195 | Mixed (III, 32.8%) | High | Serous | Tissue microarrays | CD68, CD103, CD3, CD8, CD25, FoxP3, PD-L1 | Intratumoral | ≥1 cells/HPF |
| Santoiemma 2016 (33) | Italy | 89 | Mixed | High | Mixed (Serous, 65.9%) | FFPE | CD3, CD4, CD8, CD20, TIA-1, PD-1, PD-L1, FoxP3 | Peritumoral /Intraepithelial | a grouping of number of cells |
| Baş 2019 | Turkey | 94 | Mixed (III-IV, 64.9%) | High | Serous | FFPE | CD3, CD4, CD8, PD-1, PD-L1 | Peritumoral /Intraepithelial | ≥5 cells/HPF |
Hamanishi et al. [34], have stated that PD-L1 expression in HGSOC was found to be an important prognostic factor and was inversely related to intratumoral CD8+ TILs ratio, an immunologically defined prognostic indicator. Darb-Esfahani et al. [2] have reported that there was a significant increase in PD-1 and PD-L1 expression in cancer cells in HGSOC, as well as progression-free survival in patients with high levels of PD-1 and PD-L1 mRNA. Also, Webb et al. [8] have shown that TILs and PD-L1 expression in HGSOC was associated with favorable prognosis. This immunological condition indicates that more research is needed in terms of prognostic factors [34]. There are also very few reports on the relationship between PD-L1 expression and TILs in tumor cells in ovarian cancer. In the some study are indicated that the location, subtype, and density of TILs are major determinants of the prognostic value of TILs in ovarian cancer [5]. In our study, PD-L1 positive tumor cells were associated with intratumoral CD3+ and CD8+ immune cells and peritumoral stromal CD8+ immune cells. PD-L1 staining increased the intratumoral CD3 and CD8 score and the peritumoral stromal CD8 score. In the most commonly stained 5 different 20X HPFs, as PD-L1 intratumoral TILs score increased, CD8+ cell number increased. In the study of Wang et al. [1], the PD-L1 positive group had a significantly higher number of stromal CD8+ TILs than the PD-L1 negative group. The association between tumor PD-L1 expression and stromal CD8+ TILs has been observed in T cell-rich area of tumor in particular at the invasive margin. In addition, life expectancy was shorter in cases with higher PD-L1 peritumoral stromal TILs score. In the study of Darb-Esfahani et al. [2], PD-1 positive TILs and/or PD-L1 positive cancer cells have a positive relationship with the survival of patients with HGSOC. In the study of Li et al. [5], intraepithelial CD3+, CD8+ or CD103+ TILs alone were indicated as an indicator of survival improvement in ovarian cancer. In the study of Sato et al. [6] were demonstrated neither intraepithelial nor stromal CD4+ TILs were associated with improved survival. In Table 2, PD-L1 peritumoral stromal and PD-L1 intratumoral TIL scores were higher in the surviving patients than in the dying patients. However, intratumoral CD3+ scores were found to be higher in surviving patients than in dying patients. Teng et al. [35] have reported that HGSOC patients with PD-L1 expression and higher peritumoral stromal and/or intraepithelial CD8+ TILs counts may be suitable for PD-1/PD-L1 blockade. So far, the effectiveness of PD-1/PD-L1 blockade in ovarian cancer is relatively low compared to RCC, melanoma, or non-small cell lung cancer [35].
The fact that the prognostic value of PD-L1 in ovarian cancer is still being discussed can be explained for various reasons. Different study populations and regional differences may lead to inconsistency. In the metaanalysis study of Huang et al. [16] on the prognostic parameter of ovarian TILs, it was declared that studies related to the regional differences in results, with greater prognostic effects seen among studies done in Japan and Europe than in North America. Other factors that may contribute to heterogenity in PD-L1 assays include antibody clones, platforms, targets (i.e. tumor or immune cells) and mathematical ratios for positivity. With technical issues in IHC, and temporal and spatial factors should be taken into consideration when evaluating PD-L1 in cancers [18]. Considering the differences between test methods, it is likely that PD-L1 tests will show differences in the classification of patients as PD-L1 positive or negative [36]. Depending on the heterogenity of the tumor itself, different staining patterns may be observed even at different biopsy sites from the same sample. Because of the more focal staining of PD-L1 expression in many tumors, microscopic interpretation may result in bias compared to pathologists [18,37,38]. In this study, taking into account this situation, when sampling tumor tissue, the sections containing the most dense cancer tissue were selected in the widest area (Fig. 1AB). Tumor diameters were determined as 2.9 mm, 1 mm, 0.6 mm and 1.5 mm in the studies of Chowdhury et al. [39], Stanske et al. [40], Santoiemma et al. [33] and Koppel et al. [41], respectively. In our study, the diameter of tumor samples is 6 mm.
In conclusion, when our cases were evaluated, we observed two positive staining patterns, patchy/diffuse and patchy/focal patterns, in 24 (25.5%) cases. Considering the threshold value ≥5%, we demonstrated patchy/diffuse PD-L1 expression in a ratio of 9.6%. Our study concluded that PD-L1 was not extensively expressed in HGSOC cells at a high percentage. This should not imply that the presence of focal and low PD-L1 expressing cells in HGSOC tissue is not significantly associated with HGSOC and host immune behavior. However an appropriate rate for treatment was determined in 9.6% cases. PD-L1 positive peritumoral stromal and intratumoral TILs scores were higher, in surviving compared to those who died. PD-L1 positive cases were associated with both intratumoral and peritumoral stromal CD8+ immune cells. Evaluation of PD-L1 expression and CD8+ TILs may be useful in classification of HGSOC patients for PD-1/PD-L1 blockade treatment, but further studies are needed to support this assumption, particularly on HGSOC cases.
Author contributions statement
Yılmaz Baş: Formal analysis, Investigation, Supervision, Project administration. Nermin Koç, Yılmaz Baş: Conceptualization Ideas, Methodology. Cem Koçak: Software, Visualization. Kaan Helvacı: Data Curation. Havva Hande Keser Şahin: Validation. Nermin Koç, Kaan Helvacı: Study Data. Yılmaz Baş, Raşit Akdeniz, Havva Hande Keser Şahin: Resources. Yılmaz Baş, Havva Hande Keser Şahin: Writing - Review & Editing.
Funding
The authors declare that they did not receive funding for this research from any source.
Compliance with ethical standards
Ethics committee approval was received from Hitit University Faculty of Medicine Clinical Research Ethics Committee (2017-156).
Declaration of Competing Interest
The authors declare that they have no known competing financial interestsor personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Yılmaz Baş, Email: yilbas@yahoo.com.
Nermin Koç, Email: nerminkoc@yahoo.com.
Kaan Helvacı, Email: kaanhelvaci@gmail.com.
Cem Koçak, Email: cemkocak@hotmail.com.
Raşit Akdeniz, Email: drrasitakdeniz@hotmail.com.
Havva Hande Keser Şahin, Email: havvahande.keser@saglik.gov.tr.
References
- 1.Wang Q, Lou W, Di W, Wu X. Prognostic value of tumor PD-L1 expression combined with CD8+ tumor infiltrating lymphocytes in high grade serous ovarian cancer. Int. Immunopharmacol. 2017;52:7–14. doi: 10.1016/j.intimp.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C, Braicu I, Jöhrens K. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 4.Pinto MP, Balmaceda C, Bravo ML, Kato S, Villarroel A, Owen GI, Roa JC, Cuello MA, Ibañez C. Patient inflammatory status and CD4+/CD8+ intraepithelial tumor lymphocyte infiltration are predictors of outcomes in high-grade serous ovarian cancer. Gynecol. Oncol. 2018;151(1):10–17. doi: 10.1016/j.ygyno.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Li J, Wang J, Chen R, Bai Y. Lu X The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth AA, Frosina D, Gnjatic S, Ambrosone C, Kepner J, Odunsi T, Ritter G, Lele S, Chen YT, Ohtani H, Old LJ, Odunsi K. Intraepithelial CD8+ tumor-infltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu X, Lang J. Programmed death-1 pathway blockade produces a synergistic antitumor effect: combined application in ovarian cancer. J. Gynecol. Oncol. 2017;28(5):e64. doi: 10.3802/jgo.2017.28.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol. Oncol. 2016;141(2):293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Darb-Esfahani S, Kolaschinski I, Trillsch F, Mahner S, Concin N, Vergote I, Van Nieuwenhuysen E, Achimas-Cadariu P, Glajzer J, Woopen H, Wienert S, Taube ET, Stanske M, Kulbe H, Denkert C, Sehouli J, Braicu EI. Morphology and tumour-infiltrating lymphocytes in high-stage, high-grade serous ovarian carcinoma correlated with long-term survival. Histopathology. 2018;73(6):1002–1012. doi: 10.1111/his.13711. [DOI] [PubMed] [Google Scholar]
- 10.Webb JR, Milne K, Nelson BH. PD-1 and CD103 are widely coexpressed on prognostically favorable intraepithelial CD8 T cells in human ovarian cancer. Cancer Immunol. Res. 2015;3:926–935. doi: 10.1158/2326-6066.CIR-14-0239. [DOI] [PubMed] [Google Scholar]
- 11.Mills AM, Peres LC, Meiss A, Ring KL, Modesitt SC, Abbott SE, Alberg AJ, Bandera EV, Barnholtz-Sloan J, Bondy ML, Cote ML, Funkhouser E, Moorman PG, Peters ES, Schwartz AG, Terry PD, Wallace K, Schildkraut JM. Targetable immune regulatory molecule expression in high-grade serous ovarian carcinomas in African American women: a study of PD-L1 and IDO in 112 cases from the African American cancer epidemiology study (AACES) Int. J. Gynecol. Pathol. 2019;38(2):157–170. doi: 10.1097/PGP.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J. Immunother. Cancer. 2018;6(1):8. doi: 10.1186/s40425-018-0316-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malpica A, Deavers MT, Lu K, Bodurka DC, Atkinson EN, Gershenson DM, Silva EG. Grading ovarian serous carcinoma using a two-tier system. Am. J. Surg. Pathol. 2004;28(4):496–504. doi: 10.1097/00000478-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Belhadj H, Berek J, Bermudez A, Bhatla N, Cain J, Denny L, Fujiwara K, Hacker N, Åvall-Lundqvist E, Mutch D, Odicino F, Pecorelli S, Prat J, Quinn M, Seoud MA, Shrivastava SK, Prat J, FIGO Committee on Gynecologic Oncology Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014;124(1):1–5. doi: 10.1016/j.ijgo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 15.Drakes ML, Mehrotra S, Aldulescu M, Potkul RK, Liu Y, Grisoli A, Joyce C, O'Brien TE, Stack MS, Stiff PJ. Stratification of ovarian tumor pathology by expression of programmed cell death-1 (PD-1) and PD-ligand- 1 (PD-L1) in ovarian cancer. J. Ovarian Res. 2018;11(1):43. doi: 10.1186/s13048-018-0414-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang LJ, Deng XF, Chang F, Wu XL, Wu Y, Diao QZ. Prognostic significance of programmed cell death ligand 1 expression in patients with ovariancarcinoma: a systematic review and meta-analysis. Medicine (Baltimore). 2018;97(43):e12858. doi: 10.1097/MD.0000000000012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hutarew G. PD-L1 testing, fit for routine evaluation? From a patholo gist's point of view. Memo. 2016;9(4):201–206. doi: 10.1007/s12254-016-0292-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ring KL, Pakish J, Jazaeri AA. Immune checkpoint inhibitors in the treatment of gynecologic malignancies. Cancer J. 2016;22(2):101–107. doi: 10.1097/PPO.0000000000000179. [DOI] [PubMed] [Google Scholar]
- 20.Deng F, Xu X, Lv M, Ren B, Wang Y, Guo W, Feng J, Chen X. Age is associated with prognosis in serous ovarian carcinoma. J. Ovarian Res. 2017;10(36):1–9. doi: 10.1186/s13048-017-0331-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin. Cancer Res. 2007;13(6):1757–1761. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]
- 22.Badoual C, Hans S, Merillon N, Van Ryswick C, Ravel P, Benhamouda N, Levionnois E, Nizard M, Si-Mohamed A, Besnier N, Gey A, Rotem-Yehudar R, Pere H, Tran T, Guerin CL, Chauvat A, Dransart E, Alanio C, Albert S, Barry B, Sandoval F, Quintin-Colonna F, Bruneval P, Fridman WH, Lemoine FM, Oudard S, Johannes L, Olive D, Brasnu D, Tartour E. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPVassociated head and neck cancer. Cancer Res. 2013;73(1):128–138. doi: 10.1158/0008-5472.CAN-12-2606. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt LH, Kummel A, Gorlich D, Mohr M, Brockling S, Mikesch JH, Grunewald I, Marra A, Schultheis AM, Wardelmann E, Müller-Tidow C, Spieker T, Schliemann C, Berdel WE, Wiewrodt R, Hartmann W. PD-1 and PD-L1 expression in NSCLC indicate a favorable prognosis in defined subgroups. PLoS. One. 2015;10(8) doi: 10.1371/journal.pone.0136023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raspollini MR, Castiglione F, Rossi DD, Amunni G, Villanucci A, Garbini F, Baroni G, Taddei GL. Tumourinfltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann. Oncol. 2005;16:590–596. doi: 10.1093/annonc/mdi112. [DOI] [PubMed] [Google Scholar]
- 25.Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, Hirsch MS, Matulonis UA, Liu J, Birrer MJ, Berkowitz RS, Mok SC. Increased HLADMB expression in the tumor epithelium is associated with increased CTL infltration and improved prognosis in advanced-stage serous ovarian cancer. Clin. Cancer Res. 2008;14:7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stumpf M, Hasenburg A, Riener MO, Jutting U, Wang C, Shen Y, Orlowska-Volk M, Fisch P, Wang Z, Gitsch G, Werner M, Lassmann S. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes. Br. J. Cancer. 2009;101:1513–1521. doi: 10.1038/sj.bjc.6605274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N, Rubin SC, Boyd J, Gimotty PA, Coukos G. Intraepithelial T cells and tumor proliferation: impact on the beneft from surgical cytoreduction in advanced serous ovarian cancer. Cancer Am. Cancer Soc. 2009;115:2891–2902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmayr-Heyda A, Aust S, Heinze G, Polterauer S, Grimm C, Braicu EI, Sehouli J, Lambrechts S, Vergote I, Mahner S, Pils D, Schuster E, Thalhammer T, Horvat R, Denkert C, Zeillinger R, Castillo-Tong DC. Prognostic impact of tumor infltrating CD8+ T cells in association with cell proliferation in ovarian cancer patients–a study of the OVCAD consortium. BMC Cancer. 2013;13:422. doi: 10.1186/1471-2407-13-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeLeeuw RJ, Kroeger DR, Kost SE, Chang PP, Webb JR, Nelson BH. CD25 identifes a subset of CD4(+) FoxP3(-) TIL that are exhausted yet prognostically favorable in human ovarian cancer. Cancer Immunol. Res. 2015;3:245–253. doi: 10.1158/2326-6066.CIR-14-0146. [DOI] [PubMed] [Google Scholar]
- 30.Knutson KL, Maurer MJ, Preston CC, Moysich KB, Goergen K, Hawthorne KM, Cunningham JM, Odunsi K, Hartmann LC, Kalli KR, Oberg AL, Goode EL. Regulatory T cells, inherited variation, and clinical outcome in epithelial ovarian cancer. Cancer Immunol. Immunother. 2015;64:1495–1504. doi: 10.1007/s00262-015-1753-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bösmüller HC, Wagner P, Peper JK, Schuster H, Pham DL, Greif K, Beschorner C, Rammensee HG, Stevanović S, Fend F, Staebler A. Combined Immunoscore of CD103 and CD3 Identifies Long-Term Survivors in High-Grade Serous Ovarian Cancer. Int. J. Gynecol. Cancer. 2016;26(4):671–679. doi: 10.1097/IGC.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 32.Strickland KC, Howitt BE, Shukla SA, Rodig S, Ritterhouse LL, Liu JF, Garber JE, Chowdhury D, Wu CJ, D'Andrea AD, Matulonis UA, Konstantinopoulos PA. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santoiemma PP, Reyes C, Wang LP, McLane MW, Feldman MD, Tanyi JL, Powell DJ., Jr Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol. Oncol. 2016;143(1):120–127. doi: 10.1016/j.ygyno.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 34.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc. Natl. Acad. Sci. U.S.A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res. 2015;75(11):2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ionescu DN, Downes MR, Christofides A, Tsao MS. Harmonization of PD-L1 testing in oncology: a Canadian pathology perspective. Curr. Oncol. 2018;25(3):e209–e216. doi: 10.3747/co.25.4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gerlinger M, Rowan AJ, Horswell S, Math M, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 2012;366(10):883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madore J, Vilain RE, Menzies AM, Kakavand H, Wilmott JS, Hyman J, Yearley JH, Kefford RF, Thompson JF, Long GV, Hersey P, Scolyer RA. PD-L1 expression in melanoma shows marked heterogeneity within and between patients: implications for anti-PD-1/PD-L1 clinical trials. Pigment Cell Melanoma Res. 2005;28(3):245–253. doi: 10.1111/pcmr.12340. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhury F, Dunn S, Mitchell S, Mellows T, Ashton-Key M, Gray JC. PD-L1 and CD8+ PD1+ lymphocytes exist as targets in the pediatric tumor microenvironment for immunomodulatory therapy. OncoImmunology. 2015;4(10) [Google Scholar]
- 40.Stanske M, Wienert S, Castillo-Tong DC, Kreuzinger C, Vergote I, Lambrechts S, Gabra H, Gourley C, Ganapathi RN, Kolaschinski I, Budczies J, Sehouli J, Ruscito I, Denkert C, Kulbe H, Schmitt W, Jöhrens K, Braicu I, Darb-Esfahani S. Dynamics of the intratumoral immune response during progression of high grade serous ovarian cancer. Neoplasia. 2018;20(3):280–288. doi: 10.1016/j.neo.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koppel C, Schwellenbach H, Zielinski D, Eckstein S, Martin-Ortega M, D'Arrigo C, Schildhaus HU, Rüschoff J, Jasani B. Optimization and validation of PD-L1 immunohistochemistry staining protocols using the antibody clone 28-8 on different staining platforms. Mod. Pathol. 2018;31(11):1630–1644. doi: 10.1038/s41379-018-0071-1. [DOI] [PubMed] [Google Scholar]