Abstracts
The residents of the Eastern part of Indonesia, specifically, Papua and West Papua provinces, are dependent on traditional medicines with the use of plants, which includes treating malaria. However, there are limited information on the diversity of medicinal plants in Papua Island. Hence, the Indonesian Ministry of Health put together a database of all the natural plant-based raw materials in Indonesia, to address part of the issues encountered as a result of the limited information on the diversity of plants. Based on this background, the aim of the research was to analyze the information on medicinal plants used by the traditional healers in Papua Island based on the results of research on medicinal plants and Jamu (RISTOJA), especially in treating malaria. Data were obtained through ethnomedicine research conducted in 2012 and 2017 involving 54 ethnicities in Papua. Based on the results, 72 species of medicinal plants from 67 genera and 40 families were used traditionally in treating malaria on Papua Island. The most common medicinal plants used as traditional antimalarial concoction are Alstonia scholaris (L.) R. Br., Carica papaya L., Andrographis paniculata (Burm. f.) Nees, and Physalis minima L. Similar to other ethnobotany research, the leaves were the most used plant parts in preparing the various traditional concoctions.
Keywords: Antimalarial, Medicinal plant, Ethnomedicine, Papua, West Papua, Indonesia, Bioactive plant product, Pharmaceutical science, Natural product, Alternative medicine, Evidence-based medicine
Antimalarial; Medicinal Plant; Ethnomedicine; Papua; West Papua; Indonesia; Bioactive Plant Product; Pharmaceutical Science; Natural product; Alternative Medicine; Evidence-Based Medicine
1. Introduction
Indonesia is among the developing countries in ASEAN with the high cases of malaria. Until 2007, about 80% of villages and cities in the country were declared malaria-free. However, the incidence of malaria reduced from 2.9% in 2007 to 1.9% in 2013. Five provinces with the highest incidence of malaria include Papua, West Papua, East Nusa Tenggara, Central Sulawesi, and Maluku. Also, Papua and West Papua are the two major provinces in Papua Island, Indonesia, with annual parasite incidence (API) indicator of about 67%. Five districts with the highest API indicator in Papua province include Keerom, Jayapura, Timika, Sarmi, and Boven Digoel Regency. Meanwhile, Manokwari, South Manokwari, and Wondama Bay Regency are the three districts in West Papua with the highest API indicator [1, 2, 3, 4, 5].
High cases of malaria in Papua and West Papua are due to several factors such as the resistance of the malaria-causing parasites, low level of government programs, especially in the underdeveloped areas, as well as limited health facilities in remote areas [1, 2, 6]. The government's efforts in eradicating malaria have been ongoing for a decade. The Minister of Health launched a four-phase of malaria elimination program, namely eradication, pre-elimination, elimination, and maintaining the elimination status. The program was planned for islands and provinces, which began in 2010 and expected to be fully accomplished by 2030. This was specifically targeted at Papua, West Papua, and several provinces in eastern Indonesia to be malaria-free in 2030 [2,7].
Kumar and Preetha (2012) suggested the need for each community to maximize the current technology development in the health sector in order to reduce the morbidity of the disease. Also, the government should be able to collaborate with the community in terms of organizing programs to eliminate it in various communities. Papua society is made up of various ethnic with diverse cultures and numerous knowledge in overcoming health problems. The knowledge of the society as regards health is based on the use of local approach, including traditional healers, also known as dukun or hattra. Also, a majority of these traditional healers make use of plants as medicine [8, 9].
Various plants have been known to possess medicinal properties from ancient times. The use of plants as medicine became popular considering the fact that they are more efficient, safe, and economical. Also, the use of plants for treating malaria has been carried out for thousands of years. The forerunner of the antimalarial plant-derived compounds include quinine, lapachol, and artemisinin [6, 10, 11]. The discovery and development of plants for drug innovation became one of the priorities in the Malaria Eradication Research Agenda and ethnopharmacology is a promising approach in handling malaria management in the future [10, 12]. According to Supiandi et al. (2019), there is need for the inventory of local wisdom related to the use of medicinal plants for the prevention, as well as in treating diseases, and also highlighted the need to preserve and develop it. Traditional local wisdom is the basis for developing innovative and technological advancement currently experienced, as well as in the future [13].
Based on recent studies, plants diversity in Papua Island ranged from 20,000 to 25,000 species. Several researchers have reviewed the status of exploration and documentation of plants in the island, however, the data collected were unable to accurately describe the plant diversity Research on plant diversity is expected to take a long time to be fully resolved, while some studies put it at 2150 [14,15].
The Health Ministry, through the National Institute of Health Research and Development (NIHRD) in collaboration with some leading universities, have conducted research on medicinal plants and Jamu (hereafter RISTOJA). RISTOJA is an ethnomedicinal research which explores the utilization of medicinal plants based on ethnics in Indonesia. About 54 ethnics were observed in Papua and West Papua provinces, and the traditional ingredients successfully recorded from the two provinces was around 3,303 traditional concoctions from 2,930 plant species. Also, the traditional concoctions for malaria in Papua Island were the most prominent three used from 74 types of diseases in RISTOJA [16, 17, 18]. In addition, Widiyastuti and Widayat (2013) reported the importance of properly documenting the variety of medicinal plants in Papua Island since the data related to these plants were improperly collected [19]. Based on the background, the aim of this study was to analyze the information on medicinal plants used by the traditional healers in Papua Island based on the results of RISTOJA, especially in malaria treatment. This information is expected to be further explored and developed to bring better innovation in malaria drugs, which would be beneficial in overcoming the problems with disease resistance.
2. Material and methods
2.1. Location and time of study
This study used the metadata of RISTOJA collected in 2012 and 2017, through an electronic questionnaire created using Microsoft Access software. The results of the electronic questionnaire were sent through e-mail to the Laboratory Data Management of NIHRD, which were then added to the data of each province. The data of each province was subsequently processed through cleaning and coding, then compiled into a database. However, access to the RISTOJA database is not open through the website, the usage of data is only possible by submitting proposal to the Head of NIHRD, Ministry of Health of the Republic of Indonesia.
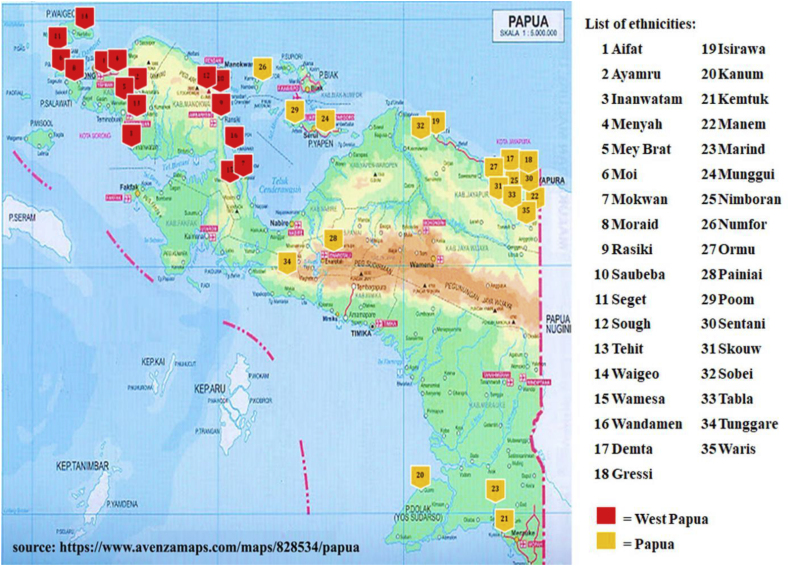
The research was conducted between October and November 2012, involving 6 and 18 ethnics in Papua and West Papua provinces, respectively. Also, in May 2017, 10 and 20 ethnics were observed in Papua and West Papua provinces, respectively, as shown in Figure 1. The ethnics used in this research were selected through purposive sampling method based on the criteria of the ethnic having a minimum population 1000 people according to Statistics Indonesia Database [16, 17]. This criteria was based on that ethnicity with a minimum population of 1000 people has long existed and has a wealth of local wisdom information which could be further developed, especially the traditional knowledge of medicinal plant for treating malaria.
Figure 1.
Distribution of research location in Papua island.
Papua Island is located in the eastern part of Indonesia between 0o19′-10o45′ south latitude and 130o45′-141o48′ east longitude. The northern part of the island is surrounded by the Pacific Sea, the Maluku islands, and the Banda ocean in the west, the Indian sea, the Gulf of Carpentaria, the Arafuru ocean, and the Australian continent in the south, while the east borders directly with Papua New Guinea. In terms of topographical conditions, Papua Island is mostly tropical rain forests, with valleys, shorelines, streams, lowlands, highlands or mountains difficult to access. Its climatic conditions include relatively high rainfall from 1800 to 3000 mm high humidity of over 80% and a temperature within a range of 19–28 °C depending on the nature of the area, with higher locations having lower air temperature. In addition, the highest location on the island is known as Puncak Jaya Regency in the Jayawijaya mountains, and is frequently covered with snow [20, 21].
2.2. Data collection
Data collection was conducted through direct interviews with traditional healers/hattra engaged in providing the services in each local ethnicity and the used medicinal plant specimens were collected. The traditional healers used as informants, were selected through purposive sampling method from the data obtained from the Regional Health Offices, the local community, or the ethnic leaders. The RISTOJA was also used as inclusion criteria in selecting the traditional healers, including those having the knowledge and ability to treat malaria using medicinal plants, as well as truly practicing traditional treatment, and widely known in the community. Traditional healers who did not meet these criteria were excluded. The total traditional healers which has been interviewed in Papua Island are 270 informants, respectively 5 individuals for each ethnicity. All of these traditional healers were unregistered to the regulatory of Ministry of Health but their traditional treatment experiences have been trusted by the local people.
Interviews were conducted using structured guidelines to collect data related to the use of plant species as medicine. The medicinal plant species were recorded based on local names, habitus, parts of plants used, and the use of the plants. Several plants were collected as specimens, identified by plant taxonomist, documented and stored in Herbarium Tawangmanguensis, MPTMRDC.
2.3. Data analysis
The data collected were analyzed qualitatively and quantitatively. Qualitative data analysis was carried out descriptively and supported with literatures review on the plants. Then, the quantitative analysis was conducted by measuring the value of Family Importance Value (FIV), Relative Frequency of Citation (RFC), and Plant Part Value (PPV). The FIV was calculated to identify the significance of plant families and it is an essential index in ethnobotany in estimating the amount of plant taxon. This value was calculated using a formula cited by Sulaiman et al. (2020) [22]:
| (1) |
FC (family) = the number of informants mentioning the family
N = total of informant interviewed
The RFC was calculated to determine which species of plant used as traditional malaria medicine. Also, this value was calculated using the formula cited by Tardío and Pardo-De-Santayana (2008) [23]:
| (2) |
FC = the number of informants who mentioned a certain species
N = total of informant interviewed
The percentage of plant parts that are used as medicinal ingredients for malaria was calculated using the formula cited by Gomez-Beloz (2002) [24]:
| (3) |
∑RU(plant part) = total number of use cited for each plant part
∑RU = total all plant part
3. Results and discussion
3.1. The distribution of malaria herbal concoction in Papua Island
The clinical symptoms of malaria used as operational definitions in RISTOJA include high fever, chills, muscular aching, abdominal pain, nausea, and loss of appetite [17]. Based on the RISTOJA data, 35 out of 54 ethnics in the provinces of Papua and West Papua widely used traditional antimalarial treatments, with a total of 89 concoctions. West Papua was with more antimalarial concoctions compared with Papua province, with 65 concoctions. Also, Waigeo, Wamesa, and Sough were the ethnics in West Papua with the highest number of concoctions. Additionally, there were diverse antimalarial concoctions among ethnics in the province, with 47 single and 18 compound concoctions, while 16 single and 8 compound concoctions recorded in Papua province.
The ethnics with antimalarial concoction are broadly distributed in the province, both in locations accessible and remote areas. This supports the fact that malaria is still a common problem in Papua Island. The Basic Health Research 2018 (RISKESDAS) also showed the prevalence of malaria in the provinces of Papua and West Papua, both ranking the first two among 34 provinces in Indonesia [25]. Ethnics in Papua province with antimalarial concoctions are more dominant in Jayapura Regency, while in West Papua province, antimalarial concoctions are more dominant in Sorong and Manokwari regencies. This is in line with the reports of Health Service (Dinas Kesehatan (DINKES)) showing that the two provinces with the highest API value in Papua Island were Jayapura and Manokwari districts [4].
More people in West Papua are dependent on traditional medicine due to the fact that most locations are difficult to reach by modern medicine. The high diversity of herbs among ethnicities in the province of West Papua shows that local wisdom regarding traditional medicine is still more embraced compared with the province of Papua. Locations in Papua province are more easily accessible, with ease of communication and entry of information from outside, including modern medicine. However, these conditions could lead to changes in ecosystems, environment, cultural values, and local wisdom, one of which is related to traditional medicine [26].
Despite these conditions, people in Papua Island still depend on traditional medicine. According to Triratnawati (2017), traditional medical practices are hereditary and dominant since many areas have not been reached with the modern health system. Also, one of the local wisdom is the use of plant-based materials in treating various diseases. RISKESDAS 2018 also showed the preference of people in both provinces in preparing their traditional medicine for treating various diseases. This is shown by the high proportion in the use of traditional homemade concoctions, which was approximately 81.7% in Papua and 70.8% in West Papua [17, 25, 27].
3.2. Diversity of plant species used for treating malaria
Table 1 shows the plant species usually used by traditional healers in treating malaria in Papua Island. It shows the family, scientific names, local names of the ethnic groups using the plants, the parts of plants used, habitat, and the qualitative values of RFC and FIV.
Table 1.
List of inventory antimalarial plants used in traditional treatment in Papua Island, Indonesia.
| No | Family | Scientific name of plant | Ethnic local name of plant | Part Used | Habit | FIV | FC | RFC |
|---|---|---|---|---|---|---|---|---|
| 1 | Acanthaceae | Andrographis paniculata (Burm. f.) Nees | Sambiloto (Moraid, Wamesa, Gresi, Nimboran, Skouw, Tabla); Sambiroto (Demta, Marind), Bedesna/samiroto (Rasiki) | leaves, stem, herb | herb | 15.73 | 3 | 0.12 |
| 2 | Acanthaceae | Dicliptera chinensis (L.) Juss. | Mogrovog (Hattam) | leaves | herb | 15.73 | 0 | 0.01 |
| 3 | Acanthaceae | Graptophyllum pictum (L.) Griff. | Mosmoga (Meyah) | leaves | herb | 15.73 | 0 | 0.01 |
| 4 | Acanthaceae | Justicia gendarussa Burm. f. | Gandarusa (Waigeo) | leaves | herb | 15.73 | 0 | 0.01 |
| 5 | Acoraceae | Acorus calamus L. | Hayau (Aifat) | rhizome | herb | 1.12 | 0 | 0.01 |
| 6 | Amaranthaceae | Iresine herbstii Hook. | Aregahani (Sough) | leaves | herb | 1.12 | 0 | 0.01 |
| 7 | Amaryllidaceae | Proiphys amboinensis (L.) Herb. | Biji malaria (Waigeo) | leaves | herb | 1.12 | 0 | 0.01 |
| 8 | Anacardiaceae | Mangifera indica L. | Mangga (Wamesa) | bark | tree | 1.12 | 0 | 0.01 |
| 9 | Apocynaceae | Alstonia scholaris (L.) R. Br. | Arasue (Aifat); Pohon susu (Ayamru); Kayu susu (Mey Brat, Wamesa, Wandamen, Manem, Skouw); Igeii (Moi); Epalom (Seget); Yede (Tehit); Aikahahe (Munggui); Ibuong (Nimboran); Yepaa (Tabla) | leaves, stem, bark | tree | 21.35 | 5 | 0.21 |
| 10 | Araceae | Colocasia antiquorum Schott | Sunggagahani (Mokwam) | stem | herb | 4.49 | 0 | 0.01 |
| 11 | Araceae | Pothos scandens L. | Ngges ngrup (Saubeba) | leaves | herb | 4.49 | 0 | 0.01 |
| 12 | Araceae | Schismatoglottis wallichii Hook. f. | Serbou (Saubeba) | leaves | herb | 4.49 | 0 | 0.01 |
| 13 | Araceae | Xanthosoma sagittifolium (L.) Schott | Keladi (Sough) | tuber | herb | 4.49 | 0 | 0.01 |
| 14 | Araliaceae | Osmoxylon novoguineense (Scheff.) Becc. | Lebe (Moi); Koskogoi (Mokwam) | bark | tree | 2.25 | 0 | 0.02 |
| 15 | Arecaceae | Areca catechu L. | Aku (Nimboran); Pinang (Tunggare) | root, fruit | tree | 7.87 | 0 | 0.02 |
| 16 | Arecaceae | Cocos nucifera L. | Kim srang (Kemtuk) | root | tree | 7.87 | 0 | 0.01 |
| 17 | Arecaceae | Metroxylon sagu Rottb. | Sagu (Wamesa); Mo (Nimboran) | stem, root, whole part | tree | 7.87 | 1 | 0.04 |
| 18 | Asparagaceae | Dracaena angustifolia (Medik.) Roxb. | Susmoro (Mokwam) | bark | herb | 1.12 | 0 | 0.01 |
| 19 | Balsaminaceae | Impatiens balsamina L. | Ugera tiga (Sough) | herb | herb | 1.12 | 0 | 0.01 |
| 20 | Brassicaceae | Cardamine flexuosa With. | Merbetamog (Sough) | leaves | herb | 1.12 | 0 | 0.01 |
| 21 | Caricaceae | Carica papaya L. | Peceren (Aifat); Eteradado (Inanwatam); Monofo (Meyah); Pepaya(Sough, Waigeo, Wandamen, Nimboran, Tabla); Labseren (Tehit); Ogoseren (Tehit); Cacaveka (Isirawa); Mbikin (Kanum); Senene ranau (Tunggare); Pepaya bllah (Waris) | leaves, root, flower | tree | 21.35 | 5 | 0.21 |
| 22 | Combretaceae | Terminalia catappa L. | Ketapang (Wamesa, Wandamen) | bark | tree | 3.37 | 1 | 0.03 |
| 23 | Crassulaceae | Bryophyllum daigremontianum (Raym.-Hamet & H. Perrier) A. Berger | Brimomog (Sough) | herb | herb | 1.12 | 0 | 0.01 |
| 24 | Cycadaceae | Cycas rumphii Miq. | Inyom (Mokwam) | bark | shrubs | 1.12 | 0 | 0.01 |
| 25 | Dioscoreaceae | Dioscorea bulbifera L. | Audub (Mokwam) | tuber | liana | 1.12 | 0 | 0.01 |
| 26 | Euphorbiaceae | Endospermum moluccanum (Teijsm. & Binn.) Kurz | Inyabi (Mokwam) | bark | tree | 8.99 | 0 | 0.02 |
| 27 | Euphorbiaceae | Euphorbia heterophylla L. | Arectmot (Mokwam); Kastroli (Waigeo); Kastol (Gresi) | leaves, root | herb | 8.99 | 1 | 0.03 |
| 28 | Euphorbiaceae | Homalanthus populifolius Graham | Hinougomog (Mokwam) | leaves | shrubs | 8.99 | 0 | 0.01 |
| 29 | Euphorbiaceae | Ricinus communis L. | Domi (Rasiki); Domimog (Sough) | leaves | tree | 8.99 | 0 | 0.02 |
| 30 | Fabaceae | Caesalpinia bonduc (L.) Roxb. | Neibi (Sough) | leaves | shrubs | 4.49 | 0 | 0.01 |
| 31 | Fabaceae | Falcataria moluccana (Miq.) Barneby & J.W.Grimes | Mosofos (Mokwam) | bark | tree | 4.49 | 0 | 0.01 |
| 32 | Fabaceae | Inocarpus papuanus Kosterm. | Gayang (Wandamen) | bark | tree | 4.49 | 0 | 0.01 |
| 33 | Fabaceae | Sesbania grandiflora (L.) Pers. | Harimah (Skouw) | leaves | tree | 4.49 | 0 | 0.01 |
| 34 | Gesneriaceae | Aeschynanthus radicans Jack | Pomeric (Sough) | leaves | herb | 1.12 | 0 | 0.01 |
| 35 | Goodeniaceae | Scaevola taccada (Gaertn.) Roxb. | Anas (Numfor) | leaves | shrubs | 1.12 | 0 | 0.01 |
| 36 | Hanguanaceae | Hanguana malayana (Jack) Merr. | Illis hutan (Sough) | leaves | herb | 1.12 | 0 | 0.01 |
| 37 | Lamiaceae | Orthosiphon aristatus (Blume) Miq. | Kumis kucing (Moraid, Waigeo, Wamesa, Marind, Tunggare) | leaves, herb | herb | 6.74 | 1 | 0.06 |
| 38 | Lamiaceae | Plectranthus scutellarioides (L.) R.Br. | Mayana (Mey Brat) | leaves | herb | 6.74 | 0 | 0.01 |
| 39 | Lauraceae | Cassytha filiformis L. | Tali kuning (Tabla) | leaves | liana parasite | 1.12 | 0 | 0.01 |
| 40 | Loranthaceae | Dendrophthoe pentandra (L.) Miq. | Iramoub (Mokwam) | leaves | shrubs parasite | 1.12 | 0 | 0.01 |
| 41 | Malvaceae | Melochia umbellata (Houtt.) Stapf | Mendewa (Ormu) | leaves | tree | 1.12 | 0 | 0.01 |
| 42 | Meliaceae | Azadirachta indica A.Juss. | Pohon india (Marind) | leaves | shrubs | 7.87 | 0 | 0.01 |
| 43 | Meliaceae | Lansium parasiticum (Osbeck) K.C.Sahni & Bennet | Langsat (Mey Brat); Rukun (Mey Brat); Lokum ayno (Waigeo); Sandipori (Wamesa) | leaves, stem, bark, seed | tree | 7.87 | 1 | 0.06 |
| 44 | Meliaceae | Swietenia mahagoni (L.) Jacq. | Mahoni (Marind) | seed | tree | 7.87 | 0 | 0.01 |
| 45 | Menispermaceae | Anamirta cocculus (L.) Wight & Arn. | Emmbait (Tehit) | stem | liana | 11.24 | 0 | 0.01 |
| 46 | Menispermaceae | Arcangelisia flava (L.) Merr. | Kiluf'un (Moi); Aidaga geiho (Mokwam); Kuigwep (Seget); Batang kwiguep (Seget); Tenggang yanggu (Nimboran) | stem, bark | liana | 11.24 | 1 | 0.06 |
| 47 | Menispermaceae | Fibraurea tinctoria Lour. | Bai (Rasiki); Giwi saknge (Manem) | stem | liana | 11.24 | 0 | 0.02 |
| 48 | Menispermaceae | Tinospora crispa (L.) Hook. f. & Thomson | Tali pahit (Moraid) | stem | liana | 11.24 | 0 | 0.01 |
| 49 | Menispermaceae | Tinospora sinensis (Lour.) Merr. | Tali malaria (Inanwatam) | leaves | liana | 11.24 | 0 | 0.01 |
| 50 | Moraceae | Artocarpus altilis (Parkinson) Fosberg | Igbi (Rasiki) | exudate | tree | 2.25 | 0 | 0.01 |
| 51 | Moraceae | Ficus variegata Blume | Adurakop (Mokwam) | exudate | tree | 2.25 | 0 | 0.01 |
| 52 | Musaceae | Musa x paradisiaca L. | Pisang (Inanwatam) | leaves | herb | 1.12 | 0 | 0.01 |
| 53 | Myrtaceae | Psidium guajava L. | Giawas (Paniai) | leaves | tree | 1.12 | 0 | 0.01 |
| 54 | Orchidaceae | Coelogyne asperata Lindl. | Boumohotmog (Mokwam) | fruit | epiphyte | 1.12 | 0 | 0.01 |
| 55 | Phyllanthaceae | Glochidion ferdinandi (Müll.Arg.) F.M. Bailey | Sautrame (Mokwam) | bark | shrubs | 10.11 | 0 | 0.01 |
| 56 | Phyllanthaceae | Glochidion lutescens Blume | Sampari (Numfor) | leaves | tree | 10.11 | 0 | 0.01 |
| 57 | Phyllanthaceae | Phyllanthus niruri L. | Belakang babiji (Wandamen, Skouw); Kakarvaya (Isirawa); Mesumgag hijau (Nimboran); Momokho akha (Sentani) | leaves, herb, whole part | herb | 10.11 | 1 | 0.07 |
| 58 | Phyllanthaceae | Phyllanthus urinaria L. | Aibaba kakopi (Poom) | herb | herb | 10.11 | 0 | 0.01 |
| 59 | Piperaceae | Piper betle L. | Sirih hutan (Waigeo) | leaves | shrubs | 1.12 | 0 | 0.01 |
| 60 | Poaceae | Imperata cylindrica (L.) Raeusch. | Udong (Kemtuk); Alang-alang (Tabla) | root, whole part | herb | 2.25 | 0 | 0.02 |
| 61 | Rubiaceae | Morinda citrifolia L. | Fayu (Mey Brat); Sanyin (Waigeo); Mengkudu (Skouw) | leaves, root, fruit, seed | tree | 10.11 | 1 | 0.07 |
| 62 | Rubiaceae | Nauclea orientalis (L.) L. | Nggal (Marind) | bark | tree | 10.11 | 0 | 0.01 |
| 63 | Rubiaceae | Neolamarckia macrophylla (Roxb.) Bosser | Kayu kuning (Wandamen) | bark | tree | 10.11 | 0 | 0.01 |
| 64 | Rubiaceae | Uncaria gambir (W. Hunter) Roxb. | Ausie (Aifat) | stem | tree | 10.11 | 0 | 0.01 |
| 65 | Sapindaceae | Allophylus cobbe (L.) Forsyth f. | Sah (Aifat) | stem | tree | 4.49 | 0 | 0.01 |
| 66 | Sapindaceae | Dodonaea viscosa Jacq. | Arwob (Saubeba); Waimu (Sough) | leaves, stem | shrubs | 4.49 | 1 | 0.03 |
| 67 | Solanaceae | Physalis minima L. | Pak pak (Tehit, Waigeo); Toki (Wamesa); Daun nipon (Wamesa); Cemplokan (Marind); Seberi-nembai (Sobei) | leaves, stem, root, bark, whole part | herb | 10.11 | 2 | 0.10 |
| 68 | Urticaceae | Dendrocnide moroides (Wedd.) Chew | Kafa (Tehit) | leaves | shrubs | 7.87 | 0 | 0.01 |
| 69 | Urticaceae | Dendrocnide stimulans (L.f.) Chew | Khu/kuh (Manem); Skuah (Nimboran); Daun gatal (Tunggare) | leaves | shrubs | 7.87 | 1 | 0.07 |
| 70 | Zingiberaceae | Hornstedtia conica Ridl. | Mewega esisu (Meyah) | leaves | herb | 3.37 | 0 | 0.01 |
| 71 | Zingiberaceae | Hornstedtia scottiana (F.Muell.) K.Schum. | Irisme (Mokwam) | rhizome | herb | 3.37 | 0 | 0.01 |
| 72 | Zingiberaceae | Kaempferia galanga L. | Sihor (Aifat) | rhizome | herb | 3.37 | 0 | 0.01 |
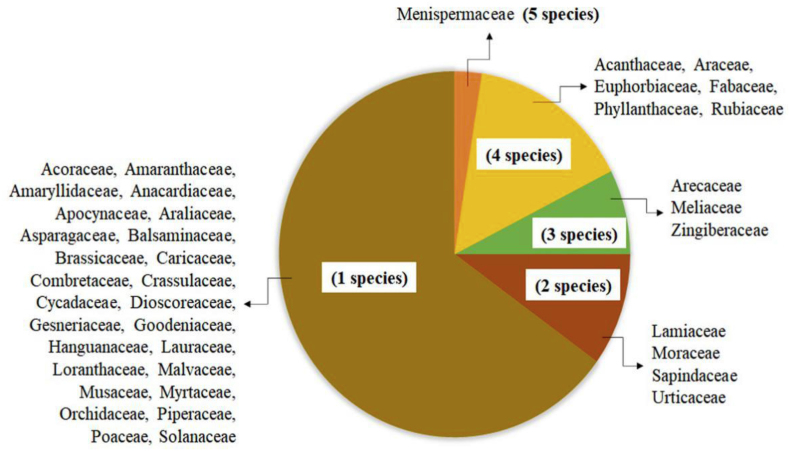
Based on the results, 72 plant species from 40 families were identified being used as traditional antimalarial medicine in 35 ethnics in the provinces of Papua and West Papua. These plant species belong to 67 genera and 40 families: Menispermaceae (5 species), Acanthaceae (4 species), Araceae (4 species), Euphorbiaceae (4 species), Fabaceae (4 species), Phyllanthaceae (4 species), Rubiaceae (4 species), Arecaceae (3 species), Meliaceae (3 species), Zingiberaceae (3 species), Lamiaceae (2 species), Moraceae (2 species), Sapindaceae (2 species), Urticaceae (2 species) and other families were represented by one species, as shown in Figure 2.
Figure 2.
Medicinal plant family and number of family species.
Considering the number of identified species, Menispermaceae were the most represented plant family, with a total of 5 species used as antimalarial medicine in Papua Island, as shown in Figure 2. This Menispermaceae family is made up of 70 genera with 420 species. In addition, some survey literature in Africa showed that about 21 genera have used as medicine, while other continents used 29 different genera. Some of the genera used as medicine include; Arcangelisia, Cissampelos, Cocculus, Cyclea, Diploclisia, Dioscoreophyllum, Fibraurea, Jateorhiza, Menispermum, Sinomenium, Sphenocentrum, Stephania, Tiliacora, Tinospora, and Triclisia. The most active compound in Menispermaceae widely used in treating various diseases is bisbenzylisoquinoline alkaloids. Most species of Menispermaceae have detoxification, antidysentery, antigastroenteritis, antioxidant, antimicrobial, antimelanogenesis, hepatoprotective, analgesic, anti-inflammatory, antidiabetic, anticancer, and antimalarial properties. Some genera of Menispermaceae are the main ingredients contained in most traditional herbs used as medicine in various countries, such as Cocculus and Tinospora in Bangladesh [28], Arcangelisia and Fibraurea in South Vietnam [29], Stephania in Asia and Cambodia, Triclisia in Tanzania [30], and Albertisia and Cissampelos in South Africa [31]. The data of RISTOJA in Papua Island support the potential use of Menispermaceae family in the development of antimalarial.
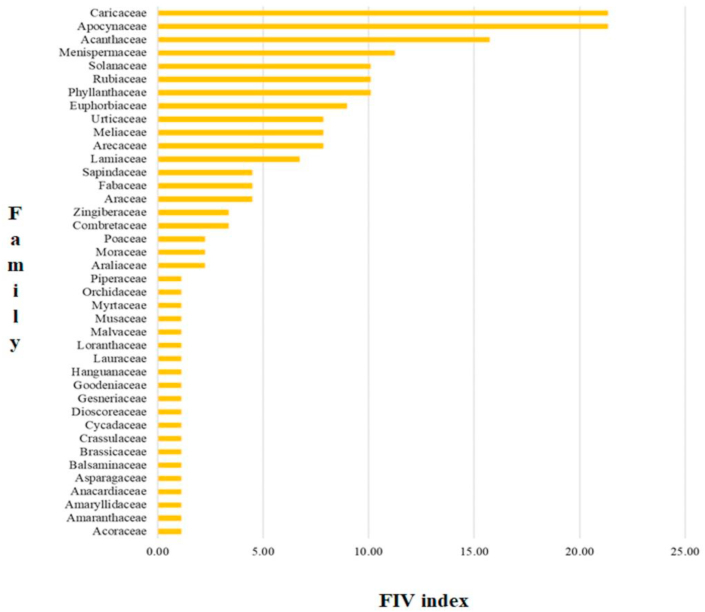
Figure 3 shows that based on FIV index, Apocynaceae and Caricaceae are families of medicinal plants commonly used in the treatment of malaria in Papua and West Papua, with index values of 21.35, respectively. This is followed by other families, such as Acanthaceae (15.73 with four species), Menispermaceae (11.24 with five species), Phyllanthaceae and Rubiaceae (10.11 each with four species), and Solanaceae (10.11 with one species) in addition to other families with FIV index above 10. The families with FIV index below 10 include, Euphorbiaceae (8.99 with four species), Arecaceae and Meliaceae (7.87 each with four species), Urticaceae (7.87 with two species), Lamiaceae (6.74 with two species), Araceae and Fabaceae (4.49 each with four species), Sapindaceae (4.49 with two species), Combretaceae (3.37 with one species), Zingiberaceae (3.37 with three species), Araliaceae and Poaceae (2.25 each with one species), and Moraceae (2.25 with two species). Also, twenty other families have a minimal FIV index, at 1.21 with one species.
Figure 3.
Family importance value (FIV) of medicinal plants used for malaria traditional treatment in Papua Island.
Apocynaceae and Caricaceae were the most cited plant families with the FIV index of 21.35, respectively, as shown in Figure 2. Apocynaceae is made up of 250 genera and 2000 species of tropical trees, shrubs, and vines. Based on literature, Apocynaceae species are generally used as traditional medicine in treating fever, malaria, pain, diabetes, digestive disorders, skin diseases, or ailments caused by parasites. Alstonia, Calotropis, Dyera, Kopsia, and Vallaris are among the genera known to have antimalarial activity. Active compounds isolated from the Apocynaceae family include alkaloids, cardenolides, triterpenoids, iridoids, pregnanes, flavonoids, and phenolic acids. The triterpenoid compounds are present in majority of the Apocynaceae family, which has the antimalarial potential [32, 33].
One species of the Caricaceae family known to have antimalarial potential is Carica papaya. This family is made up of around 35 species in 6 genera and widely distributed in Africa and Neotropics [34]. Research on Caricaceae family is predominantly specific to the C. papaya species. Some ethnobotany research documented the common use of the plant in the treatment of dengue, malaria, digestive disorders, cancer, inflammation, rheumatoid arthritis, skin diseases as well as acting as antibacterial substances. The contents of flavonoid, tannins, alkaloids, triterpenes, and carbohydrates also play important role in determining the bioactive nature of the Caricaceae family [35].
3.3. Most used plant species for traditional treatment of malaria
The relative use of the medicinal plant species by traditional healers is shown through the Relative Frequency of Citation (RFC) value. The assessment results showed RFC values within a range of 0.01–0.21. The highest RFC value was obtained from Alstonia scholaris and Carica papaya, each with a value of 0.21, followed by Andrographis paniculata at 0.12 and Physalis minima at 0.10. The four medicinal plant species occupying the first four positions are the most widely used among the 72 plant species. Other medicinal plant species have RFC values below 0.10, ranging from 0.07 to 0.01. Generally, medicinal plant species with the highest RFC values are the ones abundantly used traditionally in the treatment of malaria in Papua. The abundant use of these medicinal plant species is an indication that the knowledge on the use of the plants is widely distributed.
Table 1 shows that A. scholaris and C. papaya were with the second-highest RFC values among the 72 medicinal plant species traditionally used treating malaria in Papua Island, Indonesia. Based on this, these two species are the commonly used in the traditional treatment of malaria on the island. The tree of A. scholaris is as high as 40 m with bark-like tessellated cork and grayish-brown color. It is a native plant from Australia, with wide distribution in Southeast Asia, China, India, Nepal, Solomon Islands, Papua New Guinea, and America [36, 37]. The bark, flowers, leaves, and roots contain alkaloids as the most dominant compound, with approximately 180 types. Other typical compounds successfully isolated from the bark of A. scholaris include several types of ditamine and echitamine alkaloids, chitenine, and echicaoutchin. The leaves contain anilinoacrylate alkaloids, scholaricine, dihydrocondylocarpine alkaloids, vallesamine, lagunamine, angustilobine B acid, losbanine, alstonamine, rhazimanine, akuammiginone, and other indole alkaloid compounds. These alkaloids contents and their derivatives determine the biological activity of these plants [37, 38, 39]. Traditionally, A. scholaris are commonly used to treat diseases such as fever, malaria, fever, diarrhea, dysentery, dyspepsia, abdominal disorders, skin diseases, pruritus, tumors, asthma, bronchitis, helminthiasis, as well as antidepressants, anticancer, antituberculosis, immunostimulating, hepatoprotective etc. Also, the plant is used in commercial herbs mixture Ayush, Ayurvedic, Siddha/Tamil, and Unani [37, 38]. Its antimalarial potential has been proven scientifically by testing it against Plasmodium falciparum K1 strain, with a very high activity category. The bisalole alkaloids, namely villalstonine and macrocarpamine, are the bioactive components of these plants responsible for these activities [40]. A research by Rezeki et. al (2012) showed the use of A. scholaris in effectively reducing the levels of malaria parasites in some patients at the Local Hospital of Manokwari, West Papua. This treatment was also proven to reduce the clinical symptoms of malaria such as fever, headache, muscle aches, and diarrhea [41].
C. papaya has a wide distribution area owing to the fact that it is very easy to grow, especially in tropical regions. It is a native plant of America introduced to India in the 16th century. The tree of C. papaya is recognized through its morphology, with a delicate and weak trunk, generally unbranched, produce sap, and with the capacity of growing as high as 20 m [35,42,43]. Traditionally, this plant is commonly used in the treatment of a wide range of ailments. Each part of the plant could be used for different illnesses, as the leaves are generally used for treating malaria, dengue, jaundice, as antiviral and immunomodulatory. Its flowers are commonly utilized to treat digestive disorder, diuretics, and as tonics, while the roots are used as antifungal, for treating piles, and bruises. Also, different chemical contents are found in different parts of this plant, including flavonoids, alkaloids, phenolic compounds, β-carotene, lycopene, anthraquinone glycosides, etc [35, 43]. Teng et al. (2019) carried out a scientific research on the potential use of this plant as an antimalarial agent testing it against P. falciparum 3D7 parasites and Dd2 strains. The results showed an antimalarial activity in the medium category. The compound responsible for this activity is carpaine, which belongs to the alkaloids group. Several studies have also proven the plant to have some anti-inflammatory, antitumor, and antihypertensive properties as well as being used in treating hypoglycemic, fertility, and in wound healing [35].
Two other species worthy of consideration are A. paniculata and P. minima, with RFC values of 0.12 and 0.1 respectively. A. paniculata is one of the herbs within the family of Acanthaceae, widely cultivated in South and Southeast Asia. Traditionally, this plant is widely used in the treatment of a broad spectrum of ailments or as herbs mixture in preventing various health issues. This plant has also been commercialized in Ayurvedic, Unani, and Siddha formulas [44, 45]. The andrographolide and its derivatives are the bioactive chemical contents of A. paniculata. Andrographolide is a diterpenic lactone compound known with its antiviral, anti-inflammatory, and anticancer properties [46, 47]. According to Mishra et al. (2011), andrographolide compounds have antimalarial activity, both in vitro (P. falciparum) and in vivo (P. berghei).
Then, P. minima is an annual herbaceous, upright, semi-shrub, perennial species with 0.5–1.5 m height, belonging to the Solanaceae family. This plant is widely distributed in Asia, Africa, Australia, North and South America. Also, the plant was reported as one of the important medicinal plants in the Indian Traditional System of Medicines [48, 49]. According to Angamuthu et al. (2014), P. minima is traditionally used as analgesic, and in treating diuretic issues, anthelmintic, as tonic, and purgative. Also, steroidal lactones contained in this plant, have been reported to possess anti-inflammatory, antibacterial, analgesic, antipyretic, antimalarial, hypoglycemic, and antioxidant properties [50].
Literatures have shown that several medicinal plants traditionally used in the treatment of malaria in Papua, have modern scientific back up. According to de la Torre et al. (2012), the ethnobotany study and traditional knowledge of plants are essential parts of its development into medicine in the future [51]. This has a significant contribution to the modern medicine as seen today. In the present study, 25% of herbal medicines in modern pharmacopeia were plant-based while others are synthetic drugs using chemical compounds also isolated from plants [52]. Therefore, the recording and documentation of traditional knowledge are necessary in preserving this abundant information for the next generation. Documenting traditional knowledge provides a comprehensive database for the development of new phytochemical, pharmacological, and clinical studies. It also protects the culture and ecological value of a plant.
3.4. Part of plant species used in traditional treatment of malaria
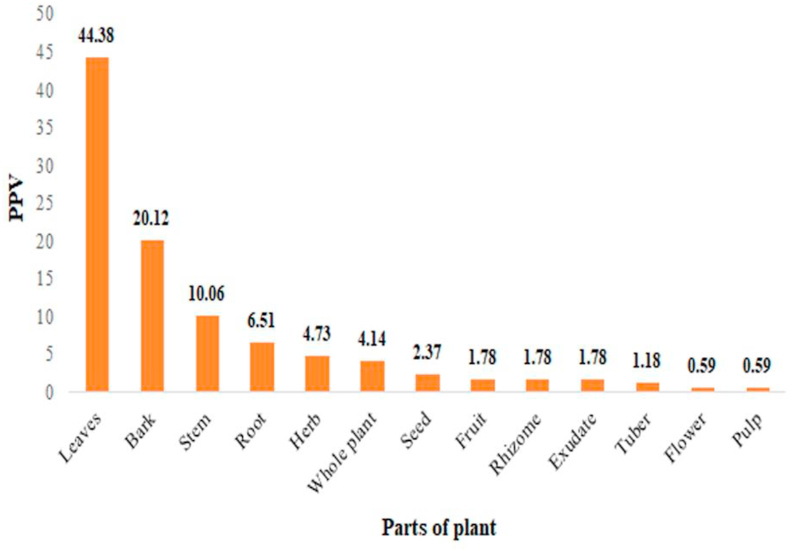
The concoctions traditionally used in treatment of malaria in Papua Island are composed of several types of plant parts. Based on the PPV results, majority of people, put at 44.38%, used leaves in Papua as their ingredients for traditional treatment of malaria. This was followed by the bark at 20.12%, stem at 10.06%, roots at 6.51%, herbs at 4.73%, whole plant at 4.14%, and seeds at 2.37%. In addition, each of fruit, rhizome, and exudate at 1.78%, tubers at 1.18%, and each of flower and fresh fruit at 0.59%, as shown in Figure 4.
Figure 4.
Part of plant value (PPV) of medicinal plants used for malaria traditional treatment in Papua Island.
In general, the parts of plants usually used in traditional medicine include leaves, bark, stem, fruit, roots, and tubers [53]. These results are similiar with many antimalarial ethnobotany studies which also showed that leaves are more used than other plant parts [54, 55, 56, 57, 58, 59, 60, 61]. The high frequency in the use of leaves as a medicinal ingredient is due to its abundance, availability, high productivity, and the fact that it is more comfortable to obtain compared with other plant parts [13, 62, 63]. Additionally, leaves are the most exploited part of plants because it provides advantages in terms of the technical acquisition, processing, and suitable for earth conservation [57, 64]. Furthermore, each part of the plant has different effects depending on its chemical composition. Since the leaves are the center of photochemical reactions, they tend to store more organic resources which could be metabolized into secondary metabolites, such as alkaloids, glycosides, flavonoid, phenolic compounds, steroid and terpenoid [13, 65, 66, 67]. According to Uzor (2020), these several secondary metabolites could become the medicinal properties for antimalaria [68].
4. Conclusion
The traditional knowledge and traditional medical practice with the use of plant-based medicine, especially in treating malaria, is a vital part of the people in Papua Island. This is probably due to the high cases of malaria and the remoteness of areas in both Papua and West Papua provinces. The ease and simplicity in finding and preparing plant concoctions are also an important reason why people depend more on traditional medicine.
The results of this study showed that the Western Part of Papua Island in Indonesia has a wide diversity of medicinal plants used in treating malaria. The traditional healers provided in detail the plant species and parts used in preparing the antimalarial concoctions. The data from traditional healers widely distributed in 35 ethnicities in Papua and West Papua, reported 72 species from 40 plant families. The most widely used plants for malaria treatment was Alstonia scholaris from Apocynaceae family and Carica papaya from Caricaceae family, which could be subjected to further research. There is need to carry out a systematic review in order to select the plant species from the data in this study, with high priority for further development.
Moreover, there is a work is in progress in analyzing numbers of plant species selected from Papua Island and to screen these antimalarial activities through in vitro assay used antiplasmodial bioassays and Heme Polymerization Inhibition Assay. However, the results would be published separately.
Declarations
Author contribution statement
M. Budiarti: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Maruzy: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
R. Mujahid: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data.
A.N. Sari: Contributed reagents, materials, analysis tools or data.
W. Jokopriyambodo, T. Widayat and S. Wahyono: Conceived and designed the experiments; Performed the experiments.
Funding statement
This work was supported by the National Institute of Health Research and Development (NIHRD), of the Republic of Indonesia.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are grateful to the local communities and hattra for their contributions to the success of this work. The efforts of the RISTOJA 2012 and 2017 team members are also appreciated, as well as the plant taxonomists for identifying the specimens. Finally, the authors wish to thank the Laboratory Data Management NIHRD team, Ministry of Health of the Republic of Indonesia, for providing the subset data.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
HELIYON-D-20-03238R1 supplementary material
References
- 1.Sorontou Y., Tukayo I.J.H., Pakpahan A. Relationship between malaria severity and parasite density of P. Falciparum againts demographic factirs from asymptomatic and symptomatic patients in Papua Indonesia. Int J Med Clin Res [Internet] 2015;6(2):331–336. https://bioinfopublication.org/files/articles/6_2_1_IJMCR.pdf Available from: [Google Scholar]
- 2.Roosihermiatie B., Paramita A., Nugroho A. Malaria self-care in nimboran subdistrict, Jayapura district, Papua province, Indonesia. Southeast Asian J. Trop. Med. Publ. Health. 2017;48(1):1–8. [PubMed] [Google Scholar]
- 3.Desideria B. 2018. BERITA PAPUA: Manokwari Posisi Teratas Kasus Malaria Se-Papua Barat [Internet]. Liputan 6.https://www.liputan6.com/health/read/3629728/manokwari-posisi-teratas-kasus-malaria-se-papua-barat [cited 2019 Aug 26]; Available from: [Google Scholar]
- 4.Dinkes . Dinas Kesehat. Papua; 2018. Dinas Kesehatan Provinsi Papua: Lima Kabupaten Masih Endemis Tinggi Malaria [Internet]http://dinkespapua.com/2018/05/06/dinkes-papua-lima-kabupaten-masih-endemis-tinggi-malaria [cited 2018 Aug 26];Available from: [Google Scholar]
- 5.Mediani M. CNN Indones; 2018. Kemenkes :Papua, Papua Barat, Dan NTT Endemis Tinggi Malaria [Internet]https://www.cnnindonesia.com/nasional/20180423203417-20-292975/kemenkes-papua-papua-barat-dan-ntt-endemis-tinggi-malaria [cited 2018 Aug 26];Available from: [Google Scholar]
- 6.Sarimole E., Martosupono M., Semangun H., Mangimbulude J.C., Studi P., Biologi M. Raja Ampat and Future Humanity (As a World Heritage) 2014. Pengobatan penyakit malaria dengan menggunakan beberapa jenis tumbuhan nabati di Kabupaten Raja ampat; pp. 1–6. [Google Scholar]
- 7.Dale P., Sipe N., Anto S., Hutajulu B., Ndoen E., Papayungan M. Malaria in Indonesia: a summary of recent research into its environmental relationship. Southeast Asian J. Trop. Med. Publ. Health. 2005;36(1):1–13. [PubMed] [Google Scholar]
- 8.Dumatubun A.E. Kebudayaan, kesehatan orang Papua dalam perspektif antropologi kesehatan. Antropol. Papua. 2002;1(1) [Google Scholar]
- 9.Kumar S., Preetha Health promotion: an effective tool for global health. Indian J. Community Med. 2012;37(3):5–12. doi: 10.4103/0970-0218.94009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wells T.N.C. Natural products as starting points for future anti-malarial therapies: going back to our roots? Malar. J. 2011;10(Suppl 1):1–12. doi: 10.1186/1475-2875-10-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh K., Hasan A., Ahmad A., Mir S.S. Anti-malarial treatment: herbal medicine a ray of hope. Int. J. Herb. Med. 2016;4(4):119–125. [Google Scholar]
- 12.Willcox M.L., Bodeker G. Traditional herbal medicines for malaria. BMJ [Internet] 2004;329(7475):1156–1159. doi: 10.1136/bmj.329.7475.1156. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid527695&toolpmcentrez&rendertypeabstract Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Supiandi M.I., Mahanal S., Zubaidah S., Julung H. Ethnobotany of traditional medicinal plants used by dayak desa community in sintang, west kalimantan, Indonesia. Biodiversitas. 2019;20(5):1264–1270. [Google Scholar]
- 14.Supriatna J., De fretes Y., Mack A., Yeager C.P., Olivieri S., Burnett J.B. Conservation International; Washington, D.C.: 1999. The Irian Jaya Biodiversity Conservation Priority-Setting Workshop. Final Report. [Internet]https://books.google.co.id/books?idap3IGwAACAAJ Available from: [Google Scholar]
- 15.Takeuchi W. Plant discoveries from PABITRA related exploration in Papua New Guinea. Org. Divers. Evol. 2003;3:77–84. [Google Scholar]
- 16.Wahyono S., Dkk . 2012. Laporan Nasional: Ekplorasi Pengetahuan Lokal Etnomedisin dan Tumbuhan Obat di Indonesia Berbasis Komunitas 2012. Jakarta. [Google Scholar]
- 17.Wahyono S., Jokopriyambodo W., Rahmawati N., Maruzy A., Widowati L., Subositi D. 2017. Laporan Nasional: Ekplorasi Pengetahuan Lokal Etnomedisin dan Tumbuhan Obat di Indonesia Berbasis Komunitas 2017. Jakarta. [Google Scholar]
- 18.Maruzy A., Mujahid R. Conservation status of medicinal plants from Papua and West Papua province (Indonesia) Media Konserv. 2019;24(2):114–123. [Google Scholar]
- 19.Widiyastuti Y., Widayat T. Inventarisasi tanaman obat di Kabupaten Jayapura, propinsi Papua. J. Tumbuh Obat. Indones. 2013;6(2):117–126. [Google Scholar]
- 20.Geosh Flysh. Kondisi geografis pulau Papua: batas, topografi, iklim dan keadaan alam [internet] Geologinesia. 2019 https://www.geologinesia.com/2019/04/kondisi-geografis-pulau-papua.html [cited 2020 May 14];Available from: [Google Scholar]
- 21.Sosilawati, Nababan M.L., Wahyudi A.R., Mahendra Z.A., Massudi W., Utami S. Kementerian Pekerjaan Umum dan Perumahan Rakyat; 2017. Sinkronisasi Program Dan Pembiayaan Pembangunan Jangka Pendek 2018-2020. Jakarta: Pusat Pemograman Dan Evaluasi Keterpaduan Infrastruktur PUPR, Badan Pengembangan Infrastruktur Wilayah. [Google Scholar]
- 22.Sulaiman, Shah Sikandar, Khan Sheharyar, Bussmann Rainer, W., Ali Maroof, Hussain Dildar, Hussain Wahid. Quantitative ethnobotanical study of indigenous knowledge on medicinal plants used by the tribal communities of Gokand Valley, District Buner, Khyber Pakhtunkhwa, Pakistan. Plant. 2020;9(8):1–29. doi: 10.3390/plants9081001. https://www.mdpi.com/2223-7747/9/8/1001 In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tardío J., Pardo-De-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of southern cantabria (northern Spain) Econ. Bot. 2008;62(1):24–39. [Google Scholar]
- 24.Gomez-Beloz A. Plant use knowledge of the winikina warao: the case for questionnaires in ethnobotany. Econ. Bot. 2002;56(6):231–241. [Google Scholar]
- 25.Tim Riskesdas 2018. Laporan Nasional Riskesdas 2018; Jakarta: 2018. [Google Scholar]
- 26.Mabel Y., Simbala H., Koneri R. Identifikasi dan pemanfaatan tumbuhan obat suku dani di Kabupaten Jayawijaya Papua. J. MIPA Univ. Sam. Ratulangi. 2016;5(2):103–107. [Google Scholar]
- 27.Triratnawati A. Dominasi medis modern atas medis tradisional suku sumuri, teluk bintuni, Papua barat. Masyarakat. Kebud dan Polit. 2017;30(2):174. [Google Scholar]
- 28.Jahan R., Khatun M.A., Nahar N., Jahan F.I., Chowdhury A.R., Nahar A. Use of Menispermaceae family plants in folk medicine of Bangladesh. Adv. Nat. Appl. Sci. 2010;4(1):1–9. [Google Scholar]
- 29.Huy N.T., Maeda A., Uyen D.T., Trang D.T.X., Sasai M., Shiono T. Alcohols induce beta-hematin formation via the dissociation of aggregated Heme and reduction in interfacial tension of the solution. Acta Trop. 2007;101:130–138. doi: 10.1016/j.actatropica.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Murebwayire S., Diallo B., Luhmer M., Vanhaelen-Fastré R., Vanhaelen M., Duez P. Alkaloids and amides from Triclisia sacleuxii. Fitoterapia. 2006;77(7–8):615–617. doi: 10.1016/j.fitote.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wet H., De, Wyk B Van. An ethnobotanical survey of southern african Menispermaceae. South Afr. J. Bot. 2008;74:2–9. [Google Scholar]
- 32.Wong S.K., Lim Y.Y., Abdullah N.R., Nordin F.J. Assessment of antiproliferative and antiplasmodial activities of five selected Apocynaceae species. BMC Compl. Alternative Med. 2011;11(3):1–8. doi: 10.1186/1472-6882-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong S.K., Lim Y.Y., Chan E.W.C. Botany, uses, phytochemistry and pharmacology of selected Apocynaceae species: a review. Pharmacogn. Commun. 2013;3(3):2–11. [Google Scholar]
- 34.Carvalho F.A., Renner S.S. Genetics and Genomics of Papaya. Springer Science; New York: 2015. The Phylogeny of the Caricaceae; pp. 81–92. [Google Scholar]
- 35.Yogiraj V., Goyal P.K., Chauhan C.S. Carica papaya linn: an overview. Int. J. Herb. Med. [Internet] 2015;2(5):1–8. http://www.florajournal.com/archives/2014/vol2issue5/PartA/2-4-12.1.pdf Available from: [Google Scholar]
- 36.Wiart C., Pharm D. Taylor & Francis; United States: 2006. Medicinal Plants of Asia and the Pacific. [Google Scholar]
- 37.Pratap B., Chakraborthy G.S., Mogha N. Complete aspects of Alstonia scholaris. Int. J. PharmTech. Res. 2013;5(1):17–26. [Google Scholar]
- 38.Dey A. Alstonia scholaris R.Br. (Apocynaceae): phytochemistry and pharmacology: a concise review. J. Appl. Pharm. Sci. 2011;1(6):51–57. [Google Scholar]
- 39.Salim A.A., Garson M.J., Craik D.J. New indole alkaloids from the bark of Alstonia scholaris. J. Nat. Prod. 2004;3(4):1591–1594. doi: 10.1021/np0498612. [DOI] [PubMed] [Google Scholar]
- 40.Keawpradub N., Kirby G.C., Steele J.C.P., Houghton P.J. 1999. Antiplasmodial Activity of Extracts and Alkaloids of Three Alstonia Species from Thailand; pp. 690–694. [DOI] [PubMed] [Google Scholar]
- 41.Rezeki R.S., Saragih A., Bahri S. Observasi klinis seduhan serbuk kulit batang kayu susu (Alstonia scholaris (L.) R. Br.) sebagai antimalaria di Manokwari. J. Pharm. Pharmacol. [Internet] 2012;1(2):95–103. http://download.portalgaruda.org/article.php?article58845&val4145 Available from: [Google Scholar]
- 42.Vij T., Prashar Y. A review on medicinal properties of Carica papaya linn. Asian Pacific J. Trop. Dis. [Internet] 2015;5(1):1–6. [Google Scholar]
- 43.Teng W.-C., Chan W., Suwanarusk R., Ong A., Ho H.-K., Russell B. In Vitro antimalarial evaluation and cytotoxicity investigations of Carica papaya leaves and carpaine. Nat. Prod. Commun. 2019;14(1):33–36. [Google Scholar]
- 44.Panneerselvam C., Ponarulselvam S., Murugan K. Potential anti-plasmodial activity of synthesized silver nanoparticle using Andrographis paniculata nees (Acanthaceae) Arch. Appl. Sci. Res. 2011;3(6):208–217. [Google Scholar]
- 45.Niranjan A., Tewari S.K., Lehri A. Biological activities of Kalmegh (Andrographis paniculata Nees) and its active principles - a review. Indian J. Nat. Prod. Resour. 2010;1(June):125–135. [Google Scholar]
- 46.Bharati B.D., Sharma P.K., Kumar N., Dudhe R., Bansal V. Pharmacological activity of Andrographis paniculata: a brief review. Pharmacologyonline. 2011;2:1–10. [Google Scholar]
- 47.Mishra K., Dash A.P., Dey N. Andrographolide: a novel antimalarial diterpene lactone compound from Andrographis paniculata and its interaction with curcumin and artesunate. J. Trop. Med. [Internet] 2011;2011:579518. doi: 10.1155/2011/579518. http://www.ncbi.nlm.nih.gov/pubmed/21760808 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mirzaee F. Therapeutic activities and phytochemistry of Physalis species based on traditional and modern medicine. Res. J. Pharmacogn. 2019;6(4):79–96. [Google Scholar]
- 49.Chotani D.L., Vaghasiya H. A phyto-pharmacological overview on Physalis minima linn. Indian J. Nat. Prod. Resour. 2012;3(4):477–482. [Google Scholar]
- 50.Angamuthu J., Ganapathy M., Evanjelene V.K., Ayyavuv N. Evaluation of phytochemical analysis and antimicrobial activity of leaf and fruit extracts of Physalis minima. Int. Emerg. Technol. Adv. Eng. 2014;4(1):462–465. [Google Scholar]
- 51.de la Torre L., Cerón C.E., Balslev H., Borchsenius F. A biodiversity informatics approach to ethnobotany: meta-analysis of plant use patterns in Ecuador. Ecol. Soc. 2012;17(1):15. [Google Scholar]
- 52.Umair M., Altaf M., Abbasi A.M. An ethnobotanical survey of indigenous medicinal plants in hafizabad district, Punjab-Pakistan. PloS One. 2017;12(6):1–22. doi: 10.1371/journal.pone.0177912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Putri L.S.E., Dasumiati Kristiyanto, Mardiansyah, Malik C., Leuvinadrie L.P. Ethnobotanical study of herbal medicine in ranggawulung urban forest, subang district, west java, Indonesia. Biodiversitas. 2016;17(1):172–176. [Google Scholar]
- 54.Dania Ogbe F.M., Eruogun O.L., Uwagboe M. Plants used for female reproductive health care in Oredo local government area, Nigeria. Sci. Res. Essays. 2008;4(3):120–130. [Google Scholar]
- 55.Rahmatullah M., Hossan S., Khatun A., Seraj S., Jahan R. Medicinal plants used by various tribes of Bangladesh for treatment of malaria. Malar Res. Treat. 2012;2012:1–5. doi: 10.1155/2012/371798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soerjani M., Kostermans A.J.G.H., Tjitroscepomo G. Balai Pustaka; Jakarta: 1987. Weeds of rice in Indonesia. [Google Scholar]
- 57.Bekalo T.H., Woodmatas S.D., Woldemariam Z.A. An ethnobotanical study of medicinal plants used by local people in the lowlands of Konta Special Woreda, southern nations, nationalities and peoples regional state, Ethiopia. J. Ethnobiol. Ethnomed. 2009;5:26. doi: 10.1186/1746-4269-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yineger H., Yewhalaw D. Traditional medicinal plant knowledge and use by local healers in Sekoru District, Jimma Zone, Southwestern Ethiopia. J. Ethnobiol. Ethnomed. 2007;3(February):1–8. doi: 10.1186/1746-4269-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giday M., Ameni G. An ethnobotanical survey of plants of veterinary importance in two woredas of Southern Tigray, Northern Ethiopia. SINET Ethiop. J. Sci. 2005;26(2):123–136. [Google Scholar]
- 60.Asase A., Oteng-Yeboah A.A., Odamtten G.T., Simmonds M.S.J. Ethnobotanical study of some Ghanaian anti-malarial plants. J. Ethnopharmacol. 2005;99(2):273–279. doi: 10.1016/j.jep.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 61.Ayyanar M., Ignacimuthu S. Traditional knowledge of kani tribals in kouthalai of tirunelveli hills, Tamil nadu, India. J. Ethnopharmacol. 2005;102(2):246–255. doi: 10.1016/j.jep.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 62.Handayani A. Prosiding Seminar Nasional Masyarakat Biodiversitas Indonesia. 2015. Pemanfaatan tumbuhan berkhasiat obat oleh masyarakat sekitar cagar alam gunung simpang, jawa barat; pp. 1425–1432. [Google Scholar]
- 63.Okello D., Kang Y. Review article: exploring antimalarial herbal plants across communities in Uganda based on electronic data. Evid. Based Complement Altern. Med. 2019:1–27. doi: 10.1155/2019/3057180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ngarivhume T., Van C.I.E.A., De Jong J.T.V.M., Van Der Westhuizen J.H. Medicinal plants used by traditional healers for the treatment of malaria in the Chipinge district in Zimbabwe. J. Ethnopharmacol. [Internet] 2015;159:224–237. doi: 10.1016/j.jep.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 65.Budiarti M., Jokopriyambodo W., Isnawati A. Karakterisasi minyak atsiri Dari simplisia basah ranting dan daun sebagai alternatif subtitusi kulit batang cinnamomum burmannii blume. J. Kefarmasian Indones. 2018;8(2):125–136. [Google Scholar]
- 66.Zougagh S., Belghiti A., Rochd T., Zerdani I., Mouslim J. Medicinal and aromatic plants used in traditional treatment of the oral pathology: the ethnobotanical survey in the economic capital casablanca, Morocco (North Africa) Nat. Products Bioprospect. [Internet] 2019;9(1):35–48. doi: 10.1007/s13659-018-0194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pagare S., Bhatia M., Tripathi N., Pagare S., Bansal Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015;9(3):293–304. [Google Scholar]
- 68.Uzor P.F. Alkaloids from plants with antimalarial activity: a review of recent studies. Evid. base Compl. Alternative Med. 2020:2020. doi: 10.1155/2020/8749083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
HELIYON-D-20-03238R1 supplementary material