Abstract
Aim
We report molecular subtype impact on 1325 early breast cancer (BCa) patients treated with whole breast hypofractionated (WBH) adjuvant forward-planned intensity modulated radiotherapy (F-IMRT) without boost.
Methods and materials
From 02/2009-05/2017 1325 patients with pTis-pT3, pNx-N1aM0 BCa who underwent breast conservation surgery were treated with WBHF-IMRT in our institute, to a total dose of 40 Gy/15 fractions, without boost. Median age: 62 (interquartile range-IQR-:51.14–70.53) years. Histology: 8% in situ carcinoma (ISC), 92% invasive tumors. Molecular subtypes (invasive tumors): 49.9% Luminal A, 33.1% Luminal B Her2 negative (−), 6.2% Luminal B Her2 positive (+), 3.6% Hormone Receptor (HR)- Her2+, 7.1% Triple negative (TNBC), and 0.2% HR+. Chemotherapy (CT) was prescribed in 28% of patients, hormonal therapy in 80.3%, monoclonal antibodies (MAb) in 86.8% of Luminal B Her2+ and 97.7% of HR- Her2+ patients.
Results
Median follow up was 72.43 (IQR: 44.63–104.13) months. The 5-year Kaplan-Meier estimates of local relapse-free survival (LRFS) was 97.8%, regional-(RRFS) 98.6%, loco-regional- (LRRFS) 96.9%, distant- (DRFS) 96.6%, disease-free survival (DFS) 94.8% and overall survival (OS) 95.5%. Considering molecular subtypes, 5-year LRFS was: 99.8% for Luminal A, 96.7% for Luminal B Her2-, 94.1% for Luminal B Her2+, 87.9% for HR- Her2+, 95.1% for TNBC and 99.1% for in situ carcinoma.
Conclusion
While the overall estimated probability of LR within 5 years after WBHF-IMRT without boost is good (2.2%), molecular subtypes have a strong impact, despite MAb therapy in Her2+ patients, and CT for TNBC patients, and could be used as a parameter in deciding the boost prescription.
Keywords: Early stage breast cancer, Breast cancer conservative treatment, Hypofractionated whole breast radiotherapy, Tumor bed boost, Breast cancer molecular subtypes
Highlights
-
•
Hypofractionated three-weeks radiotherapy ensures good local control whitout boost.
-
•
In 1325 early stage breast cancers 5-year local relapse without boost was 2.2%.
-
•
Molecular subtypes have a strong impact on estimated probability of local relapse.
-
•
5-year local control (LC) was 99.8% for Luminal A vs 87.9% for HR- Her2+.
-
•
5-year LC was 96.7% for Luminal B Her2-, 94.1% for Luminal B Her2+, 95.1% for TNBC.
1. Introduction
Radiotherapy (RT) is essential in conservative breast cancer (BCa) treatment, reducing the risk of recurrence in non-invasive and invasive BCa [[1], [2], [3], [4]], and increasing overall survival (OS) [5,6]. A subsequent boost to the tumoral bed increases local control (LC) without an impact on OS, worsening aesthetic outcome [7,8].
The UK Standardisation of Breast Radiotherapy (START) Trial B and the Ontario Canadian Trial of Breast Radiotherapy results imposed a three-week course of RT as a standard adjuvant treatment because of the similar LC to standard fractionation, with lower acute toxicity [9,10].
St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer highlighted the importance of molecular subtypes of BCa, which are used for systemic therapy recommendations [11].
Here we explore the differences in efficacy based on molecular subtypes on a large cohort of 1325 consecutive patients homogenously treated with hypofractionated whole-breast forward-planned intensity modulated radiotherapy (WBHF-IMRT) without boost.
2. Material and methods
From February 2009 to May 2017 1325 consecutive BCa patients who underwent breast conservative surgery for a pTis-pT3 pNx-pN1a M0 disease were treated with WBHF-IMRT to a total dose of 40 Gy in 15 fractions without boost in our institute, and were included in this analysis. All patients signed an informed consent for treatment and permission for publication of disease-related information in accordance with the Helsinki Declaration. The retrospective revision of patient outcomes was approved by the institutional ethics committee together with a prospective study of quality of life of BCa patients treated with hypofractionation, registered to ClinicalTrials.gov (NCT03077191: Toxicity and Outcome of Whole Breast Hypofractionated Radiotherapy: a Single Institution Experience.
Twenty-four patients presented bilateral disease, sequential or concomitant. Patient and treatment characteristics are summarized in Table 1.
Table 1.
Patient characteristics.
| Total nr. of patients | 1325 |
|---|---|
| Age groups (years): | |
| <45 | 133 (10%) |
| 45–55 | 312 (23.5%) |
| 55–65 | 322 (24.3%) |
| >65 | 558 (42.1%) |
| Age at diagnosis (median [IQR]) | 62.00 [51.14–70.53] |
| Time between surgery and RT start (months): median (IQR) | 2.55 [1.95–4.17] |
| Side: right vs left | Right 640 (48.3%) Left 685 (51.7%) |
| Histology in situ | 106 (8%) |
| DIC | 961 (72.5%) |
| LIC | 116 (8.8%) |
| Mixed | 43 (3.2%) |
| Other | 100 (7.5%) |
| pT stage | |
| is | 104 (7.8%) |
| 1mic+1a | 58 (4.4%) |
| 1b | 306 (23.1%) |
| 1c | 615 (46.4%) |
| 2 + 3 yis | 194 (14.6%) |
| y0 | 4 (0.3%) |
| y1mic + y1a | 10 (0.8%) |
| y1b | 8 (0.6%) |
| y1c | 9 (0.7%) |
| y2 | 12 (0.9%) 5 (0.4%) |
| pN stage: | |
| 0 | 937 (70.7%) |
| 1mic+1a | 241 (18.2%) |
| X y0 | 99 (7.5%) |
| y1a | 37 (2.8%) |
| yx | 7 (0.5%) |
| Number of positive lymph nodes for pN 1mic+1a (n = 241) | 4 (0.3%) |
| 1mic | 65 (27.00%) |
| 1 | 111 (46.1%) |
| 2 | 50 (20.7%) |
| 3 | 15 (6.2%) |
| Tumor grade: | |
| G1 | 313 (23.6%) |
| G2 | 651 (49.2%) |
| G3 | 359 (27.1%) |
| NA | 2 (0.1%) |
| Molecular subtypes (patients with invasive tumors): Luminal A Luminal B Her2-Luminal B Her2+ HR- Her2+ TNBC HR+ (no data on Her2 and ki67) |
1219 608 (49.9%) 403 (33.1%) 76 (6.2%) 44 (3.6%) 86 (7.1%) 2 (0.2%) |
| Positive/close margins (<2 mm) | |
| No | 1235 (93.2%) |
| Yes | 90 (6.8%) |
| Second surgery for positive margins | |
| No | 1260 (95.1%) |
| Yes | 65 (4.9%) |
| In situ histology accompanying invasive tumors | |
| No | 773 (58.3%) |
| DCIS | 483 (36.5%) |
| LCIS | 50 (3.8%) |
| Mixed | 12 (0.9%) |
| Other | 7 (0.5%) |
| Vascular Invasion | |
| No | 1175 (88.7%) |
| Yes | 150 (11.3%) |
| Chemotherapy | |
| No | |
| Yes | 954 (72%) |
| Chemotherapy Type (371 patients) | 371 (28%) |
| Anthracycline based | 170 (45.8%) |
| Anthracycline based + Taxanes | 125 (33.7%) |
| Taxane based | 53 (14.3%) |
| Other (CMF, Capecitabine, etc.) | 23 (6.2%) |
| Monoclonal antibodies | |
| No | 1216 (91.8%) |
| Yes | 109 (8.2%) |
| Monoclonal antibodies in Her2 + | 76 patients |
| No | 10 (13.2%) |
| Yes | 66 (86.8%) |
| Monoclonal antibodies in HR- Her2+ | 44 patients |
| No | 1 (2.3%) |
| Yes | 43 (97.7%) |
| Hormonal Therapy | |
| No | 261 (19.7%) |
| Yes | 1064 (80.3%) |
| AI | 664 (62.4%) |
| TAM | 158 (14.8%) |
| TAM + AI | 26 (2.4%) |
| Fulvestrant | 1 (0.1%) |
| LHRH + AI | 27 (2.5%) |
| LHRH + TAM | 169 (15.9%) |
| LHRH + TAM + AI | 18 (1.7%) |
| LHRH + Fulvestrant | 1 (0.1%) |
| Breast size: | |
| Small | 232 (17.5%) |
| Medium | 423 (31.9%) |
| Big | 459 (34.6%) |
| Very Big | 211 (15.9%) |
| Obesity | |
| No | 968 (73.1%) |
| Yes | 315 (23.8%) |
| NA | 42 (3.1%) |
| Diabetes | |
| No | 1129 (85.2%) |
| Yes | 78 (5.9%) |
| NA | 118 (8.9%) |
| Smoke | |
| Smokers and former smokers | 263 (19.8%) |
| Non smokers | 1062 (80.2%) |
| Family history of breast tumor | |
| No | 923 (69.7%) |
| Yes | 402 (30.3%) |
| Alcohol consumption (declared) | |
| No | 1267 (95.6%) |
| Yes | 58 (4.4%) |
| Hypertension | |
| No | 761 (57.4%) |
| Yes | 451 (34%) |
| NA | 113 (8.5%) |
| Thyroid illnesses | |
| No | 1025 (77.4%) |
| Yes | 181 (13.7%) |
| NA | 119 (9%) |
IQR = interquartile range, DIC = Ductal Invasive Carcinoma, LIC = Lobular Invasive Carcinoma, mic = microinvasive, Her2- = Her2 negative, Her2+ = Her 2 positive, HR-Her2+ = Hormone Receptor Negative Her2 Positive, TNBC = Triple Negative Breast Cancer, HR+ = Hormone Receptor Positive, DCIS = Ductal Carcinoma In Situ, LCIS = Lobular Carcinoma In Situ, AI = Aromatase Inhibitors, TAM = Tamoxifen, LHRH = luteinizing hormone-releasing hormone analogue, NA = not available.
All 1229 invasive cancer patients were classified into one of the five molecular subtypes, designating ki-67 and Progesterone Receptor (PgR) cut-offs at 20% [11]. There were 608 (49.9%) Luminal A patients, 403 (33.1%) Luminal B Her2 negative (−) patients, 76 (6.2%) Luminal B Her2 positive (+) patients, 44 (3.6%) Hormone receptor negative (HR-) Her2+ patients and 86 (7.1%) Triple negative (TNBC) patients. Two patients operated on in another hospital could not be classified into a molecular subtype due to unavailability of Her2 data, and were classified only as hormone receptor positive (HR+) category. Two patients with in situ tumor had a punctiform microinvasion that could not be analyzed due to the lack of material. Thus 106 (8%) patients with in situ histology were considered, while 104 (7.8%) were pTis patients, two being included in the pT1microinvasive (mic)+pT1a category. Rare BCa histologies (tubular, papillary, mucinous, etc.) were included in a single class (Other). “Other” histologies and pT1mic+1a stage were grouped together for purposes of statistical analysis.
Monoclonal antibodies (trastuzumab, pertuzumab) were prescribed in all Her2+ patients except for those with comorbidities that contraindicated the prescription. For TNBC, Luminal B Her2+, HR-Her2+, and some Luminal B Her2-patients at high risk of relapse (determined in recent years with Oncotype Dx recurrence score), different chemotherapy schemes were used, depending on molecular subtype, comorbidities, participation in prospective randomized trials, cardiac, neurotoxicity and allergies. The chemotherapy schemes were summarized as much as possible in Table 1 and approximately 93% were anthracycline and/or taxane based.
2.1. Radiation therapy
Patients were treated in supine position on a posiboard, with arms above the head. Clinical target volume (CTV) was defined as the whole breast on computed tomography (CT) scan images acquired with a 5 mm step. Planning target volume (PTV) was generated by the expansion of CTV with a 15 mm margin in all directions except lung (5 mm); a crop to the body was made to eliminate the first 5 mm of skin. None of the patients had a regional lymph-node (LN) irradiation prescription. Heart, lungs and contralateral breast were delineated and the body was automatically generated.
A median number of 4 segments was used (2-11) within a tangential two field three-dimensional (3D)-conformal irradiation technique in order to obtain a homogeneous dose, with hot spots ≤ 108% on PTV, covered by at least 95% isodose, and with 3% of the heart ≤ 40 Gy, and ≤20% of ipsilateral lung ≤ 17 Gy. Eleven patients were treated with arcs. Dual energies (6 MV and 18 MV) with different weighting factors and wedges were used as needed. Due to the complexity and the number of fields, the technique was called F-IMRT.
2.2. Follow-up
Patients were visited at half and at the end of the treatment. The first follow-up visit was scheduled at six months, then once a year. Loco-regional and distant failures were defined on specific image examinations and whenever possible histologically confirmed. Disease free survival (DFS) was defined as the time from the end of RT to any breast cancer-related event (local, regional, distant relapse) or death from cancer progression (PD), whichever occurred first. Overall survival (OS) was defined as the time from the end of RT to death or last FU. Cause-specific survival (CSS) was estimated as well considering breast cancer progression as event of interest.
2.3. Statistical analysis
Median and interquartile range (IQR) have been reported for continuous variables, while frequency distribution and percentages are reported for categorical variables.
The length of follow-up was calculated as the time from the end of RT until the last follow-up or first event assessment. Patients were still evaluable for loco-regional relapse (LRR) even when they presented distant relapse (DR) but were censored at date of death.
The Kaplan-Meier approach was used to estimate local relapse-free survival (LRFS), regional relapse-free survival (RRFS), loco-regional relapse-free survival (LRRFS), distant-relapse-free survival (DRFS), disease free survival (DFS) and OS. A log-rank test was used to compare survival of groups of patients defined based on demographic/clinical characteristics or medical treatments/therapies. Whenever of interest, the log-rank test was followed by a post-hoc analysis for pairwise comparisons of survival curves along with Bonferroni’s adjustment for multiple comparisons. Univariate and multivariate Cox regression models were estimated, after testing for proportionality assumption, to estimate the impact of risk factors on survival outcomes of interest.
The subgroup of patients treated with neoadjuvant chemotherapy were eliminated from the uni- and multivariate T and N stage analyses (ypT and ypN groups) due to the paucity of patients and events detected.
Backward selection procedures were applied to obtain a small set of significant covariates. The only variables which were not included in the multivariate analyses were the number of lymph nodes and therapies (these variables have been graphically separated in the tables by double lines). Grading, for example, was included in the multivariate analysis but was excluded by the backward procedure as non-significant. Other factors, like number of lymph nodes or therapy, were not analyzed due to potential collinearity and confounding issues (for example, number of lymph-nodes was partially superimposed with pN stage, which was preferred; monoclonal antibody therapy was performed only in Her2+ patients). Moreover, with reference to alcohol use, due to the absence or very low number of events (1 event) for outcomes other than death, it was decided to examine the impact of this factor only at the univariate level and only for overall survival. Hence, all the multivariate analyses were carried out under the same conditions, i.e., using the same set of covariates.
Estimated hazard ratios (HR) along with 95% confidence intervals have been reported and graphically displayed using forest plots. All the analyses were performed using R statistical software (version 3.5.2, https://cran.r-project.org/index.html). The significance level was set at 0.05.
3. Results
Median follow up of the whole cohort of 1325 patients was 72.43 (IQR: 44.63–104.13) months. The majority of patients (99.5%) received a total dose (TD) of 40.05 Gy in 15 fractions (2.67 Gy/fraction). One patient received a lower TD of 37.38 Gy, because she asked to stop the treatment after the 14th fraction. Four patients received a TD of 42.72 Gy and one a TD of 45.34 Gy, with 1–2 compensating fractions because of treatment interruptions for 1–3 weeks due to other pathologies.
During the follow up 34 (2.6%) patients experienced LR, 19 (1.4%) regional relapse (RR), 48 (3.6%) distant relapse (DR) and 6 (0.45%) all three breast cancer-related events (LR, RR and DR).
Overall, 91 (6.9%) patients died; considering the available information on cause of death (n = 1317 patients), 31 (2.4%) patients died from disease progression and 52 (3.9%) from other causes. In 8 patients (0.6%) the cause of death was unknown.
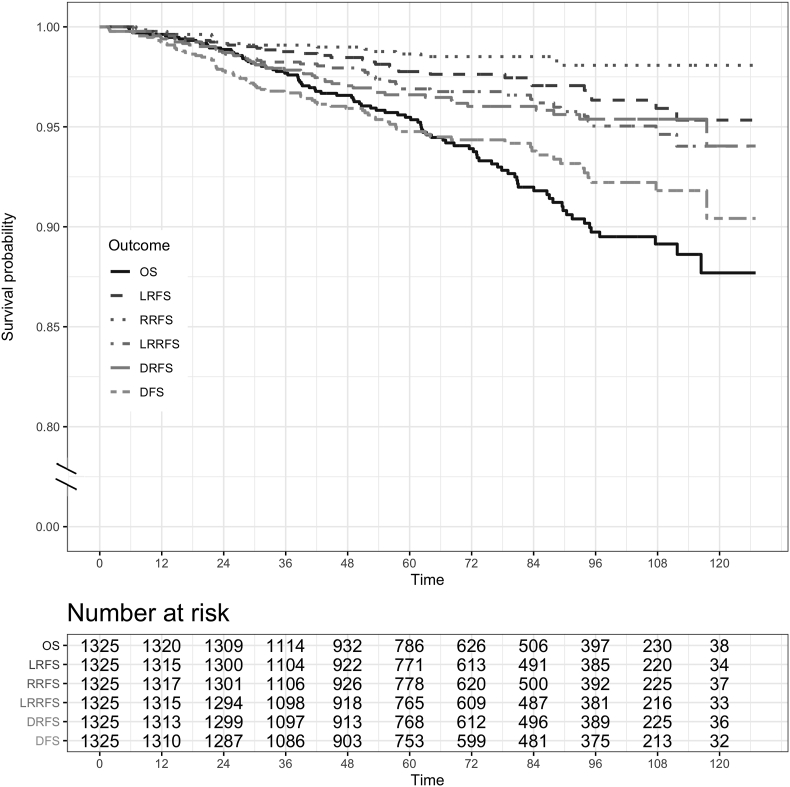
The 5-year Kaplan-Meier estimate of LRFS was 97.8%, RRFS 98.6%, LRRFS 96.9%, DRFS 96.9%, DFS 94.8% and OS 95.5% (see Fig. 1). Five-year CSS was estimated at 98.3%.
Fig. 1.
Overall (OS), local relapse-free survival (LRFS), regional relapse-free survival (RRFS), loco-regional relapse-free survival (LRRFS), distant relapse-free survival (DRFS) and disease-free survival (DFS).
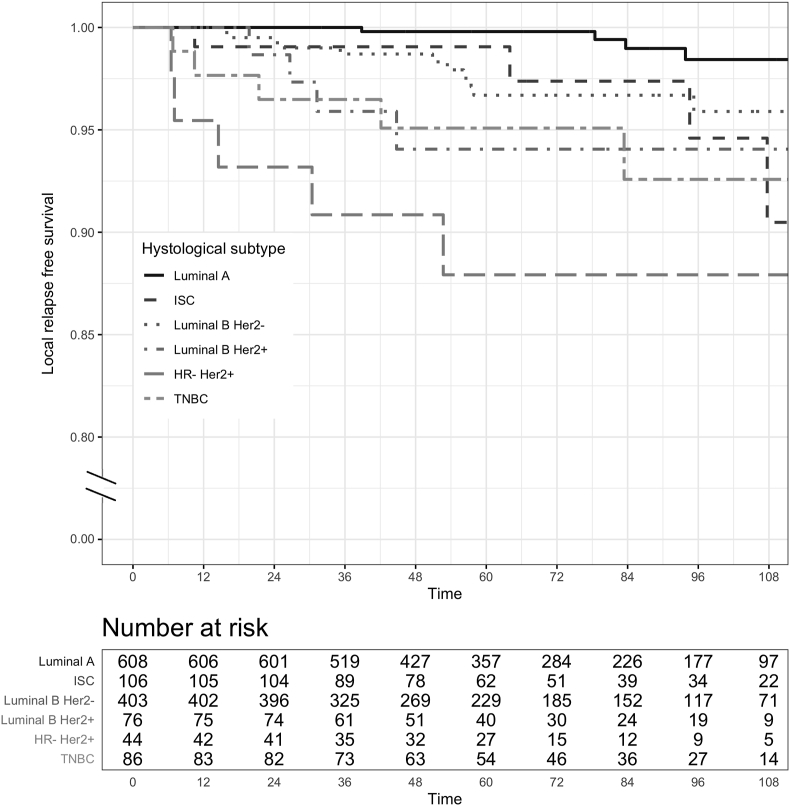
When considering the molecular subtypes and in situ carcinoma (ISC) as a single group of non-invasive tumor, estimated 5-year (95% CI) LRFS was: 99.8 (99.4–100)% for Luminal A, 96.7 (94.6–98.8)% for Luminal B Her2-, 94.1 (88.5–100)% for Luminal B Her2+, 87.9 (78.5–98.5)% for HR- Her2+, 95.1 (90.5–99.9) % for TNBC and 99.1 (97.2–100)% for ISC (see Fig. 2).
Fig. 2.
Local relapse-free survival (LRFS) for molecular subtypes of invasive tumors and for in situ carcinoma (ISC) group of patients. Although all the data have been used for statistical analyses, here, for graphic purposes only, the plot was curtailed at 9 years, since the proportion of patients experiencing the event after 120 months was negligible.
From the post-hoc analysis, it emerged that, compared to Luminal A subtype, LRFS risk was significantly different for Luminal B Her2+ (adjusted p = 0.0037), HR- Her2+ (adjusted p < 0.0001), and TNBC (adjusted p = 0.0022) patients.
Estimated RRFS at 5-years was also statistically significantly different for the five histological subtypes and patients with ISC (p < 0.0001, long–rank test): 99.8 (99.5–100)% for Luminal A, 98.5 (97.1–99.8)% for Luminal B Her2-, 100% for Luminal B Her2+, 95.5 (89.5–100)% for HR- Her2+, 92.7 (87.3–98.5)% for TNBC, and 97.5 (94.1–100)% for ISC.
Estimated 5-year DRFS was 98.6 (97.6–99.7)% for Luminal A, 95.1 (92.8–97.5)% for Luminal B Her2-, 96.9 (92.7–100)% for Luminal B Her2+, 92.6 (84.9–100)% for HR- Her2+, 89.2 (82.7–96.1)% for TNBC and 98 (95.3–100)% for ISC.
3.1. Univariate analysis
The 106 patients with pTis were removed from univariate and multivariate Cox regression models, because this subgroup of patients appeared in the stage T and subtype variables as a separate category, thus creating an overlap when considering the variables in the multivariate analysis jointly and leading to collinearity problems. Thus, only the 1229 patients with invasive tumors were considered in these analyses.
Variables significantly influencing OS at univariate analysis were older age at diagnosis, positive or unknown LN, molecular subtypes, hormonal therapy (reducing the risk of death) and diabetes (see Table 2).
Table 2.
Univariate and multivariate analysis of variables influencing overall survival.
| Univariate |
Multivariate (final selected model) |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | Reference category | |
| Overall Survival | |||||
| Age at diagnosis (cat) [45–55] | 1.3107 (0.2645–6.4947) | 0.7404 | 0.951 (0.1739–5.1995) | 0.9537 | ref <45 |
| Age at diagnosis (cat) [55–65] | 5.0751 (1.1962–21.5315) | 0.0276 | 4.9895 (1.1714–21.2524) | 0.0297 | |
| Age at diagnosis (cat) ≥65 | 7.0341 (1.7169–28.8175) | 0.0067 | 6.7727 (1.6466–27.8569) | 0.0080 | |
| pT (no y) 2 + 3 | 1.5778 (0.9549–2.607) | 0.0751 | – | – | ref 1 (1mic+1a/1b/1c) |
| pN (no y) 1a+1mi | 2.1272 (1.3319–3.3974) | 0.0016 | 1.8799 (1.1731–3.0127) | 0.0087 | ref pN = 0 |
| pN (no y) x | 3.1582 (1.4301–6.9743) | 0.0044 | 2.6776 (1.2044–5.9526) | 0.0157 | |
| Histology: LIC | 1.5917 (0.8636–2.9335) | 0.1362 | – | – | ref DIC + other |
| Histology: mixed | 1.2665 (0.4627–3.4669) | 0.6456 | – | – | |
| Grading G2 | 1.4755 (0.8072–2.697) | 0.2062 | – | – | ref G1 |
| Grading G3 | 2.0313 (1.0805–3.8186) | 0.0278 | – | – | |
| In situ histology yes | 0.8089 (0.5086–1.2866) | 0.3705 | – | – | ref no |
| Vascular invasion yes | 0.5942 (0.2744–1.2867) | 0.1867 | – | – | ref no |
| Mol. sub.: Luminal B Her2- | 1.8277 (1.1082–3.0144) | 0.0181 | 1.7757 (1.0621–2.9686) | 0.0285 | ref Luminal A |
| Mol. sub.:Luminal B Her2+ | 1.7622 (0.7296–4.256) | 0.2079 | 2.5225 (1.0298–6.1788) | 0.0430 | |
| Mol. sub.:HR- Her2+ | 2.4369 (0.9408–6.3125) | 0.0666 | 3.0638 (1.1746–7.9912) | 0.0221 | |
| Mol. sub.:TNBC | 3.568 (1.9054–6.6814) | 0.0001 | 3.668 (1.9193–7.01) | 0.0001 | |
| Breast size big | 1.1329 (0.6089–2.1079) | 0.6936 | – | – | ref small |
| Breast size medium | 1.1617 (0.6247–2.1603) | 0.6357 | – | – | |
| Breast size very big | 1.0828 (0.5146–2.2784) | 0.8339 | – | – | |
| Obesity yes | 1.2883 (0.8079–2.0544) | 0.2873 | – | – | ref no |
| Diabetes yes | 2.4485 (1.3524–4.433) | 0.0031 | – | – | ref no |
| Hypertension yes | 1.3116 (0.8488–2.0266) | 0.2219 | – | – | ref no |
| Thyroid illness yes | 0.976 (0.529–1.8007) | 0.9380 | – | – | ref no |
| Smoking (current + ex) | 1.2834 (0.7795–2.1132) | 0.3267 | – | – | ref no |
| Family history yes | 0.9889 (0.6255–1.5634) | 0.9618 | – | – | ref no |
| Nr. of lymph nodes:1 (including 1mic, no x, no y) Nr. of lymph nodes: 2 + 3 |
1.7874 (1.0178–3.1389) 2.9100 (1.5142–5.5923) |
0.0432 0.0014 |
ref = 0 | ||
| Chemotherapy yes | 1.2456 (0.8014–1.9359) | 0.3290 | ref no | ||
| Hormonal therapy yes | 0.5686 (0.3509–0.9213) | 0.0219 | ref no | ||
| Monoclonal antibodies yes | 1.3479 (0.6976–2.6043) | 0.3743 | ref no | ||
| Alcohol consumption yes | 1.6525 (0.7212–3.7865) | 0.2351 | ref no | ||
DIC = Ductal Invasive Carcinoma, LIC = Lobular Invasive Carcinoma, mic = microinvasive, Her2- = Her2 negative, Her2+ = Her 2 positive, HR-Her2+ = Hormone Receptor Negative Her2 Positive, TNBC = Triple Negative Breast Cancer, ref = reference, Mol. Sub. = Molecular Subtype, Nr. = number.
Cox univariate regression for LRFS highlighted the significant role of grading with an increased risk of local relapse for patients with G3 vs G1, molecular subtypes with all subtypes having an increased risk compared to Luminal A patients, chemotherapy, hormonal therapy (strong protective factor), monoclonal antibody therapy (see Table 3).
Table 3.
Univariate and multivariate analysis of variables influencing local and loco-regional relapse.
| Univariate |
Multivariate (final selected model) |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | Reference category | |
| Local relapse | |||||
| Age at diagnosis (cat) [45–55] | 0.6504 (0.1835–2.306) | 0.5053 | – | – | ref <45 |
| Age at diagnosis (cat) [55–65] | 1.1906 (0.3787–3.7436) | 0.7653 | – | – | |
| Age at diagnosis (cat) ≥65 | 0.5449 (0.1676–1.7714) | 0.3128 | – | – | |
| pT (no y) 2 + 3 | 2.2634 (0.9838–5.2071) | 0.0547 | – | – | ref 1 (1mic+1a/1b/1c) |
| pN (no y) 1mic+1a | 1.8182 (0.7846–4.2132) | 0.1632 | – | – | ref pN = 0 |
| pN (no y) x | 1.2672 (0.1684–9.5353) | 0.8181 | – | – | |
| Histology: LIC | 1.5263 (0.531–4.3868) | 0.4325 | – | – | ref DIC + other |
| Histology: mixed | 0.9311 (0.1261–6.8737) | 0.9442 | – | – | |
| Grading G2 | 1.4569 (0.3944–5.382) | 0.5725 | – | – | ref G1 |
| Grading G3 | 5.7811 (1.7029–19.6265) | 0.0049 | – | – | |
| In situ histology yes | 1.976 (0.9645–4.0482) | 0.0627 | 2.2766 (1.1045–4.6926) | 0.0258 | ref no |
| Vascular invasion yes | 1.3958 (0.5338–3.6497) | 0.4965 | – | – | ref no |
| Mol. sub.: Luminal B Her2-- | 4.2049 (1.3389–13.2063) | 0.0139 | 4.3379 (1.3807–13.6285) | 0.0120 | ref Luminal A |
| Mol. sub.: Luminal B Her2+ | 8.5595 (2.1403–34.2318) | 0.0024 | 8.8256 (2.2056–35.3149) | 0.0021 | |
| Mol. sub.: HR- Her2+ | 22.3353 (6.2978–79.2127) | <0.0001 | 23.4632 (6.6127–83.2518) | <0.0001 | |
| Mol. sub.: TNBC | 8.6227 (2.315–32.1172) | 0.0013 | 10.0357 (2.6729–37.6794) | 0.0006 | |
| Breast size big | 1.3757 (0.4307–4.3945) | 0.5903 | – | – | ref small |
| Breast size medium | 1.7009 (0.5481–5.2782) | 0.3579 | – | – | |
| Breast size very big | 1.2012 (0.2998–4.813) | 0.7957 | – | – | |
| Obesity yes | 1.1242 (0.5005–2.5254) | 0.7768 | – | – | ref no |
| Diabetes yes | 2.1111 (0.7344–6.0683) | 0.1655 | – | – | ref no |
| Hypertension yes | 0.9825 (0.4639–2.0809) | 0.9632 | – | – | ref no |
| Thyroid illness yes | 1.1906 (0.4543–3.1207) | 0.7227 | – | – | ref no |
| Smoking (current + ex) | 0.8707 (0.3332–2.2751) | 0.7775 | – | – | ref no |
| Family history yes | 1.3614 (0.6478–2.8613) | 0.4156 | ref no | ||
| Nr. of lymph nodes:1 (including 1mic, no x, no y) Nr. of lymph nodes: 2 + 3 |
2.2535 (0.9343–5.4352) 0.7704 (0.1025–5.7898) |
0.0705 0.7999 |
ref = 0 | ||
| Chemotherapy yes | 2.7582 (1.3457–5.6533) | 0.0056 | ref no | ||
| Hormonal therapy yes | 0.1795 (0.0877–0.3672) | <0.0001 | ref no | ||
| Monoclonal antibodies yes | 3.9507 (1.7567–8.885) | 0.0009 | ref no | ||
| Locoregional relapse | |||||
| Age at diagnosis (cat) [45–55] | 0.5184 (0.1582–1.6993) | 0.2781 | – | – | ref <45 |
| Age at diagnosis (cat) [55–65] | 1.1223 (0.3998–3.1506) | 0.8266 | – | – | |
| Age at diagnosis (cat) ≥65 | 0.7784 (0.2849–2.1266) | 0.6251 | – | – | |
| pT (no y) 2 + 3 | 2.5782 (1.2891–5.1566) | 0.0074 | – | – | ref 1 (1mic+1a/1b/1c) |
| pN (no y) 1a+1mic | 2.104 (1.0412–4.2513) | 0.0382 | – | – | ref pN = 0 |
| pN (no y) x | 1.9721 (0.463–8.3994) | 0.3584 | – | – | |
| Histology: LIC | 1.4436 (0.5634–3.6986) | 0.4444 | – | – | ref DIC + other |
| Histology: mixed | 1.4395 (0.3453–6.0003) | 0.6170 | – | – | |
| Grading G2 | 1.2622 (0.45–3.5408) | 0.6581 | - | - | ref G1 |
| Grading G3 | 4.262 (1.6139–11.2549) | 0.0034 | - | - | |
| In situ histology yes | 1.7632 (0.9468–3.2837) | 0.0738 | 2.0477 (1.0933–3.8354) | 0.0252 | ref no |
| Vascular invasion yes | 0.9864 (0.3862–2.5194) | 0.9771 | – | ref no | |
| Mol.sub.: Luminal B Her2 - | 4.0794 (1.5962–10.4257) | 0.0033 | 4.181 (1.6355–10.6882) | 0.0028 | ref Luminal A |
| Mol.sub.: Luminal B Her2+ | 5.6461 (1.5931–20.0104) | 0.0073 | 5.7861 (1.632–20.5133) | 0.0066 | |
| Mol.sub.: HR- Her2+ | 17.6526 (5.9293–52.5551) | <0.0001 | 18.6251 (6.2504–55.4989) | <0.0001 | |
| Mol.sub.: TNBC | 8.2035 (2.7566–24.4132) | 0.0002 | 9.3608 (3.1229–28.0586) | 0.0001 | |
| Breast size big | 1.1769 (0.4467–3.1008) | 0.7418 | - | - | ref small |
| Breast size medium | 1.3029 (0.5004–3.3926) | 0.5879 | - | - | |
| Breast size very big | 1.387 (0.4653–4.1347) | 0.5572 | - | - | |
| Obesity yes | 0.8906 (0.424–1.8707) | 0.7596 | – | – | ref no |
| Diabetes yes | 2.0279 (0.7915–5.196) | 0.1408 | – | – | ref no |
| Hypertension yes | 0.7426 (0.3746–1.4721) | 0.3940 | – | – | ref no |
| Thyroid illness yes | 1.539 (0.7055–3.3571) | 0.2787 | – | – | ref no |
| Smoking (current + ex) | 0.7616 (0.3197–1.8144) | 0.5386 | – | – | ref no |
| Family history yes | 1.4103 (0.7434–2.6752) | 0.2926 | ref no | ||
| Nr. of lymph nodes:1 (including 1mic, no x, no y) Nr. of lymph nodes: 2 + 3 |
2.2310 (1.0271–4.8459) 1.7914 (0.5361–5.9862 |
0.0426 0.3436 |
ref = 0 | ||
| Chemotherapy yes | 2.6737 (1.4371–4.9746) | 0.0019 | ref no | ||
| Hormonal therapy yes | 0.2169 (0.1163–0.4044) | <0.0001 | ref no | ||
| Monoclonal antibodies yes | 3.1435 (1.4955–6.6079) | 0.0025 | ref no | ||
DIC = Ductal Invasive Carcinoma, LIC = Lobular Invasive Carcinoma, mic = microinvasive, Her2- = Her2 negative, Her2+ = Her 2 positive, HR-Her2+ = Hormone Receptor Negative Her2 Positive, TNBC = Triple Negative Breast Cancer, ref = reference, Mol. Sub. = Molecular Subtype, Nr. = number.
The majority of the patient cohorts with early-stage disease had negative lymph-nodes. Among pN1a patients more than half (63.1%) had one positive LN, and only 8.5% had three positive LN. Moreover, the first level of axillary nodes is contained within the tangential fields, with, consequently, few regional events in the 6 years of median follow up. Thus, LRRFS was analyzed together, even if partially overlapping with LRFS analysis (see Table 3).
Factors influencing DRFS were: pT with patients pT2+3 at higher risk for distant relapse than pT1 patients, pN with pN1mic+1a patients at higher risk than pN0 patients, grading with G3 patients at higher risk when compared to G1 patients, molecular subtypes, chemotherapy, and hormonal therapy. The same factors and monoclonal antibody therapy influenced DFS (see Table 4).
Table 4.
Univariate and multivariate analysis of variables influencing distant relapse free survival and disease free survival.
| Univariate |
Multivariate (final selected model) |
||||
|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | Reference category | |
| Distant relapse | |||||
| Age at diagnosis (cat) [45–55] | 0.6394 (0.1804–2.2664) | 0.4885 | – | – | ref <45 |
| Age at diagnosis (cat) [55–65] | 1.3827 (0.4506–4.2428) | 0.5711 | – | – | |
| Age at diagnosis (cat) ≥65 | 1.3561 (0.4689–3.9222) | 0.5740 | – | – | |
| pT (no y) 2 + 3 | 2.5108 (1.3267–4.7517) | 0.0047 | – | – | ref 1 (1mic+1a/1b/1c) |
| pN (no y) 1a+1mic | 3.248 (1.7418–6.0565) | 0.0002 | 2.9933 (1.6013–5.5954) | 0.0006 | ref pN = 0 |
| pN (no y) x | 2.8995 (0.867–9.6968) | 0.0839 | 3.4692 (1.0329–11.6516) | 0.0442 | |
| Histology LIC | 0.9211 (0.3298–2.5726) | 0.8754 | – | – | ref DIC + other |
| Histology mixed | 0.5681 (0.0781–4.131) | 0.5765 | – | – | |
| Grading G2 | 2.4362 (0.8326–7.1278) | 0.1040 | – | – | ref G1 |
| Grading G3 | 5.2541 (1.8104–15.2481) | 0.0023 | – | – | |
| In situ histology yes | 0.8336 (0.4445–1.5633) | 0.5704 | – | – | ref no |
| Vascular invasion yes | 0.8825 (0.3486–2.2338) | 0.7919 | – | – | ref no |
| Mol. sub.: Luminal B Her2 - | 4.1932 (1.8664–9.4211) | 0.0005 | 3.8414 (1.6961–8.7005) | 0.0013 | ref Luminal A |
| Mol. sub.: Luminal B Her2 + | 3.0923 (0.8204–11.6564) | 0.0954 | 2.1048 (0.4451–9.9521) | 0.3478 | |
| Mol. sub.: HR- Her2+ | 5.2091 (1.3817–19.6385) | 0.0148 | 6.9879 (1.8476–26.4296) | 0.0042 | |
| Mol. sub.: TNBC | 8.7861 (3.4664–22.2697) | <0.0001 | 8.5622 (3.2949–22.2501) | <0.0001 | |
| Breast size big | 0.6881 (0.3015–1.5705) | 0.3746 | – | – | ref small |
| Breast size medium | 0.9332 (0.4271–2.0388) | 0.8623 | – | – | |
| Breast size very big | 0.6734 (0.2445–1.8541) | 0.4441 | – | – | |
| Obesity yes | 1.4027 (0.7462–2.6368) | 0.2934 | – | – | ref no |
| Diabetes yes | 1.4488 (0.5163–4.0652) | 0.4813 | – | – | ref no |
| Hypertension yes | 1.2673 (0.6835–2.3497) | 0.4521 | – | – | ref no |
| Thyroid illness yes | 1.1773 (0.5218–2.6563) | 0.6943 | – | – | ref no |
| Smoking (current + ex) | 0.9259 (0.4318–1.9851) | 0.8431 | – | – | ref no |
| Family history yes | 1.2427 (0.6773–2.2801) | 0.4829 | ref no | ||
| Nr. of lymph nodes:1 (including 1mic, no x, no y) Nr. of lymph nodes: 2 + 3 |
2.7939 (1.3544–5.7633) 4.3753 (1.8651–10.2637) |
0.0054 0.0007 |
ref = 0 | ||
| Chemotherapy yes | 2.6163 (1.4664–4.6678) | 0.0011 | ref no | ||
| Hormonal therapy yes | 0.3404 (0.1855–0.6245) | 0.0005 | ref no | ||
| Monoclonal antibodies yes | 1.5668 (0.6638–3.6979) | 0.3054 | ref no | ||
| Disease free | |||||
| Age at diagnosis (cat) [45–55] | 0.6657 (0.258–1.7176) | 0.4001 | – | – | ref <45 |
| Age at diagnosis (cat) [55–65] | 1.1516 (0.4839–2.7404) | 0.7497 | – | – | |
| Age at diagnosis (cat) ≥65 | 1.0803 (0.4767–2.4484) | 0.8531 | – | – | |
| pT (no y) 2 + 3 | 2.4591 (1.4467–4.1801) | 0.0009 | – | – | ref 1 (1mic+1a/1b/1c) |
| pN (no y) 1a+1mic | 2.6996 (1.6057–4.5387) | 0.0002 | 2.4563 (1.4585–4.1368) | 0.0007 | ref pN = 0 |
| pN (no y) x | 2.464 (0.8749–6.9396) | 0.0878 | 3.1137 (1.0986–8.8252) | 0.0326 | |
| Histology LIC | 0.9288 (0.4014–2.1491) | 0.8630 | – | – | ref DIC + other |
| Histology mixed | 0.7708 (0.1884–3.1528) | 0.7172 | – | – | |
| Grading G2 | 1.8883 (0.8223–4.3364) | 0.1340 | – | – | ref G1 |
| Grading G3 | 4.9257 (2.1878–11.0899) | 0.0001 | – | – | |
| In situ histology yes | 1.1552 (0.7093–1.8813) | 0.5622 | – | – | ref no |
| Vascular invasion yes | 1.0714 (0.5315–2.1593) | 0.8472 | – | – | ref no |
| Mol. sub.: Luminal B Her2 - | 3.9812 (2.0443–7.7532) | <0.0001 | 3.6008 (1.8324–7.0757) | 0.0002 | ref Luminal A |
| Mol. sub.: Luminal B Her2+ | 4.241 (1.5916–11.3007) | 0.0039 | 2.9015 (0.9321–9.0325) | 0.0660 | |
| Mol. sub.: HR- Her2+ | 8.88 (3.495–22.5622) | <0.0001 | 10.5116 (3.9193–28.1927) | <0.0001 | |
| Mol. sub.: TNBC | 7.8001 (3.5586–17.0973) | <0.0001 | 7.8017 (3.4974–17.4032) | <0.0001 | |
| Breast size big | 0.8131 (0.4042–1.6355) | 0.5617 | – | – | ref small |
| Breast size medium | 1.0539 (0.539–2.0606) | 0.8781 | – | – | |
| Breast size very big | 0.9667 (0.4327–2.1597) | 0.9342 | – | – | |
| Obesity yes | 1.197 (0.7047–2.033) | 0.5059 | – | – | ref no |
| Diabetes yes | 1.4365 (0.6189–3.3342) | 0.3992 | – | – | ref no |
| Hypertension yes | 1.0185 (0.6107–1.6986) | 0.9440 | – | – | ref no |
| Thyroid illness yes | 1.2413 (0.6469–2.3817) | 0.5157 | – | – | ref no |
| Smoking (current + ex) | 0.7352 (0.376–1.4373) | 0.3685 | – | – | ref no |
| Family history yes | 1.3345 (0.8167–2.1805) | 0.2494 | ref no | ||
| Nr. of lymph nodes:1 (including 1mic, no x, no y) Nr. of lymph nodes: 2 + 3 |
2.6953 (1.5096–4.8122) 2.7093 (1.2023–6.1048 |
0.0008 0.0162 |
ref = 0 | ||
| Chemotherapy yes | 2.3754 (1.4812–3.8095) | 0.0003 | ref no | ||
| Hormonal therapy yes | 0.2897 (0.178–0.4715) | <0.0001 | ref no | ||
| Monoclonal antibodies yes | 2.0519 (1.0765–3.9112) | 0.0290 | ref no | ||
DIC = Ductal Invasive Carcinoma, LIC = Lobular Invasive Carcinoma, mic = microinvasive, Her2- = Her2 negative, Her2+ = Her 2 positive, HR-Her2+ = Hormone Receptor Negative Her2 Positive, TNBC = Triple Negative Breast Cancer, ref = reference, Mol. Sub. = Molecular Subtype, Nr. = number.
Multivariate analysis: All the variables considered in the univariate analysis, with the exception of treatments (overlap with molecular subtypes), number of positive lymph-node (N stage was analyzed) and alcohol consumption (paucity of events) were then introduced into the multivariate analysis.
After backward selection, when considering OS, age, pN and molecular subtypes were found to be significant factors, with patients in the age-class categories 55–65 and >65 years at higher risk versus patients younger than 45 years; pN1mic+1a patients at higher risk versus pN0 patients and an increased risk was found for patients with molecular subtype different from the reference group (Luminal A). For LRFS and LRRFS, a significant role emerged for molecular subtypes as well as for in situ histology accompanying invasive tumors. To conclude, pN and molecular subtype emerged as significant risk factors for DRFS and DFS (see Table 2, Table 3, Table 4).
4. Discussion
Our early stage patient selection was similar to the START B trial, and 5-year estimates of local control (LC) for all 1325 patients was comparable (97.8%), although more than 40% of START B trial patients received a boost.
Medical oncology adopts a personalized approach in order to prevent systemic recurrence and increase survival among intrinsic subtypes, while neither partial/whole breast irradiation nor boost prescription take molecular subtypes into account [[12], [13], [14], [15], [16]]. Randomized controlled trials demonstrated that tumor bed boost increases LC, but did not include molecular subtype analysis [7,8,17].
There is some evidence that molecular subtypes predict local control. Bane and co-workers performed an unplanned retrospective analysis of immunohistochemistry for 989 (80.1%) of the 1234 node negative participants in the Ontario trial to determine whether molecular subtypes predict response to hypofractionated versus standard RT [18]. Tumors were classified as Luminal A (46.6%), Luminal B (27.2%), Her2 enriched (Luminal B Her2+ and HR- Her2+ considered together = 3.9%), TNBC (12.6%), and unclassified (9.6%). Median follow up was 12 years and 10-year cumulative incidence of LR was 4.5% for Luminal A and TNBC, 7.9% for Luminal B and 16.9% for Her2 enriched tumors (p < 0.01). As in our cohort, none of their patients received tumor bed boost (neither patients treated with hypofractionation, nor patients treated with standard fractionation). Chemotherapy was prescribed for only 12.2% of patients and hormonal therapy for 42.2% of patients. In the Her2 enriched group none were treated with trastuzumab. Other studies observed that Her2 enriched tumors have a higher rate of LR but none of these studies included patients treated with monoclonal antibodies [[19], [20], [21], [22], [23]]. The low LR rate registered for patients with TNBC was considered to be due to the systemic therapy, containing cyclophosphamide, methotrexate and fluorouracil (CMF), or to the association with their known DNA repair deficiency, and their consequent relative radiosensitivity [24]. The TNBC subtype was associated with higher risk of LR, contralateral and systemic relapse [23,[25], [26], [27]].
We acknowledge that the median follow-up of this series is probably not long enough for a proper assessment of late recurrences, even though a large proportion of patients have a follow-up of 7–8 years, with no significant impact on the differences observed at 5 years among subgroups. Peak incidence for recurrence in TNBC has been reported in the first 1–3 years [28,29] earlier than in Luminal B Her2-and Her2 enriched tumors, both of which present relapse within 5 years after treatment, while Luminal A tumors are more indolent [19,20,22,30].
In the last decade, patients with HER2+ tumors received monoclonal antibodies, improving the outcomes of this molecular subtype [25,31].
In our cohort of patients local relapse rate was influenced by molecular subtype, and at five years was 0.2% for Luminal A, 3.3% for Luminal B Her2-, 5.9% for Luminal B Her2+, 12.1% for HR- Her2+ (despite monoclonal antibody therapy), 4.9% for TNBC and 0.9% for ISC. Molecular subtype proportions in our cohort were those expected for a group of patients with early-stage BCa [32].
The following factors: tumor size, presence and number of positive lymph nodes, high-grade, hormonal and chemo-therapy were found to influence relapse free survival (LRRFS, DRFS, DFS, OS), an observation similar to that of other studies with a large number of patients [18,19,33].
As in our study, Nguyen et al. observed, on 956 patients treated in hospitals of four geographical regions of the United States, an increased risk of mortality with increasing age, except for patients younger than 40 years [33].
Our study presents several inherent limitations due to the absence of randomization, its retrospective nature and the lack of homogeneity between molecular subgroups, leading to fewer patients for some subgroups. However, it is by far the largest cohort of consecutive (real life) patients treated homogeneously with WBHF-IMRT without boost, with modern chemotherapy and monoclonal antibodies in all fit patients. The percentages of molecular subgroups are those expected for a cohort of patients with early-stage BCa and are sufficient for analysis.
In this analysis we observed that even the use of modern chemotherapies and monoclonal antibody therapy in TNBC and Her2+ cases, respectively, while improving OS and DFS, cannot sufficiently compensate for the absence of local control, 5-year LRR being high in Luminal B Her2+ and TNBC, and the highest in HR- Her2+ subtype. Given the proven correlation between local control and survival, provided by the recent Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) overview analysis of randomized trials, which demonstrated that for every four local recurrences prevented, approximately one death may be avoided, this is a worrying fact [1,2,6,[34], [35], [36], [37]]. Local dose escalation with a boost has already been shown to significantly reduce local recurrence rate by 41% overall and even more for young patients [7], [8], [17]. None of these studies stratified the patients based on molecular subtypes, which have shown an important correlation with local control (and not only) in retrospective studies. We hypothesize that the addition of a molecular subtype driven boost to the tumoral bed could lead to improved outcomes (local control, quality of life) and cost reduction, and perhaps others in some histotypes. Thus, we consider that a randomized trial to compare the results of RT with molecular subtype driven boost prescription for BCa is recommended.
There is no prospective data on the current use of improved systemic therapies and especially their neoadjuvant prescription in early-stage breast cancer, or the effect on local control. An old trial established the sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer [38]. Chemotherapy first favoured distant control with 25% relapse versus 36% with radiotherapy first (p = 0.05). Overall survival difference was not statistically significant. The five-year crude rates of local recurrence were 5% for radiotherapy first and 14% for chemotherapy first while for distant or regional recurrence or both was 32% and 20% with borderline statistical significance (p = 0.07). Thus, a 12-week course of chemotherapy followed by radiation therapy after breast conserving therapy in stage I-II breast cancer was the preferred sequence. The chemotherapy scheme included methotrexate, leucovorin, florouracil, cyclophosphamide and doxorubicin every 21 days for four cycles, with different dosages than nowadays, while radiotherapy was delivered to whole breast up to 45 Gy in 25 fractions with a tumor bed boost of 16–18 Gy, with a 10% and 20% variation permitted, respectively. Regional node irradiation was allowed, at the discretion of the radiation oncologist. A similar trial with more modern chemotherapy (to better understand the real impact of improved systemic therapies on local control) was not repeated, and the up-date of the old trial with 135 months of follow up did not modify the results [39]. This impact is undeniable, but at the same time we have benefited from improvements in imaging, with a better selection of early-stage disease [40,41], in the attention given to positive margins [42,43], and in the delivery of radiotherapy itself, with higher precision and superior conformation to the target area. Thus, we have seen a reduction in toxicity and an increase in local control in all molecular subtypes, including Luminal A, not treated with chemotherapy, with a consequent higher proportional impact of radiation therapy, as affirmed by McGale et al. in their meta-analysis [44,45]. Moreover, radiotherapy has a different mechanism of action from chemotherapy, and local and spatial cooperation were demonstrated when combined with new systemic therapies [[46], [47], [48]]. Therefore, efforts to improve results through the personalization of the indications, given the signs that some subgroups have a greater resistance to irradiation, must also be made by radiation oncologists.
5. Conclusion
The overall estimated probability of local relapse within 5 years of 2.2% after WBHF-IMRT without boost is good, but molecular subtypes have a strong impact on the estimated probability of LR, as well as on other outcomes. Results were good for Luminal A, Luminal B Her2-and ISC pts (0.2%, 3.3% and 0.9%, respectively), but insufficient for Luminal B Her2+ and HR- Her2+ (5.9% and 12.1%, respectively), despite MAb therapy, and for TNBC (4.9%), even if a modern chemotherapy was prescribed. Thus, molecular subtypes should be used as a parameter in deciding boost prescription. A randomized trial to compare the results of RT with or without boost for BCa molecular subtypes is recommended.
Declaration of competing interest
Dr. Andrei Fodor reports speaker honoraria from Accuray (AERO Academy) and Janssen (Janssen Academy); personal fees for Advisory Board from Sandoz Italia, and reimbursement for travel and accommodations for congresses from Ipsen, AB Medica, Astellas, unrelated to this work. Prof. Dr. Nadia Gisella Di Muzio reports speaker honoraria from Accuray (AERO Academy) and travel and accommodation for a congress from Ipsen, unrelated to this work. Dr. Giampaolo Bianchini reports Consultancy/Advisory role for Roche, Pfizer, AstraZeneca, Lilly, Novartis, Amgen, MSD, Chugai, Sanofi, Daiichi Sankyo, EISAI, Exact Sciences, Neopharm, unrelated to this work. All the other authors have nothing to disclose. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Fisher B., Stewart A., Bryant J. Twenty-year follow up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Veronesi U., Cascinelli N., Mariani L. Twenty-year follow-up of a randomized study comparing breast conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 3.Vinh Hung V., Verschraegen C. Breast conserving surgery with or without radiotherapy: pooled analysis for risk of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst. 2004;21(96):115. doi: 10.1093/jnci/djh013. [DOI] [PubMed] [Google Scholar]
- 4.Bijker N., Meijnen P., Peterse J.L. Breast-conserving treatment with or without radiotherapy in ductal carcinoma-in-situ: ten-year results of European organisation for research and treatment of cancer randomized phase III trial 10853 – a study by the EORTC breast cancer cooperative group and EORTC radiotherapy group. J Clin Oncol. 2006;24(21):3381–3387. doi: 10.1200/JCO.2006.06.1366. [DOI] [PubMed] [Google Scholar]
- 5.Darby S., McGale P., Correa C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Romestaing P., Lehingue Y., Carrie C. Role of a 10-Gy boost in the conservative treatment of early breast cancer: results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink H., Maingon P., Poortmans P. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol. 2015;16:47–56. doi: 10.1016/S1470-2045(14)71156-8. [DOI] [PubMed] [Google Scholar]
- 9.Whelan T.J., Pignol J.P., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 10.Haviland J.S., Owen R.J., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldhirsch A., Winer E.P., Coates A.S. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international Expert Consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hennigs A., Riedel F., Gondos A. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Canc. 2016;16(1):734. doi: 10.1186/s12885-016-2766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z., Hu P., Tu J., Yu N. Luminal B breast cancer: patterns of recurrence and clinical outcome. Oncotarget. 2016;7(40):1–10. doi: 10.18632/oncotarget.11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehinger A., Malmström P., Bendahl P.-O. Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013. Acta Oncol (Madr) 2017;56(1):68–74. doi: 10.1080/0284186X.2016.1237778. [DOI] [PubMed] [Google Scholar]
- 15.García Fernández A., Chabrera C., García Font M. Differential patterns of recurrence and specific survival between luminal A and luminal B breast cancer according to recent changes in the 2013 St Gallen immunohistochemical classification. Clin Transl Oncol. 2015;17(3):238–246. doi: 10.1007/s12094-014-1220-8. [DOI] [PubMed] [Google Scholar]
- 16.Gregucci F., Fozza A., Falivene S. Present clinical practice of breast cancer radiotherapy in Italy: a nationwide survey by the Italian Society of Radiotherapy and Clinical Oncology (AIRO) Breast Group. Radiol Med. 2020;125:674–682. doi: 10.1007/s11547-020-01147-5. [DOI] [PubMed] [Google Scholar]
- 17.Antonini N., Jones H., Horiot J.C. Effect of age and radiation dose on local control after breast conserving treatment : EORTC trial 22881-10882. Radiother Oncol. 2007;82:265–271. doi: 10.1016/j.radonc.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Bane A.L., Whelan T.J., Pond G.R. Tumor factors predictive of response to hypofractionated radiotherapy in a randomized trial following breast conservative therapy. Ann Oncol. 2014;25:992–998. doi: 10.1093/annonc/mdu090. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen P.L., Taghian A.G., Katz M.S. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 20.Voduc K.D., Cheang M.C.U., Tyldesley S., Gelmon K., Nielsen T.O., Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 21.Arvold N.D., Taghian A.G., Niemierko A. Age, breast cancer subtype approximation, and local recurrence after breast-conserving therapy. J Clin Oncol. 2011;29:3885–3891. doi: 10.1200/JCO.2011.36.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert J.M., Gonzalez-Angulo A.M., Guray M. Estrogen/Progesterone receptor negativity and HER2 positivity predict locoregional recurrence in patients with T1a,b N0 breast cancer. Int J Radiat Oncol. 2010;77(5):1296–1302. doi: 10.1016/j.ijrobp.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Lowery A.J., Kell M.R., Glynn R.W., Kerin M.J., Sweeney K.J. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Canc Res Treat. 2012;133(3):831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 24.Turner N.C., Reis-Filho J.S., Russell A.M. BRCA1 dysfunction in sporadic basal like breast cancer. Oncogene. 2007;26:2126–2132. doi: 10.1038/sj.onc.1210014. [DOI] [PubMed] [Google Scholar]
- 25.Tsoutsou P.G., Vozenin M.-C., Durham A.-D., Bourhis J. How could breast cancer molecular features contribute to locoregional treatment decision making? Crit Rev Oncol Hematol. 2017;110:43–48. doi: 10.1016/j.critrevonc.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 26.Haffty B.G., Yang Q., Reiss M. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24(36):5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 27.Bessonova L., Taylor T.H., Mehta R.S., Zell J.A., Anton-Culver H. Risk of a second breast cancer associated with hormone-receptor and HER2/neu status of the first breast cancer. Cancer Epidemiol Biomarkers Prev. 2011;20(2):389–396. doi: 10.1158/1055-9965.EPI-10-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jatoi I., Anderson W.F., Jeong J.-H., Redmond C.K. Breast cancer adjuvant therapy: time to consider its time-dependent effects. J Clin Oncol. 2011;29(17):2301–2304. doi: 10.1200/JCO.2010.32.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang E.H., Tucker S.L., Strom E.A. Predictors of locoregional recurrence in patients with locally advanced breast cancer treated with neoadjuvant chemotherapy, mastectomy, and radiotherapy. Int J Radiat Oncol. 2005;62(2):351–357. doi: 10.1016/j.ijrobp.2004.09.056. [DOI] [PubMed] [Google Scholar]
- 30.Millar E.K.A., Graham P.H., O’Toole S.A. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27(28):4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 31.Yin W., Jiang Y., Shen Z., Shao Z., Lu J. Trastuzumab in the adjuvant treatment of HER2-positive early breast cancer patients: a meta-analysis of published randomized controlled trials. PloS One. 2011;6(6) doi: 10.1371/journal.pone.0021030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vuong D., Simpson P.T., Green B., Cummings M.C., Lakhani S.R. Molecular classification of breast cancer. Virchows Arch. 2014;465(1):1–14. doi: 10.1007/s00428-014-1593-7. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen D., Yu J., Reinhold W.C., Yang S.S. Association of independent prognostic factors and treatment modality with survival and recurrence outcomes in breast cancer. JAMA Network Open. 2020;3(7) doi: 10.1001/jamanetworkopen.2020.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Overgaard M., Jensen M.-B., Overgaard J. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 35.Ragaz J., Olivotto I.A., Spinelli J.J. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British columbia randomized trial. JNCI J Natl Cancer Inst. 2005;97(2):116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 36.Abe O., Abe R., Enomoto K. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 37.Vrieling C., van Werkhoven E., Maingon P. Prognostic factors for local control in breast cancer after long-term follow-up in the EORTC boost vs No boost trial: a randomized clinical trial. JAMA Oncol. 2017 Jan 1;3(1):42–48. doi: 10.1001/jamaoncol.2016.3031. [DOI] [PubMed] [Google Scholar]
- 38.Recht A., Come S.E., Henderson I.C. The sequencing of chemotherapy and radiation therapy after conservative surgery for early-stage breast cancer. N Engl J Med. 1996;334(21):1356–1361. doi: 10.1056/NEJM199605233342102. [DOI] [PubMed] [Google Scholar]
- 39.Bellon J.R., Come S.E., Gelman R.S. Sequencing of chemotherapy and radiation therapy in early-stage breast cancer : updated results of a prospective randomized trial. J Clin Oncol. 2005;23(9):1934–1940. doi: 10.1200/JCO.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 40.Morrow M., Waters J., Morris E. MRI for breast cancer screening, diagnosis and treatment. Lancet. 2011;378:1804–1811. doi: 10.1016/S0140-6736(11)61350-0. [DOI] [PubMed] [Google Scholar]
- 41.Bernsdorf M., Berthelsen Ak, Wielenga V.T. Preoperative PET/CT in early-stage breast cancer. Ann Oncol. 2012;23:2277–2282. doi: 10.1093/annonc/mds002. [DOI] [PubMed] [Google Scholar]
- 42.Jobsen J.J., van der Palen J., Ong F., Meerwaldt J.H. Differences in outcome for positive margins in a large cohort of breast cancer patients treated with breast-conserving therapy. Acta Oncol. 2007;46:172–180. doi: 10.1080/02841860600891325. [DOI] [PubMed] [Google Scholar]
- 43.Morrow M., Van Zee K.J., Solin L.J. Society of surgical oncology- American society for radiation oncology Consensus guideline on margins for breast- conserving surgery with whole -breast irradiation in ductal carcinoma in situ. J Clin Oncol. 2016;34(33):4040–4046. doi: 10.1200/JCO.2016.68.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darby S., McGale P., Correa C. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGale P., Taylor C., Correa C. Effect of Radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality : meta-analysis of individual patient data for 8135 women in 22 randomised tirals. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Horton J.K., Halle J., Ferraro M. Radiosensitizaztion of chemotherapy-refractory, locally advanced or locally recurrent breast cancer with trastuzumab : a phase II trial. Int J Radiat Oncol Biol Phys. 2010;76(4):998–1004. doi: 10.1016/j.ijrobp.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 47.Ho A.Y., Wright J.L., Blitzblau R.C. Optimizing Radiation Therapy to boost systemic immune responses in breast cancer : a critical review for breast radiation oncologists. Int J Radiat Oncol Biol Phys. 2020;108(1):227–241. doi: 10.1016/j.ijrobp.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Césaire M., Thariat J., Candias S.M., Stefan D., Saintigny Y., Chevalier F. Combining PARP inhibition, radiation, and immunotherapy: a possible strategy to improve the treatment of cancer? Int J Mol Sci. 2018;19(12):3793. doi: 10.3390/ijms19123793. [DOI] [PMC free article] [PubMed] [Google Scholar]