Hypoxaemia is a key indicator for hospital admission with coronavirus disease 2019 (COVID-19) [1, 2]. Controversy surrounds the pathophysiology underlying hypoxaemia, with intrapulmonary shunt, mismatch in ventilation-to-perfusion (V′A/Q′) ratio, endothelial injury, microvascular coagulation and host inflammatory response hypothesised to play a role [3–6]. It has recently been proposed that COVID-19 pneumonia may exist as two phenotypes dependent on the preservation of lung mechanics and the relative contribution of V′A/Q′ mismatch and intrapulmonary shunting to hypoxaemia [7]. We hypothesised that V′A/Q′ mismatch and intrapulmonary shunting are present in COVID-19 pneumonia and aimed to assess their effect on outcome. A mathematical model was used to construct oxygen–haemoglobin dissociation curves (ODC) [8] to determine the degree of shunt and V′A/Q′ mismatch in a cohort of patients with severe COVID-19. Factors contributing to shunt and mortality were identified.
Short abstract
Using simple bedside pulse oximetry to create oxygen–haemoglobin desaturation curves may be useful in triaging patients with COVID-19. Intrapulmonary shunting is associated with worse outcomes in COVID-19, and the degree of shunt may predict outcomes. https://bit.ly/2KVv0m2
To the Editor:
Hypoxaemia is a key indicator for hospital admission with coronavirus disease 2019 (COVID-19) [1, 2]. Controversy surrounds the pathophysiology underlying hypoxaemia, with intrapulmonary shunt, mismatch in ventilation-to-perfusion (V′A/Q′) ratio, endothelial injury, microvascular coagulation and host inflammatory response hypothesised to play a role [3–6]. It has recently been proposed that COVID-19 pneumonia may exist as two phenotypes dependent on the preservation of lung mechanics and the relative contribution of V′A/Q′ mismatch and intrapulmonary shunting to hypoxaemia [7]. We hypothesised that V′A/Q′ mismatch and intrapulmonary shunting are present in COVID-19 pneumonia and aimed to assess their effect on outcome. A mathematical model was used to construct oxygen–haemoglobin dissociation curves (ODC) [8] to determine the degree of shunt and V′A/Q′ mismatch in a cohort of patients with severe COVID-19. Factors contributing to shunt and mortality were identified.
All patients presenting to our hospital in March 2020 with a diagnosis of COVID-19 through real-time PCR were included [1]. The study was approved by the local patient safety and quality control committee. The Medical Research Council ethics decision tool indicates that this research does not require review by an NHS Research Ethics Committee in England. As all patient data was anonymised, informed consent was not deemed necessary for this study, in line with local guidance.
Epidemiological, clinical, laboratory and radiological characteristics were collected in addition to level of oxygen therapy required and outcome. Unless anticoagulated prior to admission, all patients received venous thromboembolism prophylaxis. The NEWS2 score was used for all patients, a validated aggregate scoring system of patient physiological observations [9].
We retrospectively collected fingertip pulse oximetry data documented by paramedics and the emergency department. Two saturations at different inspired oxygen fraction (FIO2) values taken on admission and less than 6 h apart were used to construct ODCs. In 10 patients, the model was unable to derive a curve for the calculation of V′A/Q′. This was likely because these patients deteriorated rapidly on admission, skewing the data. However, the model was still able to calculate intrapulmonary shunt for these patients using a single data point.
The construction of ODCs has been described in detail elsewhere (figure 1) [7, 8]. The method uses a two-compartment model [10] and corrects for haemoglobin concentration. Shunting reduces arterial oxygen saturation through arteriovenous admixture which cannot be corrected by increasing FIO2. The shunt can therefore be calculated from depression of the ODC [10]. V′A/Q′ reduction decreases post-alveolar blood oxygen content, shifting the ODC rightwards [10]. This is reversible by increasing FIO2. These parameters are quantified by comparison to a reference curve.
FIGURE 1.
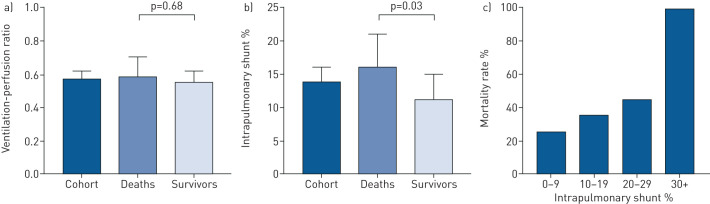
a) Ventilation-to-perfusion ratio in the death and survivor groups. b) Intrapulmonary shunt in the death and survivor groups. c) Mortality rate at increasing level of intrapulmonary shunt.
Spearman rank correlation co-efficient was used to examine the relationship between shunt and clinical parameters.
In March 2020, 108 patients were admitted with confirmed COVID-19 [1]. 14 not requiring oxygen and seven without adequate data available were excluded. The remaining 87 were included. Mean±sd age was 68.3±1.8 years and 41% (n=36) were female. Body mass index was 28.3±0.1 kg·m−2. 65 patients (75%) were white British; eight (9%) white other; eight (9%) black, Asian, or minority ethnic (BAME); and six (7%) unknown ethnicity. 46% of patients were ex- or current smokers. Cardiovascular diseases (hypertension (46%), stroke (16%), ischaemic heart disease (13%)), diabetes (25%) and respiratory diseases (asthma (15%), COPD (15%)) were the most common comorbidities. 24% of patients had previous or current cancer.
Baseline blood tests showed an activated inflammatory response (median C-reactive protein (CRP) 82, interquartile range (IQR) 49–156; lactate dehydrogenase (LDH) 628, 528–807; ferritin 926, 357–1620) and coagulation cascade (median D-dimer 1100, 663–1550). One patient had a possible pulmonary embolus on computed tomography pulmonary angiogram in addition to severe COVID-19 changes. 31 patients (36%) died.
The median shunt was 14% (IQR 4–21%) and V′A/Q′ was 0.58 (IQR 0.50–0.68) (figure 1). Shunt was 45% higher (p=0.03) in patients that died (16%, IQR 6–23%) than in those who survived (11%, IQR 1–17%) (figure 1). There was no difference (p=0.69) between the median V′A/Q′ ratio of patients that died (0.59, IQR 0.49–0.70) and those that survived (0.50, IQR 0.56–0.68). Mortality rate increased with shunt severity (figure 1). All five patients with a shunt greater than 30% died.
Shunt was related to admission NEWS2 score (Spearman correlation coefficient 0.33, p=0.0002), CRP (0.38, p=0.001), LDH (0.45, p=0.002) and urea (0.29, p=0.007), as well as duration of continuous positive airway pressure (CPAP) therapy (0.40, p=0.001) and length of hospital stay (0.25, p=0.02). V′A/Q′ mismatch was not related to any measured parameters.
The pathophysiology underlying hypoxaemia in COVID-19 is controversial [3–5]. This study identifies intrapulmonary shunt as a major pathophysiological mechanism. Shunt severity was predictive of worse outcome: longer length of stay; longer CPAP duration; and higher mortality. Furthermore, shunt correlated with CRP and LDH, but not D-dimer. V′A/Q′ mismatch, although present, was not prognostic.
Micro- and macrovascular thromboses within the pulmonary architecture [5] have been implicated in the pathogenesis of COVID-19 hypoxaemia. Dual-energy computed tomography scans identified profound perfusion abnormalities with shunting of blood to areas of lung with impaired gas exchange [6]. Our study confirms that pulmonary vascular shunting may play a significant role in the development of hypoxaemia in COVID-19. From a pathophysiological perspective, the strong correlation of CRP with intrapulmonary shunt and outcomes such as length of CPAP and death implies that a more profound inflammatory response correlates with more severe shunting, which in turn is related to worse clinical outcomes.
It has been hypothesised that COVID-19 pneumonia may exist on a spectrum between two phenotypes [7]. The less severe form (“type L”) may be associated with preserved lung mechanics with hypoxia proposed to be secondary to localised pulmonary vascular inflammation impairing hypoxic pulmonary vasoconstriction and physiological V′A/Q′ matching. The more severe form (“type H”) may be associated with a more “typical” acute respiratory distress syndrome picture: reduced lung compliance, enhanced inflammation and shunting. This theoretical model was derived from clinician observation and, to date, has not been formally confirmed. Our study provides evidence in favour of this hypothesis by confirming that more severe shunting is associated with worse outcomes, whilst V′A/Q′ mismatching does not correlate with severity of disease. The pathophysiology underlying a shunt means hypoxaemia cannot be completely reversed by increasing FIO2. This may provide an explanation for why these patients have worse outcomes. Further research may also provide insight into the underlying mechanisms responsible for “silent hypoxia” described in COVID-19 and the role of intrapulmonary shunting.
This study also demonstrates that oxygen saturations at two different FIO2 values can be used to construct ODCs useful for predicting outcome. A simple computer-based algorithm was used that can be performed at the bedside on admission and may help prioritise treatment pathways. The strength of this method is that the ODC is a predictable physical property of haemoglobin, which even in fluctuating clinical situations allows the objective and accurate measurement of shunting and V′A/Q′ mismatch. It performs well against more complex methods [11].
This study is limited by the small sample size. However, numbers are in excess of studies using this technique in other clinical conditions (E. Russell-Jones and co-workers, unpublished results). Furthermore, the retrospective design means that oxygen saturations were taken up to 6 h apart. Future prospective studies will be able to collect oxygen saturations at different FIO2 in a shorter time period, reducing the risk of patient deterioration in the interval period.
This study highlights the utility of simple clinical measurements to construct an ODC quantifying shunt and V′A/Q′ mismatch in patients with COVID-19. We also show that the degree of shunt appears to predict outcome. Furthermore, these observations add to our understanding of the pathophysiological mechanisms responsible for hypoxaemia in COVID-19. Our study has significant clinical applicability. Our noninvasive method of early calculation of shunt could assist decisions on triaging and risk stratifying. Given, though, the limited number of subjects and retrospective nature of our study, the next phase of our research will aim to validate these results in a larger, prospective cohort.
Shareable PDF
Acknowledgements
We sincerely thank all the doctors, nurses, and health care staff involved in the management of patients with COVID-19 at the Royal Surrey NHS Foundation Trust.
Footnotes
Data sharing statement: Anonymised study data is available upon reasonable request.
Author contributions: A. Kotwica, H. Knights, N. Mayor, E. Russell-Jones and D. Russell-Jones drafted the manuscript. A. Kotwica, T. Dassios and H. Knights performed the data analysis. All authors contributed to study conception and design, revision of the manuscript, data collection, and patient enrolment. D. Russell-Jones is corresponding author, had access to all the data in the study, and had final responsibility for the decision to submit for publication. A patient advisory group consisting of four current inpatients not included in the study cohort commented on the findings and contributed to the discussion and dissemination plan.
Conflict of interest: A. Kotwica has nothing to disclose.
Conflict of interest: H. Knights has nothing to disclose.
Conflict of interest: N. Mayor has nothing to disclose.
Conflict of interest: E. Russell-Jones has nothing to disclose.
Conflict of interest: T. Dassios has nothing to disclose.
Conflict of interest: D. Russell-Jones has nothing to disclose.
References
- 1.Knights H, Mayor N, Millar K, et al. Characteristics and outcomes of patients with COVID-19 at a district general hospital in Surrey, United Kingdom. Clin Med 2020; 20: e148–e153. doi: 10.7861/clinmed.2020-0303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J 2020; 55: 2000524. doi: 10.1183/13993003.00524-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mcgonagle D, O'donnell JS, Sharif K, et al. Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol 2020; 2: e437–e445. doi: 10.1016/S2665-9913(20)30121-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Camporota L, Vasques F, Sanderson B, et al. Identification of pathophysiological patterns for triage and respiratory support in COVID-19. Lancet Respir Med 2020; 8: 752–754. doi: 10.1016/S2213-2600(20)30279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price LC, McCabe C, Garfield B, et al. Thrombosis and COVID-19 pneumonia: the clot thickens! Eur Respir J 2020; 56: 2001608. doi: 10.1183/13993003.01608-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis 2020; 20: 1365–1366. doi: 10.1016/S1473-3099(20)30367-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46: 1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roe PG, Jones JG. Analysis of factors which affect the relationship between inspired oxygen partial pressure and arterial oxygen saturation. Br J Anaesth 1993; 71: 488–494. doi: 10.1093/bja/71.4.488 [DOI] [PubMed] [Google Scholar]
- 9.Royal College of Physicians. NEWS2 and deterioration in COVID-19. www.rcplondon.ac.uk/news/news2-and-deterioration-covid-19 Date last accessed: 8 Oct 2020.
- 10.Dassios T, Curley A, Morley C, et al. Using measurements of shunt and ventilation-to-perfusion ratio to quantify the severity of bronchopulmonary dysplasia. Neonatology 2015; 107: 283–288. doi: 10.1159/000376567 [DOI] [PubMed] [Google Scholar]
- 11.Lockwood GG, Fung NLS, Jones JG. Evaluation of a computer program for non-invasive determination of pulmonary shunt and ventilation-perfusion mismatch. J Clin Monit Comput 2014; 28: 581–590. doi: 10.1007/s10877-014-9554-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03841-2020.Shareable (325.1KB, pdf)