Graphical abstract
Keywords: Organophosphate insecticide, Macro-invertebrates, Phytoplankton and zooplankton
Highlights
-
•
Effects of fenitrothion on community level and functional endpoints of freshwater ecosystems in microcosms were studied.
-
•
No consistent significant effects were observed for most of the phytoplankton taxa.
-
•
There was a significant decrease for most of the species composition of zooplankton and macro-invertebrates.
-
•
Several taxa were sensitive to even the lowest concentration of fenitrothion.
Abstract
The organophosphate pesticide fenitrothion is widely used as an agricultural pesticide to control tiger bug in larval rearing for aquaculture. The objectives of the present study were to assess the effects of fenitrothion on certain structural (phytoplankton, zooplankton, macro-invertebrates and periphyton) and functional (organic matter decomposition) endpoints of freshwater microcosms. Fenitrothion 50 EC was applied in 12 microcosms (PVC tanks having 400 L of dechlorinated tap water) providing concentrations of 0, 25, 50 and 100 μg/L at a 4-day interval over a period of 4 weeks. Each of the experimental treatment was executed in three replicates. The results indicated the consistent significant effects for most of the species composition of zooplankton and macro-invertebrates. Univariate analysis showed a significant decrease in abundance (p < 0.05) of all identified insects (i.e. Notonecta sp., Gerris sp., Ranatra linearis and Chironomid larvae), when compared to control in all sampling days throughout the treatment period (no observed effect concentration; NOEC = < 25 μg/L). No consistent significant effects were observed for most of the phytoplankton taxa and organic matter decomposition and water quality variables (dissolved oxygen, free CO2, pH, nitrate etc.). However, several taxa of different endpoints were found sensitive to even the lowest concentration of fenitrothion (25 μg/L). Further studies with acute and chronic conditions are recommended involving more local species exposed to < 25 μg/L of fenitrothion.
1. Introduction
Agricultural intensifications are inevitable to fulfill the food and nutritional demand of burgeoning population coping with ever reducing available arable land in the world [1]. Further, natural calamities including sudden flood, seasonal water scarcity, salinity intrusion into coastal land, and cyclones and storms are constant threats for agricultural crop production which limit may the agricultural intensification. While dealing with these adversities and for progressing the agricultural production, farmers cultivate various high-yielding cultivars of different crops. But these high-yielding cultivars are most vulnerable to diseases and pests [2]. Therefore, the uses of various pesticides in agricultural land to control pests as well as to increase crops output per acre of land is a common practice in Bangladesh. Furthermore, the agricultural administration also inspires the farmers to use pesticides to increase the output per acre of land through providing subsidy [3].
In Bangladesh, nearly 84 pesticides are registered with 242 trade names under various chemical groups, such as organophosphate, organochlorine, carbamates and pyrethroids to protect various crops [4]. Among them, the use of organophosphate group has become increasingly popular in most of the developing countries like Bangladesh [[5], [6], [7]]. Fenitrothion, a type II organophosphate synthetic pesticide, is extensively used in agriculture to protect various crops including cereals, cotton, rice, vegetables and top fruits [8]. This pesticide was first introduced in 1959 by Sumitomo Chemical Company and Bayer Leverkusen and later by American Cyanamid Company [9].
The primary purpose of using fenitrothion is to remove tiger bug prior to stocking fish larvae in fish ponds. Moreover, the use of fenitrothion in agriculture may reach to the aquatic environments through direct spray, runoff, leaching, and disposal and washing of containers and equipment in water [6]. After reaching into the waterbodies, fenitrothion may affect the non-target aquatic organisms belonging to different trophic levels, when exceeding the threshold level [6]. Due to its’ potential toxic effects, it is recommended that formulations containing fenitrothion as an active ingredient must have the signal word “caution” on their label [10].
In the past, a number of studies have been conducted in assessing the effects of fenitrothion on non-target aquatic organisms. Most of the studies have focused on the single species laboratory tests on phytoplankton [11,12] zooplankton [[13], [14], [15], [16]], macro-invertebrates [9,[17], [18], [19]] and fish [10,[20], [21], [22], [23], [24], [25], [26], [27], [28]] using sumithion as a test compound. Sabater and Carrasco [11] estimated the 96-h no observed effect concentration (NOEC) values of fenitrothion for Chlorella vulgaris and Chlorella saccharophila to be 4600 and 1700 μg/L, respectively. Leboulanger et al. [29] estimated the 48-h EC50 (1840 μg/L) of fenitrothion for one of the copepods Mesocyclops spp. In fish, fenitrothion altered blood parameters and histopathology of different organs [22,[21], [22], [23], [24], [25], [26], [27], [28]].
Over the past decades, model ecosystem studies, such as microcosms and mesocosms are using as important techniques to assess the risk of pesticides [[30], [31], [32], [33]] and veterinary medicines [34]. There are a number of advantages of using microcosm for toxicity studies, i.e. microcosms allow replications, experimental set-up and ecological realism in a controlled environment [35].
To date, two microcosm studies have been performed to elucidate the toxic effects of fenitrothion on the plankton [15] and soil microorganisms [36]. However, there is paucity of information in different parts of the world focusing on the effects of fenitrothion on both structural (phytoplankton, zooplankton, macro-invertebrates and periphyton) and functional (organic matter decomposition) endpoints in aquatic ecosystems. Hence, the present study aimed at assessing the effects of fenitrothion on certain structural and functional endpoints of freshwater ecosystems in Bangladesh.
2. Materials and methods
2.1. Experimental design
The materials and methods of this study were followed a study described by Sumon et al. [33]. This study was conducted in twelve freshwater microcosms at the laboratory of fish Ecophysiology, Bangladesh Agricultural University, Bangladesh from July to October 2018 (temperature; 25−30 °C). Twelve polyvinyl chloride (PVC) tanks (diameter: 170 cm; total height: 75 cm) were used as microcosms, having four treatments, each with three replications. Each microcosm was filled with 4.5 cm of sediment and 400 L of tap water. Sediment samples were collected from nearby ponds where agricultural activities have not been practiced for many years. To provide sufficient oxygen, an aeration system was installed in each microcosm. Concentrated plankton (equal volumes) and macro-invertebrates (equal numbers) were collected from the ponds where sediment was collected and stocked in each microcosm. The plankton and macro-invertebrates were allowed to establish themselves over a pre-treatment period of 4 weeks prior to sumithion exposure. About 20 % water of the tanks was exchanged every two weeks among the microcosms to homogenize the structure of communities in the systems. Urea (1.4 mg/L) as source of nitrogen and triple super phosphate (0.18 mg/L) as source of phosphorus was used every two weeks in the microcosms during the whole experimental period according to the recommendations described by Daam and Van den Brink [37].
2.2. Application of sumithion
Fenitrothion (formulation: fenitrothion; active ingredient: 50 EC; manufacturer: Sumitomo Chemical Company Limited, Japan) was purchased from a local pesticide seller. After 4 weeks of pre-treatment period, fenitrothion was poured and mixed thoroughly in each microcosm at concentrations of 0, 25, 50 and 100 μg/L at 4-day interval over a period of 4 weeks. The stock solution of 1 L was prepared by dissolving the weighed amount in distilled water to get the desired concentration (500 g/L) of fenitrothion.
2.3. Plankton sampling
Plankton was sampled on day 1 and day 7 before fenitrothion application, and on days 7, 14, 21 and 28 after start application of fenitrothion. Water samples (5 L) were collected using Perspex tube in a plastic bucket and passed through plankton net with a mesh size of 20 μm for phytoplankton and 55 μm for zooplankton [33]. The 5 L water samples were concentrated to a volume of 100 mL. Subsequently, the concentrated samples were preserved in plastic bottle with 10 % buffered formalin solution and stored at 4 °C until further identification. Three sub-samples (1 mL) of the concentrated phytoplankton and zooplankton samples were analyzed at an inverted microscope (MICROS-MCX100, Austria) with a magnification of 100 × . Phytoplankton and zooplankton were analyzed to the lowest practical level, and the species or genus densities were recalculated as the number of individuals per liter of microcosm water.
2.4. Macro-invertebrates sampling
The diversity and abundance of macro-invertebrates were assessed by using two artificial substrates in each microcosm. Bamboo made basket (height- 30 cm and diameter- 20 cm) were used as an artificial substrate. Two baskets were positioned on the sediments in each of the microcosm and they were allowed to have a colonization period of 14 days. Macro-invertebrates were sampled on day 1 and day 7 before application of the fenitrothion and on days 7, 14, 21 and 28 after the first application of fenitrothion. Two baskets were sampled alternately from each of the microcosm. On each sampling date, one of the baskets was lifted from the sediment and directly enclosed by nylon net. The substrate was gently shaken inside the net to collect the invertebrates. Furthermore, the net was passed through the water column next to the tanks’ wall covering approximately one-quarter of the walls’ surface in order to catch swimming macro-invertebrates. The invertebrates (Chironomid larvae and Tubifex tubifex) inhabiting into the sediment were collected by a core sediment sampler (inner diameter: around 8 cm) and transferred to a white plastic tray, identified, counted and stocked them into their original microcosms.
2.5. Water quality parameters monitoring
Temperature, dissolved oxygen (DO), free CO2, pH, total alkalinity, nitrate and phosphate were monitored at 10 a.m. on day 1 and day 7 before the application of fenitrothion, and on days 7, 14, 21 and 28 after the first application of fenitrothion. A digital thermometer, DO meter (Model DO5509, Lutron Taiwan) and a portable pH meter (Model-RI02895, HANNA) were used to measure water temperature, dissolved oxygen and pH, respectively. Free CO2 was measured using phenolphthalein indicator and 0.0227 N NaOH titrant, and total alkalinity was measured using methyl orange indicator and 0.02 N H2SO4 titrant.
2.6. Chlorophyll-a
The chlorophyll-a content was measured on day 7, 14, 21 and 28 after first fenitrothion exposure according to the method described by Greenberg et al. [38]. In brief, 50 mL of water sample was filtered in microfilter paper through a vacuum filter. Microfilter paper was cut into small pieces and put into a graduated plastic tube containing 20 mL acetone. The filter paper was mixed well using a tissue homogenizer and wrapped with aluminum foil and kept overnight at 4 °C. On the following day, after thawing at ambient temperature the tube was centrifuged at 3000 rpm for 5 min and the supernatant was taken in the cuvette. Then reading was taken at 664, 647 and 630 mm using UV spectrophotometer and chlorophyll-a content was calculated using the following formula:
Chlorophyll-a (mg/L) = 11.85 (OD 664) – 1.54 (OD 647) – 0.08 (OD 630) [38].
2.7. Organic matter decomposition
In the present study, we examined the effects of sumithion on organic matter decomposition (OMD). Four litter bags containing 2 g of banana leaves were placed into each microcosm 1 day before the start of the application of fenitrothion. The banana leaves were dried in oven at 40 °C for 48 h after washed under tap water for 48 h. Then 2 g of dried banana leaves were put in each litter bag and the bags were suspended at 30 cm water depth in the microcosms. After the application of fenitrothion, one litter bag was collected from each microcosm on days 7, 14, 21 and 28. The collected material was weighted after dried in an oven at 40 °C for 48 h. The percentage of OMD was calculated by comparing the final dry weight after the incubation period with the initial dry weight of banana leaves.
2.8. Statistical analyses
Statistical analysis of all data was performed by using SPSS software (version 23; SPSS Inc., Chicago, IL, USA). A one-way analysis of variance (one-way ANOVA) and Tukey’s post-hoc test were used to assess the significant differences among the treatments. Differences were considered to be significant if p < 0.05. No observed effect concentrations (NOECs) were calculated as the highest fenitrothion concentration that did not show significant effects as compared to the control [39].
3. Results and discussion
3.1. Primary producers and chlorophyll-a
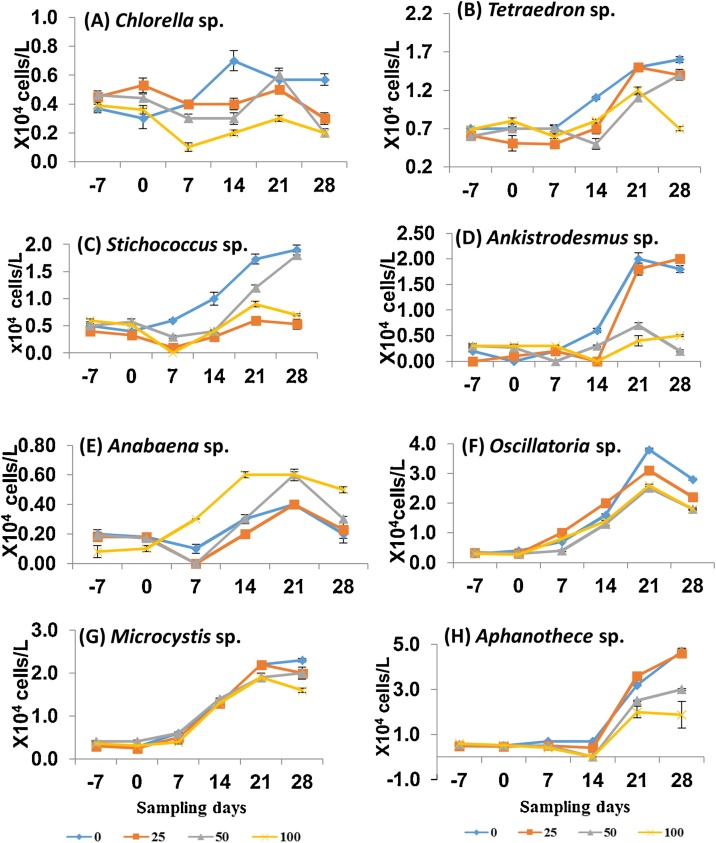
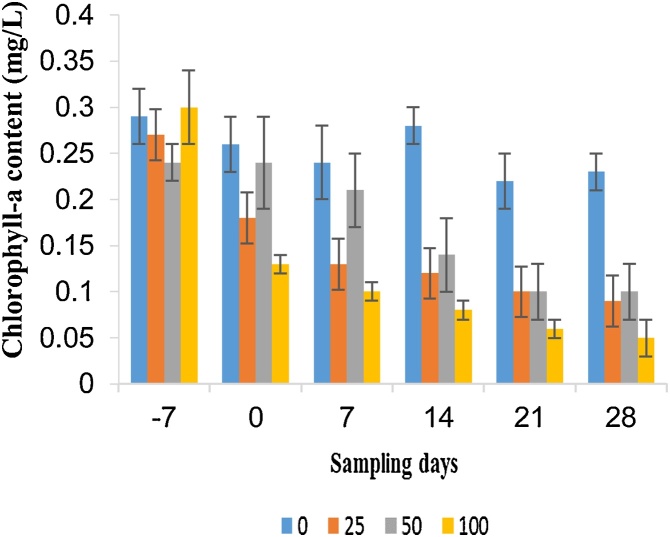
A total of 25 phytoplankton species were identified in the present study. The community of phytoplankton was dominated by Chlorophyceae (13 taxa), followed by Bacillariophyceae (7 taxa), Cyanophyceae (4 taxa) and Euglenophyceae (1 taxon) (Table 1). Among the 13 taxa of Cholorophyceae, only three taxa i.e. Chlorella sp., Tetraedron sp., and Stichococcus sp. were negatively affected from day 7 onwards (Table 1; Fig. 1). There was a significant decrease (p < 0.05) in abundance of Aphanothece sp. for all sampling days during the treatment period with a consistent NOEC of ≤ 25 μg/L. In this study, we found the phytoplankton taxa more sensitive to fenitrothion than the previous studies. For instance, Sabater and Carrasco [11] estimated the 96 h NOEC values of fenitrothion for Chlorella vulgaris and Chlorella saccharophila to be 4600 and 1700 μg/L, respectively; which is much higher than we calculated 7d NOEC (25 μg/L) for Chlorella sp. Kent and Currie [40] reported the 96 h EC50 value of sumithion for one of Cholorpyceans (Chlamydomonas segnis) as 6600 μg/L, which is again two hundred folds higher than our study. On the other hand, photosynthesis is inhibited by more than 75 % by fenitrothion in Anabaena and Aulosira [41]. Moreover, it has been reported that fenitrothion bio-concentrated (2- to 10-fold) from water to algae [42]. In the present study, results showed consistent significant decrease in chlorophyll-a content from day 14 onwards at all treatments as compared to control (NOEC = <25 μg/L) (Fig. 2).
Table 1.
No Observed Effect Concentrations (NOECs) for phytoplankton taxa expressed in terms of nominal single-dose of sumithion concentrations (μg/L) measured on each sampling day (One-way ANOVA; p < 0.05).
| Endpoints | Sampling days |
|||||
|---|---|---|---|---|---|---|
| −7 | 0 | 7 | 14 | 21 | 28 | |
| Chlorophyceae | ||||||
| Actinestrum sp. | > | > | 25 (+) | 50 (-) | > | > |
| Chlorella sp. | > | > | 25 (-) | <25 (-) | 50 (-) | <25 (-) |
| Pediastrum sp. | > | > | NP | <25 (-) | 25 (+) | 25 (+) |
| Pleurococcus sp. | > | > | <25 (+) | <25 (-) | <25 (+) | > |
| Scenedesmus sp. | > | > | > | 50 (+) | > | <25 (+) |
| Spirogyra sp. | NP | NP | NP | <25 (-) | 50 (-) | 25 (+) |
| Tetraedron sp. | > | > | 50 (-) | <25 (-) | 25 (-) | <25 (-) |
| Stichococcus sp. | > | > | <25 (-) | <25 (-) | <25 (-) | 50 (-) |
| Ulothrix sp. | NP | 25 (+) | NP | <25 (-) | <25 (-) | > |
| Cosmerium sp. | > | > | <25 (-) | 50 (-) | 50 (-) | > |
| Ankistrodesmus sp. | > | <25 (+) | 50 (+) | <25 (-) | 25 (-) | <25 (-) |
| Volvox sp. | > | > | > | <25 (-) | <25 (+) | 50 (-) |
| Closterium sp. | > | > | > | <25 (+) | > | 50 (-) |
| Cyanophyceae | ||||||
| Anabaena sp. | > | > | <25 (-) | 50 (+) | 25 (+) | 50 (+) |
| Oscillatoria sp. | > | > | 50 (+) | <25 (-) | <25 (-) | <25 (-) |
| Microcystis sp. | > | > | 50 (-) | 25 (+) | 25 (-) | <25 (-) |
| Aphanothece sp. | > | > | <25 (-) | <25 (-) | <25 (-) | 25 (-) |
| Bacillariophyceae | ||||||
| Cyclotella sp. | > | > | > | 25 (-) | <25 (-) | <25 (-) |
| Fragillaria sp. | NP | NP | <25 (-) | <25 (-) | > | > |
| Navicula sp. | > | > | <25 (+) | 50 (-) | > | <25 (-) |
| Nitzschia sp. | > | > | > | > | 25 (-) | 50 (-) |
| Surirella sp. | NP | NP | NP | <25 (-) | <25 (-) | <25 (-) |
| Tabellaria sp. | > | > | > | <25 (-) | > | 50 (-) |
| Asterrionella sp. | NP | NP | NP | NP | <25 (-) | <25 (-) |
| Euglenophyceae | ||||||
| Euglena sp. | NP | NP | <25 (+) | > | > | 25 (+) |
> = no significant effect (NOEC ≥ 100 μg/L); NP = not present (taxa not present in any of the microcosms); significant increase (+) or decrease (-) compared to control.
Fig. 1.
The population dynamics of the phytoplankton taxa; (A) Chlorella sp., (B) Tetraedron sp., (C) Stichococcus sp., (D) Ankistrodesmus sp., (E) Anabaena sp., (F) Oscillatoria sp., (G) Microcystis sp. and (H) Aphanothece sp. under the four concentrations (0, 25, 50 and 100 μg/L) of fenitrothion. Only the taxa that showed a significant response under treatment period for all sampling days are included.
Fig. 2.
Chlorophyll-a content different sampling days under four concentrations (0, 25, 50 and 100 μg/L) of fenitrothion.
3.2. Invertebrates
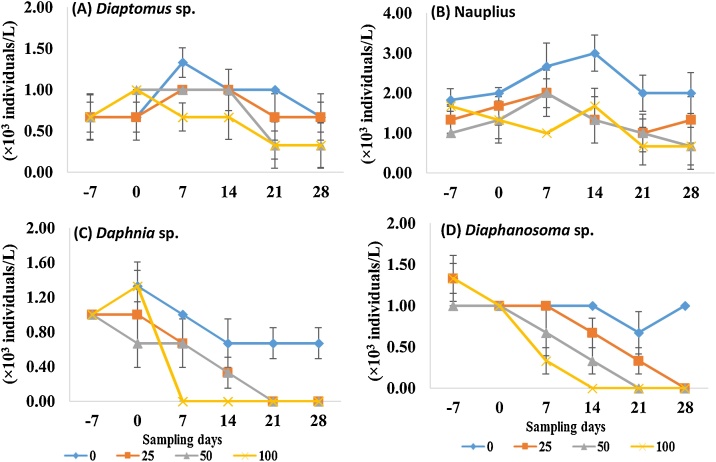
A total of 8 zooplankton taxa were identified during the experimental period. The taxonomic group was dominated by Copepoda (3 taxa), followed by Cladocera (3 taxa) and Rotifera (2 taxa). Significant (p < 0.05) decrease was recorded in abundance for most of the zooplankton species from day 7 of the first fenitrothion exposure (Table 2). Four zooplankton taxa i.e. Diaptomus sp., Nauplius, Daphnia sp. and Diaphanosoma sp. were negatively affected from day 7 till the end of the experiment. Because 7−28-d NOEC (50 μg/L) was calculated for Diaptomus sp., 7-d NOEC (50 μg/L), 14−21-d NOEC (< 25 μg/L) and 28-d NOEC (25 μg/L) were calculated for Nauplius, 7−14-d NOEC (50 μg/L) and 21−28-d NOEC (< 25 μg/L) were calculated for Daphnia sp., and 7−28-d NOEC (50 μg/L) was calculated for Diaphanosoma sp.; while Cyclops sp. was negatively affected from day 21 onwards (NOEC of 25 μg/L) (Table 2; Fig. 3). The lower abundance of zooplankton in this study might be due to toxic nature of fenitrothion to zooplankton.
Table 2.
No Observed Effect Concentrations (NOECs) for zooplankton and macroinvertebrates taxa expressed in terms of nominal single-dose of sumithion concentrations (μg/L) measured on each sampling day (One-way ANOVA; p < 0.05).
| Endpoints | Sampling days |
|||||
|---|---|---|---|---|---|---|
| −7 | 0 | 7 | 14 | 21 | 28 | |
| Copepoda | ||||||
| Cyclops sp. | > | > | > | > | 25 (-) | <25 (-) |
| Diaptomus sp. | > | > | 50 (-) | 50 (-) | 50 (-) | 50 (-) |
| Nauplius | > | > | 50 (-) | <25 (-) | <25 (-) | 25 (-) |
| Rotifera | ||||||
| Brachionus sp. | > | > | > | <25 (-) | <25 (-) | <25 (-) |
| Keratella sp. | > | > | > | <25 (-) | <25 (-) | <25 (-) |
| Cladocera | ||||||
| Moina sp. | > | > | > | 25 (-) | <25 (-) | <25 (-) |
| Daphnia sp. | > | > | 50 (-) | 50 (-) | <25 (-) | <25 (-) |
| Diaphanosoma sp. | > | > | 50 (-) | 50 (-) | 50 (-) | 50 (-) |
| Molluscs | ||||||
| Melanoides tuberculatus (adult) | > | > | > | > | > | > |
| Melanoides tuberculatus (juvenile) | > | > | <25 (-) | <25 (-) | <25 (-) | <25(-) |
| Viviparus bengalensis | > | 25(-) | 25(-) | <25 (-) | <25 (-) | 25 (-) |
| Lamellidens marginalis | > | > | 50 (-) | > | 25 (-) | 25 (-) |
| Annelid | ||||||
| Tubifex tubifex | > | > | 50 (-) | > | <25 (-) | 50 (-) |
| Insects | ||||||
| Notonecta sp. | > | > | <25 (-) | <25 (-) | <25 (-) | <25 (-) |
| Gerris sp. | > | > | <25 (-) | <25 (-) | <25 (-) | <25 (-) |
| Ranatra linearis | > | > | <25 (-) | <25 (-) | <25 (-) | <25 (-) |
| Chironomid larvae | > | > | <25 (-) | <25 (-) | <25 (-) | <25 (-) |
> = no significant effect (NOEC ≥ 100 μg/L); significant decrease (-) compared to control.
Fig. 3.
The population dynamics of the zooplankton taxa; (A) Diaptomus sp., (B) Nauplius, (C) Daphnia sp. and (D) Diaphanosoma sp. under the four concentrations (0, 25, 50 and 100 μg/L) of fenitrothion. Only the taxa that showed a significant response under treatment period for all sampling days are included.
Unfortunately, the toxicity data (e.g. NOEC value) for the affected zooplankton taxa could not be found in open literature, and therefore the direct comparison is impossible. However, a study by Damasio et al. [43] calculated the 48-h IC50 value of fenitrothion for Daphnia magna as 1.87 μg/L, which is about 26 times lower than the NOEC value we calculated for Daphnia sp. (7-d NOEC = 50 μg/L). Leboulanger et al. (2011b) estimated the 48-h EC50 of fenitrothion for one of the copepods Mesocyclops sp. (1840 μg/L), which is approximately 18 times higher than we calculated for Cyclops sp. (7-d NOEC = >100 μg/L). In our study, the Rotifer Brachionus sp. and Keratella sp. were negatively affected from day 14 of the first exposure onwards with a consistent NOEC value of < 25 μg/L. Lv et al. [14] found less toxicity of fenitrothion on Brachionus calyciflorus than our study because they calculated a 4-d NOEC value of 1000 μg/L, which is several times higher than we reported in our study. Marcial et al. [13] reported similar range of results for another species of rotifer Brachionus plicatilis when fenitrothion was used as test compound. However, the variations in the toxicity of fenitrothion may be due to differences of species tested and formulation variation of chemicals [44].
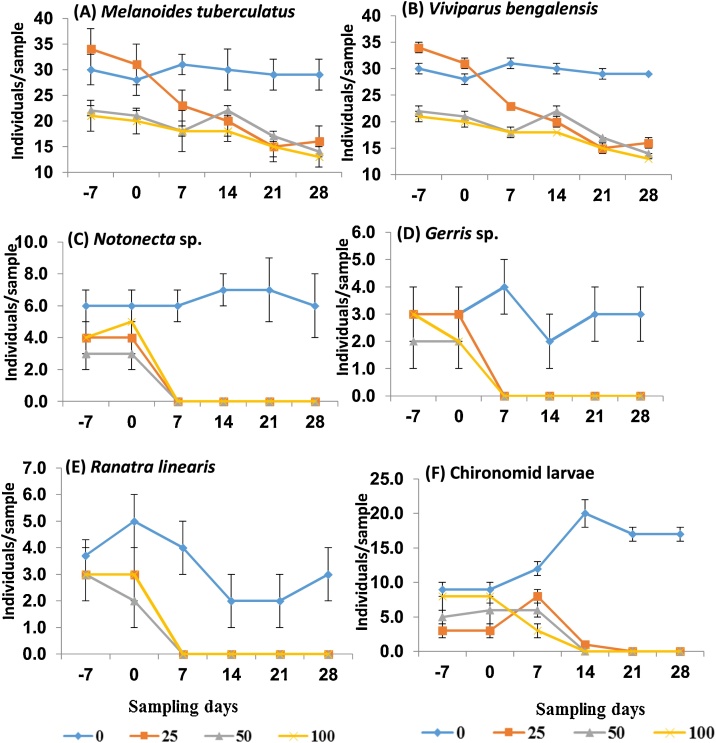
There were 9 macro-invertebrates identified in the present study. The most abundant taxonomic group was Insecta (5 taxa), followed by Mollusca (3 taxa) and Annelida (1 taxa) (Table 2). All taxa belonging to Mollusca were negatively affected from day 7 at different concentrations of fenitrothion except Melanoides tuberculatus (adult). Results did not show consistent significant decrease for Tubifex tubifex for all sampling days during treatment period (Table 2). Univariate analysis showed significant decrease in abundance values (p < 0.05) for all identified insects (i.e. Notonecta sp., Gerris sp., Ranatra linearis and Chironomid larvae), because they were negatively affected at all treatments as compared to control for all sampling days during the treatment period (NOEC = < 25 μg/L) (Table 2; Fig. 4). It has been reported that combined exposure of neonicotinoid, organophosphate and herbicide caused oxidative injury in zebrafish [45]. Forcella et al. [18] observed a significant effect of fenitrothion on AChE inhibition of Chironomus riparius when exposed to different fenitrothion concentrations (0−100 μg/L). Almost similar degree of the AChE inhibition of C. riparious exposed to fenitrothion was observed by Choi et al. [46], but they also did not calculate any threshold values (e.g. NOEC, EC50, etc.).
Fig. 4.
The population dynamics of the macro-invertebrate taxa: (A) Melanoides tuberculatus (juvenile), (B) Viviparus bengalensis, (C) Notonecta sp., (D) Gerris sp., (E) Ranatra linearis and (F) Chironomid larvae under the four concentrations (0, 25, 50 and 100 μg/L) of fenitrothion. Only the taxa that showed a significant response under treatment period for all sampling days are included.
3.3. Water quality parameters
Except on day 28 (NOEC = 25 μg/L), no significant effect was recorded on dissolved oxygen. No significant effects were observed for free CO2 and nitrate for any of the sampling days during the whole experimental period. A significant decrease was observed for pH on day 14. Total alkalinity levels decreased significantly for three consecutive sampling days (from day 7 to day 21), while phosphate concentrations significantly decreased on day 14 onwards in the treatment period (Table 3). The limited variation in water quality parameters in this study might be due to continuous use of aerator in the system. Unfortunately, we have not found any relevant literature contrasting our findings.
Table 3.
No Observed Effect Concentrations (NOECs) for water quality parameters and Organic Matter Decomposition (OMD) expressed in terms of nominal single-dose of sumithion concentrations (μg/L) measured on each sampling day (One-way ANOVA; p < 0.05).
| Water quality Parameters | Sampling days |
|||||
|---|---|---|---|---|---|---|
| −7 | 0 | 7 | 14 | 21 | 28 | |
| Dissolved oxygen | > | > | > | > | > | 25 (-) |
| pH | > | 50 (-) | > | 50 (-) | > | > |
| Total alkalinity | > | > | 50 (-) | 25 (-) | <25 (-) | > |
| Free CO2 | > | > | > | > | > | > |
| Nitrate | > | > | > | > | > | > |
| Phosphate | > | > | > | 50 (-) | 25 (-) | 25 (-) |
| OMD | ND | ND | > | <25 (+) | > | > |
> = no significant effect (NOEC ≥ 100 μg/L); significant increase (+) or decrease (-) compared to control; ND = not determined.
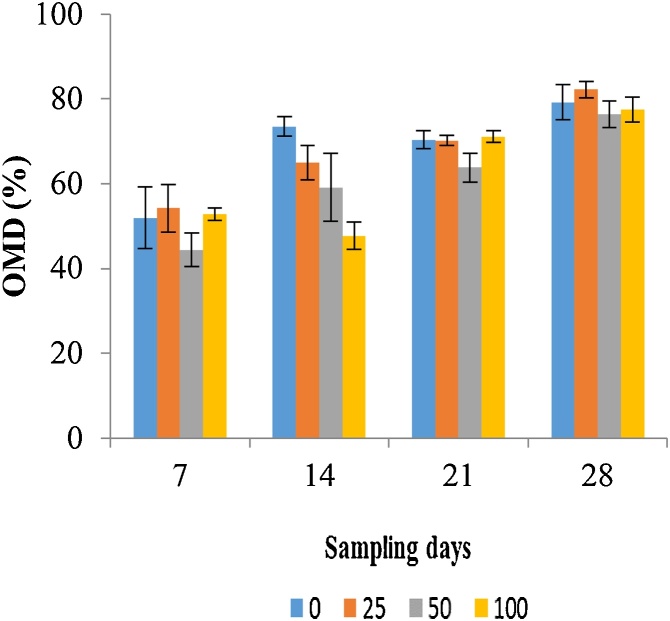
3.4. Organic matter decomposition
In the present study, decomposition rates of banana leaves were 52 %, 74 %, 70 % and 79 % on day 7, 14, 21 and 28, respectively in the control group. Decomposition of banana leaves increased slightly over time during the treatment period. The statistical analysis, however, did not show a consistent significant increase except on day 14 of the first fenitrothion exposure (NOEC = <25 μg/L) (Table 3; Fig. 5). One of the earlier studies showed that there was no significant effect of one the neonicotinoid pesticide imidacloprid on the decomposition of banana leaves in their microcosm study [33].
Fig. 5.
Organic matter decomposition (OMD) of banana leaves in different sampling days under four concentrations (0, 25, 50 and 100 μg/L) of fenitrothion.
4. Conclusion
This is the first report in assessing the toxicity of fenitrothion on structural and functional endpoints of freshwater microcosms. The present study revealed significant effects of fenitrothion on the abundances of most of the zooplankton, macro-invertebrate and some phytoplankton taxa except organic matter decomposition. We derived safe environmental concentrations of fenitrothion for different taxa through the derivation of NOECs, which would be useful for future ecological risk assessment of sub-tropical aquatic ecosystems. In the present study, as we observed several taxa were sensitive to even the lowest concentration of fenitrothion, more acute and chronic investigations are recommended including more species when exposed to < 25 μg/L of fenitrothion.
Data availability statement
The corresponding author [Md. Shahjahan] is responsible to provide the supporting data for the findings of this investigation.
Authors’ contributions
Mohammad Shadiqur Rahman conducted research and collected data; Kizar Ahmed Sumon drafted the manuscript; Md. Jasim Uddin edited manuscript; Md. Shahjahan designed and supervised the experiment, and edited the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
University Grants Commission of Bangladesh is gratefully acknowledged for financial support (2018/666/UGC) to conduct this study to the corresponding author (Md. Shahjahan).
References
- 1.Sumon K.A., Rico A., Ter Horst M.M., Van den Brink P.J., Haque M.M., Rashid H. Risk assessment of pesticides used in rice-prawn concurrent systems in Bangladesh. Sci. Total Environ. 2016;568:498–506. doi: 10.1016/j.scitotenv.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 2.Ali M.H., Sumon K.A., Sultana M., Rashid H. Toxicity of cypermethrin on the embryo and larvae of Gangetic mystus, Mystus cavasius. Environ. Sci. Pollut. Res. 2018;25:3193–3199. doi: 10.1007/s11356-017-9399-1. [DOI] [PubMed] [Google Scholar]
- 3.Rahman S. Pesticide consumption and productivity and the potential of IPM in Bangladesh. Sci. Total Environ. 2013;445:48–56. doi: 10.1016/j.scitotenv.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Ara A.G., Haque W., Hasanuzzaman M. Detection of Organochlorine and organophosphorus pesticides residues in water samples of Taragong thana in Rangpur district in Bangladesh. Res. J. Environ. Earth Sci. 2014;6:85–89. [Google Scholar]
- 5.Uddin M.H., Shahjahan M., Amin A.K.M.R., Haque M.M., Islam M.A., Azim M.E. Impacts of organophosphate pesticide, sumithion on water quality parameters and benthic invertebrates in aquaculture ponds. Aquacult. Rep. 2016;3:88–92. [Google Scholar]
- 6.Sumon K.A., Rashid H., Peeters E.T., Bosma R.H., Van den Brink P.J. Environmental monitoring and risk assessment of organophosphate pesticides in aquatic ecosystems of north-west Bangladesh. Chemosphere. 2018;206:92–100. doi: 10.1016/j.chemosphere.2018.04.167. [DOI] [PubMed] [Google Scholar]
- 7.Hossain M.I., Rahman M.S., Amin A.K.M.R., Ahmed S.I., Shahjahan M. Effects of sumithion on growth and production of phytoplankton and zooplankton in aquaculture pond. Iranian J. Fish. Sci. 2019;18:307–318. [Google Scholar]
- 8.Lewis K.A., Tzilivakis J., Warner D.J., Green A. An international database for pesticide risk assessments and management. Hum. Ecol. Risk Assess. 2016;22:1050–1064. [Google Scholar]
- 9.Sarιkaya R., Sepici‐Dinçel A., Caǧlan Karasu Benli A., Selvi M., Erkoç F. The acute toxicity of fenitrothion on narrow‐clawed crayfish (Astacus leptodactylus Eschscholtz, 1823) in association with biomarkers of lipid peroxidation. J. Biochem. Mol. Toxicol. 2011;25:169–174. doi: 10.1002/jbt.20373. [DOI] [PubMed] [Google Scholar]
- 10.Benli A.Ç.K., Özkul A. Acute toxicity and histopathological effects of sublethal fenitrothion on Nile tilapia, Oreochromis niloticus. Pestic. Biochem. Physiol. 2010;97:32–35. [Google Scholar]
- 11.Sabater C., Carrasco J.M. Effects of the organophosphorus insecticide fenitrothion on growth in five freshwater species of phytoplankton. Environ. Toxicol. 2001;16:314–320. doi: 10.1002/tox.1038. [DOI] [PubMed] [Google Scholar]
- 12.Kaonga C.C., Takeda K., Sakugawa H. Antifouling agents and Fenitrothion contamination in seawater, sediment, plankton, fish and selected marine animals from the Seto Inland Sea. Japan. Geochem. J. 2015;49:23–37. [Google Scholar]
- 13.Marcial H.S., Hagiwara A., Snell T.W. Effect of some pesticides on reproduction of rotifer Brachionus plicatilis Müller. Hydrobiologia. 2005;546:569–575. [Google Scholar]
- 14.Lv L., Snell T.W., Yang J., Li S., Zhu W. Effects of fenitrothion on life history parameters of the rotifer Brachionus calyciflorus. J. Freshwater Ecol. 2010;25:589–598. [Google Scholar]
- 15.Leboulanger C., Bouvy M., Carré C., Cecchi P., Amalric L., Bouchez A., Sarazin G. Comparison of the effects of two herbicides and an insecticide on tropical freshwater plankton in microcosms. Arch. Environ. Contam. Toxicol. 2011;61:599–613. doi: 10.1007/s00244-011-9653-3. [DOI] [PubMed] [Google Scholar]
- 16.Mano H., Tanaka Y. Spatial difference in genetic variation for fenitrothion tolerance between local populations of Daphnia galeata in Lake Kasumigaura. Japan. Ecotoxicology. 2017;26:1358–1365. doi: 10.1007/s10646-017-1860-x. [DOI] [PubMed] [Google Scholar]
- 17.Berra E., Forcella M., Giacchini R., Rossaro B., Parenti P. Biomarkers in caddisfly larvae of the species Hydropsyche pellucidula(Curtis, 1834) (Trichoptera: Hydropsychidae) measured in natural populations and after short term exposure to fenitrothion. Bull. Environ. Contam. Toxicol. 2006;76:863–870. doi: 10.1007/s00128-006-0998-7. [DOI] [PubMed] [Google Scholar]
- 18.Forcella M., Berra E., Giacchini R., Rossaro B., Parenti P. Increased alanine concentration is associated with exposure to fenitrothion but not carbamates in Chironomus riparius larvae. Ecotoxicol. Environ. Saf. 2007;66:326–334. doi: 10.1016/j.ecoenv.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 19.Chitgar M.G., Hajizadeh J., Ghadamyari M., Karimi-Malati A., Sharifi M., Hoda H. Cellular energy allocation in the predatory bug, Andrallus spinidens Fabricius (Hemiptera: pentatomidae), following sublethal exposure to diazinon, fenitrothion, and chlorpyrifos. J. Plant Prot. Res. 2014;54:78–84. [Google Scholar]
- 20.Almeida J.R., Oliveira C., Gravato C., Guilhermino L. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax L. Exposed to the organophosphate pesticide fenitrothion. Ecotoxicology. 2010;19:1369–1381. doi: 10.1007/s10646-010-0523-y. [DOI] [PubMed] [Google Scholar]
- 21.Sepici-Dincel A., Sahin D., Karasu Benli A.C., Sarikaya R., Selvi M., Erkoc F., Altan N. Genotoxicity assessment of carp (Cyprinus carpio L.) fingerlings by tissue DNA damage and micronucleus test, after environmental exposure to fenitrothion. Toxicol. Mech. Methods. 2011;21:388–392. doi: 10.3109/15376516.2010.551553. [DOI] [PubMed] [Google Scholar]
- 22.Salam M.A., Shahjahan M., Sharmin S., Haque F., Rahman M.K. Effects of sub-lethal doses of an organophosphorus insecticide sumithion on some hematological parameters in common carp, Cyprinus carpio. Pakistan J. Zool. 2015;47:1487–1491. [Google Scholar]
- 23.Ahmed S.I., Ahmmed M.K., Ghosh S.K., Islam M.M., Shahjahan M. Histo-architectural changes of intestinal morphology in Zebra fish (Danio rerio) exposed to Sumithion. Res. Agric., Livest. Fish. 2015;2:499–506. [Google Scholar]
- 24.Ahmed S.I., Zahangir M.M., Haque F., Ahmmed M.K., Shahjahan M. Alteration of blood glucose and hemoglobin levels in zebrafish exposed to sumithion. Progress. Agril. 2016;27:216–221. [Google Scholar]
- 25.Hossain S., Khatun M.H., Rahman M.K., Shahjahan M. Impacts of sumithion on blood glucose and some hematological parameters in common carp. Int. J. Environ. 2015;5:8–13. [Google Scholar]
- 26.Hossain S., Miah M.I., Islam M.S., Shahjahan M. Changes in hepatosomatic index and histoarchitecture of liver in common carp exposed to organophosphate insecticide sumithion. Asian J. Med. Biol. Res. 2016;2:164–170. [Google Scholar]
- 27.Shahjahan M., Kabir M.F., Sumon K.A., Bhowmik L.R., Rashid H. Toxicity of organophosphorous pesticide sumithion on larval stages of stinging catfish Heteropneustes fossilis. Chin. J. Oceanol. Limnol. 2017;35:109–114. [Google Scholar]
- 28.Islam S.M.M., Rahman M.A., Nahar S., Uddin M.H., Haque M.M., Shahjahan M. Acute toxicity of an organophosphate insecticide sumithion to striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 2019;6:957–962. doi: 10.1016/j.toxrep.2019.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leboulanger C., Schwartz C., Somville P., Diallo A.O., Pagano M. Sensitivity of two Mesocyclops (Crustacea, Copepoda, Cyclopidae), from tropical and temperate origins, to the herbicides, diuron and paraquat, and the insecticides, temephos and fenitrothion. Bull. Environ. Contam. Toxicol. 2011;87:487. doi: 10.1007/s00128-011-0406-9. [DOI] [PubMed] [Google Scholar]
- 30.Daam M.A., Crum S.J., Van den Brink P.J., Nogueira A.J.A. Fate and effects of the insecticide chlorpyrifos in outdoor plankton‐dominated microcosms in Thailand. Environ. Toxicol. Chem. 2008;27:2530–2538. doi: 10.1897/07-628.1. [DOI] [PubMed] [Google Scholar]
- 31.Hayasaka D., Korenaga T., Suzuki K., Saito F., Sánchez-Bayo F., Goka K. Cumulative ecological impacts of two successive annual treatments of imidacloprid and fipronil on aquatic communities of paddy mesocosms. Ecotoxicol. Environ. Saf. 2012;80:355–362. doi: 10.1016/j.ecoenv.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Hua J., Relyea R. Chemical cocktails in aquatic systems: pesticide effects on the response and recovery of &20 animal taxa. Environ. Pollut. 2014;189:18–26. doi: 10.1016/j.envpol.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Sumon K.A., Ritika A.K., Peeters E.T., Rashid H., Bosma R.H., Rahman M.S., Van den Brink P.J. Effects of imidacloprid on the ecology of sub-tropical freshwater microcosms. Environ. Pollut. 2018;236:432–441. doi: 10.1016/j.envpol.2018.01.102. [DOI] [PubMed] [Google Scholar]
- 34.Rico A., Dimitrov M.R., Van Wijngaarden R.P., Satapornvanit K., Smidt H., Van den Brink P.J. Effects of the antibiotic enrofloxacin on the ecology of tropical eutrophic freshwater microcosms. Aquat. Toxicol. 2014;147:92–104. doi: 10.1016/j.aquatox.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 35.Van den Brink P.J. Assessing aquatic population and community level risks of pesticides. Environ. Toxicol. Chem. 2013;32:972–973. doi: 10.1002/etc.2210. [DOI] [PubMed] [Google Scholar]
- 36.Hong Q., Zhang Z., Hong Y., Li S. A microcosm study on bioremediation of fenitrothion-contaminated soil using Burkholderia sp. FDS-1. Int. Biodeterior. Biodegradation. 2007;59:55–61. [Google Scholar]
- 37.Daam M.A., Van den Brink P.J. Conducting model ecosystem studies in tropical climate zones: lessons learned from Thailand and way forward. Environ. Pollut. 2011;159:940–946. doi: 10.1016/j.envpol.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 38.Greenberg A.E., Trussell R.R., Clesceri S L., editors. American Public Health Association, American Water Works Association and Water Pollution Control Federation. 16th ed. 1989. Standard methods for the examination of water and wastewater. 1268 pp. [Google Scholar]
- 39.Rico A., Sabater C., Castillo M.Á. Lethal and sub-lethal effects of five pesticides used in rice farming on the earthworm Eisenia fetida. Ecotoxicol. Eenviron. Saf. 2016;127:222–229. doi: 10.1016/j.ecoenv.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Kent R.A., Currie D. Predicting algal sensitivity to a pesticide stress. Environ. Toxicol. Chem. 1995;14:983–991. [Google Scholar]
- 41.Lal S., Saxena D.M., Lal R. Effects of DDT, fenitrothion and chlorpyrifos on growth, photosynthesis and nitrogen fixation in Anabaena (Arm 310) and Aulosira fertilissima. Agric. Ecosyst. Environ. 1987;19:197–209. [Google Scholar]
- 42.Favari L., Lopez E., Martinez-Tabche L., Diaz-Pardo E. Effect of insecticides on plankton and fish of Ignacio Ramirez reservoir (Mexico): a biochemical and biomagnification study. Ecotoxicol. Environ. Saf. 2002;51:177–186. doi: 10.1006/eesa.2002.2142. [DOI] [PubMed] [Google Scholar]
- 43.Damásio J., Guilhermino L., Soares A.M., Riva M.C., Barata C. Biochemical mechanisms of resistance in Daphnia magna exposed to the insecticide fenitrothion. Chemosphere. 2007;70:74–82. doi: 10.1016/j.chemosphere.2007.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Hayasaka D., Korenaga T., Suzuki K., Sanchez-Bayo F., Goka K. Differences in susceptibility of five cladoceran species to two systemic insecticides, imidacloprid and fipronil. Ecotoxicology. 2012;21:421–427. doi: 10.1007/s10646-011-0802-2. [DOI] [PubMed] [Google Scholar]
- 45.Shukla S., Jhamtani R.C., Dahiya M.S., Agarwal R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017;17:240–244. doi: 10.1016/j.toxrep.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi J., Caquet T., Roche H. Multilevel effects of sublethal fenitrothion exposure in Chironomus riparius Mg. (Diptera, Chironomidae) larvae. Environ. Toxicol. Chem. 2002;21:2725–2730. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The corresponding author [Md. Shahjahan] is responsible to provide the supporting data for the findings of this investigation.