Summary
Bats are reservoirs for a large number of viruses which have potential to cause major human disease outbreaks, including the current coronavirus disease 2019 (COVID-19) pandemic. Major efforts are underway to understand bat immune response to viruses, whereas much less is known about their immune responses to bacteria. In this study, MR1-restricted T (MR1T) cells were detected through the use of MR1 tetramers in circulation and tissues of Pteropus alecto (Pa) bats. Pa MR1T cells exhibited weak responses to MR1-presented microbial metabolites at resting state. However, following priming with MR1-presented agonist they proliferated, upregulated critical transcription factors and cytolytic proteins, and gained transient expression of Th1/17-related cytokines and antibacterial cytotoxicity. Collectively, these findings show that the Pa bat immune system encompasses an abundant and functionally conserved population of MR1T cells with mucosal-associated invariant T-like characteristics, suggesting that MR1 and MR1T cells also play a significant role in bat immune defense.
Subject Areas: Biological Sciences, Immunology, Components of the Immune Systems
Graphical Abstract
Highlights
-
•
MR1T cells are present in Pa bats and react to MR1-presented microbial metabolites
-
•
Pa MR1T cells upregulate Prf and MAIT-associated TFs upon culture with MR1 agonists
-
•
Upon stimulation, Pa MR1T cells rapidly and transiently express TNF and IL-17
-
•
Pa MR1T cells kill E. coli and MR1 agonist-pulsed cells in an MR1-dependent manner
Biological Sciences; Immunology; Components of the Immune Systems
Introduction
Bats are a large group of mammals in the order of Chiroptera and the only mammals capable of sustained and powered flights. Bats are divided into the suborders Yinpterochiroptera and Yangochiroptera (Tsagkogeorga et al., 2013), which formerly included megabats and most microbats, respectively. Apart from other unique features including long life spans (Wilkinson and South, 2002) and low rate of tumorigenesis (Wang et al., 2011), bats are reservoirs for pathogenic viruses of animal and human health significance, such as severe acute respiratory syndrome (SARS), Ebola virus, and Nipah virus (Luis et al., 2013; Wynne and Wang, 2013). The novel SARS coronavirus 2 (SARS-CoV-2), the causative agent of coronavirus disease 2019 (COVID-19), appears to originate from bats (Andersen et al., 2020; Zhou et al., 2020), highlighting the important role of bats in emerging infectious diseases. While bats are natural carriers of multiple pathogenic viruses, they do not usually display signs of disease (Luis et al., 2013; Wynne and Wang, 2013). Data from a broad comparative genomic study indicate that bats are the only mammals to have lost the entire pyrin and HIN domain gene family, which codes for DNA sensors important for dsDNA-triggered inflammation (Ahn et al., 2016). There are also lower levels of apoptosis-associated speck-like protein containing a CARD, caspase-1, as well as interleukin (IL)-1β secretion in bat immune cells following infection by various zoonotic viruses (Ahn et al., 2019; Goh et al., 2020). In addition, nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3) activity appear to be reduced in the bat immune cells compared to human and murine counterparts (Ahn et al., 2019). These studies suggest that bats have a dampened innate immune system (Xie et al., 2018). This may be linked to the evolutionary adaptation to powered flight (Zhang et al., 2013).
Studies into bat immune systems have been hampered by the lack of bat-specific reagents. Nevertheless, comparative genomic studies have previously identified conserved immune marker genes, facilitating the identification and characterization of bat immune cells (Gamage et al., 2020; Martinez Gomez et al., 2016; Periasamy et al., 2019). By using cross-reactive antibodies to specific markers for immune cells in other mammals, and flow cytometry-fluorescence in situ hybridization (Flow-FISH) technology for gene expression at the mRNA level, both CD4+ and CD8+ T cells can be identified in pteropodid bats (Martinez Gomez et al., 2016). In addition, subsets of B cells have been described in pteropodid bats by employing a similar strategy of screening for suitable species cross-reactive antibodies (Periasamy et al., 2019). Lastly, several subsets of blood monocytes and alveolar macrophages were identified using a similar approach (Gamage et al., 2020).
The major histocompatibility complex (MHC)-Ib related protein (MR1) presents vitamin B2-related antigens (Corbett et al., 2014; Kjer-Nielsen et al., 2012), to a group of unconventional innate-like T cell populations expressing semi-invariant T cell receptor (TCR) rearrangements collectively known as MR1-restricted T (MR1T) cells (Godfrey et al., 2019; Huang et al., 2005; Treiner et al., 2003). MR1 displays an extraordinary level of evolutionary conservation among eutherian mammals (Huang et al., 2009; Mondot et al., 2016; Riegert et al., 1998; Tsukamoto et al., 2013), with the corresponding invariant TCR rearrangements and MR1T cell populations in eutherians where MR1 is present (Boudinot et al., 2016; Goldfinch et al., 2010; Rahimpour et al., 2015; Xiao et al., 2019). In contrast, specific lack of the corresponding invariant TCR rearrangements and MR1T cell populations are noted in some mammals where the gene for MR1 is absent, such as mammals belonging to the orders of Carnivora and Lagomorpha, as well as the armadillos (Xenarthra) (Boudinot et al., 2016). Overall, these observations suggest that MR1 and MR1T cells have co-evolved under selection pressure and implicate their importance in the immune system of eutherian mammals (Boudinot et al., 2016; Godfrey et al., 2019; Huang et al., 2009).
In mice and humans, classical mucosal-associated invariant T (MAIT) cells expressing an invariant TCR with the TRAV1-2 segment represent the vast majority of MR1T cells (Godfrey et al., 2019). MAIT cells recognize vitamins B2 (riboflavin) and B9 (folic acid)-related microbial metabolites and are an antimicrobial T cell population present in high abundance in tissues and circulation (Godfrey et al., 2019). MAIT cells rapidly perform a range of effector responses following MR1-restricted recognition of antigen, including cytokine production, cytotoxicity, antimicrobial activity, and tissue repair (Boulouis et al., 2020b; Constantinides et al., 2019; Dusseaux et al., 2011; Gibbs et al., 2017; Hinks et al., 2019; Lamichhane et al., 2019; Leeansyah et al., 2013; Leng et al., 2019; Meierovics et al., 2013). MAIT cell development and functional characteristics are under the control of the master transcription factor promyelocytic leukemia zinc finger (PLZF, or zinc finger and BTB domain containing 16, ZBTB16), the Th17-associated retinoid-related orphan receptor (ROR) γt, as well as the T cell transcription factors eomesodermin (eomes) and T box transcription factor 21 (TBX21, or T-bet) (Dusseaux et al., 2011; Koay et al., 2016, 2019; Leeansyah et al., 2014, 2015; Martin et al., 2009; Savage et al., 2008). Evidence from human in vitro and challenge studies, as well as murine and non-human primate models of infectious diseases, strongly support an important role of MAIT cells in various bacterial and viral infections (Boulouis et al., 2020b; Ellis et al., 2020; Howson et al., 2018; Le Bourhis et al., 2010; Meierovics et al., 2013; Salerno-Goncalves et al., 2017; van Wilgenburg et al., 2018; Wang et al., 2018, 2019). Interestingly, this includes the recent observation that MAIT cells respond strongly to SARS-CoV-2 infection in humans (Flament et al., 2020; Jouan et al., 2020; Parrot et al., 2020). Altogether, these characteristics strongly support the notion that MR1 and MR1T cells perform critical functions in the immune system of placental mammals.
In the current study, we investigated the phenotypic and functional characteristics of MR1T cells in a model pteropodid bat, the fruit-eating black flying fox Pteropus alecto (Pa) belonging to the suborder Yinpterochiroptera. We provide here the first evidence of a highly abundant MR1T cell population with MAIT cell-like characteristics in the pteropodid bats, including reactivity, proliferation, cytokine production, and cytotoxicity in response to stimulation by an agonist MR1-binding ligand and the model microbe Escherichia coli. The execution of MAIT-like effector functionality of Pa MR1T cells is tightly regulated with rapid onset of action and rapid resolution, potentially minimizing the risk of immune pathology, and supporting the notion of enhanced innate immune tolerance in bats. The abundance of MAIT cell-like MR1T cells in this species supports the concept that MR1 and MR1T cells may play important roles in bat immune systems.
Results
MR1 Is Highly Conserved in Various Bat Species
The human hMR1 whole genomic and translated full-length amino acid sequences were compared with those of bat species from the suborder Yinpterochiroptera and Yangochiroptera, as well as with those of various other placental mammals (Figures S1A–S1C). Strong gene and protein sequence similarity (85–88% and 80–83% homology, respectively) was noted for MR1 from various Yinpterochiropterans and Yangochiropterans versus human origin (Figures S1B and S1C). Furthermore, MR1 genes in the various bat species displayed greater sequence similarity with hMR1 than with those of house mouse (Mus musculus), brown rat (Rattus norvegicus), and cattle (Bos taurus) (Figures S1B and S1C). MR1 sequences from bats also tended to cluster within their own respective suborders of Yinpterochiroptera and Yangochiroptera (Figure S1A). The closest MR1 sequence phylogenetic distance to the bats belong to those of the odd-toed ungulate horse (Equus caballus), in agreement with the thymocyte transcript data set on the relative genetic similarity between pteropodid bats and the horse (Papenfuss et al., 2012), followed by those of the even-toed ungulates cattle and pig (Sus scrofa), and then those of human and chimpanzee (Pan troglodytes) (Figure S1A). The rodent and amphibian MR1 sequences were phylogenetically more distant to MR1 sequences from bats (Figure S1A). Overall, these results suggest the presence of the riboflavin and folic acid-related metabolite restriction element MR1 in various bat species and are consistent with the notion of high conservation of MR1 among placental mammals (Huang et al., 2009; Mondot et al., 2016; Riegert et al., 1998; Tsukamoto et al., 2013). Importantly, these results highlight the relatively close sequence similarities between hMR1 and MR1 from the various bat species.
MR1T Cells Are Present in the Circulation and Tissues of Pteropus alecto
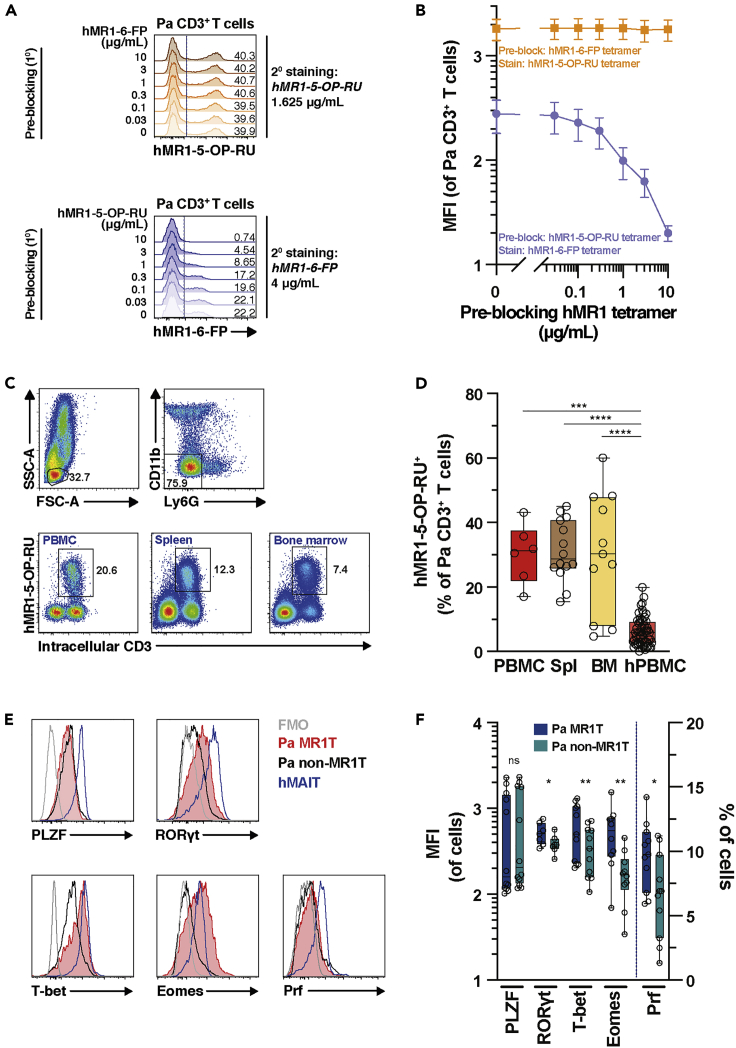
Because the MR1 sequence is present in various bat species, the presence of T cells reactive to the restriction element MR1 was investigated using a model pteropid bat, the fruit-eating black flying fox Pa. Due to the higher sequence similarity of Pa MR1 at the amino acid level with that of human than mouse (Figure S1C), hMR1 tetramers loaded with the MR1 ligands 5-(2-oxopropylideneamino)-6-d-ribitylaminouracil (5-OP-RU) or 6-formyl pterin (6-FP) were used to stain mononuclear cells (MNCs) derived from Pa peripheral blood (PB), spleen, and bone marrow (BM), compared with PBMC controls from healthy adult humans. Initially, Pa spleen MNCs were stained with titrated concentrations of hMR1-5-OP-RU tetramer to determine an optimal staining concentration. Human MR1-5-OP-RU tetramer staining occurred at high dilutions (Figure S2A), suggesting specific and high-affinity binding of the 5-OP-RU-loaded hMR1 tetramer. While hMAIT cells predominantly did not bind the hMR1-6-FP tetramer, the Pa hMR1-5-OP-RU+ T cells stained weakly positive for hMR1-6-FP tetramer (Figure S2B). The staining with hMR1-6-FP tetramer was gradually lost at lower concentrations (Figure S2C). The specificity of hMR1-5-OP-RU tetramer binding was confirmed in pre-blocking experiments with titrated hMR1-6-FP tetramer followed by staining with a fixed concentration of hMR1-5-OP-RU tetramer. Here, pre-blocking with hMR1-6-FP tetramers had no detectable effect on hMR1-5-OP-RU tetramer binding to Pa CD3+ T cells (Figure 1A, top panel and 1B). In contrast, pre-blocking with titrated hMR1-5-OP-RU tetramer completely abolished hMR1-6-FP binding at the highest hMR1-5-OP-RU tetramer concentration (Figure 1A, bottom panel and 1B). These results indicate that the weak binding of Pa CD3+ T cells to 6-FP-loaded hMR1 tetramer was of low-affinity, whereas the strong binding to 5-OP-RU-loaded hMR1 tetramer was highly specific.
Figure 1.
MR1T Cells Are Present in Circulation and Tissues of P. alecto
(A) Representative histogram of Pa bone marrow (BM) mononuclear cells (MNCs) either pre-blocked with titrated concentrations of hMR1-6-FP or hMR1-5-OP-RU tetramer as indicated, followed by incubation with a fixed concentration of hMR1-5-OP-RU tetramer (top panel) or hMR1-6-FP tetramer (bottom panel), respectively (n = 3).
(B) The mean fluorescent intensity (MFI) levels of hMR1-5-OP-RU tetramer following hMR1-6-FP pre-blocking, and hMR1-6-FP teramer following hMR1-5-OP-RU pre-blocking (n = 3). See also Figures S2A–S2C.
(C and D) (C) Identification and (D) enumeration of MR1T cells in the circulation, spleen (spl), and BM of Pa (n = 6-14). Human peripheral blood (PB) MAIT cells were included as a comparison (n = 57). See also Figures S2D and S2E.
(E and F) (E) Representative histrograms and (F) expression of the transcription factors PLZF, RORγt, T-bet, and Eomes as well as the cytolytic protein perforin (Prf) in MR1T cells and non-MR1T cells in BM tissues of Pa bats (n = 7-12) and in human MAIT cells. See also Figure S2F. Data presented as a line graph with error bars represents the mean and standard error. Box and whisker plots show all data points, median, and the interquartile range. See also Figure S2. Statistical significance was determined using the Kruskal-Wallis ANOVA followed by Dunn's multiple comparison test followed post-hoc test (D), Wilcoxon's signed-rank test (E; PLZF), or paired t test (F; RORγt, T-bet, Eomes, Prf). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001,∗∗p < 0.01,∗p < 0.05. ns, not significant.
By using hMR1-5-OP-RU tetramer, 5-OP-RU-reactive cells were detected within the CD3+ T cell population of the wild Pa PB, BM, and spleen (Figures 1C and 1D), at levels higher than those observed for PB hMAIT cells (Figure 1D). The vast majority of hMR1-5-OP-RU+ T cells in humans are TRAV1-2+ (TCR Vα7.2+) MAIT cells. Prior to the discovery of the MR1 ligands, hMAIT cells were routinely identified by the co-expression of TCR Vα7.2 and high levels of the C-type lectin receptor CD161 on T cells (Martin et al., 2009). However, the anti-Vα7.2 mAb clone 3C10 did not stain Pa hMR1-5-OP-RU+ T cells (Figure S2D). Furthermore, neither of the two mAb clones commonly used to detect hCD161, DX12 and HP-3G10, stained Pa hMR1-5-OP-RU+ T cells (Figure S2E). Altogether, these findings indicate that the hMR1 tetramers can identify MR1T cells in the circulation and tissues of the pteropid bat. Throughout this study, Pa MR1T cells are defined by hMR1-5-OP-RU tetramer staining on Pa CD3+ T cells, irrespective of binding toward hMR1-6-FP tetramer. Moreover, because of the limited availability of Pa PB and spleen tissue samples, BM tissue samples were selected to characterize MR1T cell phenotypic and functional characteristics hereafter, with spleen tissues included as a comparison where available.
Pa MR1T Cells Express Classical and Innate-Like T-Cell-Associated Transcription Factors
Human MAIT cells express the innate-like T cell transcription factor PLZF, alongside with the Th17 master transcription factor RORγt and the classical T cell transcription factors T-bet and Eomes (Dias et al., 2017a; Gherardin et al., 2016; Leeansyah et al., 2015) (Figure 1E). Pa BM and spleen MR1T cells also expressed these transcription factors (Figures 1E, 1F, and S2F). MR1T cells in both spleen and BM of Pa expressed higher levels of RORγt, T-bet, and Eomes compared to those of Pa non-MR1T cells (Figures 1E, 1F, and S2F). However, PLZF was detected at similar levels between Pa MR1T and non-MR1T cells. Pa ex vivo MR1T cells expressed low levels of the pore-forming protein perforin (Prf), although significantly more than Pa non-MR1T cells (Figures 1E, 1F, and S2F). This is reminiscent of hMAIT cell Prf expression (Kurioka et al., 2015; Le Bourhis et al., 2013; Leeansyah et al., 2015) and suggests that Pa MR1T cells may have poor cytotoxic capacity at resting state. Taken together, these findings indicate that Pa MR1T cells resemble hMAIT cells with regards to expression of Prf as well as innate-like and classical T cell-associated transcription factors.
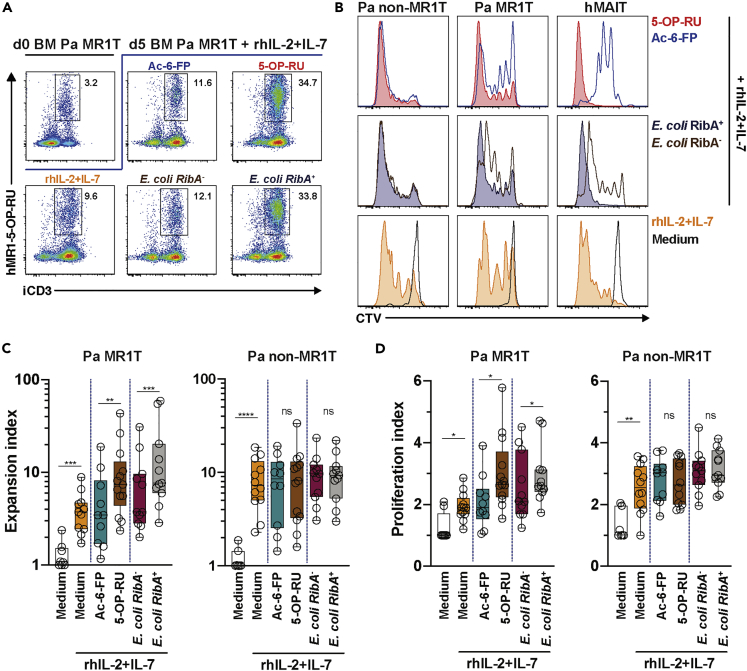
Pa MR1T Cells Proliferate following Stimulation with Synthetic Riboflavin-Related MAIT Cell Antigen and Riboflavin Synthesis-Competent E. coli
Given that Pa MR1T cells bind strongly to 5-OP-RU-loaded and weakly to 6-FP-loaded hMR1 tetramers, Pa MR1T cells may potentially be reactive to both riboflavin (5-OP-RU)-related and folic acid (6-FP)-related metabolites. To evaluate this possibility, MNCs from Pa BM and spleen were cultured in the presence of 5-OP-RU (Mak et al., 2017), acetyl(Ac)-6-FP, or mildly fixed riboflavin synthesis – competent RibA+ and – incompetent RibA− strains of E. coli. Due to the unavailability of commercial Pa-specific T cell-supportive cytokines, cultures were supplemented with recombinant human (rh)IL-2 and (rh)IL-7. Assessments on day 5 post-stimulation demonstrated that Pa MR1T cells specifically proliferated in response to stimulation with the riboflavin-related intermediate 5-OP-RU and RibA+ riboflavin synthesis-competent strains of E. coli but significantly less so to the folic acid metabolite Ac-6-FP and a RibA− riboflavin-synthesis-deficient strain of E. coli (Figures 2A–2D). Further analyses indicated that the low-level Pa MR1T cell proliferation to Ac-6-FP and a RibA− strain of E. coli stimulations was likely due to the proliferative response to rhIL-2+IL-7 supplementation alone (Figures 2B–2D). The Pa MR1T cell proliferative pattern was similar to that of hMAIT cells (Figure 2B), although hMAIT cells appeared to have stronger proliferative responses toward 5-OP-RU and RibA+ E. coli (Figures S2G and S2H). Interestingly, Pa non-MR1T cells displayed relatively strong proliferative responses to rhIL-2+IL-7 supplementation regardless of antigenic stimulations (Figures 2B–2D). Collectively, these findings indicate that Pa MR1T cells display a specific proliferative response toward riboflavin-related metabolite antigens similar to that of hMAIT cells. Moreover, despite the weak binding to hMR1-6-FP tetramer, Pa MR1T cells did not appear to proliferate in response to folic acid-related metabolite stimulation.
Figure 2.
Pa MR1T Cells Specifically Proliferate to Riboflavin-Related Metabolite MR1 ligands.
(A and B) (A) Identification of Pa MR1T cells and (B) representative histograms of CellTrace Violet (CTV) dilution after 5 days in vitro culture with the MR1 ligands 5-OP-RU and Ac-6-FP, as well as riboflavin synthesis-autotroph E. coli RibA+ 1100-2 and riboflavin synthesis-deficient E. coli RibA− BSV18 in the presence of recombinant human (rh)IL-2 and IL-7 or control medium.
(C and D) (C) Expansion and (D) proliferation indices of Pa MR1T cells and non-MR1T cells following 5 days culture with various MR1 ligands and E. coli RibA+ EC120S and 1100-2 and E. coli RibA− BSV18 based on CTV dilution using the FlowJo v 9.9 software (n = 7-13). See also Figures S2G and S2H. Box and whisker plots show all data points, median, and the interquartile range. Statistical significance was determined using the Mann-Whitney test for comparisons between control medium and medium supplemented with rhIL-2+IL-7, and between Ac-6-FP and 5-OP-RU (C and D). The Wilcoxon's signed-rank test was used to determine significance between E. coli RibA+ and RibA− strains (C and D). ∗∗∗∗p < 0.0001, ∗∗∗p < 0.001,∗∗p < 0.01,∗p < 0.05. ns, not significant. iCD3, intracellular CD3.
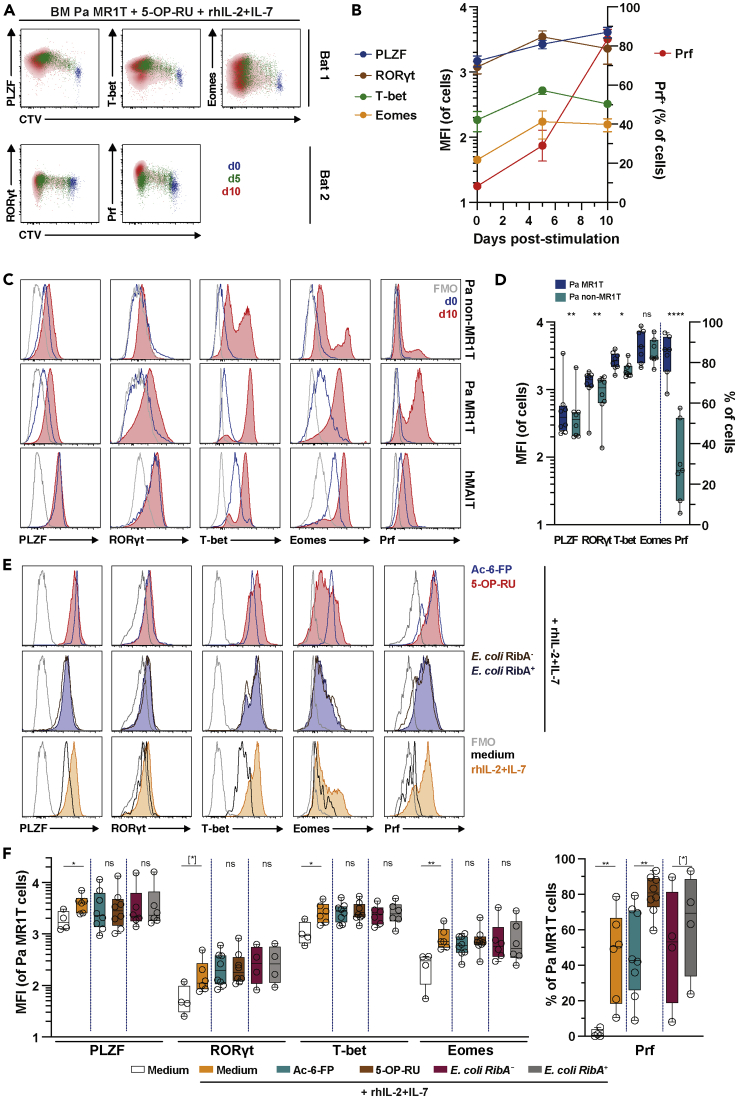
Pa MR1T Cells Upregulate Effector Transcription Factors and Perforin following Agonist MR1 Ligand and Cytokine Stimulation
In humans, MAIT cells upregulate critical transcription factors as well as cytolytic effector proteins following antigen-specific and cytokine stimulations (Leeansyah et al., 2015). Pa BM MR1T cells progressively over the 10-day culture period upregulated PLZF, RORγt, T-bet, Eomes, and Prf following proliferation in response to 5-OP-RU stimulation in the presence of exogenous hIL-2+IL-7 (Figures 3A and 3B). Pa non-MR1T cells did not upregulate these transcription factors and Prf to the same extent (Figures 3C and 3D), indicating higher sensitivity of Pa MR1T cells to this stimulation. Further analyses indicated that cytokine stimulation alone appeared to be sufficient to induce the upregulation of these transcription factors and Prf in Pa MR1T cells (Figures 3E and 3F), consistent with the pattern observed in hMAIT cells (Kurioka et al., 2015; Leeansyah et al., 2015). Interestingly, cognate TCR stimulations further increased Prf expression, but not PLZF, RORγt, T-bet, or Eomes (Figures 3E and 3F). Taken together, these data indicate that Pa MR1T cells resemble hMAIT cells in their expression of transcription factors and the cytolytic protein Prf following TCR and cytokine stimulation.
Figure 3.
Pa MR1T Cells Upregulate Effector Transcription Factors Following Proliferation.
(A and B) (A) Flow cytometry plots and (B) expression of the transcription factors PLZF, T-bet, and Eomes as well as RORγt and the cytolytic protein Prf from separate, independent experiments, respectively, along with CTV dilution by Pa MR1T cells on day 0, 5, and 10 following stimulation with 5-OP-RU supplemented with rhIL-2+IL-7 (n = 4-9).
(C and D) (C) Histograms of the transcription factors PLZF, RORγt, T-bet, and Eomes, and the cytolytic protein Prf at resting and following culture with 5-OP-RU supplemented with rhIL-2+IL-7 and (D) expression of the same effector transcription factors and cytolytic protein by Pa MR1T cells and Pa non-MR1T cells on day 10 of culture (n = 7-8).
(E and F) (E) Histograms and (F) levels (MFI) of the transcription factors PLZF, RORγt, T-bet, and Eomes, and the cytolytic protein Prf at day 7–10 of culture in medium alone, medium supplemented with rhIL-2+IL-7, or with various MR1 ligands, riboflavin-producing or -deficient E. coli strains in the presence of rhIL-2+IL-7 (n = 47-8). Data presented as a line graph with error bars represent the mean and standard error. Box and whisker plot shows all data points, median, and the interquartile range. Statistical significance was determined using the paired t test (RORγt, Eomes, Prf) (D), or Wilcoxon's signed-rank test (PLZF and T-bet) (D), between Ac-6-FP and 5-OP-RU (F), and between E. coli RibA+ and RibA− strains (F), or Mann-Whitney's test between medium and rhIL-2+IL-7 (F). ∗∗∗∗p < 0.0001, ∗∗p < 0.01,∗p < 0.05, [∗] p < 0.1. FMO, fluorescence minus one; ns, not significant.
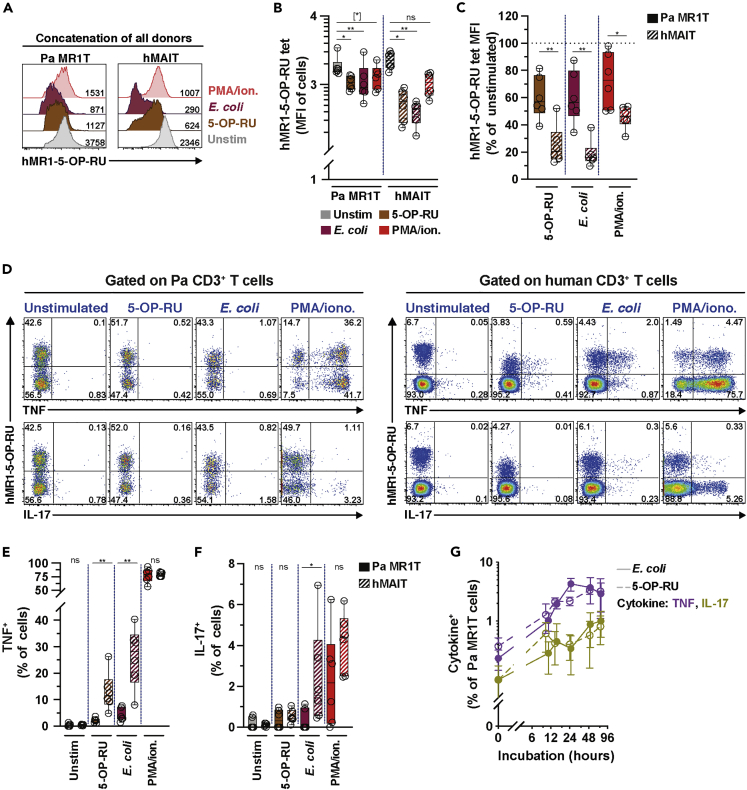
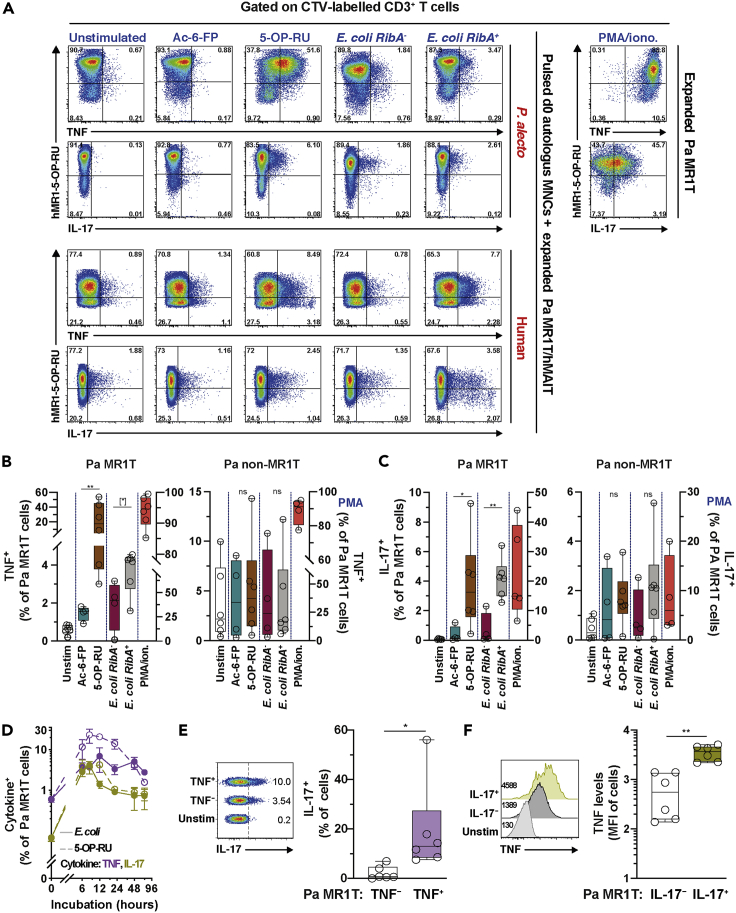
Antigen-Expanded Pa MR1T Cells Produce TNF and IL-17
In humans and mice, MAIT cells produce Th1/Th17-like cytokines, including IFNγ, TNF, and IL-17 (Godfrey et al., 2019). To evaluate whether Pa MR1T cells also produce these cytokines, resting ex vivo Pa MR1T cells without previous priming or expansion were incubated with the riboflavin-related metabolite 5-OP-RU or mildly fixed RibA+ E. coli overnight. The downregulation of the hMAIT cell surface TCR can be used as an alternative means to measure hMAIT cell activation following cognate TCR-dependent stimulations (Dias et al., 2016, 2017a). Pa MR1T cells exhibited modest TCR downregulation following cognate stimulations (Figures 4A and 4B). The levels of TCR downregulation by Pa MR1T cells were significantly less than those of hMAIT cells (Figure 4C), suggesting less activation of unprimed Pa MR1T cells following cognate stimulations. Short-term incubations with phorbol ester and calcium ionophore did not significantly down-regulate surface TCR expression on either Pa MR1T or hMAIT cells (Figures 4A and 4B), but the degree of such downregulation was still weaker in Pa MR1T cells than that of hMAIT cells (Figure 4C). Surprisingly, very little TNF or IL-17 expression was detected in unprimed ex vivo Pa MR1T cells stimulated with 5-OP-RU or riboflavin-producing E. coli (Figures 4D–4F and S3A), despite uptake of E. coli by Pa BM MNC (Figure S3B) and surface TCR downregulation (Figures 4A–4C). However, under identical stimulations conditions, unprimed hMAIT cells produced significant amounts of TNF and IL-17 (Figures 4D–4F and S3A). Negligible cytokine production by Pa MR1T and hMAIT cells was observed following Ac-6-FP and riboflavin-deficient E. coli stimulations (Figure S3A). Nevertheless, strong stimulation using phorbol ester and calcium ionophore provoked TNF and IL-17 expression by resting, non-expanded Pa MR1T cells at comparable levels to those of hMAIT cells in this limited set of experiments (Figures 4D–4F and S3A). Furthermore, the resting unprimed Pa MR1T cells also did not express TNF and IL-17 at higher bacterial doses (Figure S3C), although non-significantly increased levels were observed during longer incubations (Figure 4G). Expression of caspase 3 appeared to increase in unprimed Pa MR1T cells stimulated with 5-OP-RU- or mildly-fixed RibA+ E. coli, but this increase was comparable to what was observed in hMAIT cells (Figure S3D). Altogether, these findings indicate a lack of robust pro-inflammatory cytokine expression by resting unprimed Pa MR1T cells following agonistic stimulations.
Figure 4.
Unprimed Pa MR1T Cells Were Incapable in Producing TNF and IL-17 following Stimulation with Agonist MR1 ligands.
(A and B) (A) Concatenation and levels (B) of Pa MR1T or hMAIT cell hMR1-5-OP-RU staining intensity (MFI) from following stimulation with 5-OP-RU, riboflavin synthesis-competent E. coli, or PMA/ionomycin (n = 6 each).
(C) Comparison of hMR1-5-OP-RU staining intensity (MFI) between Pa MR1T cells and hMAIT cells following stimulations relative to unstimulated controls (n = 6). (D) Representative flow cytometry plots of (E) TNF and (F) IL-17 expression by freshly thawed (day 0) Pa BM MR1T cells or hMAIT cells following 24 h culture with MR1 ligand 5-OP-RU or E. coli EC120S (n = 6). PMA/ionomycin treatment for 6 h were used as positive controls. (G) TNF and IL-17 production by freshly thawed Pa BM MR1T cells at indicated time points following incubation with MR1 ligand 5-OP-RU or E. coli EC120S (n = 2-6). See also Figures S3A–S3D. Data presented as a line graph with error bars represent the mean and standard error. Box and whisker plot shows all data points, median, and the interquartile range. Statistical significance was determined using the Friedman test followed with Dunn's multiple comparison test (B), and Mann-Whitney's test (C, E, and F). ∗∗p < 0.01,∗p < 0.05, [∗] p < 0.1. ns, not significant.
Next, whether the relative lack of cytokine production in resting Pa MR1T cells also extends to antigen- and cytokine-primed and proliferating Pa MR1T cells was evaluated. To that end, expansion cultured Pa MR1T cells were incubated with MNC from the same Pa animals pre-pulsed with the riboflavin-related metabolite 5-OP-RU, the folate-related metabolite Ac-6-FP, or riboflavin–autotroph RibA+ and –auxotroph RibA− strains of E. coli. The addition of autologous MNC was necessary to provide antigen-presenting cells (APCs), which were lost during culture (Figure 2A). Using this approach (Figure S3E), the expansion cultured and primed Pa MR1T cells expressed TNF and IL-17 already at 8 h following stimulation with APC pulsed with 5-OP-RU or riboflavin-autotroph RibA+ strains of E. coli (Figures 5A–5C, S3F, and S3G). No significant cytokine expression was detected from those stimulated with Ac-6-FP or riboflavin-deficient RibA− strains of E. coli (Figures 5A–5C). This pattern resembled that of expanded hMAIT cells, albeit hMAIT cells produced somewhat less TNF and IL-17 using this experimental approach (Figure 5A). Notably, the peak of cytokine expression by expanded Pa MR1T cells occurred relatively early between 6 and 12 h post-stimulation, and decreased rapidly over the 24–48 h period (Figure 5D). No commercially available anti-IFNγ was able to detect IFNγ production by Pa MR1T cells, with a representative clone shown in Figure S3H.
Figure 5.
Expanded Pa MR1T Cells Produce TNF and IL-17 following Stimulation with Agonist MR1 Ligands.
(A–D) (A) Representative flow cytometry plots of (B) TNF and (C) IL-17 expression by Pa MR1T cells, Pa non-MR1T cells, or hMAIT cells following 8 h co-culture or (D) at indicated time points of expanded (day 15–17) Pa BM MNC or hMAIT cells with freshly-thawed, autologous resting Pa BM MNC or hPBMC, respectively, that had been pulsed with MR1 ligands Ac-6-FP or 5-OP-RU, or E. coli strains EC120S, 1100-2 (both RibA+), or BSV18 (RibA-) (n = 4-7 (B and C), 2–6 (D)). PMA/ionomycin treatment for 8 h on expanded Pa BM MNC alone were used as positive controls.
(E) Flow cytometry plots and expression of IL-17 in TNF− and TNF+ Pa MR1T cells co-cultured with freshly-thawed, autologous resting Pa BM MNC pre-pulsed with 5-OP-RU (n = 6).
(F) Histograms and levels of TNF (mean fluorescence intensity; MFI) within IL-17- TNF+ and IL-17+ TNF+ Pa MR1T cell subsets following co-culture with freshly thawed, autologous resting Pa BM MNC pre-pulsed with 5-OP-RU (n = 6). See also Figures S3E–S3J. Box and whisker plot shows all data points, median, and the interquartile range. Data presented as a line graph with error bars represent the mean and standard error. Statistical significance was determined using the Mann-Whitney test (B and C), Wilcoxon's signed-rank test (E), or paired t test (F). ∗∗p < 0.01,∗p < 0.05, [∗] p < 0.1. ns, not significant.
Further analyses of 5-OP-RU-stimulated expanded Pa MR1T cells indicated that IL-17 was mostly expressed by TNF+ Pa MR1T cells (Figure 5E). Moreover, IL-17+ Pa MR1T cells produced higher levels of TNF (Figure 5F), suggesting a pro-inflammatory propensity of IL-17-producing Pa MR1T cells. Pa non-MR1T cells did not respond to the bacterial riboflavin- and folate-related metabolite stimulations used (Figures 5A–5C). Polyclonal stimulation using PMA/ionomycin triggered expression of TNF by both Pa MR1T and non-MR1T cells (Figures 5A and 5B). However, IL-17 expression following PMA/ionomycin stimulation was predominantly observed in Pa MR1T cells (Figures 5A and 5C), suggesting Pa MR1T cells are programmed toward higher IL-17 production. Finally, there was a similar level of TNF production by Pa splenic MR1T cells following PMA/ionomycin stimulation to that of Pa BM MR1T cells (Figures S3I and S3J). Overall, these findings indicate that Pa tissue MR1T cells display a Th1/17-like functional profile in response to TCR-dependent recognition of riboflavin-related bacterial metabolite antigens.
Antigen-Expanded Pa MR1T Cells Kill Cell Lines of Bat and Human Origin Pulsed with Riboflavin-Related Bacterial Metabolites
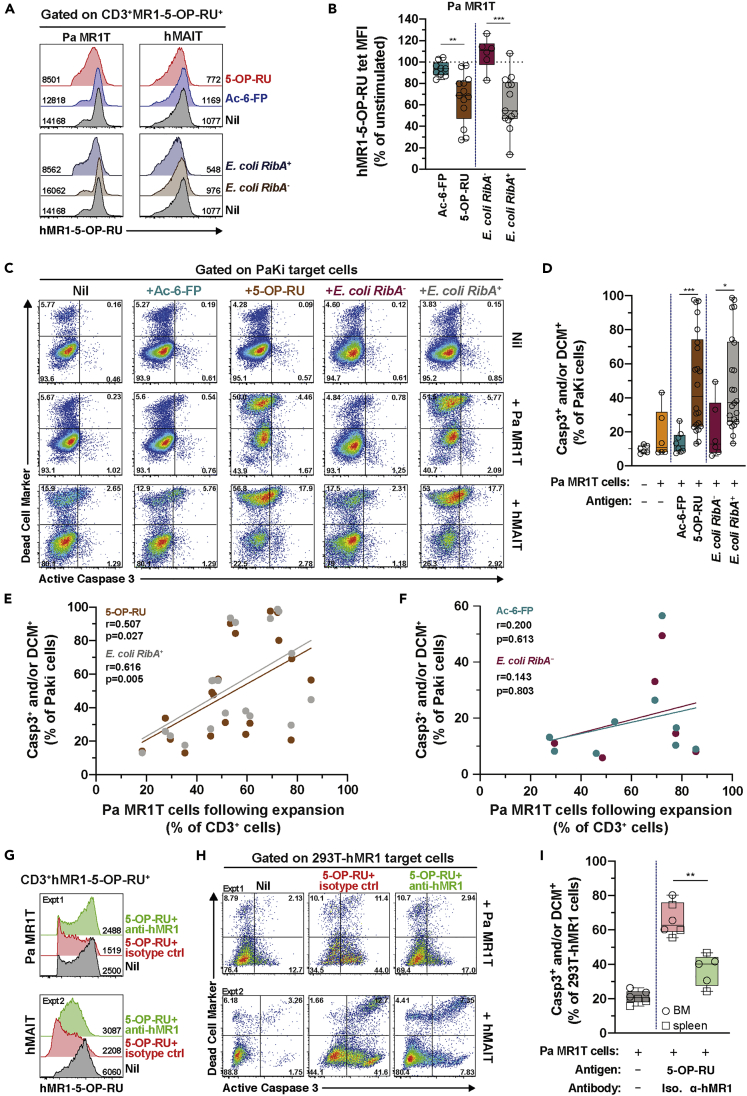
Pa MR1T cells readily expressed Prf following 5-OP-RU-stimulation and proliferation (Figure 3), suggesting the capacity to mediate cytotoxicity. Therefore, the next set of experiments evaluated the ability of Pa MR1T cells to kill multiple epithelial cell lines of bat and human origin pulsed with 5-OP-RU, folate-related metabolite Ac-6-FP, and riboflavin-synthesis-competent RibA+ or riboflavin-synthesis-deficient RibA− strains of E. coli. Human MAIT cells release cytolytic proteins to kill infected cells, a process readily detectable by measuring the levels of the degranulation marker CD107a (Boulouis et al., 2020b; Dias et al., 2016; Leeansyah et al., 2015). However, while hMAIT cells strongly degranulated when co-cultured with 5-OP-RU- or RibA+ E. coli-pulsed Pa kidney (PaKi) cell line, no CD107a expression was detected in Pa MR1T cells using the same anti-human CD107a mAb, likely due to lack of mAb cross-reactivity (Figure S4A). Thus, Pa MR1T cell activation was assessed by measuring surface TCR downregulation. By using this approach, Pa MR1T cells clearly downregulated TCR expression following stimulation with PaKi cell line pulsed with 5-OP-RU or riboflavin-synthesis-autotroph RibA+ strains of E. coli, but not in response to Ac-6-FP or riboflavin-synthesis-deficient RibA− strains of E. coli (Figures 6A and 6B). Likewise, hMAIT cells downregulated the TCR expression following similar stimulations (Figure 6A), consistent with the upregulation of CD107a (Figure S4A). These findings indicate that TCR downregulation can be used to measure TCR-dependent activation of Pa MR1T cells.
Figure 6.
Expanded Pa MR1T Cells Kill Target Epithelial Cell Lines Pulsed with Agonist MR1 Ligands.
(A) Histograms and (B) levels (MFI) of the MR1-5-OP-RU tetramer staining of expanded (d15-17) Pa MR1T cells or hMAIT cells following 24 h co-culture with Pa kidney (PaKi) cell line fed with Ac-6-FP, 5-OP-RU, E. coli RibA− (BSV18), or E. coli RibA+ (1100-2 or EC120S) (n = 6-13).
(C and D) (C) Flow cytometry plots and (D) expression of active caspase (Casp)3 and amine-reactive cytoplasmic dye (dead cell marker; DCM) by the PaKi target cells fed with various antigens as indicated following 24 h co-culture with expanded (d15-17) Pa MR1T or hMAIT cells (n = 6-22).
(E and F) Correlation between frequency of Pa MR1T cells within the expanded culture and frequency of Casp3+ and/or DCM + PaKi cell lines fed with (E) 5-OP-RU or E. coli RibA+ (1100-2 or EC120S) (n = 19), or (F) Ac-6-FP or E. coli RibA− (BSV18) (n = 6-9).
(G, H, and I) Histograms and MFI of the MR1-5-OP-RU tetramer staining of expanded Pa MR1T cells or hMAIT cells (G) and 5-OP-RU-pulsed 293T-hMR1 target cells apoptosis (H and I) following 24 h co-culture, in the presence of anti-hMR1 mAb or IgG2a isotype control (n = 5-6). See also Figure S4. Box and whisker plot shows all data points, median, and the interquartile range. Statistical significance was determined using unpaired t test (B) and the Mann-Whitney test (D and I). Correlations were assessed using the Spearman rank correlation (E and F). ∗∗∗p < 0.001, ∗∗p < 0.01,∗p < 0.05. ns, not significant.
In line with these observations, Pa MR1T cells directly killed PaKi cells pulsed with 5-OP-RU or riboflavin synthesis-competent strains of E. coli, but only minimally killed PaKi cells pulsed with Ac-6-FP or a riboflavin synthesis-deficient strain of E. coli (Figures 6C and 6D). Similar results were observed when using Pa lung (PaLu) cell line as the target cells (Figure S4B), suggesting that Pa MR1T cells are able to kill multiple cell types of Pa origin. Interestingly, Pa MR1T cells also efficiently killed epithelial cell lines of human origin pulsed with 5-OP-RU or riboflavin-autotrophic strains of E. coli, including HeLa cells expressing endogenous levels of hMR1 and 293T cells over-expressing hMR1 (293T-hMR1) (Figures S4C and S4D). Thus, Pa MR1T cells were capable of mediating cytotoxicity against cell lines of both Pa and human origins pulsed with riboflavin-related bacterial metabolite antigens, but not when pulsed with folic acid-related metabolites (Figure S4E). The ability of Pa MR1T cells to mediate cytotoxicity was not restricted by the tissues of origin, as Pa MR1T cells isolated from BM and spleen were equally capable in killing antigen- or bacteria-pulsed cells (Figure S4F). Activated hMAIT cells likewise efficiently killed PaKi cells expressing endogenous level of Pa MR1 pulsed with the riboflavin-related metabolite 5-OP-RU and riboflavin-synthesis competent strains of E. coli (Figure 6C), consistent with strong degranulation (Figure S4A) and TCR downregulation (Figure 6A) following identical stimulations. Intriguingly, resting ex vivo Pa MR1T cells were not capable of mediating cytotoxicity within the duration of the assay, despite their apparent activation as suggested by the mild down-regulation of the cognate TCR levels (Figures S4G–S4I). This observation is consistent with the lack of the pore-forming cytolytic protein Prf expression by resting, ex vivo Pa MR1T cells (Figures 1E and 1F). Furthermore, this was in line with the observation that unprimed Pa MR1T cells expressed very low levels of TNF and IL-17 following similar stimulations (Figure 4).
Further analyses showed killing of PaKi cells pulsed with 5-OP-RU or riboflavin synthesis-competent E. coli strains correlated well with the proportion of Pa MR1T cells present in the co-culture following the in vitro expansion (Figure 6E). However, no such correlation was observed for PaKi cells pulsed with Ac-6-FP or riboflavin synthesis-deficient E. coli (Figure 6F). This suggested that killing of antigen- or bacteria-pulsed target cells was mediated by bona fide Pa MR1T cells, and not by residual non-MR1T cells present in the co-culture. Finally, to determine the MR1 dependence of Pa MR1T cell cytotoxicity, 5-OP-RU-pulsed 293T-hMR1 cells were used as target cells due to the ability of hMR1 to present cognate antigens to Pa MR1T cells (Figures S4C–S4E). Blocking MR1 using mAb clone 26.5 specific to hMR1 markedly reduced Pa MR1T cell activation and killing of 5-OP-RU-pulsed 293T-hMR1 cells (Figures 6G–6I), similar to the degree of inhibition of hMAIT cell-mediated cytotoxicity (Figures 6G and 6H). Blocking mAb specific to hMR1 did not appear to inhibit Pa MR1T or hMAIT cell activation and killing of 5-OP-RU-pulsed PaKi cells (Figures S4J–S4L), suggesting that the anti-hMR1 mAb clone 26.5 has little cross-reactivity to the Pa MR1. Collectively, these findings indicate that Pa MR1T cells mediate effector function and cellular cytotoxicity in a predominantly TCR-dependent manner against cells of Pa and human origins presenting the cognate riboflavin-related metabolite antigens via the restriction element MR1 (see Graphical Abstract).
Discussion
Bats are one of the most diverse mammalian orders, belonging to an ancient extant lineage of Eutheria in a phylogenetic lineage distinct from other higher eutherian mammals (Burgin et al., 2018; Zhang et al., 2013). Bats are natural reservoirs to numerous viruses, capable of causing zoonotic diseases in other mammals, without showing pathological signs of disease themselves (Letko et al., 2020). In recent years, bats are also increasingly recognized as significant reservoirs for antimicrobial-resistant bacterial organisms of human and animal health significance (Claudio et al., 2018; Garces et al., 2019; McDougall et al., 2019; Nowak et al., 2017; Nowakiewicz et al., 2020). Combined with the bats' ability of powered and sustained flight, their geographical ranges, and interactions with other animals, bats are therefore considered a significant threat for the spread of antimicrobial-resistant bacteria and emergence of zoonotic viruses capable of causing disease in humans and domesticated animals. The current COVID-19 pandemic caused by SARS-CoV-2, believed to have originated from the horseshoe bats (Boni et al., 2020; Zhou et al., 2020), acutely illustrates severe consequences to human health from interspecies transmission potential of zoonotic pathogens from bats.
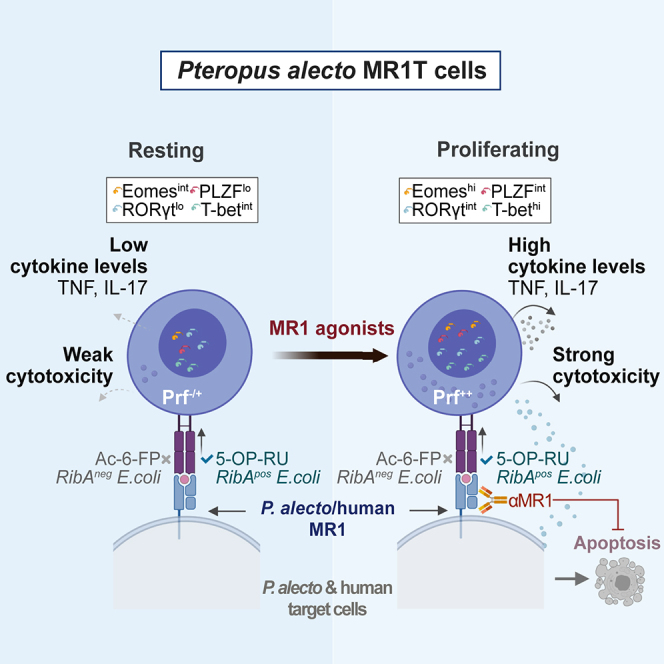
Studies of the immune system in bats are critical to understanding their responses and tolerance to microbial pathogens. In the current study, by using a set of tools and framework established in our laboratory, we investigated in detail the MR1T cell immune compartments in a pteropodid bat model, the fruit-eating black flying fox Pa. Consistent with the existence of the putative MR1 gene in Pa, we demonstrate for the first time the presence of an abundant population of MR1T cells in circulation and tissues of Pa. The Pa MR1T cells display phenotypic and functional characteristics that are consistent with those of hMAIT cells, including the expression of the master transcription factors PLZF and RORγt, as well as the T cell transcription factors T-bet and Eomes. Selective and vigorous proliferation was also observed following culture with the agonist MR1 ligand 5-OP-RU or riboflavin synthesis-competent strains of E. coli. Antigen-activated Pa MR1T cells expressed the pro-inflammatory cytokines TNF and IL-17, and strikingly, killed epithelial cell lines of both pteropodid bat and human origins pulsed with agonist MR1 ligand and riboflavin-producing E. coli. The ability of Pa MR1T cells to also kill target cell lines of human origin is consistent with high MR1 sequence homology between Pa and humans, and the MR1T cell functional xenoreactivity to various eutherian MR1 orthologs, as previously observed in hMAIT cells (Huang et al., 2009). Finally, blocking experiments established that Pa MR1T cell cytotoxicity is predominantly dependent on the restriction element MR1. Collectively, the presence of functionally-conserved MR1T cells in the pteropodid bats with hMAIT cell-like characteristics supports their important roles in the bat immune system. The strong evolutionary conservation of MR1 and MR1T cells in both humans and pteropodid bats presents also the possibility of a significant overlap in the selection pressure that maintains MR1 and MR1T cell population in humans and pteropodid bats.
The reason why pteropodid bats have hMAIT cell-like MR1T cell population beyond the putative selection and co-evolution with MR1 (Boudinot et al., 2016) is unclear. Previous studies show that frugivorous bats, including pteropodid bats, harbor large numbers of bacteria in their gastrointestinal tract dominated by large families of gram-negative bacteria, including Enterobacteriaceae, Moraxellaceae, and Pasteurellaceae (Buckles, 2015; Claudio et al., 2018; Heard et al., 1997; Henry et al., 2018). Members of these gram-negative families are capable in riboflavin biosynthesis (Constantinides et al., 2019; Gutierrez-Preciado et al., 2015; Schmaler et al., 2018; Tastan et al., 2018), a prerequisite for the production of the riboflavin-related metabolites (Corbett et al., 2014) driving MAIT cell development, expansion and activation (Constantinides et al., 2019; Legoux et al., 2019; Schmaler et al., 2018; Tastan et al., 2018). Interestingly, the transdermal application of sterile riboflavin-related antigenic metabolites alone is sufficient to induce MAIT cell development and promote their effector function in germ-free mice (Constantinides et al., 2019; Legoux et al., 2019). Thus, it is possible that dominance of riboflavin metabolite-producing bacteria in the gastrointestinal tract of frugivorous pteropodid bats promote the development and expansion of MR1T cells in their circulation and tissues.
Bats can be susceptible to a range of bacterial pathogens (Buckles, 2015; Helmick et al., 2004; Lei and Olival, 2014; Muhldorfer et al., 2011a, 2011b). Many of these bacteria can synthesize riboflavin, and therefore, it is tempting to speculate that the functionally-conserved MR1T cells in pteropodid bats may protect them from such microbial infections. These large families of gram-negative bacteria also often harbor strong antimicrobial resistance, and bats are suspected to serve as reservoirs for such antimicrobial-resistant bacterial pathogens of human health significance (Claudio et al., 2018; McDougall et al., 2019; Nowak et al., 2017; Nowakiewicz et al., 2020). While it is unclear whether the hMAIT cell-like MR1T cells in pteropid bats can mediate antimicrobial activity, the recent discovery that hMAIT cells mount potent antimicrobial activity and control antimicrobial-resistant bacterial pathogens (Boulouis et al., 2020b), posits the potential importance of the MR1T cell population in limiting the size of the drug-resistant bacterial reservoirs in bats.
Currently, there is a poor understanding how bat-borne pathogens rarely cause disease in bats. Several studies have proposed a more balanced host defense and tolerance system to viral infections in bats including the ‘flight as fever’ hypothesis (O'Shea et al., 2014; Zhang et al., 2013) and an ‘always on’ IFN system (Schountz et al., 2017), as well as an increased immune tolerance through dampened NLRP3-mediated inflammation (Ahn et al., 2019), reduced stimulator of interferon genes-dependent IFN response (Xie et al., 2018), and an overall inhibitory state of natural killer cells inferred from the genome analysis (Pavlovich et al., 2018). Our group previously demonstrated a dampened NLRP3-mediated inflammatory response to various of RNA viruses in bat immune cells, without any influence to viral infectivity (Ahn et al., 2019). This suggests an overall state of host-directed immune tolerance to minimize immunopathology and clinical signs of disease. Consistent with this hypothesis, we found that resting Pa MR1T cells at steady state failed to express significant levels of pro-inflammatory cytokines and did not mediate cytotoxicity following recognition of antigen. This is in contrast to hMAIT cells which readily mediate these effector functions, as shown here and elsewhere (Boulouis et al., 2020a; Dias et al., 2017b; Leeansyah et al., 2013, 2015). In humans, full activation of MAIT cells requires additional immune signaling beyond that of MR1-TCR interaction (Hinks and Zhang, 2020; Lamichhane et al., 2019; Slichter et al., 2016). These include stimuli by inflammasome-derived cytokines produced by myeloid cells, including IL-1β and IL-18 (Tang et al., 2013; Ussher et al., 2014). In Pa, there is a dampened inflammasome signaling and subsequent inflammasome-derived cytokine production by myeloid cells following stimulations with lipopolysaccharide (Ahn et al., 2019; Goh et al., 2020), a major cell wall component of E. coli. Thus, it is tempting to speculate that the subdued inflammasome response by Pa myeloid APC may account for the lack of immediate effector responses by resting Pa MR1T cells.
In MR1 ligand- and cytokine-primed Pa MR1T cells, there was robust production of pro-inflammatory cytokines and strong cytotoxicity in response to antigen, in contrast to resting Pa MR1T cells. This suggests that prolonged antigenic and supportive cytokine stimulations may break the immune tolerance ‘threshold’ of Pa MR1T cells and/or the Pa myeloid cells. Surprisingly, the expression of pro-inflammatory cytokines by previously primed Pa MR1T cells occurred in a relative burst of activity before rapidly declining to baseline levels within 12–24 h. This is in stark contrast to what is normally observed in hMAIT cells where there is persistent pro-inflammatory cytokine production beyond 24 h post-stimulation (Boulouis et al., 2020a; Dias et al., 2016; Lamichhane et al., 2019; Sattler et al., 2015; Tang et al., 2013). Thus, while primed Pa MR1T cells are functional and mediate effector functions, such activity is limited in duration. It is tempting to speculate that this may reduce the risk of collateral damage and immunopathology, while may still allowing effective control of pathogen loads.
In summary, our present study suggests that the highly conserved and abundant MR1T cells in circulation and tissues of Pa bats recognize MR1-restricted antigens and display a dampened MR1-dependent effector functionality, supporting the hypothesis of an enhanced innate immune tolerance in bats to minimize pathology and disease in bats as reservoir hosts.
Limitations of the Study
There are several questions remaining to be answered from the current study. Firstly, in the present study, CD3+ T cells in Pa bats were identified by intracellular staining with anti-CD3 mAb clone CD3-12, which detects a highly conserved epitope on the cytoplasmic domain of CD3ε (Martinez Gomez et al., 2016). Therefore, we were unable to FACS-sort live and highly pure Pa MR1T cell population to ascertain the invariant TCR usage of the Pa MR1T cells. Furthermore, there are challenges and technical difficulties in the assembly of hypervariable regions of the bat genome, including the MHC and TCR. Thus, the full repertoire of TCR sequences remains to be elucidated. Secondly, due to the lack of cross-reactive antibodies to pteropodid bats, the αβ and γδTCR as well as the CD4 and CD8 co-receptor usage, and expression of several other MAIT cell-related phenotypic and functional markers, including CD161, CD107a, GrzB, and IFNγ, could not be identified and measured.
Despite these limitations, all our available phenotypic and functional data on MR1 restriction, reactivity, and functional response strongly indicate that the T cell population in Pa circulation and tissues identified using the hMR1 tetramers indeed represent a bona fide MR1T cell population. In the present study, we further identified additional sets of tools that will help advance immunological studies in bats, which are often associated with technical challenges described herein. These include advances in the in vitro culture and expansion of Pa conventional T and MR1T cells through the use of human cytokines IL-2 and IL-7, and cytotoxicity assays that can be used in future studies investigating the bat cellular immune responses to various pathogens. It is currently unknown whether these human T cell homeostatic cytokines have differential effects on Pa MR1T and conventional T cell expansion and function. Ideally, this should be examined in conjunction with Pa-derived IL-2 and IL-7 that are not available for the present study. Going forward, it will be important to investigate the presence of MR1T cells in other bat species given their putative MR1 conservation, ideally using species-specific MR1 tetramers that are currently unavailable. Furthermore, the use of unloaded MR1 tetramer control should be included to confirm binding specificity of the riboflavin-related metabolite antigens to MR1T cells of bat origins. Finally, given that MAIT cells in humans respond to various viral infections (Provine and Klenerman, 2020), including to SARS-CoV-2 (Flament et al., 2020; Jouan et al., 2020; Parrot et al., 2020), future studies should explore whether these MR1T cell populations play a key role in controlling viral pathogens and dampening overt inflammatory responses to viral infections in bats, as well as their overall contribution to the bat immune system.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Edwin Leeansyah (edwin.leeansyah@sz.tsinghua.edu.cn)
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
No datasets or code were generated or analyzed in this study. All raw data associated with this study are available upon request from the Lead Contact. All software is commercially available.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Dr. Ted Hansen for the kind gift of the 293T-hMR1 cell line. The MR1 tetramer technology was developed jointly by Dr. James McCluskey, Dr. Jamie Rossjohn, and Dr. David Fairlie; and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne. The authors thank the following for help with bat sampling: Crameri Research Consulting, J. Meers, H. Field and Duke-NUS team members (for a detailed listing see Text S1). The research was supported by Swedish Research Council Grant 2015-00174, Marie Skłodowska-Curie Actions, Cofund, Project INCA 600398, the Jonas Söderquist Foundation for Virology and Immunology, and the Petrus and Augusta Hedlund Foundation (to EL), Singapore National Resarch Foundation grant (NRF2012NRF-CRP001-056), Singapore Ministry of Health (MOE2019-T2-2-130), Singapore National Medical Research Council (MOH-000386; OFIRG19NOV-0050) (to L-FW). Further support came from the Swedish Research Council Grant 2016–03052, Swedish Cancer Society Grant CAN 2017/777, and National Institutes of Health Grant R01DK108350 (to JKS), and CoSTAR-HS ARG Seed Fund 2018/02, –NMRC Collaborative centre grant NMRC/CG/C005B/2017_SGH (to ALHK). DPF acknowledges an ARC grant (CE140100011) and an NHMRC SPR Fellowship (1117017).
Author Contributions
Y.Y.H., W.R.S., J.H.J.N., M.Y.G., and C.B., M.A. performed the experiments. E.L., Y.Y.H., W.R.S., J.H.J.N., and Z.F. analyzed the data. E.L., Y.Y.H., and W.R.S. designed the experiments. J.Y.W.M., D.P.F., A.L.H.K., J.K.S., and L.-F.W. provided critical reagents. E.L. conceived the study. E.L. and L.-F.W. managed the study. E.L., Y.Y.H., W.R.S., J.K.S., and L.-F.W. wrote the paper, which was edited and approved by all authors.
Declaration of Interests
D.P.F. is an inventor on a patent application (PCT/AU2013/000742, WO2014005194) and J.Y.W.M. and D.P.F. are inventors on another patent application (PCT/AU2015/050148, WO2015149130) involving MR1 ligands for MR1-restricted MAIT cells owned by University of Queensland, Monash University and University of Melbourne. The other authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101876.
Contributor Information
Edwin Leeansyah, Email: edwin.leeansyah@sz.tsinghua.edu.cn.
Lin-Fa Wang, Email: linfa.wang@duke-nus.edu.sg.
Supplemental Information
References
- Ahn M., Anderson D.E., Zhang Q., Tan C.W., Lim B.L., Luko K., Wen M., Chia W.N., Mani S., Wang L.C. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat. Microbiol. 2019;4:789–799. doi: 10.1038/s41564-019-0371-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn M., Cui J., Irving A.T., Wang L.F. Unique loss of the PYHIN gene family in bats amongst mammals: implications for inflammasome sensing. Sci. Rep. 2016;6:21722. doi: 10.1038/srep21722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K.G., Rambaut A., Lipkin W.I., Holmes E.C., Garry R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020;26:450–452. doi: 10.1038/s41591-020-0820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni M.F., Lemey P., Jiang X., Lam T.T., Perry B.W., Castoe T.A., Rambaut A., Robertson D.L. Evolutionary origins of the SARS-CoV-2 sarbecovirus lineage responsible for the COVID-19 pandemic. Nat. Microbiol. 2020;5:1408–1417. doi: 10.1038/s41564-020-0771-4. [DOI] [PubMed] [Google Scholar]
- Boudinot P., Mondot S., Jouneau L., Teyton L., Lefranc M.P., Lantz O. Restricting nonclassical MHC genes coevolve with TRAV genes used by innate-like T cells in mammals. Proc. Natl. Acad. Sci. U S A. 2016;113:E2983–E2992. doi: 10.1073/pnas.1600674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulouis C., Gorin J.B., Dias J., Bergman P., Leeansyah E., Sandberg J.K. Opsonization-enhanced antigen presentation by MR1 activates rapid polyfunctional MAIT cell responses acting as an effector arm of humoral antibacterial immunity. J. Immunol. 2020;205:67–77. doi: 10.4049/jimmunol.2000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulouis C., Sia W.R., Gulam M.Y., Teo J.Q.M., Png Y.T., Phan T.K., Mak J.Y.W., Fairlie D.P., Poon I.K.H., Koh T.H. Human MAIT cell cytolytic effector proteins synergize to overcome carbapenem resistance in Escherichia coli. PLoS Biol. 2020;18:e3000644. doi: 10.1371/journal.pbio.3000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckles E.L. Chiroptera (bats) Fowler's Zoo Wild Anim. Med. 2015;8:281–290. [Google Scholar]
- Burgin C.J., Colella J.P., Kahn P.L., Upham N.S. How many species of mammals are there? J. Mammal. 2018;99:1–14. [Google Scholar]
- Claudio V.C., Gonzalez I., Barbosa G., Rocha V., Moratelli R., Rassy F. Bacteria richness and antibiotic-resistance in bats from a protected area in the Atlantic Forest of Southeastern Brazil. PLoS One. 2018;13:e0203411. doi: 10.1371/journal.pone.0203411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides M.G., Link V.M., Tamoutounour S., Wong A.C., Perez-Chaparro P.J., Han S.J., Chen Y.E., Li K., Farhat S., Weckel A. MAIT cells are imprinted by the microbiota in early life and promote tissue repair. Science. 2019;366:eaax6624. doi: 10.1126/science.aax6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett A.J., Eckle S.B., Birkinshaw R.W., Liu L., Patel O., Mahony J., Chen Z., Reantragoon R., Meehan B., Cao H. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature. 2014;509:361–365. doi: 10.1038/nature13160. [DOI] [PubMed] [Google Scholar]
- Dias J., Leeansyah E., Sandberg J.K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl. Acad. Sci. U S A. 2017;114:E5434–E5443. doi: 10.1073/pnas.1705759114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias J., Sandberg J.K., Leeansyah E. Extensive phenotypic analysis, transcription factor profiling, and effector cytokine production of human MAIT cells by flow cytometry. Methods Mol. Biol. 2017;1514:241–256. doi: 10.1007/978-1-4939-6548-9_17. [DOI] [PubMed] [Google Scholar]
- Dias J., Sobkowiak M.J., Sandberg J.K., Leeansyah E. Human MAIT-cell responses to Escherichia coli: activation, cytokine production, proliferation, and cytotoxicity. J. Leukoc. Biol. 2016;100:233–240. doi: 10.1189/jlb.4TA0815-391RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusseaux M., Martin E., Serriari N., Peguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood. 2011;117:1250–1259. doi: 10.1182/blood-2010-08-303339. [DOI] [PubMed] [Google Scholar]
- Ellis A.L., Balgeman A.J., Larson E.C., Rodgers M.A., Ameel C., Baranowski T., Kannal N., Maiello P., Juno J.A., Scanga C.A. MAIT cells are functionally impaired in a Mauritian cynomolgus macaque model of SIV and Mtb co-infection. PLoS Pathog. 2020;16:e1008585. doi: 10.1371/journal.ppat.1008585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flament H., Rouland M., Beaudoin L., Toubal A., Bertrand L., Lebourgeois S., Gouda Z., Rousseau C., Soulard P., Hurtado-Nedelec M. Outcome of SARS-CoV-2 infection linked to MAIT cell activation and cytotoxicity: evidence for an IL-18 dependent mechanism. medRxiv. 2020 [Google Scholar]
- Gamage A.M., Zhu F., Ahn M., Foo R.J.H., Hey Y.Y., Low D.H.W., Mendenhall I.H., Dutertre C.A., Wang L.F. Immunophenotyping monocytes, macrophages and granulocytes in the Pteropodid bat Eonycteris spelaea. Sci. Rep. 2020;10:309. doi: 10.1038/s41598-019-57212-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garces A., Correia S., Amorim F., Pereira J.E., Igrejas G., Poeta P. First report on extended-spectrum beta-lactamase (ESBL) producing Escherichia coli from European free-tailed bats (Tadarida teniotis) in Portugal: a one-health approach of a hidden contamination problem. J. Hazard. Mater. 2019;370:219–224. doi: 10.1016/j.jhazmat.2017.12.053. [DOI] [PubMed] [Google Scholar]
- Gherardin N.A., Keller A.N., Woolley R.E., Le Nours J., Ritchie D.S., Neeson P.J., Birkinshaw R.W., Eckle S.B., Waddington J.N., Liu L. Diversity of T cells restricted by the MHC class I-related molecule MR1 facilitates differential antigen recognition. Immunity. 2016;44:32–45. doi: 10.1016/j.immuni.2015.12.005. [DOI] [PubMed] [Google Scholar]
- Gibbs A., Leeansyah E., Introini A., Paquin-Proulx D., Hasselrot K., Andersson E., Broliden K., Sandberg J.K., Tjernlund A. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 2017;10:35–45. doi: 10.1038/mi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey D.I., Koay H.F., McCluskey J., Gherardin N.A. The biology and functional importance of MAIT cells. Nat. Immunol. 2019;20:1110–1128. doi: 10.1038/s41590-019-0444-8. [DOI] [PubMed] [Google Scholar]
- Goh G., Ahn M., Zhu F., Lee L.B., Luo D., Irving A.T., Wang L.F. Complementary regulation of caspase-1 and IL-1beta reveals additional mechanisms of dampened inflammation in bats. Proc. Natl. Acad. Sci. U S A. 2020;117:28939–28949. doi: 10.1073/pnas.2003352117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinch N., Reinink P., Connelley T., Koets A., Morrison I., Van Rhijn I. Conservation of mucosal associated invariant T (MAIT) cells and the MR1 restriction element in ruminants, and abundance of MAIT cells in spleen. Vet. Res. 2010;41:62. doi: 10.1051/vetres/2010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Preciado A., Torres A.G., Merino E., Bonomi H.R., Goldbaum F.A., Garcia-Angulo V.A. Extensive identification of bacterial riboflavin transporters and their distribution across bacterial species. PLoS One. 2015;10:e0126124. doi: 10.1371/journal.pone.0126124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heard D.J., De Young J.L., Goodyear B., Ellis G.A. Comparative rectal bacterial flora of four species of flying fox (Pteropus sp.) J. Zoo. Wildl. Med. 1997;28:471–475. [PubMed] [Google Scholar]
- Helmick K.E., Heard D.J., Richey L., Finnegan M., Ellis G.A., Nguyen A., Tucker L., Weyant R.S. A Pasteurella-like bacterium associated with pneumonia in captive megachiropterans. J. Zoo. Wildl. Med. 2004;35:88–93. doi: 10.1638/01-083. [DOI] [PubMed] [Google Scholar]
- Henry R., Galbraith P., Coutts S., Prosser T., Boyce J., McCarthy D.T. What's the risk? Identifying potential human pathogens within grey-headed flying foxes faeces. PLoS ONE. 2018;13:e0191301. doi: 10.1371/journal.pone.0191301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks T.S.C., Marchi E., Jabeen M., Olshansky M., Kurioka A., Pediongco T.J., Meehan B.S., Kostenko L., Turner S.J., Corbett A.J. Activation and in vivo evolution of the MAIT cell transcriptome in mice and humans reveals tissue repair functionality. Cell Rep. 2019;28:3249–3262.e5. doi: 10.1016/j.celrep.2019.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinks T.S.C., Zhang X.W. MAIT cell activation and functions. Front. Immunol. 2020;11:1014. doi: 10.3389/fimmu.2020.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howson L.J., Napolitani G., Shepherd D., Ghadbane H., Kurupati P., Preciado-Llanes L., Rei M., Dobinson H.C., Gibani M.M., Teng K.W.W. MAIT cell clonal expansion and TCR repertoire shaping in human volunteers challenged with Salmonella Paratyphi A. Nat. Commun. 2018;9:253. doi: 10.1038/s41467-017-02540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Gilfillan S., Cella M., Miley M.J., Lantz O., Lybarger L., Fremont D.H., Hansen T.H. Evidence for MR1 antigen presentation to mucosal-associated invariant T cells. J. Biol. Chem. 2005;280:21183–21193. doi: 10.1074/jbc.M501087200. [DOI] [PubMed] [Google Scholar]
- Huang S., Martin E., Kim S., Yu L., Soudais C., Fremont D.H., Lantz O., Hansen T.H. MR1 antigen presentation to mucosal-associated invariant T cells was highly conserved in evolution. Proc. Natl. Acad. Sci. U S A. 2009;106:8290–8295. doi: 10.1073/pnas.0903196106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jouan Y., Guillon A., Gonzalez L., Perez Y., Boisseau C., Ehrmann S., Ferreira M., Daix T., Jeannet R., Francois B. Phenotypical and functional alteration of unconventional T cells in severe COVID-19 patients. J. Exp. Med. 2020;217:e20200872. doi: 10.1084/jem.20200872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjer-Nielsen L., Patel O., Corbett A.J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–723. doi: 10.1038/nature11605. [DOI] [PubMed] [Google Scholar]
- Koay H.F., Gherardin N.A., Enders A., Loh L., Mackay L.K., Almeida C.F., Russ B.E., Nold-Petry C.A., Nold M.F., Bedoui S. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 2016;17:1300–1311. doi: 10.1038/ni.3565. [DOI] [PubMed] [Google Scholar]
- Koay H.F., Su S., Amann-Zalcenstein D., Daley S.R., Comerford I., Miosge L., Whyte C.E., Konstantinov I.E., d'Udekem Y., Baldwin T. A divergent transcriptional landscape underpins the development and functional branching of MAIT cells. Sci. Immunol. 2019;4:eaay6039. doi: 10.1126/sciimmunol.aay6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka A., Ussher J.E., Cosgrove C., Clough C., Fergusson J.R., Smith K., Kang Y.H., Walker L.J., Hansen T.H., Willberg C.B. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 2015;8:429–440. doi: 10.1038/mi.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane R., Schneider M., de la Harpe S.M., Harrop T.W.R., Hannaway R.F., Dearden P.K., Kirman J.R., Tyndall J.D.A., Vernall A.J., Ussher J.E. TCR- or cytokine-activated CD8(+) mucosal-associated invariant T cells are rapid polyfunctional effectors that can coordinate immune responses. Cell Rep. 2019;28:3061–3076.e5. doi: 10.1016/j.celrep.2019.08.054. [DOI] [PubMed] [Google Scholar]
- Le Bourhis L., Dusseaux M., Bohineust A., Bessoles S., Martin E., Premel V., Core M., Sleurs D., Serriari N.E., Treiner E. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLoS Pathog. 2013;9:e1003681. doi: 10.1371/journal.ppat.1003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourhis L., Martin E., Peguillet I., Guihot A., Froux N., Core M., Levy E., Dusseaux M., Meyssonnier V., Premel V. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 2010;11:701–708. doi: 10.1038/ni.1890. [DOI] [PubMed] [Google Scholar]
- Leeansyah E., Ganesh A., Quigley M.F., Sonnerborg A., Andersson J., Hunt P.W., Somsouk M., Deeks S.G., Martin J.N., Moll M. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood. 2013;121:1124–1135. doi: 10.1182/blood-2012-07-445429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeansyah E., Loh L., Nixon D.F., Sandberg J.K. Acquisition of innate-like microbial reactivity in mucosal tissues during human fetal MAIT-cell development. Nat. Commun. 2014;5:3143. doi: 10.1038/ncomms4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeansyah E., Svard J., Dias J., Buggert M., Nystrom J., Quigley M.F., Moll M., Sonnerborg A., Nowak P., Sandberg J.K. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLoS Pathog. 2015;11:e1005072. doi: 10.1371/journal.ppat.1005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legoux F., Bellet D., Daviaud C., El Morr Y., Darbois A., Niort K., Procopio E., Salou M., Gilet J., Ryffel B. Microbial metabolites control the thymic development of mucosal-associated invariant T cells. Science. 2019;366:494–499. doi: 10.1126/science.aaw2719. [DOI] [PubMed] [Google Scholar]
- Lei B.R., Olival K.J. Contrasting patterns in mammal-bacteria coevolution: bartonella and leptospira in bats and rodents. PLoS Negl. Trop. Dis. 2014;8:e2738. doi: 10.1371/journal.pntd.0002738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng T., Akther H.D., Hackstein C.P., Powell K., King T., Friedrich M., Christoforidou Z., McCuaig S., Neyazi M., Arancibia-Carcamo C.V. TCR and inflammatory signals tune human MAIT cells to exert specific tissue repair and effector functions. Cell Rep. 2019;28:3077–3091.e5. doi: 10.1016/j.celrep.2019.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M., Seifert S.N., Olival K.J., Plowright R.K., Munster V.J. Bat-borne virus diversity, spillover and emergence. Nat. Rev. Microbiol. 2020;18:461–471. doi: 10.1038/s41579-020-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luis A.D., Hayman D.T., O'Shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R., Mills J.N., Timonin M.E., Willis C.K., Cunningham A.A. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special? Proc. Biol. Sci. 2013;280:20122753. doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak J.Y., Xu W., Reid R.C., Corbett A.J., Meehan B.S., Wang H., Chen Z., Rossjohn J., McCluskey J., Liu L. Stabilizing short-lived Schiff base derivatives of 5-aminouracils that activate mucosal-associated invariant T cells. Nat. Commun. 2017;8:14599. doi: 10.1038/ncomms14599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E., Treiner E., Duban L., Guerri L., Laude H., Toly C., Premel V., Devys A., Moura I.C., Tilloy F. Stepwise development of MAIT cells in mouse and human. PLoS Biol. 2009;7:e54. doi: 10.1371/journal.pbio.1000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Gomez J.M., Periasamy P., Dutertre C.A., Irving A.T., Ng J.H., Crameri G., Baker M.L., Ginhoux F., Wang L.F., Alonso S. Phenotypic and functional characterization of the major lymphocyte populations in the fruit-eating bat Pteropus alecto. Sci. Rep. 2016;6:37796. doi: 10.1038/srep37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall F., Boardman W., Gillings M., Power M. Bats as reservoirs of antibiotic resistance determinants: a survey of class 1 integrons in Grey-headed Flying Foxes (Pteropus poliocephalus) Infect. Genet. Evol. 2019;70:107–113. doi: 10.1016/j.meegid.2019.02.022. [DOI] [PubMed] [Google Scholar]
- Meierovics A., Yankelevich W.J., Cowley S.C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl. Acad. Sci. U S A. 2013;110:E3119–E3128. doi: 10.1073/pnas.1302799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondot S., Boudinot P., Lantz O. MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules? Immunogenetics. 2016;68:537–548. doi: 10.1007/s00251-016-0927-9. [DOI] [PubMed] [Google Scholar]
- Muhldorfer K., Schwarz S., Fickel J., Wibbelt G., Speck S. Genetic diversity of Pasteurella species isolated from European vespertilionid bats. Vet. Microbiol. 2011;149:163–171. doi: 10.1016/j.vetmic.2010.10.002. [DOI] [PubMed] [Google Scholar]
- Muhldorfer K., Speck S., Kurth A., Lesnik R., Freuling C., Muller T., Kramer-Schadt S., Wibbelt G. Diseases and causes of death in European bats: dynamics in disease susceptibility and infection rates. PLoS One. 2011;6:e29773. doi: 10.1371/journal.pone.0029773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak K., Fahr J., Weber N., Lubke-Becker A., Semmler T., Weiss S., Mombouli J.V., Wieler L.H., Guenther S., Leendertz F.H. Highly diverse and antimicrobial susceptible Escherichia coli display a naive bacterial population in fruit bats from the Republic of Congo. PLoS One. 2017;12:e0178146. doi: 10.1371/journal.pone.0178146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakiewicz A., Zieba P., Gnat S., Troscianczyk A., Osinska M., Lagowski D., Kosior-Korzecka U., Puzio I. Bats as a reservoir of resistant Escherichia coli: a methodical view. Can we fully estimate the scale of resistance in the reservoirs of free-living animals? Res. Vet. Sci. 2020;128:49–58. doi: 10.1016/j.rvsc.2019.10.017. [DOI] [PubMed] [Google Scholar]
- O'Shea T.J., Cryan P.M., Cunningham A.A., Fooks A.R., Hayman D.T., Luis A.D., Peel A.J., Plowright R.K., Wood J.L. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 2014;20:741–745. doi: 10.3201/eid2005.130539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papenfuss A.T., Baker M.L., Feng Z.P., Tachedjian M., Crameri G., Cowled C., Ng J., Janardhana V., Field H.E., Wang L.F. The immune gene repertoire of an important viral reservoir, the Australian black flying fox. BMC Genom. 2012;13:261. doi: 10.1186/1471-2164-13-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot T., Gorin J.B., Ponzetta A., Maleki K.T., Kammann T., Emgard J., Perez-Potti A., Sekine T., Rivera-Ballesteros O., Karolinska C.-S.G. MAIT cell activation and dynamics associated with COVID-19 disease severity. Sci. Immunol. 2020;5:eabe1670. doi: 10.1126/sciimmunol.abe1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovich S.S., Lovett S.P., Koroleva G., Guito J.C., Arnold C.E., Nagle E.R., Kulcsar K., Lee A., Thibaud-Nissen F., Hume A.J. The Egyptian rousette genome reveals unexpected features of bat antiviral immunity. Cell. 2018;173:1098–1110.e18. doi: 10.1016/j.cell.2018.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Periasamy P., Hutchinson P.E., Chen J., Bonne I., Shahul Hameed S.S., Selvam P., Hey Y.Y., Fink K., Irving A.T., Dutertre C.A. Studies on B cells in the fruit-eating black flying fox (Pteropus alecto) Front. Immunol. 2019;10:489. doi: 10.3389/fimmu.2019.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provine N.M., Klenerman P. MAIT cells in health and disease. Annu. Rev. Immunol. 2020;38:203–228. doi: 10.1146/annurev-immunol-080719-015428. [DOI] [PubMed] [Google Scholar]
- Rahimpour A., Koay H.F., Enders A., Clanchy R., Eckle S.B., Meehan B., Chen Z., Whittle B., Liu L., Fairlie D.P. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 2015;212:1095–1108. doi: 10.1084/jem.20142110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegert P., Wanner V., Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J. Immunol. 1998;161:4066–4077. [PubMed] [Google Scholar]
- Salerno-Goncalves R., Luo D., Fresnay S., Magder L., Darton T.C., Jones C., Waddington C.S., Blohmke C.J., Angus B., Levine M.M. Challenge of humans with wild-type salmonella enterica serovar typhi elicits changes in the activation and homing characteristics of mucosal-associated invariant T cells. Front. Immunol. 2017;8:398. doi: 10.3389/fimmu.2017.00398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler A., Dang-Heine C., Reinke P., Babel N. IL-15 dependent induction of IL-18 secretion as a feedback mechanism controlling human MAIT-cell effector functions. Eur. J. Immunol. 2015;45:2286–2298. doi: 10.1002/eji.201445313. [DOI] [PubMed] [Google Scholar]
- Savage A.K., Constantinides M.G., Han J., Picard D., Martin E., Li B., Lantz O., Bendelac A. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29:391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaler M., Colone A., Spagnuolo J., Zimmermann M., Lepore M., Kalinichenko A., Bhatia S., Cottier F., Rutishauser T., Pavelka N. Modulation of bacterial metabolism by the microenvironment controls MAIT cell stimulation. Mucosal Immunol. 2018;11:1060–1070. doi: 10.1038/s41385-018-0020-9. [DOI] [PubMed] [Google Scholar]
- Schountz T., Baker M.L., Butler J., Munster V. Immunological control of viral infections in bats and the emergence of viruses highly pathogenic to humans. Front. Immunol. 2017;8:1098. doi: 10.3389/fimmu.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichter C.K., McDavid A., Miller H.W., Finak G., Seymour B.J., McNevin J.P., Diaz G., Czartoski J.L., McElrath M.J., Gottardo R. Distinct activation thresholds of human conventional and innate-like memory T cells. JCI Insight. 2016;1:e86292. doi: 10.1172/jci.insight.86292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X.Z., Jo J., Tan A.T., Sandalova E., Chia A., Tan K.C., Lee K.H., Gehring A.J., De Libero G., Bertoletti A. IL-7 licenses activation of human liver intrasinusoidal mucosal-associated invariant T cells. J. Immunol. 2013;190:3142–3152. doi: 10.4049/jimmunol.1203218. [DOI] [PubMed] [Google Scholar]
- Tastan C., Karhan E., Zhou W., Fleming E., Voigt A.Y., Yao X., Wang L., Horne M., Placek L., Kozhaya L. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T-cell presentation. Mucosal Immunol. 2018;11:1591–1605. doi: 10.1038/s41385-018-0072-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature. 2003;422:164–169. doi: 10.1038/nature01433. [DOI] [PubMed] [Google Scholar]
- Tsagkogeorga G., Parker J., Stupka E., Cotton J.A., Rossiter S.J. Phylogenomic analyses elucidate the evolutionary relationships of bats. Curr. Biol. 2013;23:2262–2267. doi: 10.1016/j.cub.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Tsukamoto K., Deakin J.E., Graves J.A., Hashimoto K. Exceptionally high conservation of the MHC class I-related gene, MR1, among mammals. Immunogenetics. 2013;65:115–124. doi: 10.1007/s00251-012-0666-5. [DOI] [PubMed] [Google Scholar]
- Ussher J.E., Bilton M., Attwod E., Shadwell J., Richardson R., de Lara C., Mettke E., Kurioka A., Hansen T.H., Klenerman P. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 2014;44:195–203. doi: 10.1002/eji.201343509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wilgenburg B., Loh L., Chen Z., Pediongco T.J., Wang H., Shi M., Zhao Z., Koutsakos M., Nussing S., Sant S. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 2018;9:4706. doi: 10.1038/s41467-018-07207-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., D'Souza C., Lim X.Y., Kostenko L., Pediongco T.J., Eckle S.B.G., Meehan B.S., Shi M., Wang N., Li S. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun. 2018;9:3350. doi: 10.1038/s41467-018-05202-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kjer-Nielsen L., Shi M., D'Souza C., Pediongco T.J., Cao H., Kostenko L., Lim X.Y., Eckle S.B.G., Meehan B.S. IL-23 costimulates antigen-specific MAIT cell activation and enables vaccination against bacterial infection. Sci. Immunol. 2019;4:eaaw0402. doi: 10.1126/sciimmunol.aaw0402. [DOI] [PubMed] [Google Scholar]
- Wang L.F., Walker P.J., Poon L.L. Mass extinctions, biodiversity and mitochondrial function: are bats 'special' as reservoirs for emerging viruses? Curr. Opin. Virol. 2011;1:649–657. doi: 10.1016/j.coviro.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G.S., South J.M. Life history, ecology and longevity in bats. Aging Cell. 2002;1:124–131. doi: 10.1046/j.1474-9728.2002.00020.x. [DOI] [PubMed] [Google Scholar]
- Wynne J.W., Wang L.F. Bats and viruses: friend or foe? PLoS Pathog. 2013;9:e1003651. doi: 10.1371/journal.ppat.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X., Li K., Ma X., Liu B., He X., Yang S., Wang W., Jiang B., Cai J. Mucosal-associated invariant T cells expressing the TRAV1-TRAJ33 chain are present in pigs. Front. Immunol. 2019;10:2070. doi: 10.3389/fimmu.2019.02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J., Li Y., Shen X., Goh G., Zhu Y., Cui J., Wang L.F., Shi Z.L., Zhou P. Dampened STING-dependent interferon activation in bats. Cell Host Microbe. 2018;23:297–301.e4. doi: 10.1016/j.chom.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Cowled C., Shi Z., Huang Z., Bishop-Lilly K.A., Fang X., Wynne J.W., Xiong Z., Baker M.L., Zhao W. Comparative analysis of bat genomes provides insight into the evolution of flight and immunity. Science. 2013;339:456–460. doi: 10.1126/science.1230835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets or code were generated or analyzed in this study. All raw data associated with this study are available upon request from the Lead Contact. All software is commercially available.