Highlights
-
•
Cardiovascular health decline occurs during adolescence.
-
•
Black-white disparities begin before early adolescence.
-
•
Disparities are associated with differential cardiovascular health trajectories.
-
•
Modifying early adolescent behaviors may prevent cardiovascular health decline.
Keywords: Cardiovascular disease, Risk factors, Pediatrics, Women
Abbreviations: CVH, cardiovascular health; CVD, cardiovascular disease; BMI, body mass index; NGHS, National Growth and Health Study; AHA, American Heart Association; MET, metabolic equivalent; HEI, Health Eating Index
Abstract
Adolescence is a critical time for the preservation or loss of cardiovascular health. We aimed to describe trajectories of cardiovascular health in adolescent girls and identify early adolescent factors associated with cardiovascular health in young adulthood. We used data from the National Growth and Health Study, a longitudinal cohort of 2,379 girls followed annually from ages 9–19 years. We classified participants as having ideal, intermediate, or poor levels of the seven cardiovascular health metrics at four developmental stages: early (ages 9–11), middle (ages 12–14), and late (ages 15–17) adolescence, and early young adulthood (ages ≥ 18). We calculated total cardiovascular health scores (range 0–14) at each stage and empirically identified patterns of cardiovascular health trajectories. We examined associations between trajectory group membership and various demographic, behavioral, and physiological factors. Mean cardiovascular health scores declined with age from 10.8 to 9.4 in white girls and 10.3 to 8.9 in black girls; 17% of white girls and 23% of black girls had low cardiovascular health (score < 8) by early young adulthood. We identified five cardiovascular health trajectories: high-stable (14% of participants), high-to-moderate (48%), high-to-low (20%), moderate-stable (10%), and moderate-to-low (8%). Exceeding 14 h per week of television in early adolescence and teen pregnancy were associated with higher odds of being in several less healthy trajectory groups. In conclusion, cardiovascular health declines during adolescence and black-white disparities begin before early adolescence. Key targets for improving cardiovascular health in adolescent girls may include reductions in sedentary behavior and prevention of teen pregnancy.
1. Introduction
Adults who reach middle age with ideal cardiovascular health (CVH) live longer, healthier lives (Wilkins et al., 2012) free from cardiovascular disease (CVD) (Fang et al., 2016) and other chronic illnesses (Fang et al., 2016, Kulshreshtha et al., 2013, Muntner et al., 2013, Colangelo et al., 2011, Ogunmoroti et al., 2017). Ideal CVH includes seven metrics: optimal dietary patterns, regular physical activity, abstinence from tobacco, a healthy body mass index (BMI), and optimal levels of blood pressure, blood cholesterol, and blood glucose (Lloyd-Jones et al., 2010). While 45% of children in the US under age 20 meet ideal levels of at least five CVH metrics, the prevalence drops to 31% for adults ages 20–39 and 10% for adults over age 40 (Benjamin et al., 2019). A pooled analysis of five prospective cohort studies spanning from childhood to adulthood recently identified distinct trajectories of CVH loss, with earlier loss of CVH associated with subclinical atherosclerosis in middle age (Allen et al., 2016).
In this same pooled cohort analysis, late adolescence appeared to be a key period for CVH loss (Allen et al., 2018). Many changes occur during adolescence that affect CVH including changes in fat distribution (De Ridder et al., 1992), insulin resistance (Hannon et al., 2006), blood pressure (Shankar et al., 2005), and lipid levels (Eissa et al., 2016). Neurobiological changes during puberty and young adulthood (Gogtay et al., 2004) alter adolescents’ cognitive and affective states, decision making, and risk taking behaviors (Guyer et al., 2016). Psychologically, adolescents demonstrate emerging independence (Arnett, 2000) and a strong peer group orientation (Gardner and Steinberg, 2005). Together these changes can affect the CVH metrics of diet, physical activity, and tobacco use. Additionally, many conditions known to affect CVH first occur during adolescence, including pregnancy (Amin et al., 2017) and depression (Avenevoli et al., 2015).
The National Growth and Health Study (NGHS), a US-based cohort of 2379 girls followed annually from ages 9–19 years during 1987–1997, presents a unique opportunity to study more detailed CVH trajectories in adolescence. The cohort was established to identify the emergence of racial disparities in obesity and related cardiovascular risk factors in adolescent girls (Obesity and cardiovascular disease, 1992). Key findings from NGHS include greater increases in body fat (Kimm et al., 2004) and obesity (Kimm et al., 2004) and greater declines in physical activity (Kimm et al., 2002) in black compared to white girls. In this study, we aimed to leverage the rich repeated measures data in NGHS in order to 1) describe trajectories of CVH in adolescent girls ages 9–19 years in the NGHS cohort, and 2) identify early and late adolescent factors associated with CVH trajectories.
2. Methods
The NGHS enrolled 1213 black girls and 1166 white girls from Cincinnati, OH, Richmond, CA, and Washington, DC in 1987–1988. The original NGHS protocol was approved by the institutional review board at each participating center; parents provided informed consent and participants provided informed assent. Girls were ages 9–10 at recruitment and were followed annually with in-person examinations for ten years. All NGHS visits were scheduled at least 4 months post-partum for participants experiencing pregnancy during the study. The current study uses publicly available data from the National Heart, Lung, and Blood Institute’s Biolincc repository; the Boston Children’s Hospital Committee on Clinical Investigation and Biolincc approved the use of the data for this study. Further study information is available at https://biolincc.nhlbi.nih.gov/studies/nghs/.
2.1. Definitions of cardiovascular health metrics
We used the American Heart Association (AHA) definitions for CVH metrics for children (Lloyd-Jones et al., 2010), with minor adaptations based on variables collected in NGHS (supplementary Table 1). Girls were asked whether they had ever tried a cigarette starting at visits 1–5, with more detailed smoking histories taken at visits 6–10. Physical activity was measured at visits 1, 3, 5, 7–10 using a Habitual Activity Questionnaire; the metabolic equivalent (MET) and frequency of each reported activity were used to calculate an average score of MET-times per week. Girls were classified as having ideal (≥20 MET-times per week), intermediate (10 ≤ MET-times per week < 20), or poor (<10 MET-times per week) physical activity based on previous NGHS publications defining these groups as active, moderately active, and inactive, respectively (Kimm et al., 2005). Dietary intake was measured with a three-day food diary after participant training by a certified nutritionist at visits 1–5, 7, 8, 10 and analyzed for nutrient composition using the Nutrition Data System from the University of Minnesota’s Nutrition Coordinating Center. Food codes were matched to the United States Department of Agriculture database to produce daily intakes of major food groups used to calculate a 2015 Health Eating Index (HEI-2015) (Krebs-Smith et al., 2018). We used the top decile of HEI-2015 score for the study population from the first study visit to set the score for ideal diet throughout the remaining visits, given that < 1% of adolescents report an ideal diet based on the AHA definition (Benjamin et al., 2019); the 2nd-5th deciles defined the intermediate diet score and the bottom five deciles defined the poor diet score.
Height and weight were measured at each visit with a standard stadiometer and electronic scale; BMI was calculated as kg/m2 and classified according to the Centers for Disease Control 2000 BMI percentiles for children. Blood pressure was measured annually by a trained technician using a standard mercury sphygmomanometer; the mean of the second and third measurement was entered as the blood pressure for that visit. Fasting total cholesterol was measured at visits 1, 3, 5, 7, and 10; fasting glucose was measured at visis 7 and 10.
We classified each participant as having ideal (score = 2), intermediate (score = 1), or poor (score = 0) levels of each of the seven metrics (Supplementary Table 1) at each available study visit. Given the high proportion of girls (≥97%) with ideal glucose levels at study visit 7, we assigned ideal glucose status to all girls at earlier visits when glucose was not measured. For each of the other six metrics, if the metric was unavailable at a given visit we imputed the metric by substituting the lowest of the two surrounding visits. If no data were available for the given metric at the visit before or after, we considered the metric missing. The number of girls with measured, imputed, and missing data for each of the seven metrics at each visit year is reported in Supplementary Table 2. We then summed the scores on the seven individual metrics to create a total CVH score ranging from 0 to 14 for each participant at each visit. We included participants who had at least one summed total CVH score during any study visit, giving an analytic sample size of 2259 girls.
We created four developmental stage groupings representing early (ages 9–11), middle (ages 12–14), and late (ages 15–17) adolescence and early young adulthood (ages ≥ 18). If a participant had a total CVH score recorded at more than one study visit during a given developmental stage, we used the lower (less ideal) CVH score as the score for that stage. We classified the total CVH score for each developmental stage as low (0–7), moderate (8–11), or high (12–14) based on previous studies in adults demonstrating a graded relationship between these classifications and subclinical atherosclerosis (Polonsky et al., 2017).
2.2. Definitions of covariates
We defined early menarche as occurrence of the first menstrual period before age 12 (Kaplowitz and Oberfield, 1999). We defined participants as regular breakfast eaters in early adolescence if they reported “yes” to the question “On school days do you usually eat breakfast?” at the first study visit. We defined participants as exceeding the recommended amount of television viewing in early adolescence if they reported watching more than 14 h of television per week at the first study visit, based on American Academy of Pediatrics recommendations to limit screen time to <2 h per day (Education, 1999). We defined depressive symptoms as a score of >20 on the Center for Epidemiological Studies Depression scale (Vilagut et al., 2016) when assessed at visits 8 or 10. We defined teen pregnancy as any pregnancy occurring during the study period. Parents reported their highest level of education and total family income at the baseline study visit.
2.3. Statistical approach
We used latent class trajectory modeling to identify and categorize NGHS participants based on patterns of longitudinal change in the total CVH score during the four pre-specified developmental periods. A total of 1971 participants with a CVH score from at least three study visits were eligible for the trajectory analysis. Using a customized SAS macro, PROC TRAJ, we explored the distinct patterns in longitudinal change in the total CVH score over time. First, we determined CVH score trajectories as a function of age using PROC TRAJ. Next, we used Bayesian Information Criterion (BIC) posterior probabilities to evaluate the fit of the most parsimonious model, choosing the best model as the one with the smallest negative BIC. We assigned participants to the trajectory class for which they had the highest Posterior Predicted Probability (PPP). Our trajectory model had high PPPs ranging from 0.74 to 0.90, indicating that each study participant had a high probability of belonging to one particular trajectory, and a low probability of belonging to the others (Supplementary Table 3).
We compared demographic characteristics and covariates for each of the CVH trajectory groups using chi-square tests and t-tests. We created multinomial logistic regression models to estimate the odds of CVH trajectory group membership based on each of the covariates of interest, with the most favorable CVH trajctory group serving as the referent. We further adjusted the models for demographic characteristics and formally tested for interaction between each covariate and race. A p-value < 0.05 was considered statistically significant. All analyses used SAS® 9.4 (SAS Institute, Cary, N.C., USA)
3. Results
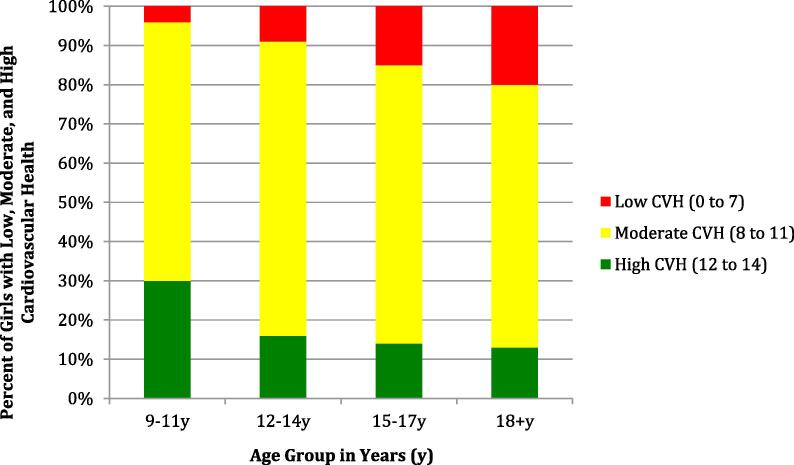
Demographic characteristics and key health indicators for study participants are available in Table 1; all comparisons by race were statistically significant. The mean CVH score declined from 10.6 (SD 1.6) in early adolescence to 9.9 (SD 1.7) in middle adolescence, to 9.5 (SD 1.9) in late adolescence and finally to 9.1 (SD 2.1) in young adulthood. At each developmental stage, black girls had a mean CVH score approximately 0.5 points lower than white girls (p < 0.001 for comparison by race at each time point). Only 30% of girls entered adolescence with high CVH scores of 12–14 and the prevalence of high CVH declined at each subsequent developmental stage (Fig. 1). This loss of total CVH was related to worsening of all CVH metrics except cholesterol, with the greatest loss of ideal CVH seen in the blood pressure, physical activity, and smoking metrics (supplemental Fig. 1a–g). Compared to white participants, black participants were more likely to lose ideal status on each metric except smoking.
Table 1.
Characteristics of 2259 girls enrolled in the National Growth and Health Study, 1987–1997.
| Characteristic | White Participants N = 1105 | Black Participants N = 1154 | p-value |
|---|---|---|---|
| Demographics | |||
| Age at enrollment in years (mean, 95% CI) | 10.0 (9.9–10.0) | 10.1 (10.0–10.1) | <0.0001 |
| Years of follow-up (mean, 95% CI) | 8.6 (8.5–8.7) | 8.8 (8.7–8.8) | 0.0127 |
| Parent’s Highest Level of Education, n (%) | <0.0001 | ||
| High school or less | 209 (19.0%) | 360 (31.2%) | |
| Some college | 337 (30.5%) | 547 (47.5%) | |
| College graduate | 558 (50.5%) | 246 (21.3%) | |
| Family Income, n (%) | <0.0001 | ||
| Less than $10,000 | 74 (7.0%) | 295 (27.3%) | |
| $10,000–19,999 | 97 (9.2%) | 208 (19.3%) | |
| $20,000–39,000 | 343 (32.6%) | 320 (29.6%) | |
| More than $40,000 | 539 (51.2%) | 257 (23.8%) | |
| Early Adolescent Health Variables | |||
| Reports eating breakfast regularly, n (%) | 988 (89.4%) | 881 (76.3%) | <0.0001 |
| Hours of TV viewing per week (mean, 95%CI) | 24.9 (23.9–45.9) | 36.4 (35.4–37.3) | <0.0001 |
| Exceeds 14 h per week of TV viewing, n (%)a | 831 (76.7%) | 992 (89.94) | <0.0001 |
| Age at menarche in years (mean, 95% CI) | 13.6 (13.5–13.7) | 12.7 (12.6–12.8) | <0.0001 |
| Early menarche before age 12, n (%)a | 115 (10.7%) | 302 (26.5%) | <0.0001 |
| Late Adolescent Health Variables | |||
| Pregnant during study period, n (%) | 217 (19.6%) | 509 (44.1%) | <0.0001 |
| CESD Depression score at age 15 years (mean, 95% CI) | 16.5 (15.7–17.4) | 14.7 (13.8–15.6) | 0.0044 |
| CESD Depression score ≥ 20 at age 15 years, n (%)a | 166 (33.4%) | 117 (24.7%) | 0.0028 |
TV = television. CESD = Center for Epidemiological Studies Depression scale.
Missing observations were not included in calculating the percentage for these characteristics.
Fig. 1.
Total Cardiovascular Health (CVH) Score in Girls enrolled in the National Growth and Health Study, 1987–1997. Total CVH score (0–14) reflects the summation of scores on the seven individual cardiovascular health metrics for each participant during each developmental period.
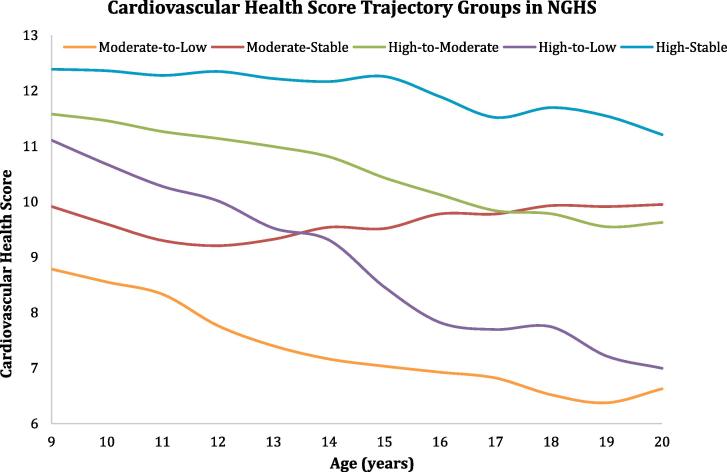
Based on observed patterns of changes in the CVH score over time, we identified and labeled five CVH trajectories (Fig. 2). The most common trajectory was high-to-moderate (45.1%) followed by high-to-low (20.1%) and high-stable (14.9%). The decline in CVH in the high-to-low group was most prominent in middle to late adolescence (ages 14–17) whereas the moderate-to-low group had a more prominent decline in CVH in early to middle adolescence (ages 11–14). The high-to-moderate and moderate-stable groups, and the high-to-low and moderate-to-low groups, both ended up with similar CVH scores in young adulthood (10 and 7, respectively). Only the high-stable group retained a mean ideal CVH score ≥ 11 into young adulthood.
Fig. 2.
Empirically-derived Trajectories of the Cardiovascular Health Score over 10 years for Adolescent Girls in the National Growth and Health Study, 1987–1997. Five trajectory classes identified and labeled.
We found statistically significant differences in trajectory group membership for all demographic and health indicators tested with the exception of high depression scores (p = 0.051) (Table 2). Girls who retained the highest CVH scores throughout adolescence (those in the high-stable group) were more likely to be white (72%), have parents with the highest education (65%) and income (63%) levels, eat breakfast regularly in early adolescence (90%), and were least likely to experience a teen pregnancy (15%). Girls with the lowest CVH scores at the end of adolescence (those in the high-to-low and moderate-to-low groups) had the highest mean hours of television watching in early adolescence (34.6 and 35.9 h per week), the lowest mean age at menarche (12.8 years), and the highest depression scores (17.2 and 16.9).
Table 2.
Associations of adolescent characteristics with five cardiovascular health trajectories identified in girls enrolled in the National Growth and Health Study, 1987–1997
| Characteristic | Trajectory Group |
p-value | ||||
|---|---|---|---|---|---|---|
| High-Stable n (%) | High-to-Moderate n (%) | High-to-Low n (%) | Moderate-Stable n (%) | Moderate-to-Low n (%) | ||
| Overall N, % of total sample | 275 (14%) | 954 (48%) | 392 (20%) | 198 (10%) | 152 (8%) | |
| Demographic factors | ||||||
| Race | <0.001 | |||||
| White | 197 (72%) | 490 (51%) | 154 (39%) | 71 (36%) | 43 (28%) | |
| Black | 78 (28%) | 464 (49%) | 238 (61%) | 127 (64%) | 109 (72%) | |
| Parent’s Highest Level of Education | <0.001 | |||||
| High school or less | 25 (9%) | 223 (23%) | 119 (30%) | 44 (22%) | 58 (38%) | |
| Some college | 72 (26%) | 376 (40%) | 185 (47%) | 82 (41%) | 63 (42%) | |
| College graduate | 178 (65%) | 354 (37%) | 88 (23%) | 72 (37%) | 31 (20%) | |
| Family Income | <0.001 | |||||
| Less than $10000 | 16 (6%) | 134 (15%) | 86 (23%) | 27 (14%) | 29 (20%) | |
| $10000–19999 | 19 (7%) | 118 (13%) | 67 (18%) | 18 (10%) | 39 (27%) | |
| $20000–39000 | 64 (24%) | 314 (35%) | 110 (30%) | 60 (32%) | 42 (30%) | |
| More than $40000 | 165 (63%) | 340 (37%) | 104 (29%) | 84 (44%) | 33 (23%) | |
| Early Adolescent Health Variables | ||||||
| Reports eating breakfast regularly | 247 (90%) | 805 (84%) | 313 (80%) | 157 (79%) | 121 (80%) | 0.0026 |
| Mean hours of TV viewing per week (95% CI) | 24.1 (22.2–26.1) | 30.2 (29.1–31.3) | 34.6 (32.9–36.3) | 30.5 (28.2–32.8) | 35.9 (33.2–38.7) | <0.001 |
| Exceeds 14 h/week of TV viewing | 188 (69%) | 778 (84%) | 338 (88%) | 172 (88%) | 135 (92%) | <0.001 |
| Mean age at menarche (95% CI) | 13.4 (13.2–13.6) | 13.0 (12.9–13.1) | 12.8 (12.7–12.9) | 13.0 (12.8–13.2) | 12.8 (12.6–13.1) | <0.001 |
| Menarche < 12y | 31 (11%) | 173 (18%) | 105 (27%) | 32 (16%) | 36 (24%) | <0.001 |
| Late Adolescent Health Variables | ||||||
| Teen pregnancy | 42 (15%) | 322 (34%) | 152 (39%) | 67 (34%) | 45 (30%) | <0.001 |
| Mean CESD Depression score (95% CI) | 14.9 (13.1–16.7) | 15.0 (14.0–15.9) | 17.2 (15.7–18.7) | 15.6 (13.6–17.6) | 16.9 (14.5–19.2) | 0.0967 |
| CESD Depression score ≥ 20 (n, %) | 34 (28%) | 107 (25%) | 65 (37%) | 29 (30%) | 23 (32%) | 0.0507 |
CESD = Center for Epidemiological Studies Depression; CI = Confidence Interval.
In unadjusted models, girls who identified as black, whose parents did not graduate from college, and whose family income was <$40,000 had higher odds of being in any of the less favorable CVH trajectory groups relative to the high-stable group (Table 3), as did girls who exceeded 14 h per week of television in early adolescence, who experienced menarche before age 12, and who experienced a teen pregnancy. In models adjusted for race, parental education and income, exceeding 14 h per week of television in early adolescence and experiencing a teen pregnancy remained associated with higher odds of being in the high-to-moderate, high-to-low, and moderate-stable CVH groups relative to the high-stable CVH group. Early menarche remained associated with higher odds of being in the high-to-low CVH group. The associations with television viewing, teen pregnancy, and early menarche were similar for both black and white participants and were not modified by race.
Table 3.
Odds of belonging to one of five cardiovascular health trajectories identified in girls enrolled in the National Growth and Health Study based on demographic factors and adolescent health variables
| Trajectory Group |
|||||
|---|---|---|---|---|---|
| Characteristic | High-Stable | High-to-Moderate OR (95% CI) | High-to-Low OR (95% CI) | Moderate-Stable OR (95% CI) | Moderate-to-Low OR (95% CI) |
| Model 1: Unadjusted | |||||
| Black Race * | Ref | 2.4 (1.8–3.2) | 3.9 (2.8–5.4) | 4.5 (3.1–6.7) | 6.4 (4.1–9.9) |
| Parent did not graduate college + | Ref | 3.1 (2.3–4.1) | 6.3 (4.5–8.9) | 3.2 (2.2–4.7) | 7.2 (4.5–11.4) |
| Family income <$40,000 ‡ | Ref | 2.8 (2.1–3.7) | 4.2 (3.0–5.9) | 2.1 (1.4–3.0) | 5.6 (3.5–8.8) |
| Does not report regular breakfast eating | Ref | 1.6 (1.1–2.5) | 2.2 (1.4–3.5) | 2.3 (1.4–3.9) | 2.3 (1.3–3.9) |
| Exceeds 14 h/week of TV | Ref | 2.2 (1.6–3.1) | 3.4 (2.3–5.1) | 3.2 (1.9–5.2) | 5.4 (2.8–10.5) |
| Menarche < 12y | Ref | 1.7 (1.2–2.6) | 2.9 (1.9–4.5) | 1.5 (0.8–2.6) | 2.4 (1.4–4.1) |
| Teen pregnancy | Ref | 2.8 (1.8–4.4) | 3.5 (2.4–5.2) | 2.8 (1.9–4.4) | 2.3 (1.4–3.8) |
| CESD score ≥ 20 | Ref | 0.8 (0.5–1.2) | 1.5 (0.9–2.5) | 1.1 (0.6–2.0) | 1.2 (0.6–2.3) |
| Model 2: Adolescent Health Variables each individually adjusted for race, parental education, and family income | |||||
| Does not report regular breakfast eating | Ref | 1.2 (0.7–1.9) | 1.3 (0.8–2.2) | 1.5 (0.9–2.7) | 1.4 (0.8–2.5) |
| Exceeds 14 h/week of TV | Ref | 1.6 (1.1–2.2) | 2.0 (1.2–3.1) | 2.1 (1.2–3.5) | 2.7 (1.4–5.4) |
| Menarche < 12y | Ref | 1.5 (0.9–2.3) | 2.2 (1.4–3.6) | 1.0 (0.6–1.8) | 1.6 (0.9–2.8) |
| Teen pregnancy | Ref | 1.7 (1.2–2.6) | 1.7 (1.1–2.6) | 1.8 (1.1–2.8) | 1.0 (0.6–1.7) |
| CESD Depression score ≥ 20 | Ref | 0.8 (0.5–1.3) | 1.5 (0.9–2.6) | 1.3 (0.7–2.4) | 1.4 (0.7–2.8) |
CESD = Center for Epidemiological Studies Depression; OR = Odds Ratio; CI = Confidence Interval;
Ref = Reference Group.
All statistically significant associations are in bold.
White race is referent group.
Parent graduated college is referent group.
Family income ≥$40,000 is referent group.
4. Discussion
Our analysis demonstrates that adolescence is an important developmental period for the loss or preservation of CVH in girls. Notably, almost all adolescents in NGHS demonstrated some decline in CVH. We empirically identified five distinct patterns of CVH loss in this historical cohort of over 2000 black and white girls. Mean CVH scores for each trajectory group varied widely at age 9, highlighting the importance of even earlier life influences on CVH including the perinatal period and early childhood. The largest declines in CVH were seen from early to middle adolescence, coinciding with the physical transition of puberty and the social transition from primary to secondary school. Our results are consistent with prior studies in NGHS documenting dramatic declines in physical activity (Kimm et al., 2002) and increases in the prevalence of obesity (Kimm et al., 2004), sugar sweetened beverage intake (Striegel-Moore et al., 2006), and poor dietary quality throughout adolescence (Moore et al., 2012).
Unsurprisingly, girls who maintained the most favorable CVH scores were more likely to have high parental income and education, as well as better breakfast eating and lower television viewing in early adolescence. Girls who entered adolescence with moderate CVH and declined further into the low CVH range were more likely to be from lower socioeconomic strata and report less healthy behaviors. Girls in the moderate-stable group shared some features with both the high-declining and moderate-declining groups and represent an intriguing group for future study, as they were the were the only group to maintain their CVH score throughout adolescence.
Race was associated with both CVH score at the beginning of adolescence and CVH trajectories. Black girls were more likely to enter adolescence with only moderate CVH, and to end up with moderate or low CVH, even if they started adolescence with high CVH. These differences may be due to disparities in income and education between black and white families, as well as unmeasured factors such as access to healthy environments, rooted in differential treatment by race. These remain important areas for future investigation.
Excessive television viewing was associated with CVH decline. Extensive research shows associations between television viewing and lower physical activity (Marshall et al., 2004), poorer dietary habits (Wiecha et al., 2006), and obesity (Marshall et al., 2004) in youth. Furthermore, media use introduces exposure to advertising for unhealthy food (Wiecha et al., 2006) and tobacco products (B.F. New media and tobacco control, 2012). Thus it is not surprising that this behavior was associated with worsening CVH trajectories. The impact of the current media landscape on adolescent CVH - including ubiquitous smartphone use, streaming video, and social media – is an area ripe for investigation.
Earlier menarche, an important marker of later life CVD risk in women, was also associated with lower CVH in early adolescence and with being in the trajectory group with the most CVH loss. Similar associations between early menarche and lower CVH have been shown in post-menopausal women (Cao et al., 2015). Early menarche is associated with childhood obesity (Rosenfield et al., 2009) and may be a marker of poor diet, physical activity, and BMI in pre-adolescence. The termination of linear growth soon after menarche in those already predisposed to weight gain may result in further weight gain and accelerated loss of ideal status for blood pressure, cholesterol, and glucose.
Teen pregnancy was also associated with less optimal CVH trajectories. Pregnancy is associated with numerous physiological changes that may affect later CVH. Post-partum weight retention is common and is typically greater for adolescent compared to adult women (Whelan et al., 2017). Previous studies have found that NGHS participants who gave birth during adolescence had greater accrual of overall and central adiposity compared to participants who did not give birth during the study period (Gunderson et al., 2009). Pregnancies complicated by gestational hypertension, diabetes, or pre-eclampsia are associated with future CVD risk (Fraser et al., 2012). Unfortunately, more detailed pregnancy histories were not available in the public NGHS dataset for analysis. It is also plausible that the same unmeasured social determinants of health that led some adolescents to experience a teen pregnancy led to accelerated loss of CVH in this population.
The prevalence of ideal CVH metrics in this study differ somewhat from contemporary cross-sectional data from adolescents ages 12–19 years old participating in the 2015–2016 National Health and Nutrition Examination Surveys (NHANES) (Benjamin et al., 2019). The prevalence of ideal smoking status is much higher in today’s adolescents (93.6%) compared to in NGHS. Adolescent smoking prevalence in the US reached a peak of 40% in 1997 before declining to historic lows in the past few years; it is noteworthy that this trend is now reversing with the onset of the vaping epidemic (Johnston et al., 2018). The prevalence of ideal cholesterol in today’s adolescent girls (71.2%) is also higher than in NGHS, consistent with favorable trends in childhood lipid levels seen in NHANES data from 1999 to 2016 (Perak et al., 2019). The overall pattern of cholesterol improvement throughout adolescence seen in the NGHS data is consistent with the known effects of pubertal hormones on lipid metabolism in early puberty (Eissa et al., 2016). The prevalence of ideal BMI in adolescents is lower now (60.1%) compared to in NGHS; the dramatic weight gain seen during NGHS captures the onset of the current obesity epidemic (Kimm et al., 2004). Finally the prevalence of ideal physical activity in adolescents ages 12–19 years old in NHANES (25.5%) is similar to the prevalence of ideal physical activity in the early and mid adolescent years of NGHS but markedly different than in late adolescence in NGHS. Similar declines in physical activity in girls are seen in contemporary NHANES cohorts, with black females reporting the least physical activity by young adulthood (Armstrong et al., 2018). Examining longitudinal patterns by developmental stage in cohorts such as NGHS is necessary to identify critical windows for intervention to promote maintenance of CVH metrics.
Other historical longitudinal cohorts have investigated the key role of CVH in adolescence on development of cardiovascular risk factors and surrogate markers of CVD in adulthood. The International Childhood Cardiovascular Cohort Consortium (i3C), which includes data from 5785 participants in five international cohort studies, found that the number of ideal CVH metrics in adolescence was inversely associated with carotid intima media thickness in adulthood (Oikonen et al., 2013). The Special Turku Coronary Risk Factor Intervention Project for Children (STRIP) study found that a randomized dietary intervention from infancy through adolescence led to higher CVH and lower aortic intima-media thickness in young adulthood, demonstrating that CVH is not static and can be improved at this key stage in the life course (Pahkala et al., 2013). Our results complement these findings by revealing more detailed CVH trajectories in adolescent girls. While our analyses are observational, they reinforce the importance of maximizing healthy dietary patterns, minimizing sedentary behavior in children as they enter adolescence, and encouraging adolescents to abstain from initiating smoking. Unfortunately interventions to improve physical activity (Metcalf et al., 2012) and dietary patterns (Salam et al., 2016) among youth over the past three decades have made little impact and thus novel population health and individual-based strategies are needed. Girls with early menarche or those experiencing a teen pregnancy may especially benefit from these interventions. More work is needed on even earlier life CVH trajectories to identify opportunities for ensuring more children enter adolescence with ideal CVH. Similarly, detailed assessment of CVH trajectories as adolescents age into young adulthood are needed to understand the impact of the many transitions in education, housing, relationships, and employment that occur in emerging adults.
Limitations to this study include the reliance on self-reported diet, physical activity, and smoking data and the collection of data approximately 30 years ago, when, for example, teen pregnancy rates were significantly higher than currently (Pazol et al., 2011). We used different diet and physical activity metrics from those in the standard AHA definitions of CVH, although we chose metrics previously shown to have high construct validity for moderate to vigorous physical activity (Kimm et al., 2005) and exemplary dietary patterns (Reedy et al., 2018) as defined by the AHA. We chose to impute missing data based on the lower of the next available metrics given prior studies showing overall loss of CVH in adolescence; this may have biased our results toward greater CVH loss, with the exception of glucose where we assigned those with missing data as ideal. Even with these imputations, missing data was common in the latter years and may have impacted the reliability of the late adolescent/young adult estimates. Finally, NGHS chose to sample only girls identifying as white or black from three urban communities in the United States; in contrast to nationally representative studies, our results may not be generalizable beyond this specific sample.
5. Conclusion
Despite these limitations, NGHS contains one of the most detailed, frequent measurements of the CVH metrics in adolescence and thus we believe these findings are still relevant today. Future research is needed on modern cohorts that span from early childhood through adolescence to young adulthood in order to better define critical windows for CVH loss. These findings could shape both population and individual level interventions to improve the cardiovascular health of youth.
Sources of Funding
This work was supported by a NIH National Heart, Lung, and Blood Institute Career Development Award to Dr. Gooding [grant number K23-HL12236]. Dr Marma is supported by NIH/NHLBI K23HL145101.
Disclosures
None.
CRediT authorship contribution statement
Holly C. Gooding: Conceptualization, Writing - original draft, Visualization, Funding acquisition. Hongyan Ning: Methodology, Formal analysis, Visualization, Writing - review & editing. Amanda M. Perak: Writing - review & editing. Norrina Allen: Methodology, Writing - review & editing. Donald Lloyd-Jones: Resources, Writing - review & editing, Supervision. Lynn L. Moore: Resources, Writing - review & editing. Martha R. Singer: Software, Data curation. Sarah D. Ferranti: Writing - review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2020.101276.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Wilkins J.T., Ning H., Berry J., Zhao L., Dyer A.R., Lloyd-Jones D.M. Lifetime risk and years lived free of total cardiovascular disease. JAMA. 2012;308(17):1795–1801. doi: 10.1001/jama.2012.14312. doi:1388927 10.1001/jama.2012.14312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang N., Jiang M., Fan Y. Ideal cardiovascular health metrics and risk of cardiovascular disease or mortality: a meta-analysis. Int. J. Cardiol. Published online. 2016 doi: 10.1016/j.ijcard.2016.03.210. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha A., Vaccarino V., Judd S.E. Life’s Simple 7 and risk of incident stroke: the reasons for geographic and racial differences in stroke study. Stroke. 2013;44(7):1909–1914. doi: 10.1161/STROKEAHA.111.000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muntner P., Judd S.E., Gao L. Cardiovascular risk factors in CKD associate with both ESRD and mortality. J. Am. Soc. Nephrol. 2013;24(7):1159–1165. doi: 10.1681/ASN.2012070642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colangelo L., Whooley M.A., TV T.-H., Siddique J., Reis J.P., Liu K. Ideal cardiovascular health and development of depressive symptoms: the CARDIA study. Circulation Published online. 2011 [Google Scholar]
- Ogunmoroti O., Oni E., Michos E.D. Life’s simple 7 and incident heart failure: the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. Published online. 2017 doi: 10.1161/JAHA.116.005180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D.M., Hong Y., Labarthe D. Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation. 2010;121(4):586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. doi:CIRCULATIONAHA.109.192703 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- Benjamin E.J., Muntner P., Alonso A. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation Published online. 2019 doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Allen N.B., Krefman A., Labarthe D.R. Abstract 17479: early life cardiovascular health trajectories and their associationw ithi subclinical atherosclerosis. Circulation. 2016;134(suppl_1):A17479. [Google Scholar]
- Allen N.B., Krefman A., Labarthe D.R. Abstract 015: Critical periods in cardiovascular health across the life course: a pooled cohort analysis. Circulation. 2018;137(suppl_1):A015. [Google Scholar]
- De Ridder C.M., Thijssen J.H.H., Brüing P.F., Den Brande J.L.V., Zonderland M.L., Erich W.B.M. Body fat mass, body fat distribution, and pubertal development: a longitudinal study of physical and hormonal sexual maturation of girls. J. Clin. Endocrinol. Metab. Published online. 1992 doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- Hannon T.S., Janosky J., Arslanian S.A. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. Published online. 2006 doi: 10.1203/01.pdr.0000246097.73031.27. [DOI] [PubMed] [Google Scholar]
- Shankar R.R., Eckert G.J., Saha C., Tu W., Pratt J.H. The change in blood pressure during pubertal growth. J. Clin. Endocrinol. Metab. Published online. 2005 doi: 10.1210/jc.2004-0926. [DOI] [PubMed] [Google Scholar]
- Eissa M.A., Mihalopoulos N.L., Holubkov R., Dai S., Labarthe D.R. Changes in fasting lipids during puberty. J. Pediatr. Published online. 2016 doi: 10.1016/j.jpeds.2015.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. Published online. 2004 doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Silk J.S., Nelson E.E. The neurobiology of the emotional adolescent: from the inside out. Neurosci. Biobehav. Rev. Published online. 2016 doi: 10.1016/j.neubiorev.2016.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnett J.J. Emerging adulthood. A theory of development from the late teens through the twenties. Am. Psychol. 2000;55(5):469–480. [PubMed] [Google Scholar]
- Gardner M., Steinberg L. Peer influence on risk taking, risk preference, and risky decision making in adolescence and adulthood: an experimental study. Dev Psychol. Published online. 2005 doi: 10.1037/0012-1649.41.4.625. [DOI] [PubMed] [Google Scholar]
- Amin R., Decesare J.Z., Hans J., Roussos-Ross K. Epidemiologic surveillance of teenage birth rates in the United States, 2006–2012. Obstetr. Gynecol. 2017 doi: 10.1097/AOG.0000000000001897. [DOI] [PubMed] [Google Scholar]
- Avenevoli S., Swendsen J., He J.P., Burstein M., Merikangas K.R. Major depression in the national comorbidity survey-adolescent supplement: prevalence, correlates, and treatment. J. Am. Acad. Child Adolesc. Psychiatr. Published online. 2015 doi: 10.1016/j.jaac.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1992. Obesity and cardiovascular disease risk factors in black and white girls: the NHLBI Growth and Health Study. Am. J. Public Health. 82(12), 1613–1620. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1456335. [DOI] [PMC free article] [PubMed]
- Kimm S.Y.S., Barton B.A., Obarzanek E. Racial divergence in adiposity during adolescence: the NHLBI growth and health study. Pediatrics Published online. 2004 doi: 10.1542/peds.107.3.e34. [DOI] [PubMed] [Google Scholar]
- Kimm S.Y.S., Barton B.A., Obarzanek E. Obesity development during adolescence in a biracial cohort: the NHLBI growth and health study. Pediatrics Published online. 2004 doi: 10.1542/peds.110.5.e54. [DOI] [PubMed] [Google Scholar]
- Kimm S.Y., Glynn N.W., Kriska A.M. Decline in physical activity in black girls and white girls during adolescence. N. Engl. J. Med. 2002;347(10):709–715. doi: 10.1056/NEJMoa003277347/10/709. [DOI] [PubMed] [Google Scholar]
- Kimm S.Y.S., Glynn N.W., Obarzanek E. Relation between the changes in physical activity and body-mass index during adolescence: a multicentre longitudinal study. Lancet Published online. 2005 doi: 10.1016/S0140-6736(05)66837-7. [DOI] [PubMed] [Google Scholar]
- Krebs-Smith S.M., Pannucci T.R.E., Subar A.F. Update of the healthy eating index: HEI-2015. J. Acad. Nutr. Diet. Published online. 2018 doi: 10.1016/j.jand.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky T.S., Ning H., Daviglus M.L. Association of cardiovascular health with subclinical disease and incident events: the multi-ethnic study of atherosclerosis. J. Am. Heart Assoc. Published online. 2017 doi: 10.1161/JAHA.116.004894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz P.B., Oberfield S.E. Reexamination of the age limit for defining when puberty is precocious in girls in the United States: implications for evaluation and treatment. Pediatrics Published online. 1999 doi: 10.1542/peds.104.4.936. [DOI] [PubMed] [Google Scholar]
- Education AA of PC on P Media education. Pediatrics. 1999;104(2 Pt 1):341–343. [PubMed] [Google Scholar]
- Vilagut G., Forero C.G., Barbaglia G., Alonso J. Screening for depression in the general population with the center for epidemiologic studies depression (ces-d): a systematic review with meta-analysis. PLoS One Published online. 2016 doi: 10.1371/journal.pone.0155431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striegel-Moore R.H., Thompson D., Affenito S.G. Correlates of beverage intake in adolescent girls: the National Heart, Lung, and Blood Institute Growth and Health Study. J. Pediatr. Published online. 2006 doi: 10.1016/j.jpeds.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Moore L.L., Singer M.R., Mustafa Qureshi M., Loring Bradlee M., Daniels S.R. Food group intake and micronutrient adequacy in adolescent girls. Nutrients Published online. 2012 doi: 10.3390/nu4111692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall S.J., Biddle S.J.H., Gorely T., Cameron N., Murdey I. Relationships between media use, body fatness and physical activity in children and youth: a meta-analysis. Int. J. Obes. 2004;28(10):1238–1246. doi: 10.1038/sj.ijo.0802706. [DOI] [PubMed] [Google Scholar]
- Wiecha J.L., Peterson K.E., Ludwig D.S., Kim J., Sobol A., Gortmaker S.L. When children eat what they watch: impact of television viewing on dietary intake in youth. Arch. Pediatr. Adolesc. Med. 2006;160(4):436–442. doi: 10.1001/archpedi.160.4.436. [DOI] [PubMed] [Google Scholar]
- 2012. B. F. New media and tobacco control. Tob Control. 21(2), 139–144. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=emed10&NEWS=N&AN=22345236. [DOI] [PubMed]
- Cao X., Zhou J., Yuan H., Chen Z. Cumulative effect of reproductive factors on ideal cardiovascular health in postmenopausal women: a cross-sectional study in central south China. BMC Cardiovasc. Disord. 2015;15:176. doi: 10.1186/s12872-015-0172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfield R.L., Lipton B.R., Drum M.L. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics. 2009;123(1):84–88. doi: 10.1542/peds.2008-0146. [DOI] [PubMed] [Google Scholar]
- Whelan E., Armson B., Ashley-Martin J., MacSween K., Woolcott C. Gestational weight gain and interprepregnancy weight change in adolescent mothers. J. Pediatr. Adolesc. Gynecol. 2017;30(3):356–361. doi: 10.1016/j.jpag.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Gunderson E.P., Striegel-Moore R., Schreiber G. Longitudinal study of growth and adiposity in parous compared with nulligravid adolescents. Arch. Pediatr. Adolesc. Med. Published online. 2009 doi: 10.1001/archpediatrics.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser A., Nelson S., Macdonald-Wallis C. Associations of pregnancy complications with calculated cardiovascular disease risk and cardiovascular risk factors in middle age: the Avon Longitudinal Study of Parents and Children. Circulation. 2012;125(11):1367–1380. doi: 10.1161/CIRCULATIONAHA.111.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston L.D., Miech R.A., O’Malley P.M., Bachman J.G., Schulenberg J.E., Patrick M.E. Monitoring the Future: National survey results on drug use 1975–2018. Overview: key findings on adolescent drug use. Natl. Inst. Drug Abus. Natl. Institutes Heal. Published online 2019. 2018 [Google Scholar]
- Perak A.M., Ning H., Kit B.K. Trends in levels of lipids and apolipoprotein B in US youths aged 6 to 19 years, 1999–2016. JAMA – J. Am. Med. Assoc. 2019 doi: 10.1001/jama.2019.4984. Published online 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong S., Wong C.A., Perrin E., Page S., Sibley L., Skinner A. Association of physical activity with income, race/ethnicity, and sex among adolescents and young adults in the United States findings from the national health and nutrition examination survey, 2007–2016. JAMA Pediatr. Published online. 2018 doi: 10.1001/jamapediatrics.2018.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikonen M., Laitinen T.T., Magnussen C.G. Ideal cardiovascular health in young adult populations from the United States, Finland, and Australia and its association with cIMT: the International Childhood Cardiovascular Cohort Consortium. J. Am. Hear Assoc. 2013;2(3) doi: 10.1161/JAHA.113.000244. doi:jah3234 10.1161/JAHA.113.000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahkala K., Hietalampi H., Laitinen T.T. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (The Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] Study) Circulation. 2013;127(21):2088–2096. doi: 10.1161/CIRCULATIONAHA.112.000761. doi:CIRCULATIONAHA.112.000761 10.1161/CIRCULATIONAHA.112.000761. [DOI] [PubMed] [Google Scholar]
- Metcalf B., Henley W., Wilkin T. Effectiveness of intervention on physical activity of children: systematic review and meta-analysis of controlled trials with objectively measured outcomes (EarlyBird 54) BMJ. Published online. 2012 doi: 10.1136/bmj.e5888. [DOI] [PubMed] [Google Scholar]
- Salam R.A., Hooda M., Das J.K. Interventions to improve adolescent nutrition: a systematic review and meta-analysis. J. Adolesc. Heal. Published online. 2016 doi: 10.1016/j.jadohealth.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazol K., Warner L., Gavin L. Vital signs: teen pregnancy - United States, 1991–2009. Morb Mortal Wkly Rep. 2011;60(13):414–420. [PubMed] [Google Scholar]
- Reedy J., Lerman J.L., Krebs-Smith S.M. Evaluation of the healthy eating index-2015. J. Acad. Nutr. Diet. Published online. 2018 doi: 10.1016/j.jand.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.