Summary
Traumatic brain injury (TBI) involves complex secondary injury processes following the primary injury. The secondary injury is often associated with rapid metabolic shifts and impaired brain function immediately after the initial tissue damage. Magnetic resonance spectroscopic imaging (MRSI) coupled with hyperpolarization of 13C-labeled substrates provides a unique opportunity to map the metabolic changes in the brain after traumatic injury in real-time without invasive procedures. In this report, we investigated two patients with acute mild TBI (Glasgow coma scale 15) but no anatomical brain injury or hemorrhage. Patients were imaged with hyperpolarized [1-13C]pyruvate MRSI 1 or 6 days after head trauma. Both patients showed significantly reduced bicarbonate (HCO3–) production, and one showed hyperintense lactate production at the injured sites. This study reports the feasibility of imaging altered metabolism using hyperpolarized pyruvate in patients with TBI, demonstrating the translatability and sensitivity of the technology to cerebral metabolic changes after mild TBI.
Subject Areas: Biological Sciences, Neuroscience, Molecular Neuroscience, Clinical Neuroscience, Techniques in Neuroscience
Graphical Abstract
Highlights
-
•
Clinical translation of hyperpolarized pyruvate to TBI was demonstrated
-
•
Patients with mild TBI were imaged with hyperpolarized [1-13C]pyruvate
-
•
Altered lactate and HCO3– production in the brain nearest the site of trauma
Biological Sciences; Neuroscience; Molecular Neuroscience; Clinical Neuroscience; Techniques in Neuroscience
Introduction
Traumatic brain injury (TBI) causes mechanical damage and disruption of normal metabolism in the brain (Corps et al., 2015). A major challenge for clinicians is managing complex secondary processes following the primary injury. After the primary trauma, the surviving tissue undergoes metabolic shifts, resulting in the development of potentially hazardous secondary metabolites and further damage. The secondary events develop over a timescale after the primary injury, providing a potential window of opportunity for detection and therapeutic intervention. Moreover, TBI is suspected to contribute to a variety of chronic degenerative processes such as chronic traumatic encephalopathy, Alzheimer disease, and Parkinson disease (Smith et al., 2013). Numerous therapies have been proposed to reduce or prevent secondary brain damage, directly impacting long-term patient outcome (Xiong et al., 2015). Therefore, the noninvasive detection and characterization of TBI pathophysiology during the acute and sub-acute stages will have critical clinical implications and will be vital for identifying and developing effective therapies.
Altered glucose metabolism and mitochondrial dysfunction are features of the pathophysiologic events subsequent to TBI (Brooks and Martin, 2015; Kim et al., 2017). Invasive microdialysis methods have been reported to study brain metabolism in these patients, but in spite of the critical importance of directly detecting mitochondrial function, no specific noninvasive methods exist. Positron emission tomography (PET) detects uptake of radioactively labeled compounds such as glucose or acetate (e.g., [18F]FDG, [11C]acetate) in the human brain. Previous studies demonstrating cerebral hyperglycolysis in patients with TBI using [18F]fluorodeoxyglucose ([18F]FDG) PET measured cellular glucose uptake but could not measure the metabolic fate of glucose (Bergsneider et al., 1997). Another [18F]FDG-PET study that combined with microdialysis of patients with TBI reported that the rate of glucose metabolism correlates with microdialysate lactate and pyruvate concentrations but not with the lactate-to-pyruvate ratio (Hutchinson et al., 2009), suggesting an increase in glucose metabolism to both lactate and pyruvate, as opposed to a shift toward anaerobic metabolism. Other studies indicated that the increased glucose uptake is directly related to the upregulated pentose phosphate pathway rather than to the rest of the glycolysis (Dusick et al., 2007).
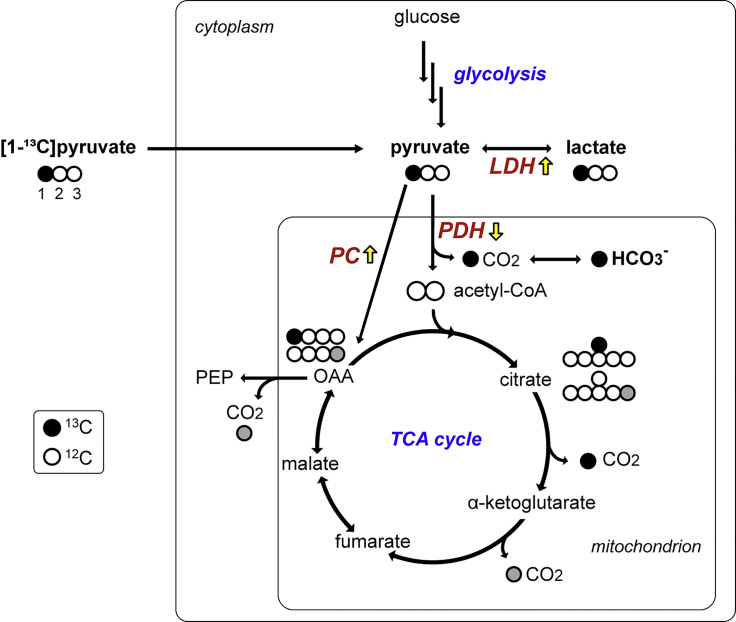
Alternative way of imaging in vivo metabolism is using dynamic nuclear polarization (DNP) and rapid dissolution technique (Ardenkjaer-Larsen et al., 2003). Commercially available DNP polarizer can achieve more than 100,000-fold signal amplification of magnetic resonance-detectable (e.g., 13C-labeled) substrates in liquid state. Administration of hyperpolarized 13C-labeled substrates in coordination with 13C magnetic resonance spectroscopy imaging (MRSI) opened new opportunities to assess in vivo metabolic processes of individual enzyme-catalyzed reactions in various organs including the brain. In particular, previous animal studies using hyperpolarized [1-13C]pyruvate demonstrated that increased [1-13C]lactate production in the injured brain tissue was associated with microglial activation (DeVience et al., 2017; Guglielmetti et al., 2017). Metabolites that are typically detectable in the cerebral 13C spectrum using hyperpolarized [1-13C]pyruvate are [1-13C]lactate and [13C]bicarbonate (HCO3–) as shown in Figure 1. The yellow arrows indicate metabolic shifts in acute TBI (DeVience et al., 2017). In this study, we translated these preclinical discoveries to demonstrate metabolic abnormalities in the brain of patients after acute mild TBI.
Figure 1.
Metabolic Fate of Hyperpolarized [1-13C]Pyruvate in the Brain
[1-13C]Pyruvate (black circle: 13C, white circle: 12C) is converted either to [1-13C]lactate via LDH or acetyl-CoA and 13CO2 (and [13C]HCO3-) through PDH. Possibly, [1-13C]pyruvate can be also converted into [1-13C]oxaloacetate (OAA) through PC, eventually releasing the labeled carbon (13C) as CO2. The gray circle indicates 13C after backward scrambling of OAA to fumarate and malate in the TCA cycle. PEP: phosphoenolpyruvate; LDH: lactate dehydrogenase; PDH: pyruvate dehydrogenase; PC: pyruvate carboxylase.
Results
Two patients with acute TBI were recruited from the Parkland Health and Hospital System Emergency Room. Computed tomographic (CT) scans at admission confirmed that neither patient had underlying fractures, hemorrhage, or other anatomical injury. Patient #1 was a daily smoker, and Patient #2 was diabetic. Otherwise, both subjects were generally healthy and had no history of mental illness or alcoholism. Both patients tolerated the examination well.
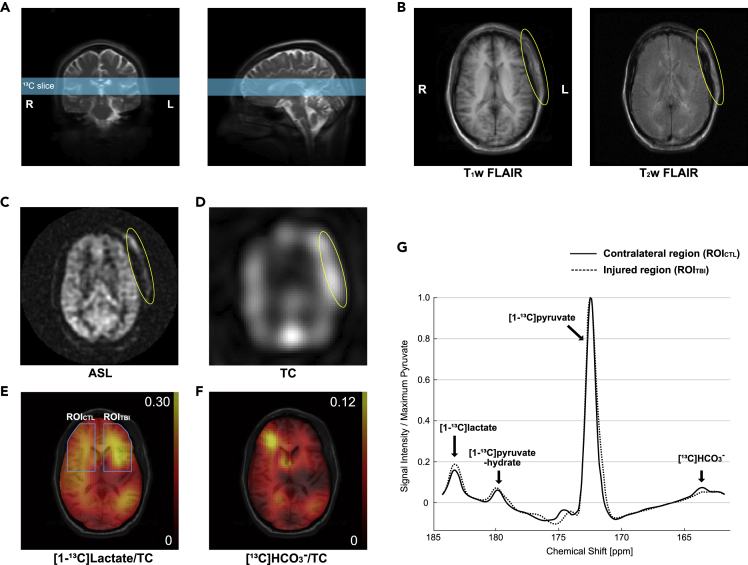
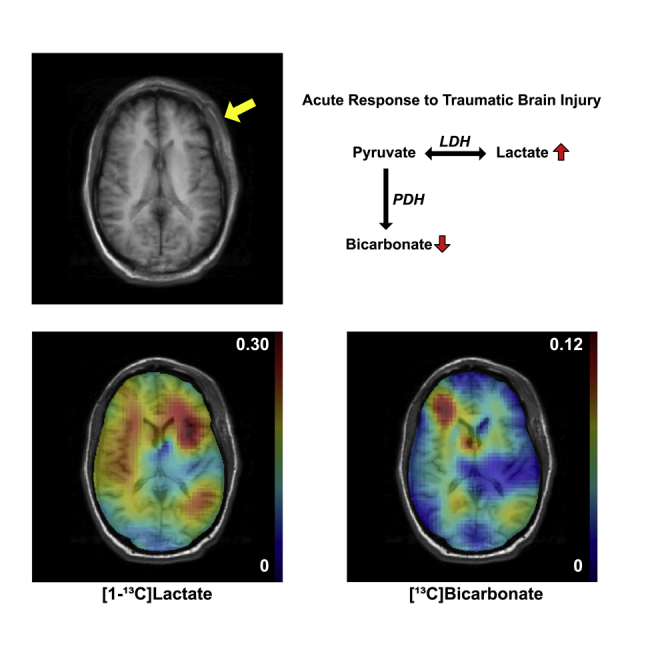
The first patient was a 35-year-old African American male (88 kg, 168 cm) with a 2-cm laceration to the left frontal scalp due to blunt force trauma (whipped by a metal gun) with a Glasgow Coma Scale (GCS) score of 15 and no loss of consciousness (LOC). Figure 2 shows axial images of this patient 28 h after the head trauma. Besides left frontal scalp cutaneous edema/ecchymosis, no cerebral contusion or anatomical brain damage was identified in the T1-weighted and T2-weighted fluid-attenuated inversion recovery (FLAIR) images. Arterial spin labeling (ASL) detected a hyperintense region at the site of the left frontal scalp injury but unremarkable appearance of cerebral perfusion. Likewise, total hyperpolarized 13C signal was hyperintense in the injured scalp (Figure 2D) but comparable between the left and right brain hemispheres. In contrast, [13C]HCO3– and [1-13C]lactate production from hyperpolarized [1-13C]pyruvate was altered in the injured side of the brain (left frontal lobe, underlying the scalp injury) when compared with the contralateral side (Figures 2E and 2F). [13C]HCO3– signal, averaged over the injured region of interest and normalized with total 13C (TC) signal (ROITBI, HCO3–/TC = 0.027), was 54.0% lower than that in the contralateral ROI (ROICTL, HCO3–/TC = 0.059). Conversely, lactate production in the injured region (lactate/TC = 0.236) was larger by 23.7% than the unimpacted contralateral brain region (lactate/TC = 0.191).
Figure 2.
Metabolism of Acute TBI in Patient #1 Imaged by Hyperpolarized 13C Pyruvate
The patient (35 years old, male), injured on the left frontal scalp (GCS 15, no LOC), was imaged 28 h after the injury.
(A) An axial slice that includes the injured region was prescribed for 13C imaging.
(B and C) (B) T1-weighted and T2-weighted 1H FLAIR and (C) ASL images showed swelling and increased perfusion in the injured site outside the skull (yellow circle), whereas no cerebral contusion, hemorrhage, or hypoperfusion was detected.
(D) Region with increased total hyperpolarized 13C (TC) signal image matched to the scalp edema.
(E and F) (E) Increased [1-13C]lactate conversion and (F) decreased [13C]HCO3– production were detected in the brain tissues of the injured hemisphere.
(G) Averaged spectra over the injured brain region (ROITBI, dotted spectra) and the contralateral normal-appearing brain region (ROICTL, solid spectra).
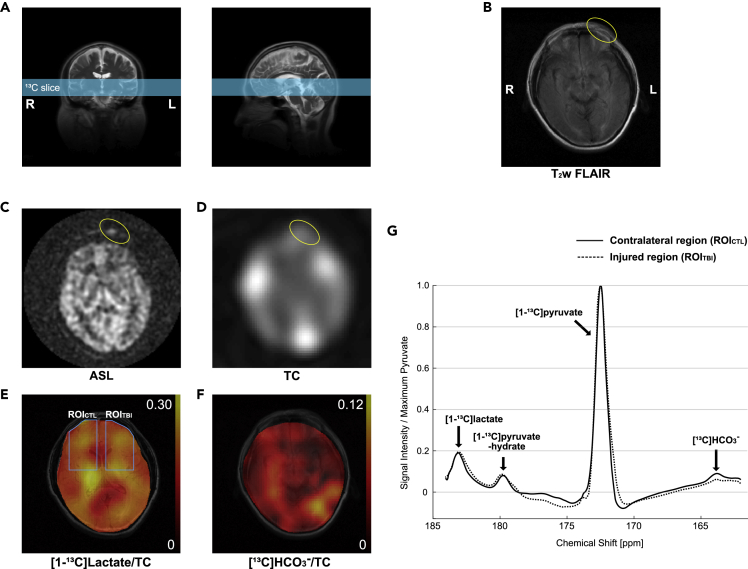
The second patient (Patient #2; 48 years old, 77 kg, 160 cm, Hispanic, male) sustained a head injury as a consequence of an ∼6-m fall from a construction site scaffolding (GCS 15, 2 min LOC). When recruited, he had a 2-cm laceration to the left medial eyebrow and 5-cm subcutaneous hematoma extending laterally from the laceration. He was studied using the same 1H/13C magnetic resonance imaging (MRI) protocol 6 days after the initial injury. Similar to Patient #1, no structural brain damage was found in the 1H MRI, but smaller [13C]HCO3– was observed in the injured region (HCO3–/TC = 0.025) than the contralateral side of the brain (0.047) (Figure 3). [1-13C]Lactate level was comparable (lactate/TC = 0.164 for the injured region and 0.162 for the contralateral region).
Figure 3.
Hyperpolarized 13C Pyruvate Imaging of Patient #2
The patient had a 2-cm laceration to the left medial eyebrow and 5-cm hematoma extending laterally from the laceration from a head injury (GCS 15, 2 min LOC). Images were acquired 6 days from the injury.
(A–C) (A) 13C slice was prescribed to include the laceration. Axial 1H images of (B) T2-weighted FLAIR and (C) ASL showed the injured region outside the skull (yellow circle).
(D) Besides the scalp hematoma, no abnormal distribution of total hyperpolarized 13C signals (TC).
(E and F) (E) Lactate was comparable between the impacted region and the contralateral side of the brain, whereas (F) decreased HCO3– signal was observed in the impacted brain region.
(G) Averaged spectra over the injured brain region (ROITBI, dotted spectra) and the contralateral normal-appearing brain region (ROICTL, solid spectra).
Discussion
A common feature reported in patients with TBI is the increase of glucose consumption rate with no parallel increase in mitochondrial oxidative phosphorylation, known as hyperglycolysis (Bergsneider et al., 1997). Hyperglycolysis coupled with mitochondrial dysfunction should favor metabolism of pyruvate, the end product of glycolysis, to lactate via lactate dehydrogenase, rather than oxidation to acetyl-CoA via pyruvate dehydrogenase (PDH). The hyperintense [1-13C]lactate signal in the impacted region from Patient #1 is consistent with previous in vivo preclinical imaging studies. DeVience et al. showed significant increase of [1-13C]lactate production from hyperpolarized [1-13C]pyruvate 4 h post-injury using a controlled cortical impact rat model (DeVience et al., 2017), and Guglielmetti et al. reported longitudinal changes of lactate production using this technique (Guglielmetti et al., 2017). The absence of hyperglycolysis in Patient #2 could be due to the severity of the injury (Bergsneider et al., 1997) or metabolic transition from hyperglycolysis to hypometabolic stage (Greco et al., 2020; Jalloh et al., 2015).
Mitochondrial dysfunction plays a key role in the pathophysiology of TBI (Kim et al., 2017). [13C]HCO3– production from hyperpolarized [1-13C]pyruvate reflects PDH flux. As PDH is an enzyme complex that is integrated into the inner membrane of the mitochondria, the decreased [13C]HCO3– production in the injured brain region implies mitochondrial injury/dysfunction in spite of normal anatomy, which is consistent with previous animal study (DeVience et al., 2017). It should be noted that [13C]HCO3– can be also produced via an anaplerotic pathway into the tricarboxylic acid cycle, which is exclusively achieved by pyruvate carboxylase (PC), an astrocyte-specific enzyme (Shank et al., 1985), as shown in Figure 1. Pyruvate carboxylation in astrocyte increases during the secondary injury process, whereas pyruvate utilization via PDH is downregulated to protect neurons (Bartnik-Olson et al., 2013).
As shown in the Figures 2D and 3D, hyperpolarized 13C signals were predominant in the gray matter rather than the white matter or subcutaneous tissues. These are consistent with previous human brain studies using hyperpolarized pyruvate (Gordon et al., 2019; Lee et al., 2019). Besides the directly impacted brain regions, the lactate and HCO3– maps showed larger variation in the patients when compared with those acquired from healthy subjects in previous studies. For instance, reduced HCO3– production was observed in the right posterior brain region of Patient #1, indicating possible contrecoup injury in the region.
Both patients were overweight. Patient #1 was very muscular with body mass index (BMI) of 31.2 but did not have a diagnosis of type 2 diabetes and was not on any hypoglycemic medications. Patient #2 (BMI = 30.1) did have a diagnosis of type 2 diabetes on metformin (500 mg orally twice daily). Plasma glucose levels of the patients were well within 160 mg/dL (108 for patient #1, 133 for patient #2), which is representative of normal brain biochemistry for clinical purposes as measured by PET (Varrone et al., 2009; Viglianti et al., 2017). Although diffuse changes in pyruvate metabolism could be postulated due to hyperglycemia, it seems unlikely that regional abnormalities as observed here would occur.
The specific timing of this glucose dysmetabolism is currently not known. Longitudinal monitoring with a larger patient population will be needed to further evaluate the utility of hyperpolarized pyruvate for noninvasive assessment of the TBI metabolism. If this result can be verified, it would have a major impact on the evaluation of patients involved in sports, military activities, or those who suffer from assault or accidents. Considering that this technique is safe and well-tolerated by patients, this technology may prove valuable for managing patients experiencing secondary injury processes, identifying early treatment response, and accelerating drug development efforts. In fact, pyruvate has been suggested as a neuro-protective substrate with therapeutic effect (Moro et al., 2016). Patients with moderate and severe TBI are expected to have larger metabolic alteration with potential challenges of imaging data interpretation due to morphological distortion and hemorrhage. Beyond acute and sub-acute TBI, the imaging methods and associated biomarkers to be developed under this application will be likely also applicable to concussion.
In this study, we investigated cerebral metabolism in patients with acute TBI using hyperpolarized [1-13C]pyruvate. We found altered cerebral metabolism in the brain nearest the site of trauma despite no visible anatomical damage in the brain on MRI, demonstrating the sensitivity of hyperpolarized pyruvate to altered metabolism in TBI. The acute metabolic changes in [1-13C]lactate and [13C]HCO3– images in the injured region indicate the potential clinical utility of hyperpolarized [1-13C]pyruvate in managing patients with TBI and provides objective evidence of injury even when conventional studies with CT and MRI are unrevealing.
Limitations of the Study
This study demonstrated the feasibility of hyperpolarized [1-13C]pyruvate for imaging metabolic changes following a mild brain injury in humans. Although we could detect clear alteration of pyruvate metabolism in the injured brain, biological interpretation of the imaging biomarker needs to be careful and requires further clarification. First, production of [1-13C]lactate signal reflects both metabolic flux from pyruvate to lactate and isotopic chemical exchange between pyruvate and lactate (Kennedy et al., 2012). The latter can be significantly affected by the intrinsic lactate pool size (Hurd et al., 2013). Second, unlike [13C]HCO3–, [1-13C]lactate signal detected in the brain is not exclusively produced by brain tissues as [1-13C]lactate produced in the vasculature or other organs (Wespi et al., 2018; Xu et al., 2011) can be delivered to the brain. Moreover, although lactate is a preferred energy substrate for neurons (Bélanger et al., 2011; Magistretti and Allaman, 2018), it is likely that the excessive cerebral presence of hyperpolarized pyruvate results in cellular transport of pyruvate (and lactate) into both neurons and glia via monocarboxylate transporters. The large spatial resolution is another limitation of the study. Although the large voxel size was helpful to achieve reliable detection of HCO3-, it was unavoidable to experience partial volume effects in the 13C images. Imaging with improved spatial resolution will be required for accurate regional assessment of TBI metabolism and to reduce the partial volume effects. The statistical analysis was not performed in this pilot study primarily due to the small number of subjects and the difficulty of patient recruitments in such an acute stage. Considering the diversity of brain injury types and the complexity of secondary injuries, studies with a larger number of patients with longitudinal follow-ups will be necessary for further evaluation of the imaging technique and for systematic characterization of the metabolic alterations.
Resource Availability
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Jae Mo Park (jaemo.park@utsouthwestern.edu).
Materials Availability
This study did not generate new unique reagents or materials.
Data and Code Availability
Original/source data or images in the paper is available upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
Funding: The Texas Institute for Brain Injury and Repair of Peter O'Donnell Jr. Brain Institute; The Mobility Foundation; National Institutes of Health of the United States (1R01NS107409-01A1, 5P41EB015908-32, 1S10OD018468-01); The Welch Foundation (I-2009-20190330). Personnel Support: We appreciate the research nurses and the MR technicians of the Advanced Imaging Research Center at UT Southwestern—Lucy Christie, Jeannie Baxter, Kelley Derner, Maida Tai, and Salvador Pena.
Author Contributions
M.C.P., C.J.M., and J.M.P. designed research; E.P.H., M.C.P., S.B., C.J.M., and J.M.P. recruited patients; E.P.H., C.E.H., J.L., J.R., G.D.R., C.R.M., and J.M.P. performed research; E.P.H., M.C.P., C.J.M., and J.M.P. analyzed data; E.P.H., M.C.P., C.R.M., C.J.M., and J.M.P. wrote the paper.
Declaration of Interests
G.D.R. is an employee of GE Healthcare.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101885.
Supplemental Information
References
- Ardenkjaer-Larsen J.H., Fridlund B., Gram A., Hansson G., Hansson L., Lerche M.H., Servin R., Thaning M., Golman K. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U S A. 2003;100:10158–10163. doi: 10.1073/pnas.1733835100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnik-Olson B.L., Harris N.G., Shijo K., Sutton R.L. Insights into the metabolic response to traumatic brain injury as revealed by (13)C NMR spectroscopy. Front. Neuroenergetics. 2013;5:8. doi: 10.3389/fnene.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsneider M., Hovda D.A., Shalmon E., Kelly D.F., Vespa P.M., Martin N.A., Phelps M.E., McArthur D.L., Caron M.J., Kraus J.F., Becker D.P. Cerebral hyperglycolysis following severe traumatic brain injury in humans: a positron emission tomography study. J. Neurosurg. 1997;86:241–251. doi: 10.3171/jns.1997.86.2.0241. [DOI] [PubMed] [Google Scholar]
- Bélanger M., Allaman I., Magistretti P.J. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- Brooks G.A., Martin N.A. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front. Neurosci. 2015;8:408. doi: 10.3389/fnins.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corps K.N., Roth T.L., McGavern D.B. Inflammation and neuroprotection in traumatic brain injury. JAMA Neurol. 2015;72:355–362. doi: 10.1001/jamaneurol.2014.3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVience S.J., Lu X., Proctor J., Rangghran P., Melhem E.R., Gullapalli R., Fiskum G.M., Mayer D. Metabolic imaging of energy metabolism in traumatic brain injury using hyperpolarized [1-13C]pyruvate. Sci. Rep. 2017;7:1907. doi: 10.1038/s41598-017-01736-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusick J.R., Glenn T.C., Lee W.N.P., Vespa P.M., Kelly D.F., Lee S.M., Hovda D.A., Martin N.A. Increased pentose phosphate pathway flux after clinical traumatic brain injury: a [1,2-13C2]glucose labeling study in humans. J. Cereb. Blood Flow Metab. 2007;27:1593–1602. doi: 10.1038/sj.jcbfm.9600458. [DOI] [PubMed] [Google Scholar]
- Gordon J.W., Chen H.-Y., Autry A., Park I., Van Criekinge M., Mammoli D., Milshteyn E., Bok R., Xu D., Li Y. Translation of Carbon-13 EPI for hyperpolarized MR molecular imaging of prostate and brain cancer patients. Magn. Reson. Med. 2019;81:2702–2709. doi: 10.1002/mrm.27549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco T., Vespa P.M., Prins M.L. Alternative substrate metabolism depends on cerebral metabolic state following traumatic brain injury. Exp. Neurol. 2020;329:113289. doi: 10.1016/j.expneurol.2020.113289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guglielmetti C., Chou A., Krukowski K., Najac C., Feng X., Riparip L.-K., Rosi S., Chaumeil M.M. In vivo metabolic imaging of Traumatic Brain Injury. Sci. Rep. 2017;7:17525. doi: 10.1038/s41598-017-17758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd R.E., Spielman D., Josan S., Yen Y.-F., Pfefferbaum A., Mayer D. Exchange-linked dissolution agents in dissolution-DNP (13)C metabolic imaging. Maregn Reson. Med. 2013;70:936–942. doi: 10.1002/mrm.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson P.J., O'Connell M.T., Seal A., Nortje J., Timofeev I., Al-Rawi P.G., Coles J.P., Fryer T.D., Menon D.K., Pickard J.D., Carpenter K.L.H. A combined microdialysis and FDG-PET study of glucose metabolism in head injury. Acta Neurochir (Wien) 2009;151:51–61. doi: 10.1007/s00701-008-0169-1. discussion 61. [DOI] [PubMed] [Google Scholar]
- Jalloh I., Carpenter K.L.H., Helmy A., Carpenter T.A., Menon D.K., Hutchinson P.J. Glucose metabolism following human traumatic brain injury: methods of assessment and pathophysiological findings. Metab. Brain Dis. 2015;30:615–632. doi: 10.1007/s11011-014-9628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy B.W.C., Kettunen M.I., Hu D.-E., Brindle K.M. Probing lactate dehydrogenase activity in tumors by measuring hydrogen/deuterium exchange in hyperpolarized l-[1-(13)C,U-(2)H]lactate. J. Am. Chem. Soc. 2012;134:4969–4977. doi: 10.1021/ja300222e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Han S.C., Gallan A.J., Hayes J.P. Neurometabolic indicators of mitochondrial dysfunction in repetitive mild traumatic brain injury. Concussion. 2017;2:CNC48. doi: 10.2217/cnc-2017-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.Y., Soliman H., Geraghty B.J., Chen A.P., Connelly K.A., Endre R., Perks W.J., Heyn C., Black S.E., Cunningham C.H. Lactate topography of the human brain using hyperpolarized 13C-MRI. Neuroimage. 2019;204:116202. doi: 10.1016/j.neuroimage.2019.116202. [DOI] [PubMed] [Google Scholar]
- Magistretti P.J., Allaman I. Lactate in the brain: from metabolic end-product to signalling molecule. Nat. Rev. Neurosci. 2018;19:235–249. doi: 10.1038/nrn.2018.19. [DOI] [PubMed] [Google Scholar]
- Moro N., Ghavim S.S., Harris N.G., Hovda D.A., Sutton R.L. Pyruvate treatment attenuates cerebral metabolic depression and neuronal loss after experimental traumatic brain injury. Brain Res. 2016;1642:270–277. doi: 10.1016/j.brainres.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank R.P., Bennett G.S., Freytag S.O., Campbell G.L. Pyruvate carboxylase: an astrocyte-specific enzyme implicated in the replenishment of amino acid neurotransmitter pools. Brain Res. 1985;329:364–367. doi: 10.1016/0006-8993(85)90552-9. [DOI] [PubMed] [Google Scholar]
- Smith D.H., Johnson V.E., Stewart W. Chronic neuropathologies of single and repetitive TBI: substrates of dementia? Nat. Rev. Neurol. 2013;9:211–221. doi: 10.1038/nrneurol.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varrone A., Asenbaum S., Vander Borght T., Booij J., Nobili F., Någren K., Darcourt J., Kapucu O.L., Tatsch K., Bartenstein P., Van Laere K., European Association of Nuclear Medicine Neuroimaging Committee EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur. J. Nucl. Med. Mol. Imaging. 2009;36:2103–2110. doi: 10.1007/s00259-009-1264-0. [DOI] [PubMed] [Google Scholar]
- Viglianti B.L., Wong K.K., Wimer S.M., Parameswaran A., Nan B., Ky C., Townsend D.M., Rubello D., Frey K.A., Gross M.D. Effect of hyperglycemia on brain and liver 18F-FDG standardized uptake value (FDG SUV) measured by quantitative positron emission tomography (PET) imaging. Biomed. Pharmacother. 2017;88:1038–1045. doi: 10.1016/j.biopha.2017.01.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wespi P., Steinhauser J., Kwiatkowski G., Kozerke S. Overestimation of cardiac lactate production caused by liver metabolism of hyperpolarized [1-13 C]pyruvate. Magn. Reson. Med. 2018;80:1882–1890. doi: 10.1002/mrm.27197. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang Y., Mahmood A., Chopp M. Investigational agents for treatment of traumatic brain injury. Expert Opin. Investig. Drugs. 2015;24:743–760. doi: 10.1517/13543784.2015.1021919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T., Mayer D., Gu M., Yen Y.-F., Josan S., Tropp J., Pfefferbaum A., Hurd R., Spielman D. Quantification of in vivo metabolic kinetics of hyperpolarized pyruvate in rat kidneys using dynamic 13C MRSI. NMR Biomed. 2011;24:997–1005. doi: 10.1002/nbm.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original/source data or images in the paper is available upon request.