Highlights
-
•
Head and neck squamous cell carcinomas (HNSC) is one of the most common malignant tumors with high incidence, relapse and mortality rate.
-
•
STAT proteins are implicated in various biological processes, including cell proliferation, metastasis, and immune regulation.
-
•
The mRNA level of STAT1/2/4/5A/6 were significantly upregulated in HNSC tissues. Genetic alteration revealed that STAT1/2/3/4/5A/5B/6 were altered in the queried TCGA HNSC samples.
-
•
Immune infiltrations analysis suggested a significant association between STAT5A expression and the abundance of specific immune cells.
-
•
Several kinase targets and transcription factor targets of STAT5A in HNSC were also identified.
-
•
Enrichment analysis suggested that STAT5A and co-expression genes were mainly responsible for adaptive immune response, T cell activation, cytokine-cytokine receptor interaction, chemokine signaling pathway, cell adhesion molecules, ribosome, and RNA transport.
Keywords: Head and neck squamous cell carcinomas, STAT family, Biomarker, Bioinformatics analysis
Abstract
Background
Head and neck squamous cell carcinomas (HNSC) are among the most common malignant tumors with high incidence, relapse, and mortality rate. STAT proteins are implicated in various biological processes, including cell proliferation, metastasis, and immune regulation.
Method
Various bioinformatics tools were used to explore the role of the STAT family in HNSC.
Result
The mRNA levels of STAT1/2/4/5A/6 were significantly upregulated in HNSC tissues. The levels of STAT1/2/4/5A/6 could be used for the detection of HNSC. HNSC patients with a high level of STAT5A had a poor overall survival and relapse-free survival. A moderate to high correlation was obtained between the STAT family and HNSC. Genetic alteration revealed that STAT1/2/3/4/5A/5B/6 were altered in 6%, 5%, 7%, 8%, 6%, 6%, and 4% of the queried TCGA HNSC samples, respectively. Immune infiltrations analysis suggested a significant association between STAT5A expression and abundance of specific immune cells. Further, copy number alteration of STAT5A could certainly inhibit infiltration level. Moreover, a close correlation was obtained between STAT5A level and the expression of immune markers in HNSC. Several kinase targets and transcription factor targets of STAT5A in HNSC were also identified. Enrichment analysis suggested that STAT5A and co-expression genes were mainly responsible for adaptive immune response, T cell activation, cytokine-cytokine receptor interaction, chemokine signaling pathway, cell-adhesion molecules, and ribosome and RNA transport.
Conclusion
Our results provided additional data for the expression and clinical significance of the STAT family in HNSC, and further study should be performed to verify these.
Introduction
Head and neck cancer (HNC) is a collection of malignant tumors occurring in the upper gastrointestinal tract, salivary glands, and thyroid [1]. Over 830,000 people are estimated to be diagnosed with HNC, and over 430,000 people are estimated to die of HNC annually globally [2]. About 90% of HNCs are head and neck squamous cell carcinomas (HNSC) [3]. Though aggressive therapies, including surgery, chemoradiotherapy, and immunotherapy, were applied for HNSC, over 50% curative patients were present with relapse [4]. Worse still, the mortality of HNSC is as high as 40–50% [5]. Once HNSC patients present with recurrence and/or metastasis, the median overall survival (OS) hardly exceeds 12 months [6]. These sobering findings demonstrate an urgent need for novel approaches to HNSC.
STAT proteins are implicated in various biological processes, including cell proliferation, metastasis, and immune regulation [7]. Seven members have been identified, including STAT1/2/3/4/5A/5B/6. Data increasingly reveal that variant STAT proteins and abnormally activated STAT pathways are linked to various diseases, such as malignancies, asthma and immune-related disease [7, 8]. Some proteins of the STAT family were suggested as therapy targets for certain cancers due to their significance in tumor initiation and progression [9]. Moreover, some proteins of the STAT family were also suggested as the biomarkers for the diagnosis and prognosis of cancers, including STAT1 and STAT2 for pancreatic cancer and STAT5B and STAT6 for lung cancer [10, 11].
Several studies have been performed to clarify the expression or the functions of certain STAT family members in HNSC. For example, inhibition of STAT3 could lead to greater cetuximab sensitivity [12]. Activation of STAT4 could potentially mitigate lymphatic metastasis in HNSC [13]. Moreover, gene-expression signature associated with recurrent disease in HNSC was profiled to clarify the gene expression profile of HNSC[14]. However, the role of the STAT family in HNSC was far from fully illuminated. Therefore, we will systematically explore and analyze STAT family expression and their diagnostic and prognostic value, as well as their related regulatory networks in HNSC. Our findings will provide additional data about the significant function of the STAT family in the tumorigenesis and progress of HNSC.
Methods
Patient information and datasets
Gene expression HNSC dataset GSE2379 [15] of Oncomine was extracted and analyzed in our study (May 8, 2020). The gene expression profiles (level 3 data) of primary HNSC patients were extracted from the TCGA database (https://tcga-data.nci.nih.gov/tcga/) (May 8, 2020). Clinical data such as gender, age, survival, and outcome were also downloaded from the TCGA data portal. Further analyses were performed with following bioinformatics analysis portals (TCGA visualization portals).
Oncomine
Oncomine (https://www.oncomine.org/) is a cancer microarray database which could be used for gene-expression profiles mining [16]. The mRNA level of the STAT family in HNSC patients and healthy people was analyzed using Oncomine. The fold-change was set as 2, and the P-value was set as 0.05.
UALCAN
UALCAN (http://ualcan.path.uab.edu/index.html) is the Cancer Genome Atlas (TCGA) database visual web portal [17]. The mRNA level of the STAT family in HNSC patients and healthy people and its correlation with clinical pathological parameters, including gender and tumor stage, was analyzed using UALCAN. These analyses were performed using TCGA HNSC dataset (n = 520), and p-value <0.05 indicated that the results were statistically significant.
Kaplan Meier-plotter
Kaplan Meier-plotter (KM-plotter, http://www.kmplot.com/) is a comprehensive meta-analysis web port for the discovery and validation of survival biomarkers [18]. The role of the STAT family in the prognosis of HNSC patients was analyzed using the KM-plotter with the medium STAT family expression as the group cut-off value. These analyses were performed using the TCGA HNSC dataset (n = 520), and p-value <0.05 indicated that the results were statistically significant.
cbioportal
cbioportal (https://www.cbioportal.org/) is a TCGA database visual web portal for genomics analysis [19]. The genetic alteration and gene co-expression of STAT family in HNSC were explored with cbioportal using the TCGA HNSC dataset (n = 520). Mutation data were obtained from whole exome sequencing. Mutation packager: HNSC/20160128/gdac.broadinstitute.org_HNSC.Mutation_Packager_Raw_Calls.Level_3.2016012800.0.0.tar.gz. Genomic alterations of the STAT family in HNSC, including mutations, CNAs (amplifications and homozygous deletions), and changes in gene expression or protein abundance, were analyzed. We also analyzed the effects of genetic alteration of the STAT family on patients’ prognosis in the “survival” module of cbioportal. p-value <0.05 indicated that the results were statistically significant.
TIMER
TIMER (http://cistrome.org/TIMER/) is a TCGA database visual web portal for analyses of tumor immunity [20]. The correlation analysis between STAT5A with immune cells infiltrations (“Gene” module) as well as immune biomarker level (“correlation” module) in HNSC were acquired in TIMER, which is performed by spearman analysis. These analyses were performed using the TCGA HNSC dataset (n = 520), and p-value <0.05 indicated that the results were statistically significant.
Linkedomics
LinkedOmics (http://www.linkedomics.org/ login.php) is a TCGA database visual web portal for genomics analysis [21]. The genes significantly correlated with STAT5A in HNSC were explored with the “LinkFinder” module using Spearman's correlation coefficient. Moreover, the enrichment analyses, including those of GO, KEGG pathways, kinase targets, miRNA targets and transcription factor-targets of STAT5A, and correlated genes, were explored with the “Link-Interpreter” module using GSEA analysis. The “Link-Interpreter” module of LinkedOmics could perform pathway and network analyses of differentially expressed genes. Web-based Gene SeT AnaLysis Toolkit (WebGestalt) is one of the most comprehensive functional category databases [22]. These analyses were performed using the TCGA HNSC dataset (n = 520). The rank criterion was an FDR <0.05, and 500 simulations were performed.
Result
Defining the STAT family in HNSC
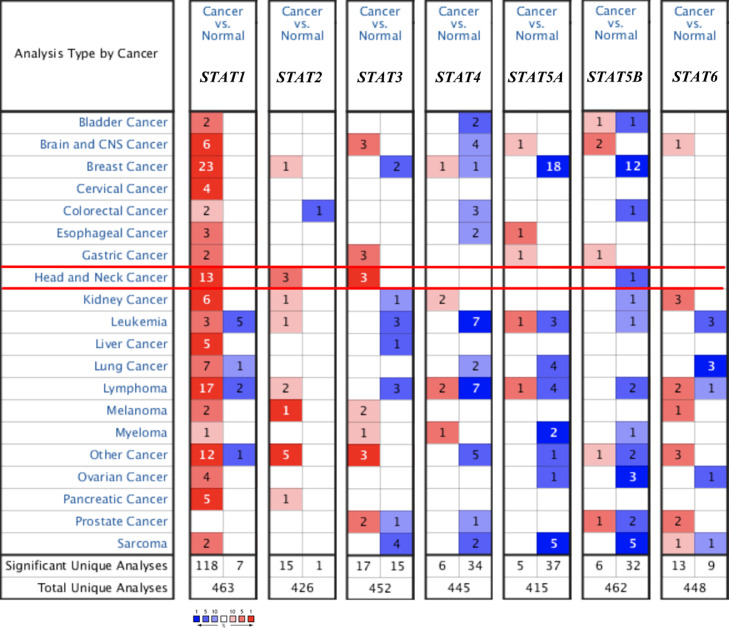
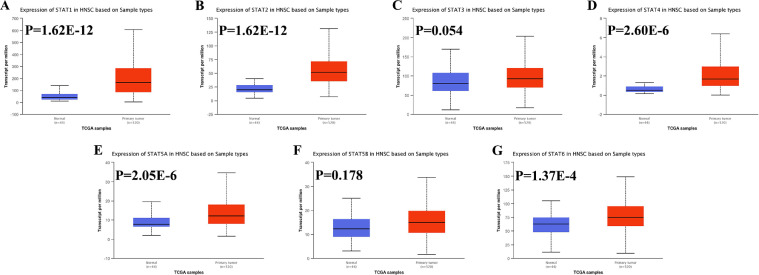
The mRNA level of STAT family in HNSC was got from Oncomine and UALCAN. As shown in Fig. 1, the data of Oncomine revealed that the mRNA level of STAT family was increased or decreased in different types of cancers, including HNSC. Ginoset al. found that the STAT1 mRNA level was upregulated with a fold-change of 3.090 (P = 9.25E-11, Table 1) [14]. Another study also revealed that the STAT1 mRNA level was 3.104 times in tumor tissues compared with normal tissues [15]. However, there is no data about the expression of the other STAT family proteins. According to the data from UALCAN, the mRNA level of STAT1(Fig. 2A, P = 1.62E-12), STAT2 (Fig. 2B, P = 1.62E-12), STAT4 (Fig. 2D, P = 2.69E-6), STAT5A (Fig. 2E, P = 2.05E-6), and STAT6 (Fig. 2G, P = 1.37E-4) were significantly upregulated in HNSC tissues.
Fig 1.
STAT family level in HNSC. The number in the figure was the number of datasets with statistically significant (p<0.01) mRNA over-expression (red) or down-expression (blue) of the STAT family, which was obtain with the P-value of 0.05 and fold change of 2.
Table 1.
The mRNA levels of STAT family in HNSC (ONCOMINE).
Fig 2.
STAT family level in HNSC. The relative level of STAT1(A), STAT2 (B), STAT3(C), STAT4(D), STAT5A(E), STAT5B(F), and STAT6(G) in HNSC tissues and normal tissues.
The expression of the STAT family in subgroup group of HNSC patients
The above data suggest that STAT1/2/4/5A/6 were significantly upregulated in HNSC tissues. Thus, we further detected the correlation between the STAT family and clinical pathological features in HNSC. The pathological features were comprised of race, gender, age, tumor grade, HPV status, nodal metastasis status, TP53 mutation status, and cancer stage. As expected, STAT1 and STAT2 were upregulated in HNSC patients in contrast to healthy individuals in subgroup analyses based on race, gender, age, tumor grade, HPV status, nodal metastasis status, TP53 mutation status, and cancer stage (Supplemental Fig. 1). Interestingly, the mRNA level of STAT4 (Supplemental Fig. 2A), STAT5A (Supplemental Fig. 2B), and STAT6 (Supplemental Fig. 2C) were upregulated in HNSC patients in contrast to healthy individuals in subgroup analyses. Therefore, the levels of STAT1/2/4/5A/6 could be used for the detection of HNSC.
Prognostic value of the STAT family in HNSC
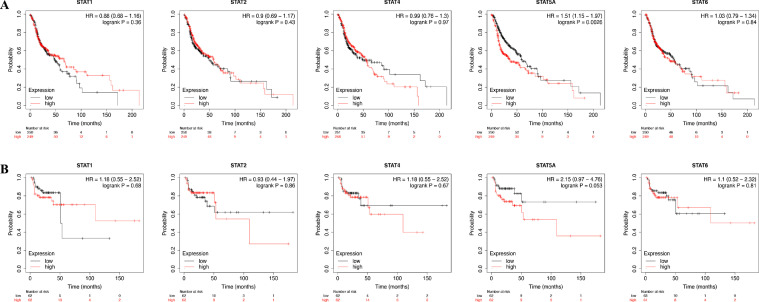
The prognostic value of different expressed STAT family in HNSC were analyzed with KM-plotter. The results demonstrated that HNSC patients with high level of STAT5A (HR=1.51, 95%:1.15–1.97, P = 0.0026) had a poor overall survival (OS) (Fig. 3A). Interestingly, we found that HNSC patients with a high level of STAT5A (HR=2.15, 95%:0.97–4.76, P = 0.053) had a poor relapse-free survival (RFS); however, the P-value is over 0.05 (Fig. 3B). Further, STAT1/2/4/6 had no effect on the OS and RFS of HNSC patients. Therefore, the level of STAT5A could be used for predicting the prognosis of HNSC patients.
Fig 3.
The prognostic value of the STAT family in HNSC. (A) The overall survival curve of HNSC patients with high and low level of the STAT family. (B) The relapse-free survival curve of HNSC patients with high and low level of STAT family.
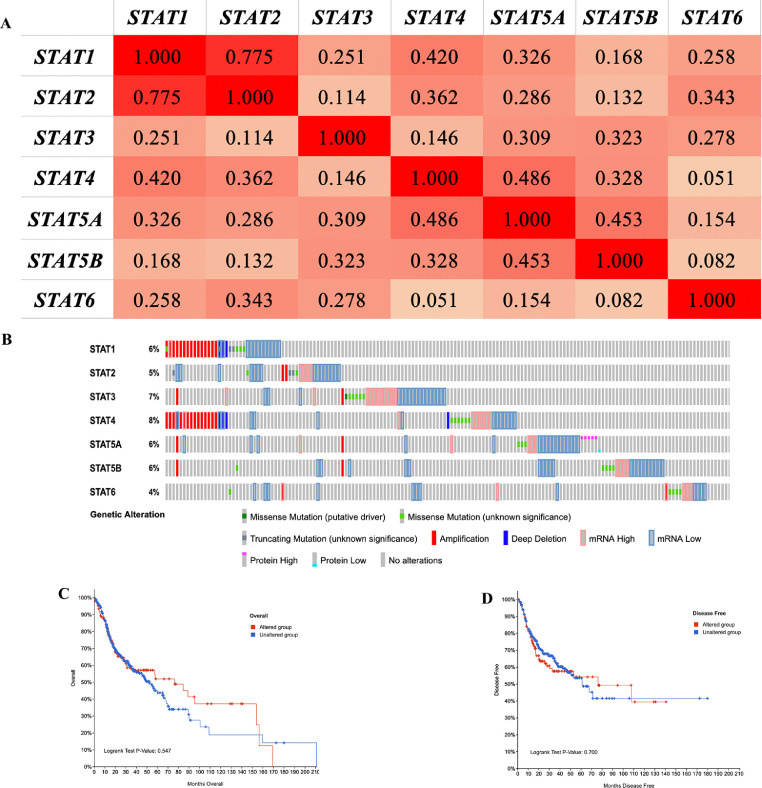
Co-expression and genetic alteration of the STAT family in HNSC
Fig. 4A shows the result of co-expression of the STAT family in HNSC. A moderate to high correlation was obtained in the STAT family. Genetic alteration revealed that STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B, and STAT6 were altered in 6%, 5%, 7%, 8%, 6%, 6%, and 4% of the queried TCGA HNSC samples, respectively (Fig. 4B). Further, genetic alteration forms of the STAT family were composed of missense mutation, truncating mutation, amplification, deep deletion, mRNA high, mRNA low, protein high and protein low (Fig. 4B).However, these genetic alterations would not affect the OS (Fig. 4C, P = 0.547) and disease-free survival (Fig. 4D, P = 0.700) of HNSC patients.
Fig 4.
Co-expression and genetic alteration analysis of the STAT family in HNSC. (A) Co-expression heat map of STAT family in HNSC. (B) Summary of alterations of STAT family in HNSC. (C,D) Kaplan–Meier plots comparing disease-free survival and overall survival in cases with/without STAT family alterations.
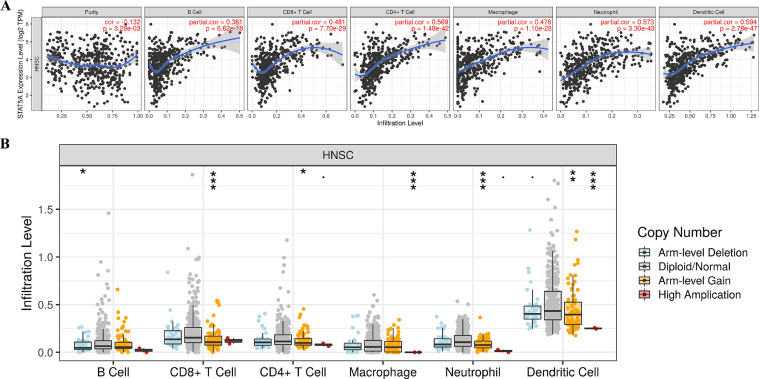
Immune infiltrations analysis of STAT5A in HNSC
The above results revealed that STAT5A level was increased in HNSC tissues and correlated with clinical pathological features. Moreover, the level of STAT5A could be used for predicting the prognosis of HNSC patients. Therefore, STAT5A was selected for further analysis. Previous studies have suggested the significance of STAT signaling in immunology [23]. Here, we conducted immune infiltrations analysis of STAT5A in HNSC. The data suggested a significant association between STAT5A expression and the abundance of B cells (Cor=0.381, P = 6.62e-18), CD8+ T cells (Cor=0.481, P = 7.70e-29), CD4+ T cells (Cor=0.569, P = 1.48e-42), Macrophages (Cor=0.476, P = 1.10e-28), Neutrophils (Cor=0.573, P = 3.30e-43), and Dendritic cells (Cor=0.594, P = 2.78e-47) (Fig. 5A). Additionally, we also found that copy number alteration of STAT5A could certainly inhibit infiltration level (Fig. 5B). Interestingly, there is a close correlation between STAT5A level and the expression of immune markers in HNSC (Table 2). Therefore, STAT5A may exert a specific function in immune infiltration of HNSC microenvironment.
Fig 5.
Immune infiltration of STAT5A in HNSC. (A) The correlation between STAT5A and abundance of different immune cell levels in HNSC. (B) The correlation between copy number alteration of STAT5A and immune cell infiltration in HNSC.
Table 2.
The Kinase, miRNA and transcription factor-target networks of STAT5A in HNSC (LinkedOmics).
| Enriched category | Geneset | LeadingEdgeNum | P value |
|---|---|---|---|
| Kinase Target | Kinase_LCK Kinase_SYK Kinase_TYN Kinase_LYN Kinase_BCR |
20 14 23 22 6 |
0 0 0 0 0 |
| miRNA Target | CTGTTAC, MIR-194 TTGGAGA, MIR-515-5P, MIR-519E GTAAACC, MIR-299-5P AGTCTAG, MIR-151 ATAACCT, MIR-154 |
20 36 10 4 16 |
0 0 0 0 0 |
| Transcription Factor Target | V$IRF_Q6 V$ELF1_Q6 V$PU1_Q6 V$PEA3_Q6 V$IRF_Q6 |
57 62 56 60 66 |
0 0 0 0 0 |
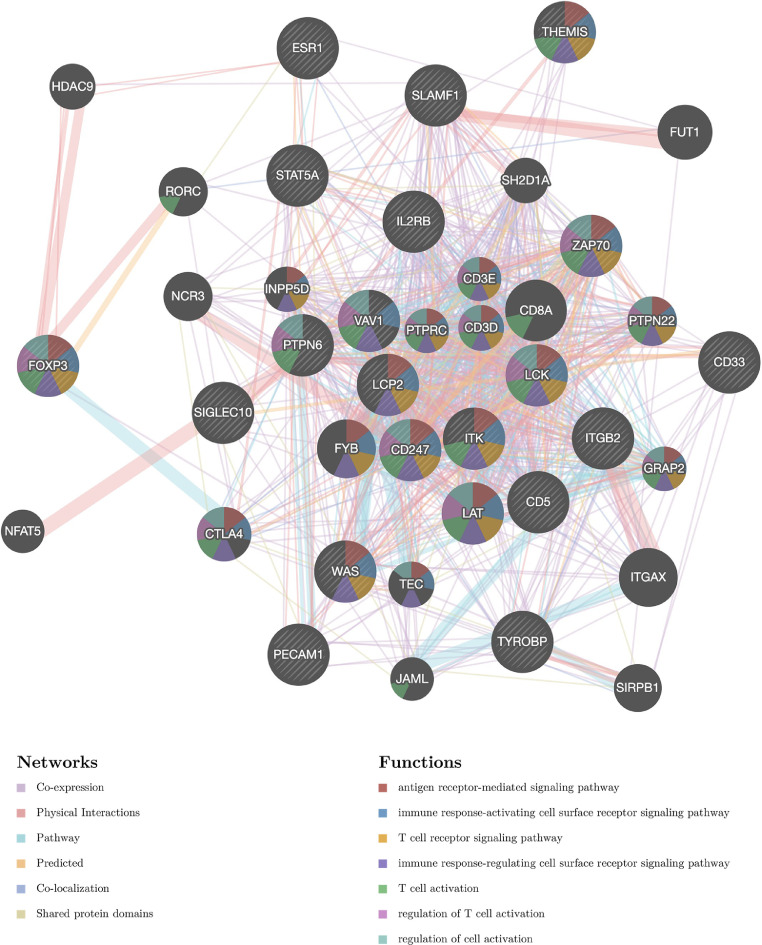
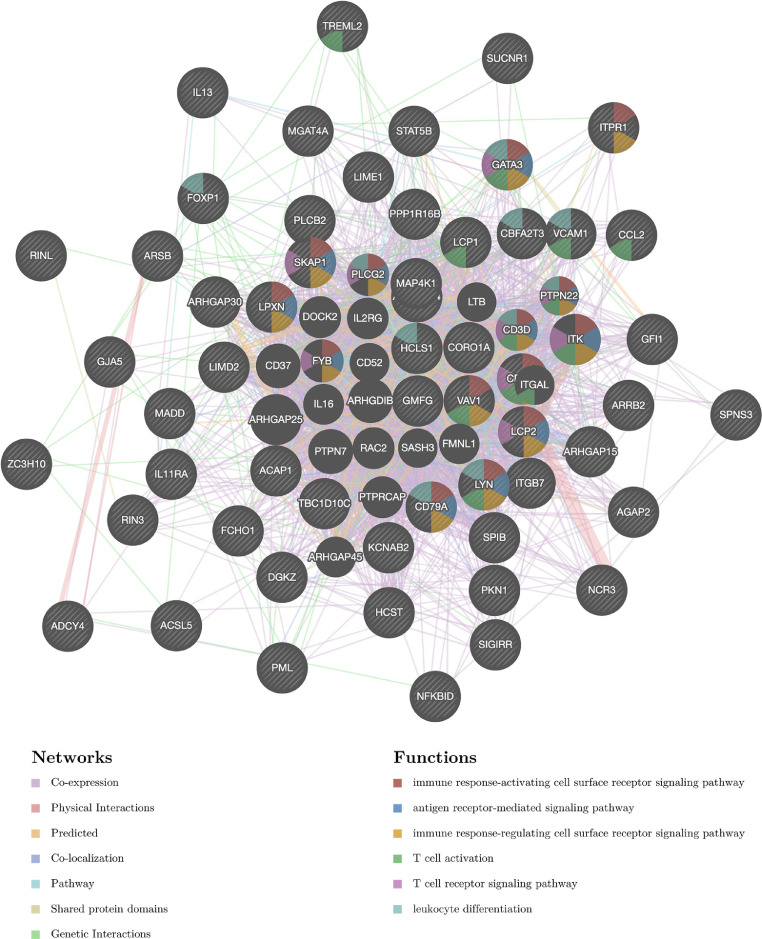
Kinase targets and transcription factor targets of STAT5A in HNSC
The above results suggested a significant role of STAT5A in HNSC. Thus, we further explored the Kinase targets and transcription factor (TF) targets of STAT5A in HNSC. As shown in Table 3, the most significant five Kinase targets of STAT5A in HNSC are Kinase_LCK, Kinase_SYK, Kinase_TYN, Kinase_LYN, and Kinase_BCR. Moreover, the gene sets enriched for kinase LCK are mainly linked to antigen receptor-mediated signaling pathway, immune response, T cell receptor signaling pathway, and T cell activation (Fig. 6). With regard to the TF targets of STAT5A in HNSC, the results suggested V$IRF_Q6, V$ELF1_Q6, V$PU1_Q6, V$PEA3_Q6, and V$IRF_Q6 as the top 5 significant targets. Interestingly, the gene sets enriched for TF V$IRF_Q6 are mainly linked to immune response, antigen receptor-mediated signaling pathway, T cell receptor signaling pathway, T cell activation, and leukocyte differentiation (Fig. 7).
Table 3.
Correlation analysis between STAT5A and gene biomarkers of immune cells in HNSC (TIMER).
| Immune cells | Biomarkers | None | Purity | ||
|---|---|---|---|---|---|
| Cor | P-value | Cor | P-value | ||
| CD8+ T cell | CD8A CD8B |
0.593 0.595 |
*** *** |
0.585 0.584 |
*** *** |
| T cell (general) | CD3D CD3E CD2 |
0.621 0.66 0.657 |
*** *** *** |
0.616 0.655 0.649 |
*** *** *** |
| B cell | CD19 CD79A |
0.465 0.448 |
*** *** |
0.448 0.43 |
*** *** |
| Monocyte | CD86 CD115(CSF1R) |
0.542 0.597 |
*** *** |
0.527 0.585 |
*** *** |
| TAM | CCL2 CD68 IL10 |
0.451 0.297 0.408 |
*** *** *** |
0.434 0.282 0.39 |
*** *** *** |
| M1 Macrophage | INOS (NOS2) IRF5 COX2(PTGS2) |
0.289 0.409 −0.042 |
*** *** 0.344 |
0.288 0.416 −0.033 |
*** *** 0.464 |
| M2 Macrophage | CD163 VSIG4 MS4A4A |
0.451 0.403 0.468 |
*** *** *** |
0.443 0.393 0.455 |
*** *** *** |
| Neutrophils | CD66b (CEACAM8) CD11b (ITGAM) CCR7 |
0.089 0.515 0.57 |
* *** *** |
0.059 0.492 0.566 |
0.191 *** *** |
| Natural killer cell | KIR2DL1 KIR2DL3 KIR2DL4 KIR3DL1 KIR3DL2 KIR3DL3 KIR2DS4 |
0.256 0.377 0.442 0.346 0.487 0.232 0.28 |
*** *** *** *** *** *** *** |
0.264 0.371 0.443 0.334 0.482 0.22 0.264 |
*** *** *** *** *** *** *** |
| Dendritic cell | HLA-DPB1 HLA-DQB1 HLA-DRA HLA-DPA1 BDCA-1(CD1C) BDCA-4(NRP1) CD11c (ITGAX) |
0.632 0.493 0.611 0.62 0.438 0.28 0.485 |
*** *** *** *** *** *** *** |
0.624 0.473 0.601 0.609 0.414 0.269 0.473 |
*** *** *** *** *** *** *** |
| Th1 | T-bet (TBX21) STAT4 STAT1 IFN-g (IFNG) TNF-a (TNF) |
0.623 0.537 0.397 0.479 0.269 |
*** *** *** *** *** |
0.611 0.528 0.388 0.463 0.255 |
*** *** *** *** *** |
| Th2 | GATA3 STAT6 STAT5A IL13 |
0.345 0.361 - 0.373 |
*** *** - *** |
0.333 0.387 - 0.365 |
*** *** - *** |
| Tfh | BCL6 IL21 |
0.206 0.445 |
*** *** |
0.231 0.424 |
*** *** |
| Th17 | STAT3 IL17A |
0.494 0.273 |
*** *** |
0.494 0.258 |
*** *** |
| Treg | FOXP3 CCR8 STAT5B TGFb (TGFB1) |
0.618 0.52 0.574 0.014 |
*** *** *** 0.752 |
0.607 0.501 0.575 −0.003 |
*** *** *** 0.944 |
| T cell exhaustion | PD-1 (PDCD1) CTLA4 LAG3 TIM-3 (HAVCR2) GZMB |
0.621 0.574 0.568 0.603 0.52 |
*** *** *** *** *** |
0.613 0.567 0.562 0.592 0.509 |
*** *** *** *** *** |
Fig 6.
PPI network of kinase_LCK networks. PPI network and functional analysis about the gene sets of kinase_LCK networks. The different colors for the network nodes indicate the biological functions of the set of enrichment genes.
Fig 7.
PPI network of transcription factor IRF networks. PPI network and functional analysis about the gene sets of transcription factor IRF networks. The different colors for the network nodes indicate the biological functions of the set of enrichment genes.
Enrichment analysis of STAT5A in HNSC
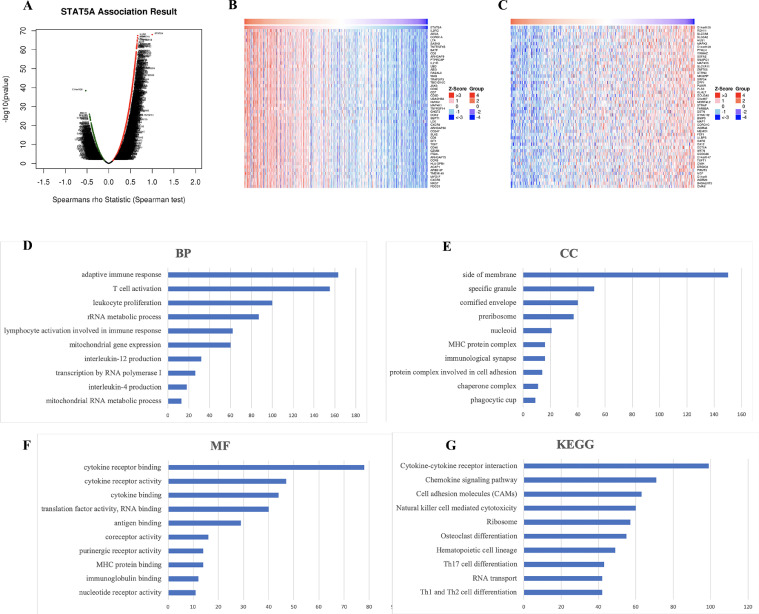
The above results suggested a significant role of STAT5A in HNSC. Thus, we conducted enrichment analysis of STAT5A in HNSC. We first explored co-expression genes correlated with STAT5A with the data of TCGA HNSC patients with LinkedOmics. The results in a volcano plot found that 10,151 genes were correlated with STAT5A were obtained in HNSC, including 6132 genes that were positively linked to STAT5A and 4042 genes that were negatively linked to STAT5A (Fig. 8A). The heat map in Fig. 8B and C shows the 50 significant gene sets positively and negatively linked to STAT5A (Fig. 8B and C). GO analysis conducted by GSEA suggested that STAT5A and co-expression genes were mainly responsible for adaptive immune response, T cell activation, leukocyte proliferation, rRNA metabolic process, cytokine receptor binding and activity, translation factor activity, antigen binding, and immunoglobulin binding (Fig. 8D–F). Further, KEGG analysis conducted by GSEA suggested that STAT5A and co-expression genes were mainly responsible for cytokine-cytokine receptor interaction, chemokine signaling pathway, cell adhesion molecules, ribosome, Th17 cell differentiation, and RNA transport (Fig. 8G).
Fig. 8.
The enrichment analysis of STAT5A in HNSC. (A) The genes positively and negatively correlated with STAT5A in HNSC. (B) The top 50 genes positively correlated with STAT5A in HNSC. (C) The top 50 genes negatively correlated with STAT5A in HNSC. (D–F) Gene Ontology-enriched terms. (C,D) Kyoto Encyclopedia of Genes and Genomes (KEGG)-enriched terms.
Discussion
The STAT family has been found to be involved in human cancer tumorigenesis, progression, metastasis, survival, and resistance to treatment [24]. In gastric cancer, STAT5A could facilitate the tumorigenesis of tumor cells [25]. STAT3 was suggested as a novel drug target for cancer therapy [26]. In HNSC, inhibition of STAT3 could lead to greater cetuximab sensitivity [12]. Moreover, activation of STAT4 could potentially mitigate lymphatic metastasis in HNSC [13]. However, the role of STAT family in HNSC was far from fully illuminated. Therefore, our study was performed.
The level of STAT family in HNSC was first detected, which suggested that the mRNA levels of STAT1/2/4/5A/6 were significantly upregulated in HNSC tissues. We also found that the level of STAT1/2/4/5A/6 could be used for the detection of HNSC. Moreover, HNSC patients with high levels of STAT5A had a poor overall survival and relapse-free survival. As a matter of fact, some of the STAT family had been found to be as biomarkers for cancers. In breast cancer, individual STATs may function as a biomarker predicting favorable prognosis [27]. Pang et al. found that STAT2 was a marker significantly associated with the prognosis of pancreatic cancer [11]. Moreover, high STAT4 level demonstrated a better disease-free survival in gastric cancer [28].
Another important finding of our study was that STAT5A was associated with immune cell infiltrations and that copy number alteration of STAT5A could certainly inhibit infiltration level. Our result was consistent with previous data. Rani et al. found that STAT5A was involved in the development of Tregs [29]. Moreover, sustained activation of STAT5A was linked to anti-tumor immunosuppression [29]. In hepatocellular carcinoma, STAT5A was associated with immune-related biological processes [30]. In fact, certain immune cells or immune biomarkers associated with STAT5A had been suggested as the immune-therapy targets for HNSC. PD-L1 expression could predict benefit from checkpoint inhibitor therapy in HNSC [31]. Another study demonstrated CTLA4 as a novel therapeutic strategy in HNSCC [31]. Therefore, STAT5A may exert a specific function in immune infiltration of HNSC microenvironment.
Several kinases targets, including Kinase_LCK, Kinase_SYK, Kinase_TYN, Kinase_LYN, and Kinase_BCR, were also identified. Interestingly, these kinases were associated with cell-cycle arrest, apoptosis, mitosis, and immune response [[32], [33]–34]. Additionally, kinase LCK is a positive regulator of inflammatory signaling and is suggested as the potential target for cancer treatment [35]. Moreover, LCK is the major contributor to the development and activation of T-cells [36]. Thus, STAT5A regulated immune infiltration in HNSC through kinase LCK.
We also identified several transcription factor targets of STAT5A in HNSC, including V$IRF_Q6, V$ELF1_Q6, V$PU1_Q6, V$PEA3_Q6, and V$IRF_Q6. These transcription factor targets in tumor cells could result in cell cycle disorder and affect cell aberrant proliferation, decreased differentiation, decreased apoptosis, and rapid multiplication and development [37]. In nasopharyngeal carcinoma, ELF1 could promote tumor cell proliferation and metastasis [38]. In colorectal carcinoma, Pea3 facilitate tumor cell invasion and metastasis. However, these transcription factor targets have rarely been studied in HNSC. Therefore, STAT5A and these transcription factors may also regulate cell proliferation and invasion in HNSC.
There are some limitations in this study. The sample data were basically derived from the TCGA database, and it would be better to verify the result with other databases. In addition, we did not perform experimental verification.
Conclusion
Overall, our results provided additional data for the expression and clinical significance of the STAT family in HNSC, and further study should be performed to verify these findings.
CRediT authorship contribution statement
Haosheng Ni: Data curation, Formal analysis, Visualization, Writing - original draft. Hui Sun: Data curation, Formal analysis, Visualization, Writing - original draft. Miaosen Zheng: Methodology. Tingting Bian: Software. Jian Liu: Investigation, Project administration, Resources. Xiaoli Li: Validation. Jianguo Zhang: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing - review & editing. Yifei Liu: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This study was funded by grants from Jiangsu Post-doctoral Foundation Research Project, China (No. 2020Z136), Six talent peaks project in Jiangsu Province, China (No. WSN-059), Scientific research topic of Jiangsu provincial health and Family Planning Commission, China (No. H201626), Key talents of Medical Science in Jiangsu Province, China (No. QNRC2016682), Key Scientific and Technological Projects in Nantong City, Jiangsu, China (No. MS22018001, MS22019015), Jiangsu Post-doctoral Foundation Research Project, China (No. 2019Z142), Scientific and Technological Project in Nantong City, Jiangsu, China (No.JCZ19103).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2020.100976.
Contributor Information
Jianguo Zhang, Email: 13815212431@163.com.
Yifei Liu, Email: ntdxliuyifei@sina.com.
Appendix. Supplementary materials
References
- 1.Cramer J.D., Burtness B., Le Q.T., Ferris R.L. The changing therapeutic landscape of head and neck cancer. Nat. Rev. Clin. Oncol. 2019;16(11):669–683. doi: 10.1038/s41571-019-0227-z. [DOI] [PubMed] [Google Scholar]
- 2.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Wyss A., Hashibe M., Chuang S.C., Lee Y.C., Zhang Z.F., Yu G.P., Winn D.M., Wei Q., Talamini R., Szeszenia-Dabrowska N., Sturgis E.M., Smith E., Shangina O., Schwartz S.M., Schantz S., Rudnai P., Purdue M.P., Eluf-Neto J., Muscat J., Morgenstern H., Michaluart P., Jr., Menezes A., Matos E., Mates I.N., Lissowska J., Levi F., Lazarus P., La Vecchia C., Koifman S., Herrero R., Hayes R.B., Franceschi S., Wünsch-Filho V., Fernandez L., Fabianova E., Daudt A.W., Dal Maso L., Curado M.P., Chen C., Castellsague X., de Carvalho M.B., Cadoni G., Boccia S., Brennan P., Boffetta P., Olshan A.F. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the international head and neck cancer epidemiology consortium. Am. J. Epidemiol. 2013;178(5):679–690. doi: 10.1093/aje/kwt029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill S.J., D'Andrea A.D. Predictive potential of head and neck squamous cell carcinoma organoids. Cancer Discov. 2019;9(7):828–830. doi: 10.1158/2159-8290.CD-19-0527. [DOI] [PubMed] [Google Scholar]
- 5.Moskovitz J., Moy J., Ferris R.L. Immunotherapy for head and neck squamous cell carcinoma. Curr. Oncol. Rep. 2018;20(2):22. doi: 10.1007/s11912-018-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saada-Bouzid E., Peyrade F., Guigay J. Molecular genetics of head and neck squamous cell carcinoma. Curr. Opin. Oncol. 2019;31(3):131–137. doi: 10.1097/CCO.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 7.Miklossy G., Hilliard T.S., Turkson J. Therapeutic modulators of STAT signalling for human diseases. Nat. Rev. Drug Discov. 2013;12(8):611–629. doi: 10.1038/nrd4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bromberg J., Darnell J.E., Jr. The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19(21):2468–2473. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 9.Thomas S.J., Snowden J.A., Zeidler M.P., Danson S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br. J. Cancer. 2015;113(3):365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M., Chen H., Zhou L., Chen K., Su F. Expression profile and prognostic values of STAT family members in non-small cell lung cancer. Am. J. Transl. Res. 2019;11(8):4866–4880. [PMC free article] [PubMed] [Google Scholar]
- 11.Pang C., Gu Y., Ding Y., Ma C., Yv W., Wang Q., Meng B. Several genes involved in the JAK-STAT pathway may act as prognostic markers in pancreatic cancer identified by microarray data analysis. Medicine. 2018;97(50):e13297. doi: 10.1097/MD.0000000000013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner J.A., Yang E.S., Trummell H.Q., Nowsheen S., Willey C.D., Raisch K.P. Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother. Oncol. 2011;99(3):339–343. doi: 10.1016/j.radonc.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 13.Anderson K., Ryan N., Volpedo G., Varikuti S., Satoskar A.R., Oghumu S. Immune suppression mediated by STAT4 deficiency promotes lymphatic metastasis in HNSCC. Front. Immunol. 2019;10:3095. doi: 10.3389/fimmu.2019.03095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginos M.A., Page G.P., Michalowicz B.S., Patel K.J., Volker S.E., Pambuccian S.E., Ondrey F.G., Adams G.L., Gaffney P.M. Identification of a gene expression signature associated with recurrent disease in squamous cell carcinoma of the head and neck. Cancer Res. 2004;64(1):55–63. doi: 10.1158/0008-5472.can-03-2144. [DOI] [PubMed] [Google Scholar]
- 15.Cromer A., Carles A., Millon R., Ganguli G., Chalmel F., Lemaire F., Young J., Dembélé D., Thibault C., Muller D., Poch O., Abecassis J., Wasylyk B. Identification of genes associated with tumorigenesis and metastatic potential of hypopharyngeal cancer by microarray analysis. Oncogene. 2004;23(14):2484–2498. doi: 10.1038/sj.onc.1207345. [DOI] [PubMed] [Google Scholar]
- 16.Rhodes D.R., Yu J., Shanker K., Deshpande N., Varambally R., Ghosh D., Barrette T., Pandey A., Chinnaiyan A.M. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6(1):1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandrashekar D.S., Bashel B., Balasubramanya S.A.H., Creighton C.J., Ponce-Rodriguez I., Chakravarthi B., Varambally S. UALCAN: a portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia. 2017;19(8):649–658. doi: 10.1016/j.neo.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou G.X., Liu P., Yang J., Wen S. Mining expression and prognosis of topoisomerase isoforms in non-small-cell lung cancer by using oncomine and Kaplan-Meier plotter. PLoS One. 2017;12(3) doi: 10.1371/journal.pone.0174515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Q., Sun S., Li Y., Li X., Li Z., Liang H. Identification of therapeutic targets and prognostic biomarkers among CXC chemokines in the renal cell carcinoma microenvironment. Front. Oncol. 2019;9:1555. doi: 10.3389/fonc.2019.01555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li B., Severson E., Pignon J.C., Zhao H., Li T., Novak J., Jiang P., Shen H., Aster J.C., Rodig S., Signoretti S., Liu J.S., Liu X.S. Comprehensive analyses of tumor immunity: implications for cancer immunotherapy. Genome Biol. 2016;17(1):174. doi: 10.1186/s13059-016-1028-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vasaikar S.V., Straub P., Wang J., Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res. 2018;46(D1):D956–d963. doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Vasaikar S., Shi Z., Greer M., Zhang B. WebGestalt 2017: a more comprehensive, powerful, flexible and interactive gene set enrichment analysis toolkit. Nucleic Acids Res. 2017;45(W1):W130–w137. doi: 10.1093/nar/gkx356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen K.L., Brockwell N.K., Parker B.S. JAK-STAT signaling: a double-edged sword of immune regulation and cancer progression. Cancers. 2019;11(12) doi: 10.3390/cancers11122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhoeven Y., Tilborghs S., Jacobs J., De Waele J., Quatannens D., Deben C., Prenen H., Pauwels P., Trinh X.B., Wouters A., Smits E.L.J., Lardon F., van Dam P.A. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020;60:41–56. doi: 10.1016/j.semcancer.2019.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Dong S.R., Ju X.L., Yang W.Z. STAT5A reprograms fatty acid metabolism and promotes tumorigenesis of gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2019;23(19):8360–8370. doi: 10.26355/eurrev_201910_19147. [DOI] [PubMed] [Google Scholar]
- 26.Lee H., Jeong A.J., Ye S.K. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep. 2019;52(7):415–423. doi: 10.5483/BMBRep.2019.52.7.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang S., Yu L., Shi W., Li X., Yu L. Prognostic roles of signal transducers and activators of transcription family in human breast cancer. Biosci. Rep. 2018;38(6) doi: 10.1042/BSR20171175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishi M., Batsaikhan B.E., Yoshikawa K., Higashijima J., Tokunaga T., Takasu C., Kashihara H., Ishikawa D., Shimada M. High STAT4 expression indicates better disease-free survival in patients with gastric cancer. Anticancer Res. 2017;37(12):6723–6729. doi: 10.21873/anticanres.12131. [DOI] [PubMed] [Google Scholar]
- 29.Rani A., Murphy J.J. STAT5 in cancer and immunity. J. Interferon Cytokine Res. 2016;36(4):226–237. doi: 10.1089/jir.2015.0054. [DOI] [PubMed] [Google Scholar]
- 30.Dong Z., Chen Y., Yang C., Zhang M., Chen A., Yang J., Huang Y. STAT gene family mRNA expression and prognostic value in hepatocellular carcinoma. OncoTargets Ther. 2019;12:7175–7191. doi: 10.2147/OTT.S202122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon B., Young R.J., Rischin D. Head and neck squamous cell carcinoma: genomics and emerging biomarkers for immunomodulatory cancer treatments. Semin. Cancer Biol. 2018;52(Pt 2):228–240. doi: 10.1016/j.semcancer.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 32.al-Ramadi B.K., Zhang H., Bothwell A.L. Cell-cycle arrest and apoptosis hypersusceptibility as a consequence of Lck deficiency in nontransformed T lymphocytes. Proc. Natl. Acad. Sci. USA. 1998;95(21):12498–12503. doi: 10.1073/pnas.95.21.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prinos P., Garneau D., Lucier J.F., Gendron D., Couture S., Boivin M., Brosseau J.P., Lapointe E., Thibault P., Durand M., Tremblay K., Gervais-Bird J., Nwilati H., Klinck R., Chabot B., Perreault J.P., Wellinger R.J., Elela S.A. Alternative splicing of SYK regulates mitosis and cell survival. Nat. Struct. Mol. Biol. 2011;18(6):673–679. doi: 10.1038/nsmb.2040. [DOI] [PubMed] [Google Scholar]
- 34.Feng S., Cheng X., Zhang L., Lu X., Chaudhary S., Teng R., Frederickson C., Champion M.M., Zhao R., Cheng L., Gong Y., Deng H., Lu X. Myeloid-derived suppressor cells inhibit T cell activation through nitrating LCK in mouse cancers. Proc. Natl. Acad. Sci. USA. 2018;115(40):10094–10099. doi: 10.1073/pnas.1800695115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bommhardt U., Schraven B., Simeoni L. Beyond TCR signaling: emerging functions of Lck in cancer and immunotherapy. Int. J. Mol. Sci. 2019;20(14) doi: 10.3390/ijms20143500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palacios E.H., Weiss A. Function of the Src-family kinases, Lck and Fyn, in T-cell development and activation. Oncogene. 2004;23(48):7990–8000. doi: 10.1038/sj.onc.1208074. [DOI] [PubMed] [Google Scholar]
- 37.Saleeb R., Kim S.S., Ding Q., Scorilas A., Lin S., Khella H.W., Boulos C., Ibrahim G., Yousef G.M. The miR-200 family as prognostic markers in clear cell renal cell carcinoma. Urol. Oncol. 2019;37(12):955–963. doi: 10.1016/j.urolonc.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Chen C.H., Su L.J., Tsai H.T., Hwang C.F. ELF-1 expression in nasopharyngeal carcinoma facilitates proliferation and metastasis of cancer cells via modulation of CCL2/CCR2 signaling. Cancer Manag. Res. 2019;11:5243–5254. doi: 10.2147/CMAR.S196355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.