Abstract
Genetic instability is a striking feature of human cancers, with an impact on the genesis, progression and prognosis. The clinical importance of genomic instability and aneuploidy is underscored by its association with poor patient outcome in multiple cancer types, including breast and colon cancer. Interestingly, there is growing evidence that prognostic gene expression signatures simply reflect the degree of genomic instability. Additionally, also the proteome is affected by aneuploidy and has therefore become a powerful tool to screen for new targets for therapy, diagnosis and prognostication. In this context, the chapter presents the impact of genomic instability on disease prognostication occurring in human cancers.
Keywords: Genetic instability, Prognosis, Genomics, Proteomics, Cancer
1. Genomic Instability and Disease Prognostication in Colorectal Cancer
Colorectal cancer is globally the fourth leading cause of cancer mortality, with about 1.2 million new cases and 608,000 deaths worldwide per year (Jemal et al. 2011). The incidence of colorectal cancer is higher in developed countries although the disease is rarely diagnosed before the age of 40. Most patients with R0 resection of node-negative CRC are cured of their cancer by surgery, but an unacceptable number of patients experience relapse due to regional recurrence or to distant metastasis, or both. Today, Dukes classification of CRC and the Tumor-Node-Metastasis (TNM) classification system for solid tumors are the routine staging systems and the basis to evaluate patient prognosis in CRC. However, in terms of prognosis it has been shown that genomic instability plays an important role as do various demographical, pathological and molecular characteristics: next to age (Kearney et al. 1993; Cascinu et al. 1996), tumor stage (Sun 2006; Gerling et al. 2010), tumor location (Zarbo et al. 1997), histopathological grade (Schillaci et al. 1990), disease free survival and overall survival (Garrity et al. 2004; Chen et al. 2002), several studies have shown that patients with aneuploid tumors had a worse outcome compared to patients with euploid tumors (Witzig et al. 1991; Sinicrope et al. 2006; Bosari et al. 1992). Similarly, Gerling et al. (2010) presented survival data of CRC patients showing that advanced stage but diploid carcinomas had a similar prognosis as compared with early stage tumors, but the outlook for aneuploid carcinoma is typically unfavorable, indicating that aneuploidy in CRC more strongly impacts on prognosis than the tumor stage itself.
In one of the most comprehensive meta-analyses of 10,126 patients, Walther et al. (2008) demonstrated that genomic instability is associated with worse prognosis in CRC and that it could be used to stratify patient prognosis, in addition to pathological staging: CRC patients with aneuploid tumor cells—quantified by either flow cytometry (n = 9,526 patients) or image cytometry (n = 600 patients)—appeared to have a poorer survival irrespective of their ethnic background, anatomical location and treatment with 5-fluoroucacil (5-FU)-based adjuvant chemotherapy. In line with this, Guastadisegni et al. (2010) confirmed the association between MSI and favorable prognosis. Thirty-one eligible studies reporting survival for 12,782 patients characterized for MSI indicated that MSI has the potential to be used in the clinical setting as a prognostic and predictive marker. Being part of the meta-analysis, the study by Sinicrope et al. (2006) found that DNA ploidy was the strongest prognostic marker.
Interestingly, the pattern of chromosomal aneuploidy in sporadic (SCC) and ulcerative (UCC) colitis-associated colorectal carcinomas seems to be strinkingly conserved. Nevertheless, in a single cohort of 31 UCCs and 257 SCCs Gerling et al. (2010) associated the frequency of aneuploidy to clinical parameters and showed that UCCs have a higher frequency of aneuploidy compared to SCCs (100 % versus 74.6 %; p < 0.006). A logistic regression analysis assessed age, sex, UICC stage, T- and N-status, histologic tumor grading, underlying inflammation, and DNA ploidy status. Out of these features, only age and DNA ploidy status were significant contributing parameters, indicating both patients of higher age at diagnosis and patients with aneuploid malignancy have a poor survival prognosis. Additional logistic regression analysis comprising these two significant parameters only confirmed age [odds ratio (OR), 1.05; 95 % CI, 1.02–1.09; p = 0.003] and DNA ploidy (OR, 4.07; 95 % CI, 1.46–11.36; p = 0.007) to be independent prognostic parameters. Among those, DNA aneuploidy with an OR of 4.07 seemed to be the strongest independent prognostic marker for R0-resected colorectal cancer patients overall. The dominance of aneuploidy as an independent poor prognostic predictor in patients with SCC and UCC was further supported by the fact that patients with diploid tumors at advanced stages (UICC stage III/IV) did present a survival comparable to that of patients with aneuploid tumors at early stages. The latter finding might even suggest that the presence of aneuploid tumor cell populations may influence the patient’s prognosis more dominantly than tumor stage. This was in part supported by Laubert et al. (2013) who could demonstrate that aneuploidy and elevated CEA levels, apart from increasing T category, could predict metachronous metastases and thus assist individual risk assessment.
In this context, other authors report a comparable incidence of DNA aneuploidy in SCC. Interestingly, the high incidence of aneuploidy is not restricted to late-stage lesions but is found in more than 50 % of stage I CRC tumors. This was evaluated on the basis of single tumor samples and did not take into account that the intra-tumor heterogeneity could lead to an underestimation of the true occurrence of chromosomal aneuploidy and genomic instability, respectively (Flyger et al. 1999; Bondi et al. 2009).
The essential etiologic element of CRC is widely accepted to lie in genetic changes of epithelial cells in the colonic mucosa. Morphologic changes from normal mucosa and adenomatous polyps to cancer with accumulation of genetic aberrations are well documented (Fearon and Vogelstein 1990). However, individual colorectal adenomas and carcinomas have different propensities to progress to malignancy. In this context, genome, transcriptome and proteome analysis with respect to DNA ploidy data may yield aneuploidy-associated biomarkers that could assess the individual progression risk to malignancy. On the genome/transcriptome level, fluorescence in situ hybridization (FISH) with specific probe sets was used to screen a total of 47 samples [centromere probes for chromosomes 17 and 18 (CEP17 and CEP18), SMAD7 (SMAD family member 7; 18q21.1), EGFR (epidermal growth factor; 7p12), NCOA3 (nuclear receptor coactivator 3; 20q12), TP53 (Tumor protein 53; 17p13.1), MYC (v-myc avian myelocytomatosis viral oncogene homolog; 8q24.21), and RAB20 (member RAS oncogene family; 13q34)]. These samples reflected different stages during colorectal cancer development and included 18 adenomas of patients without synchronous or subsequent carcinoma, 23 adenomas of carcinoma patients, and 6 matched carcinomas (Habermann et al. 2011a). In summary, Habermann et al. concluded that genomic instability in colorectal adenomas is reflected by genomic amplification of the oncogenes EGFR, MYC, NCOA3, and RAB20. For NCOA3 it could be shown that a diploid signal count of that gene is associated with a longer adenoma recurrence-free observation time (p = 0.042).
On the proteome level, a comprehensive proteomic analysis of diploid and aneuploid colorectal cancer cell lines and clinical tissues was carried out (Gemoll et al. 2011). Two proteins, HDAC2 (histone deacetylase 2) and TXNL1 (thioredoxin-like 1), were not only significantly expressed in two-dimensional gel electrophoresis (2-DE) analysis and validated by Western blotting, but showed expression differences also in clinical samples, discerning aneuploid from diploid CRCs (Fig. 1). It seems that HDAC2 is overexpressed in colorectal cancer and associated with reduced survival (Ashktorab et al. 2009; Weichert et al. 2008). Furthermore, HDAC2 overexpression could be induced by a loss of the anaphase-promoting complex (APC), favoring the development of genomic instability. This is in line with the finding of HDAC2-overexpression in patients with aneuploid tumors by Gemoll et al. (2011). In contrast, TXNL1 is involved in the cellular response to sugar starvation stress and regulates the redox equilibrium in higher eukaryotes (Jimenez et al. 2006; Manandhar et al. 2009). TXNL1 binds to the transcription factor B-MYb and is overexpressed in diploid as compared to aneuploid carcinomas, thus potentially maintaining genomic stability (Gemoll et al. 2011). Interestingly, TXNL1 was also expressed at low levels in aneuploid endometrial malignancies (Gemoll et al. 2012).
Fig. 1.
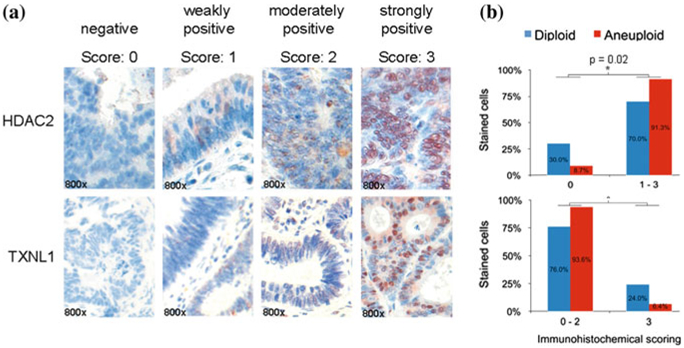
a HDAC2 and TXNL1 immunohistochemical detection in colorectal cancer specimens based on a tissue microarray. Image examples are given at 800-fold magnification. b Tissue-microarray-based immunohistochemical evaluation of HDAC2 and TXNL1 comparing diploid versus aneuploid colorectal carcinoma specimens. Immunoreactivity was scored with “0” showing no positivity, “1” presenting up to 20 % immunopositive cells, “2” up to 50 %, and “3” above 50 % stained cells. Bar plots of the TMA analysis confirmed HDAC2 and TXNL1 as significantly (asterisk) differentially expressed proteins between diploid and aneuploid tumors. Figure adapted from Gemoll et al. (2011)
2. Genomic Instability and Disease Prognostication in Breast Cancer
Breast cancer is one of the major causes of morbidity and mortality in females all over the world (Jemal et al. 2011). Despite the fact that tremendous progress has been achieved in chemotherapy and radiation therapy, breast cancer is still one of the most frequent malignancies with poor prognosis. The effects of independent prognostic factors for survival of breast cancer patients, including estrogen receptor/progesterone receptor (ER/PR) status, HER2 gene amplification and/or overexpression, tumor size, lymph node status, histological grade, and age have been thoroughly recognized (Ferguson et al. 2013). Especially the tumor, node, and metastasis (TNM) system has been extensively used. However, breast cancer is a malignant disease with multiple driving factors involved, and it has been reported that molecular mechanisms may affect tumor growth and progression, thereby potentially limiting the prognostic value of the TNM system (Coradini and Daidone 2004; Song et al. 2013).
Aneuploidy is, in general, correlated to cell proliferation and poor differentiation but not disease stage (Silvestrini 2000). However, Fallenius et al. (1988) demonstrated that node positive non-aneuploid tumors exhibited a better survival than node negative but aneuploid tumors, indicating that ploidy in this study cohort was a stronger prognostic marker than node assessment.
In 2006, Kronenwett et al. (2006) introduced a new concept to measure a tumor cell population with high levels of clonal heterogeneity. The stemline-scatter-index (SSI) is computed with the sum of the proliferation index, the variance of the diploid G0/G1 peak, and the 5c exceeding rate (5cER). Primarily based on the ploidy classification by Auer et al. (1980), the SSI is able to divide cytometrically assessed diploid, tetraploid and aneuploid samples into genomically stable and unstable subtypes. A total of 890 invasive breast cancer patients with a mean follow-up of 8.9 years were evaluated by using this algorithm and showed a significantly better survival of genomically stable subtypes compared with the unstable subtype within each ploidy category (0.04 < p < 0.004).
To evaluate potential differences in gene expression patterns between genomically stable and unstable breast tumors, Habermann et al. (2009) examined 17 diploid genomic stable, 15 aneuploid genomic stable, and 16 aneuploid genomic unstable breast carcinomas. A 12-gene expression signature associated with genomic instability in breast cancer was defined and demonstrated a biological and prognostic value across multiple different cancer entities (Habermann et al. 2009; Mettu et al. 2010). For breast cancer, genomic unstable carcinomas in patient cohorts from Sorlie et al. (2003), van de Vijver et al. (2002), Sotiriou et al. (2003) were associated with distinct shorter relapse-free survival and metastasis-free survival (p < 0.04; Fig. 2). All three studies were not analyzed regarding genomic stability/instability of the samples, initially. However, it was shown that the 12-gene signature is independent of clinicopathological factors such as lymph node status, the NIH criteria, the St. Gallen criteria, and grading used for breast cancer prognostication. In addition, gene sets of the MammaPrint® (van de Vijver et al. 2002; van’t Veer et al. 2002) and Oncotype DX® (Paik et al. 2004) tests—two clinically used breast cancer prognostic gene expression signatures—were used to predict genomic instability: 84 % (MammaPrint®) and 91 % (Oncotype DX®) of all cases were correctly classified. Along this line, Swanton et al. (2009) corroborated the importance of genomic instability by showing a link between aneuploidy-associated gene expression and poor response to taxane, a microtubule-stabilizing (MTS) agent. A pre-therapeutic assessment of genomic instability could therefore even optimize treatment stratification.
Fig. 2.
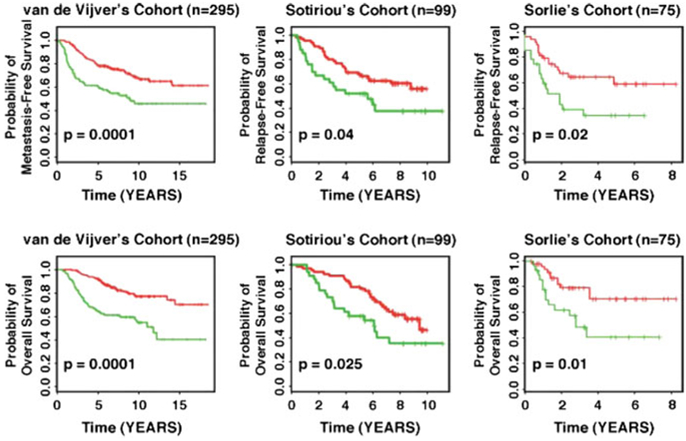
Applying the 12-gene genomic instability signature for prediction of disease-free and overall survival in independent datasets using Kaplan-Meier analyses. The curves in red reflect carcinoma patients harboring the genomically stable signature, the curves in green represent patients with the one implying genomic instability. For all three examples, statistically significant association of genomic instability with shorter disease-free and overall survival was observed. Figure adapted from Habermann et al. (2011a, b)
In 2007, Yildrim-Assaf et al. (2007) published another example for histogram reclassification: Based on thresholds in the categories of 5cER (>10 aneuploid cells) and 9cER (>1 aneuploid cell), patients with node negative and positive breast cancers can be stratified into a high-risk subgroup with unfavorable prognosis. In total, 370 breast cancer patients showed histology grade, lymph node status, and the above-mentioned binary DNA histogram classification to be the three strongest relapse predictors in a Cox multivariate analysis. The significance of rare-event nuclei (5cER and 9cER) was emphasized by the work of Sidoni et al. (2001) who examined fresh scrape smears from 599 breast carcinomas. According to their results, single cell aneuploidy is a marker for genetic instability with an increased risk of tumor recurrences despite otherwise favorable prognostic parameters. The data seem in accordance with the concept of progressive genetic evolutionary changes in solid tumors (Bartek et al. 1990) and with the unfavorable prognostic significance of DNA hypertetraploidy component as demonstrated in both image (Siitonen et al. 1993) and flow cytometry studies (Pinto et al. 1997).
3. Genomic Instability and Disease Prognostication in Other Cancers
The relationship between genomic instability and cancer prognosis has been explored across a range of cancer types. Next to breast and colorectal cancer (see above), several retrospective studies, summarized in Table 1, consistently associated genomic instability with poor prognosis and demonstrated that it provided additional prognostic information beyond conventional clinical parameters (McGranahan et al. 2012).
Table 1.
Summary of flow and image cytometry studies relating to genomic instability in various cancer types
| Cancer type | Method of measuring genomic instability |
Number of patients |
Outcome Shorter survival/poor prognosis of aneuploid/genomic instable tumors |
Reference |
|---|---|---|---|---|
| Colorectal cancer | Flow cytometry | 694 | Higher survival rate of diploid tumors | Witzig et al. (1991) |
| Flow cytometry | 528 | Higher survival rate of diploid tumors | Sinicrope et al. (2006) | |
| Image cytometry | 213 | Higher survival rate of diploid tumors without metastasis (Dukes’stage A & B) | Bosari et al. (1992) | |
| Image cytometry | 288 | Higher survival rate of diploid tumors; Aneuploidy strongest prognostic marker for CRC | Gerling et al. (2010) | |
| Flow and image cytometry | 10,126 | Higher survival rate of diploid tumors | Walther et al. (2008) | |
| Image cytometry | 217 | Higher survival rate of diploid tumors | Laubert et al. (2013) | |
| Genotyping of MSI markers | 12,782 | Higher survival rate of MSI tumors | Guastadisegni et al. (2010) | |
| Flow cytometry | 163 | Higher survival rate of diploid tumors | Flyger et al. (1999) | |
| Image cytometry | 219 | Higher survival rate of euploid tumors | Bondi et al. (2009) | |
| Image cytometry | 47 | Diploid signal count of NCOA3 is associated with a longer adenoma recurrence-free surveillance | Habermann et al. (2011a, b) | |
| Image cytometry | 78 | Higher survival rate of diploid tumors; HDAC2 & TXNL1 marker for genomic stability | Gemoll et al. (2011) | |
| Breast cancer | Image cytometry | 227 | Higher survival rate of diploid tumors | Fallenius et al. (1988) |
| Image cytometry | 890 | Higher survival rate of genomically stable subtypes | Kronenwett et al. (2006) | |
| Image cytometry | 112 | Higher survival rate of diploid tumors | Auer et al. (1980) | |
| Image cytometry | 48 | 12-gene signature predict degree of genomic instability and disease prognostication | Habermann et al. (2009), Mettu et al. (2010) | |
| Image cytometry | 370 | Lower survival rate of highly aneuploid tumors | Yildrim-Assaf et al. (2007) | |
| Image cytometry | 599 | Single cell aneuploidy as marker for genomic instability and biologic aggressiveness | Sidoni et al. (2001) | |
| Image cytometry | 134 | Lower survival rate of tumors with cancer cells with >5c DNA content | Siitonen et al. (1993) | |
| Flow cytometry | 860 | Hypertetraploidy as marker for biologic aggressiveness | Pinto et al. (1997) | |
| Endometrial cancer | Flow cytometry | 256 and 203 | Higher survival rate of diploid tumors | Britton et al. (1989, 1990) |
| Flow cytometry | 76 | Higher survival rate of diploid tumors | Ikeda et al. (1993) | |
| Image cytometry | 358 | Higher survival rate of diploid tumors | Lundgren et al. (2002) | |
| Flow cytometry | 174 | Higher survival rate of diploid tumors | Susini et al. (2007) | |
| Flow cytometry | 363 | Higher survival rate of diploid tumors | Wik et al. (2009) | |
| Ovarian cancer | Flow cytometry | 682 | Higher survival rate of diploid tumors | Akeson et al. (2009) |
| Image cytometry | 284 | Higher survival rate of diploid tumors | Kristensen et al. (2003) | |
| Image cytometry | 47 | Higher survival rate of diploid tumors | Kildal et al. (2004) | |
| Large B-cell lymphoma | H&E staining | 54 | Lower survival rate of patients with chromosomal instability | Bakhoum et al. (2011) |
| Oral squamous cancer | FISH | 77 | Lower survival rate of patients with chromosomal instability | Sato et al. (2010) |
| Synovial sarcoma | CGH | 22 | Lower survival rate of patients with specific chromosomal instability | Nakagawa et al. (2006) |
In endometrial cancer, genomic instability has been quantified by either image cytometry or flow cytometry (Evans and Podratz 1996). Next to traditional phenotypic variables, including stage, histologic grade and subtype, Britton et al. (1989, 1990) showed prognostic significance in univariate analysis of 256 and 203 endometrial carcinomas. A more detailed assessment revealed DNA ploidy as an independent prognostic factor by Ikeda et al. (1993). In 2002, Lundgren et al. (2002) published a study of relapse free survival following surgical treatment in 358 consecutive patients and found that DNA diploidy predicted disease free-survival. Likewise, prospective and multivariate studies successfully indicated the status of genomic instability as an independent prognostic variable (Susini et al. 2007; Wik et al. 2009). In this context, it seems that the grade of genomic instability correlates with a recurrent pattern of chromosomal imbalances and dominates specific gene and protein expression changes, irrespective of the histopathological subtypes in endometrial cancers. In order to identify the impact of chromosomal aberrations on protein expression, Gemoll et al. mapped genomic imbalances with associated gene and protein expression changes of endometrial cancer patients (Gemoll et al. 2012; Habermann et al. 2011b): Next to recurrent genomic imbalances of the chromosome arms 1q, 3q, 8q, 4q, and 15q, two proteins, AKR7A2 (aflatoxin B1 aldehyde reductase member 2) and ANXA2 (Annexin A2), showed translational alterations in consistence with transcriptional changes. While AKR7A2 is involved in the detoxification of aldehydes and ketones, there is evidence that ANXA2 facilitates the reorganization of the extracellular matrix in physiological and pathological processes such as tumor invasion (Mai et al. 2000).
Furthermore, in a multivariate analysis of 682 and 284 ovarian cancers, genomic instability was found to be associated with a worse prognosis. Here, Akeson et al. (2009) determined age, stage, presence of residual tumor, histological subtype, CA125, and DNA ploidy status as univariate predictors of survival time. Along the same line, Kristensen et al. (2003) showed the predictive power of genomic instability in multivariate analysis with a hazard ration of 10.3. These findings are supported by the study of Kildal et al. (2004) that found clinical stage to be the strongest prognostic feature, followed by the extent of residual tumor, and DNA ploidy status.
Furthermore, studies in synovial and oral squamous cell carcinomas as well as diffuse B-cell lymphoma, have suggested that genomic instability is associated with poor prognosis (Mettu et al. 2010; Bakhoum et al. 2011; Sato et al. 2010; Nakagawa et al. 2006).
4. Conclusion
Genomic instability is a defining feature of human cancers. It has an impact on the expression levels of resident genes but in addition also on associated protein expression. Such aneuploidy-associated protein expression patterns could reveal novel diagnostic and therapeutic targets. The evidence for the selective contribution of genomic instability on prognosis is supported by several studies in which patients with aneuploid tumors had a worse outcome compared to patients with euploid tumors. Overall, the assessment of nuclear aneuploidy by image or flow cytometry could become a routine practice to assist in predicting individual cancer risk and in disease prognostication in solid tumors.
Contributor Information
Timo Gemoll, Section for Translational Surgical Oncology and Biobanking, Department of Surgery, University of Lübeck & University Medical Center Schleswig-Holstein, Lübeck, Germany.
Gert Auer, Karolinska Biomic Center, Karolinska Institutet, Stockholm, Sweden.
Thomas Ried, Genetics Branch, Center for Cancer Research, National Cancer Institute, NIH, Bethesda, USA.
Jens K. Habermann, Section for Translational Surgical Oncology and Biobanking, Department of Surgery, University of Lübeck & University Medical Center Schleswig-Holstein, Lübeck, Germany
References
- Akeson M, Jakobsen AM, Zetterqvist BM, Holmberg E, Brannstrom M, Horvath G (2009) A population-based 5-year cohort study including all cases of epithelial ovarian cancer in western Sweden: 10-year survival and prognostic factors. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc 19:116–123 [DOI] [PubMed] [Google Scholar]
- Ashktorab H, Belgrave K, Hosseinkhah F, Brim H, Nouraie M, Takkikto M, Hewitt S, Lee EL, Dashwood RH, Smoot D (2009) Global histone H4 acetylation and HDAC2 expression in colon adenoma and carcinoma. Dig Dis Sci 54:2109–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer GU, Caspersson TO, Wallgren AS (1980) DNA content and survival in mammary carcinoma. Analytical and quantitative cytology 2:161–165 [PubMed] [Google Scholar]
- Bakhoum SF, Danilova OV, Kaur P, Levy NB, Compton DA (2011) Chromosomal instability substantiates poor prognosis in patients with diffuse large B-cell lymphoma. Clin Cancer Res: Off J Am Assoc Cancer Res 17:7704–7711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Vojtesek B, Staskova Z, Rejthar A, Kovarik J, Lane DP (1990) Patterns of expression of the p53 tumour suppressor in human breast tissues and tumours in situ and in vitro. Int J Cancer (Journal international du cancer) 46:839–844 [DOI] [PubMed] [Google Scholar]
- Bondi J, Pretorius M, Bukholm I, Danielsen H (2009) Large-scale genomic instability in colon adenocarcinomas and correlation with patient outcome. APMIS acta pathologica, microbiologica, et immunologica Scandinavica 117:730–736 [DOI] [PubMed] [Google Scholar]
- Bosari S, Lee AK, Wiley BD, Heatley GJ, Hamilton WM, Silverman ML (1992) DNA quantitation by image analysis of paraffin-embedded colorectal adenocarcinomas and its prognostic value. Mod Pathol 5:324–328 [PubMed] [Google Scholar]
- Britton LC, Wilson TO, Gaffey TA, Lieber MM, Wieand HS, Podratz KC (1989) Flow cytometric DNA analysis of stage I endometrial carcinoma. Gynecol Oncol 34:317–322 [DOI] [PubMed] [Google Scholar]
- Britton LC, Wilson TO, Gaffey TA, Cha SS, Wieand HS, Podratz KC (1990) DNA ploidy in endometrial carcinoma: major objective prognostic factor. Mayo Clin Proc 65:643–650 [DOI] [PubMed] [Google Scholar]
- Cascinu S, Del Ferro E, Grianti C, Ligi M, Catalano G (1996) S-phase fraction and tumor aneuploidy in colorectal carcinoma of young patients. Cancer 78:1857–1860 [DOI] [PubMed] [Google Scholar]
- Chen HS, Sheen-Chen SM, Lu CC (2002) DNA index and S-phase fraction in curative resection of colorectal adenocarcinoma: analysis of prognosis and current trends. World J Surg 26:626–630 [DOI] [PubMed] [Google Scholar]
- Coradini D, Daidone MG (2004) Biomolecular prognostic factors in breast cancer. Curr Opin Obstet Gynecol 16:49–55 [DOI] [PubMed] [Google Scholar]
- Evans MP, Podratz KC (1996) Endometrial neoplasia: prognostic significance of ploidy status. Clin Obstet Gynecol 39:696–706 [DOI] [PubMed] [Google Scholar]
- Fallenius AG, Franzen SA, Auer GU (1988) Predictive value of nuclear DNA content in breast cancer in relation to clinical and morphologic factors. A retrospective study of 227 consecutive cases. Cancer 62:521–530 [DOI] [PubMed] [Google Scholar]
- Fearon ER, Vogelstein B (1990) A genetic model for colorectal tumorigenesis. Cell 61:759–767 [DOI] [PubMed] [Google Scholar]
- Ferguson NL, Bell J, Heidel R, Lee S, Vanmeter S, Duncan L, Munsey B, Panella T, Orucevic A (2013) Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J 19:22–30 [DOI] [PubMed] [Google Scholar]
- Flyger HL, Larsen JK, Nielsen HJ, Christensen IJ (1999) DNA ploidy in colorectal cancer, heterogeneity within and between tumors and relation to survival. Cytometry 38:293–300 [DOI] [PubMed] [Google Scholar]
- Garrity MM, Burgart LJ, Mahoney MR, Windschitl HE, Salim M, Wiesenfeld M, Krook JE, Michalak JC, Goldberg RM, O’Connell MJ, Furth AF, Sargent DJ, Murphy LM, Hill E, Riehle DL, Meyers CH, Witzig TE (2004) Prognostic value of proliferation, apoptosis, defective DNA mismatch repair, and p53 overexpression in patients with resected Dukes B2 or C colon cancer: a north central cancer treatment group study. J Clin Oncol 22:1572–1582 [DOI] [PubMed] [Google Scholar]
- Gemoll T, Roblick UJ, Szymczak S, Braunschweig T, Becker S, Igl BW, Bruch HP, Ziegler A, Hellman U, Difilippantonio MJ, Ried T, Jornvall H, Auer G, Habermann JK (2011) HDAC2 and TXNL1 distinguish aneuploid from diploid colorectal cancers. Cell Mol Life Sci CMLS 68:3261–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemoll T, Habermann JK, Lahmann J, Szymczak S, Lundgren C, Bundgen NK, Jungbluth T, Nordstrom B, Becker S, Lomnytska MI, Bruch HP, Ziegler A, Hellman U, Auer G, Roblick UJ, Jornvall H (2012) Protein profiling of genomic instability in endometrial cancer. Cell Mol Life Sci CMLS 69:325–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerling M, Meyer KF, Fuchs K, Igl BW, Fritzsche B, Ziegler A, Bader F, Kujath P, Schimmelpenning H, Bruch HP, Roblick UJ, Habermann JK (2010) High frequency of aneuploidy defines ulcerative colitis-associated carcinomas: a comparative prognostic study to sporadic colorectal carcinomas. Ann Surg [DOI] [PubMed] [Google Scholar]
- Guastadisegni C, Colafranceschi M, Ottini L, Dogliotti E (2010) Microsatellite instability as a marker of prognosis and response to therapy: a meta-analysis of colorectal cancer survival data. Eur J Cancer 46:2788–2798 [DOI] [PubMed] [Google Scholar]
- Habermann JK, Doering J, Hautaniemi S, Roblick UJ, Bundgen NK, Nicorici D, Kronenwett U, Rathnagiriswaran S, Mettu RK, Ma Y, Kruger S, Bruch HP, Auer G, Guo NL, Ried T (2009) The gene expression signature of genomic instability in breast cancer is an independent predictor of clinical outcome. Int J Cancer (Journal international du cancer) 124:1552–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann JK, Brucker CA, Freitag-Wolf S, Heselmeyer-Haddad K, Kruger S, Barenboim L, Downing T, Bruch HP, Auer G, Roblick UJ, Ried T (2011a) Genomic instability and oncogene amplifications in colorectal adenomas predict recurrence and synchronous carcinoma. Mod Pathol 24:542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habermann JK, Bundgen NK, Gemoll T, Hautaniemi S, Lundgren C, Wangsa D, Doering J, Bruch HP, Nordstroem B, Roblick UJ, Jornvall H, Auer G, Ried T (2011b) Genomic instability influences the transcriptome and proteome in endometrial cancer subtypes. Molecular cancer 10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674 [DOI] [PubMed] [Google Scholar]
- Ikeda M, Watanabe Y, Nanjoh T, Noda K (1993) Evaluation of DNA ploidy in endometrial cancer. Gynecol Oncol 50:25–29 [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- Jimenez A, Pelto-Huikko M, Gustafsson JA, Miranda-Vizuete A (2006) Characterization of human thioredoxin-like-1: potential involvement in the cellular response against glucose deprivation. FEBS Lett 580:960–967 [DOI] [PubMed] [Google Scholar]
- Kearney TJ, Price EA, Lee S, Silberman AW (1993) Tumor aneuploidy in young patients with colorectal cancer. Cancer 72:42–45 [DOI] [PubMed] [Google Scholar]
- Kildal W, Kaern J, Kraggerud SM, Abeler VM, Sudbo J, Trope CG, Lothe RA, Danielsen HE (2004) Evaluation of genomic changes in a large series of malignant ovarian germ cell tumors—relation to clinicopathologic variables. Cancer Genet Cytogenet 155:25–32 [DOI] [PubMed] [Google Scholar]
- Kristensen GB, Kildal W, Abeler VM, Kaern J, Vergote I, Trope CG, Danielsen HE (2003) Large-scale genomic instability predicts long-term outcome for women with invasive stage I ovarian cancer. Ann Oncol 14:1494–1500 [DOI] [PubMed] [Google Scholar]
- Kronenwett U, Ploner A, Zetterberg A, Bergh J, Hall P, Auer G, Pawitan Y (2006) Genomic instability and prognosis in breast carcinomas. Cancer Epidemiol Biomark Prev 15:1630–1635(A publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology)
- Laubert T, Bente V, Freitag-Wolf S, Voulgaris H, Oberlander M, Schillo K, Kleemann M, Burk C, Bruch HP, Roblick UJ, Habermann JK (2013) Aneuploidy and elevated CEA indicate an increased risk for metachronous metastasis in colorectal cancer. Int J Colorectal Dis 28:767–775 [DOI] [PubMed] [Google Scholar]
- Lundgren C, Auer G, Frankendal B, Moberger B, Nilsson B, Nordstrom B (2002) Nuclear DNA content, proliferative activity, and p53 expression related to clinical and histopathologic features in endometrial carcinoma. Int J Gynecol Cancer: Off J Int Gynecol Cancer Soc 12:110–118 [DOI] [PubMed] [Google Scholar]
- Mai J, Waisman DM, Sloane BF (2000) Cell surface complex of cathepsin B/annexin II tetramer in malignant progression. Biochim Biophys Acta 1477:215–230 [DOI] [PubMed] [Google Scholar]
- Manandhar G, Miranda-Vizuete A, Pedrajas JR, Krause WJ, Zimmerman S, Sutovsky M, Sutovsky P (2009) Peroxiredoxin 2 and peroxidase enzymatic activity of mammalian spermatozoa. Biol Reprod 80:1168–1177 [DOI] [PubMed] [Google Scholar]
- McGranahan N, Burrell RA, Endesfelder D, Novelli MR, Swanton C (2012) Cancer chromosomal instability: therapeutic and diagnostic challenges. EMBO Rep 13:528–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettu RK, Wan YW, Habermann JK, Ried T, Guo NL (2010) A 12-gene genomic instability signature predicts clinical outcomes in multiple cancer types. Int J Biol Mark 25:219–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Numoto K, Yoshida A, Kunisada T, Ohata H, Takeda K, Wai D, Poremba C, Ozaki T (2006) Chromosomal and genetic imbalances in synovial sarcoma detected by conventional and microarray comparative genomic hybridization. J Cancer Res Clin Oncol 132:444–450 [DOI] [PubMed] [Google Scholar]
- Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, Baehner FL, Walker MG, Watson D, Park T, Hiller W, Fisher ER, Wickerham DL, Bryant J, Wolmark N (2004) A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 351:2817–2826 [DOI] [PubMed] [Google Scholar]
- Pinto AE, Andre S, Nogueira M, Mendonca E, Soares J (1997) Flow cytometric DNA hypertetraploidy is associated with unfavourable prognostic features in breast cancer. J Clin Pathol 50:591–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ried T, Heselmeyer-Haddad K, Blegen H, Schrock E, Auer G (1999) Genomic changes defining the genesis, progression, and malignancy potential in solid human tumors: a phenotype/genotype correlation. Genes Chromosom Cancer 25:195–204 [DOI] [PubMed] [Google Scholar]
- Sato H, Uzawa N, Takahashi K, Myo K, Ohyama Y, Amagasa T (2010) Prognostic utility of chromosomal instability detected by fluorescence in situ hybridization in fine-needle aspirates from oral squamous cell carcinomas. BMC Cancer 10:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schillaci A, Tirindelli DD, Ferri M, Teodori L, Mauro F, Nicolanti V, Stipa S (1990) Flow cytometric analysis in colorectal carcinoma: prognostic significance of cellular DNA content. Int J Colorectal Dis 5:223–227 [DOI] [PubMed] [Google Scholar]
- Sidoni A, Cavaliere A, D’Amico GA, Brachelente G, Bucciarelli E (2001) Biopathological significance of single cell DNA aneuploidy measured by static cytometry in breast cancer. Breast 10:325–329 [DOI] [PubMed] [Google Scholar]
- Siitonen SM, Kallioniemi OP, Helin HJ, Isola JJ (1993) Prognostic value of cells with more than 5c DNA content in node-negative breast cancer as determined by image cytometry from tissue sections. Hum Pathol 24:1348–1353 [DOI] [PubMed] [Google Scholar]
- Silvestrini R (2000) Relevance of DNA-ploidy as a prognostic instrument for solid tumors. Ann Oncol 11:259–261 [DOI] [PubMed] [Google Scholar]
- Sinicrope FA, Rego RL, Halling KC, Foster N, Sargent DJ, La Plant B, French AJ, Laurie JA, Goldberg RM, Thibodeau SN, Witzig TE (2006) Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 131:729–737 [DOI] [PubMed] [Google Scholar]
- Song J, Su H, Zhou YY, Guo LL (2013) Prognostic value of survivin expression in breast cancer patients: a meta-analysis. Tumour Biol: J Int Soc Oncodev Biol Med 34:2053–2062 [DOI] [PubMed] [Google Scholar]
- Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D (2003) Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA 100:8418–8423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotiriou C, Neo SY, McShane LM, Korn EL, Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL, Liu ET (2003) Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA 100:10393–10398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XF (2006) Clinicopathological and biological features of DNA tetraploid colorectal cancers. Cancer J 12:501–506 [DOI] [PubMed] [Google Scholar]
- Susini T, Amunni G, Molino C, Carriero C, Rapi S, Branconi F, Marchionni M, Taddei G, Scarselli G (2007) Ten-year results of a prospective study on the prognostic role of ploidy in endometrial carcinoma: dNA aneuploidy identifies high-risk cases among the so-called ‘low-risk’ patients with well and moderately differentiated tumors. Cancer 109:882–890 [DOI] [PubMed] [Google Scholar]
- Swanton C, Nicke B, Schuett M, Eklund AC, Ng C, Li Q, Hardcastle T, Lee A, Roy R, East P, Kschischo M, Endesfelder D, Wylie P, Kim SN, Chen JG, Howell M, Ried T, Habermann JK, Auer G, Brenton JD, Szallasi Z, Downward J (2009) Chromosomal instability determines taxane response. Proc Natl Acad Sci USA 106:8671–8676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Vijver MJ, He YD, van’t Veer LJ, Dai H, Hart AA, Voskuil DW, Schreiber GJ, Peterse JL, Roberts C, Marton MJ, Parrish M, Atsma D, Witteveen A, Glas A, Delahaye L, van der Velde T, Bartelink H, Rodenhuis S, Rutgers ET, Friend SH, Bernards R (2002) A gene-expression signature as a predictor of survival in breast cancer. N Engl J Med 347:1999–2009 [DOI] [PubMed] [Google Scholar]
- van’t Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, Mao M, Peterse HL, van der Kooy K, Marton MJ, Witteveen AT, Schreiber GJ, Kerkhoven RM, Roberts C, Linsley PS, Bernards R, Friend SH (2002) Gene expression profiling predicts clinical outcome of breast cancer. Nature 415:530–536 [DOI] [PubMed] [Google Scholar]
- Walther A, Houlston R, Tomlinson I (2008) Association between chromosomal instability and prognosis in colorectal cancer: a meta-analysis. Gut 57:941–950 [DOI] [PubMed] [Google Scholar]
- Weichert W, Roske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, Gekeler V, Boehm M, Beckers T, Denkert C (2008) Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res 14:1669–1677 (an official journal of the American Association for Cancer Research) [DOI] [PubMed] [Google Scholar]
- Wik E, Trovik J, Iversen OE, Engelsen IB, Stefansson IM, Vestrheim LC, Haugland HK, Akslen LA, Salvesen HB (2009) Deoxyribonucleic acid ploidy in endometrial carcinoma: a reproducible and valid prognostic marker in a routine diagnostic setting. Am J Obstet Gynecol 201(603):e601–e607 [DOI] [PubMed] [Google Scholar]
- Witzig TE, Loprinzi CL, Gonchoroff NJ, Reiman HM, Cha SS, Wieand HS, Katzmann JA, Paulsen JK, Moertel CG (1991) DNA ploidy and cell kinetic measurements as predictors of recurrence and survival in stages B2 and C colorectal adenocarcinoma. Cancer 68:879–888 [DOI] [PubMed] [Google Scholar]
- Yildirim-Assaf S, Coumbos A, Hopfenmuller W, Foss HD, Stein H, Kuhn W (2007) The prognostic significance of determining DNA content in breast cancer by DNA image cytometry: the role of high grade aneuploidy in node negative breast cancer. J Clin Pathol 60:649–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarbo RJ, Nakhleh RE, Brown RD, Kubus JJ, Ma CK, Mackowiak P (1997) Prognostic significance of DNA ploidy and proliferation in 309 colorectal carcinomas as determined by two-color multiparametric DNA flow cytometry. Cancer 79:2073–2086 [PubMed] [Google Scholar]


