Abstract
Introduction
Insulin pumps are increasingly being used as a method of insulin delivery in patients with type 1 diabetes mellitus (T1DM). Diabetic ketoacidosis (DKA) is a serious complication of T1DM. This study aims to identify the causes of DKA in patients with T1DM on continuous subcutaneous insulin infusion (CSII) and to compare these with patients with T1DM on multiple daily insulin injections (MDIIs).
Research design and methods
This is a prospective observational study between January and June 2019 at the Cleveland Clinic Fairview Hospital. Demographic, clinical, and biochemical data were obtained from chart review. A questionnaire to explore additional clinical data relating to DKA was administered, with additional items for patients on the insulin pump.
Results
Seventy-four patients were admitted with a diagnosis of DKA between the period of January and June 2019. Of these, 45 met the inclusion criteria and 43 consented. These were divided into two groups: group 1 included patients on MDII and group 2 included CSII. Overall, the most common precipitating factor for developing DKA was insulin non-adherence, seen in 51.2% of the cases. The most common cause of DKA in group 2 was pump/tubing related to 55% of the cases.
Conclusion
Despite non-adherence being common in both CSII and MDII, a combination of social factors, education and insulin pump malfunction, such as pump/tubing problems, might be playing a pivotal role in DKA etiology in young adults with T1DM, especially in CSII users. Continued education on pump use may reduce the rate of DKA in pump users.
Keywords: DKA, insulin pump, insulin-deficient type 1 diabetes, adults
Significance of this study.
What is already known about this subject?
Diabetic ketoacidosis (DKA) is a life-threatening but preventable complication of diabetes, which leads to an increase rate of hospital admissions.
Previous studies focused on describing DKA trends in children; however, about 80% of patients with DKA are adults.
Further studies to understand the etiology of DKA in adults using multiple daily insulin injectionI and continuous subcutaneous insulin infusion are needed.
What are the new findings?
The most common precipitating factor for developing DKA is insulin non-compliance, defined as a one or more missed insulin dose 1 week prior to admission.
Despite the major triggering factor for DKA in pump users being pump/tubing problems, recurrence of DKA is still likely multifactorial, including a combination of social factors, lack of education, and insulin pump malfunction.
How might these results change the focus of research or clinical practice?
Continued patient education and healthcare provider support in managing the pump and tubing might help reduce the rate of DKA in this population.
Efforts to understand and address other factors contributing to DKA, such as demographics, clinical characteristics, and social risk factors should be a focus of future DKA prevention strategies and studies.
Introduction
Diabetic ketoacidosis (DKA) is a potentially life-threatening metabolic complication, typically marked by metabolic acidosis, ketosis, and uncontrolled hyperglycemia. It is a common, costly, and dangerous complication of type 1 diabetes mellitus (T1DM).1 The most common risk factor for DKA is poor adherence to insulin treatment and poor glycemic control.2 With the advent of continuous subcutaneous insulin infusion (CSII) or insulin pumps, it was initially observed that DKA was more frequent after its initiation compared with patients who were on conventional insulin injections, often attributed to pump malfunction or technical problems with insulin delivery.3 4 Since then, issues with insulin delivery have improved, and insulin treatment via CSII has been shown to provide better glycemic control for patients with T1DM.5
There is a paucity of recent studies on adult patients with T1DM using CSII and the causes of DKA.6 We, therefore, aimed to identify the causes of DKA in adult patients with T1DM on CSII and compare these with patients on insulin therapy using multiple daily insulin injections (MDIIs).
Methods
Setting and participants
This was a single-center prospective observational study conducted between January and June 2019 at the Cleveland Clinic Fairview Hospital, a regional hospital of the Cleveland Clinic Health System. Confidentiality was maintained through the study. Informed consent was obtained from patients by the investigation team, and the participants signed consent forms before being enrolled in the study.
The eligible study population consisted of all patients who were 18 years or older with T1DM and admitted with the diagnosis of DKA between January and June 2019. Because of our hospital protocol that all patients with DKA should be admitted to the Medical Intensive Care Unit (MICU), all patients included in the study were initially admitted to the MICU regardless of DKA severity. MICU management for DKA patients is protocol driven, and there were no changes to protocols during the study. We included patients who were either on an insulin pump or MDII >12 weeks before study enrollment. Patients who declined to participate and patients on the insulin pump for less than 12 weeks were excluded. T1DM was defined as exclusive insulin therapy since diagnosis. DKA was defined according to the revised biochemical criteria of the American Diabetic Association (ADA), which includes: hyperglycemia (blood glucose >250 mg/dL (≈13.9 mmol/L)); arterial pH <7.30 or serum bicarbonate <18 mEq/L and ketonemia (blood beta-hydroxybutyrate >3 mmol/L) or positive urine ketones. The severity of DKA (mild, moderate, or severe) was done based on the classification of the ADA, which is based on plasma glucose, arterial pH, serum bicarbonate level, urine and serum ketones, effective serum osmolality, anion gap, and alteration in sensorial or mental status.7 Poor glycemic control was defined by hemoglobin A1c (HbA1c) >9%. More than two episodes defined recurrent DKA in the last 12 months.
On admission, a questionnaire was provided to explore the etiology of DKA in patients with MDII and CSII. The questionnaire was targeted to adults with T1DM admitted with DKA’s diagnosis, with content experts from internal medicine and endocrinology. The questionnaire included multiple-choice questions and open-ended questions regarding the patient’s history of diabetes, awareness of DKA, acute and chronic diabetes complications, and diabetes management. Additional questions regarding pump function were added for patients on insulin pump, (online supplemental file 1). Insulin pump data were downloaded from the electronic device and assessed by the investigator team at the DKA diagnosis time to assure there was no discrepancy in the collected data.
bmjdrc-2020-001329supp001.pdf (1.2MB, pdf)
Demographic characteristics, clinical, and laboratory data were retrieved from the electronic medical records.
Statistical analysis
Patient information was described using means with SD for continuous variables, and median with quartiles, counts, and percentages for all categorical variables. The study population was divided into two groups (CSII and MDII users). Kruskal-Wallis test was used to compare the non-normally distributed continuous variables. A χ2 test was used to compare the categorical variables. Fisher’s exact test was conducted when one or more of the cells had an expected five or less frequency. All analyses were performed using SAS V.9.4 for Linux. The level of statistical significance was set at p<0.05 (two tailed).
Results
A total of 74 patients were admitted with a DKA diagnosis between the period of January and June 2019. Of these, 45 met the inclusion criteria and 43 consented. These were divided into two groups: group 1 included patients on MDII, and group 2 included patients on CSII. There were 23 patients (53.5%) in group 1 and 20 patients (46.5%) in group 2.
Baseline characteristics
Among the evaluated patients, 53.5% were female with a median age of 32 years (IQR: 23.0–43.0). Female sex was significantly more prevalent in the CSII users compared with the MDII users (75.0% vs 34.8%, p=0.008). The Caucasian race was more prevalent (79.1%) in the studied cohort as compared with other races. Neither age, race, body mass index, nor educational level presented a significant difference between the group with insulin pump and the group without insulin pump (table 1).
Table 1.
Baseline characteristics of MDII and CSII users
| Overall (N=43) |
MDII users (N=23) |
CSII users (N=20) |
P value* | |
| Agem, median (IQR), years | 32.0 (23.0–43.0) | 38.0 (25.0–43.0) | 27.0 (22.0–43.5) | 0.25† |
| Female, n (%) | 23 (53.5) | 8 (34.8) | 15 (75.0) | 0.008‡ |
| Body mass index, n (%), kg/m2 | 0.19§ | |||
| <18.5 | 3 (7.0) | 3 (13.0) | 0 (0.0) | |
| 18.5–25 | 23 (53.5) | 12 (52.2) | 11 (55.0) | |
| 25–30 | 8 (18.6) | 3 (13.0) | 5 (25.0) | |
| 30–35 | 7 (16.3) | 5 (21.7) | 2 (10.0) | |
| >35 | 2 (4.7) | 0 (0.0) | 2 (10.0) | |
| Caucasian race, n (%) | 34 (79.1) | 16 (69.6) | 18 (90) | 0.14§ |
| Education level, n (%) | 0.20§ | |||
| High school | 20 (47.6) | 12 (54.5) | 8 (40.0) | |
| Bachelors | 19 (45.3) | 10 (45.5) | 9 (45.0) | |
| Masters | 3 (7.1) | 0 (0.0) | 3 (15.0) |
Statistics presented as mean±SD, median (P25, P75) or n (column %).
*The p values correspond to the comparison between with pump and without pump groups.
†Kruskal-Wallis test.
‡Pearson's χ2 test.
§Fisher’s exact test.
CSII, continuous subcutaneous insulin infusion; MDII, multiple daily insulin injection.
Clinical and biochemical characteristics
Of the 43 patients enrolled in the study, 66.6% had a baseline HbA1c >9% at the time of admission. Poor glycemic control (HbA1c >9%) was more prevalent in patients with MDII compared with patients with CSII (82.6% vs 47.0%, p=0.062). The duration of T1DM was found to be more than 10 years in 74% of the studied cohort. Due to the chronicity and the duration of diabetes, microvascular complications were expected. Patients on MDII had a significantly higher incidence of peripheral neuropathy than patients with an insulin pump (60.9% vs 30.0%, p=0.043). There was no significant difference between MDII and CSII groups in terms of nephropathy and retinopathy. Among the studied cohort, only 25.6% used continuous glucose monitoring (CGM) before admission. As expected, CGM use was significantly higher in the group with an insulin pump than the group without an insulin pump (40% vs 13%, p=0.043). The use of non-insulin glucose-lowering medications in addition to insulin was uncommon across both groups.
Regarding DKA episodes, most of the admitted cases were classified as mild (39.5%). Severe cases were more prevalent in the CSII group compared with the MDII group (35% vs 13%, p=0.21). A higher recurrence number of DKA episodes were more frequent in the group using MDII when compared with the group using CSII (52.2% vs 25.0%, p=0.052). Only 34.9% of the enrolled participants knew about hyperglycemic sick day management and DKA prevention. Overall, the group with an insulin pump had more awareness of DKA prevention (50% vs 21.7%, p=0.052).
As for diabetes management, most patients with T1DM admitted with DKA and using CSII skipped their insulin bolus dose at prescribed times, especially premeals, compared with patients with T1DM using MDII (90% vs 30.4%, p<0.001).
The use of insulin pump was significantly associated with a prolonged MICU length of stay (LOS) compared with no insulin pump use (mean days 1.9±0.62 v. 1.3±0.71, p=0.001); however, the overall duration of hospitalization was not significantly different. Rest of characteristics are described in table 2.
Table 2.
Clinical and biochemical characteristics of MDII and CSII users
| Overall (N=43) |
MDII users (N=23) |
CSII users (N=20) |
P value | |
| Hemoglobin g/dL, median (IQR) | 12.5 (10.7–14.) | 12.2 (10.9–14.) | 12.5 (10.4–14.) | 0.94* |
| GFR ≥60 mL/min/1.73 m2, n (%) | 35 (81.4) | 19 (82.6) | 16 (80.0) | 0.99† |
| HbA1c %, n (%) | 0.062† | |||
| <7 | 2 (4.8) | 1 (4.3) | 1 (5.3) | |
| 7–9 | 12 (28.6) | 3 (13) | 9 (47.4) | |
| >9 | 28 (66.6) | 19 (82.6) | 9 (47.4) | |
| Severity of DKA, n (%) | 0.21‡ | |||
| Mild | 17 (39.5) | 11 (47.8) | 6 (30.0) | |
| Moderate | 16 (37.2) | 9 (39.1) | 7 (35.0) | |
| Severe | 10 (23.3) | 3 (13.0) | 7 (35.0) | |
| No diabetic complications, n (%) | 16 (37.2) | 6 (26.1) | 10 (50.0) | 0.11‡ |
| Diabetic retinopathy, n (%) | 9 (20.9) | 6 (26.1) | 3 (15.0) | 0.47† |
| Diabetic nephropathy, n (%) | 13 (30.2) | 6 (26.1) | 7 (35.0) | 0.53‡ |
| Diabetic neuropathy, n (%) | 20 (46.5) | 14 (60.9) | 6 (30.0) | 0.043‡ |
| Duration of DM, years, n (%) | 0.78† | |||
| <5 | 5 (11.6) | 3 (13.0) | 2 (10.0) | |
| 5–10 | 6 (14.0) | 4 (17.4) | 2 (10.0) | |
| >10 | 32 (74.4) | 16 (69.6) | 16 (80.0) | |
| Number of DKA episodes over last 12 months | 0.052† | |||
| None | 9 (20.9) | 6 (26.1) | 3 (15.0) | |
| 1 | 13 (30.2) | 3 (13.0) | 10 (50.0) | |
| 2 | 4 (9.3) | 2 (8.7) | 2 (10.0) | |
| >2 | 17 (39.5) | 12 (52.2) | 5 (25.0) | |
| Number of hypoglycemic events in last 6 months | 0.68‡ | |||
| <5 | 12 (28.6) | 6 (27.3) | 6 (30.0) | |
| 5–10 | 13 (31.0) | 6 (27.3) | 7 (35.0) | |
| 10–20 | 12 (28.6) | 8 (36.4) | 4 (20.0) | |
| >20 | 5 (11.9) | 2 (9.1) | 3 (15.0) | |
| Number of hypoglycemic events needing another person’s assistance in last 6 months | 0.079‡ | |||
| <1 | 22 (51.2) | 11 (47.8) | 11 (55.0) | |
| 1–3 | 16 (37.2) | 7 (30.4) | 9 (45.0) | |
| >3 | 5 (11.6) | 5 (21.7) | 0 (0.0) | |
| Patient with recent changes in insulin regimen or settings | 4 (9.3) | 3 (13.0) | 1 (5.0) | 0.61† |
| Patients using CGM, n (%) | 11 (25.6) | 3 (13) | 8 (40) | 0.043‡ |
| Patients who takes all of his or her insulin bolus at prescribed times, n (%) | 25 (58.1) | 7 (30.4) | 18 (90.0) | <0.001‡ |
| Patients who lack insulin supplies, n (%) | 6 (14.0) | 4 (17.4) | 2 (10.0) | 0.67† |
| Patients who check insulin expiration date prior to use, n (%) | 31 (72.1) | 15 (65.2) | 16 (80.0) | 0.28‡ |
| Patients who know about hyperglycemic sick day management/DKA prevention, n (%) | 15 (34.9) | 5 (21.7) | 10 (50.0) | 0.052‡ |
| Patients who receive non-insulin medication for DM, n (%) | 2 (4.7) | 2 (8.7) | 0 (0.0) | 0.49† |
| Patients who know how to count carbs, n (%) | 37 (86.0) | 18 (78.3) | 19 (95.0) | 0.19† |
| Patients who know how to use correctional insulin, n (%) | 40 (93.0) | 21 (91.3) | 19 (95.0) | 0.99† |
| Hospital length of stay (days, mean+SD) | 2.7±1.5 | 2.5±0.99 | 2.9±1.9 | 0.49* |
| ICU length of stay (days, mean+SD) | 1.6±0.73 | 1.3±0.71 | 1.9±0.62 | 0.001* |
Statistics presented as N (column %).
*P values.
†Fisher’s exact test.
‡Pearson’s χ2 test.
CGM, continuous glucosemonitor; CSII, continuous subcutaneous insulin infusion; DKA, diabetes ketoacidosis; DM, diabetes mellitus; GFR, glomerular filtration rate; HbA1c, hemoglobin A1c; ICU, intensive care unit; MDII, multiple daily insulin injection.
Etiology of DKA
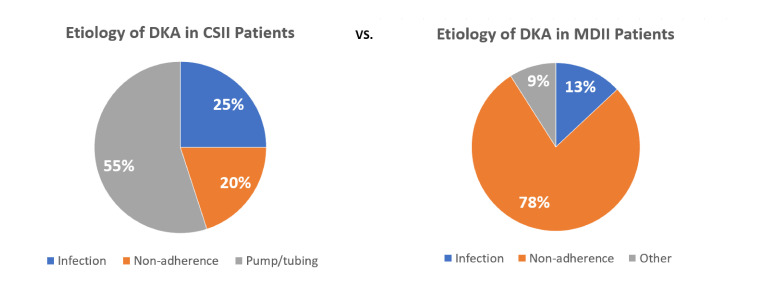
The causes for developing DKA and percentage of occurrence are summarized in figure 1.
Figure 1.
Graph pie portraying and comparing etiologies of DKA in CSII and MDII users. CSII, continuous subcutaneous insulin infusion; DKA, diabetic ketoacidosis; MDII, multiple daily insulin injection.
Overall, regardless of the use or not of an insulin pump, the most common precipitating factor for developing DKA was insulin non-adherence (51.2%), defined as one or more missed insulin doses in the last week prior admission. Non-adherence was more prevalent in patients without insulin pump compared with patients with insulin pump (78.3% vs 20.0%).
Among the subset of patients with T1DM using an insulin pump, 55% of the DKA cases were associated with a pump/tubing defect, which mainly encompassed kinking and air bubbles in the tubing that prevented the pump from delivering insulin properly. Underling infection was present in 25% of the cases, followed by non-adherence by a 20%.
Characteristics of insulin pump users
In our cohort, most of our participants used Medtronic pumps (70%), 15% Tandem Diabetes, 10% Animas, and 5% Omnipod. More than half of our patients (55%) had an insulin pump for more than 5 years and were trained mostly at their physician’s office (55%).
When evaluating if further action was done with hyperglycemic events or when presenting DKA symptoms, most of the patients (90%) will correct hyperglycemia with the insulin pump. Other preventive measures such as hydrating and checking ketones will be done by 30% and 20% of the participants, respectively (table 3).
Table 3.
Descriptive data of insulin pump users
| Total (N=20) | |
| Pump device, n (%) | |
| Animas | 2 (10.0) |
| Medtronic | 14 (70.0) |
| Omnipod | 1 (5.0) |
| Tandem Diabetes | 3 (15.0) |
| Current pump is older than 5 years, n (%) | 7 (35.0) |
| Length in years of having the pump, n (%) | |
| <5 | 9 (45.0) |
| 5–10 | 4 (20.0) |
| >10 | 7 (35.0) |
| How the patient was trained on the pump, n (%) | |
| MD office | 11 (55.0) |
| Pump company | 8 (40.0) |
| Other | 1 (5.0) |
| Patient knows his or her pump settings, n (%) | 15 (75.0) |
| If yes, patient knows the current basal rates | 12 (63.2) |
| If yes, patient can demonstrate a basal rate | 13 (68.4) |
| If yes, patient uses bolus wizard or bolus | 17 (85.0) |
| If yes, patient shows how to look up current carb ratio | 11 (55.0) |
| Patient can demonstrate suspending insulin delivery | 20 (100.0) |
| What action did patient take when blood glucose continued to increase/had symptoms of DKA? | |
| Corrected through pump | 18 (90.0) |
| Injected subcutaneous insulin | 4 (20.0) |
| Checked ketones | 4 (20.0) |
| Hydrated | 6 (30.0) |
| Other | 4 (20.0) |
| Patient has a back-up plan if pump malfunctions | 17 (89.5) |
| How often does the patient change pump insertion site? | |
| 2–3 days | 10 (50.0) |
| 4–5 days | 10 (50.0) |
Statistics presented as N (column %).
DKA, diabetes ketoacidosis; MD, medical degree.
Discussion
DKA is a life threatening but preventable complication of diabetes that occurs most frequently in persons with T1DM.8 It is associated with an overall mortality of <1% in adults with T1DM.9 After a slight decline from 2000 to 2009, Centers for Disease Control and Prevention’s US Diabetes Surveillance System indicated an increase in hospitalization rates for DKA during 2009–2014, most notably in persons aged <45 years.10 This epidemiology trend is reflected in our results, where most of the patients of the study cohort who developed DKA were young (median 32.0 years; IQR 23.0–43.0), white females, with an overall poor glycemic control (HbA1c >9%), and recurrent DKA episodes (>2 in last 12 months). The recurrence of DKA episodes was seen more in the MDII group compared with CSII. These findings go along with the systematic literature review done by Farsani et al,2 which reported a higher prevalence of DKA in women and patients treated with insulin injections compared with men and patients using CSII pumps. The above was as well supported by a cross-sectional study of adult patients with DKA admitted in a hospital in Atlanta, Georgia, where recurrent DKA episodes were frequently seen in young women with T1DM and was caused mostly by the omission of insulin treatment.11
Previous studies focused on describing DKA trends in children12 13; however, about 80% of patients with DKA are adults.14 In this study, we aimed to identify factors contributing to DKA in adult insulin pump users that may help future preventive measures.
Among the 43 adults enrolled in our study with DKA, 20 (46.5%) were using an insulin pump device at admission. A Medtronic pump device was used by more than half of the participants (70%). Of these, 75% were female, Caucasian race, median age 27.0 years (22.0–43.5), duration of T1DM for more than 10 years (80%), and with poor underlying glycemic control. Compared with the population characteristics described in the updated US Type 1 Diabetes Exchange Clinic Registry from 2016 to 2018, our population shared similar baseline characteristics. Based on the registry data, the population between 26 and 49 years old were mostly females (50%), non-Hispanic whites (89%), and with T1DM duration of 27.7 years±10.1. Insulin pump device was present in 64% of this subgroup and a Medtronic pump device was mostly used (53%).15 16
In our study, CSII users had a lower DKA frequency compared with MDII users, which is supported by previous studies where treatment with CSII is associated with a low incidence of DKA.17 Based on our data, pump/tubing-related problems were the most important reason for insulin pump users for DKA development. Similar data were found in a Danish survey where kinking of the tubing and/or leakage of insulin at the infusion site was the most likely contributor to DKA development.18 This is in contrast to the results described by Hanas et al,19 which found that the most common causes of DKA in patients using CSII were missed insulin doses (48.6%), followed by gastroenteritis (14.1%) and technical pump problems (12.7%).
Although recurrence of DKA events, as mentioned above, are higher in the MDII group, the overall recurrent rate in our CSII group was higher (25%) when compared with the national data obtained from the T1D Exchange Clinic Registry. Per T1D Exchange Clinical registry, only 1% of the pump users among the age group 26–49 years old experienced more than one DKA event in prior 3 months.15 This result depicts a unique population of adults with T1DM that is not representative of the typical T1DM population with CSII. This group of patients needs a special consideration since are known to have an increased risk for mortality20 and not the overall low mortality reported by the CDC in prior studies. Also, this population represents a higher rate of readmissions within 30 days and a higher healthcare cost.21
The explanation for a higher recurrent rate of DKA in our CSII users is most likely multifactorial and not only attributed to an insulin pump malfunction. One of the factors contributing to an increased risk of DKA in this group is the underlying poor glycemic control at the time of admission, determined by elevated HbA1c. This result stresses poor compliance of diabetic treatment regimen among CSII users. In fact, 90% of the patients using an insulin pump mentioned in the questionnaire to skip insulin bolus doses.
Another explanation behind the higher rates of DKA in our CSII users may be related to the infrequent change of the infusion site. The manufacturers of CSII pumps usually recommend changing infusion sites every 2–3 days to avoid skin and infusion problems.22 In our cohort, 50% admitted to exchange the infusion site after more than 3 days. Also, 72% of our studied cohort did not check insulin expiration date, possibly administrating expired insulin.
Other reasons to consider is education, especially regarding insulin pump use and recognition of DKA symptoms. Per Dogan et al18 most of the episodes of DKA occurs early after the pump is initiated, suggesting a learning curve occurs in all new forms of treatment. Based on our questionnaire, most of the patients had been using insulin pumps for at least 5 years, 75% new about their settings, and 76% recognized malfunction. These data are less suggestive of a lack of expertise but instead the problem seems to rely on the lack of knowledge regarding further actions after recognizing a pump malfunction.
There has been scant literature on the assessment of patient knowledge while already on the insulin pump. One study tried to assess patient knowledge during the hospital stay to discern gaps in knowledge and to fill in those deficiencies to ensure a safe hospital stay and included items for the avoidance of DKA for outpatient care (such as knowledge on delivering a correction bolus or having back-up insulin for pump malfunction).23 This tool can potentially be modified for use during outpatient visits to prevent DKA.
Also, education in regards to DKA symptoms recognition is poor. In our cohort, only 20% applied other preventive measures such as checking ketones. This is backed up by data reported in the T1D exchange clinical registry, where checking ketones was more common in child than adults, and blood ketones were uncomely check among all age groups. Only 20% recognized having a blood ketone meter.16
Although pump user education and re-education on DKA avoidance are ideally done in the outpatient setting, educating should be considered in hospitals. Several attempts have been made to augment providers’ knowledge of insulin pump use.24–26 Perhaps teaching hospital providers how to use insulin pumps might improve further outcomes related to DKA, such as shortening hospital LOS. Since based on our results, patients with CSII had prolonged MICU LOS compared with a patient with MDII.
Despite the novel data provided from the questionnaire, the study has inherent limitations. First, this was a single-center prospective observational study with a small sample size; therefore, findings may not be generalized to other settings. However, to our knowledge, this is one of the few studies that aimed to look at causes of DKA in the adult population with T1DM involving pump users and non-users. Second, we did not examine the injection site for skin abnormalities (eg, lipohypertrophy) or the pump itself for defects. Instead, we used a questionnaire and the pump data download for information. Third, we did not obtain data on medical insurance, social risk factors, or compliance with outpatient follow-up that may affect treatment and pump compliance. Finally, although the majority of the cases had mild DKA and in other hospitals will be managed in non-MICU settings, our hospital DKA management protocol provided us the opportunity to have a better view and understanding of DKA behavior in the adult population; information that will be missed if moderate or severe DKA were only included. Additionally, T1DM was not confirmed by standard diagnostic criteria.
Conclusions
Although CSII use has helped overcome most of the challenges in T1DM management over the last years, complications such as DKA are still a burden. In this study, we portrayed the etiology of DKA in adults with T1DM with CSII and MDII, and as well as described a unique population with recurrent DKA events. The major triggering factor for DKA remains treatment non-adherence. In addition to non-adherence, pump device malfunction such as pump/tubing defects, is a significant leading factor for DKA development in the CSII population. The recurrence of DKA events, especially in our CSII users, is likely multifactorial. A combination of social factors, education, and insulin pump malfunction, such as pump/tubing problems, might be playing a pivotal role in DKA recurrence in young adults with T1DM.
Continued patient education and healthcare provider support in managing the pump and tubing and further pump development might help reduce DKA’s rate in this population. Efforts to understand and address other factors contributing to DKA, such as the demographic and clinical characteristics of young adults, symptom recognition, self-management skills, and social risk factors, including access to care, insurance coverage, or cost of treatment, should be a focus for future DKA prevention strategies and studies.
Footnotes
Contributors: MF organized and collected data, drafted and revised the paper. MA designed study protocol, data collection tools, and monitored data collection. SA designed study protocol, data collection tools, and monitored data collection. RA did data collection. ML analyzed the data and wrote the statistical plan. XW analyzed the data and wrote the statistical plan. MCL designed the study protocol, data collection tools, monitored data collection for the whole study, and drafted and revised the paper. MJA-J designed the study protocol, data collection tools, monitored data collection for the whole study, and drafted and revised the paper.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The Cleveland Clinic Institutional Review Board (IRB) reviewed and approved the study. IRB confirmation number 17–1680.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request. All data relevant to the study are included in the article or uploaded as supplemental information. All data is available on REDCap, will be available on request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Realsen J, Goettle H, Chase HP. Morbidity and mortality of diabetic ketoacidosis with and without insulin pump care. Diabetes Technol Ther 2012;14:1149–54. 10.1089/dia.2012.0161 [DOI] [PubMed] [Google Scholar]
- 2.Fazeli Farsani S, Brodovicz K, Soleymanlou N, et al. Incidence and prevalence of diabetic ketoacidosis (DKA) among adults with type 1 diabetes mellitus (T1D): a systematic literature review. BMJ Open 2017;7:e016587. 10.1136/bmjopen-2017-016587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mecklenburg RS, Benson EA, Benson JW, et al. Acute complications associated with insulin infusion pump therapy. Report of experience with 161 patients. JAMA 1984;252:3265–9. [PubMed] [Google Scholar]
- 4.Peden NR, Braaten JT, McKendry JB. Diabetic ketoacidosis during long-term treatment with continuous subcutaneous insulin infusion. Diabetes Care 1984;7:1–5. 10.2337/diacare.7.1.1 [DOI] [PubMed] [Google Scholar]
- 5.Jeitler K, Horvath K, Berghold A, et al. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia 2008;51:941–51. 10.1007/s00125-008-0974-3 [DOI] [PubMed] [Google Scholar]
- 6.Pettus JH, Zhou FL, Shepherd L, et al. Incidences of severe hypoglycemia and diabetic ketoacidosis and prevalence of microvascular complications stratified by age and glycemic control in U.S. adult patients with type 1 diabetes: a real-world study. Diabetes Care 2019;42:2220–7. 10.2337/dc19-0830 [DOI] [PubMed] [Google Scholar]
- 7.Kitabchi AE, Umpierrez GE, Murphy MB, et al. Hyperglycemic crises in diabetes. Diabetes Care 2004;27 Suppl 1:S94–102. 10.2337/diacare.27.2007.s94 [DOI] [PubMed] [Google Scholar]
- 8.Ejaz S, Wilson T. Managing type 1 diabetes ‑ a journey from starvation to insulin pump. Minerva Endocrinol 2013;38:123–31. [PubMed] [Google Scholar]
- 9.Giessmann LC, Kann PH. Risk and relevance of insulin pump therapy in the aetiology of ketoacidosis in people with type 1 diabetes. Exp Clin Endocrinol Diabetes 2020;128:745-751. 10.1055/a-0654-5134 [DOI] [PubMed] [Google Scholar]
- 10.Benoit SR, Zhang Y, Geiss LS, et al. Trends in Diabetic Ketoacidosis Hospitalizations and In-Hospital Mortality - United States, 2000-2014. MMWR Morb Mortal Wkly Rep 2018;67:362–5. 10.15585/mmwr.mm6712a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Randall L, Begovic J, Hudson M, et al. Recurrent diabetic ketoacidosis in inner-city minority patients: behavioral, socioeconomic, and psychosocial factors. Diabetes Care 2011;34:1891–6. 10.2337/dc11-0701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Rewers A, Stafford JM, et al. Trends in the prevalence of ketoacidosis at diabetes diagnosis: the search for diabetes in youth study. Pediatrics 2014;133:e938–45. 10.1542/peds.2013-2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolfsdorf J, Glaser N, Sperling MA, et al. Diabetic ketoacidosis in infants, children, and adolescents: a consensus statement from the American diabetes association. Diabetes Care 2006;29:1150–9. 10.2337/dc06-9909 [DOI] [PubMed] [Google Scholar]
- 14.Kitabchi AE, Umpierrez GE, Miles JM, et al. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009;32:1335–43. 10.2337/dc09-9032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care 2015;38:971–8. 10.2337/dc15-0078 [DOI] [PubMed] [Google Scholar]
- 16.Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016-2018. Diabetes Technol Ther 2019;21:66–72. [published correction appears in Diabetes Technol Ther. 2019 Apr;21(4):230]. 10.1089/dia.2018.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stratigou T, Vallianou N, Vlassopoulou B, et al. DKA cases over the last three years: has anything changed? Diabetes Metab Syndr 2019;13:1639–41. 10.1016/j.dsx.2019.03.022 [DOI] [PubMed] [Google Scholar]
- 18.Dogan ADA, Jørgensen UL, Gjessing HJ. Diabetic ketoacidosis among patients treated with continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2017;11:631–2. 10.1177/1932296816668375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanas R, Lindgren F, Lindblad B. A 2-yr national population study of pediatric ketoacidosis in Sweden: predisposing conditions and insulin pump use. Pediatr Diabetes 2009;10:33–7. 10.1111/j.1399-5448.2008.00441.x [DOI] [PubMed] [Google Scholar]
- 20.Gibb FW, Teoh WL, Graham J, et al. Risk of death following admission to a UK hospital with diabetic ketoacidosis. Diabetologia 2016;59:2082–7. 10.1007/s00125-016-4034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurtado CR, Lemor A, Vallejo F, et al. Causes and predictors for 30-day re-admissions in adult patients with diabetic ketoacidosis in the United States: a nationwide analysis, 2010-2014. Endocr Pract 2019;25:242–53. 10.4158/EP-2018-0457 [DOI] [PubMed] [Google Scholar]
- 22.Heinemann L, Krinelke L. Insulin infusion set: the Achilles heel of continuous subcutaneous insulin infusion. J Diabetes Sci Technol 2012;6:954–64. 10.1177/193229681200600429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kannan S, Satra A, Calogeras E, et al. Insulin pump patient characteristics and glucose control in the hospitalized setting. J Diabetes Sci Technol 2014;8:473–8. 10.1177/1932296814522809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Delgado-Hurtado JJ, Armstrong A, Correa RR, et al. Perception of graduating endocrinology, diabetes, and metabolism fellows on the benefits of wearing a continuous glucose monitor and/or insulin pump for their education. Endocr Pract 2019;25:423–6. 10.4158/EP-2018-0357 [DOI] [PubMed] [Google Scholar]
- 25.Marks BE, Wolfsdorf JI, Waldman G, et al. Pediatric endocrinology trainees' education and knowledge about insulin pumps and continuous glucose monitors. Diabetes Technol Ther 2019;21:105–9. 10.1089/dia.2018.0331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweeney TJ, Kenny DJ, Schubert CC. Inpatient insulin pump therapy: assessing the effectiveness of an educational program. J Nurses Prof Dev 2013;29:84–9. 10.1097/NND.0b013e318286c5da [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjdrc-2020-001329supp001.pdf (1.2MB, pdf)