Abstract
A 66-year-old woman with a remote history of breast cancer and prior tobacco use presented to the hospital with chest pain. She was found to have an elevated troponin consistent with a diagnosis of a non-ST segment elevation myocardial infarction (NSTEMI). A left heart catheterisation revealed non-obstructive coronary disease, and subsequent transthoracic and transoesophageal echocardiograms demonstrated vegetations on both the mitral and aortic valves. Multiple blood cultures showed no growth raising suspicion for non-bacterial thrombotic endocarditis (NBTE). A CT of the chest, abdomen and pelvis was obtained that was consistent with metastatic pancreatic cancer. Her hospital course was complicated by recurrent embolic strokes leading to a rapid clinical deterioration. As a result, she was transitioned to comfort measures and passed away shortly thereafter. To our knowledge, this is the first reported case of an NSTEMI as the initial presentation of NBTE due to underlying malignancy.
Keywords: cardiovascular medicine, valvar diseases, cardiovascular system, oncology, venous thromboembolism
Background
NBTE was first described as fibrin depositions on cadaveric cardiac valves by Zeigler in 1888.1 In 1923, Libman labelled this finding as marantic endocarditis, stemming from the Greek work marantikos, or wasting away.1 This term was later changed by Gross and Friedberg to non-bacterial thrombotic endocarditis in 1936.2 Although this condition was initially seen only in postmortem cases, advances in cardiac imaging have increased recognition in antemortem cases over the past 30 years. The first antemortem diagnosis of NBTE was made in 19763 using M-mode transthoracic echocardiography (TTE). However, with the advent of transoesophageal echocardiography (TEE), physicians have been able to diagnose NBTE more readily.
The pathogenesis of NBTE remains poorly defined, but it is theorised to be caused by migration of inflammatory mononuclear cells that form thrombi with platelets, fibrin and immune complexes. These thrombi can proceed to form sterile vegetations on previously undamaged valves. Of note, the vegetations in NBTE tend to be extremely friable and have a higher propensity to embolise compared with those of infective endocarditis.1 As such, thromboembolic events (particularly stroke) are a common presentation for NBTE.
Despite many published cases of antemortem NBTE, there is a notable absence of literature to review the demographics of this condition. There have been several case series4–7 describing the incidence of NBTE to range from 0.3% to 9.3% among all autopsies. In the largest review to date, Lopez et al1 reported the postmortem prevalence of NBTE to be 1.3% based on a total of 82 676 autopsies. In this same cohort, Lopez et al1 noted the presence of NBTE in about 4% of patients with advanced malignancy. In addition, Edoute et al8 performed echocardiographic screening on 200 patients with solid tumours in the absence of bacterial endocarditis, prosthetic valves or rheumatic heart disease. Approximately 19% of patients in this study8 were found to have valvular vegetations. Of note, this study used TTE rather than TEE so the observed prevalence was likely underreported.
NBTE has been historically associated with advanced mucin-secreting adenocarcinoma but can be seen with any malignancy. It has also been observed with other conditions such as antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis. Patients of all ages can be affected, but most are between 40 and 80 years of age.1 Men and women are affected equally.1
Case presentation
A 66-year-old woman with a history of tobacco use and breast cancer treated with mastectomy and adjuvant chemotherapy presented to the hospital with dizziness and chest pain for several hours. Her initial evaluation was notable for an elevated troponin I of 0.48 ng/mL (reference range:≤0.04 ng/mL) consistent with a diagnosis of a non-ST segment elevation myocardial infarction. The patient underwent a cardiac catheterisation, which revealed non-obstructive coronary disease. Subsequent TTE showed a reduced left ventricular ejection fraction of 33%. Multiple mobile, oscillating echodensities were observed on the mitral valve as well as severe mitral regurgitation and severe aortic stenosis. The echodensities were further evaluated with a TEE, which showed multiple mobile masses on both the mitral (figures 1 and 2) and aortic valves (figure 3).
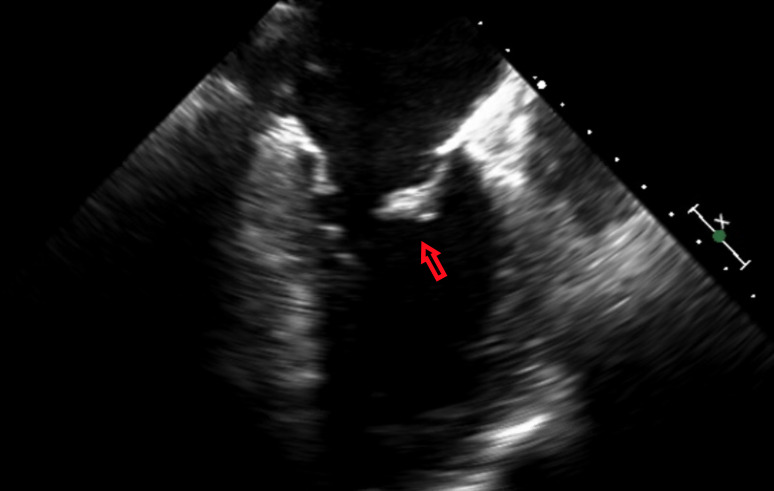
Figure 1.
A large oscillating vegetation noted on the mitral valve on transesophageal echocardiography.
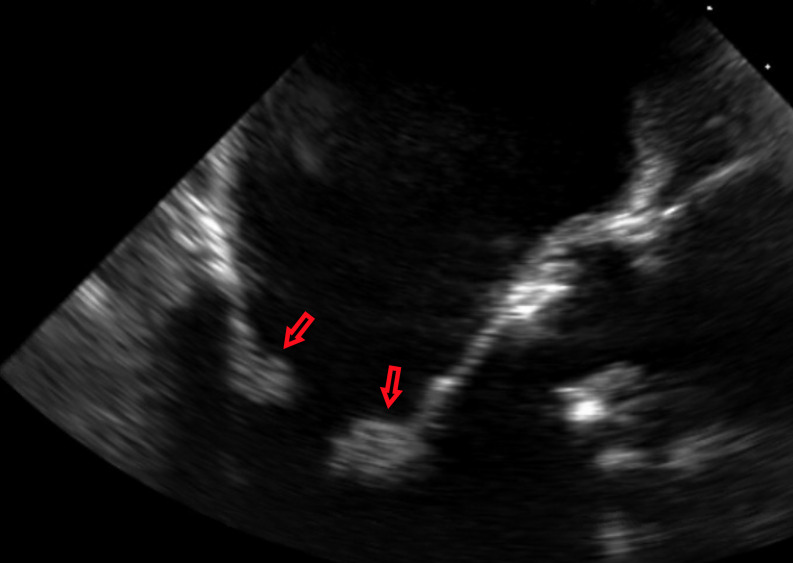
Figure 2.
Multiple vegetations noted on the both cusps of the mitral valve on transoesophageal echocardiography.
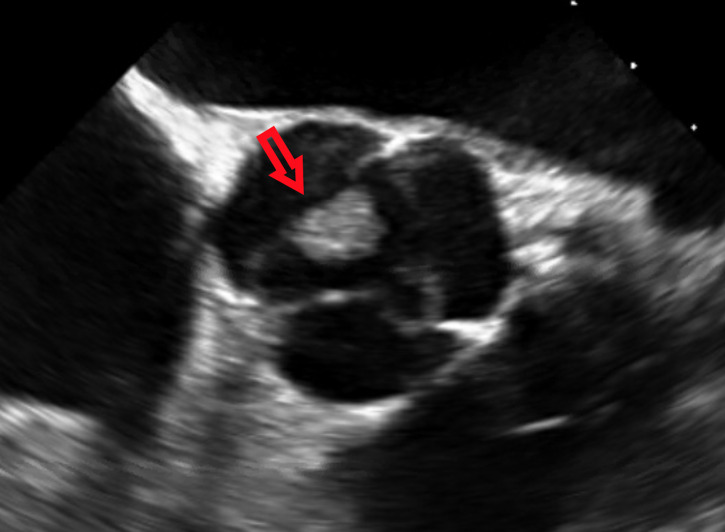
Figure 3.
A large vegetation noted on the non-coronary cusp of the aortic valve on transoesophageal echocardiography.
Our initial differential diagnosis for these echodensities included infective endocarditis versus NBTE due to Libman-Sacks endocarditis, antiphospholipid syndrome or malignancy. Infective endocarditis was thought to be unlikely as the patient was afebrile, had no leucocytosis, and multiple blood cultures showed no growth. Additional rheumatologic workup including antinuclear and antiphospholipid antibodies was also unrevealing. Of note, the patient reported persistent fatigue and unintentional weight loss prior to admission raising suspicion for an underlying malignancy. CT of the chest, abdomen and pelvis revealed a 2.3 cm mass in the head of the pancreas with partial encasement of the coeliac artery (figure 4), multiple splenic and renal infarcts and innumerable low-density masses in the liver consistent with metastases.
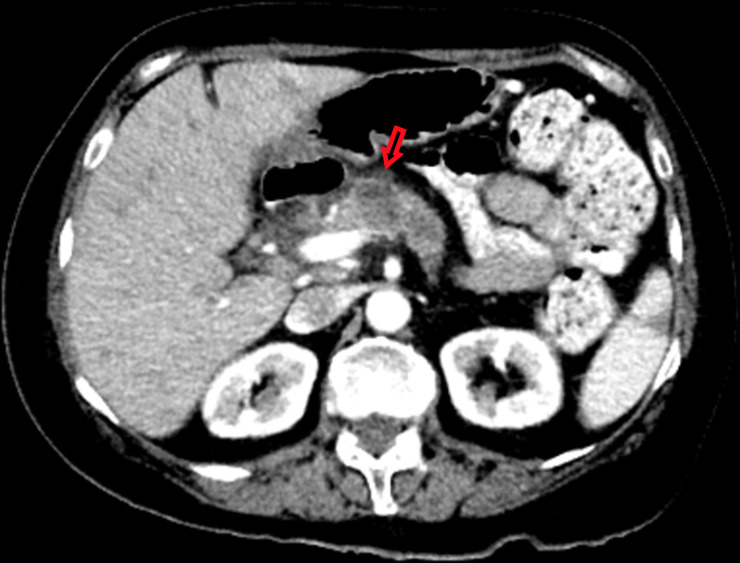
Figure 4.
2.3×2.2 cm mass noted on the pancreatic head on CT abdomen.
The CT and TEE findings were felt to be consistent with a diagnosis of metastatic pancreatic cancer with concomitant NBTE involving the mitral and aortic valves. The patient’s hospital course was complicated by altered mental status, left-sided haemiplegia, dysarthria and facial droop raising concern for embolic stroke. Subsequent MRI of the brain demonstrated a large right middle cerebral artery stroke. The patient was started on anticoagulation but experienced a rapid deterioration due to recurrent strokes.
Outcome and follow-up
Given her rapid clinical deterioration, the patient was ultimately transitioned to comfort care and passed away shortly thereafter.
Discussion
We performed a literature review of antemortem cases of NBTE published from 1990 to 2020 using as a PubMed search with the terms ‘non-bacterial thrombotic endocarditis’ or ‘marantic endocarditis’. This search generated 182 cases of NBTE that were reviewed. Information regarding baseline characteristics, including age, gender, valvular involvement, complications (ie, stroke, thromboembolic events), mortality and life expectancy from time of diagnosis, were collected and summarised in table 1.
Table 1.
Aetiologies and outcomes of published antemortem cases of non-bacterial thrombotic endocarditis
| Aetiology | Total cases | Cerebrovascular stroke | Other thromboembolic events |
| Malignancy | 108 (59%) | 89 (82%) | 54 (50%) |
| Breast | 5 (3%) | 5 (100%) | 2 (40%) |
| Colorectal | 4 (2%) | 2 (50%) | 1 (25%) |
| Oesophagogastroduodenal | 11 (6%) | 11 (100%) | 4 (36%) |
| Hepatobiliary | 4 (2%) | 4 (100%) | 0 (0%) |
| Haematologic | 5 (3%) | 1 (20%) | 2 (40%) |
| Lung | 30 (16%) | 25 (83%) | 14 (47%) |
| Ovarian | 16 (9%) | 15 (94%) | 13 (81%) |
| Pancreas | 14 (8%) | 9 (64%) | 10 (71%) |
| Adenocarcinoma of unknown primary | 7 (4%) | 5 (71%) | 3 (43%) |
| Uterus | 7 (4%) | 7 (100%) | 3 (43%) |
| Other malignancies* | 6 (3%) | 5 (83%) | 2 (33%) |
| Antiphospholipid syndrome | 29 (16%) | 10 (34%) | 8 (28%) |
| Systemic lupus erythematosus | 15 (8%) | 4 (27%) | 5 (33%) |
| Rheumatoid arthritis | 3 (2%) | 2 (67% | 0 (0%) |
| Giant cell arteritis | 2 (1%) | 0 (0%) | 0 (0%) |
| Other† | 25 (14%) | 10 (40%) | 6 (24%) |
| All | 182 | 115 (63%) | 73 (40%) |
Aetiologies are expressed as a number (%).
*Aetiologies included bladder, cervical, prostate and thyroid cancers.
†Aetiologies included adenomyosis, Crohn’s disease, hypereosinophilic syndrome, hyperhomocysteinaemia, Behcets, bone marrow transplant, mesenteric vein thrombosis, systemic sclerosis, Still’s disease, temporal arteritis, Wegner’s granulomatosis and unclear aetiology.
Our literature search revealed that NBTE most commonly affects the mitral valve (61%), followed by the aortic (36%), tricuspid (9%) and pulmonic (2%). Eighty six per cent of cases involved one valve, 10% involved two valves and only 3% involved three or more. While the vast majority of these cases involved native valves, to our knowledge, there have been at least three cases with NBTE affecting prosthetic valves as well. Of all 182 cases reviewed, 108 (59.3%) were associated with malignancy and 42% of patients were men. The most common malignancies associated with NBTE were lung, ovarian, pancreatic and oesophagogastroduodenal, which in total compromised of 33% of all cases of NBTE. Among the cases of NBTE that were not associated with malignancy, antiphospholipid syndrome and systemic lupus erythematosus were the most common aetiologies. The average age of patients with NBTE was 52. However, patients with NBTE associated with malignancy were significantly older than those with non-malignancy associated NBTE (57 vs 45 years old).
Echocardiography has been the diagnostic modality of choice in recognising NBTE. Although TTE is an excellent initial diagnostic modality, its image resolution limits the ability to detect small vegetations (<3 mm). With infective endocarditis, TEE has been shown to be superior in evaluating cardiac anatomy and valvular structure.9 Since a majority of NBTE cases have multiple, small verrucous lesions,1 TEE would theoretically be a more sensitive modality for the diagnosis of NBTE. If there is a clinical suspicion of NBTE based on echocardiography findings, patients should be evaluated for infective endocarditis and an underlying rheumatologic disorder. Further assessment for an underlying malignancy is warranted if infective endocarditis and rheumatologic disease are excluded.
The diagnosis of NBTE is often suspected in patients with symptomatic cerebral infarction without findings suggestive of atherosclerotic disease or cardioembolic disease due to atrial fibrillation or infective endocarditis. Due to the small friable verrucous valvular lesions in NBTE, patients are at increased risk of thromboembolic phenomenon.1 Rarely, thromboembolic events may even precede the diagnosis of the underlying aetiology itself. Our review shows 82% of malignancy-associated NBTE and 63% of all NBTE cases were associated with stroke. In the following study, Singhal et al10 used diffusion-weighted MRI to compare stroke patterns in patients with infective endocarditis and NBTE. They identified four predominant patterns including single lesion, territorial infarction, disseminated punctate lesions and numerous lesions in multiple territories ranging from <10 mm to >30 mm. All patients with stroke in the setting of NBTE exhibited the last pattern, but patients with infective endocarditis displayed all four patterns.10 Approximately 40% of patients within our review also had a non-cerebrovascular thromboembolic event including, but not limited to: pulmonary embolism, splenic infarct, peripheral vascular embolism, mesenteric ischaemia and rarely, coronary thromboembolism.
The management of NBTE remains largely undefined due to the lack of prospective studies comparing treatment modalities in NBTE. Thus, the mainstay of therapy involves treating the underlying disease. Since advanced malignancy is the most common cause of NBTE, it is imperative to diagnose and initiate oncologic therapy quickly. Older reports and retrospective studies have shown that long-term anticoagulation, particularly unfractionated or low-molecular weight heparin, can be used with varying levels of success with low risk for intracerebral haemorrhage. Anticoagulation should be continued lifelong if possible, since discontinuation of therapy has been linked with increased risk of embolic events.11 12 Although anticoagulation may decrease thromboembolic complications, to our knowledge, there have not been any prospective studies done to confirm this.
Guidelines on surgical management of endocarditis are directed towards infective endocarditis but may be extrapolated to NBTE. Consideration of surgical intervention should be highly individualised taking into account the patient’s age, comorbidities, severity of valvular dysfunction and overall prognosis. Valvular repair or valvular replacement may be considered should the patient develop heart failure in the setting of severe valvular disease, high-grade heart block or recurrent thromboembolic phenomenon despite optimal medical therapy.13
Due to its association with advanced malignancy, NBTE portends a poor prognosis. In our review, 6-month follow-up data were available for 75 patients with malignancy-associated NBTE. These patients had a mortality rate of 87%, with an average time from diagnosis to death of 34 days. There was 6-month follow-up data available for only 19 patients with non-malignancy-associated NBTE. The mortality rate in this group was considerably lower at only 16%. All of the patients who died in this group had an underlying diagnosis of antiphospholipid syndrome. The average time from diagnosis to death in this cohort was 15 days.
Our literature search is the first to analyse a large cohort of NBTE cases that were diagnosed antemortem and highlights a stark contrast in outcomes with malignancy-associated NBTE and non-malignancy-associated NBTE. However, there are limitations to this review, as consistent long-term data were not available in all the case reports. Further studies are needed to better assess mortality rates and long-term outcomes in patients with NBTE.
Learning points.
Non-bacterial thrombotic endocarditis (NBTE) is most commonly associated with malignancy, particularly in patients with lung, ovarian, pancreatic and oesophagogastroduodenal cancers. Other causes include antiphospholipid syndrome, systemic lupus erythematosus and rheumatoid arthritis.
Reports of NBTE most commonly involve thromboembolic phenomena, particularly stroke.
Treatment of the underlying disorder is imperative, and although treatment modalities have not been well studied in NBTE, anticoagulation and surgery should be considered.
Prognosis of malignancy-associated non-bacterial thrombotic endocarditis is extremely grim with an observed 6-month mortality of 87% and an average time from diagnosis to death of 34 days.
Footnotes
Twitter: @MaulinPatelDO
Contributors: MJP wrote the manuscript with support from JE. Additionally, JE helped supervise the project.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Lopez JA, Ross RS, Fishbein MC, et al. . Nonbacterial thrombotic endocarditis: a review. Am Heart J 1987;113:773–84. 10.1016/0002-8703(87)90719-8 [DOI] [PubMed] [Google Scholar]
- 2.Gross L, Friedberg CK. Nonbacterial thrombotic endocarditis: classification and general description. Archives of Internal Medicine 1936;58:620–40. [Google Scholar]
- 3.Estevez CM, Corya BC. Serial echocardiographic abnormalities in nonbacterial thrombotic endocarditis of the mitral valve. Chest 1976;69:801–4. 10.1378/chest.69.6.801 [DOI] [PubMed] [Google Scholar]
- 4.Angrist A, Marquiss J. The changing morphologic picture of endocarditis since the advent of chemotherapy and antibiotic agents. Am J Pathol 1954;30:39–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Macdonald RA, Robbins SL. The significance of nonbacterial thrombotic endocarditis: an autopsy and clinical study of 78 cases. Ann Intern Med 1957;46:255. 10.7326/0003-4819-46-2-255 [DOI] [PubMed] [Google Scholar]
- 6.Barron KD, Siqueira E, HIRANO A. Cerebral embolism caused by nonbacterial thrombotic endocarditis. Neurology 1960;10:391. 10.1212/WNL.10.4.391 [DOI] [PubMed] [Google Scholar]
- 7.Rohner RF, Prior JT, Sipple JH. Mucinous malignancies, venous thrombosis and terminal endocarditis with emboli: a syndrome. Cancer 1966;19:1805–12. [DOI] [PubMed] [Google Scholar]
- 8.Edoute Y, Haim N, Rinkevich D, et al. . Cardiac valvular vegetations in cancer patients: a prospective echocardiographic study of 200 patients. Am J Med 1997;102:252–8. 10.1016/S0002-9343(96)00457-3 [DOI] [PubMed] [Google Scholar]
- 9.Mügge A, Daniel WG, Frank G, et al. . Echocardiography in infective endocarditis: reassessment of prognostic implications of vegetation size determined by the transthoracic and the transesophageal approach. J Am Coll Cardiol 1989;14:631–8. 10.1016/0735-1097(89)90104-6 [DOI] [PubMed] [Google Scholar]
- 10.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke 2002;33:1267–73. 10.1161/01.str.0000015029.91577.36 [DOI] [PubMed] [Google Scholar]
- 11.Sack GH, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine 1977;56:1–37. [PubMed] [Google Scholar]
- 12.Rogers LR, Cho ES, Kempin S, et al. . Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746–56. 10.1016/0002-9343(87)90908-9 [DOI] [PubMed] [Google Scholar]
- 13.Nishimura R, Otto C, Bonow R, et al. . AHA/ACC guideline for the management of patients with valvular heart disease: Executive summary: a report of the American College of Cardiology/American heart association Task force on practice guidelines. Circulation 2014;2014:2440–92. [DOI] [PubMed] [Google Scholar]