Abstract
Background
Cognition is commonly affected in brain disorders. Non-invasive brain stimulation (NIBS) may have procognitive effects, with high tolerability. This meta-analysis evaluates the efficacy of transcranial magnetic stimulation (TMS) and transcranial Direct Current Stimulation (tDCS) in improving cognition, in schizophrenia, depression, dementia, Parkinson's disease, stroke, traumatic brain injury, and multiple sclerosis.
Methods
A PRISMA systematic search was conducted for randomized controlled trials. Hedges' g was used to quantify effect sizes (ES) for changes in cognition after TMS/tDCS v. sham. As different cognitive functions may have unequal susceptibility to TMS/tDCS, we separately evaluated the effects on: attention/vigilance, working memory, executive functioning, processing speed, verbal fluency, verbal learning, and social cognition.
Results
We included 82 studies (n = 2784). For working memory, both TMS (ES = 0.17, p = 0.015) and tDCS (ES = 0.17, p = 0.021) showed small but significant effects. Age positively moderated the effect of TMS. TDCS was superior to sham for attention/vigilance (ES = 0.20, p = 0.020). These significant effects did not differ across the type of brain disorder. Results were not significant for the other five cognitive domains.
Conclusions
Our results revealed that both TMS and tDCS elicit a small trans-diagnostic effect on working memory, tDCS also improved attention/vigilance across diagnoses. Effects on the other domains were not significant. Observed ES were small, yet even slight cognitive improvements may facilitate daily functioning. While NIBS can be a well-tolerated treatment, its effects appear domain specific and should be applied only for realistic indications (i.e. to induce a small improvement in working memory or attention).
Key words: Brain disorder, cognitive dysfunction, non-invasive brain stimulation, prefrontal cortex, repetitive transcranial magnetic stimulation, transcranial direct current stimulation
Introduction
Cognitive functioning is affected in many brain disorders (Robbins, 2011). The observed impairment can be profound, as in Alzheimer's disease or Parkinson's disease (PD), or relatively mild, as in depression (Brown & Marsden, 1990; Caviness et al., 2007; Douglas et al., 2018; Petersen et al., 2001). Other brain disorders, such as multiple sclerosis (MS), schizophrenia or traumatic brain injury (TBI) can co-occur with more varying levels of cognitive performance, with either mild or severe cognitive dysfunction (Chiaravalloti & DeLuca, 2008; Heinrichs & Zakzanis, 1998; Sun, Tan, & Yu, 2014; Walker & Tesco, 2013). Impairments may affect multiple cognitive domains, including information processing, working memory, executive functioning and attention (Caviness et al., 2007; Higuchi et al., 2017; Maloni, 2018). These disturbances can profoundly impact daily functioning and quality of life (Papakostas et al., 2004; Schrag, Jahanshahi, & Quinn, 2000). Cognition determines, to a considerable extent, one's social and professional success and the ability to live independently (Audet, Hebert, Dubois, Rochette, & Mercier, 2007; Benedict & Zivadinov, 2006; Green, Kern, Braff, & Mintz, 2000). Moreover, for people in their working age, current society demands high adaptability, resistance to stress and continuous learning, which is impeded by cognitive dysfunction. This refrains many patients, even those with minor cognitive impairments, from holding employments at the level they were trained for. As amelioration of cognitive abilities could lead to improvements in daily life functioning, many studies have tried to improve cognitive functioning using various techniques (Bell & Bryson, 2001; Perneczky et al., 2006; Shin, Carter, Masterman, Fairbanks, & Cummings, 2005).
Current treatment options include rehabilitation, cognitive remediation, physical exercise, cognitive enhancing medication, and various brain stimulation techniques (Cicerone et al., 2005; Leroi, McDonald, Pantula, & Harbishettar, 2012; Wallace, Ballard, Pouzet, Riedel, & Wettstein, 2011), yet treatment effects vary and effect sizes (ES) are generally low. Non-invasive brain stimulation (NIBS) entails the modulation of brain excitability and activity (Ziemann et al., 2008) and consists of different methods, such as transcranial magnetic stimulation (TMS) and transcranial direct current stimulation (tDCS). TMS and tDCS have been used most commonly in an attempt to improve cognition in people with brain disorders. It is generally considered that anodal tDCS (AtDCS) increases the function of the underlying areas of the cortex, whereas cathodal tDCS has a suppressive effect (Nitsche & Paulus, 2011). TMS can either lead to an increase or decrease in cortical excitability depending on the stimulation frequency, varying from 1 to 50 Hertz. High tolerability with few side-effects is considered an important advantage over medication and, in the case of tDCS, has a potential to be applied at home (Aleman, Sommer, & Kahn, 2007, 2018; Chervyakov, Chernyavsky, Sinitsyn, & Piradov, 2015; Lage, Wiles, Shergill, & Tracy, 2016; Rossi et al., 2009; Slotema, Aleman, Daskalakis, & Sommer, 2012). Furthermore, contrasting cognitive remediation or practice and drill, NIBS demands little effort from patients, which is an important advantage for those who also suffer from fatigue, apathy, or diminished motivation.
Until now, the efficacy of NIBS is unclear and some have questioned whether these techniques have any effect on the brain at all (Vöröslakos et al., 2018). Skepticism is increased by lack of clear theories and evidence on working mechanisms of both interventions, which hampers smart applications (Chase, Boudewyn, Carter, & Phillips, 2020; Singh, Erwin-Grabner, Goya-Maldonado, & Antal, 2019) Nevertheless, many studies have applied NIBS techniques in an attempt to improve cognitive function in different diagnostic groups. Although some studies showed promising findings, the results of studies on the effect of NIBS on cognition remain inconsistent and the field would benefit from an overarching systematic overview of findings so far.
Cognition is a broad concept and different cognitive functions are subserved by different cerebral and cerebellar circuits, which may be more or less susceptible to stimulation techniques. As both TMS and tDCS are thought to affect mainly the outer layers of the brain, cognitive circuits that rely on midbrain and other deep structures such as the cingulate gyrus and the hippocampus may be expected to be insensitive to NIBS. In addition, since NIBS is usually applied to the cerebral cortex, cognitive functions that rely highly on subcortical or cerebellar circuits may not be expected to be responsive either. As the dorsolateral prefrontal cortex (DLPFC) is laterally located and often close to the area of stimulation, working memory may be a cognitive function expected to benefit from NIBS (Brunoni & Vanderhasselt, 2014; Miniussi & Rossini, 2011; Pope & Chris Miall, 2014; Tracy et al., 2015). Thus, the results of TMS and tDCS may become less heterogeneous when analyzed within the specific cognitive domains (attention/vigilance, working memory, executive functioning, processing speed, verbal fluency, verbal learning, and social cognition).
The aim of the current review and meta-analysis is therefore to quantitatively investigate the procognitive effect of NIBS (i.e. TMS and tDCS) in a domain-specific way, across different brain disorders in which cognitive dysfunction is a common problem. We evaluate all randomized controlled trials (RCTs) assessing the effect of the two most commonly applied types of NIBS (i.e. TMS and tDCS) for cognitive dysfunction in schizophrenia, depression, dementia, PD, MS, stroke, and TBI.
Methods
Search strategy
Following the Preferred Reporting for Systematic Reviews and Meta-analysis (PRISMA) Statement (Moher, Liberati, Tetzlaff, & Altman, 2009), a systematic search was performed in PubMed (Medline), EMBASE, Web of Science, and Cochrane Database of Systematic Reviews, using the following search terms: [‘cognition’ OR ‘cognitive functioning’] AND [‘transcranial magnetic stimulation’, ‘pulsed electromagnetic field therapy’, ‘low field magnetic stimulation’, ‘transcranial electrical stimulation’ OR ‘transcranial direct current stimulation’] AND [‘randomized controlled trial’, ‘RCT’ OR ‘randomized controlled study’], for each brain disorder (schizophrenia, depression, dementia, PD, MS, stroke, and TBI; exact terms described in online Supplementary Methods). No year or language limits were applied. Review articles and meta-analyses were examined for cross-references. The search cutoff date was 1 May 2019.
Study inclusion
-
(1)
RCTs investigating the effects of TMS or tDCS treatment on cognition measured with a neuropsychological test (battery);
-
(2)
Studying patients affected by one of our conditions of interest;
-
(3)
Single/double-blind studies comparing the treatment to a patient control group receiving sham stimulation. In case of combined interventions, the control group received the same non-brain stimulation component of the intervention (e.g. brain stimulation + medication v. sham + medication). Studies applying stimulation on a control stimulation site instead of sham were also included;
-
(4)
Studies reporting sufficient information to compute common ES statistics [i.e. mean and standard deviations (s.d.), exact F-, p-, t, or z-values] or corresponding authors provided these data upon request;
-
(5)
If multiple publications were retrieved describing the same cohort, the sample with the largest overall sample size and/or original data was included;
-
(6)
Studies were published in an international peer-reviewed journal.
Measures
For each identified study, we included pre- and post-assessments of cognitive functioning for the active v. sham condition. To facilitate cross-comparisons, cognitive outcomes were categorized into neurocognitive domains (Lage et al., 2016). We selected seven cognitive domains based on the two cognitive batteries commonly used in studies evaluating cognitive performance in psychiatric and neurological patient populations: the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia; Green et al., 2004) and the Movement Disorder Society Task Force (Litvan et al., 2012): attention/vigilance, working memory, executive functioning, processing speed, verbal learning, verbal fluency, and social cognition. When a study applied multiple cognitive tests to assess the same cognitive domain, we included the primary outcome measure as defined by authors. When the primary outcome was not defined, we selected the test most relevant to the defined cognitive domain. If studies reported multiple outcomes for a single cognitive test (e.g. reaction time and number of errors), the outcome most relevant to the cognitive domain was included for analysis.
Statistical analyses
We separately evaluated the effects of TMS and tDCS across the seven brain disorders, including all relevant study samples for the seven cognitive domains. Study samples were grouped according to brain disorder. We evaluated whether the effect varied per specific brain disorder and performed subanalyses when k ⩾ 3.
Calculations
All analyses were performed using Comprehensive Meta-Analysis Version 2.0 (Borenstein, Hedges, Higgins, & Rothstein, 2009). Details are provided in online Supplementary Methods. In short, Hedges' g was used to quantify ES for changes in cognitive performance, where a positive ES represented a superior effect of brain stimulation v. sham. Studies with multiple treatment groups (e.g. different stimulation intensity) and one sham group were entered as individual study samples (k). Single-dose (i.e. challenge) studies were included, sensitivity analyses were run with and without these challenge studies.
ES of p < 0.05 (two-tailed) were considered statistically significant, 0.2 reflecting a small, 0.5 a medium, and ⩾0.8 a large effect (Cohen, 1988). To investigate whether studies could be combined to share a common population ES, the Q-value and I2-statistic were evaluated. I2-values of 25, 50, and 75% are considered as low, moderate, and high heterogeneity, respectively (Cohen, 1988; Higgins, Thompson, Deeks, & Altman, 2003). Outlier studies were evaluated when heterogeneity was significant (p < 0.05), defined as standardized residual Z-scores of ES exceeding ±1.96 (p < 0.05, two-tailed).
For (trend-)significant results, potential publication bias was investigated by means of a visual inspection of the funnel plot and Egger's test (p < 0.1, two-tailed) (Egger, Smith, Schneider, & Minder, 1997). Rosenthal's fail-safe number (NR) was calculated, estimating the number of unpublished studies with non-significant results needed to bring an observed result to non-significance (NR ⩾ 5k + 10 to rule out a file drawer problem) (Rosenthal, 1979).
Sensitivity analyses were performed for (trend-)significant results, correcting for inflated control groups, single-dose studies, and moderator effects of the number of treatment sessions, mean age, and gender (online Supplementary Methods).
Results
The literature search following PRISMA guidelines is depicted in Fig. 1. Demographic information on the included studies is provided in Table 1 for TMS and Table 2 for tDCS. The total number of included studies was 82, reporting on 93 study samples (k), evaluating a grand total of 2784 patients. Table 3 depicts the mean demographical group characteristics for the 43 TMS studies and 39 tDCS studies. Forest plots of the significant results are depicted in Fig. 2, forest plots of the remaining cognitive domains are depicted in online Supplementary Figs S1–S10.
Fig. 1.
PRISMA flow diagram of the performed literature search. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses, dementia, depression, SZ (schizophrenia), MS (multiple sclerosis), PD (Parkinson's diseases), stroke and TBI (traumatic brain injury).
Table 1.
Characteristics of the included studies for Transcranial Magnetic Stimulation (TMS)
| TMS study (year) | Design | Stimulation type/site | n | Age | %F | Duration of illness | Hz | Nr. of sessions | Cognitive domain(s) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | ||||||||||
| McIntosh et al. (2004) | Double | Left TPC | 16 | 36 ± 10.9 | 56 | AAO: 24 ± 6.0 | 1 | 4 | VL | |
| Cross-over | Sham | 4 | ||||||||
| Fitzgerald et al. (2005) | Double | Left auditory TPC | 17 | 16–65 | 1 | 10 | A/V, WM, VF, VL | |||
| Sham | 16 | 16–65 | 10 | |||||||
| Mogg et al. (2007) | Double | Left DLPFC | 8 | 51 ± 14.5 | 13 | 25y ± 16.7 | 10 | 10 | EF, VF, VL | |
| Sham | 9 | 34 ± 9.8 | 0 | 9y ± 7.9 | 10 | |||||
| Mittrach et al. (2010) | Double | Left DLPFC | 18 | 35 ± 0.5 | 6y ± 5.2 | 10 | 10 | A/V, EF, PS | ||
| Sham | 14 | 34 ± 10.5 | 21 | 6y ± 8.8 | 10 | |||||
| Barr et al. (2011) | Double | Bilateral DLPFC | 12 | 47 ± 12.8 | 42 | 20 | 1 | WM, PS | ||
| Sham | 12 | 47 ± 12.8 | 42 | 1 | ||||||
| Zheng, Guo, Li, Li, and Wang (2012) | Double | Left DLPFC | 18 | 57 ± 5.4 | AAO: 34 ± 6.5 | 20 | 5 | WM, VF | ||
| Left DLPFC | 18 | 57 ± 7.4 | AAO: 32 ± 7.2 | 10 | 5 | |||||
| ITBS; left DLPFC | 19 | 56 ± 9.3 | AAO: 33 ± 8.1 | 1–50 | 5 | |||||
| Sham | 17 | 56 ± 5.8 | AAO: 33 ± 10.0 | 5 | ||||||
| Barr et al. (2013) | Double | Bilateral DLPFC | 13 | 41 ± 12.0 | 46 | 19y ± 11.7 | 20 | 20 | WM, PS | |
| Sham | 14 | 49 ± 12.4 | 21 | 25y ± 16.2 | 20 | |||||
| Guse et al. (2013) | Double | Left pMFG | 13 | 37 (22–58) | 23 | >0.5y | 10 | 15 | A/V, EF, PS | |
| Sham | 12 | 36 (20–51) | 25 | >0.5y | 15 | |||||
| Wölwer et al. (2014) | Double | Left DLPFC | 18 | 34 (22–59) | 22 | 6y ± 5.2 | 10 | 10 | A/V, EF, PS, SC | |
| Sham | 14 | 34 (22–59) | 21 | 6y ± 8.7 | 10 | |||||
| Rabany, Deutsch, and Levkovitz (2014) | Double | Left DLPFC | 20 | 33 ± 11.31 | 35 | AAO: 21 ± 9.8 | 20 | 20 | A/V, WM, EF, PS | |
| Sham | 10 | 36 ± 11.0 | 20 | AAO: 26 ± 8.3 | 20 | |||||
| Dlabac-De Lange et al. (2015) | Double | Bilateral DLPFC | 16 | 42 ± 11.6 | 13 | 16y ± 10.1 | 10 | 30 | EF, PS, VF, VL | |
| Sham | 16 | 32 ± 9.7 | 25 | 10y ± 8.9 | 30 | |||||
| Wobrock et al. (2015) | Double | Left DLPFC | 76 | 36 ± 10.5 | 18 | >1y | 10 | 15 | EF, PS | |
| Sham | 81 | 35 ± 9.1 | 31 | >1y | 15 | |||||
| Hasan et al. (2016) | Double | Left DLPFC | 77 | 36 ± 10.6 | 14 | >1y | 10 | 15 | WM, EF, VF | |
| Sham | 79 | 36 ± 9.0 | 28 | >1y | 15 | |||||
| Francis et al. (2019) | Double | Bilateral DLPFC | 9 | 23 ± 3.1 | 22 | 3y ± 1.6 | 20 | 10 | VL, WM, EF, PS, VF | |
| Sham | 10 | 22 ± 2.0 | 20 | 2y ± 1.1 | 10 | |||||
| Depression/bipolar | ||||||||||
| Loo et al. (2001) | Double | Left DLPFC | 9 | 48 | 50 | 10 | 20 | A/V, WM, EF, VL, VF | ||
| Sham | 9 | 48 | 50 | 20 | ||||||
| Moser et al. (2002) | Double | Left aMFG | 9 | 61 ± 10.3 | 20 | 5 | EF, PS, VF, VL | |||
| Sham | 10 | 61 ± 10.2 | 5 | |||||||
| Fitzgerald et al. (2003) | Double | HF; L PFC | 20 | 42.20 ± 9.80 | 40 | AAO: 29 ± 11.1 | 10 | 10 | A/V, WM, EF, VL | |
| LF; R PFC | 20 | 45.55 ± 11.49 | 35 | AAO: 31 ± 14.9 | 1 | 10 | ||||
| Sham | 20 | 49.15 ± 14.24 | 55 | AAO: 34 ± 11.4 | 10 | |||||
| Hausmann et al. (2004) | Double | HFL; DLPFC | 12 | 47.33 ± 13.34 | 50 | 20 | 10 | EF, PS, VF, VL | ||
| HFL/LFR; DLPFC | 13 | 45.23 ± 11.95 | 62 | 1–20 | 10 | |||||
| Sham | 13 | 47.00 ± 11.31 | 69 | 10 | ||||||
| Mosimann et al. (2004) | Double | Left DLPFC | 15 | 60.0 ± 13.4 | 33 | AAO: 36 ± 16.7 | 20 | 10 | EF, PS, VF, VL | |
| Sham | 9 | 64.4 ± 13.0 | 56 | AAO: 53 ± 14.0 | 10 | |||||
| McDonald et al. (2006) | Double | HFL DLPFC | 25 | 49 (41–55) | 72 | 10 | 10 | A/V, WM, VF | ||
| LFR DLPFC | 25 | 49 (39–54) | 36 | 1 | 10 | |||||
| Sham | 12 | 54 (47–64) | 42 | 10 | ||||||
| Loo, Mitchell, McFarquhar, Malhi, and Sachdev (2007) | Double | Left PFC | 19 | 49.8 ± 2.5 | 53 | AAO: 28 ± 16.4 | 10 | 20 | A/V, WM, PS, EF, VF, VL | |
| Sham | 19 | 45.7 ± 15.0 | 42 | AAO: 32 ± 13.2 | 20 | |||||
| Vanderhasselt, de Raedt, Baeken, Leyman, and D'Haenen (2009) | Double | Left DLPFC | 15 | 45.6 ± 5.87 | 60 | AAO: 38 ± 16.4 | 10 | 10 | EF | |
| Cross-over | Sham | 10 | ||||||||
| Ullrich, Kranaster, Sigges, Andrich, and Sartorius (2012) | Double | Left DLPFC | 22 | 56.9 ± 10.2 | 69 | 7y ± 3.4 | 30 | 15 | PS, WM | |
| Sham | 21 | 54.1 ± 7.8 | 57 | 6y ± 6.0 | 15 | |||||
| Wajdik et al. (2014) | Single | Left DLPFC | 32 | 21–65 | 10 | 15 | A/V, WM, EF, PS, VF, VL | |||
| Sham | 31 | 21–65 | 15 | |||||||
| Cheng et al. (2016) | Double | cTBS; right DLPFC | 15 | 21–70 | 1–50 | 10 | EF | |||
| iTBS; left DLPFC | 15 | 21–70 | 1–50 | 10 | ||||||
| c + iTBS; bilateral DLPFC | 15 | 21–70 | 1–50 | 10 | ||||||
| Sham | 15 | 21–70 | 10 | |||||||
| Kaster et al. (2018) | Double | DLPFC, VLPFC | 25 | 65 ± 5.5 | 32 | AAO: 33 ± 18.0 | 18 | 20 | A/V, WM, EF, VF | |
| Sham | 27 | 65 ± 5.5 | 44 | AAO: 30 ± 18.6 | 20 | |||||
| Kavanaugh et al. (2018) | Double | Left DLPFC | 43 | 46 ± 11.6 | NR | 10 | 20 | A/V, WM, PS | ||
| Sham | 41 | 48 ± 12.8 | 20 | |||||||
| Myczkowski et al. (2018) | Double | Left DLPFC | 20 | 41 ± 11.7 | 75 | 12y ± 14.7 | 18 | 20 | A/V, WM, EF, PS, VF, VL | |
| Sham | 23 | 41 ± 9.0 | 78 | 11y ± 10.4 | 20 | |||||
| Dementia | ||||||||||
| Eliasova et al. (2014) | Double | IFG | 10 | 75 ± 7.5 | 40 | 4 ± 1.6 | 10 | 2 | EF, SP | |
| Cross-over | Sham | 2 | ||||||||
| Zhao et al. (2017) | Double | P3/P4, T5/T6 | 17 | 69 ± 5.8 | 59 | NR | 20 | 30 | VL | |
| Sham | 13 | 71 ± 5.2 | 54 | 30 | ||||||
| Koch et al. (2018) | Double | Precuneus | 14 | 70 ± 5.1 | 50 | 14mo ± 5.1 | 20 | 10 | EF, PS | |
| Cross-over | Sham | 10 | ||||||||
| Padala et al. (2018) | Double | Left DLPFC | 4 | 68 ± 10.0 | 0 | NR | 10 | 20 | EF, PS | |
| Cross-over | Sham | 5 | 64 ± 9.0 | 20 | 10 | |||||
| Parkinson's disease | ||||||||||
| Sedláčková, Rektorová, Srovnalová, and Rektor (2009) | Double | Left PMCd | 10 | 64 ± 6.7 | 10 | 8y ± 6.5 | 10 | 1 | A/V, WM, PS | |
| Cross-over | Left DLPFC | 1 | ||||||||
| Sham | 1 | |||||||||
| Pal, Nagy, Aschermann, Balazs, and Kovacs (2010) | Double | Left DLPFC | 12 | 68.5 (median) | 50 | 6y (median) | 5 | 10 | EF | |
| Sham | 10 | 67.5 (median) | 50 | 7y (median) | 10 | |||||
| Benninger et al. (2011) | Double | iTBS, Bilateral DLPFC | 13 | 62 ± 6.9 | 46 | 11y ± 7.1 | 1–50 | 8 | SP | |
| Sham | 13 | 66 ± 9.0 | 15 | 7y ± 3.4 | 8 | |||||
| Srovnalova, Marecek, and Rektorova (2011) | Double | Bilateral IFG | 10 | 66 ± 6.0 | 40 | 5y ± 2.5 | 25 | 1 | SP, EF | |
| Cross-over | Sham | 1 | ||||||||
| Benninger et al. (2012) | Double | Bilateral PMC | 13 | 65 ± 9.1 | 15 | 9y ± 4.1 | 50 | 8 | SP | |
| Sham | 13 | 64 ± 8.3 | 31 | 9y ± 6.8 | 8 | |||||
| Dagan, Herman, Mirelman, Giladi, and Hausdorff (2017) | Double | Mpfc | 7 | 76 ± 6.47 | 0 | 10y ± 3.8 | 10 | 8 | EF | |
| Cross-over | Sham | 6 | 10y ± 3.8 | 8 | ||||||
| Buard et al. (2018) | Double | Bilateral DLPFC | 22 | 67 ± 7.2 | 27 | 20 | 10 | A/V, EF, PS, VL, VF | ||
| Sham | 24 | 70 ± 8.0 | 29 | 10 | ||||||
| Stroke | ||||||||||
| Fregni, Boggio, Nitsche, Rigonatti, and Pascual-Leone (2006a, 2006b) | Double | PMC unaffected side | 10 | 58 ± 11.3 | 20 | 4y ± 2.9 | 1 | 5 | A/V, WM, EF, PS | |
| Sham | 5 | 53 ± 12.6 | 40 | 4y ± 2.6 | 5 | |||||
| Kim, Kim, Ho Chun, Hwa Yi, and Sung Kwon (2010) | Double | HF; left DLPFC | 6 | 54 ± 16.9 | 33 | 241da ± 42.5 | 10 | 10 | A/V, WM, EF, PS, VL | |
| LF; left DLPFC | 6 | 68 ± 7.4 | 67 | 404da ± 71.7 | 1 | 10 | ||||
| Sham | 6 | 67 ± 17.2 | 33 | 70da ± 39.0 | 10 | |||||
| Sun (2015) | Double | Right DLPFC | 22 | 43 ± 12.3 | 37 | 67da (30–365) | 1 | 20 | VL | |
| Sham | 22 | 47 ± 11.8 | 38 | 56da (30–296) | 20 | |||||
| Traumatic Brain Injury | ||||||||||
| Lee and Kim (2018) | Single | Right DLPFC | 7 | 42 ± 11.3 | 29 | 4mo ± 1.7 | 1 | 10 | EF | |
| Sham | 6 | 41 ± 11.0 | 33 | 4mo ± 1.9 | 10 | |||||
Hz, Hertz; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; PMC, premotor cortex; TPC, temporo-parietal cortex; VLPFC; ventrolateral prefrontal cortex; a, anterior; m, medial; p, posterior; d, dorsal; HF, high frequency; LF, low frequency; iTBS, intermitted theta burst stimulation; cTBS, continuous theta burst stimulation; c + iTBS, continuous and intermittent theta burst stimulation; AAO, age at onset; y, years; mo, months; da, days; A/V, attention/vigilance; EF, executive functioning; PS, processing speed; VF, verbal fluency; VL, verbal learning; WM, working memory.
Year of publication (Year), study design (Design), stimulation type and/or site, number of participants per group (n), age (mean ± standard deviation), proportion of females (%F), duration of illness (mean ± standard deviation if available), stimulation frequency (Hz), number (nr.) of sessions and cognitive domains(s) are specified for each study.
Table 2.
Characteristics of the included studies for transcranial direct current stimulation (tDCS)
| tDCS study (year) | Design | Stimulation type/site | n | age ± s.d. | %F | Duration of illness | mA | Nr. of sessions | Cognitive domain(s) |
|---|---|---|---|---|---|---|---|---|---|
| Schizophrenia | |||||||||
| Smith et al. (2015) | Double | A: left DLPFC, C: right SO ridge | 17 | 47 ± 11.1 | 18 | 2 | 5 | A/V, WM, EF, PS, VL, SC | |
| Sham | 16 | 45 ± 9.2 | 38 | 5 | |||||
| Rassovsky et al. (2015) | Single | A: DLPFC, C: right SO | 12 | 46 ± 11.2 | 17 | 20y ± 13.8 | 2 | 1 | SC |
| C: DLPFC, A: right SO | 12 | 48 ± 7.5 | 50 | 25y ± 10.9 | 1 | SC | |||
| Sham | 12 | 42 ± 10.3 | 33 | 15y ± 7.7 | 1 | ||||
| Palm et al. (2016) | Double | A: left DLPFC, C: right SO | 10 | 38 ± 12.9 | 50 | 7y ± 6.1 | 2 | 10 | WM, EF, PS, |
| Sham | 10 | 34 ± 10.7 | 0 | 14y ± 12.1 | 10 | ||||
| Gögler et al. (2017) | Double | A: left DLPFC, C: right SO | 20 | 37 ± 9.2 | 35 | 7y ± 5.9 | 2 | 1 | WM, EF, PS |
| Sham | 20 | 32 ± 8.3 | 50 | 1 | |||||
| Orlov et al. (2017) | Double | A: left DLPFC, C: right SO | 22 | 35 ± 9.4 | 14 | 13y ± 7.3 | 2 | 2 | WM, VL |
| Sham | 25 | 39 ± 9.4 | 16 | 17y ± 9.2 | 2 | ||||
| Gomes et al. (2018) | Double | A: left DLPFC, C: right DLPFC | 12 | 39 ± 9.3 | 17 | 16y ± 11.6 | 2 | 10 | A/V, WM, EF, PS, VL |
| Sham | 12 | 34 ± 12.1 | 42 | 10y ± 7.3 | 10 | ||||
| Jeon et al. (2018) | Double | A: left DLPFC, C: right DLPFC | 25 | 40 ± 9.4 | 50 | 13y | 2 | 10 | A/V, WM, EF, PS, VL, SC |
| Sham | 27 | 40 ± 12.4 | 57 | 14y | 10 | ||||
| Mellin et al. (2018) | Double | A: Left DLPFC, C: left TPJ | 7 | 30 ± 11.0 | 2 | 10 | WM, EF, PS, VF, VL | ||
| Sham | 7 | 39 ± 10.0 | 10 | ||||||
| Papazova et al. (2018) | Double | A: left DLPFC, C: right deltoid muscle | 20 | 37 ± 10.6 | 45 | 9y ± 7.6y | 1 | 1 | WM |
| Cross-over | A: left DLPFC, C: right deltoid muscle | 2 | 1 | ||||||
| Sham | 1 | ||||||||
| Lindenmayer et al. (2019) | Double | A: left DLPFC, C: left AC | 15 | 40 ± 10.7 | 13 | 3y ± 4.5 | 2 | 40 | A/V, WM, EF, PS, VL, SC |
| Sham | 13 | 40 ± 10.7 | 15 | 3y ± 4.5 | 40 | ||||
| Depression/bipolar | |||||||||
| Fregni et al. (2006a, 2006b) | Double | A: left DLPFC, C: right SO | 9 | 48 ± 10.4 | 56 | 10y ± 5.9 | 1 | 5 | A/V, WM, EF, PS |
| Sham | 9 | 45 ± 9.3 | 67 | 9y ± 4.2 | 5 | ||||
| Boggio et al. (2007) | Double | A: left DLPFC, C: right SO | 12 | 48 ± 9.9 | 50 | 24y ± 7.4 | 2 | 1 | SC |
| Sham | 7 | 47 ± 10.4 | 71 | 22y ± 10.6 | 1 | ||||
| Loo et al. (2010) | Double | A: left DLPFC C:right lat. O | 20 | 49 ± 10.0 | 55 | AAO: 31y ± 14.1 | 1 | 5 | A/V, WM, EF, PS, VF, VL |
| Sham | 20 | 46 ± 12.5 | 55 | AAO: 32y ± 14.7 | 5 | ||||
| Loo et al. (2012) | Double | A: left DLPFC, C:right lat. O | 31 | 48 ± 12.5 | 45 | AAO: 28y ± 12.6 | 2 | 15 | A/V, WM, EF, VF, VL |
| Sham | 29 | 49 ± 12.6 | 48 | AAO: 28y ± 12.5 | 15 | ||||
| Palm et al. (2012) | Double | A: left DLPFC, C: right SO; active first | 11 | 56 ± 12.0 | 55 | AAO: 44y ± 10.0 | 0.5 | 10 | WM, VF, VL |
| Cross-overa | A: left DLPFC, C: right SO; sham first | 11 | 58 ± 12.0 | 73 | AAO: 4y ± 15.0 | 0.5 | 10 | ||
| Segrave, Arnold, Hoy and Fitzgerald (2014) | Double | A: left DLPFC, C: right lat. O; CCT | 9 | 43 ± 18.3 | 22 | AAO: 17y ± 9.1 | 2 | 5 | WM, PS |
| Sham; CCT | 9 | 45 ± 10.2 | 44 | AAO: 16y ± 5.9 | 5 | ||||
| Bennabi et al. (2015) | Double | A: left DLPFC, C: right SO | 12 | 60 ± 12.0 | 83 | 2 | 10 | PS, EF, VL | |
| Sham | 11 | 60 ± 5.4 | 46 | 10 | |||||
| Moreno et al. (2015) | Single | A: left DLPFC, C: right DLPFC | 10 | 35 ± 4.1 | 50 | 2 | 1 | WM, PS, SC | |
| Sham | 10 | 32 ± 4.7 | 50 | 1 | |||||
| Brunoni et al. (2016) | Double | A: left DLPFC, C: right DLPFC | 26 | 41 ± 12.0 | 81 | 2 | 12 | A/V, WM, EF, PS | |
| Sham | 26 | 46 ± 14.0 | 77 | 12 | |||||
| Bersani et al. (2017) | Double | A: left DLPFC, C: right CBC | 21 | 48 ± 10.7 | 71 | 19y ± 11.0 | 2 | 15 | EF, PS |
| Sham | 21 | 29 ± 10.2 | 38 | 12y ± 6.6 | 15 | ||||
| Brunoni et al. (2017) | Double | A: left DLPFC, C: right DLPFC; oral placebo | 72 | 45 ± 11.8 | 68 | AAO: 26y ± 11.7 | 2 | 22 | A/V, WM, EF, PS, VF |
| Sham; oral placebo | 55 | 41 ± 12.9 | 68 | AAO: 26y ± 11.3 | 22 | ||||
| Salehinejad, Ghanavai, Rostami, and Nejati (2017) | Single | A: left DLPFC, C: right DLPFC | 12 | 27 ± 7.1 | 58 | 2 | 10 | A/V, WM | |
| Sham | 12 | 26 ± 4.6 | 67 | 10 | |||||
| Pavlova et al. (2018) | Single | A: left DLPFC, C: right SO; 20 min | 21 | 36 ± 10.8 | 81 | 0.5 | 10 | A/V, WM, PS, VF | |
| A: left DLPFC, C: right SO; 30 min | 27 | 37 ± 8.8 | 63 | 0.5 | 10 | ||||
| Sham | 20 | 40 ± 12.2 | 75 | 10 | |||||
| Dementia | |||||||||
| Ferrucci et al. (2008) | Double | A: TP area, C: right deltoid muscle | 10 | 75 ± 7.3 | 70 | 1.5 | 1 | A/V | |
| Cross-over | Sham | 70 | 1 | ||||||
| Boggio et al. (2012) | Double | A: bilateral TC; C: right deltoid muscle | 15 | 79 ± 8.1 | 40 | 4y ± 1.7 | 2 | 5 | A/V, VL |
| Cross-over | Sham | 4y ± 1.7 | 5 | ||||||
| Suemoto et al. (2014) | Double | A: left DLPFC, C: right SO | 20 | 79 ± 7.1 | 75 | 2 | 6 | A/V, WM | |
| Sham | 20 | 82 ± 8.0 | 65 | 6 | |||||
| Biundo et al. (2015) | Double | A: left DLPFC, C: right SO | 7 | 69 ± 7.6 | 14 | 2 | 10 | A/V, PS, VF, VL | |
| Sham | 9 | 72 ± 4.1 | 11 | 10 | |||||
| André et al. (2016) | Single | A: left DLPFC, C: right SO | 13 | 63–94 | 2 | 1 | EF, WM | ||
| Sham | 8 | 63–94 | 1 | ||||||
| Parkinson's disease | |||||||||
| Manenti et al. (2018) | Double | A: left DFPFC, C: right SO; CT | 11 | 66 ± 6.4 | 55 | 6y ± 3.9 | 2 | 10 | A/V, EF, PS, VF, VL |
| Sham; CT | 11 | 64 ± 7.1 | 36 | 8y ± 3.4 | 10 | ||||
| Elder, Colloby, Firbank, McKeith, and Taylor (2019) | Double | A: right PC; C: OcC | 19 | 76 ± 8.8 | 21 | 1.2 | 10 | PS | |
| Sham | 17 | 74 ± 7.0 | 29 | 10 | |||||
| Stroke | |||||||||
| Jo et al. (2009) | Single | A: left DLPFC, C: right SO | 10 | 48 ± 8.7 | 30 | 72da ± 30.0 | 2 | 1 | WM |
| Cross-over | Sham | 1 | |||||||
| Yun, Chun, and Kim (2015) | Double | A: left aTL; C: right SO | 15 | 61 ± 12.9 | 60 | 42da ± 31.9 | 2 | 15 | A/V, WM, PS, VL |
| A: right aTL, C: left SO | 15 | 59 ± 15.0 | 53 | 38da ± 27.0 | 2 | 15 | |||
| Sham | 15 | 69 ± 14.6 | 53 | 40da ± 29.6 | 15 | ||||
| Park, Koh, Choi, and Ko (2013) | Double | A: bilateral PFC, C: non-dominant arm | 6 | 65 ± 14.3 | 67 | 29da ± 18.7 | 2 | 15 | A/V, WM, PS |
| Sham | 5 | 66 ± 10.8 | 40 | 25da ± 17.5 | 15 | ||||
| Traumatic brain injury | |||||||||
| Kang, Kim, and Paik (2012) | Double | A: left DLPFC, C: right SO | 9 | 50 ± 7.2 | 11 | 18mo ± 4.3 | 2 | 1 | A/V |
| Cross-over | Sham | 1 | |||||||
| Leśniak, Polanowska, Seniów, and Członkowska (2014) | Double | A: left DLPFC, C: right SO; CT | 14 | 28 ± 9 | 33 | 11mo ± 5.8 | 1 | 15 | A/V, WM, VL |
| Sham; CT | 12 | 29 ± 7.7 | 18 | 13mo ± 6.4 | 15 | ||||
| Multiple sclerosis | |||||||||
| Hanken et al. (2016) | Double | A: right PC, C: left forehead | 20 | 51 ± 9.4 | 65 | 1.5 | 1 | A/V | |
| Sham | 20 | 49 ± 9.7 | 40 | 1 | |||||
| Mattioli et al. (2016) | Double | A: left DLPFC, C: right shoulder | 10 | 38 ± 10.0 | 70 | 7y ± 6.1 | 2 | 10 | A/V, EF, PS, VL |
| Sham | 10 | 47 ± 10.4 | 90 | 11y ± 6.5 | 10 | ||||
| Chalah et al. (2017) | Double | A: left DLPFC, C: right SO | 10 | 41 ± 11.2 | 40 | 14y ± 9.9 | 2 | 5 | EF |
| Cross-over | A: right pPC, C: Cz | 5 | |||||||
| Sham | 5 | ||||||||
| Fiene et al. (2018) | Single | A: left DLPFC C: right shoulder | 15 | 43 ± 15.0 | 53 | 10y ± 8.6 | 1.5 | 1 | A/V |
| Cross-over | Sham | 1 | |||||||
mA, miliÀmpère; A, anodal; C, cathodal; CT, cognitive therapy; CCT, cognitive control therapy; AC, auditory cortex; CBC; cerebellar cortex; Cz, central midline; DLPFC, dorsolateral prefrontal cortex; IFG, inferior frontal gyrus; lat. O, lateral aspect of the orbit; OcC, occipital cortex; PC, parietal cortex; PMC, premotor cortex; SO, supraorbital area; TC, temporal cortex; TL, temporal lobe; TP, temporo-parietal; TPJ, temporo-parietal junction; VLPFC, ventrolateral prefrontal cortex; a, anterior; m, medial; p, posterior; min, minutes; AAO, age at onset; y, years; mo, months; da, days; A/V, attention/vigilance; EF, executive functioning; PS, processing speed; SC, social cognition; VF, verbal fluency; VL, verbal learning; WM, working memory.
Year of publication (Year), study design (Design), stimulation type and/or site, number of participants per group (n), age (mean ± standard deviation), proportion of females (%F), duration of illness (mean ± standard deviation if available), stimulation intensity (mA), number (nr.) of sessions and cognitive domains(s) are specified for each study.
Within-group cross-over.
Table 3.
Mean group characteristics for the included TMS and tDCS studies
| TMS | tDCS | |
|---|---|---|
| Number of studies included, N | 43 | 39 |
| Number of study-samples included, k | 49 | 44 |
| Participants, total n | 1591 | 1193 |
| Study-sample size, mean (s.d.) | 18 (±15.0) | 19 (±15.2) |
| Age in years, mean (s.d.) | 49.0 (±9.88) | 45.7 ± 10.91 |
| Gender, proportion of females (s.d.) | 37 (±18.2) | 48 (±21.5) |
Fig. 2.
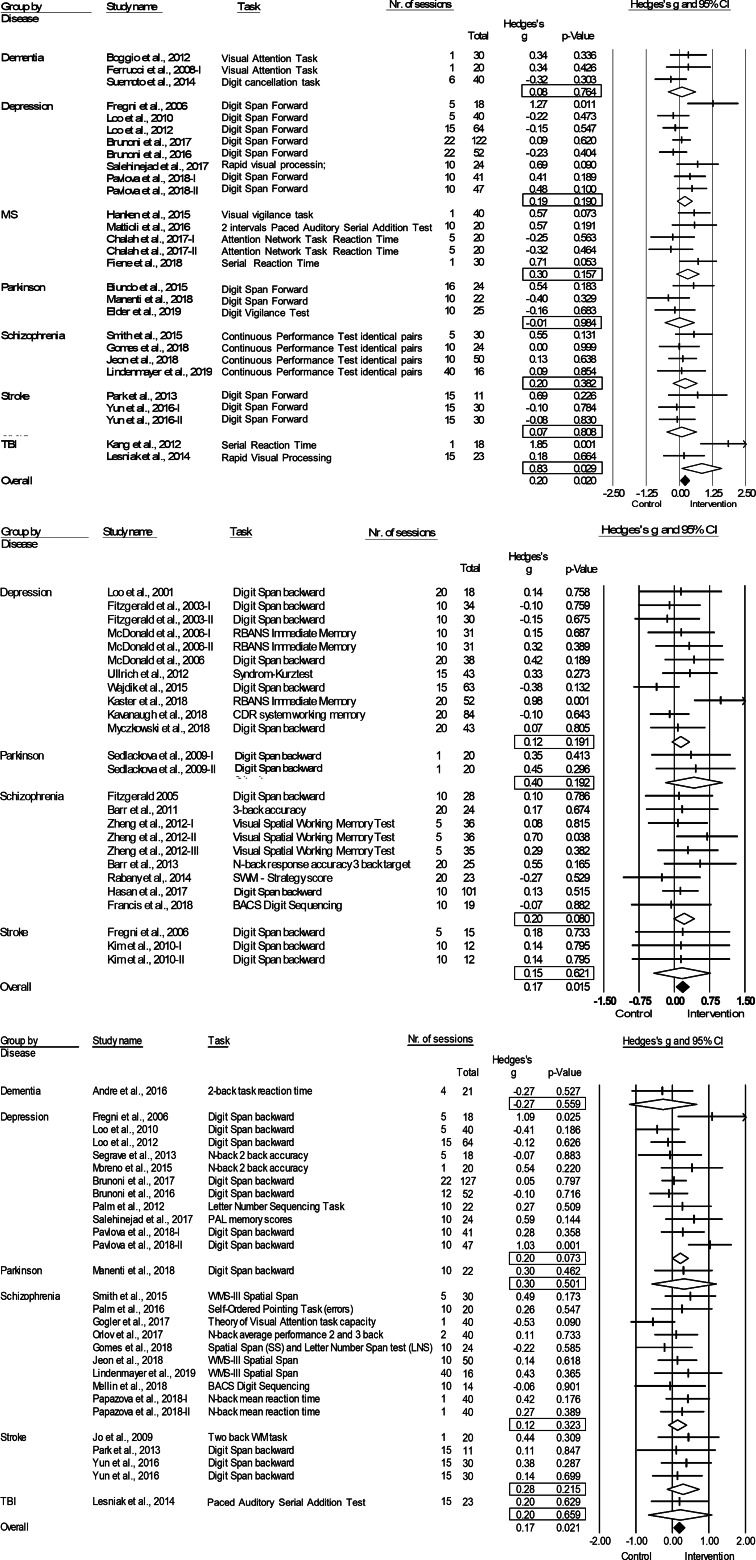
Forest plots of the effect of tDCS and TMS on working memory and tDCS on attention/vigilance. Results are summarized for all studies, sorted by brain disorder. (a) Forest plot of the effect of tDCS on attention/vigilance, outlier excluded. (b) Forest plot of the effect of TMS on working memory. (c) Forest plot of the effect of tDCS on working memory. BACS, Brief Assessment of Cognition in Schizophrenia; CDR, Cognitive Drug Research Computerized Assessment System; PAL, Paired Associate Learning; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SWM, Spatial Working Memory; WMS, Wechsler Memory Scale; WM, Working Memory.
Attention/vigilance
TMS: Combining 21 study samples (n = 680), TMS did not differ from sham in the effect on attention/vigilance, studies were homogeneous (ES = 0.10, p = 0.210; Table 4). ES did not differ between the four different brain disorders included (online Supplementary Table S1).
Table 4.
Effects of Transcranial Magnetic Stimulation (TMS) and transcranial Direct Current Stimulation (tDCS) across brain disorders for the seven cognitive domains
| Cognitive domain | Type | k | n | Hedges' g | 95% CI | p value | Q-statistic (df) | I2 (%) | NR |
|---|---|---|---|---|---|---|---|---|---|
| Attention/Vigilance | TMS | 21 | 679 | 0.10 | (−0.078 to 0.263) | 0.210 | Q(20) = 19.15, p = 0.512 | 0 | |
| tDCS | 29 | 980 | 0.27 | (0.074 to 0.457) | 0.006 | Q(28) = 58.43, p = 0.001 | 52.08 | 93 | |
| tDCS without outlier | 28 | 931 | 0.20 | (0.032 to 0.367) | 0.020 | Q(27) = 39.64; p = 0.055 | 31.90 | 45 | |
| Working Memory | TMS | 25 | 873 | 0.17 | (0.030 to 0.299) | 0.015 | Q(24) = 22.47, p = 0.551 | 4.10 | 18 |
| tDCS | 28 | 939 | 0.17 | (0.026 to 0.319) | 0.021 | Q(27) = 29.90, p = 0.319 | 9.70 | 28 | |
| Executive Functioning | TMS | 40 | 1145 | 0.07 | (−0.046 to 0.184) | 0.243 | Q(39) = 38.36, p = 0.499 | 0 | |
| tDCS | 19 | 662 | 0.04 | (−0.201 to 0.288) | 0.726 | Q(18) = 36.52, p = 0.006 | 50.72 | ||
| tDCS without outliers | 17 | 622 | 0.00 | (−0.154 to 0.156) | 0.992 | Q(16) = 11.51, p = 0.777 | 0 | ||
| Processing Speed | TMS | 30 | 941 | −0.01 | (−0.134 to 0.118) | 0.900 | Q(29) = 23.71, p = 0.743 | 0 | |
| tDCS | 24 | 754 | 0.24 | (−0.030 to 0.565) | 0.099 | Q(23) = 76.87, p < 0.05 | 70.55 | ||
| tDCS without outliers | 21 | 732 | −0.02 | (−0.163 to 0.123) | 0.784 | Q(20) = 20.71, p = 0.414 | 3.45 | ||
| Verbal Fluency | TMS | 21 | 747 | −0.05 | (−0.187 to 0.097) | 0.538 | Q(20) = 14.12, p = 0.825 | 0 | |
| tDCS | 9 | 399 | 0.14 | (−0.170 to 0.351) | 0.193 | Q(8) = 7.26, p = 0.509 | 0 | ||
| Verbal Learning | TMS | 21 | 690 | 0.08 | (−0.147 to 0.241) | 0.635 | Q(20) = 32.21, p = 0.041 | 37.91 | |
| TMS without outliers | 20 | 651 | −0.01 | (−0.165 to 0.139) | 0.866 | Q(19) = 17.82, p = 0.534 | 0 | ||
| tDCS | 18 | 551 | 0.05 | (−0.137 to 0.233) | 0.609 | Q(17) = 16.44, p = 0.493 | 0 | ||
| Social Cognition | tDCS | 7 | 171 | 0.27 | (−0.023 to 0.566) | 0.070 | Q(6) = 1.72, p = 0.190 | 0 | 2 |
k, number of study samples; n, number of studies; df, degrees of freedom; I2, heterogeneity; NR, Rosenthal's Fail-safe number.
Note: Bold values indicate significant test-statistic (p < 0.05).
tDCS: tDCS (k = 29; n = 980) was superior to sham in improving performance on attention/vigilance tasks (ES = 0.27, p = 0.006; Table 4; Fig. 2a). The number of null-studies needed to render this effect non-significant (NR) was 93, with no evidence for publication bias (online Supplementary Fig. S11). However, heterogeneity was moderate and the I2 of 52.08 indicated that 48.92% of the dispersion reflects the difference in the true ES while the remaining 52.08% can be attributed to random sampling error (Table 4). One outlier study was identified (Z-score>1.96; Orlov et al., 2017). This pilot study combined two sessions on day 1 and day 14, with four sessions of cognitive therapy in-between. After exclusion, ES remained significant and heterogeneity decreased (ES = 0.20, p = 0.020; Table 4; Fig. 2b). However, the funnel plot indicated potential publication bias (p = 0.047, online Supplementary Fig. S11). The effect of tDCS did not differ across disorders (online Supplementary Table S1). Regarding the sensitivity analyses, four studies compared multiple interventions to the same control group, yet the mean weighted ES did not change significantly after splitting these shared placebo groups to prevent inflated ES (ES = 0.20, p = 0.022). However, when excluding challenge/single-dose studies, the effect of tDCS on attention/vigilance was no longer significant (ES = 0.12, p = 0.140). Meta-regressions did not reveal significant moderation effects for the number of treatment sessions, age, or gender (online Supplementary Table S2).
Working memory
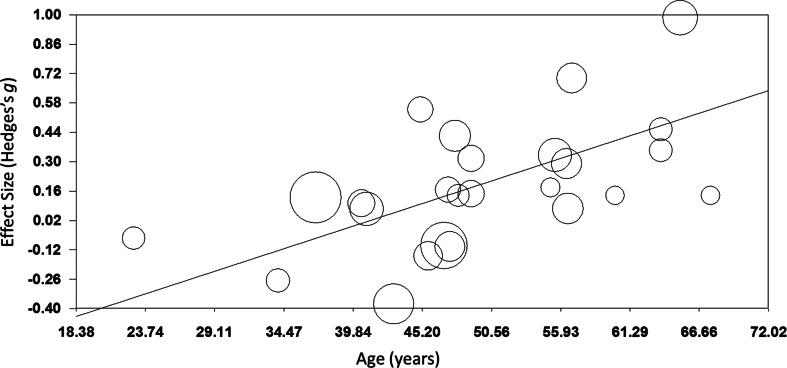
TMS: TMS (k = 25; n = 873) showed a small yet significant effect, with low heterogeneity (ES = 0.17, p = 0.015; Table 4; Fig. 2b). ES did not differ between brain disorders (online Supplementary Table S1) and publication bias was low (online Supplementary Fig. S12). This effect of TMS on working memory did not change after correcting control group sample sizes of the five studies that compared multiple interventions (ES = 0.16, p = 0.032) or after excluding one single-dose study (ES = 0.15, p = 0.049). Notably, age was a positive moderator, as study samples with a higher mean age showed more improvement in working memory after TMS in a linear fashion as depicted in Fig. 3 (slope coefficient = 0.020, p = 0.005). The number of treatment sessions and gender did not moderate ES (online Supplementary Table S2).
Fig. 3.
Meta-regression of the effect of TMS on working memory. Study-samples depicted by circles proportional to their sample size. The x-axis represents the mean age of the study-samples in years, y-axis depicts the effect size (Hedges' g).
tDCS: A small significant effect on working memory was detected for tDCS, studies were homogeneous (k = 28; n = 939; ES = 0.17, p = 0.021; Table 4; Fig. 2c) and there was no evidence for publication bias (online Supplementary Fig. S13). This effect did not differ across different brain disorders (online Supplementary Table S1). ES remained significant when adjusting control group sample sizes for the three studies with multiple intervention groups (ES = 0.15, p = 0.038) and when excluding five single-dose studies (ES = 0.17, p = 0.038). No significant moderators were identified (online Supplementary Table S2).
Executive functioning
TMS: Overall, TMS (k = 40; n = 1145) was not superior to sham in improving executive functioning, with low heterogeneity (ES = 0.07, p = 0.243; Table 4), ES did not differ between disorders (online Supplementary Table S1).
tDCS: tDCS (k = 19, n = 662) did not differ from sham, with moderate heterogeneity (ES = 0.04, p = 0.726; Table 4). ES did not differ across disorders (online Supplementary Table S1). Mattioli, Bellomi, Stampatori, Capra, and Miniussi (2016) and the DLPFC-condition of Chalah, Créange, Lefaucheur, and Ayache (2017) were both identified as outliers. Mattioli et al. (2016) was a positive outlier (Z-score >1.96) and the only study using total errors of the Wisconsin's Card Sorting Test as an outcome measure. In line with the positive effect that was found for right PPC stimulation in the same study (Table 1), Chalah et al. (2017) relate their strong negative effect (Z-score <1.96) to the phenomenon of cerebral lateralization in MS. After exclusion, heterogeneity decreased and ES remained non-significant (ES = 0.00, p = 0.992; Table 4).
Processing speed
TMS: No superior effect was found for TMS (k = 30; n = 941) v. sham, with low heterogeneity (ES = −0.01, p = 0.900; Table 4), without any differences across the included disorders (online Supplementary Table S1).
tDCS: No difference was found in the effect of tDCS (k = 24; n = 794) v. sham (ES = 0.24, p = 0.099, Table 4), ES did not vary between disorders (online Supplementary Table S1). Heterogeneity was moderate to high (Table 4), three outlier studies were identified. Segrave et al. (2014) reported remarkable strong improvements after sham compared to tDCS (N-back RT, Z-score <−1.96). Diverging from other included studies, both Moreno et al. (2015) (Z-score >1.96) and Biundo et al. (2015) (Z-score >1.96) used the RBANS written coding task. After exclusion, heterogeneity decreased and ES remained non-significant (ES = −0.02, p = 0.784; Table 4). Notably, the effect of tDCS then varied between different brain disorders [Q(4) = 12.62; p = 0.013; online Supplementary Table S1] yet subgroup analyses showed that tDCS was not superior for depression (k = 8; n = 389; ES = −0.00, 95% CI −0.20 to 0.20, p = 0.997), schizophrenia (k = 7; n = 194; ES = −0.02, 95% CI −0.29 to 0.26, p = 0.894), and stroke (k = 3; n = 71; ES = 0.10, 95% CI −0.35 to 0.55, p = 0.654). The number of studies on PD (k = 2) and MS (k = 1) was insufficient to perform subgroup analyses.
Verbal fluency
TMS: No superior effect was found for TMS (k = 21; n = 747) on verbal fluency, with low heterogeneity (ES = −0.05, p = 0.538; Table 4) and no differences across disorders (online Supplementary Table S1).
tDCS: No significant difference was found between tDCS (k = 9; n = 399) v. sham, heterogeneity was low (ES = 0.14, p = 0.193; Table 4), ES did not vary across disorders (online Supplementary Table S1).
Verbal learning
TMS: Overall, TMS (k = 21; n = 690) showed no positive effect v. sham but heterogeneity was high (ES = 0.08, p = 0.635; Table 4). Significant differences in effect between disorders were detected (p = 0.029; online Supplementary Table S1), subgroup analyses showed an effect for stroke [k = 3; n = 64; ES = 0.677, Q(2) = 7.01, p = 0.005]. After excluding one outlier study (Z-score >1.96) (Sun, Lu, Zhang, Wen, & Sun, 2015), heterogeneity decreased and overall ES remained non-significant (Table 4). Notably, the difference in the effect of TMS on verbal learning between disorders was then no longer observed (online Supplementary Table S1).
tDCS: tDCS did not differ from sham (k = 18; n = 550), included studies were homogeneous (ES = 0.05, p = 0.609; Table 4) and ES did not differ across disorders (online Supplementary Table S1).
Social cognition
TMS: As we retrieved only one study investigating the effect of TMS on social cognition (Wölwer et al., 2014), no meta-analysis could be conducted.
tDCS: tDCS (k = 7; n = 171) showed a trend-significant effect on social cognition, with low heterogeneity (ES = 0.27, p = 0.070; Table 4). The funnel plot and Egger's regression test indicated potential publication bias (p = 0.09; online Supplementary Fig. S13), yet the power of this test is limited due to the small number of studies included (Egger et al., 1997). The difference in ES between disorders was trend-significant (online Supplementary Table S1). The studies on schizophrenia showed a non-significant effect (k = 5; n = 132; ES = 0.17, p = 0.326), although the two studies on depression were positive they could not be combined in meta-analysis.
Discussion
We investigated the procognitive effects of TMS and tDCS in 83 randomized, double- or single-blind sham-controlled studies in schizophrenia, depression, dementia, MS, PD, stroke, and TBI. Using a domain-specific approach, we found a positive effect of both TMS (ES = 0.17) and tDCS (ES = 0.17) as compared to sham on working memory. The finding that both NIBS techniques elicit a similar effect in the same domain may suggest that the circuitry underlying working memory is readily accessible for activation using NIBS. In addition, this is the first meta-analysis reporting improvements in attention/vigilance in tDCS as compared to placebo stimulation (ES = 0.19), while the ES of 0.10 for TMS was not significant. This suggests that tDCS may be superior to TMS in improving attention/vigilance, although firm conclusions can only be drawn by making direct comparisons within one study randomizing participants across conditions. Other cognitive domains did not benefit from either TMS or tDCS as compared to sham.
Our findings largely corroborate with two quantitative reviews that have specifically evaluated the effects of NIBS on working memory, both including a lower number of studies (Brunoni & Vanderhasselt, 2014; Hill, Fitzgerald, & Hoy, 2016; Martin, McClintock, Forster, & Loo, 2016). Hill et al. (2016) (16 AtDCS studies; 182 neuropsychiatric patients and 170 controls) showed that AtDCS significantly improved online accuracy in patients (ES = 0.77) and offline reaction time in healthy individuals (ES = 0.16) as compared to sham, although no overall effect was found for accuracy or reaction time. Brunoni and Vanderhasselt (2014) (12 TMS and tDCS RCTs, 805 psychiatric patients and healthy controls) identified medium effects of NIBS, on accuracy (ES correct responses = 0.25; ES error rates = 0.29) and reaction time (ES = −0.22), yet meta-regressions revealed that accuracy only improved in participants receiving TMS and not tDCS. Both of the abovementioned reviews did not evaluate differences across disorders. Moreover, Hill et al. (2016) only selected studies that applied the N-back, Sternberg, or digit-span task, while Brunoni and Vanderhasselt (2014) included NIBS studies specifically targeting the DLPFC and implementing the N-back task. A third review by Martin et al. (2016) evaluating multiple cognitive domains reported a significant ES of 0.51 (95% CI 0.18–0.83) after pooling three rTMS studies for working memory in secondary analyses.
Interestingly, we found a significant moderator effect of age for TMS on working memory, indicating that the potential procognitive effect of TMS is larger at a higher age. A possible explanation for this finding could be that the skull thickness decreases over age, making it easier to stimulate the cortex underneath due to a shorter distance of the cortex from the coil. However, this finding is not supported by conventional theories emphasizing that the distance between the cortex and the coil increases with age due to brain atrophy, negatively affecting TMS efficacy (McConnell et al., 2001). Also, there might be more potential for cognitive improvement in an older population as younger patients may show ceiling effects in cognitive functioning (Lillie, Urban, Lynch, Weaver, & Stitzel, 2016; Schulte-Geers et al., 2011). Although we found no differences in effect between disorders, the effect of age might be an indirect reflection of subtle differences in treatment effect between disorders. Where dementia and PD usually strike at a high age, schizophrenia and MS overall affect a relatively young population. Furthermore, the diseases with late age of onset generally have more detrimental effects on cognition. More research is needed to gain more insight into the potential associations between TMS efficacy and age and potential ceiling effects to explain this unexpected effect.
One of the possible mechanisms behind the positive effects of NIBS on working memory and of tDCS on attention/vigilance may include an increase in dopamine release. Recently, Fonteneau et al. (2018) have demonstrated that a single session of bifrontal tDCS induced dopamine release in the ventral striatum in healthy individuals (n = 32). Striatal dopamine function not only links to neural efficiency of the (dorsolateral) striatum but also of the prefrontal cortex and associated higher-order cognitive functioning, including attention switching and working memory updating (Cools, 2011; Landau, Lal, O'Neil, Baker, & Jagust, 2009; Stelzel, Basten, Montag, Reuter, & Fiebach, 2010). For the five other cognitive domains investigated in the present meta-analysis, we did not find any procognitive effects of either TMS or tDCS. It is possible that these domains rely more on subcortical, cerebellar, medial, and fusiform areas, being not directly accessible using NIBS techniques (Demirtas-Tatlidede, Vahabzadeh-Hagh, & Pascual-Leone, 2013; Kim, Hong, Kim, & Yoon, 2019). It is also possible that a longer duration of stimulation or a higher stimulation frequency or intensity is required to influence these domains. Alternatively, a combination of cognitive training and NIBS may be needed to gain improvement. Although not included in the present meta-analysis, a positive effect of NIBS on global cognitive function and verbal fluency was recently demonstrated in a quantitative review on patients with mild cognitive impairment (Xu et al., 2019) in addition to a small positive effect on executive function (11 RCTs, 367 patients).
Overall, we found no evidence for the effect of either TMS or tDCS to differ across brain disorders. Although TMS showed a positive effect on verbal learning for stroke only (k = 3) and tDCS on processing speed for MS (k = 1) and PD (k = 2) only, no definite conclusions could be drawn regarding the nature of these differences due to the limited number of studies that could be included for the relevant brain disorders. The existence of common shared pathophysiologic substrates regarding decreased plasticity across brain disorders has been proposed to underlie cognitive decline in different brain disorders, which may suggest that patients with different brain disorders could benefit from the same procognitive interventions (Demirtas-Tatlidede et al., 2013; Kim et al., 2019), which has indeed been shown to be the case for exercise (Dauwan et al., 2019; Herold, Törpel, Schega, & Müller, 2019).
Strengths and limitations
An important strength of this study is the inclusion of seven different cognitive domains that were analyzed separately, uncovering the differences in effects across different domains. In our opinion, this approach is relevant, as cognition is subserved by different cerebral and cerebellar circuits. Different circuits in the brain are responsible for the proper functioning of different domains, which follows that the effect of NIBS may be inconsistent across domains. This inconsistency has been reported in prior studies (Lindenmayer et al., 2019; Loo et al., 2010; Manenti et al., 2018). Splitting up cognition into relevant separate domains elucidates effects that might be hidden when looking at global cognition.
Also, this meta-analysis provides an answer to the need for a comparison between the two most commonly used types of NIBS. The relevance of such a comparison is supported by the existing debate about which of the two is the most effective and suitable for clinical use (Brunoni & Vanderhasselt, 2014; Inukai et al., 2016). Our meta-analysis reveals that both seem to have a profound effect on a specific and corresponding cognitive domain, namely working memory. Although tDCS also impacted attention/vigilance, the effects of both types are nonetheless absent in the other domains. While tDCS and TMS are different types of stimulation with different working mechanisms, our findings indicate that they might trigger comparable effects on cognition.
Our literature search revealed that many studies on the effect of NIBS did not primarily study its effect on cognition, but included cognitive tests as a secondary outcome of interest. Instead, prior studies have primarily focused on utilizing neuropsychological tests as a means to observe the deterioration of cognitive abilities. As a consequence, the included tests may lack sensitivity to detect cognitive improvement, and only a few studies could be included for certain analyses which affected the power of those analyses (e.g. tDCS studies for stroke and PD; tDCS and TMS studies for TBI).
Although we aimed to avoid major differences in methodology as far as possible by careful inspection of the included studies, heterogeneity is inevitably present. The precise nature of this heterogeneity can be due to numerous factors varying across participants, data sets, studies, and brain disorders. Technical factors include the type of coil, sham technique, coil positioning, and stimulation protocol (Tables 1 and 2) (Imburgio & Orr, 2018; Lage et al., 2016; Woods et al., 2016). For example, as reflected in our retrieved studies (Tables 1 and 2) the DLPFC is one of the key anatomical regions that is most frequently targeted to improve cognition. However, we also found other (adjacent) regions to be stimulated across the included studies (e.g. Eliasova, Anderkova, Marecek, & Rektorova, 2014; Guse et al., 2013). Provided that the included studies had chosen an appropriate stimulation site based on the available literature, all stimulation sites were included in our meta-analysis.
Notably, a broad range of the cognitive tests were administered throughout the included studies. To avoid that the definition of cognitive domains would be arbitrary, we based our seven domains on the guidelines of the two most commonly used cognitive test batteries (Green et al., 2004; Litvan et al., 2012). Despite this, practice effects, ceiling and/or floor effects, and test battery sensitivity may still have contributed to heterogeneity within cognitive domains. In theory, practice effects should not play a role in randomized controlled designs, being present in both active and sham treatment groups, but with small sample sizes such effects may not cancel out. We do note that positive effects as quantified by cognitive tests may not always transfer to cognitive functioning in daily life. On the other hand, meaningful changes in cognitive functioning may not always be fully quantifiable with the cognitive tasks used. Also, post-treatment cognitive testing took place right after brain stimulation and therefore the potential long-term effect of NIBS on cognition remains understudied (Cirillo et al., 2017; Gersner, Kravetz, Feil, Pell, & Zangen, 2011).
Conclusions
Overall, we found a small yet significant effect of both TMS and tDCS on working memory in patients with brain disorders as compared to sham treatment, tDCS also showed a superior effect on attention/vigilance. Results were not significant for the remaining cognitive domains. Findings were similar across the different brain disorders for both techniques, indicating that TMS and tDCS can only affect specific neural circuits if applied on frontal and temporal regions and hence can be applied to improve specific cognitive domains (i.e. working memory and attention/vigilance).
Acknowledgements
The authors thank Edith van Liemburg, Fleur Verhaag, and Lieke Jorna for their assistance in retrieving relevant articles and those authors that replied to our e-mail requests for additional data or further clarification of their results. This work was supported by the Dutch Brain Foundation (Hersenstichting, number PZ 2017.01.2, 2017), received by Professor Dr Sommer and Dr Begemann.
Financial support
This work was supported by the Dutch Brain Foundation (Hersenstichting, number PZ 2017.01.2, 2017), received by Professor Dr Sommer and Dr Begemann.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720003670.
click here to view supplementary material
Conflict of interest
The authors report no potential conflicts of interest.
References
- Aleman, A., Enriquez-Geppert, S., Knegtering, H., & Dlabac-de Lange, J. J. (2018). Moderate effects of noninvasive brain stimulation of the frontal cortex for improving negative symptoms in schizophrenia: Meta-analysis of controlled trials. Neuroscience and Biobehavioral Reviews, 89, 111–118. 10.1016/j.neubiorev.2018.02.009. [DOI] [PubMed] [Google Scholar]
- Aleman, A., Sommer, I. E., & Kahn, R. S. (2007). Efficacy of slow repetitive transcranial magnetic stimulation in the treatment of resistant auditory hallucinations in schizophrenia: A meta-analysis. Journal of Clinical Psychiatry, 68(3), 416–421. 10.4088/JCP.v68n0310. [DOI] [PubMed] [Google Scholar]
- André, S., Heinrich, S., Kayser, F., Menzler, K., Kesselring, J., Khader, P. H., … Mylius, V. (2016). At-home tDCS of the left dorsolateral prefrontal cortex improves visual short-term memory in mild vascular dementia. Journal of the Neurological Sciences, 369, 185–190. 10.1016/j.jns.2016.07.065. [DOI] [PubMed] [Google Scholar]
- Audet, T., Hebert, R., Dubois, M.-F., Rochette, A., & Mercier, L. (2007). Impact of motor, cognitive, and perceptual disorders on ability to perform activities of daily living after stroke. Stroke, 32(11), 2602–2608. 10.1161/hs1101.098154. [DOI] [PubMed] [Google Scholar]
- Barr, M. S., Farzan, F., Arenovich, T., Chen, R., Fitzgerald, P. B., & Daskalakis, Z. J. (2011). The effect of repetitive transcranial magnetic stimulation on gamma oscillatory activity in schizophrenia. PLoS ONE, 6(7), e22627 10.1371/journal.pone.0022627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr, M. S., Farzan, F., Rajji, T. K., Voineskos, A. N., Blumberger, D. M., Arenovich, T, … Daskalakis, Z. J. (2013). Can repetitive magnetic stimulation improve cognition in Schizophrenia? Pilot data from a randomized controlled trial. Biological Psychiatry, 73(6), 510–517. [DOI] [PubMed] [Google Scholar]
- Bell, M. D., & Bryson, G. (2001). Work rehabilitation in schizophrenia: Does cognitive impairment limit improvement? Schizophrenia Bulletin, 27(2), 269–279. 10.1093/oxfordjournals.schbul.a006873. [DOI] [PubMed] [Google Scholar]
- Benedict, R. H. B., & Zivadinov, R. (2006). Predicting neuropsychological abnormalities in multiple sclerosis. Journal of the Neurological Sciences, 245(1-2), 67–72. 10.1016/j.jns.2005.05.020. [DOI] [PubMed] [Google Scholar]
- Bennabi, D., Nicolier, M., Monnin, J., Tio, G., Pazart, L., Vandel, P., & Haffen, E. (2015). Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clinical Neurophysiology, 126(6), 1185–1189. 10.1016/j.clinph.2014.09.026. [DOI] [PubMed] [Google Scholar]
- Benninger, D. H., Berman, B. D., Houdayer, E., Pal, N., Luckenbaugh, D. A., Schneider, L., … Hallett, M. (2011). Intermittent theta-burst transcranial magnetic stimulation for treatment of Parkinson disease. Neurology, 76(7), 601–609. 10.1212/WNL.0b013e31820ce6bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger, D. H., Iseki, K., Kranick, S., Luckenbaugh, D. A., Houdayer, E., & Hallett, M. (2012). Controlled study of 50-Hz repetitive transcranial magnetic stimulation for the treatment of Parkinson disease. Neurorehabilitation and Neural Repair, 26(9), 1096–1105. 10.1177/1545968312445636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani, F. S., Minichino, A., Bernabei, L., Spagnoli, F., Corrado, A., Vergnani, L., … Delle Chiaie, R. (2017). Prefronto-cerebellar tDCS enhances neurocognition in euthymic bipolar patients. Findings from a placebo-controlled neuropsychological and psychophysiological investigation. Journal of Affective Disorders, 209, 262–269. 10.1016/j.jad.2016.11.037. [DOI] [PubMed] [Google Scholar]
- Biundo, R., Weis, L., Fiorenzato, E., Gentile, G., Giglio, M., Schifano, R., … Antonini, A. (2015). Double-blind randomized trial of t-DCS versus sham in Parkinson patients with mild cognitive impairment receiving cognitive training. Brain Stimulation, 8(6), 1223–1225. 10.1016/j.brs.2015.07.043. [DOI] [PubMed] [Google Scholar]
- Boggio, P. S., Bermpohl, F., Vergara, A. O., Muniz, A. L. C. R., Nahas, F. H., Leme, P. B., … Fregni, F. (2007). Go-no-go task performance improvement after anodal transcranial DC stimulation of the left dorsolateral prefrontal cortex in major depression. Journal of Affective Disorders, 101(1-3), 91–98. 10.1016/j.jad.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Boggio, P. S., Ferrucci, R., Mameli, F., Martins, D., Martins, O., Vergari, M., … Priori, A. (2012). Prolonged visual memory enhancement after direct current stimulation in Alzheimer's disease. Brain Stimulation, 5(3), 223–230. 10.1016/j.brs.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Borenstein, M., Hedges, L. V., Higgins, J. P. T., & Rothstein, H. R. (2009). Introduction to meta-analysis chapter 10–13. Chichester, UK: John Wiley & Sons, Ltd; 10.1002/9780470743386.ch13. [DOI] [Google Scholar]
- Brown, R. G., & Marsden, C. D. (1990). Cognitive function in Parkinson's disease: From description to theory. Trends in Neurosciences, 13(1), 21–29. 10.1016/0166-2236(90)90058-I. [DOI] [PubMed] [Google Scholar]
- Brunoni, A. R., Moffa, A. H., Sampaio, B., Borrione, L., Moreno, M. L., Fernandes, R. A., … Benseñor, I. M. (2017). Trial of electrical direct-current therapy versus escitalopram for depression. New England Journal of Medicine, 376(26), 2523–2533. 10.1056/NEJMoa1612999. [DOI] [PubMed] [Google Scholar]
- Brunoni, A. R., Tortella, G., Benseñor, I. M., Lotufo, P. A., Carvalho, A. F., & Fregni, F. (2016). Cognitive effects of transcranial direct current stimulation in depression: Results from the SELECT-TDCS trial and insights for further clinical trials. Journal of Affective Disorders, 202, 46–52. 10.1016/j.jad.2016.03.066. [DOI] [PubMed] [Google Scholar]
- Brunoni, A. R., & Vanderhasselt, M. A. (2014). Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: A systematic review and meta-analysis. Brain and Cognition, 86, 1–9. 10.1016/j.bandc.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Buard, I., Sciacca, D. M., Martin, C. S., Rogers, S., Sillau, S. H., Greher, M. R., … Kluger, B. M. (2018). Transcranial magnetic stimulation does not improve mild cognitive impairment in Parkinson's disease. Movement Disorders, 33(3), 489–491. 10.1002/mds.27246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caviness, J. N., Driver-Dunckley, E., Connor, D. J., Sabbagh, M. N., Hentz, J. G., Noble, B., … Adler, C. H. (2007). Defining mild cognitive impairment in Parkinson's disease. Movement Disorders, 22(9), 1272–1277. 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- Chalah, M. A., Créange, A., Lefaucheur, J.-P., & Ayache, S. S. (2017). P071 tDCS effects over the left DLPFC versus the right PPC in multiple sclerosis fatigue. Clinical Neurophysiology, 128(3), e39–e40. 10.1016/j.clinph.2016.10.196. [DOI] [Google Scholar]
- Chase, H. W., Boudewyn, M. A., Carter, C. S., & Phillips, M. L. (2020). Transcranial direct current stimulation: A roadmap for research, from mechanism of action to clinical implementation. Molecular Psychiatry, 25(2), 397–407. 10.1038/s41380-019-0499-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, C. M., Juan, C. H., Chen, M. H., Chang, C. F., Lu, H. J., Su, T. P., … Li, C. T. (2016). Different forms of prefrontal theta burst stimulation for executive function of medication-resistant depression: Evidence from a randomized sham-controlled study. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 66, 35–40. 10.1016/j.pnpbp.2015.11.009. [DOI] [PubMed] [Google Scholar]
- Chervyakov, A. V., Chernyavsky, A. Y., Sinitsyn, D. O., & Piradov, M. A. (2015). Possible mechanisms underlying the therapeutic effects of transcranial magnetic stimulation. Frontiers in Human Neuroscience, 9, 1–14. 10.3389/fnhum.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaravalloti, N. D., & DeLuca, J. (2008). Cognitive impairment in multiple sclerosis. The Lancet Neurology, 7(12), 1139–1151. 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Cicerone, K. D., Dahlberg, C., Malec, J. F., Langenbahn, D. M., Felicetti, T., Kneipp, S., … Catanese, J. (2005). Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation, 86(8), 1681–1692. 10.1016/j.apmr.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cirillo, G., Di Pino, G., Capone, F., Ranieri, F., Florio, L., Todisco, V., … Di Lazzaro, V. (2017). Neurobiological after-effects of non-invasive brain stimulation. Brain Stimulation, 10(1), 1–18. 10.1016/j.brs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- Cohen, J. (1988). Statistical Power Analysis for the Behavioural Science (2nd Edition). USA: Statistical Power Anaylsis for the Behavioral Sciences. [Google Scholar]
- Cools, R. (2011). Dopaminergic control of the striatum for high-level cognition. Current Opinion in Neurobiology. 10.1016/j.conb.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Dagan, M., Herman, T., Mirelman, A., Giladi, N., & Hausdorff, J. M. (2017). The role of the prefrontal cortex in freezing of gait in Parkinson's disease: Insights from a deep repetitive transcranial magnetic stimulation exploratory study. Experimental Brain Research, 235(8), 2463–2472. 10.1007/s00221-017-4981-9. [DOI] [PubMed] [Google Scholar]
- Dauwan, M., Begemann, M. J. H., Slot, M. I. E., Lee, E. H. M., Scheltens, P., & Sommer, I. E. C. (2019). Physical exercise improves quality of life, depressive symptoms, and cognition across chronic brain disorders: A transdiagnostic systematic review and meta-analysis of randomized controlled trials. Journal of Neurology. 10.1007/s00415-019-09493-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirtas-Tatlidede, A., Vahabzadeh-Hagh, A. M., & Pascual-Leone, A. (2013). Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology, 64, 566–578. 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dlabac-De Lange, J. J., Bais, L., Van Es, F. D., Visser, B. G. J., Reinink, E., Bakker, B., … Knegtering, H. (2015). Efficacy of bilateral repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: Results of a multicenter double-blind randomized controlled trial. Psychological Medicine, 45(6), 1263–1275. 10.1017/S0033291714002360. [DOI] [PubMed] [Google Scholar]
- Douglas, K. M., Gallagher, P., Robinson, L. J., Carter, J. D., McIntosh, V. V. W., Frampton, C. M. A., … Porter, R. J. (2018). Prevalence of cognitive impairment in major depression and bipolar disorder. Bipolar Disorders, 20(3), 260–274. 10.1111/bdi.12602. [DOI] [PubMed] [Google Scholar]
- Egger, M., Smith, G. D., Schneider, M., & Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder, G. J., Colloby, S. J., Firbank, M. J., McKeith, I. G., & Taylor, J. P. (2019). Consecutive sessions of transcranial direct current stimulation do not remediate visual hallucinations in Lewy body dementia: A randomised controlled trial. Alzheimer's Research and Therapy, 11(1), 9 10.1186/s13195-018-0465-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasova, I., Anderkova, L., Marecek, R., & Rektorova, I. (2014). Non-invasive brain stimulation of the right inferior frontal gyrus may improve attention in early Alzheimer's disease: A pilot study. Journal of the Neurological Sciences, 346(1-2), 318–322. 10.1016/j.jns.2014.08.036. [DOI] [PubMed] [Google Scholar]
- Ferrucci, R., Mameli, F., Guidi, I., Mrakic-Sposta, S., Vergari, M., Marceglia, S., … Priori, A. (2008). Transcranial direct current stimulation improves recognition memory in Alzheimer disease. Neurology, 71(7), 493–498. 10.1212/01.wnl.0000317060.43722.a3. [DOI] [PubMed] [Google Scholar]
- Fiene, M., Rufener, K. S., Kuehne, M., Matzke, M., Heinze, H. J., & Zaehle, T. (2018). Electrophysiological and behavioral effects of frontal transcranial direct current stimulation on cognitive fatigue in multiple sclerosis. Journal of Neurology, 265(3), 607–617. 10.1007/s00415-018-8754-6. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. B., Benitez, J., Daskalakis, J. Z., Brown, T. L., Marston, N. A. U., De Castella, A., … Kulkarni, J. (2005). A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. Journal of Clinical Psychopharmacology, 25(4), 358–362. 10.1097/01.jcp.0000168487.22140.7f. [DOI] [PubMed] [Google Scholar]
- Fitzgerald, P. B., Brown, T. L., Marston, N. A. U., Daskalakis, Z. J., De Castella, A., & Kulkarni, J. (2003). Transcranial magnetic stimulation in the treatment of depression: A double-blind, placebo-controlled trial. Archives of General Psychiatry, 6(10), 1002 10.1001/archpsyc.60.9.1002. [DOI] [PubMed] [Google Scholar]
- Fonteneau, C., Redoute, J., Haesebaert, F., Le Bars, D., Costes, N., Suaud-Chagny, M. F., … Brunelin, J. (2018). Frontal transcranial direct current stimulation induces dopamine release in the ventral striatum in human. Cerebral Cortex, 28(7), 2636–2646. 10.1093/cercor/bhy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis, M. M., Hummer, T. A., Vohs, J. L., Yung, M. G., Visco, A. C., Mehdiyoun, N. F., … Breier, A. (2019). Cognitive effects of bilateral high frequency repetitive transcranial magnetic stimulation in early phase psychosis: A pilot study. Brain Imaging and Behavior, 13(3), 852–861. 10.1007/s11682-018-9902-4. [DOI] [PubMed] [Google Scholar]
- Fregni, F., Boggio, P. S., Nitsche, M. A., Rigonatti, S. P., & Pascual-Leone, A. (2006a). Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression [1]. Depression and Anxiety, 23(8), 482–484. 10.1002/da.20201. [DOI] [PubMed] [Google Scholar]
- Fregni, F., Boggio, P. S., Valle, A. C., Rocha, R. R., Duarte, J., Ferreira, M. J. L., … Pascual-Leone, A. (2006b). A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke, 37(8), 2115–2122. 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Gersner, R., Kravetz, E., Feil, J., Pell, G., & Zangen, A. (2011). Long-term effects of repetitive transcranial magnetic stimulation on markers for neuroplasticity: Differential outcomes in anesthetized and awake animals. Journal of Neuroscience, 31(20), 7521–7526. 10.1523/JNEUROSCI.6751-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gögler, N., Papazova, I., Oviedo-Salcedo, T., Filipova, N., Strube, W., Funk, J., … Hasan, A. (2017). Parameter-based evaluation of attentional impairments in schizophrenia and their modulation by prefrontal transcranial direct current stimulation. Frontiers in Psychiatry, 8, 259 10.3389/fpsyt.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes, J. S., Trevizol, A. P., Ducos, D. V., Gadelha, A., Ortiz, B. B., Fonseca, A. O., … Dias, A. M. (2018). Effects of transcranial direct current stimulation on working memory and negative symptoms in schizophrenia: A phase II randomized sham-controlled trial. Schizophrenia Research: Cognition, 31(20), 7521–7526. 10.1016/j.scog.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, M. F., Kern, R. S., Braff, D. L., & Mintz, J. (2000). Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the ‘right stuff’? Schizophrenia Bulletin, 26(1), 119–136. 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green, M. F., Nuechterlein, K. H., Gold, J. M., Barch, D. M., Cohen, J., Essock, S., … Marder, S. R. (2004). Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biological Psychiatry, 56(5), 301–307. 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Guse, B., Falkai, P., Gruber, O., Whalley, H., Gibson, L., Hasan, A., … Wobrock, T. (2013). The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls-A randomized placebo-controlled, double-blind fMRI study. Behavioural Brain Research, 237, 300–307. 10.1016/j.bbr.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Hanken, K., Bosse, M., Möhrke, K., Eling, P., Kastrup, A., Antal, A., … Hildebrandt, H. (2016). Counteracting fatigue in multiple sclerosis with right parietal anodal transcranial direct current stimulation. Frontiers in Neurology, 7, 154 10.3389/fneur.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan, A., Guse, B., Cordes, J., Wölwer, W., Winterer, G., Gaebel, W., … Wobrock, T. (2016). Cognitive effects of high-frequency rTMS in schizophrenia patients with predominant negative symptoms: Results from a multicenter randomized sham-controlled trial. Schizophrenia Bulletin, 42(5), 1253–1261. 10.1093/schbul/sbv142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann, A., Pascual-Leone, A., Kemmler, G., Rupp, C. I., Lechner-Schoner, T., Kramer-Reinstadler, K., … Weiss, E. M. (2004). No deterioration of cognitive performance in an aggressive unilateral and bilateral antidepressant rTMS add-on trial. Journal of Clinical Psychiatry, 65(6), 772–782. 10.4088/JCP.v65n0608. [DOI] [PubMed] [Google Scholar]
- Heinrichs, R. W., & Zakzanis, K. K. (1998). Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology, 12(3), 426–445. 10.1037/0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Herold, F., Törpel, A., Schega, L., & Müller, N. G. (2019). Functional and/or structural brain changes in response to resistance exercises and resistance training lead to cognitive improvements – A systematic review. European Review of Aging and Physical Activity, 16(1), 10 10.1186/s11556-019-0217-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P., Thompson, S. G., Deeks, J. J., & Altman, D. G. (2003). Measuring inconsistency in meta-analyses testing for heterogeneity. BMJ (Clinical Research Ed.), 327(7414), 557–560. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi, Y., Sumiyoshi, T., Seo, T., Suga, M., Takahashi, T., Nishiyama, S., … Suzuki, M. (2017). Associations between daily living skills, cognition, and real-world functioning across stages of schizophrenia; a study with the Schizophrenia Cognition Rating Scale Japanese version. Schizophrenia Research: Cognition, 7, 13–18. 10.1016/j.scog.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill, A. T., Fitzgerald, P. B., & Hoy, K. E. (2016). Effects of anodal transcranial direct current stimulation on working memory: A systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimulation, 9(2), 197–208. 10.1016/j.brs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Imburgio, M. J., & Orr, J. M. (2018). Effects of prefrontal tDCS on executive function: Methodological considerations revealed by meta-analysis. Neuropsychologia, 117, 156–166. 10.1016/j.neuropsychologia.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Inukai, Y., Saito, K., Sasaki, R., Tsuiki, S., Miyaguchi, S., Kojima, S., … Onishi, H. (2016). Comparison of three non-invasive transcranial electrical stimulation methods for increasing cortical excitability. Frontiers in Human Neuroscience, 10, 668 10.3389/fnhum.2016.00668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon, D. W., Jung, D. U., Kim, S. J., Shim, J. C., Moon, J. J., Seo, Y. S., … Kim, Y. N. (2018). Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: A double-blind 12-week study. Schizophrenia Research, 197, 378–385. 10.1016/j.schres.2017.12.009. [DOI] [PubMed] [Google Scholar]
- Jo, J. M., Kim, Y. H., Ko, M. H., Ohn, S. H., Joen, B., & Lee, K. H. (2009). Enhancing the working memory of stroke patients using tDCS. American Journal of Physical Medicine and Rehabilitation, 88(5), 404–409. 10.1097/PHM.0b013e3181a0e4cb. [DOI] [PubMed] [Google Scholar]
- Kang, E. K., Kim, D. Y., & Paik, N. J. (2012). Transcranial direct current stimulation of the left prefrontal cortex improves attention in patients with traumatic brain injury: A pilot study. Journal of Rehabilitation Medicine, 95(4), 332–343. 10.2340/16501977-0947. [DOI] [PubMed] [Google Scholar]
- Kaster, T. S., Daskalakis, Z. J., Noda, Y., Knyahnytska, Y., Downar, J., Rajji, T. K., … Blumberger, D. M. (2018). Efficacy, tolerability, and cognitive effects of deep transcranial magnetic stimulation for late-life depression: A prospective randomized controlled trial. Neuropsychopharmacology, 43(11), 2231–2238. 10.1038/s41386-018-0121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh, B. C., Aaronson, S. T., Clarke, G. N., Holtzheimer, P. E., Johnson, C. W., McDonald, W. M., … Carpenter, L. L. (2018). Neurocognitive effects of repetitive transcranial magnetic stimulation with a 2-coil device in treatment-resistant major depressive disorder. Journal of ECT, 3(258), 265 10.1097/YCT.0000000000000494. [DOI] [PubMed] [Google Scholar]
- Kim, T. D., Hong, G., Kim, J., & Yoon, S. (2019). Cognitive enhancement in neurological and psychiatric disorders using transcranial magnetic stimulation (TMS): A review of modalities, potential mechanisms and future implications. Experimental Neurobiology, 28(1), 1–16. 10.5607/en.2019.28.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, B. R., Kim, D. Y., Ho Chun, M., Hwa Yi, J., & Sung Kwon, J. (2010). Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: A double-blind, sham-controlled trial. American Journal of Physical Medicine and Rehabilitation, 89(5), 362–368. 10.1097/PHM.0b013e3181d8a5b1. [DOI] [PubMed] [Google Scholar]
- Koch, G., Bonnì, S., Pellicciari, M. C., Casula, E. P., Mancini, M., Esposito, R., … Bozzali, M. (2018). Transcranial magnetic stimulation of the precuneus enhances memory and neural activity in prodromal Alzheimer's disease. NeuroImage, 169, 302–311. 10.1016/j.neuroimage.2017.12.048. [DOI] [PubMed] [Google Scholar]
- Lage, C., Wiles, K., Shergill, S. S., & Tracy, D. K. (2016). A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. Journal of Neural Transmission, 123(12), 1479–1490. 10.1007/s00702-016-1592-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau, S. M., Lal, R., O'Neil, J. P., Baker, S., & Jagust, W. J. (2009). Striatal dopamine and working memory. Cerebral Cortex, 19(2), 445–454. 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, S. A., & Kim, M. K. (2018). Effect of low frequency repetitive transcranial magnetic stimulation on depression and cognition of patients with traumatic brain injury: A randomized controlled trial. Medical Science Monitor, 24, 8789–8794. 10.12659/MSM.911385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroi, I., McDonald, K., Pantula, H., & Harbishettar, V. (2012). Cognitive impairment in Parkinson disease: Impact on quality of life, disability, and caregiver burden. Journal of Geriatric Psychiatry and Neurology, 25(4), 208–214. 10.1177/0891988712464823. [DOI] [PubMed] [Google Scholar]
- Leśniak, M., Polanowska, K., Seniów, J., & Członkowska, A. (2014). Effects of repeated anodal tDCS coupled with cognitive training for patients with severe traumatic brain injury: A pilot randomized controlled trial. Journal of Head Trauma Rehabilitation, 29(3), E20–E29. 10.1097/HTR.0b013e318292a4c2. [DOI] [PubMed] [Google Scholar]
- Lillie, E. M., Urban, J. E., Lynch, S. K., Weaver, A. A., & Stitzel, J. D. (2016). Evaluation of skull cortical thickness changes with age and sex from computed tomography scans. Journal of Bone and Mineral Research, 31(2), 299–307. 10.1002/jbmr.2613. [DOI] [PubMed] [Google Scholar]
- Lindenmayer, J. P., Kulsa, M. K. C., Sultana, T., Kaur, A., Yang, R., Ljuri, I., … Khan, A. (2019). Transcranial direct-current stimulation in ultra-treatment-resistant schizophrenia. Brain Stimulation, 12(1), 54–61. 10.1016/j.brs.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Litvan, I., Goldman, J. G., Tröster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., … Emre, M. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement disorder society task force guidelines. Movement Disorders, 27(3), 349–356. 10.1002/mds.24893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loo, C. K., Alonzo, A., Martin, D., Mitchell, P. B., Galvez, V., & Sachdev, P. (2012). Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. British Journal of Psychiatry, 200(1), 52–59. 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- Loo, C. K., Mitchell, P. B., McFarquhar, T. F., Malhi, G. S., & Sachdev, P. S. (2007). A sham-controlled trial of the efficacy and safety of twice-daily rTMS in major depression. Psychological Medicine, 37(3), 341 10.1017/S0033291706009597. [DOI] [PubMed] [Google Scholar]
- Loo, C., Sachdev, P., Elsayed, H., McDarmont, B., Mitchell, P., Wilkinson, M., … Gandevia, S. (2001). Effects of a 2- to 4-week course of repetitive Transcranial Magnetic Stimulation (rTMS) on neuropsychologic functioning, electroencephalogram, and auditory threshold in depressed patients. Biological Psychiatry, 49(7), 615–623. 10.1016/S0006-3223(00)00996-3. [DOI] [PubMed] [Google Scholar]
- Loo, C. K., Sachdev, P., Martin, D., Pigot, M., Alonzo, A., Malhi, G. S., … Mitchell, P. (2010). A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. International Journal of Neuropsychopharmacology, 13(1), 61–69. 10.1017/S1461145709990411. [DOI] [PubMed] [Google Scholar]
- Maloni, H. (2018). Cognitive impairment in multiple sclerosis. Journal for Nurse Practitioners, 14(3), 172–177. 10.1016/j.nurpra.2017.11.018. [DOI] [Google Scholar]
- Manenti, R., Cotelli, M. S., Cobelli, C., Gobbi, E., Brambilla, M., Rusich, D., … Cotelli, M. (2018). Transcranial direct current stimulation combined with cognitive training for the treatment of Parkinson disease: A randomized, placebo-controlled study. Brain Stimulation, 11(6), 1251–1262. 10.1016/j.brs.2018.07.046. [DOI] [PubMed] [Google Scholar]
- Martin, D. M., McClintock, S. M., Forster, J., & Loo, C. K. (2016). Does therapeutic repetitive transcranial magnetic stimulation cause cognitive enhancing effects in patients with neuropsychiatric conditions? A systematic review and meta-analysis of randomised controlled trials. Neuropsychology Review, 26(3), 295–309. 10.1007/s11065-016-9325-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattioli, F., Bellomi, F., Stampatori, C., Capra, R., & Miniussi, C. (2016). Neuroenhancement through cognitive training and anodal tDCS in multiple sclerosis. Multiple Sclerosis, 22(2), 222–230. 10.1177/1352458515587597. [DOI] [PubMed] [Google Scholar]
- Mcconnell, K., Nahas, Z., Shastri, A., Lorberbaum, J. P., Kozel, F. A., Bohning, D. E., & George, M. S. (2001). The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biological Psychiatry, 49(5), 454–459. [DOI] [PubMed] [Google Scholar]
- McDonald, W. M., Easley, K., Byrd, E. H., Holtzheimer, P., Tuohy, S., Woodard, J. L., … Epstein, C. M. (2006). Combination rapid transcranial magnetic stimulation in treatment refractory depression. Neuropsychiatric Disease and Treatment, 2(1), 85–94. [PMC free article] [PubMed] [Google Scholar]
- McIntosh, A. M., Semple, D., Tasker, K., Harrison, L. K., Owens, D. G. C., Johnstone, E. C., … Ebmeier, K. P. (2004). Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Research, 127(1-2), 9–17. 10.1016/j.psychres.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Mellin, J. M., Alagapan, S., Lustenberger, C., Lugo, C. E., Alexander, M. L., Gilmore, J. H., … Fröhlich, F. (2018). Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. European Psychiatry, 51, 25–33. 10.1016/j.eurpsy.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi, C., & Rossini, P. M. (2011). Transcranial magnetic stimulation in cognitive rehabilitation. Neuropsychological Rehabilitation, 21(5), 579–601. 10.1080/09602011.2011.562689. [DOI] [PubMed] [Google Scholar]
- Mittrach, M., Thünker, J., Winterer, G., Agelink, M. W., Regenbrecht, G., Arends, M., … Cordes, J. (2010). The tolerability of rTMS treatment in schizophrenia with respect to cognitive function. Pharmacopsychiatry, 43(3), 110–117. 10.1055/s-0029-1242824. [DOI] [PubMed] [Google Scholar]
- Mogg, A., Purvis, R., Eranti, S., Contell, F., Taylor, J. P., Nicholson, T., … McLoughlin, D. M. (2007). Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: A randomized controlled pilot study. Schizophrenia Research, 93(1-3), 221–228. 10.1016/j.schres.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Moher, D., Liberati, A., Tetzlaff, J., & Altman, D. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ (Online), 339(jul 21, 1), b2535–b2535. 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno, M. L., Vanderhasselt, M. A., Carvalho, A. F., Moffa, A. H., Lotufo, P. A., Benseñor, I. M., … Brunoni, A. R. (2015). Effects of acute transcranial direct current stimulation in hot and cold working memory tasks in healthy and depressed subjects. Neuroscience Letters, 591, 126–131. 10.1016/j.neulet.2015.02.036. [DOI] [PubMed] [Google Scholar]
- Moser, D. J., Jorge, R. E., Manes, F., Paradiso, S., Benjamin, M. L., & Robinson, R. G. (2002). Improved executive functioning following repetitive transcranial magnetic stimulation. Neurology, 58(8), 1288–1290. 10.1212/WNL.58.8.1288. [DOI] [PubMed] [Google Scholar]
- Mosimann, U. P., Schmitt, W., Greenberg, B. D., Kosel, M., Müri, R. M., Berkhoff, M., … Schlaepfer, T. E. (2004). Repetitive transcranial magnetic stimulation: A putative add-on treatment for major depression in elderly patients. Psychiatry Research, 126(2), 123–133. 10.1016/j.psychres.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Myczkowski, M. L., Fernandes, A., Moreno, M., Valiengo, L., Lafer, B., Moreno, R. A., … Brunoni, A. R. (2018). Cognitive outcomes of TMS treatment in bipolar depression: Safety data from a randomized controlled trial. Journal of Affective Disorders, 235, 20–26. 10.1016/j.jad.2018.04.022. [DOI] [PubMed] [Google Scholar]
- Nitsche, M. A., & Paulus, W. (2011). Transcranial direct current stimulation – update 2011. Restorative Neurology and Neuroscience, 29(6), 463–492. 10.3233/RNN-2011-0618. [DOI] [PubMed] [Google Scholar]
- Orlov, N. D., Tracy, D. K., Joyce, D., Patel, S., Rodzinka-Pasko, J., Dolan, H., … Shergill, S. S. (2017). Stimulating cognition in schizophrenia: A controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimulation, 10(3), 560–566. 10.1016/j.brs.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Padala, P. R., Padala, K. P., Lensing, S. Y., Jackson, A. N., Hunter, C. R., Parkes, C. M., … Sullivan, D. H. (2018). Repetitive transcranial magnetic stimulation for apathy in mild cognitive impairment: A double-blind, randomized, sham-controlled, cross-over pilot study. Psychiatry Research, 261, 312–318. 10.1016/j.psychres.2017.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal, E., Nagy, F., Aschermann, Z., Balazs, E., & Kovacs, N. (2010). The impact of left prefrontal repetitive transcranial magnetic stimulation on depression in Parkinson's disease: A randomized, double-blind, placebo-controlled study. Movement Disorders, 25(14), 2311–2317. 10.1002/mds.23270. [DOI] [PubMed] [Google Scholar]
- Palm, U., Keeser, D., Hasan, A., Kupka, M. J., Blautzik, J., Sarubin, N., … Padberg, F. (2016). Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: A double-blind, sham-controlled proof-of-concept study. Schizophrenia Bulletin, 34(2), 189–199. 10.1093/schbul/sbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm, U., Schiller, C., Fintescu, Z., Obermeier, M., Keeser, D., Reisinger, E., … Padberg, F. (2012). Transcranial direct current stimulation in treatment resistant depression: A randomized double-blind, placebo-controlled study. Brain Stimulation, 5(3), 242–251. 10.1016/j.brs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- Papakostas, G. I., Petersen, T., Mahal, Y., Mischoulon, D., Nierenberg, A. A., & Fava, M. (2004). Quality of life assessments in major depressive disorder: A review of the literature. General Hospital Psychiatry, 26(1), 13–17. 10.1016/j.genhosppsych.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Papazova, I., Strube, W., Becker, B., Henning, B., Schwippel, T., Fallgatter, A. J., … Hasan, A. (2018). Improving working memory in schizophrenia: Effects of 1 mA and 2 mA transcranial direct current stimulation to the left DLPFC. Schizophrenia Research, 202, 203–209. 10.1016/j.schres.2018.06.032. [DOI] [PubMed] [Google Scholar]
- Park, S. H., Koh, E. J., Choi, H. Y., & Ko, M. H. (2013). A double-blind, sham-controlled, pilot study to assess the effects of the concomitant use of transcranial direct current stimulation with the computer assisted cognitive rehabilitation to the prefrontal cortex on cognitive functions in patients with stroke. Journal of Korean Neurosurgical Society, 54(6), 484 10.3340/jkns.2013.54.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova, E. L., Menshikova, A. A., Semenov, R. V., Bocharnikova, E. N., Gotovtseva, G. N., Druzhkova, T. A., … Guekht, A. B. (2018). Transcranial direct current stimulation of 20- and 30-minutes combined with sertraline for the treatment of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 82, 31–38. 10.1016/j.pnpbp.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Perneczky, R., Pohl, C., Sorg, C., Hartmann, J., Komossa, K., Alexopoulos, P., … Kurz, A. (2006). Complex activities of daily living in mild cognitive impairment: Conceptual and diagnostic issues. Age and Ageing, 35(3), 240–245. 10.1093/ageing/afj054. [DOI] [PubMed] [Google Scholar]
- Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., … Winblad, B. (2001). Current concepts in mild cognitive impairment. Archives of Neurology, 58(12), 1985 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pope, P. A., & Chris Miall, R. (2014). Restoring cognitive functions using non-invasive brain stimulation techniques in patients with cerebellar disorders. Frontiers in Psychiatry, 5, 33 10.3389/fpsyt.2014.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabany, L., Deutsch, L., & Levkovitz, Y. (2014). Double-blind, randomized sham controlled study of deep-TMS add-on treatment for negative symptoms and cognitive deficits in schizophrenia. Journal of Psychopharmacology, 28(7), 686–680. 10.1177/0269881114533600. [DOI] [PubMed] [Google Scholar]
- Rassovsky, Y., Dunn, W., Wynn, J., Wu, A. D., Iacoboni, M., Hellemann, G., … Green, M. F. (2015). The effect of transcranial direct current stimulation on social cognition in schizophrenia: A preliminary study. Schizophrenia Research, 165(2–3), 171–174. 10.1016/j.schres.2015.04.016. [DOI] [PubMed] [Google Scholar]
- Robbins, T. W. (2011). Cognition: The ultimate brain function. Neuropsychopharmacology, 36(1), 1–2. 10.1038/npp.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal, R. (1979). The file drawer problem and tolerance for null results. Psychological Bulletin, 86, 638–641. doi: 10.1037/0033-2909.86.3.638. [DOI] [Google Scholar]
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A., Avanzini, G., Bestmann, S., … Ziemann, U. (2009). Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology, 120(12), 2008–2039. 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehinejad, M. A., Ghanavai, E., Rostami, R., & Nejati, V. (2017). Cognitive control dysfunction in emotion dysregulation and psychopathology of major depression (MD): Evidence from transcranial brain stimulation of the dorsolateral prefrontal cortex (DLPFC). Journal of Affective Disorders, 210, 241–248. 10.1016/j.jad.2016.12.036. [DOI] [PubMed] [Google Scholar]
- Schrag, A., Jahanshahi, M., & Quinn, N. (2000). What contributes to quality of life in patients with Parkinson's disease? Journal of Neurology Neurosurgery and Psychiatry, 69(3), 308–312. 10.1136/jnnp.69.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Geers, C., Obert, M., Schilling, R. L., Harth, S., Traupe, H., Gizewski, E. R., … Verhoff, M. A. (2011). Age and gender-dependent bone density changes of the human skull disclosed by high-resolution flat-panel computed tomography. International Journal of Legal Medicine, 125(3), 417–425. 10.1007/s00414-010-0544-3. [DOI] [PubMed] [Google Scholar]
- Sedláčková, S., Rektorová, I., Srovnalová, H., & Rektor, I. (2009). Effect of high frequency repetitive transcranial magnetic stimulation on reaction time, clinical features and cognitive functions in patients with Parkinson's disease. Journal of Neural Transmission, 116(9), 1093–1101. 10.1007/s00702-009-0259-0. [DOI] [PubMed] [Google Scholar]
- Segrave, R. A., Arnold, S., Hoy, K., & Fitzgerald, P. B. (2014). Concurrent cognitive control training augments the antidepressant efficacy of tDCS: A pilot study. Brain Stimulation, 7(2), 325–331. 10.1016/j.brs.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Shin, I. S., Carter, M., Masterman, D., Fairbanks, L., & Cummings, J. L. (2005). Neuropsychiatric symptoms and quality of life in Alzheimer disease. American Journal of Geriatric Psychiatry, 13(6), 469–474. 10.1097/00019442-200506000-00005. [DOI] [PubMed] [Google Scholar]
- Singh, A., Erwin-Grabner, T., Goya-Maldonado, R., & Antal, A. (2019). Transcranial magnetic and direct current stimulation in the treatment of depression: Basic mechanisms and challenges of two commonly used brain stimulation methods in interventional psychiatry. Neuropsychobiology, 1–11. doi: 10.1159/000502149 [DOI] [PubMed] [Google Scholar]
- Slotema, C. W., Aleman, A., Daskalakis, Z. J., & Sommer, I. E. (2012). Meta-analysis of repetitive transcranial magnetic stimulation in the treatment of auditory verbal hallucinations: Update and effects after one month. Schizophrenia Research, 142(1–3), 40–45. 10.1016/j.schres.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Smith, R. C., Boules, S., Mattiuz, S., Youssef, M., Tobe, R. H., Sershen, H., … Davis, J. M. (2015). Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: A randomized controlled study. Schizophrenia Research. 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Srovnalova, H., Marecek, R., & Rektorova, I. (2011). The role of the inferior frontal gyri in cognitive processing of patients with Parkinson's disease: A pilot rTMS study. Movement Disorders, 26(8), 1545–1548. 10.1002/mds.23663. [DOI] [PubMed] [Google Scholar]
- Stelzel, C., Basten, U., Montag, C., Reuter, M., & Fiebach, C. J. (2010). Frontostriatal involvement in task switching depends on genetic differences in D2 receptor density. Journal of Neuroscience, 30(42), 14205–14212. 10.1523/JNEUROSCI.1062-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suemoto, C. K., Apolinario, D., Nakamura-Palacios, E. M., Lopes, L., Paraizo Leite, R. E., Sales, M. C., … Fregni, F. (2014). Effects of a non-focal plasticity protocol on apathy in moderate Alzheimer's disease: A randomized, double-blind, sham-controlled trial. Brain Stimulation, 7(2), 308–313. 10.1016/j.brs.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Sun, L. (2015). Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Medical Science Monitor, 21, 761–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, L., Lu, H., Zhang, T., Wen, M., & Sun, L. (2015). Impact of repetitive transcranial magnetic stimulation on post-stroke dysmnesia and the role of BDNF Val66Met SNP. Medical Science Monitor, 21, 761–768. 10.12659/MSM.892337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, J. H., Tan, L., & Yu, J. T. (2014). Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Annals of Translational Medicine, 2(8), 80 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy, D. K., Shergill, S. S., David, A. S., Fonagy, P., Zaman, R., Downar, J., … Bhui, K. (2015). Self-harm and suicidal acts: A suitable case for treatment of impulsivity-driven behaviour with repetitive transcranial magnetic stimulation (rTMS). BJPsych Open, 1(1), 87–91. 10.1192/bjpo.bp.115.000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich, H., Kranaster, L., Sigges, E., Andrich, J., & Sartorius, A. (2012). Ultra-high-frequency left prefrontal transcranial magnetic stimulation as augmentation in severely ill patients with depression: A naturalistic sham-controlled, double-blind, randomized trial. Neuropsychobiology, 66(3), 141–148. 10.1159/000339561. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt, M. A., de Raedt, R., Baeken, C., Leyman, L., & D'Haenen, H. (2009). A single session of rTMS over the left dorsolateral prefrontal cortex influences attentional control in depressed patients. World Journal of Biological Psychiatry, 10(1), 34–42. 10.1080/15622970701816514. [DOI] [PubMed] [Google Scholar]
- Vöröslakos, M., Takeuchi, Y., Brinyiczki, K., Zombori, T., Oliva, A., Fernández-Ruiz, A., … Berényi, A. (2018). Direct effects of transcranial electric stimulation on brain circuits in rats and humans. Nature Communications, 9(1), 483 10.1038/s41467-018-02928-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajdik, C., Claypoole, K. H., Fawaz, W., Holtzheimer, P. E., Neumaier, J., Dunner, D., … Avery, D. H. (2014). No change in neuropsychological functioning after receiving repetitive transcranial magnetic stimulation treatment for major depression. Journal of ECT, 30(4), 320–324. 10.1097/YCT.0000000000000096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker, K. R., & Tesco, G. (2013). Molecular mechanisms of cognitive dysfunction following traumatic brain injury. Frontiers in Aging Neuroscience, 5, 29 10.3389/fnagi.2013.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace, T. L., Ballard, T. M., Pouzet, B., Riedel, W. J., & Wettstein, J. G. (2011). Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacology Biochemistry and Behavior, 99(2), 130–145. 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- Wobrock, T., Guse, B., Cordes, J., Wölwer, W., Winterer, G., Gaebel, W., … Hasan, A. (2015). Left prefrontal high-frequency repetitive transcranial magnetic stimulation for the treatment of schizophrenia with predominant negative symptoms: A sham-controlled, randomized multicenter trial. Biological Psychiatry, 77(11), 979–988. 10.1016/j.biopsych.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Wölwer, W., Lowe, A., Brinkmeyer, J., Streit, M., Habakuck, M., Agelink, M. W., … Cordes, J. (2014). Repetitive transcranial magnetic stimulation (rTMS) improves facial affect recognition in schizophrenia. Brain Stimulation, 7(4), 559–563. 10.1016/j.brs.2014.04.011. [DOI] [PubMed] [Google Scholar]
- Woods, A. J., Antal, A., Bikson, M., Boggio, P. S., Brunoni, A. R., Celnik, P., … Nitsche, M. A. (2016). A technical guide to tDCS, and related non-invasive brain stimulation tools. Clinical Neurophysiology, 127(2), 1031–1048. 10.1016/j.clinph.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Qiu, Z., Zhu, J., Liu, J., Wu, J., Tao, J., … Chen, L. (2019). The modulation effect of non-invasive brain stimulation on cognitive function in patients with mild cognitive impairment: A systematic review and meta-analysis of randomized controlled trials 11 Medical and Health Sciences 1103 Clinical Sciences 11 Medica. BMC Neuroscience, 20(1), 2 10.1186/s12868-018-0484-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun, G. J., Chun, M. H., & Kim, B. R. (2015). The effects of transcranial direct-current stimulation on cognition in stroke patients. Journal of Stroke, 17(3), 354–358. 10.5853/jos.2015.17.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, J., Li, Z., Cong, Y., Zhang, J., Tan, M., Zhang, H., … Shan, P. (2017). Repetitive transcranial magnetic stimulation improves cognitive function of Alzheimer's disease patients. Oncotarget, 8(20), 33864–33871. 10.18632/oncotarget.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, L., Guo, Q., Li, H., Li, C., & Wang, J. J. (2012). Effects of repetitive transcranial magnetic stimulation with different paradigms on the cognitive function and psychotic symptoms of schizophrenia patients. Beijing Da Xue Xue Bao. Yi Xue Ban = Journal of Peking University. Health Sciences, 44(5), 732–736. [PubMed] [Google Scholar]
- Ziemann, U., Paulus, W., Nitsche, M. A., Pascual-Leone, A., Byblow, W. D., Berardelli, A., … Rothwell, J. C. (2008). Consensus: Motor cortex plasticity protocols. Brain Stimulation, 1(3), 164–182. 10.1016/j.brs.2008.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291720003670.
click here to view supplementary material