Abstract
A 63-year-old woman on flecainide, furosemide, and triamterene–hydrochlorothiazide presented with weakness and diarrhoea. She had profound hyponatraemia, hypokalaemia and a pre-renal acute kidney injury (AKI). Her ECG showed a regular wide complex tachycardia concerning for monomorphic ventricular tachycardia. She was haemodynamically stable and treated with aggressive electrolyte repletion and amiodarone. Flecainide toxicity can present as a variety of arrhythmias and early recognition is crucial. This case focuses on flecainide toxicity from multiple concomitant insults: diuretic use, diarrhoea, hypokalaemia, hyponatraemia and pre-renal AKI. We emphasise the importance of close outpatient monitoring of electrolytes in a patient on diuretics and flecainide to prevent life-threatening arrhythmias. We discourage use of multiple diuretics in patients taking flecainide.
Keywords: arrhythmias, safety
Background
Flecainide has been in use for more than 30 years as a relatively safe class IC antiarrhythmic drug. It slows conduction by acting on cardiac sodium channels in a rate-dependent manner, thereby prolonging the PR interval and widening the QRS complex. However, it has a 90%–95% bioavailability, a half-life of 12–27 hours1 and a very narrow therapeutic window; characteristics that can exacerbate drug toxicity due to persistent serum drug levels. Electrolyte derangements such as hypokalaemia and hyponatraemia, as well as renal failure, are known triggers of toxicity. Flecainide toxicity can present as different types of arrhythmias.1 There are no defined treatments for flecainide toxicity, although sodium bicarbonate has been effectively used to prevent further cardiotoxic effects and is considered to be the gold standard. Amiodarone and lidocaine have been used as well, though the mechanism is unknown.2
Case presentation
Our patient is a 63-year-old woman with a history of morbid obesity, paroxysmal atrial fibrillation, hypothyroidism, hypertension, bipolar disorder and neuropathy who presented to her cardiologist’s office with persistent exertional dyspnoea. An evaluation for ischaemic cause was negative, and the patient was determined to have a structurally normal heart. Although she did have evidence of some volume overload, her overall evaluation was negative for heart failure with reduced ejection fraction. Due to the possibility that her exertional dyspnoea was secondary to atrial fibrillation, improved rhythm control was attempted. A trial of diltiazem was unsuccessful, resulting in symptomatic hypotension. Diltiazem was discontinued and flecainide was started at 100 mg two times per day. She achieved chemical cardioversion within 2 weeks of starting flecainide.
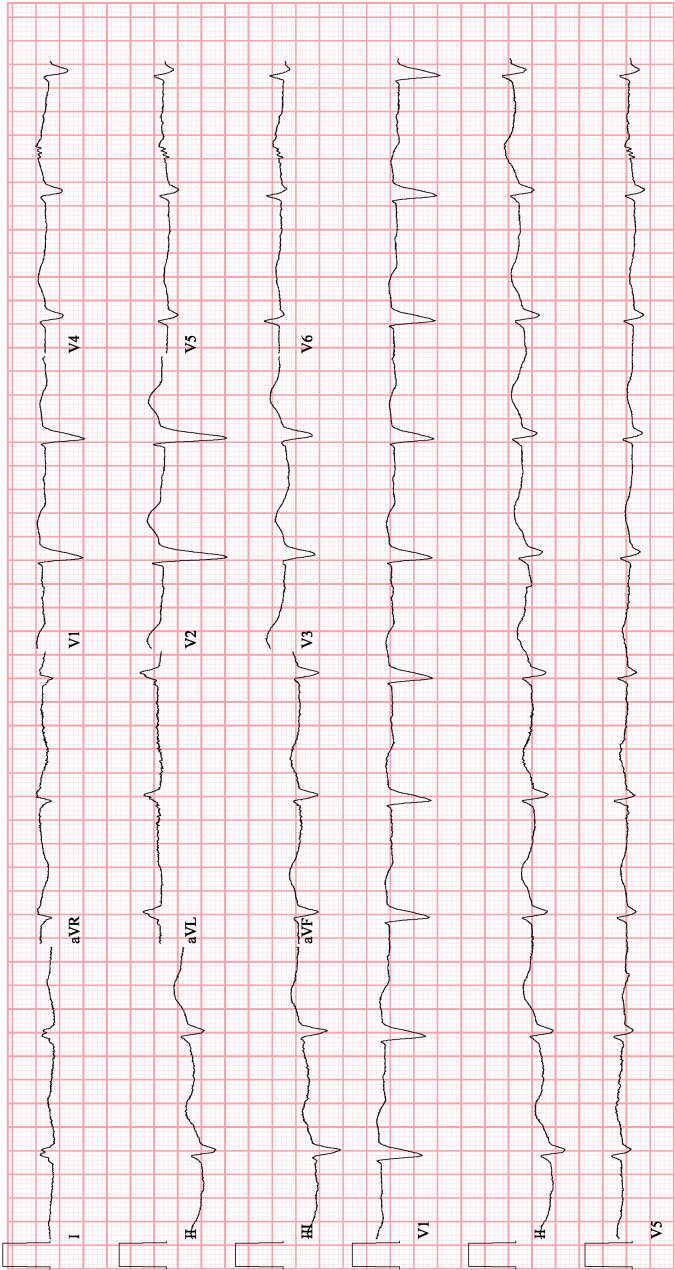
She subsequently developed severe diarrhoea and weakness. She was admitted to the hospital with findings of hypovolemic hyponatraemia, exacerbated by continuation of her diuretics in the setting of diarrhoea. At this time, her sodium (Na) level was 127 mEq/L and potassium (K) was 3.4 mEq/L. Her ECG showed sinus bradycardia with first degree atrioventricular (AV) block and left bundle branch block with a QRS duration of 178 ms (figure 1).
Figure 1.
Initial ECG while on flecainide.
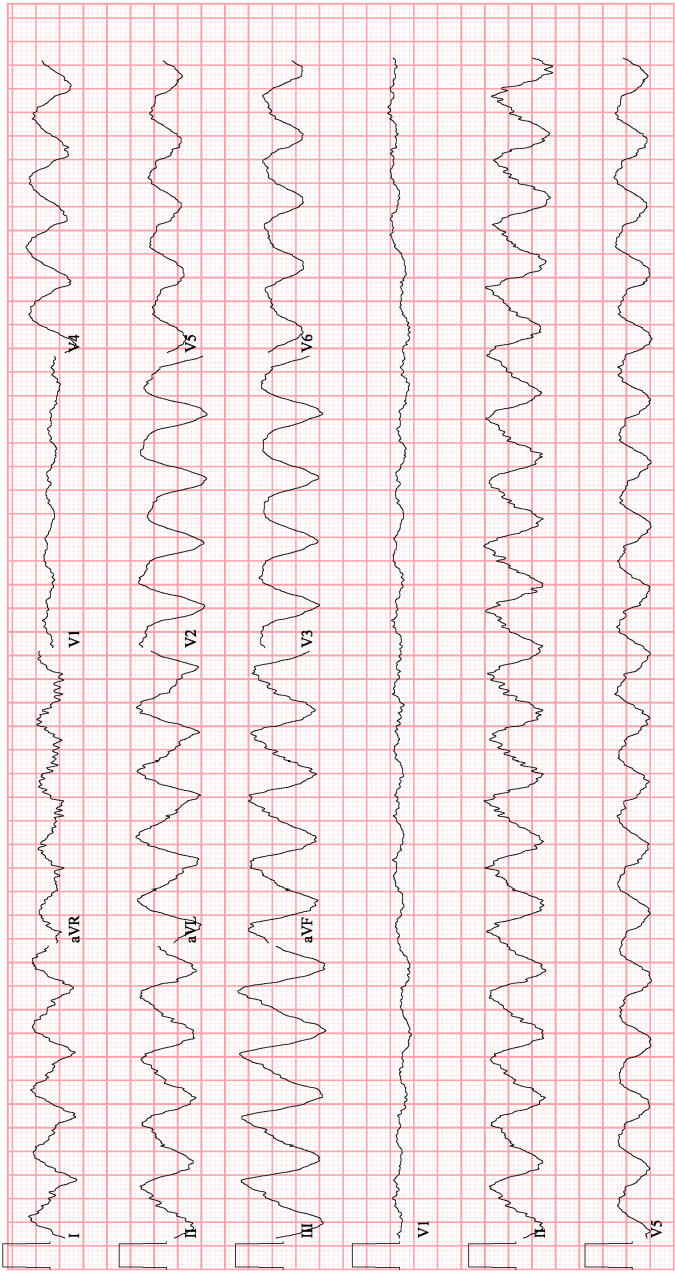
Her bradycardia initially improved with cessation of metoprolol and flecainide, however, after an electrophysiology evaluation, both flecainide and metoprolol were resumed due to their efficacy in maintaining sinus rhythm and minimising her symptomatic dyspnoea. She was discharged to a skilled nursing facility with discontinuation of her furosemide but continuation of her other diuretics. Two weeks later, she returned to the hospital after persistent daily episodes of diarrhoea, with an acute kidney injury (AKI), hypovolemic hyponatraemia 116 mg/dL and hypokalaemia 2.5 mEq/L. Her ECG during this admission showed atrial fibrillation with a slow ventricular rate of 53 beats/min (bpm), a left bundle branch block with a QRS duration of 182 ms and a QTc interval of 471 ms. Flecainide and her diuretics were again discontinued, her beta blocker was continued and she was given intravenous fluids. One day into her admission, she spontaneously developed a sustained, monomorphic wide complex tachycardia (WCT) with maintenance of haemodynamic stability (figure 2). At that time, her Na was 122 mEq/L, improved from a nadir of 115 mEq/L earlier that day, and her K was 3.1 mEq/L.
Figure 2.
Monomorphic ventricular tachycardia in the setting of flecainide toxicity.
Two troponin levels drawn 4 hours apart were negative. Given an absence of anginal symptoms and recent negative ischaemic work-up, treatment focused on evaluating for non-ischaemic causes of her arrhythmia as well as rhythm conversion.
Differential diagnosis
Our patient’s initial ECG showed a uniform wide complex rhythm at a ventricular rate of 184 bpm. While the majority of ventricular tachycardia exists in the setting of structural heart disease, our patient had an echocardiogram 2 months prior that did not show evidence of reduced ejection fraction or wall motion abnormalities. While acute ischaemic causes of her presentation were possible, it was less likely given her haemodynamic stability, absence of anginal symptoms and normal troponin levels despite sustaining this rhythm.
The differential for WCT in the absence of structural heart disease involves generally haemodynamically stable rhythms such as outflow tract ventricular tachycardia, idiopathic fascicular ventricular tachycardia or a supraventricular tachycardia with aberrancy. Hyperkalaemia was briefly considered as a potential aetiology for this rhythm due to aggressive electrolyte repletion and correction of hypokalaemia with AKI on presentation. However, her laboratory results at that time showed a serum K at 4.1 mEq/L. The use of flecainide lent consideration to the proarrhythmic effect from flecainide toxicity, a condition associated with increased mortality.3 Flecainide is known for its high affinity, dose-dependent mechanism. It has a high volume of distribution and is excreted by the kidneys. Despite discontinuation of the medication, there was concern for drug accumulation in the setting of our patient’s presentation with AKI. Several case reports describe WCTs due to flecainide toxicity.2 4–12 Prolongation of the QRS complex can result in conversion of atrial fibrillation to a slow atrial flutter with 1:1 AV conduction giving the appearance of a regular WCT.4 In our patient, positive Brugada criteria with evidence of a northwest axis and negative precordial concordance led to the diagnosis of likely monomorphic ventricular tachycardia.
Treatment
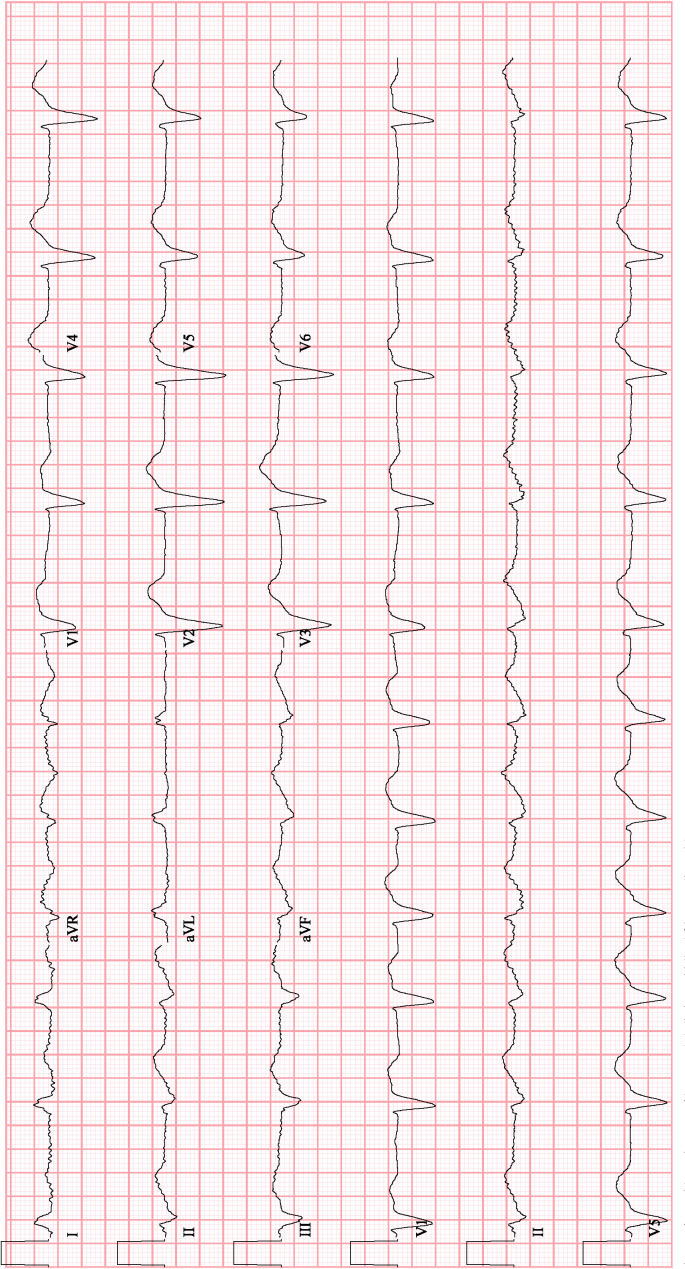
The patient was upgraded to the cardiac intensive care unit for close haemodynamic monitoring. An intravenous bolus of 150 mg of amiodarone was administered, and she converted her rhythm back to atrial fibrillation with a left bundle branch block (figure 3). At this time, her Na was 123 mEq/L and K was 4.1 mEq/L. An amiodarone infusion was continued for 36 hours and she received two more bolus doses of amiodarone due to recurrent episodes of non-sustained WCT.
Figure 3.
Conversion after administration of amiodarone.
Outcome and follow-up
Amiodarone and flecainide were discontinued at discharge with flecainide listed in the electronic health record as an allergy. She was started on propanolol for rate control of her atrial fibrillation and discharged safely to her home.
Discussion
Flecainide has demonstrated a superior safety profile and effectiveness in comparison to other antiarrhythmic drugs despite significant negative outcomes in certain populations.1 5 Shortly after approval by the Food and Drug Administration in 1984, the CAST trial showed increased incidence of sudden cardiac death in the use of class IC antiarrhythmics in those with a history of myocardial infarction or structural heart disease,13 which limited their use to patients without evidence of structural heart disease. Additionally, flecainide is characterised by a narrow therapeutic range of 0.2–1.0 mg/mL and is highly susceptible to accumulation from AKI due to its renal excretion. Strong pharmacokinetics, including high bioavailability of up to 90%, long half-life of up to 23 hours and large volume of distribution allow this medication to become toxic when supratherapeutic. This may explain the high mortality of up to 10% reported in flecainide overdose.3
Flecainide toxicity can result in several life-threatening arrhythmias, including monomorphic ventricular tachycardia, ventricular bradycardia, bradycardia degenerating into polymorphic ventricular tachycardia, polymorphic ventricular tachycardia without bradycardia and ultimately ventricular fibrillation.6–8
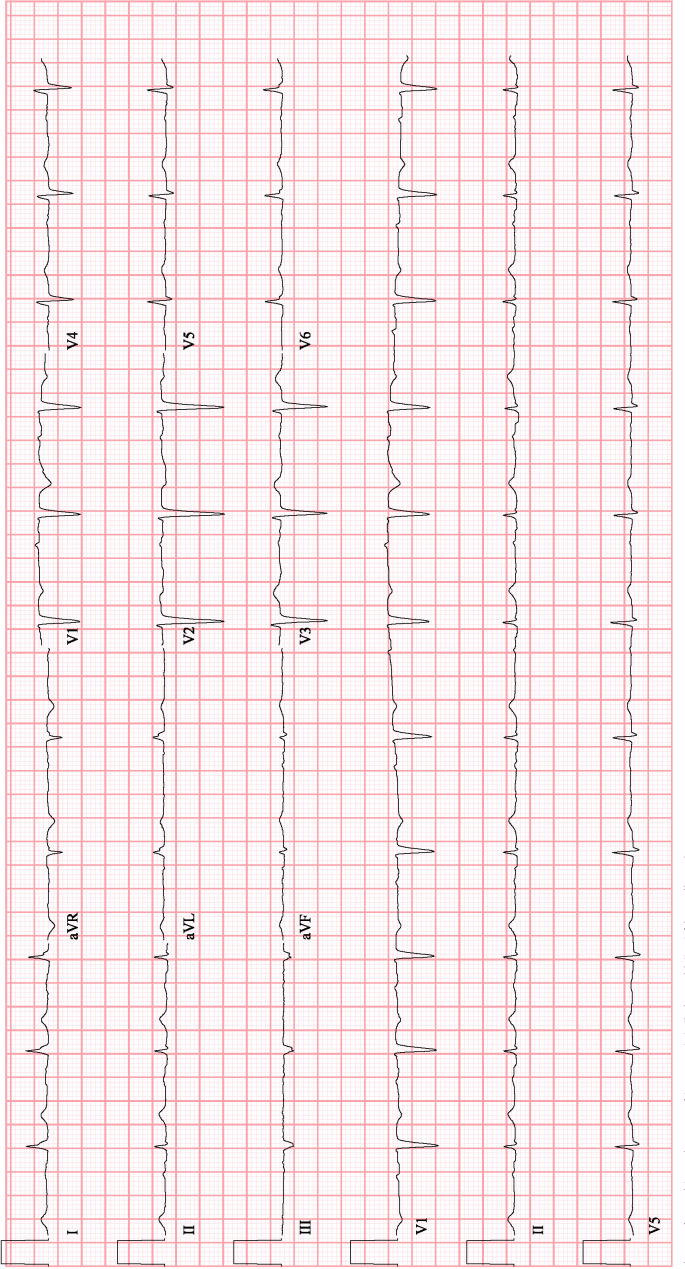
QRS prolongation correlates well with supratherapeutic drug levels and is associated with development of flecainide toxicity. Our patient’s QRS duration was 182 ms prior to development of monomorphic ventricular tachycardia and improved to 124 ms after 7 days of discontinuation of flecainide. Following hospital discharge and 10 days after discontinuation, her ECG showed a QRS duration of 98 ms with Na of 136 mEq/L and K of 4.7 mEq/L (figure 4).
Figure 4.
Patient’s baseline ECG 10 days following discontinuation of flecainide.
Significant electrolyte abnormalities, in part due to concomitant diuretic use, may potentiate toxicity independently or affect the duration of ventricular contraction.9 At least one report in the literature suggests that significant hyponatraemia can induce toxicity despite therapeutic drug level.10 Ohki et al report a case of hypokalaemia-induced QTc prolongation degenerating into Torsades de Pointes (TdP).8 Interestingly, despite our patient having hyponatraemia, hypokalaemia, QRS prolongation, QTc prolongation and bradycardia, she developed a haemodynamically stable monomorphic ventricular tachycardia as opposed to an unstable polymorphic rhythm, such as TdP. Monomorphic ventricular tachycardia is thought to develop from functional conduction blocks caused by flecainide-induced conduction delays resulting in re-entry.4 Additionally, the manufacturer’s label notes that in an extensive literature review, ventricular tachycardia was noted in 0.4% of 568 patients.14
While our case was successfully treated with intravenous amiodarone bolus and infusion, this may not always result in termination of the arrhythmia, and other treatment options must be considered. Intravenous sodium bicarbonate administered to achieve the goal of pH 7.5–7.6 is the mainstay of treatment for flecainide toxicity. The presence of sodium ions is thought to competitively displace the flecainide from the sodium channels while alkalisation of the blood reduces its free concentration in the serum. Sodium bicarbonate administration must be cautiously employed as overly rapid and aggressive sodium repletion may result in neurological consequences such as cerebral oedema. Intravenous administration of fat emulsion is a treatment option used to mitigate the large volume of distribution and decrease availability of the drug.11 There are a few cases of initiation of veno-arterial extracorporeal membrane oxygenation for treatment of the haemodynamic instability from persistent arrhythmias caused by flecainide toxicity.12 It is important to note that in haemodynamically unstable rhythms, while defibrillation should always be attempted, it is not consistently successful due to the increased fibrillation threshold caused by flecainide and several other antiarrhythmic drugs. Dialysis has not proven to be beneficial in reducing drug levels.
While generally considered to have a reliable safety profile in those without structural heart disease, several factors can lead to toxicity. In addition to screening for structural heart disease, baseline conduction abnormalities on ECG should be assessed and a meticulous review of the patient’s medications must be conducted to identify any thiazide diuretics, nephrotoxic medications and hepatotoxic medications. Electrolytes and renal function should be monitored closely in patients being treated with flecainide, and patients should also be made aware of acute conditions which may result in significant electrolyte abnormalities such as persistent diarrhoea and vomiting. Many case reports of flecainide toxicity exist in the setting of intentional overdose. It may be prudent to screen for a history of psychiatric disorders prior to initiation of treatment.
Learning points.
This case demonstrates how accumulation of several risk factors ultimately led to the development of a rare medication side effect. Our patient demonstrated several independent causes of flecainide toxicity including left bundle branch block, hyponatraemia, hypokalaemia and acute kidney injury.
While flecainide is generally safe when used appropriately, we recommend shared decision making and extensive patient education on potential side effects.
We support aggressive monitoring for the development of ischaemic heart disease, ECG changes and electrolyte abnormalities.
We advise against the use of this medication in patients who require high doses of diuretics, multiple diuretics and those who cannot consistently follow up with medical care.
Footnotes
Twitter: @DrDonthi
Contributors: ND and TC wrote the initial manuscript. The manuscript was edited and revised by LG and CD. All authors were involved in the patient’s care.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Aliot E, Capucci A, Crijns HJ, et al. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace 2011;13:161–73. 10.1093/europace/euq382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vu NM, Hill TE, Summers MR, et al. Management of life-threatening flecainide overdose: a case report and review of the literature. HeartRhythm Case Rep 2016;2:228–31. 10.1016/j.hrcr.2015.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Köppel C, Oberdisse U, Heinemeyer G. Clinical course and outcome in class Ic antiarrhythmic overdose. J Toxicol Clin Toxicol 1990;28:433–44. 10.3109/15563659009038586 [DOI] [PubMed] [Google Scholar]
- 4.Banavalikar B, Shenthar J, Padmanabhan D. Unusual wide complex tachycardia during rhythm control for atrial fibrillation. Circulation 2018;138:537–9. 10.1161/CIRCULATIONAHA.118.036071 [DOI] [PubMed] [Google Scholar]
- 5.Friberg L. Ventricular arrhythmia and death among atrial fibrillation patients using anti-arrhythmic drugs. Am Heart J 2018;205:118–27. 10.1016/j.ahj.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 6.Nogales Asensio JM, Moreno Sánchez N, Doncel Vecino LJ, Asensio JMN, Sánchez NM, Vecino LJD, et al. Torsade-de-pointes in a patient under flecainide treatment, an unusual case of proarrhythmicity. Int J Cardiol 2007;114:E65–7. 10.1016/j.ijcard.2006.07.124 [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Pak H-N, Park JS, et al. Flecainide-associated bradycardia-dependent torsade de pointes: another potential mechanism of proarrhythmia. Pacing Clin Electrophysiol 2013;36:e84-6. 10.1111/j.1540-8159.2010.02935.x [DOI] [PubMed] [Google Scholar]
- 8.Ohki R, Takahashi M, Mizuno O, et al. Torsades de pointes ventricular tachycardia induced by mosapride and flecainide in the presence of hypokalemia. Pacing Clin Electrophysiol 2001;24:119–21. 10.1046/j.1460-9592.2001.00119.x [DOI] [PubMed] [Google Scholar]
- 9.Khavandi A, Walker PR. Flecainide cardiotoxicity precipitated by electrolyte imbalance. caution with thiazide diuretics. Emerg Med J 2007;24:e26. 10.1136/emj.2006.044362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kasia C, Henry C, Santucci P. A case of electrolyte disturbances leading to flecainide toxicity at normal serum levels and pacemaker malfunction. HeartRhythm Case Rep 2019;5:448–51. 10.1016/j.hrcr.2019.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellsworth H, Stellpflug SJ, Cole JB, et al. A life-threatening flecainide overdose treated with intravenous fat emulsion. Pacing Clin Electrophysiol 2013;36:e87-9. 10.1111/j.1540-8159.2012.03485.x [DOI] [PubMed] [Google Scholar]
- 12.Mandawat A, McCullough SA, Gilstrap LG, et al. Successful treatment of flecainide overdose with sustained mechanical circulatory support. HeartRhythm Case Rep 2015;1:137–40. 10.1016/j.hrcr.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruskin JN. The cardiac arrhythmia suppression trial (cast). N Engl J Med 1989;321:386–8. 10.1056/NEJM198908103210608 [DOI] [PubMed] [Google Scholar]
- 14.RxDrugLabels Pharmaceuticals 3M. TAMBOCOR - 3M Pharmaceuticals [Internet], 2006. Available: https://rxdruglabels.com/lib/rx/rx-meds/tambocor/ [Accessed 19 May 2020].