Abstract
In this case, we present an uncommon gastrointestinal infection in an immunocompromised patient that was solely diagnosed because of close collaboration between treating physicians and microbiologists. The patient is a 42-year-old male who underwent heart transplantation 5 years earlier. He presented with fever, weight loss, diarrhoea and tiredness. Initial investigations could not elucidate the aetiology of his symptoms. The patient was referred to the department of infectious diseases for further evaluation. Serology for Yersinia species was ordered and the result was suggestive for the possibility of a Yersinia species infection. Close collaboration between treating physicians and microbiologists followed and led to additional investigations, which revealed the diagnosis of a Yersinia pseudotuberculosis infection with extensive lesions in the gastrointestinal tract. Treatment with ciprofloxacin resulted in complete resolution of symptoms and healing of the gastrointestinal lesions. In conclusion, this case underlines the need for a multidisciplinary approach to complex patients of which symptoms have yet to be understood.
Keywords: foodborne infections, endoscopy, infection (gastroenterology)
Background
This report underlines the need for a multidisciplinary approach to ensure a complete and thorough diagnostic workup. This case also exemplifies the need for clinicians to understand the laboratory process and its limitations. Yersinia pseudotuberculosis (Y. pseudotuberculosis) is an example of a pathogen that can be missed if it is not part of the routine laboratory process. Although Y. pseudotuberculosis is known to cause outbreaks, little is known about its prevalence. We hope this case will make clinicians aware of the possibility of uncommon gastrointestinal infections such as Y. pseudotuberculosis.
Case presentation
A 42-year-old immunosuppressed man was referred to the department of infectious diseases because of long-standing fever. The patient underwent heart transplantation 5 years earlier for a hypertrophic obstructive cardiomyopathy. His current immunosuppressive regimen consisted of mycophenolate mofetil and tacrolimus. The fever first presented 4 weeks prior to the consultation. He complained about long-standing fever, which on evaluation fluctuated between 38°C and 38.8°C, rhinorrhea, tiredness, decreased appetite with 3 kg weight loss, abdominal pain and a changed stool pattern with watery diarrhoea. Further medical history and physical examination were otherwise unremarkable.
Investigations
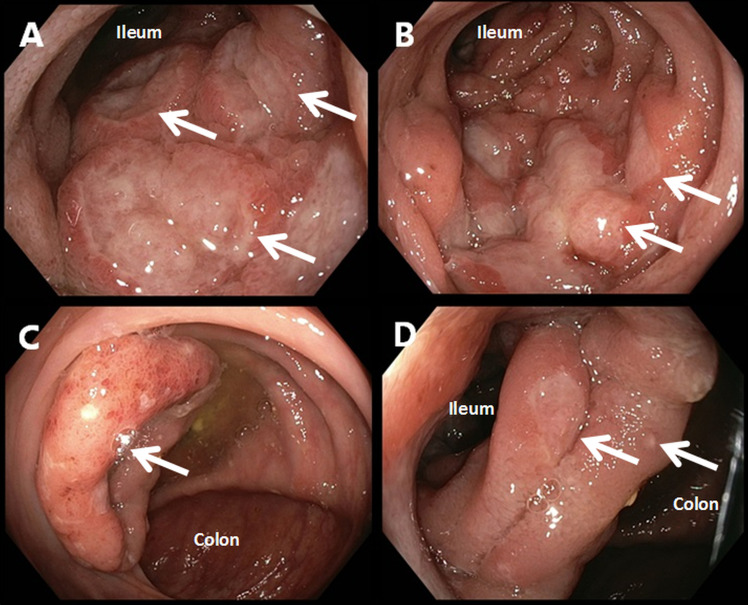
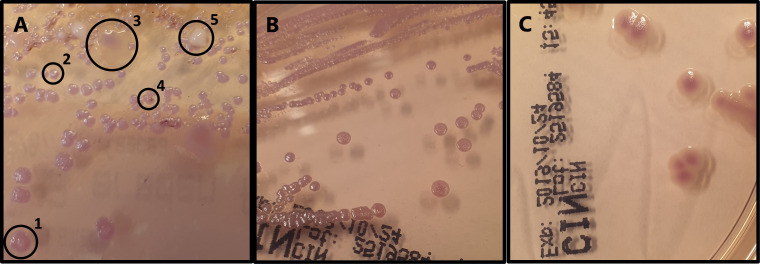
Laboratory analysis demonstrated a leukocytosis (13.6×109 cells/L; reference range 4.0–10.0×109 cells/L), elevated eosinophilic granulocytes (0.55×109 cells/L; reference range 0.0–0.4×109 cells/L), elevated neutrophil granulocytes (10.80×109 cells/L; reference range 1.60–8.30×109 cells/L) and an elevated C reactive protein (54 mg/L; reference range 0.0–10.0 mg/L). The calprotectin level in faeces was determined and found to be elevated (1755 mg/L; reference range 0–50 mg/L), indicating an inflammatory process in gastrointestinal tract. Faecal molecular diagnostics were performed using a laboratory developed PCR for Giardia lamblia, Cryptosporidium species, Dientamoeba fragilis, Entamoeba histolytica, Blastocystis hominis, Salmonella species, Shigella species, Plesiomonas shigelloides, diarrheagenic Yersinia enterocolitica biotypes (Y. enterocolitica), diarrhoea associated thermophilic Campylobacter species and Shiga toxin genes. All these PCRs were found to be negative. A positron emission tomography (PET) was performed to localise the inflammation and evaluate the possibility of post-transplant lymphoma. The PET revealed diffuse increased fludeoxyglucose uptake in a large portion of the ascending colon. Therefore, an ileocolonoscopy was performed. Ileocolonoscopy confirmed severe inflammation of the terminal ileum with multiple large elevated lesions with ulceration on top (figure 1A). In the ileum, superficial erosions of the mucosa were also observed (figure 1B). The ileocecal valve was swollen, erythematous and showed several small ulcerations (figure 1C). At the transition of the ileum to the colon, further ulceration was observed (figure 1D). Biopsies were taken of the lesions. Histological evaluation revealed a dense infiltrate with CD3 and CD20 expressing cells, which is indicative for a mixture of T and B cells, respectively. There was no evidence for B cell clonality or lymphoma. Specific immunohistochemical stains for Epstein-Barr virus and cytomegalovirus were also negative. As an inflammatory origin was suspected, an interferon-gamma-release assay for tuberculosis, a faeces PCR for Tropheryma whipplei, ova and parasite examination and Yersinia species serology were performed. All tests came back negative except for the Yersinia species serology. This test showed the presence of both an IgA and an IgG response against Yersinia species, which is indicative for an ongoing Yersinia species infection. Because of a negative Y. enterocolitica PCR, it was postulated that the patient might be suffering from a Y. pseudotuberculosis infection. A faecal sample was cultured on Cefsulodin-Irgasan-Novobiocin (CIN) agar in order to confirm a Y. pseudotuberculosis infection. Growth after 3 days showed several different colony morphologies (figure 2A). A representative sample of all red colonies with distinctive morphology was analysed by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS). Y. pseudotuberculosis was found among the colonies and was subcultured (figure 2B). Subsequent antimicrobial susceptibility testing revealed no resistance against the tested antimicrobials (table 1).
Figure 1.
Endoscopic images made during ileocolonoscopy. Scope type: GIF-HQ190 (Olympus, Tokyo, Japan). (A) Image taken at the terminal ileum with multiple large elevated lesions with ulceration on top. The white arrows illustrate these lesions. (B) Image taken in the ileum. White arrows point towards the border of superficial mucosal ulceration with normal mucosa. (C) Image taken in the colon showing a swollen and erythematous ileocecal valve with small ulcerations. The colonic mucosa has an otherwise normal appearance. The white arrow points towards the ostium of the valve. (D) Image taken at the transition of the ileum to the colon. Several superficial mucosal ileum lesions can be observed, of which two examples have been denoted with a white arrow.
Figure 2.
Culture on Cefsulodin-Irgasan-Novobiocin (CIN) agar (Thermo Fisher Scientific Oxoid, Basingstoke, UK) incubated for 72 hours at 22°C under atmospheric conditions. (A) Faecal sample of the patient. Different colony morphologies are encircled: (1) pink to red colony of intermediate size; (2) small pink colony; (3) large pale mucoid colony with a pink centre; (4) white colony of intermediate size; (5) small pink colony with a red centre. (B) Subculture of Yersinia pseudotuberculosis isolated from the patient in question, which lacks the typical ‘Bull’s eye’ colony morphology. (C) Subculture of a Yersinia enterocolitica strain showing typical ‘Bull’s eye’ colony morphology for comparison.
Table 1.
Results of antimicrobial susceptibility testing
| Antimicrobial agent | Zone diameter (mm) | Interpretation |
| Ampicillin | 40 | Susceptible |
| Amoxicillin/clavulanic acid | 39 | Susceptible |
| Piperacillin/tazobactam | 44 | Susceptible |
| Ceftriaxone | 50 | Susceptible |
| Ceftazidime | 40 | Susceptible |
| Imipenem/cilastatin | 35 | Susceptible |
| Meropenem | 41 | Susceptible |
| Gentamicin | 28 | Susceptible |
| Tobramycin | 32 | Susceptible |
| Ciprofloxacin | 34 | Susceptible |
| Trimethoprim/sulfamethoxazole | 26 | Susceptible |
Antimicrobial susceptibility testing was performed using disk diffusion in compliance with the operating procedures of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).
Outcome and follow-up
The patient was treated with ciprofloxacin 500 mg orally twice daily for 14 days. After completion of treatment, all complaints resolved, laboratory findings improved and the faecal calprotectin decreased to 122 mg/L. Two months after treatment, a follow-up ileocolonoscopy showed healed ulcerations with scarring.
Discussion
Yersinia species are Gram-negative facultative anaerobe rod-shaped bacteria. They are psychrotrophic in nature, which allows them to grow at low temperature. The Yersiniaceae are part of the Enterobacterales and are divided in several different species, of which only three are important human pathogens: Yersinia pestis (Y. pestis), Y. enterocolitica and Y. pseudotuberculosis.1 Y. pestis is responsible for plague in humans. Y. enterocolitica is the most common Yersinia species to infect humans, but only certain biotypes are known human pathogens and will cause gastrointestinal disease with ileocolitis and mesenteric lymphadenitis, which is usually self-limiting.2 Non-diarrheagenic biotypes of Y. enterocolitica could however be important in immunocompromised patients. Y. pseudotuberculosis is the known causative agent of Far East scarlet-like fever or Izumi fever. This clinical presentation is associated with the presence of the superantigen called Y. pseudotuberculosis-derived mitogen (YpM).3 The symptoms that are associated with a Y. pseudotuberculosis infection are diarrhoea, fever, abdominal pain, fatigue, vomiting and exanthema.1 Y. pseudotuberculosis is also known to cause terminal ileitis, mesenteric lymphadenitis and granulomatous disease. In some cases, Y. pseudotuberculosis can cause septic arthritis, sepsis, thrombosis of mesenteric vessels, focal abscesses, intestinal necrosis, and haemorrhage. Literature suggests a more severe course of infection in immunocompromised patients.4–6 Y. pseudotuberculosis is a common commensal inhabitant of the gastrointestinal tract of a wide variety of animals. The main hosts are rodents, wild birds and domestic animals, especially pigs and ruminants.1 Y. pseudotuberculosis is also found to be widely spread in soil and water in the environment, where it can persist for a long period of time. The environment is contaminated via infected animal faeces.1 Human infection occurs via contaminated food or water. Outbreaks have been described in Finland, Canada, Japan and a large outbreak in New Zealand.7–12 The source of infection remains to be elucidated in most studies. However, consumption of raw vegetables has been identified as a source of transmission in a large epidemic in Finland.7 The psychrotrophic nature of Yersinia species explains why refrigeration does not prevent outbreaks with these pathogens and why Y. enterocolitica can grow in blood products.2 13 14 In our case, animal contact or a foodborne source could not be identified and therefore the transmission route remains unknown. Y. pseudotuberculosis infections have been reported globally; however, little is known about their incidence. Y. enterocolitica used to be highly prevalent in Belgium and linked to consumption of pork; however, due to changes in slaughtering techniques, food hygiene and eating habits, this has steadily decreased.15 16
In this case, the diagnostic process was delayed for several reasons. In clinical practice, it can be difficult to have a complete overview of all possibilities of laboratory testing and its pitfalls.17 In this particular case, the negative faecal PCR for Y. enterocolitica was wrongly interpreted as negative for all Yersinia species. In our hospital, we use a laboratory developed PCR, which uses specific primers for Y. enterocolitica biotypes known to cause infection and therefore Y. pseudotuberculosis and Y. enterocolitica biotypes that do not regularly cause gastroenteritis are not detected. Commercial PCRs for the specific clinical diagnosis of a Y. pseudotuberculosis infection are not widely available. However, a PCR-based detection technique specifically for Y. pseudotuberculosis would be feasible and could, for example, be based on the inv gene.18
Serological examination in this case revealed the possibility of a Yersinia species infection. This result in combination with a negative Y. enterocolitica PCR raised the suspicion of a Y. pseudotuberculosis or a non-diarrheagenic Y. enterocolitica biotype infection. To confirm this diagnosis, a faecal sample was send in for specific culture. The CIN agar showed growth of several different colonies (figure 2A). However, these were initially disregarded based on the morphology of the colonies. This occurred because our laboratory technicians were initially focused on the typical ‘Bull’s eye’ morphology of Y. enterocolitica (figure 2C). In this case, the positive Yersinia species serology prompted re-evaluation of the growth on the CIN agar and revealed growth of Y. pseudotuberculosis. The colony morphology of Y. pseudotuberculosis on CIN agar is red, but smaller and can lack the typical ‘Bull’s eye’ morphology compared with Y. enterocolitica.19 Therefore, a representative sample of all red colonies with distinctive morphology should be tested to ensure no Y. pseudotuberculosis infection is missed. In our case, colonies that were suspect for Y. pseudotuberculosis were identified using MALDI-TOF MS. A limitation of MALDI-TOF MS is that the evolutionary closely related Y. pseudotuberculosis can be misidentified as Y. pestis.20 Given the clinical presentation and the epidemiology of Y. pestis in the Netherlands, a Y. pestis infection was deemed highly unlikely and therefore further confirmation was deemed unnecessary.
The histopathology was suggestive for several different etiologies, which included conditions of an inflammatory, infectious or toxic nature. Although the serological test result indicated an ongoing Yersinia species infection, no specific histopathological characteristics exist that can confirm this diagnosis. Also, additional staining on biopsies to confirm a Yersinia species infection is not routinely performed in our centre and is not readily available.
Serological examination in our case was key to the diagnosis a Y. pseudotuberculosis infection, as it prompted us to perform specific culture for Yersinia species. Serology for Yersinia species was initiated because of the clinical suspicion of a Yersinia species ileitis based on the results of the PET and ileocolonoscopy. The serological diagnosis of Yersinia species can be made with several techniques such as with an ELISA or immunoblotting. However, this ELISA is prone to false-positive results due to cross-reactive antibodies, which can be seen with thyroid-stimulating immunoglobulin in patients with Graves’ disease and with several pathogens such as Bartonella henselae, Brucella species, Borrelia burgdorferi, Chlamydia pneumoniae, Francisella tularensis and Rickettsia rickettsii. Results should therefore either be confirmed by a second test or carefully interpreted in the context of the clinical presentation.21 The sensitivity of an ELISA for Yersinia species serology compared with immunoblotting was found to be 95% in one study.21 This was significantly higher when compared with complement fixation, which only had a sensitivity of 26% when compared with immunoblotting.21 Literature regarding the sensitivity of serological testing during acute Yersinia species infection is lacking, because serology is usually only performed to evaluate arthritis.
The serological diagnosis in this case was made using the SERION ELISA classic Yersinia IgA/IgG (Institut Virion/Serion, Würzburg, Germany) kit. Confirmation was performed via the recomLine Yersinia IgA/IgG 2.0 (Mikrogen Diagnostik, Neuried, Germany) immunoblots. IgM antibody levels against Yersinia species were not determined. The presence of both an IgA and IgG response is indicative for an ongoing infection with Yersinia species.
In our case, we decided to treat the patient with antibiotic therapy because of his immunocompromised status and the severity of the ulcerations seen during ileocolonoscopy. To the best of our knowledge, no clinical protocol for the treatment of Y. pseudotuberculosis has been established, which led us to a regimen used for Y. enterocolitica infections.22 This regimen consisted of a fluoroquinolone, for which susceptibility was confirmed via disk diffusion (table 1). In our case, ciprofloxacin 500 mg orally twice daily was prescribed for 14 days.
In conclusion, we presented a case of severe ulcerative ileitis due to a Y. pseudotuberculosis infection in an immunocompromised patient. This case illustrates that in the era of faecal PCR, we have to be aware of its limitations. Therefore, serology and faecal culture will remain important mainstays in the diagnostic workup, especially in the immunocompromised host. Close collaboration between treating physicians and microbiologists is necessary for accurate interpretation of diagnostic assays such as faecal PCR. This close collaboration will result in a better diagnostic process, in particular in regard of the immunocompromised host.
Learning points.
Faecal PCR is a highly sensitive and specific method for the detection of pathogens; however, pathogens for which the targets are not included will be missed. Therefore, a Yersinia enterocolitica PCR can be a misnomer because depending on the setup and targets of the PCR, it will usually only detect diarrheagenic biotypes of Y. enterocolitica and not all Y. enterocolitica biotypes. Non-diarrheagenic biotypes of Y. enterocolitica can be of importance in the immunocompromised patient.
Yersinia species serology and faecal culture remain important mainstays in the diagnosis of a Y. pseudotuberculosis infection but require specific expertise of both treating physicians and microbiologists.
Routine interpretation of the Y. pseudotuberculosis on Cefsulodin-Irgasan-Novobiocin (CIN) agar is challenging and therefore a representative sample of all distinct red colonies on CIN agar must be further analysed.
In immunocompromised patients with persistent gastrointestinal complaints, a multidisciplinary approach and aggressive diagnostics are needed to exclude infections such as Y. pseudotuberculosis.
Footnotes
Contributors: MH has written the draft of the manuscript. ML has advised and written the draft of the case description. JM has consulted in making the final version of the manuscript. JK has specifically advised on writing the parts about testing and AB had supervised the diagnostic workup from an internal medicine point of view.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fredriksson-Ahomaa M. Yersinia enterocolitica and Yersinia pseudotuberculosis. Infectious Disease: Foodborne Diseases 2007:79–113. [Google Scholar]
- 2.Galindo CL, Rosenzweig JA, Kirtley ML, et al. . Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in Human Yersiniosis. J Pathog 2011;2011:1–16. 10.4061/2011/182051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshino K, Ramamurthy T, Nair GB, et al. . Geographical heterogeneity between Far East and Europe in prevalence of ypm gene encoding the novel superantigen among Yersinia pseudotuberculosis strains. J Clin Microbiol 1995;33:3356–8. 10.1128/JCM.33.12.3356-3358.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaasch AJ, Dinter J, Goeser T, et al. . Yersinia pseudotuberculosis bloodstream infection and septic arthritis: case report and review of the literature. Infection 2012;40:185–90. 10.1007/s15010-011-0160-2 [DOI] [PubMed] [Google Scholar]
- 5.Koornhof HJ, Smego RA, Nicol M. Yersiniosis. II: the pathogenesis of Yersinia infections. Eur J Clin Microbiol Infect Dis 1999;18:87–112. 10.1007/s100960050237 [DOI] [PubMed] [Google Scholar]
- 6.Smego RA, Frean J, Koornhof HJ. Yersiniosis I: microbiological and clinicoepidemiological aspects of plague and non-plague Yersinia infections. Eur J Clin Microbiol Infect Dis 1999;18:1–15. 10.1007/s100960050219 [DOI] [PubMed] [Google Scholar]
- 7.Nuorti JP, Niskanen T, Hallanvuo S, et al. . A widespread outbreak of Yersinia pseudotuberculosis O:3 infection from iceberg lettuce. J Infect Dis 2004;189:766–74. 10.1086/381766 [DOI] [PubMed] [Google Scholar]
- 8.Nowgesic E, Fyfe M, Hockin J, et al. . Outbreak of Yersinia pseudotuberculosis in British Columbia--November 1998. Can Commun Dis Rep 1999;25:97–100. [PubMed] [Google Scholar]
- 9.Nakano T, Kawaguchi H, Nakao K, et al. . Two outbreaks of Yersinia pseudotuberculosis 5a infection in Japan. Scand J Infect Dis 1989;21:175–9. 10.3109/00365548909039966 [DOI] [PubMed] [Google Scholar]
- 10.Toma S. Human and nonhuman infections caused by Yersinia pseudotuberculosis in Canada from 1962 to 1985. J Clin Microbiol 1986;24:465–6. 10.1128/JCM.24.3.465-466.1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsubokura M, Otsuki K, Sato K, et al. . Special features of distribution of Yersinia pseudotuberculosis in Japan. J Clin Microbiol 1989;27:790–1. 10.1128/JCM.27.4.790-791.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williamson DA, Baines SL, Carter GP, et al. . Genomic insights into a sustained national outbreak of Yersinia pseudotuberculosis. Genome Biol Evol 2016;8:3806–14. 10.1093/gbe/evw285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palonen E, Lindström M, Korkeala H. Adaptation of enteropathogenic Yersinia to low growth temperature. Crit Rev Microbiol 2010;36:54–67. 10.3109/10408410903382581 [DOI] [PubMed] [Google Scholar]
- 14.Klein HG, Dodd RY, Ness PM, et al. . Current status of microbial contamination of blood components: summary of a conference. Transfusion 1997;37:95–101. 10.1046/j.1537-2995.1997.37197176958.x [DOI] [PubMed] [Google Scholar]
- 15.Tauxe RV, Vandepitte J, Wauters G, et al. . Yersinia enterocolitica infections and pork: the missing link. Lancet 1987;1:1129–32. 10.1016/S0140-6736(87)91683-7 [DOI] [PubMed] [Google Scholar]
- 16.Verhaegen J, Charlier J, Lemmens P, et al. . Surveillance of human Yersinia enterocolitica infections in Belgium: 1967-1996. Clin Infect Dis 1998;27:59–64. 10.1086/514636 [DOI] [PubMed] [Google Scholar]
- 17.Wang EW, Bhatti M, Cantu S, et al. . Diagnosis of Yersinia enterocolitica Infection in Cancer Patients With Diarrhea in the Era of Molecular Diagnostics for Gastrointestinal Infections. Open Forum Infect Dis 2019;6:1–5. 10.1093/ofid/ofz116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley DA, Tan CE, Donaldson A, et al. . The design, validation and clinical verification of an in-house qualitative PCR to detect Yersinia enterocolitica and Yersinia pseudotuberculosis in faeces. Pathology 2019;51:733–6. 10.1016/j.pathol.2019.08.001 [DOI] [PubMed] [Google Scholar]
- 19.Tan LK, Ooi PT, Carniel E, et al. . Evaluation of a modified cefsulodin-Irgasan-novobiocin agar for isolation of Yersinia spp. PLoS One 2014;9:e106329. 10.1371/journal.pone.0106329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rudrik JT, Soehnlen MK, Perry MJ, et al. . Safety and accuracy of matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of highly pathogenic organisms. J Clin Microbiol 2017;55:3513–29. 10.1128/JCM.01023-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rawlins ML, Gerstner C, Hill HR, et al. . Evaluation of a Western blot method for the detection of Yersinia antibodies: evidence of serological cross-reactivity between Yersinia outer membrane proteins and Borrelia burgdorferi. Clin Diagn Lab Immunol 2005;12:1269–74. 10.1128/CDLI.12.11.1269-1274.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mead PS. Yersinia species (including plague) : Bennett JE, Dolin R, Blaser MJ, Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 8th edn Philadelphia, 2015: 2607–18. [Google Scholar]