Abstract
Splenic abscess is a rare entity, however if unrecognised or left untreated, it is invariably fatal. We herein report a case of splenic abscess in a 40-year-old man presenting with fever, left-sided abdominal pain, altered sensorium and vomiting. On clinical examination, hepatosplenomegaly was noted and the ultrasound of the abdomen showed multiple hypoechoic regions in the upper pole of spleen, and the diagnosis of splenic abscess was made. The patient received antimicrobial therapy and underwent an open splenectomy with full recovery. Pus aspirated from the splenic abscess grew an unusual organism named Parabacteroides distasonis. In the literature, there are only a few recorded cases of P. distasonis causing splenic abscess. Through this case report, we would like to emphasise the pathogenic role of P. distasonis in causing clinical disease, as this organism is typically known to constitute a part of the normal flora.
Keywords: gastrointestinal system, infection (gastroenterology), gastrointestinal surgery
Background
Splenic abscesses constitute an infrequent occurrence, and are possibly life-threatening with a high mortality rate if left untreated.1 2 Various causative pathogens namely Escherichia coli, Proteus mirabilis, Streptococcus spp, Klebsiella pneumoniae, Staphylococcus aureus, Salmonella spp, Enterococcus spp and Pseudomonas spp have been reported in literature.3–6 In case of immunocompromised patients or those suffering from malignancy or HIV, the aetiological agent can also be of fungal origin.4 It can also be caused by different anaerobic organisms like Peptostreptococcus spp, Bacteroides spp, Fusobacterium spp, Clostridium spp and Propionibacterium acnes.5 7 Imaging studies such as ultrasound and CT scan help in the diagnosis and management of splenic abscesses. Treatment modalities are either percutaneous drainage by aspiration or drainage by retaining a catheter in the abscess cavity or splenectomy or open drainage of abscess by laparotomy.
Described here is a rare case of splenic abscess due to Parabacteroides distasonis in a 40-year-old male patient. He was successfully treated with the intervention of medical and surgical management.
Case presentation
A 40-year-old male labourer presented with a significant medical history of diabetes mellitus and hypertension of 2 years’ duration. He hailed from the South Indian region of Cuddalore in Tamil Nadu. He had developed high-grade fever, left-sided abdominal pain, altered sensorium and vomiting (six to eight times a day) for the past 1 week. The vomitus was bilious, non-projectile and non-blood stained. For his acute reports, he was admitted at a local hospital in Cuddalore and was treated symptomatically, the details of which he is not aware of. He also claimed to be a non-alcoholic and non-smoker. As his abdominal pain failed to subside, he sought treatment at the emergency services, from where he was admitted to the Department of Surgery at the Jawaharlal Institute of Postgraduate Medical Education and Research, Puducherry, India. He described the pain as being sharp in nature, localised to the left lower side of the chest with radiation to the left shoulder tip. He said that his pain worsened with deep breathing. He had not experienced any similar pain or trauma in the past.
On physical examination, his vital signs were as follows: a high blood pressure of 175/103 mm Hg, increased heart rate of 113 beats/min, febrile with temperature of 38.7°C, respiratory rate of 22/min and oxygen saturation of 98% at room air. He however had no signs of icterus, pallor, clubbing, lymphadenopathy or generalised oedema. His oral mucosa was dry. Heart sounds S1 and S2 were heard without any murmurs, rubs or gallops. Lungs were clear on auscultation with reduced air entry noted in the left basal region. His abdomen was soft and fullness was noted in left hypochondriac and epigastric region. The umbilicus was normal in position, and on palpation, tenderness and localised guarding was present in the left hypochondriac region. There was hepatomegaly and the spleen tip was palpable. There was no free fluid felt in the abdomen. Bowel sounds were normally heard.
The patient was diagnosed to have a splenic abscess and was started empirically on intravenous ceftriaxone 2 g 12th hourly and intravenous metronidazole 500 mg 8th hourly for 3 days. The abscess however, did not resolve in the given duration of the antibiotic therapy and the patient continued to have frequent fever spikes. Moreover, as he was a diabetic with a multiloculated abscess without any window for pigtail insertion by ultrasound, he was taken for surgical removal of the spleen. Open splenectomy was done and resulted in 500 mL blood loss.
Intraoperative findings also did not show any free fluid collection in the abdomen. Mild splenomegaly was present, with multiple abscess cavities reaching the splenic surface, and dense adhesions were present between the spleen, retroperitoneum and diaphragm. Anterior wall of the stomach towards the greater curvature side was adherent to the abscess in the superior pole of the spleen.
Investigations
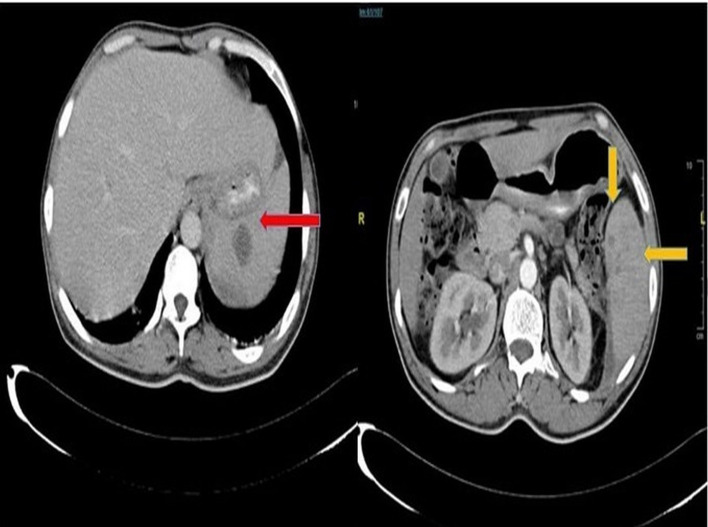
Laboratory examination of the blood showed only leucocytosis with white cell count of 21×109/L, while other routine haematological and biochemical investigations were normal. Ultrasonography of the abdomen showed hepatosplenomegaly—spleen size was 13.3 cm, mildly enlarged with rounded margins, multiple hypoechoic lesions were seen in the region of the upper pole, with the largest lesion measuring around 3.6×1.8 cm. The liver size was 17.7 cm, enlarged with normal echoes. Both the gall bladder and pancreas were normal. A contrast enhanced CT scan confirmed the findings of ultrasound (figure 1).
Figure 1.
Contrast enhanced CT scan suggestive of multiple hypoenhancing lesion across the spleen with the largest being 3.7×1.4 cm (red arrow) with no free fluid and possible gas formation.
From the patient, 9 mL of blood was taken from the peripheral line and was loaded into an automated blood culture bottle of the BACT/ALERT 3D system, which flagged a positive signal after 21.5 hours. From the culture bottle, direct gram stain was done which showed Gram negative rods. Further it was subcultured on to 5% sheep blood agar and MacConkey agar. On MacConkey agar it showed pale-coloured colonies which were identified by the MALDI-TOF MS (VITEK MS, biomerieux) as Acinetobacter baumannii—resistant to all first-line antibiotics namely amikacin, ciprofloxacin, ceftazidime, while it was sensitive to cefoperazone sulbactam, piperacillin–tazobactam and meropenem.
Under aseptic precautions, 5–7 mL of pus was drained and 2–3 mL was sent to the microbiology laboratory. Pus from the splenic abscess was sent for both aerobic and anaerobic culture. The bacterial growth was that of a facultative anaerobe, producing small, grey, alpha‐haemolytic colonies on 5 % sheep blood agar. The isolate was identified as Enterococcus faecalis with a negative catalase test reaction, and positive bile‐esculin and growth in 6.5 % salt broth tests. It was sensitive only to linezolid, teicoplanin and vancomycin, and resistant to ampicillin and tetracycline.
P. distasonis was isolated from the pus sent for anaerobic culture. Anaerobic atmosphere of 80% N2, 5%–10% H2 and 10% CO2 was created by an automated anaerobic culture system (ANOXOMAT Mark 11 CTS system, MART Microbiology, The Netherlands). Gram stain of the culture smear showed gram negative, non-spore forming, non-motile rod with parallel sides and blunt ends. On Brucella blood agar with hemin and vitamin K1, small, circular, convex grey moist, non-haemolytic colonies measuring 1–2 mm were seen. Colonies on Bacteroides bile esculin agar were 1–2 mm in diameter, grey to black, circular, slightly convex and smooth with entire margin and esculin was hydrolysed (figure 2). Phenotypic biochemical tests were employed for identification of P. distasonis. It was catalase test positive which differentiated it from the catalase negative Bacteroides merdae, B. caccae and B. cellulosilyticus. Using special potency discs, the P. distasonis isolate was found resistant to kanamycin 1000 µg, vancomycin 5 µg and colistin 10 µg. MALDI-TOF MS system identified the colonies of P. distasonis with a confidence value of 99.9%. Antibiotic susceptibility testing was done by the Kirby Bauers disc diffusion following the European Committee on Antimicrobial Susceptibility Testing recommendations by Nagy et al8 and Horn et al.9 P. distasonis had susceptible zone diameter for imipenem, chloramphenicol, cefoxitin, clindamycin and metronidazole. Metronidazole susceptibility testing was also done by epsilometer test (figure 3), which showed a susceptible minimum inhibitory concentration of 0.25 µg/mL as per the M11-A9 Clinical Laboratory Standards Institute guidelines.10
Figure 2.
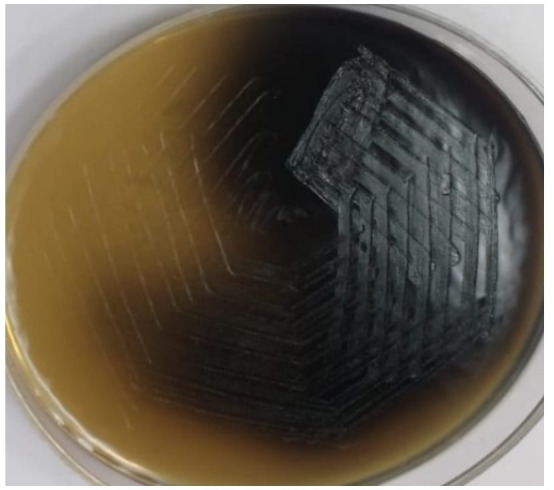
Bile esculin agar showing hydrolysis of esculin.
Figure 3.

Epsilometer test showing metronidazole susceptibility of Parabacteroides distasonis (minimum inhibitory concentration—0.25 μg/mL).
Treatment
The patient was postoperatively treated with intravenous piperacillin–tazobactum 4.5 g given 6th hourly and intravenous metronidazole 500 mg given 8th hourly. Based on the culture report, intravenous linezolid 600 mg 12th hourly was added and continued for 7 days. Two weeks following splenectomy, the patient was administered pneumococcal polysaccharide vaccine, Haemophilus influenzae type b and conjugate meningococcus C vaccine as practised in our hospital.
Outcome and follow-up
The postoperative course was uneventful and the patient exhibited a gradual improvement in clinical condition. The wound was clean and no surgical site infection was noted, and he was discharged.
Discussion
Splenic abscess is an uncommon presentation with incidence rate of 0.1%–0.7% in the autopsy series.6 The incidence rate of splenic abscess is increasing, owing to the growing figures of immunocompromised patients and extensive usage of diagnostic imaging modalities.11–13 It usually occurs in patients with neoplasia, immunodeficiency, trauma, metastatic infection, splenic infarct or diabetes.14 Splenic abscesses frequently get misdiagnosed as the signs and symptoms are always non-specific; nonetheless, with the advent of modern imaging techniques, their diagnosis has become easier. Sarr and Zuidema suggested that patients suffering from splenic abscess have a triad of fever, left upper quadrant pain and a tender mass.15
The most common organisms isolated from splenic abscess cases are particularly aerobic organisms namely the Streptococcal spp and E. coli.16 Llenas-Garcia et al stated that the aetiological agents causing splenic abscesses differed, based on the geographical location and the population studied, and found increased percentage of Mycobacterium tuberculosis in splenic abscesses.17
There exists in literature, a case of splenic abscess caused by P. distasonis in an 18-year-old boy with a history of sickle cell disease. This was reported by Jaffar et al in the year 2007, and P. distasonis was isolated from the splenic aspirate. The patient had undergone medical and surgical intervention and had full recovery.18
P. distasonis was termed after A. Distaso, a Romanian bacteriologist. The description of this organism was given by Eggerth and Gagnon (1933) and Holdeman et al (1977, 1984). Based on the ratio of major cellular fatty acids, the principal quinones (MK-9 and MK-10) and 16S rRNA gene sequence analysis, Bacteroides distasonis has been reclassified as P. distasonis.19 P. distasonis, B. goldsteinii and B. merdae though phenotypically similar to Bacteroides sensu stricto, they are phylogenetically different, and can be differentiated based on the menaquinones (MK10, MK11) present abundantly in the latter. Also, P. distasonis, B. goldsteinii and B. merdae are phylogenetically related to Tannerella forsythensis, but the ratio of fatty acids (anteiso-C15:0 to iso-C15:0) and the menaquinones composition of these three species are different from that of T. forsythensis.19
The pathogenesis of abscess formation by P. distasonis remains unclear. In contrast to B. fragilis, which induces abscess formation by capsular polysaccharides, for this non-capsulated P. distasonis, it has been hypothesised that other associated factors mediate abscess formation through a common mechanism that involves T-cell activation via the CD28-B7 pathway.20 The most probable mode of spread of the organism in this case could be from the gut via the bloodstream to the spleen.
This obligate anaerobe is usually considered a part of the beneficial commensal gut microorganism representing about 25% of the intestinal microbiota.21 This organism produces a complex substance such as bacteriocin which helps in the prevention of colonisation and invasion of the exogenous bacteria, thereby preventing certain infectious diseases.22 Although this microorganism acts as a normal commensal in the gut microbiota, it can also be responsible for infections with significant morbidity and mortality. There are few evidences in the literature that suggest P. distasonis contributes to inflammatory bowel disease.23
The MALDI-TOF MS is considered as an effective tool for the rapid identification of anaerobes. Studies, which have been done to evaluate the MALDI-TOF MS Biotyper (Microflex, Bruker Daltonik, Bremen, Germany), have shown few discordant results. This is attributable to the low numbers of related spectra in the database. The presence of related spectra in the database has led to misidentification of B. cellulosilyticus as P. distasonis with high scores leading to misreading of the interpretation.24
It is noteworthy that P. distasonis, though an important flora of lower gastrointestinal tract, can possibly be involved in the horizontal gene transfer resulting in the spread of resistance genes both among themselves and other genera. A study conducted by Boente et al found that all strains of P. distasonis were resistant to ampicillin. Cefoxitin, tetracycline and clindamycin resistance rates were high at 75%, 87.5% and 50%, respectively. The high frequency of tetracycline resistance has been attributed to the presence of the tetQ gene.25
A gold standard treatment or specific guidelines for treating splenic abscesses is not available.26 Splenic abscesses are managed either with an antibiotic therapy or splenectomy or percutaneous drainage. Though the different treatment modalities do not affect the mortality rates, the fatal outcomes are more increasingly associated with the patient’s primary underlying illness.27
Learning points.
Parabacteroides distasonis should be considered a potential pathogen with the ability of causing a splenic abscess.
Rapid identification of Parabacteroides up to the species level is facilitated by the MALDI-TOF MS and the clinical relevance of the isolate should be analysed.
The non-specific clinical presentation of the abscesses found in the spleen and their possible association with anaerobes and high mortality rates pose a dire need for early detection and treatment of the condition.
Acknowledgments
The authors fully acknowledge the contribution of the patient to the research.
Footnotes
Contributors: AG reviewed the literature, acquired and analysed laboratory data, and prepared the manuscript. RB defined the intellectual content, analysed laboratory data, edited and reviewed the manuscript. BS provided the clinical details, managed the patient, and edited and reviewed the manuscript. TPE provided the clinical details, managed the patient, and edited and reviewed the manuscript. RB, as the guarantor, accepts full responsibility for the work, had access to data and controlled the decision to publish.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Carbonell AM, Kercher KW, Matthews BD, et al. Laparoscopic splenectomy for splenic abscess. Surg Laparosc Endosc Percutan Tech 2004;14:289–91. 10.1097/00129689-200410000-00013 [DOI] [PubMed] [Google Scholar]
- 2.Llenas-García J, Fernández-Ruiz M, Caurcel L, et al. Splenic abscess: a review of 22 cases in a single institution. Eur J Intern Med 2009;20:537–9. 10.1016/j.ejim.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 3.Lee C-H, Leu H-S, Hu T-H, et al. Splenic abscess in southern Taiwan. J Microbiol Immunol Infect 2004;37:39–44. [PubMed] [Google Scholar]
- 4.Ferraioli G, Brunetti E, Gulizia R, et al. Management of splenic abscess: report on 16 cases from a single center. Int J Infect Dis 2009;13:524–30. 10.1016/j.ijid.2008.08.024 [DOI] [PubMed] [Google Scholar]
- 5.Brook I, Frazier EH. Microbiology of liver and spleen abscesses. J Med Microbiol 1998;47:1075–80. 10.1099/00222615-47-12-1075 [DOI] [PubMed] [Google Scholar]
- 6.Chang K-C, Chuah S-K, Changchien C-S, et al. Clinical characteristics and prognostic factors of splenic abscess: a review of 67 cases in a single medical center of Taiwan. World J Gastroenterol 2006;12:460–4. 10.3748/wjg.v12.i3.460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gangahar DM, Delany HM. Intrasplenic abscess: two case reports and review of the literature. Am Surg 1981;47:488–91. [PubMed] [Google Scholar]
- 8.Nagy E, Justesen US, Eitel Z, et al. Development of EUCAST disk diffusion method for susceptibility testing of the Bacteroides fragilis group isolates. Anaerobe 2015;31:65–71. 10.1016/j.anaerobe.2014.10.008 [DOI] [PubMed] [Google Scholar]
- 9.Horn R, Bourgault AM, Lamothe F. Disk diffusion susceptibility testing of the Bacteroides fragilis group. Antimicrob Agents Chemother 1987;31:1596–9. 10.1128/AAC.31.10.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wayne P. Clsi: methods for antimicrobial susceptibility testing of anaerobic bacteria: Approved standard CLSI document M11-A9, clinical and laboratory Standards Institute 2018.
- 11.de Bree E, Tsiftsis D, Christodoulakis M, et al. Splenic abscess: a diagnostic and therapeutic challenge. Acta Chir Belg 1998;98:199–202. 10.1080/00015458.1998.12098414 [DOI] [PubMed] [Google Scholar]
- 12.Ooi LL, Nambiar R, Rauff A, et al. Splenic abscess. Aust N Z J Surg 1992;62:780–4. 10.1111/j.1445-2197.1992.tb06917.x [DOI] [PubMed] [Google Scholar]
- 13.Smyrniotis V, Kehagias D, Voros D, et al. Splenic abscess. an old disease with new interest. Dig Surg 2000;17:354–7. 10.1159/000018878 [DOI] [PubMed] [Google Scholar]
- 14.Ng K-K, Lee T-Y, Wan Y-L, et al. Splenic abscess: diagnosis and management. Hepatogastroenterology 2002;49:567–71. [PubMed] [Google Scholar]
- 15.Sarr MG, Zuidema GD. Splenic abscess--presentation, diagnosis, and treatment. Surgery 1982;92:480–5. [PubMed] [Google Scholar]
- 16.Ooi LL, Leong SS. Splenic abscesses from 1987 to 1995. Am J Surg 1997;174:87–93. 10.1016/S0002-9610(97)00030-5 [DOI] [PubMed] [Google Scholar]
- 17.Llenas-García J, Fernández-Ruiz M, Caurcel L, et al. Splenic abscess: a review of 22 cases in a single institution. Eur J Intern Med 2009;20:537–9. 10.1016/j.ejim.2009.04.009 [DOI] [PubMed] [Google Scholar]
- 18.Al-Salem AH, Qaisaruddin S, Al Jam'a A, et al. Splenic abscess and sickle cell disease. Am J Hematol 1998;58:100–4. [DOI] [PubMed] [Google Scholar]
- 19.Sakamoto M, Benno Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. Int J Syst Evol Microbiol 2006;56:1599–605. 10.1099/ijs.0.64192-0 [DOI] [PubMed] [Google Scholar]
- 20.Tzianabos AO, Chandraker A, Kalka-Moll W, et al. Bacterial pathogens induce abscess formation by CD4(+) T-cell activation via the CD28-B7-2 costimulatory pathway. Infect Immun 2000;68:6650–5. 10.1128/IAI.68.12.6650-6655.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salyers AA. Bacteroides of the human lower intestinal tract. Annu Rev Microbiol 1984;38:293–313. 10.1146/annurev.mi.38.100184.001453 [DOI] [PubMed] [Google Scholar]
- 22.Nakano V, Ignacio A, Fernandes MR, et al. Intestinal Bacteroides and Parabacteroides species producing antagonistic substances. Microbiology 2006;1:61–4. [Google Scholar]
- 23.Yang F, Kumar A, Davenport KW, et al. Complete genome sequence of a Parabacteroides distasonis strain (CavFT hAR46) isolated from a gut Wall-Cavitating Microlesion in a patient with severe Crohn's disease. Microbiol Resour Announc 2019;8:e00585–19. 10.1128/MRA.00585-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coltella L, Mancinelli L, Onori M, et al. Advancement in the routine identification of anaerobic bacteria by MALDI-TOF mass spectrometry. Eur J Clin Microbiol Infect Dis 2013;32:1183–92. 10.1007/s10096-013-1865-1 [DOI] [PubMed] [Google Scholar]
- 25.Boente RF, Ferreira LQ, Falcão LS, et al. Detection of resistance genes and susceptibility patterns in Bacteroides and Parabacteroides strains. Anaerobe 2010;16:190–4. 10.1016/j.anaerobe.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 26.Thanos L, Dailiana T, Papaioannou G, et al. Percutaneous CT-guided drainage of splenic abscess. AJR Am J Roentgenol 2002;179:629–32. 10.2214/ajr.179.3.1790629 [DOI] [PubMed] [Google Scholar]
- 27.Paris S, Weiss SM, Ayers WH, et al. Splenic abscess. Am Surg 1994;60:358–61. [PubMed] [Google Scholar]