Key Points
Question
What is the household secondary attack rate for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)?
Findings
In this meta-analysis of 54 studies with 77 758 participants, the estimated overall household secondary attack rate was 16.6%, higher than observed secondary attack rates for SARS-CoV and Middle East respiratory syndrome coronavirus. Controlling for differences across studies, secondary attack rates were higher in households from symptomatic index cases than asymptomatic index cases, to adult contacts than to child contacts, to spouses than to other family contacts, and in households with 1 contact than households with 3 or more contacts.
Meaning
These findings suggest that households are and will continue to be important venues for transmission, even in areas where community transmission is reduced.
This systematic review and meta-analysis examines evidence for household transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), disaggregated by several covariates, and compares it with other coronaviruses
Abstract
Importance
Crowded indoor environments, such as households, are high-risk settings for the transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Objectives
To examine evidence for household transmission of SARS-CoV-2, disaggregated by several covariates, and to compare it with other coronaviruses.
Data Source
PubMed, searched through October 19, 2020. Search terms included SARS-CoV-2 or COVID-19 with secondary attack rate, household, close contacts, contact transmission, contact attack rate, or family transmission.
Study Selection
All articles with original data for estimating household secondary attack rate were included. Case reports focusing on individual households and studies of close contacts that did not report secondary attack rates for household members were excluded.
Data Extraction and Synthesis
Meta-analyses were done using a restricted maximum-likelihood estimator model to yield a point estimate and 95% CI for secondary attack rate for each subgroup analyzed, with a random effect for each study. To make comparisons across exposure types, study was treated as a random effect, and exposure type was a fixed moderator. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline was followed.
Main Outcomes and Measures
Secondary attack rate for SARS-CoV-2, disaggregated by covariates (ie, household or family contact, index case symptom status, adult or child contacts, contact sex, relationship to index case, adult or child index cases, index case sex, number of contacts in household) and for other coronaviruses.
Results
A total of 54 relevant studies with 77 758 participants reporting household secondary transmission were identified. Estimated household secondary attack rate was 16.6% (95% CI, 14.0%-19.3%), higher than secondary attack rates for SARS-CoV (7.5%; 95% CI, 4.8%-10.7%) and MERS-CoV (4.7%; 95% CI, 0.9%-10.7%). Household secondary attack rates were increased from symptomatic index cases (18.0%; 95% CI, 14.2%-22.1%) than from asymptomatic index cases (0.7%; 95% CI, 0%-4.9%), to adult contacts (28.3%; 95% CI, 20.2%-37.1%) than to child contacts (16.8%; 95% CI, 12.3%-21.7%), to spouses (37.8%; 95% CI, 25.8%-50.5%) than to other family contacts (17.8%; 95% CI, 11.7%-24.8%), and in households with 1 contact (41.5%; 95% CI, 31.7%-51.7%) than in households with 3 or more contacts (22.8%; 95% CI, 13.6%-33.5%).
Conclusions and Relevance
The findings of this study suggest that given that individuals with suspected or confirmed infections are being referred to isolate at home, households will continue to be a significant venue for transmission of SARS-CoV-2.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is spread via direct or indirect contact with infected people via infected respiratory droplets or saliva, fomites, or aerosols.1,2 Crowded indoor environments with sustained close contact and conversations, such as households, are a particularly high-risk setting.3
The World Health Organization China Joint Mission reported human-to-human transmission in China largely occurred within families, accounting for 78% to 85% of clusters in Guangdong and Sichuan provinces.4 Stay-at-home orders reduced human mobility by 35% to 63% in the United States,5 63% in the United Kingdom,6 and 54% in Wuhan,7 relative to normal conditions, which concomitantly increased time at home. Modeling studies demonstrated that household transmission had a greater relative contribution to the basic reproductive number after social distancing (30%-55%) than before social distancing (5%-35%).8 While current US Centers for Disease Control and Prevention recommendations are to maintain 6 feet of distance from a sick household member, this may be difficult to achieve in practice and not be fully effective.9
The household secondary attack rate characterizes virus transmissibility. Studies can collect detailed data on type, timing, and duration of contacts and identify risk factors associated with infectiousness of index cases and susceptibility of contacts. Our objective was to estimate the secondary attack rate of SARS-CoV-2 in households and determine factors that modify this parameter. We also estimated the proportion of households with index cases that had any secondary transmission. Furthermore, we compared the SARS-CoV-2 household secondary attack rate with that of other severe viruses and with that to close contacts for studies that reported the secondary attack rate for both close and household contacts.
Methods
Definitions
We estimated the transmissibility of SARS-CoV-2 within the household or family by the empirical secondary attack rate by dividing the number of new infections among contacts by the total number of contacts. Household contacts include anyone living in the same residence as the index case. Family contacts include the family members of index cases, including individuals who live outside the index case’s household. Close contact definitions varied by study and included physical proximity to an index case, exceeding a minimum contact time, and/or not wearing effective protection around index cases before the index case was tested.
Search Strategy
Following Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline, we searched PubMed using terms including SARS-CoV-2 or COVID-19 with secondary attack rate, household, close contacts, contact transmission, contact attack rate, or family transmission (eTable 1 in the Supplement) with no restrictions on language, study design, time, or place of publication. The last search was conducted October 19, 2020.
Eligibility Criteria
Eligibility criteria are described in eAppendix 1 in the Supplement. All articles with original data for estimating household secondary attack rate were included. Case reports focusing on individual households and studies of close contacts that did not report secondary attack rates for household members were excluded.
Data Extraction
One of us (Z.J.M.) extracted data from each study. Details appear in eAppendix 2 in the Supplement.
Evaluation of Study Quality and Risk of Bias
To assess the methodological quality and risk of bias of included studies of SARS-CoV-2, we used the same modified version of the Newcastle-Ottawa quality assessment scale for observational studies used by Fung et al.10,11 Studies received as many as 9 points based on participant selection (4 points), study comparability (1 point), and outcome of interest (4 points). Studies were classified as having high (≤3 points), moderate (4-6 points), and low (≥7 points) risk of bias. One of us (Z.J.M.) evaluated the study quality and assigned the quality grades.
Statistical Analysis
Meta-analyses were done using a restricted maximum-likelihood estimator model to yield Freeman-Tukey double arcsine–transformed point estimates and 95% CI for secondary attack rate for each subgroup analyzed, with a random effect for each study.12 For comparisons across covariates (ie, household or family, index case symptom status, adult or child contacts, contact sex, relationship to index case, adult or child index cases, index case sex, number of household contacts, study location, universal or symptomatic testing, dates of study) and comparisons with close contacts and other viruses, study was treated as a random effect, and the covariate was a fixed moderator. Variables had to have been collected in at least 3 studies to be included in meta-analyses. The Cochran Q test and I2 statistic are reported as measures of heterogeneity. I2 values of 25%, 50%, and 75% indicated low, moderate, and high heterogeneity, respectively.13 Stastistical significance was set at a 2-tailed α = .05. All analyses were done in R version 4.0.2 using the package metafor (R Project for Statistical Computing).14,15
When at least 10 studies were available, we used funnel plots, Begg correlation, and Egger test to evaluate publication bias, with significance set at P < .10.16,17 If we detected publication bias, we used the Duval and Tweedie trim-and-fill approach for adjustment.18
Results
We identified 54 relevant published studies that reported household secondary transmission, with 77 758 participants (eTable 1 in the Supplement).19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72 A total of 16 of 54 studies (29.6%) were at high risk of bias, 27 (50.0%) were moderate, and 11 (20.4%) were low (eTable 2 in the Supplement). Lower quality was attributed to studies with 1 or fewer test per contact (35 studies [64.8%]), small sample sizes (31 [57.4%]), and secondary attack rate not disaggregated by covariates (28 [51.9%]).
A description of index case identification period and methods and symptom status is provided in eTable 3 in the Supplement. Most studies did not describe how co–primary index cases were handled or whether secondary infections could have been acquired from outside the household, both of which can inflate the empirical secondary attack rate. Testing and monitoring strategies varied between studies, often reflecting variations in local testing guidelines implemented as part of contact tracing (eTable 4 and eAppendix 3 in the Supplement).
Figure 1 summarizes secondary attack rates for 44 studies19,20,21,22,23,24,25,26,28,29,30,32,33,34,35,36,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,57,59,61,62,63,65,66,67,69,70 of household contacts and 10 of family contacts.26,31,37,45,58,60,65,68,71,72 Estimated mean secondary attack rate for household contacts was 16.4% (95% CI, 13.4%-19.6%) and family contacts was 17.4% (95% CI, 12.7%-22.5%). One study40 restricted index cases to children (age <18 years), resulting in a substantially lower secondary attack rate of 0.5%. Excluding this outlier, the combined secondary attack rate for household and family contacts was 17.1% (95%, 14.6%-19.7%). Secondary attack rates for household and family contacts were more than 3 times higher than for close contacts (4.8%; 95% CI, 3.4%-6.5%; P < .001) (eFigure 2 in the Supplement). Significant heterogeneity was found among studies of household (I2 = 96.9%; P < .001), family (I2 = 93.0%; P < .001), and close (I2 = 97.0%; P < .001) contacts. No significant publication bias was observed for studies of household, family, or close contacts (eFigure 3 in the Supplement). Secondary attack rates were not significantly different when restricting to 38 studies19,20,22,23,26,27,28,29,30,31,34,35,36,37,38,39,40,42,44,45,46,47,48,49,50,51,54,55,56,57,60,62,63,65,67,68,69,72 with low or moderate risk of bias (15.6%; 95%, 12.8%-18.5%) (eFigure 4 in the Supplement). There were no significant differences in secondary attack rates between 21 studies in China22,27,31,36,37,39,45,46,48,58,61,62,63,64,65,66,67,68,70,71,72 and 33 studies from other countries19,20,21,23,24,25,26,28,29,30,32,33,34,35,38,40,41,42,43,44,47,49,50,51,52,53,54,55,56,57,59,60,69 (eFigure 5 in the Supplement), 18 studies that tested symptomatic contacts19,20,21,24,25,28,29,33,34,41,47,50,53,56,58,59,61,64 and 33 studies that reported testing all contacts22,23,26,27,30,31,35,36,37,38,39,40,42,43,44,45,46,48,49,51,52,54,55,57,60,63,65,66,67,69,70,71,72 (eFigure 6 in the Supplement), and 16 early studies22,23,25,31,37,39,45,58,61,63,64,65,66,68,71,72 (January-February) and 20 later studies19,24,26,29,30,32,33,34,35,38,42,44,50,53,54,55,56,59,60,69 (March-July) (eFigure 7 in the Supplement).
Figure 1. Secondary Attack Rates (SAR) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Household Contacts and Family Contacts.
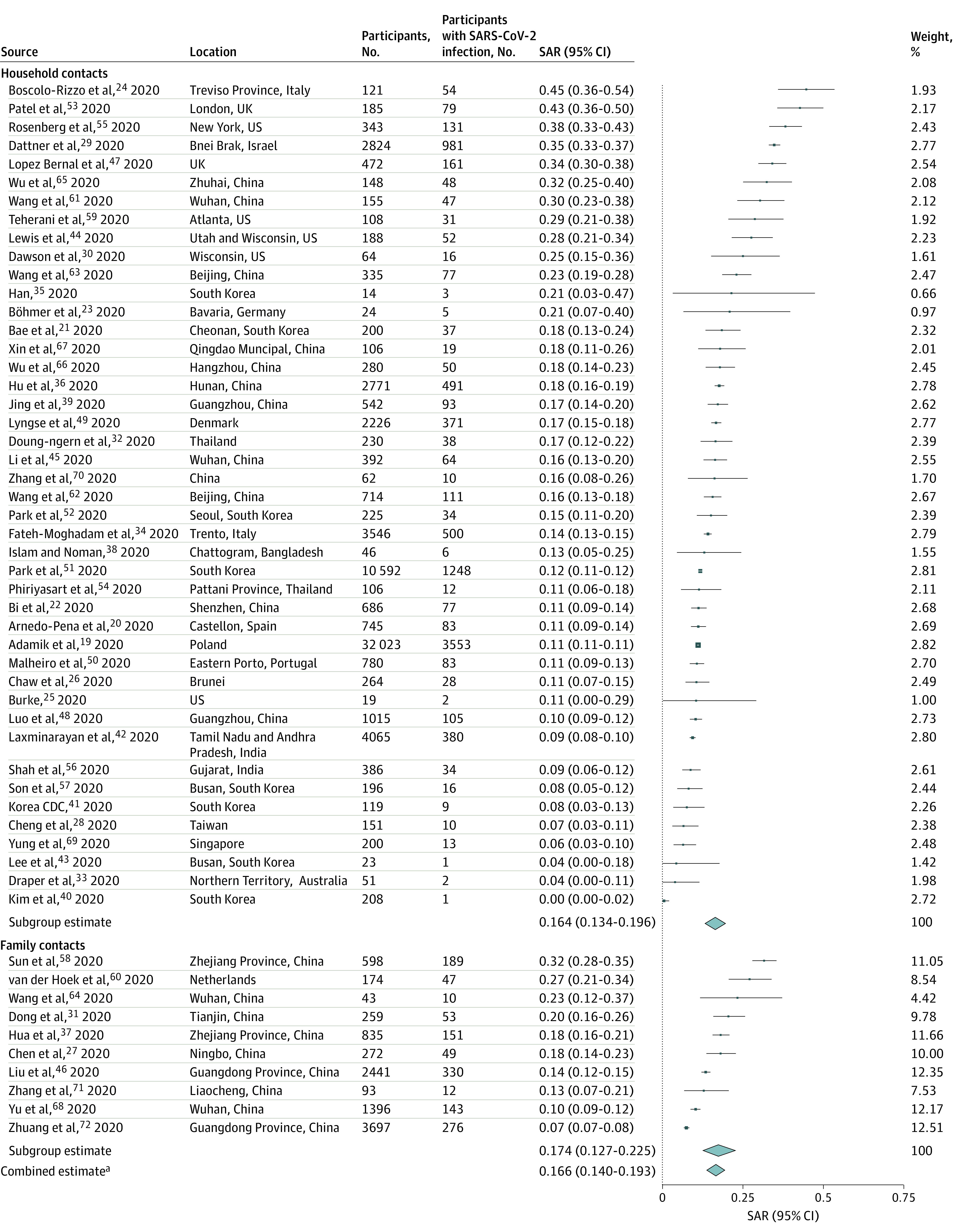
Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs. CDC indicates Centers for Disease Control and Prevention.
aWeights for the combined estimate are available in eTable 8 in the Supplement.
To study the transmissibility of asymptomatic SARS-CoV-2 index cases, eFigure 8 in the Supplement summarizes 27 studies19,20,21,23,24,25,26,30,32,33,34,44,45,47,50,52,53,54,56,59,60,61,63,64,68,69,72 reporting household secondary attack rates from symptomatic index cases and 4 studies26,43,44,52 from asymptomatic or presymptomatic index cases. Estimated mean household secondary attack rate from symptomatic index cases (18.0%; 95% CI, 14.2%-22.1%) was significantly higher than from asymptomatic or presymptomatic index cases (0.7%; 95% CI, 0%-4.9%; P < .001), although there were few studies in the latter group. These findings are consistent with other household studies28,70 reporting asymptomatic index cases as having limited role in household transmission.
There is evidence for clustering of SARS-CoV-2 infections within households, with some households having many secondary infections while many others have none.73,74,75 For example, 1 study55 reported that 26 of 103 (25.2%) households had all members test positive. This is consistent with observation of overdispersion in the number of secondary cases per index case across a range of settings.3 While most studies reported only the average number of secondary infections per index case, some also reported transmission by household.44,55,56,63,65,69 Figure 2 summarizes the proportion of households with any secondary transmission. Using an empirical analysis based on secondary attack rates and mean number of contacts per household, we found the proportion of households with any secondary transmission was lower than expected in a setting with no clustering (eg, most transmission is not characterized by a minority of infected individuals) (eTable 5 in the Supplement). Ideally, future studies will assess this formally by fitting a β binomial to quantify overdispersion in the full data.
Figure 2. Mean Number of Contacts per Household, Secondary Attack Rate (SAR) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2), and Proportion of Households Reporting Any Secondary Transmission From Index Cases.
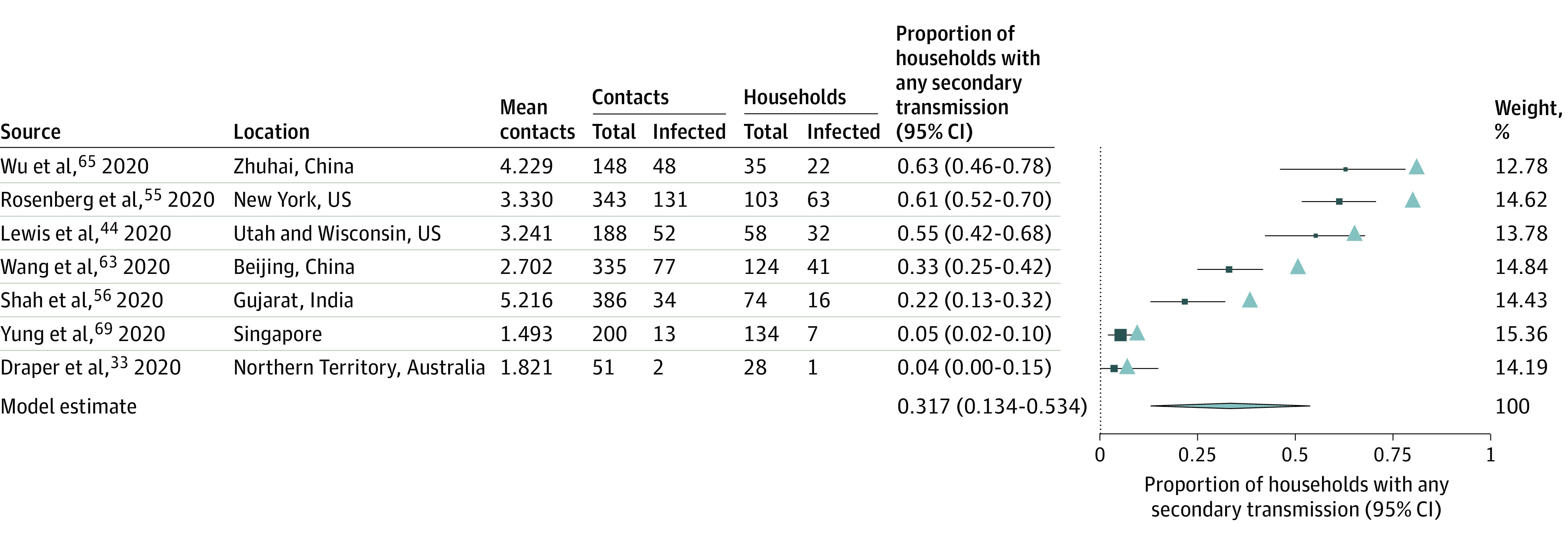
The expected proportion of households with any secondary transmission (represented by the triangles) was calculated as proportion with at least 1 secondary infection in a household = 1 − (1 −SAR)n, where n is the mean number of contacts for that study (eTable 5 in the Supplement). Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs.
A number of studies examined factors associated with susceptibility of household contacts to infection (eTable 6 in the Supplement). Age was the most examined covariate, with most studies20,29,36,37,38,39,45,46,48,49,55,63,65,68 reporting lower secondary transmission of SARS-CoV-2 to child contacts than adult contacts. In 5 studies,20,36,39,48,49 individuals older than 60 years were most susceptible to SARS-CoV-2 infection. Contact age was not associated with susceptibility in 9 studies,26,28,32,44,47,58,66,67,70 although these were typically less powered to detect a difference. Figure 3 summarizes 15 studies22,26,29,37,39,42,44,45,47,49,55,59,60,63,65 reporting separate secondary attack rates to children and adult contacts. The estimated mean household secondary attack rate was significantly higher to adult contacts (28.3%; 95% CI, 20.2%-37.1%) than to child contacts (16.8%; 95% CI, 12.3%-21.7%; P < .001). Significant heterogeneity was found among studies of adult (I2 = 96.8%; P < .001) and child contacts (I2 = 78.9%; P < .001). Begg (P = .03) and Egger (P = .03) tests were statistically significant for studies of adult but not child contacts (eFigure 9 in the Supplement). One study of adults63 had a high secondary attack rate in the forest plot. Excluding this study improved the funnel plot symmetry and resulted in a secondary attack rate to adult contacts of 26.3% (95% CI, 19.3%-33.2%).
Figure 3. Secondary Attack Rates (SAR) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Adult (≥18 Years) and Child (<18 Years) Household and Family Contacts.
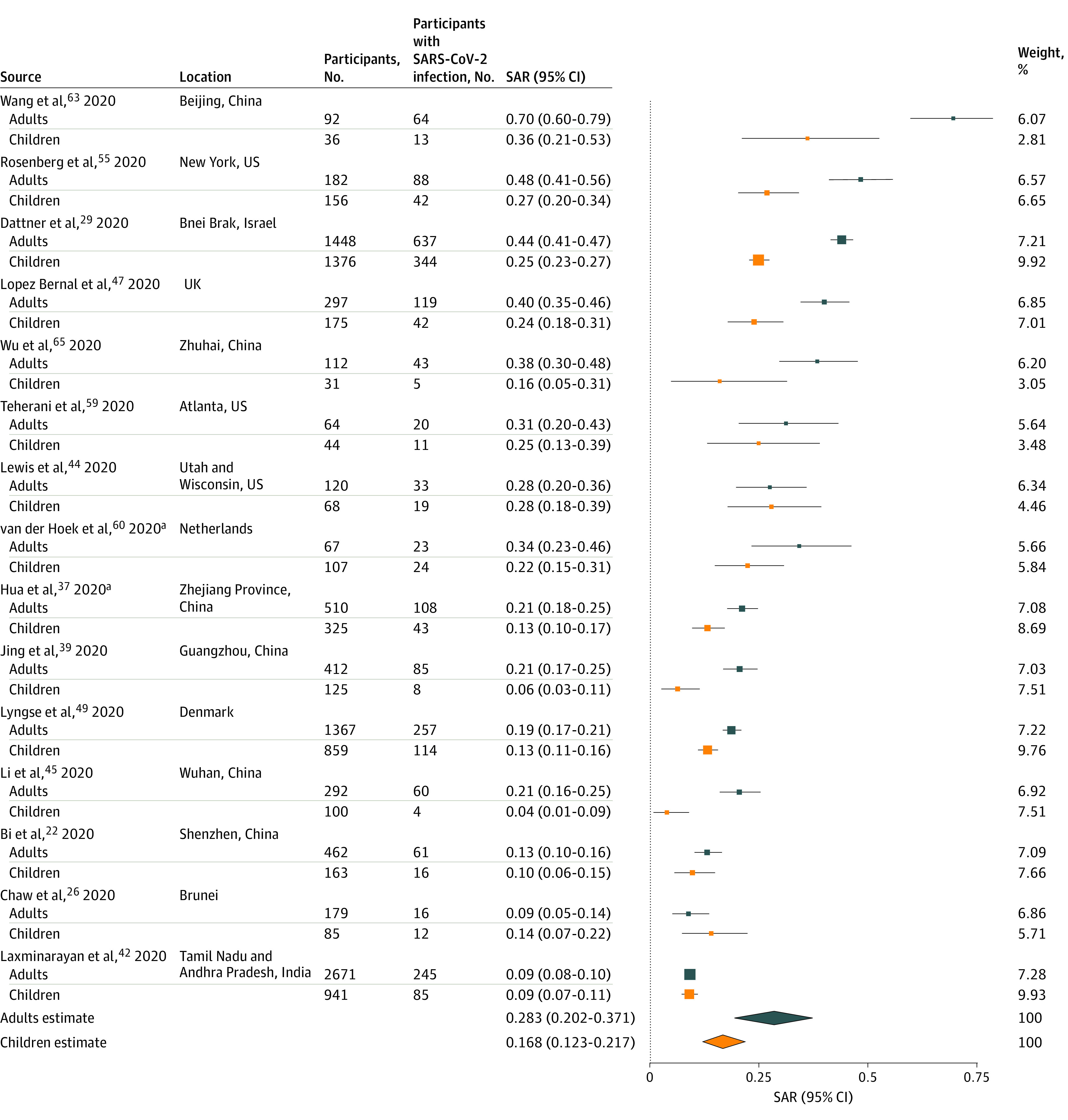
Point sizes are an inverse function of the precision of the estimates and bars correspond to 95% CIs.
aStudy of family contacts.
The second most examined factor was sex of exposed contacts, which was not associated with susceptibility for most studies20,22,26,32,36,39,44,45,47,48,49,58,65,66,67,70 except 3.38,46,68 eFigure 10 in the Supplement summarizes results from 11 studies20,39,42,44,45,47,49,58,65,67,69 reporting household secondary attack rates by contact sex. Estimated mean household secondary attack rate to female contacts (20.7%; 95% CI, 15.0%-26.9%) was not significantly different than to male contacts (17.7%; 95% CI, 12.4%-23.8%). Significant heterogeneity was found among studies of female contacts (I2 = 87.4%; P < .001) and male contacts (I2 = 87.7%; P < .001). Moderate asymmetry was observed in the funnel plots, which was significant for studies of female contacts from Egger test (P = .07) but not male contacts (eFigure 11 in the Supplement). However, imputation of an adjusted effect size using the trim-and-fill method did not significantly change the secondary attack rate to female contacts (19.7%; 95% CI, 13.9%-25.6%).
Spouse relationship to index case was associated with secondary infection in 4 studies26,45,46,58 of 6 in which this was examined.65,67 Infection risk was highest for spouses, followed by nonspouse family members and other relatives, which were all higher than other contacts.46 Figure 4 summarizes results from 7 studies26,44,45,46,58,65,67 reporting household secondary attack rates by relationship. Estimated mean household secondary attack rate to spouses (37.8%; 95% CI, 25.8%-50.5%) was significantly higher than to other contacts (17.8%; 95% CI, 11.7%-24.8%). Significant heterogeneity was found among studies of spouses (I2 = 78.6%; P < .001) and other relationships (I2 = 83.5%; P < .001).
Figure 4. Secondary Attack Rates (SAR) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Household and Family Contacts by Relationship to Index Case.
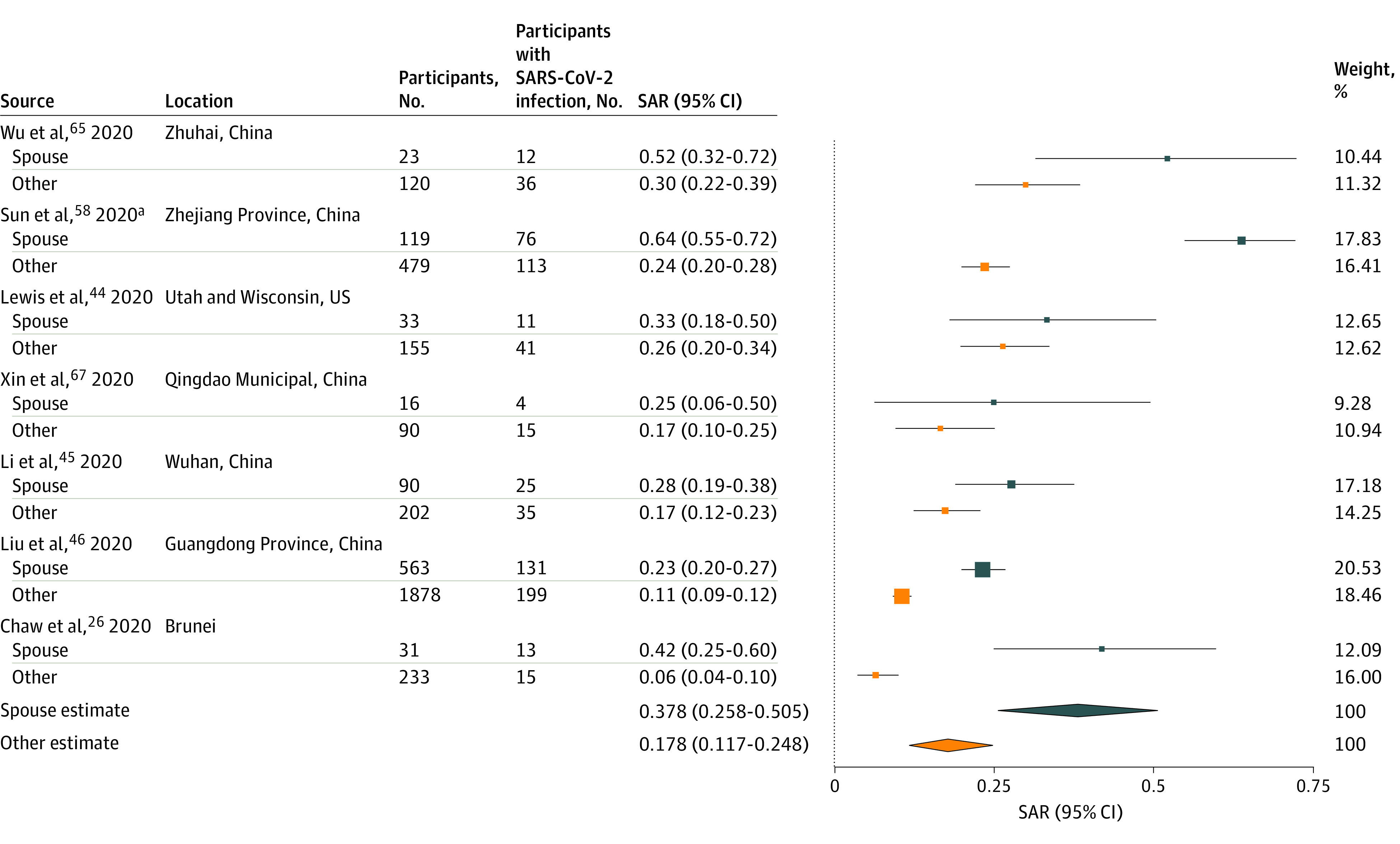
Point sizes are an inverse function of the precision of the estimates and bars correspond to 95% CIs.
aStudy of family contacts.
Several studies examined factors associated with infectiousness of index cases. Older index case age was associated with increased secondary infections in 3 studies20,47,67 of 9 in which this was examined.22,36,39,44,63,65 eFigure 12 in the Supplement summarizes results from 3 studies42,44,51 reporting household secondary attack rates by index case age. Estimated mean household secondary attack rate from adults (15.2%; 95% CI, 6.2%-27.4%) was not significantly different than that from children (7.9%; 95% CI, 1.7%-16.8%). Index case sex was associated with transmission in 3 studies42,44,67 of 9 in which this was examined.20,36,45,47,63,65 eFigure 13 in the Supplement summarizes results from 7 studies20,42,44,45,65,67,69 reporting household secondary attack rates by index case sex. Estimated mean household secondary attack rate from female contacts (16.6%; 95% CI, 11.2%-22.8%) was not significantly different than from male contacts (16.4%; 95% CI, 9.0%-25.5%).
Critically severe index case symptoms was associated with higher infectiousness in 6 studies20,38,46,47,48,67 of 9 in which this was examined.44,63,70 Index case cough was associated with infectivity in 2 studies 20,65 of 8 in which this was examined45,46,47,48,63,67 (eAppendix 4 in the Supplement).
Contact frequency with the index case was associated with higher odds of infection, specifically at least 5 contacts during 2 days before the index case was confirmed,70 at least 4 contacts and 1 to 3 contacts,63 or frequent contact within 1 meter.22,67,68 Smaller households were associated with transmission in 4 studies20,39,47,49 of 7 in which this was examined.55,63,65 Figure 5 summarizes results from 6 studies20,47,49,55,61,65 reporting household secondary attack rates by number of contacts in the household. Estimated mean household secondary attack rate for households with 1 contact (41.5%; 95% CI, 31.7%-51.7%) was significantly higher than households with at least 3 contacts (22.8%; 95% CI, 13.6%-33.5%; P < .001) but not different than households with 2 contacts (38.6%; 95% CI, 17.9%-61.6%). There was significant heterogeneity in secondary attack rates between studies with 1 contact (I2 = 52.9%; P = .049), 2 contacts (I2 = 93.6%; P < .001), or 3 or more contacts (I2 = 91.6%; P < .001). Information was not available on household crowding (eg, number of people per room).
Figure 5. Secondary Attack Rates (SAR) of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by the Number of People Living in the Same Household as the Index Case.
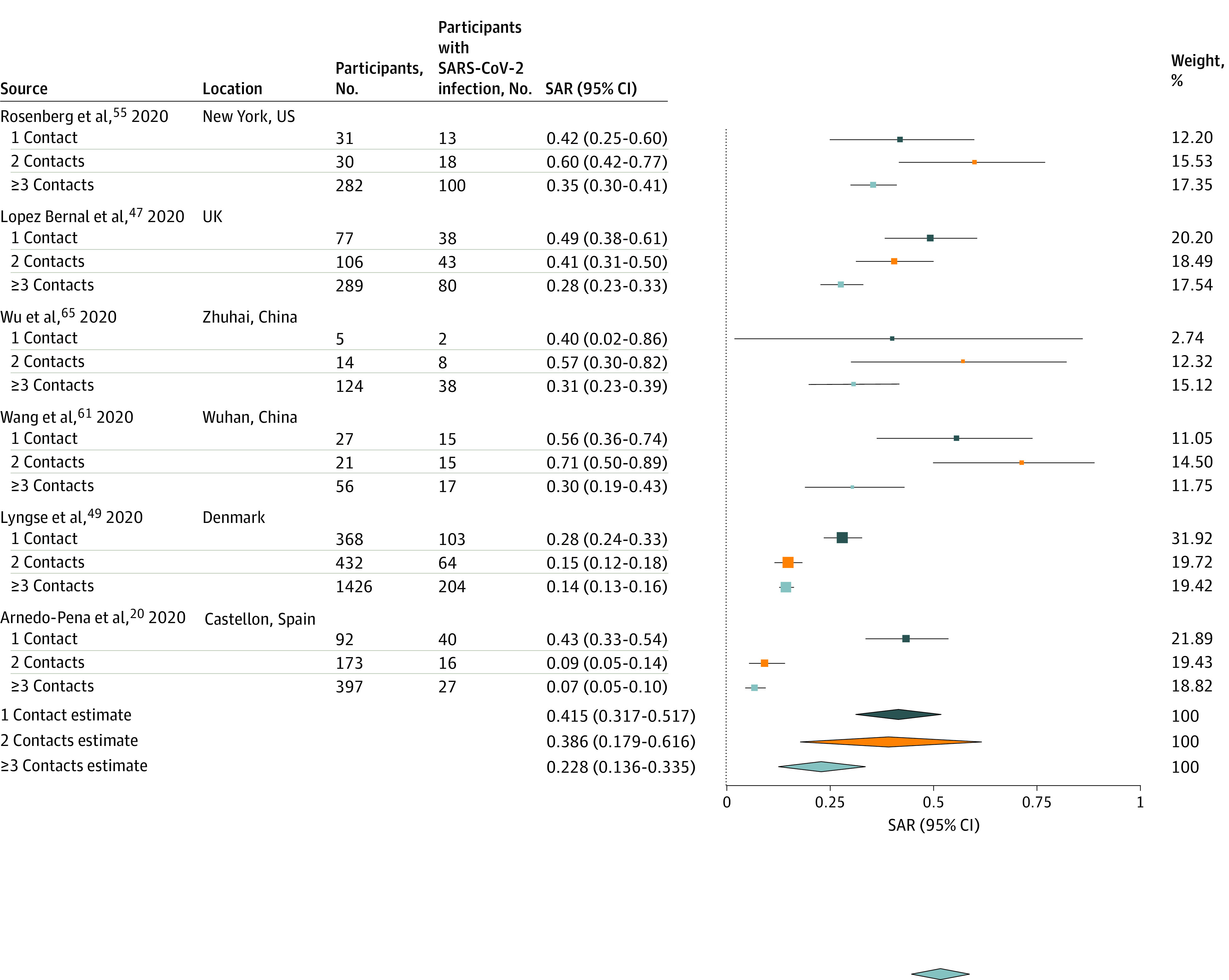
Point sizes are an inverse function of the precision of the estimates, and bars correspond to 95% CIs.
eFigure 14 in the Supplement summarizes 7 studies76,77,78,79,80,81,82 reporting household secondary attack rates for SARS-CoV, and 7 studies83,84,85,86,87,88,89 for Middle East respiratory syndrome coronavirus (MERS-CoV). Estimated mean household secondary attack rate was 7.5% (95% CI, 4.8%-10.7%) for SARS-CoV and 4.7% (95% CI, 0.9%-10.7%) for MERS-CoV (eTable 7 in the Supplement), both lower than the household secondary attack rate of 16.6% for SARS-CoV-2 in this study (P < .001). The SARS-CoV-2 secondary attack rate was also higher than secondary attack rates reported for HCoV-NL63 (0-12.6%), HCoV-OC43 (10.6-13.2%), HCoV-229E (7.2-14.9%), and HCoV-HKU1 (8.6%).90,91,92 Household secondary attack rates for SARS-CoV-2 were within the mid-range of household secondary attack rates reported for influenza, which ranged from 1% to 38% based on polymerase chain reaction–confirmed infection.93
Discussion
We synthesized the available evidence on household studies of SARS-CoV-2. The combined household and family secondary attack rate was 16.6% (95% CI, 14.0%-19.3%), although with significant heterogeneity between studies. This point estimate is higher than previously observed secondary attack rates for SARS-CoV and MERS-CoV. Households are favorable environments for transmission. They are what are known as 3Cs environments, as they are closed spaces, where family members may crowd and be in close contact with conversation.94 There may be reduced use of personal protective equipment relative to other settings.
That secondary attack rates were not significantly different between household and family contacts may indicate that most family contacts are in the same household as index cases. Household and family contacts are at higher risk than other types of close contacts, and risks are not equal within households. Spouses were at higher risk than other family contacts, which may explain why the secondary attack rate was higher in households with 1 vs 3 or greater contacts. Spouse relationship to the index case was also a significant risk factor observed in studies of SARS-CoV and H1N1.82,95 This may reflect intimacy, sleeping in the same room, or longer or more direct exposure to index cases. Further investigation is required to determine whether sexual contact is a transmission route. Although not directly assessed, household crowding (eg, number of people per room) may be more important for SARS-CoV-2 transmission than the total number of people per household, as has been demonstrated for influenza.96,97,98
The finding that secondary attack rates were higher to adult contacts than to child contacts is consistent with empirical and modeling studies.99,100 Lower infection rates in children may be attributed to asymptomatic or mild disease, reduced susceptibility from cross-immunity from other coronaviruses,101 and low case ascertainment,102 but the difference persisted in studies in which all contacts were tested regardless of symptoms. Higher transmission rates to adults may be influenced by spousal transmission. Given the increased risk to spousal contacts, future studies might compare child contacts and nonspouse adult contacts to ascertain whether this difference persists. Limited data suggest children have not played a substantive role in household transmission of SARS-CoV-2.40,103,104,105 However, a study in South Korea of 10 592 household contacts noted relatively high transmission from index cases who were aged 10 to 19 years.51 Although children seem to be at reduced risk for symptomatic disease, it is still unclear whether they shed virus similarly to adults.106
We did not find associations between household contact or index case sex and secondary transmission. The World Health Organization reports roughly even distribution of SARS-CoV-2 infections between women and men worldwide, with higher mortality in men.107
We found significantly higher secondary attack rates from symptomatic index cases than asymptomatic or presymptomatic index cases, although less data were available on the latter. The lack of substantial transmission from observed asymptomatic index cases is notable. However, presymptomatic transmission does occur, with some studies reporting the timing of peak infectiousness at approximately the period of symptom onset.108,109 In countries where infected individuals were isolated outside the home, this could further alter the timing of secondary infections by limiting contacts after illness onset.110
Household secondary attack rates were higher for SARS-CoV-2 than SARS-CoV and MERS-CoV, which may be attributed to structural differences in spike proteins,111 higher basic reproductive rates,112 and higher viral loads in the nose and throat at the time of symptom onset.113 Symptoms associated with MERS-CoV and SARS-CoV often require hospitalization, which increases nosocomial transmission, whereas less severe symptoms of SARS-CoV-2 facilitate community transmission.113 Similarly, presymptomatic transmission was not observed for MERS-CoV or SARS-CoV.114,115
Limitations
Our study had several limitations. The most notable is the large amount of unexplained heterogeneity across studies. This is likely attributable to variability in study definitions of index cases and household contacts, frequency and type of testing, sociodemographic factors, household characteristics (eg, density, air ventilation), and local policies (eg, centralized isolation). Rates of community transmission also varied across locations. Given that studies cannot always rule out infections from outside of the home (eg, nonhousehold contacts), household transmission may be overestimated. For this reason, we excluded studies that used antibody tests to diagnose household contacts. Furthermore, many analyses ignored tertiary transmission within the household, classifying all subsequent cases as secondary to the index case. Eighteen studies19,20,21,24,25,28,29,33,34,41,47,50,53,56,58,59,61,64 involved testing only symptomatic household contacts, which would miss asymptomatic or subclinical infections, although secondary attack rate estimates were similar across studies testing all vs only symptomatic contacts.
Important questions remain regarding household spread of SARS-CoV-2. Chief among them is the infectiousness of children to their household contacts and the infectiousness of asymptomatic, mildly ill, and severely ill index cases. This study did not provide additional elucidation of factors influencing intergenerational spread. People unable to work at home may have greater risk of SARS-CoV-2 exposure, which may increase transmission risk to other household members. There may be overdispersion in the number of secondary infections per index case, which could be caused by variations in viral shedding, household ventilation, or other factors.
Conclusions
The findings of this study suggest that households are and will continue to be important venues for transmission, even where community transmission is reduced. Prevention strategies, such as increased mask-wearing at home, improved ventilation, voluntary isolation at external facilities, and targeted antiviral prophylaxis, should be further explored.
eFigure 1. PRISMA Flow Diagram for Review of Household Secondary Attack of SARS-CoV-2, MERS-CoV, SARS-CoV, and Other Coronaviruses
eFigure 2. Secondary Attack Rates of SARS-CoV-2 for Studies of Close Contacts
eFigure 3. Funnel Plots of Studies Reporting Secondary Attack Rates of SARS-CoV-2 for Household, Family, and Close Contacts
eFigure 4. Household Secondary Attack Rates of SARS-CoV-2, Restricted to Studies With Low or Moderate Risk of Bias as Determined by the Modified Newcastle-Ottawa Scale
eFigure 5. Household Secondary Attack Rates of SARS-CoV-2, Grouped by Studies in China vs Other Locations
eFigure 6. Secondary Attack Rates of SARS-CoV-2, Grouped by Studies That Tested Only Symptomatic Household Contacts and Studies That Tested All Household Contacts Irrespective of Symptoms
eFigure 7. Household Secondary Attack Rates of SARS-CoV-2, Grouped by Studies Early (January-February) and Later (March-July) in the Pandemic
eFigure 8. Secondary Attack Rates of SARS-CoV-2 From Symptomatic and Asymptomatic or Presymptomatic Index Cases to Household and Family Contacts
eFigure 9. Funnel Plots of Studies Reporting Household Secondary Attack Rates of SARS-CoV-2 for Adult (≥18 Years) and Child (<18 Years) Contacts
eFigure 10. Secondary Attack Rates of SARS-CoV-2 for Household and Family Contacts by Contact Sex
eFigure 11. Funnel Plots of Studies Reporting Household Secondary Attack Rates of SARS-CoV-2 for Female and Male Contacts
eFigure 12. Secondary Attack Rates of SARS-CoV-2 to Household Contacts From Adult (≥18 Years) and Child (<18 Years) Index Cases
eFigure 13. Secondary Attack Rates of SARS-CoV-2 for Household Contacts by Index Case Sex
eFigure 14. Household Secondary Attack Rates of SARS-CoV and MERS-CoV
eTable 1. Electronic Databases and Search Strategy for Household Secondary Attack Rate of SARS-CoV-2, MERS-CoV, SARS-CoV, and Other Coronaviruses
eTable 2. Risk of Bias Assessment for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 3. Description of Index Cases for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 4. Description of Contacts for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 5. Overdispersion of the Number of Secondary Infections of SARS-CoV-2 per Household
eTable 6. Assessment of Factors Potentially Affecting Susceptibility and Infectivity of SARS-CoV-2 in Household Transmission Studies
eTable 7. Household Secondary Attack Rate Comparison With Other Viruses
eTable 8. Weights for Combined Estimate of Secondary Attack Rates of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Household Contacts and Family Contacts
eAppendix 1. Eligibility Criteria
eAppendix 2. Data Extraction
eAppendix 3. Additional Description of Studies
eAppendix 4. Additional Description of Risk Factors
eReferences.
References
- 1.World Health Organization Transmission of SARS-CoV-2: implications for infection prevention precautions. Published July 9, 2020. Accessed November 11, 2020. https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions
- 2.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173(5):362-367. doi: 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishiura H, Oshitani H, Kobayashi T, et al. Closed environments facilitate secondary transmission of coronavirus disease 2019 (COVID-19). medRxiv. Preprint published online April 16, 2020. doi: 10.1101/2020.02.28.20029272 [DOI]
- 4.World Health Organization Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). February 16-24, 2020. Accessed November 11, 2020. https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf
- 5.Badr HS, Du H, Marshall M, Dong E, Squire MM, Gardner LM. Association between mobility patterns and COVID-19 transmission in the USA: a mathematical modelling study. Lancet Infect Dis. 2020;20(11):1247-1254. doi: 10.1016/S1473-3099(20)30553-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drake TM, Docherty AB, Weiser TG, Yule S, Sheikh A, Harrison EM. The effects of physical distancing on population mobility during the COVID-19 pandemic in the UK. Lancet Digit Health. 2020;2(8):e385-e387. doi: 10.1016/S2589-7500(20)30134-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang H, Wang L, Yang Y Human mobility restrictions and the spread of the novel coronavirus (2019-nCoV) in China. National Bureau of Economic Research. Published March 2020. Accessed November 11, 2020. https://www.nber.org/papers/w26906 [DOI] [PMC free article] [PubMed]
- 8.Curmei M, Ilyas A, Evans O, Steinhardt J.. Estimating household transmission of SARS-CoV-2. medRxiv. Preprint published online June 27, 2020. doi: 10.1101/2020.05.23.20111559 [DOI]
- 9.US Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19): how to protect yourself and others. Updated November 4, 2020. Accessed November 11, 2020. https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html
- 10.Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analysis. Accessed November 11, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 11.Fung HF, Martinez L, Alarid-Escudero F, et al. ; SC-COSMO Modeling Group . The household secondary attack rate of SARS-CoV-2: a rapid review. Clin Infect Dis. Published online October 12, 2020:ciaa1558. doi: 10.1093/cid/ciaa1558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals Math Stat. 1950;21(4):607-611. doi: 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- 13.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Software. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 15.R Core Team R: a language and environment for statistical computing. Accessed November 13, 2020. https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088-1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 17.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. doi: 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 19.Adamik B, Bawiec M, Bezborodov V, et al. Bounds on the total number of SARS-CoV-2 infections: the link between severeness rate, household attack rate and the number of undetected cases. Published August 15, 2020. Accessed November 11, 2020. https://docisolation.prod.fire.glass/?guid=8579b2af-bdb0-4d1f-f538-bc52f86d984e
- 20.Arnedo-Pena A, Sabater-Vidal S, Meseguer-Ferrer N, et al. COVID-19 secondary attack rate and risk factors in household contacts in Castellon (Spain): preliminary report. Enfermedades Emergentes. 2020;19(2):64-70. Accessed November 11, 2020. https://docisolation.prod.fire.glass/?guid=45f61a53-bdcc-40ab-ded8-dd9646aa077c [Google Scholar]
- 21.Bae S, Kim H, Jung T-Y, et al. Epidemiological characteristics of COVID-19 outbreak at fitness centers in Cheonan, Korea. J Korean Med Sci. 2020;35(31):e288. doi: 10.3346/jkms.2020.35.e288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911-919. doi: 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böhmer MM, Buchholz U, Corman VM, et al. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8):920-928. doi: 10.1016/S1473-3099(20)30314-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boscolo-Rizzo P, Borsetto D, Spinato G, et al. New onset of loss of smell or taste in household contacts of home-isolated SARS-CoV-2-positive subjects. Eur Arch Otorhinolaryngol. 2020;277(9):2637-2640. doi: 10.1007/s00405-020-06066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burke RM. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January-February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245-246. doi: 10.15585/mmwr.mm6909e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaw L, Koh WC, Jamaludin SA, Naing L, Alikhan MF, Wong J. SARS-CoV-2 transmission in different settings: analysis of cases and close contacts from the Tablighi cluster in Brunei Darussalam. Emerg Infect Dis. 2020;26(11):2598-2606. doi: 10.3201/eid2611.202263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Wang AH, Yi B, et al. [Epidemiological characteristics of infection in COVID-19 close contacts in Ningbo city]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):667-671. doi: 10.3760/cma.j.cn112338-20200304-00251 [DOI] [PubMed] [Google Scholar]
- 28.Cheng HY, Jian SW, Liu DP, Ng TC, Huang WT, Lin HH; Taiwan COVID-19 Outbreak Investigation Team . Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180(9):1156-1163. doi: 10.1001/jamainternmed.2020.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dattner I, Goldberg Y, Katriel G, et al. The role of children in the spread of COVID-19: Using household data from Bnei Brak, Israel, to estimate the relative susceptibility and infectivity of children. medRxiv. Preprint published online October 11, 2020. doi: 10.1101/2020.06.03.20121145 [DOI] [PMC free article] [PubMed]
- 30.Dawson P, Rabold EM, Laws RL, et al. Loss of taste and smell as distinguishing symptoms of COVID-19. Clin Infect Dis. 2020;ciaa799. doi: 10.1093/cid/ciaa799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong XC, Li JM, Bai JY, et al. [Epidemiological characteristics of confirmed COVID-19 cases in Tianjin]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(5):638-641. doi: 10.3760/cma.j.cn112338-20200221-00146 [DOI] [PubMed] [Google Scholar]
- 32.Doung-ngern P, Suphanchaimat R, Panjagampatthana A, et al. Case-control study of use of personal protective measures and risk for SARS-CoV 2 infection, Thailand. Emerg Infect Dis. 2020;26(11):2607-2616. doi: 10.3201/eid2611.203003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Draper AD, Dempsey KE, Boyd RH, et al. The first 2 months of COVID-19 contact tracing in the Northern Territory of Australia, March-April 2020. Commun Dis Intell (2018). 2020;44:44. [DOI] [PubMed] [Google Scholar]
- 34.Fateh-Moghadam P, Battisti L, Molinaro S, et al. Contact tracing during phase I of the COVID-19 pandemic in the Province of Trento, Italy: key findings and recommendations. medRxiv. Preprint published online July 29, 2020. doi: 10.1101/2020.07.16.20127357 [DOI]
- 35.Han T. Outbreak investigation: transmission of COVID-19 started from a spa facility in a local community in Korea. Epidemiol Health. 2020;42(0):e2020056-e2020050. doi: 10.4178/epih.e2020056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu S, Wang W, Wang Y, et al. Infectivity, susceptibility, and risk factors associated with SARS-CoV-2 transmission under intensive contact tracing in Hunan, China. medRxiv. Preprint published online November 3, 2020. doi: 10.1101/2020.07.23.20160317 [DOI] [PMC free article] [PubMed]
- 37.Hua CZ, Miao ZP, Zheng JS, et al. Epidemiological features and viral shedding in children with SARS-CoV-2 infection. J Med Virol. Published online June 15, 2020. doi: 10.1002/jmv.26180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Islam SS, Noman ASM Transmission dynamics and contact tracing assessment of COVID-19 in Chattogram, Bangladesh and potential risk of close contacts at different exposure settings. Published October 12, 2020. Accessed November 11, 2020. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3677863
- 39.Jing Q-L, Liu M-J, Zhang ZB, et al. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(10):1141-1150. doi: 10.1016/S1473-3099(20)30471-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2020;archdischild-2020-319910. doi: 10.1136/archdischild-2020-319910 [DOI] [PubMed] [Google Scholar]
- 41.COVID-19 National Emergency Response Center, Epidemiology and Case Management Team, Korea Centers for Disease Control and Prevention Coronavirus disease-19: summary of 2,370 contact investigations of the first 30 cases in the Republic of Korea. Osong Public Health Res Perspect. 2020;11(2):81-84. doi: 10.24171/j.phrp.2020.11.2.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laxminarayan R, Wahl B, Dudala SR, et al. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691-697. doi: 10.1126/science.abd7672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee M, Eun Y, Park K, Heo J, Son H. Follow-up investigation of asymptomatic COVID-19 cases at diagnosis in Busan, Korea. Epidemiol Health. 2020;42:e2020046. doi: 10.4178/epih.e2020046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis NM, Chu VT, Ye D, et al. Household transmission of SARS-CoV-2 in the United States. Clin Infect Dis. Published online August 16, 2020. doi: 10.1093/cid/ciaa1166 [DOI] [Google Scholar]
- 45.Li W, Zhang B, Lu J, et al. Characteristics of household transmission of COVID-19. Clin Infect Dis. 2020;71(8):1943-1946. doi: 10.1093/cid/ciaa450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu T, Liang W, Zhong H, et al. Risk factors associated with COVID-19 infection: a retrospective cohort study based on contacts tracing. Emerg Microbes Infect. 2020;9(1):1546-1553. doi: 10.1080/22221751.2020.1787799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopez Bernal J, Panagiotopoulos N, Byers C, et al. Transmission dynamics of COVID-19 in household and community settings in the United Kingdom. medRxiv. Preprint published online August 22, 2020. doi: 10.1101/2020.08.19.20177188 [DOI] [PMC free article] [PubMed]
- 48.Luo L, Liu D, Liao X, et al. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China: a prospective cohort study. Ann Intern Med. Published August 13, 2020. doi: 10.7326/M20-2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyngse FP, Kirkeby CT, Halasa T, et al. COVID-19 transmission within Danish households: a nationwide study from lockdown to reopening. medRxiv. Preprint published online September 9, 2020. doi: 10.1101/2020.09.09.20191239 [DOI]
- 50.Malheiro R, Figueiredo AL, Magalhães JP, et al. Effectiveness of contact tracing and quarantine on reducing COVID-19 transmission: a retrospective cohort study. Public Health. 2020;189:54-59. doi: 10.1016/j.puhe.2020.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YJ, Choe YJ, Park O, et al. ; COVID-19 National Emergency Response Center, Epidemiology and Case Management Team . Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020;26(10):2465-2468. doi: 10.3201/eid2610.201315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Park SY, Kim Y-M, Yi S, et al. Coronavirus disease outbreak in call center, South Korea. Emerg Infect Dis. 2020;26(8):1666-1670. doi: 10.3201/eid2608.201274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel A, Charani E, Ariyanayagam D, et al. New-onset anosmia and ageusia in adult patients diagnosed with SARS-CoV-2 infection. Clin Microbiol Infect. Published online June 2 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phiriyasart F, Chantutanon S, Salaeh F, et al. Outbreak investigation of coronavirus disease (COVID-19) among Islamic missionaries in southern Thailand, April 2020. Outbreak, Surveillance, Investigation, and Response (OSIR) Journal. 2020;13(2). Accessed November 11, 2020. http://www.osirjournal.net/index.php/osir/article/view/195
- 55.Rosenberg ES, Dufort EM, Blog DS, et al. ; New York State Coronavirus 2019 Response Team . COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—March 2020. Clin Infect Dis. 2020;71(8):1953-1959. doi: 10.1093/cid/ciaa549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah K, Desai N, Saxena D, Mavalankar D, Mishra U, Patel GC. Household secondary attack rate in Gandhinagar district of Gujarat state from Western India. medRxiv. Preprint published online September 5, 2020. doi: 10.1101/2020.09.03.20187336 [DOI]
- 57.Son H, Lee H, Lee M, et al. Epidemiological characteristics of and containment measures for COVID-19 in Busan, South Korea. Epidemiology and Health. 2020;42:e2020035. doi: 10.4178/epih.e2020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun WW, Ling F, Pan JR, et al. [Epidemiological characteristics of COVID-19 family clustering in Zhejiang Province]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(6):625-629. doi: 10.3760/cma.j.cn112150-20200227-00199 [DOI] [PubMed] [Google Scholar]
- 59.Teherani MF, Kao CM, Camacho-Gonzalez A, et al. Burden of illness in households with SARS-CoV-2 infected children. J Pediatric Infect Dis Soc. 2020;9(5):613-616. doi: 10.1093/jpids/piaa097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Hoek W, Backer JA, Bodewes R, et al. [The role of children in the transmission of SARS-CoV-2]. Ned Tijdschr Geneeskd. 2020;164:D5140. [PubMed] [Google Scholar]
- 61.Wang Z, Ma W, Zheng X, Wu G, Zhang R. Household transmission of SARS-CoV-2. J Infect. 2020;81(1):179-182. doi: 10.1016/j.jinf.2020.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang X, Pan Y, Zhang D, et al. Basic epidemiological parameter values from data of real-world in mega-cities: the characteristics of COVID-19 in Beijing, China. BMC Infect Dis. 2020;20(1):526. doi: 10.1186/s12879-020-05251-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Tian H, Zhang L, et al. Reduction of secondary transmission of SARS-CoV-2 in households by face mask use, disinfection and social distancing: a cohort study in Beijing, China. BMJ Glob Health. 2020;5(5):e002794. doi: 10.1136/bmjgh-2020-002794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang X, Zhou Q, He Y, et al. Nosocomial outbreak of COVID-19 pneumonia in Wuhan, China. Eur Respir J. 2020;55(6):2000544. doi: 10.1183/13993003.00544-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wu J, Huang Y, Tu C, et al. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. Published online May 11, 2020;ciaa557. doi: 10.1093/cid/ciaa557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu Y, Song S, Kao Q, Kong Q, Sun Z, Wang B. Risk of SARS-CoV-2 infection among contacts of individuals with COVID-19 in Hangzhou, China. Public Health. 2020;185:57-59. doi: 10.1016/j.puhe.2020.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xin H, Jiang F, Xue A, et al. Risk factors associated with occurrence of COVID-19 among household persons exposed to patients with confirmed COVID-19 in Qingdao Municipal, China. Transbound Emerg Dis. 2020. doi: 10.1111/tbed.13743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yu HJ, Hu YF, Liu XX, et al. Household infection: the predominant risk factor for close contacts of patients with COVID-19. Travel Med Infect Dis. 2020;36:101809. doi: 10.1016/j.tmaid.2020.101809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yung CF, Kam KQ, Chong CY, et al. Household transmission of SARS-CoV-2 from adults to children. J Ped. 2020;225:249-251. doi: 10.1016/j.jpeds.2020.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Cheng W, Luo L, et al. Secondary transmission of coronavirus disease from presymptomatic persons, China. Emerg Infect Dis. 2020;26(8):1924-1926. doi: 10.3201/eid2608.201142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang JZ, Zhou P, Han DB, et al. [Investigation on a cluster epidemic of COVID-19 in a supermarket in Liaocheng, Shandong province]. Zhonghua Liu Xing Bing Xue Za Zhi. Published April 27, 2020. [DOI] [PubMed] [Google Scholar]
- 72.Zhuang YL, Zhang YT, Li M, et al. [Analysis on the cluster epidemic of coronavirus disease 2019 in Guangdong Province]. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(7):720-725. [DOI] [PubMed] [Google Scholar]
- 73.Stringhini S, Wisniak A, Piumatti G, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in Geneva, Switzerland (SEROCoV-POP): a population-based study. Lancet. 2020;396(10247):313-319. doi: 10.1016/S0140-6736(20)31304-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang K, Wang L, Li F, et al. Analysis of epidemiological characteristics of coronavirus 2019 infection and preventive measures in Shenzhen China: a heavy population city. medRxiv. Preprint published online March 3, 2020. doi: 10.1101/2020.02.28.20028555 [DOI]
- 75.Fontanet A, Grant R, Tondeur L, et al. SARS-CoV-2 infection in primary schools in northern France: A retrospective cohort study in an area of high transmission. medRxiv. Preprint published online June 29, 2020. doi: 10.1101/2020.06.25.20140178 [DOI]
- 76.Chan LY, Wong JT, Li PK, Lui SF, Fung H, Sung J. Risk of transmission of severe acute respiratory syndrome to household contacts by infected health care workers and patients. Am J Med. 2004;116(8):559-560. doi: 10.1016/j.amjmed.2003.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goh DL, Lee BW, Chia KS, et al. Secondary household transmission of SARS, Singapore. Emerg Infect Dis. 2004;10(2):232-234. doi: 10.3201/eid1002.030676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lau JT, Lau M, Kim JH, Tsui HY, Tsang T, Wong TW. Probable secondary infections in households of SARS patients in Hong Kong. Emerg Infect Dis. 2004;10(2):235-243. doi: 10.3201/eid1002.030626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson-Clark SD, Deeks SL, Gournis E, et al. Household transmission of SARS, 2003. CMAJ. 2006;175(10):1219-1223. doi: 10.1503/cmaj.050876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ou J, Li Q, Zeng G, Dun Z; Centers for Disease Control and Prevention (CDC) . Efficiency of quarantine during an epidemic of severe acute respiratory syndrome—Beijing, China, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(43):1037-1040. [PubMed] [Google Scholar]
- 81.Tuan PA, Horby P, Dinh PN, et al. ; WHO SARS Investigation Team in Vietnam . SARS transmission in Vietnam outside of the health-care setting. Epidemiol Infect. 2007;135(3):392-401. doi: 10.1017/S0950268806006996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pang X, Zhu Z, Xu F, et al. Evaluation of control measures implemented in the severe acute respiratory syndrome outbreak in Beijing, 2003. JAMA. 2003;290(24):3215-3221. doi: 10.1001/jama.290.24.3215 [DOI] [PubMed] [Google Scholar]
- 83.Al Hosani FI, Kim L, Khudhair A, et al. Serologic follow-up of Middle East respiratory syndrome coronavirus cases and contacts—Abu Dhabi, United Arab Emirates. Clin Infect Dis. 2019;68(3):409-418. doi: 10.1093/cid/ciy503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Arwady MA, Alraddadi B, Basler C, et al. Middle East respiratory syndrome coronavirus transmission in extended family, Saudi Arabia, 2014. Emerg Infect Dis. 2016;22(8):1395-1402. doi: 10.3201/eid2208.152015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Assiri A, McGeer A, Perl TM, et al. ; KSA MERS-CoV Investigation Team . Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369(5):407-416. doi: 10.1056/NEJMoa1306742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Drosten C, Meyer B, Müller MA, et al. Transmission of MERS-coronavirus in household contacts. N Engl J Med. 2014;371(9):828-835. doi: 10.1056/NEJMoa1405858 [DOI] [PubMed] [Google Scholar]
- 87.Memish ZA, Al-Tawfiq JA, Alhakeem RF, et al. Middle East respiratory syndrome coronavirus (MERS-CoV): a cluster analysis with implications for global management of suspected cases. Travel Med Infect Dis. 2015;13(4):311-314. doi: 10.1016/j.tmaid.2015.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Payne DC, Biggs HM, Al-Abdallat MM, et al. Multihospital outbreak of a Middle East respiratory syndrome coronavirus deletion variant, Jordan: a molecular, serologic, and epidemiologic investigation. Open Forum Infect Dis. 2018;5(5):ofy095. doi: 10.1093/ofid/ofy095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Van Kerkhove MD, Alaswad S, Assiri A, et al. Transmissibility of MERS-CoV infection in closed setting, Riyadh, Saudi Arabia, 2015. Emerg Infect Dis. 2019;25(10):1802-1809. doi: 10.3201/eid2510.190130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Esposito S, Bosis S, Niesters HG, et al. Impact of human coronavirus infections in otherwise healthy children who attended an emergency department. J Med Virol. 2006;78(12):1609-1615. doi: 10.1002/jmv.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Monto AS, DeJonge PM, Callear AP, et al. Coronavirus occurrence and transmission over 8 years in the HIVE cohort of households in Michigan. J Infect Dis. 2020;222(1):9-16. doi: 10.1093/infdis/jiaa161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Beale S, Lewer D, Aldridge RW, et al. Household transmission of seasonal coronavirus infections: results from the Flu Watch cohort study. Wellcome Open Research. 2020;5(145):145. doi: 10.12688/wellcomeopenres.16055.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsang TK, Lau LLH, Cauchemez S, Cowling BJ. Household transmission of influenza virus. Trends Microbiol. 2016;24(2):123-133. doi: 10.1016/j.tim.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ministry of Health, Labour, and Welfare Avoid the “Three Cs”! Accessed August 5, 2020. https://www.mhlw.go.jp/content/10900000/000619576.pdf
- 95.Pang X, Yang P, Li S, et al. Pandemic (H1N1) 2009 among quarantined close contacts, Beijing, People’s Republic of China. Emerg Infect Dis. 2011;17(10):1824-1830. doi: 10.3201/eid1710.101344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tam K, Yousey-Hindes K, Hadler JL. Influenza-related hospitalization of adults associated with low census tract socioeconomic status and female sex in New Haven County, Connecticut, 2007-2011. Influenza Other Respir Viruses. 2014;8(3):274-281. doi: 10.1111/irv.12231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chandrasekhar R, Sloan C, Mitchel E, et al. Social determinants of influenza hospitalization in the United States. Influenza Other Respir Viruses. 2017;11(6):479-488. doi: 10.1111/irv.12483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sloan C, Chandrasekhar R, Mitchel E, Schaffner W, Lindegren ML. Socioeconomic disparities and influenza hospitalizations, Tennessee, USA. Emerg Infect Dis. 2015;21(9):1602-1610. doi: 10.3201/eid2109.141861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. Published September 25, 2020. doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davies NG, Klepac P, Liu Y, Prem K, Jit M, Eggo RM; CMMID COVID-19 working group . Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205-1211. doi: 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 101.Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: kinetics, correlates of protection, and association with severity. Nat Commun. 2020;11(1):4704. doi: 10.1038/s41467-020-18450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mehta NS, Mytton OT, Mullins EWS, et al. SARS-CoV-2 (COVID-19): what do we know about children? a systematic review. Clin Infect Dis. Published May 11, 2020;ciaa556. doi: 10.1093/cid/ciaa556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Posfay-Barbe KM, Wagner N, Gauthey M, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics. 2020;146(2):e20201576. doi: 10.1542/peds.2020-1576 [DOI] [PubMed] [Google Scholar]
- 104.Lee B, Raszka WV Jr. COVID-19 transmission and children: the child is not to blame. Pediatrics. 2020;146(2):e2020004879. doi: 10.1542/peds.2020-004879 [DOI] [PubMed] [Google Scholar]
- 105.Zhu Y, Bloxham CJ, Hulme KD, et al. Children are unlikely to have been the primary source of household SARS-CoV-2 infections. medRxiv. Preprint published online March 30, 2020. doi: 10.1101/2020.03.26.20044826 [DOI]
- 106.L'Huillier AG, Torriani G, Pigny F, Kaiser L, Eckerle I. Culture-competent SARS-CoV-2 in nasopharynx of symptomatic neonates, children, and adolescents. Emerg Infect Dis. 2020;26(10):2494-2497. doi: 10.3201/eid2610.202403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.World Health Organization Gender and COVID-19. Published May 14, 2020. Accessed November 11, 2020. https://www.who.int/publications/i/item/gender-and-covid-19
- 108.Walsh KA, Jordan K, Clyne B, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81(3):357-371. doi: 10.1016/j.jinf.2020.06.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672-675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 110.Ali ST, Wang L, Lau EHY, et al. Serial interval of SARS-CoV-2 was shortened over time by nonpharmaceutical interventions. Science. 2020;369(6507):1106-1109. doi: 10.1126/science.abc9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rabaan AA, Al-Ahmed SH, Haque S, et al. SARS-CoV-2, SARS-CoV, and MERS-COV: a comparative overview. Infez Med. 2020;28(2):174-184. [PubMed] [Google Scholar]
- 112.Petersen E, Koopmans M, Go U, et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect Dis. 2020;20(9):e238-e244. doi: 10.1016/S1473-3099(20)30484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Petrosillo N, Viceconte G, Ergonul O, Ippolito G, Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26(6):729-734. doi: 10.1016/j.cmi.2020.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci U S A. 2004;101(16):6146-6151. doi: 10.1073/pnas.0307506101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cowling BJ, Park M, Fang VJ, Wu P, Leung GM, Wu JT. Preliminary epidemiological assessment of MERS-CoV outbreak in South Korea, May to June 2015. Euro Surveill. 2015;20(25):7-13. doi: 10.2807/1560-7917.ES2015.20.25.21163 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. PRISMA Flow Diagram for Review of Household Secondary Attack of SARS-CoV-2, MERS-CoV, SARS-CoV, and Other Coronaviruses
eFigure 2. Secondary Attack Rates of SARS-CoV-2 for Studies of Close Contacts
eFigure 3. Funnel Plots of Studies Reporting Secondary Attack Rates of SARS-CoV-2 for Household, Family, and Close Contacts
eFigure 4. Household Secondary Attack Rates of SARS-CoV-2, Restricted to Studies With Low or Moderate Risk of Bias as Determined by the Modified Newcastle-Ottawa Scale
eFigure 5. Household Secondary Attack Rates of SARS-CoV-2, Grouped by Studies in China vs Other Locations
eFigure 6. Secondary Attack Rates of SARS-CoV-2, Grouped by Studies That Tested Only Symptomatic Household Contacts and Studies That Tested All Household Contacts Irrespective of Symptoms
eFigure 7. Household Secondary Attack Rates of SARS-CoV-2, Grouped by Studies Early (January-February) and Later (March-July) in the Pandemic
eFigure 8. Secondary Attack Rates of SARS-CoV-2 From Symptomatic and Asymptomatic or Presymptomatic Index Cases to Household and Family Contacts
eFigure 9. Funnel Plots of Studies Reporting Household Secondary Attack Rates of SARS-CoV-2 for Adult (≥18 Years) and Child (<18 Years) Contacts
eFigure 10. Secondary Attack Rates of SARS-CoV-2 for Household and Family Contacts by Contact Sex
eFigure 11. Funnel Plots of Studies Reporting Household Secondary Attack Rates of SARS-CoV-2 for Female and Male Contacts
eFigure 12. Secondary Attack Rates of SARS-CoV-2 to Household Contacts From Adult (≥18 Years) and Child (<18 Years) Index Cases
eFigure 13. Secondary Attack Rates of SARS-CoV-2 for Household Contacts by Index Case Sex
eFigure 14. Household Secondary Attack Rates of SARS-CoV and MERS-CoV
eTable 1. Electronic Databases and Search Strategy for Household Secondary Attack Rate of SARS-CoV-2, MERS-CoV, SARS-CoV, and Other Coronaviruses
eTable 2. Risk of Bias Assessment for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 3. Description of Index Cases for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 4. Description of Contacts for Studies Included in Review of Household Transmissibility of SARS-CoV-2
eTable 5. Overdispersion of the Number of Secondary Infections of SARS-CoV-2 per Household
eTable 6. Assessment of Factors Potentially Affecting Susceptibility and Infectivity of SARS-CoV-2 in Household Transmission Studies
eTable 7. Household Secondary Attack Rate Comparison With Other Viruses
eTable 8. Weights for Combined Estimate of Secondary Attack Rates of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) for Household Contacts and Family Contacts
eAppendix 1. Eligibility Criteria
eAppendix 2. Data Extraction
eAppendix 3. Additional Description of Studies
eAppendix 4. Additional Description of Risk Factors
eReferences.


