Abstract
Objectives
India is witnessing a disturbing growth in non-communicable diseases (NCDs), including chronic kidney disease (CKD). Recently, a WHO STEPS survey was conducted in the state of Punjab, India to collect data from the adult population on NCD risk factors. We sought to compare the prevalence of CKD and its risk factors between this large state in northern India and the USA.
Setting
Samples were drawn from both locations, Punjab, India and the USA, using multistage stratified sampling designs to collect data representative of the general population.
Participants
Data from 2002 participants in the Punjab survey (2014–2015) and 5057 in the USA (National Health and Nutrition Examination Survey (NHANES; 2013–2014), between the ages of 18–69 years were examined.
Primary and secondary outcome measures
Modified Poisson regression was employed to compare prevalence between the two samples for markers of CKD and its risk factors. All analyses used sampling weights.
Results
The average age in the Punjab sample was significantly lower than the USA (38.3 vs 42.5 years, p<0.0001). While smoking and obesity were higher in the USA, hypertension was much more common in Punjab (48.2% vs 33.4%, p<0.0001). Significant differences were seen in the prevalence of CKD, with lower prevalence of eGFR <60 mL/min/1.73 m2 (2.0% vs 3.8%, p<0.0001), but markedly higher prevalence of albuminuria (46.7% vs 8.9%, p<0.0001) in Punjab. These differences could not be explained by traditional risk factors such as diabetes and hypertension.
Conclusions
We report a strikingly high prevalence of albuminuria in Punjab, India, compared with the USA. This requires further study and may have enormous public health implications for future burden of progressive CKD, end-stage kidney disease, morbidity, mortality and specifically for elevated risk or presence of cardiovascular disease in the northern state of Punjab, India.
Funding came from the National Health Mission, Punjab, India, JST and the Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Keywords: epidemiology, nephrology, adult nephrology
Strengths and limitations of this study.
Representative samples from both the State of Punjab, India and the USA.
Uniform laboratory testing for identification of kidney disease.
Comprehensive data collection on anthropomorphic measurements, laboratory measurements, comorbid conditions and health behaviours.
Cross-sectional study design cannot establish causality.
Because the sample from India was only from one state, the Punjab, we cannot generalise our findings to all of India.
Introduction
The state of Punjab—indeed all of India, similar to other low-income and middle-income countries, is witnessing a disturbing growth in NCDs.1 The country faces this epidemiological transition while continuing to grapple with the problem of communicable diseases, which still remain a significant burden.2 With this knowledge, the Department of Health and Family Welfare in Punjab, India, worked closely with the Post Graduate Institute of Medical Education and Research, Chandigarh, India, and medical colleges in the state to conduct the first representative survey of NCDs in the state of Punjab in 2014 and 2015.
The goal of this survey was to collect critical and up to date data on risk factors for NCDs in Punjab, with the hope of improving health planning and implementation of state initiatives, such as the National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular disease and Stroke.3 This survey provides a wealth of data on both risk factors for kidney disease and kidney disease itself, comparable to data collected in the USA from the National Health and Nutrition Examination Survey (NHANES).
Previous work using data from this source have shown an alarmingly high prevalence of hypertension (40.1%) and pre-hypertension (40.8%) in the region, with approximately 70% of these individuals being unaware of their condition.4 Similarly, although less prevalent, diabetes was found in 8.3% (6.3% with pre-diabetes) participants, with only 18% of individuals being aware of their disease.5 Since diabetes and hypertension are two of the key risk factors for kidney disease, we hypothesised that the state of Punjab may be experiencing or on the verge of experiencing a significant burden of kidney disease.
Therefore, in the current study, we sought to examine the prevalence of chronic kidney disease (CKD; using both low glomerular filtration rate and albuminuria criteria) and risk factors for CKD, comparing the Punjab to a representative sample of individuals from the US NHANES. In addition, we also sought to compare the magnitude of the associations between risk factors and CKD in the two samples.
Materials and methods
Study sample
The STEPS survey of non-communicable disease (NCD) risk factors was carried out from June 2014 to August 2015 in Punjab.3 A multistage stratified sampling design was used to generate representative data for two age groups (18–44, 45–69), sex, and area of residence in the state. A total of 5127 adults, ages 18–69 years, participated in the survey. The overall response rate for STEP1/2 and STEP 3 was 95% and 93%, respectively. Data were collected in three steps: sociodemographic and behavioural information was collected in step 1, physical measurements such as height, weight and blood pressure were done in step 2, and biochemical measurements were undertaken to assess salt intake, blood glucose, triglycerides and cholesterol levels in step 3. This analysis included individuals from step 3 of the survey, which was carried out in a subset of 2700 participants. The individuals used in step 3 were selected by taking a subsample of half of the study participants considering resource constraints. Every second individual contacted for steps 1 and 2 was subjected to step 3. A total of 2002 individuals who had complete data on both albuminuria and serum creatinine were analysed. Specific sample weights were available for the individuals included in step 3.
The US data for comparison were from the 2013–2014 NHANES, included 5057 individuals. Multistage stratified sampling design was used to collect data representative of the US general population.6 The NHANES is supported by the National Center for Health Statistics and was designed to assess the health and nutritional status of adults and children in the USA. The study combines interviews, physical examinations, laboratory tests and participant lifestyle surveys. Individuals between the ages of 18 and 69 years, with complete information on estimated glomerular filtration rate (eGFR) and albuminuria, were examined to match with the Punjab sample.
Patient and public involvement
The research question was assessed using existing data taken from large, representative surveys, which contained more health questions and health measures than those presented in this work. The aim of the larger studies were to assess the overall health of each region, focused on diseases of global health impact, rather than individual patient priorities. The NHANES programme began in the early 1960s, as a series of surveys focusing on different population groups or health topics over time. Participants were not involved in the design of the study, recruitment or conduct of the study. NHANES participants receive their results from their examination as a preliminary report when leaving the exam centre. A final report of findings is sent to each participant through the mail 12–16 weeks after their exam. Participants are free to discuss their results with their doctor and to keep for their own medical records.
Similarly, the Punjab STEPS survey was a state-level public health effort undertaken to estimate the burden of many NCDs in that region. The government-funded study, similar to NHANES, did not enlist patient opinion during study design, but did have a plan to provide results to participants if abnormal and warranting medical follow-up.
Measures
In the Punjab, collection of blood and urine samples were done in the mornings, after participants had fasted overnight. Samples were centrifuged using a minicentrifuge and separated serum was stored in ice boxes then transferred daily to a nearest public health institute with facility for −20°C storage. Samples were transported to the central laboratory weekly. Collection of all the biochemical tests was at household level. Urine albumin-to-creatinine ratio (ACR) was performed as a point-of-care field test using the URS 2AC strip that tests for two parameters microalbumin and creatinine (Biosense Technologies, Thane, Maharashtra, India). Calibration of the instruments and validation of field testing kits in a proportion of samples were performed by the central biochemistry laboratory at PGIMER, Chandigarh, per their standard protocol. Point-of-care field testing has been validated previously.7 8 Laboratory measurements of serum creatinine (IDMS standardised assays) were made on Modular P 800 autoanalyser (Roche Diagnostics, Germany) using commercially available kits (Roche Diagnostics, Germany). In the US NHANES sample, urine samples were processed, stored and shipped to University of Minnesota, Minneapolis, Minnesota, for analysis. Detailed instructions on specimen collection and processing are discussed in the NHANES Laboratory Procedures Manual (LPM—https://wwwn.cdc.gov/nchs/data/nhanes/2015-2016/manuals/2016_MEC_Laboratory_Procedures_Manual.pdf). Vials were stored under appropriate frozen (−30°C) conditions until they are shipped to University of Minnesota for testing. The NHANES quality assurance and quality control (QA/QC) protocols meet the 1988 Clinical Laboratory Improvement Act mandates. Detailed QA/QC instructions are discussed in the NHANES LPM. A solid-phase fluorescent immunoassay was employed for the measurement of human urinary albumin is described by Chavers et al.9 Contract laboratories randomly perform repeat testing on 2% of all specimens.
Kidney function was assessed by eGFR, calculated with using the CKD-Epi formula in both samples, employing the coefficients for white race in India.10 Albuminuria was defined as a urine ACR >30 mg/g. Kidney disease was also assessed using the KDIGO risk categories, which places individuals into four risk groups for mortality based on their eGFR and ACR levels (low risk: eGFR >60 and ACR <30; moderately high risk: eGFR 45–59 with ACR <30 or eGFR >60 with ACR 30–300; high risk: eGFR 30–44 with ACR <30, eGFR 45–59 with ACR 30–300, or eGFR >60 with ACR >300; or very high risk: eGFR <30, eGFR 30–44 with ACR >30, or eGFR 45–59 with ACR >300).11
Risk factors for kidney disease were defined similarly between the two samples. Diabetes was defined by the presence of any of the following: being told by a doctor they had diabetes, taking medication for diabetes (including medication from traditional healers in India), or fasting glucose >126 mg/dL. Hypertension was defined as any of the following: being told by a doctor they had hypertension, taking medications for hypertension, or having systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg.
Body mass index (BMI) was examined as both continuous and categorical to investigate different cut-points for identifying obesity between samples, in an attempt to account for the differences in stature. In the USA, the WHO definition was employed where underweight was defined as BMI <18.5, normal weight as BMI 18.5–24.99, overweight as BMI 25–29.99 and obese as BMI ≥30 kg/m2. In Punjab, obesity was defined using the same criteria as other published papers using this survey data with underweight being defined as BMI <18.5, normal weight as BMI 18.5–22.99, overweight as BMI 23–26.99 and obese as BMI ≥27 kg/m2.1–3
Statistical analysis
Demographic, socioeconomic, anthropometric, health status and markers of kidney disease were compared between counties using sample weighted t-tests for means or χ2 tests for categorical variables. ACR was expressed as the median value due to its highly right-skewed nature. Associations between patient characteristics and risk factors for kidney disease with laboratory markers of kidney disease were modelled using modified Poisson regression with robust errors. This modelling approach was chosen, as opposed to logistic regression, because it yields estimates of prevalence ratios (PRs), rather than ORs.12 13 PR estimates were determined for the kidney disease risk factors within each country in a single model using interactions between a country indicator variable and each measure. PR estimates for variables other than BMI, where two parameterisations were examined, were taken from the model with BMI modelled as a continuous variable.
To compare the effect of different adjustments on the association between cohort and the markers of kidney disease, models are presented unadjusted, adjusted for demographics and fully adjusted. Age and sex were considered as demographic variables. A sensitivity analysis was performed for each kidney disease marker, stratifying the models by sex.
Analysis of deidentified data received from the Punjab WHO Steps Survey for this study was deemed IRB exempt by the University of Michigan IRB. NHANES data are publically available for use by researchers and does not require an IRB approval.
Results
Many differences exist between individuals in Punjab and the USA, as shown in table 1. The mean age was approximately 4 years younger in Punjab (p<0.0001), with a higher proportion of men (58.2% vs 48.9%, p<0.0001) compared with the USA. The USA had a much higher percentage of both high school or higher education and private health insurance coverage (p<0.0001). Overall body size was very different, with Punjab residents being 6 cm shorter, weighing 18 kg less, having 10 cm smaller waist circumference, and BMI lower by 4.6 kg/m2 (all p<0.0001). Comparison of obesity by categories showed a higher percentage of individuals in Punjab as underweight (11.3% vs 1.5% in the USA) and a higher proportion of obese individuals in the USA (37.9% vs 28.9%, p<0.0001), while proportions of those in the normal or overweight categories were very similar. While smoking was higher in the USA, hypertension was much more common in Punjab (48.2% vs 33.4%, p<0.0001). No differences were seen in the prevalence of diabetes, cardiovascular disease or triglyceride levels, although the USA had higher total cholesterol levels (4.9 vs 3.9 mmol/L (189 vs 150 mg/dL) in Punjab, p<0.0001).
Table 1.
Comparison of weighted survey sample participant characteristics between the adult populations in the State of Punjab, India and the USA
| Measure | Punjab (2014–2015) | USA (2013–2014) | P value | ||
| N | Mean (SE) or % | N | Mean (SE) or % | ||
| Age (years) | 2002 | 38.3 (0.60) | 5057 | 42.5 (0.38) | <0.0001 |
| Male (%) | 2002 | 58.2% | 5057 | 48.9% | 0.0001 |
| Education to high school or above (%) | 2002 | 43.4% | 4718 | 85.3% | <0.0001 |
| Health insurance (%) | 2002 | 6.2% | 5052 | 79.8% | <0.0001 |
| Height (cm) | 1986 | 163.0 (0.37) | 5008 | 169.0 (0.31) | <0.0001 |
| Weight (Kg) | 1993 | 65.4 (0.6) | 5006 | 83.5 (0.54) | <0.0001 |
| BMI (kg/m2)* | 1982 | 24.6 (0.23) | 5000 | 29.2 (0.20) | <0.0001 |
| Underweight | 1982 | 11.3 | 5000 | 1.5 | <0.0001 |
| Normal | 29.5 | 29.1 | |||
| Overweight | 30.3 | 31.5 | |||
| Obese | 28.9 | 37.9 | |||
| Waist (cm) | 1995 | 89.0 (0.62) | 4836 | 98.8 (0.38) | <0.0001 |
| Current smoker (%) | 2002 | 7.5% | 5057 | 21.6% | <0.0001 |
| Diabetes (%) | 1043 | 7.7% | 5057 | 8.9% | 0.42 |
| Hypertension (%) | 2000 | 48.2% | 5057 | 33.4% | <0.0001 |
| CVD (%) | 1989 | 4.6% | 5057 | 3.4% | 0.08 |
| Triglyceride (mmol/L) | 2001 | 1.4 (0.04) | 2294 | 1.4 (0.04) | 0.35 |
| Total cholesterol (mmol/L) | 2002 | 3.9 (0.06) | 4812 | 4.9 (0.02) | <0.0001 |
| Serum creatinine (μmol/L) | 2002 | 61.9 (0.9) | 4798 | 77.8 (0.9) | <0.0001 |
| eGFR (mL/min/1.73 m2) | 2002 | 114.8 (1.1) | 4798 | 97.8 (0.6) | <0.0001 |
| eGFR<60 mL/min/1.73 m2 | 2002 | 2.0% | 4798 | 3.8% | <0.0001 |
| Urine albumin (g/L; median) | 1928 | 0.2 (0.03) | 4971 | 0.07 (0.002) | <0.0001 |
| Urine creatinine (μmol/L; median) | 1928 | 7242 (265) | 4971 | 9275 (292) | <0.0001 |
| ACR (mg/mmol; median)† | 1928 | 2.5 (0.25) | 4971 | 0.66 (0.007) | <0.0001 |
| ACR>3 mg/mmol | 1928 | 46.7% | 4971 | 8.9% | <0.0001 |
USA: underweight <18.5, normal = 18.5–24.9, overweight = 25–29.9, obese 30+.
India: underweight< 18, normal = 18–22.9, overweight = 23–24.9, obese 25+.
*Different body mass index (BMI) cut-points used for obesity.
†Median employed to examine differences in urine measurements due to high degree of risk skew.
ACR, urine albumin:creatinine ratio; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate.
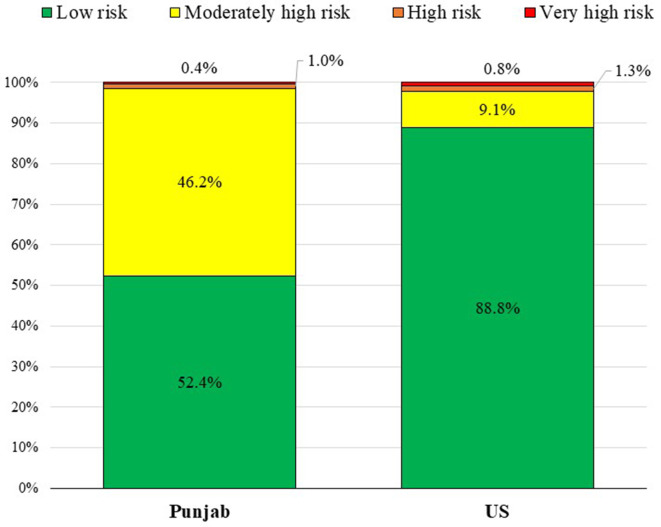
Although Punjab had a lower prevalence of eGFR <60 mL/min/1.73 m2 (2.0% vs 3.8%, p<0.0001), the prevalence of albuminuria was five times higher (46.7% vs 8.9%, p<0.0001). When assessing kidney function using the KDIGO risk categories (table 2), the high prevalence of high Urine ACR lead to 46.2% of participants in Punjab being classified as ‘moderately high risk’, compared with only 9.1% in the USA. In contrast, Punjab had only 1.4% in the ‘high risk’ or ‘extremely high risk’ groups compared with 2.1% in the USA (figure 1).
Table 2.
Prevalence of albuminuria and eGFR KDIGO Risk Categories among adults in Punjab and USA
| Punjab, India | Albuminuria categories | Total | |||||
| A1 | A2 | A3 | |||||
| Normal to mildly increased | Moderately increased | Severely increased | |||||
| <30 mg/g <3 mg/mmol |
30–300 mg/g 3–30 mg/mmol |
>300 mg/g >30 mg/mmol |
|||||
| GFR categories (mL/min/1.73 m2) | G1 | Normal to high | ≥90 | 46.7 (40.7–52.6) | 42.0 (35.3–48.7) | 0.2 (0–0.7) | 88.9 (86.0–91.8) |
| G2 | Mildly decreased | 60–89 | 5.7 (3.6–7.6) | 3.5 (2.4–4.7) | 0 | 9.2 (6.8–11.5) | |
| G3a | Mildly to mod decreased | 45–59 | 0.7 (0.1–1.3) | 0.5 (0–1.0) | 0 | 1.2 (0.3–2.1) | |
| G3b | Mod to severe decreased | 30–44 | 0.3 (0–0.7) | 0.5 (0–1.1) | 0 | 0.8 (0.1–1.5) | |
| G4 | Severely decreased | 15–29 | 0.03 (0–0.08) | 0.01 (0–0.02) | 0 | 0.04 (0–0.09) | |
| G5 | Kidney failure | <15 | 0 | 0 | 0 | 0 | |
| Total | 53.4 (46.3–60.2) | 46.5 (39.5–53.5) | 0.2 (0–0.7) | 100 | |||
| USA | |||||||
| GFR categories (mL/min/1.73 m2) | G1 | Normal to high | ≥90 | 60.7 (58.1–63.1) | 4.8 (4.0–5.6) | 0.5 (0.3–0.7) | 66.0 (63.1–68.9) |
| G2 | Mildly decreased | 60–89 | 28.1 (25.6–30.7) | 2.2 (1.5–2.7) | 0.1 (0.05–0.2) | 30.4 (27.7–33.1) | |
| G3a | Mildly to mod decreased | 45–59 | 2.1 (1.4–2.7) | 0.4 (0.2–0.7) | 0.2 (0–0.3) | 2.7 (1.9–3.5) | |
| G3b | Mod to severe decreased | 30–44 | 0.3 (0.1–0.4) | 0.2 (0.03–0.4) | 0.1 (0.02–0.2) | 0.6 (0.4–0.8) | |
| G4 | Severely decreased | 15–29 | 0.05 (0.0–0.1) | 0.05 (0.01–1.0) | 0.09 (0–0.2) | 0.2 (0.05–0.3) | |
| G5 | Kidney failure | <15 | 0 | 0.06 (0–0.2) | 0.07 (0.01–0.1) | 0.1 (0.01–0.3) | |
| Total | 91.2 (90.0–92.5) | 7.7 (6.6–8.8) | 1.1 (0.8–1.3) | 100 | |||
Green = low risk, Yellow = moderately high risk, Orange = high risk, Red = very high risk.
Green
Figure 1.
Distribution of Kidney Disease: Improving Global Outcomes Risk Categories among adults in Punjab, India and the USA.
To compare the magnitude of association between traditional risk factors for CKD between the two samples, we modelled PRs in each country within one model to allow for the associations to be compared statistically (table 3). When examining low eGFR (<60 mL/min/1.73 m2) as the outcome, male participants in Punjab showed a much lower prevalence compared with females (PR=0.22, p=0.007); while no association was seen in the USA between sex and low eGFR (PR=1.09, p=0.56). These associations were significantly different from each other with p=0.006. Another difference between the associations and outcome was seen for hypertension (p=0.008), where a non-significant lower PR was observed in Punjab (PR=0.75, p=0.43) and a strong positive association was seen in the USA (PR=2.24, p<0.0001). Similar positive associations were seen in both samples for older age, higher education level, CVD and DM on the prevalence of low eGFR (table 3A).
Table 3.
Prevalence ratios for markers of chronic kidney disease (CKD) by risk factors
| Measure | Punjab | US | P value for interaction | ||||
| PR | 95% CI | P value | PR | 95% CI | P value | ||
| (A) Low eGFR (eGFR<60 mL/min/1.73 m2) | |||||||
| USA (vs Punjab) | 1.00 | – | – | 0.05 | 0.004 to 0.71 | 0.03 | – |
| Age (per 10 years) | 1.73 | 1.29 to2.31 | 0.0002 | 2.14 | 1.82 to 2.52 | <0.0001 | 0.20 |
| Male (vs female) | 0.22 | 0.07 to 0.66 | 0.007 | 1.09 | 0.81 to 1.47 | 0.56 | 0.006 |
| Education high school + (vs no) | 1.86 | 0.84 to 4.10 | 0.13 | 1.53 | 1.06 to 2.20 | 0.02 | 0.66 |
| Current smoker (vs no) | 2.32 | 0.31to 1.74 | 0.41 | 0.92 | 0.63 to 1.34 | 0.66 | 0.38 |
| Hypertension (vs no) | 0.75 | 0.37 to 1.52 | 0.43 | 2.24 | 1.51 to 3.33 | <0.0001 | 0.008 |
| DM (vs no) | 2.75 | 1.17 to 6.48 | 0.02 | 1.76 | 1.27 to 2.44 | 0.0007 | 0.34 |
| CVD (vs no) | 1.11 | 0.34 to 3.60 | 0.87 | 1.98 | 1.20 to 1.38 | 0.0002 | 0.35 |
| Total cholesterol (per 20 mg/dL, per 0.5 mmol/L) | 1.09 | 0.73 to 1.64 | 0.67 | 0.92 | 0.76 to 1.11 | 0.36 | 0.44 |
| BMI (per 5 kg/m2) | 0.82 | 0.59 to 1.15 | 0.25 | 1.13 | 1.04 to 1.23 | 0.006 | 0.07 |
| Obesity: | |||||||
| Underweight | 1.24 | 0.26 to 5.95 | 0.79 | 1.45 | 0.36 to 5.89 | 0.60 | 0.88 |
| Healthy weight | 1.00 | – | Ref | 1.00 | – | Ref | |
| Overweight | 2.63 | 1.09 to 6.34 | 0.03 | 1.31 | 0.83 to 2.07 | 0.25 | 0.17 |
| Obese | 0.73 | 0.27t o 1.95 | 0.53 | 1.40 | 0.91 to 2.14 | 0.13 | 0.23 |
| (B) Albuminuria (ACR>30 mg/g, 3 mg/mmol) | |||||||
| USA (vs Punjab) | 1.00 | – | – | 0.18 | 0.08 to 0.38 | <0.0001 | – |
| Age (per 10 years) | 1.03 | 0.99 to 1.08 | 0.16 | 1.06 | 0.98 to 1.14 | 0.15 | 0.60 |
| Male (vs female) | 0.99 | 0.88 to 1.12 | 0.93 | 0.77 | 0.65 to 0.92 | 0.004 | 0.02 |
| Education high school + (vs no) | 0.99 | 0.88 to 1.11 | 0.80 | 0.81 | 0.67 to 0.98 | 0.03 | 0.09 |
| Current smoker (vs no) | 1.09 | 0.85 to 1.40 | 0.48 | 1.35 | 1.11 to 1.63 | 0.002 | 0.20 |
| Hypertension (vs no) | 1.19 | 1.06 to 1.34 | 0.005 | 1.93 | 1.59 to 2.36 | <0.0001 | <0.0001 |
| DM (vs no) | 1.32 | 1.12 to 1.56 | 0.0008 | 2.54 | 2.07 to 3.13 | <0.0001 | <0.0001 |
| CVD (vs no) | 1.14 | 0.92 to 1.37 | 0.24 | 1.32 | 1.16 to 0.99 | 0.06 | 0.37 |
| Total cholesterol (per 20 mg/dL, per 0.5 mmol/L) | 1.11 | 1.04 to 1.17 | 0.001 | 0.99 | 0.90 to 1.09 | 0.81 | 0.05 |
| BMI (per 5 kg/m2) | 0.99 | 0.94 to 1.03 | 0.54 | 1.04 | 0.98 to 1.10 | 0.19 | 0.16 |
| Obesity | |||||||
| Underweight | 0.90 | 0.72 to 1.11 | 0.32 | 1.20 | 0.56 to 2.56 | 0.64 | 0.47 |
| Healthy weight | 1.00 | – | Ref | 1.00 | – | Ref | – |
| Overweight | 0.95 | 0.82 to 1.10 | 0.48 | 0.93 | 0.72 to 1.18 | 0.53 | 0.85 |
| Obese | 0.95 | 0.83 to 1.09 | 0.48 | 1.01 | 0.80 to 1.27 | 0.96 | 0.68 |
| (C) CKD (low eGFR or albuminuria) | |||||||
| USA (vs Punjab) | 1.00 | – | – | 0.11 | 0.05 to 0.22 | <0.0001 | – |
| Age (per 10 years) | 1.04 | 1.00 to 1.09 | 0.06 | 1.20 | 1.12 to 1.29 | <0.0001 | 0.0007 |
| Male (vs female) | 0.97 | 0.86 to 1.09 | 0.58 | 0.81 | 0.70 to 0.94 | 0.007 | 0.0686 |
| Education high school + (vs no) | 1.00 | 0.90 to 1.12 | 0.96 | 0.91 | 0.77 to 1.09 | 0.31 | 0.379 |
| Current smoker (vs no) | 1.09 | 0.85 to 1.40 | 0.49 | 1.26 | 1.06 to 1.49 | 0.008 | 0.3526 |
| Hypertension (vs no) | 1.18 | 1.05 to 1.33 | 0.006 | 1.87 | 1.57 to 2.23 | <0.0001 | <0.0001 |
| DM (vs no) | 1.35 | 1.16 to 1.58 | 0.0002 | 2.11 | 1.77 to 2.53 | <0.0001 | 0.0002 |
| CVD (vs no) | 1.13 | 0.94 to 1.37 | 0.19 | 1.49 | 1.12 to 1.18 | 0.0006 | 0.072 |
| Total cholesterol (per 20 mg/dL, per 0.5 mmol/L) | 1.09 | 1.04 to 1.16 | 0.002 | 0.97 | 0.88 to 1.05 | 0.41 | 0.02 |
| BMI (per 5 kg/m2) | 0.98 | 0.94 to 1.03 | 0.48 | 1.06 | 1.01 to 1.12 | 0.017 | 0.025 |
| Obesity | |||||||
| Underweight | 0.89 | 0.72 to 1.10 | 0.28 | 1.31 | 0.67 to 2.54 | 0.43 | 0.28 |
| Healthy weight | 1.00 | – | Ref | 1.00 | – | Ref | – |
| Overweight | 0.98 | 0.86 to 1.13 | 0.81 | 0.98 | 0.79 to 1.22 | 0.84 | 0.97 |
| Obese | 0.95 | 0.83 to 1.09 | 0.48 | 1.06 | 0.86 to 1.29 | 0.60 | 0.41 |
BMI, body mass index; CVD, cardiovascular disease; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate.
table 3B displays the associations between patient factors and the prevalence of albuminuria. Significant differences between the samples was again seen with sex and the outcome (p=0.02). No association between sex and albuminuria was seen in Punjab, where in the US males had a lower prevalence of albuminuria (PR=0.77, p=0.004). While in both samples hypertension and DM were associated with a higher prevalence of albuminuria, the magnitude of association was much stronger in the USA (PR=1.19 in Punjab vs PR=1.93 in the USA for hypertension and PR=1.32 in Punjab vs PR=2.54 in the USA for DM). Current smoking was associated with albuminuria only in the USA (PR=1.34, p=0.002), while higher total cholesterol was associated with albuminuria in the Punjab (PR=1.11 per 0.5 mmol/L higher total cholesterol).
When combining low eGFR and albuminuria into a composite (CKD) outcome (table 3C), more differences were found between the samples in certain associations. Significantly larger associations were found in the USA for the relationship between older age, hypertension, DM and BMI, while a larger association was seen between total cholesterol and the composite CKD measure in the Punjab.
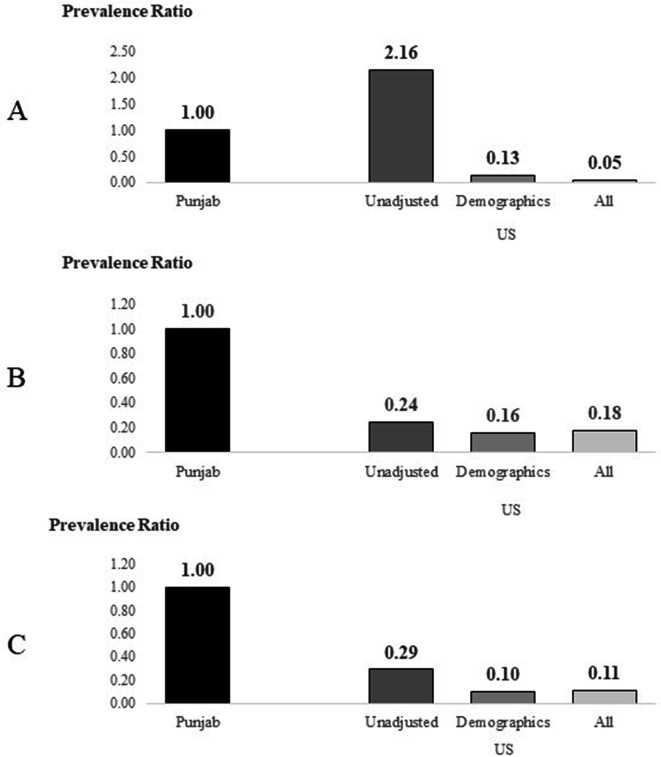
Unadjusted and less fully adjusted models are presented in online supplemental tables 1–3 for each of the kidney disease markers. As shown in figure 2, which displays the changes in PRs comparing USA to Punjab for each marker of CKD for different levels of adjustments, only low eGFR showed a marked change. Before accounting for any differences in participants in the two studies, the prevalence of low eGFR was much higher in the USA (PR=2.16), but after accounting for demographics (age and sex) and other health measures (remaining covariates), the USA has a much lower prevalence of low eGFR compared with Punjab (PR=0.13 and 0.05, respectively), suggesting that if the USA had the same patient make up as Punjab, the prevalence of low eGFR would be much lower. The findings for albuminuria and any CKD were very similar in showing that before adjustment the prevalence of either marker was much lower in the USA (PR=0.24 and 0.29, respectively) and accounting for difference in demographics and health measures between the samples changed these estimates very little. These results suggest that traditional risk factors do not entirely explain the difference in prevalence seen among markers of kidney disease between the US and Punjab.
Figure 2.
Changes in prevalence ratios between Punjab and the USA for markers of chronic kidney disease (CKD) with different levels of adjustment for risk factors. (A) Low estimated glomerular filtration rate, (B) albuminuria, (C) any CKD. Demographics=age, sex and education and all=demographics plus measures in table 3 (current smoker, hypertension, DM, cardiovascular disease, total cholesterol, obesity as body mass index categories).
bmjopen-2020-040444supp001.pdf (63.1KB, pdf)
In a sensitivity analysis, examining the association between risk factors and each kidney marker separately by sex, no significant changes in association direction or magnitude were detected (data not shown).
Discussion
In comparing two representative samples of participants from the adult population of Punjab, India and the USA, we found a very high prevalence of albuminuria in the Punjab, with almost half of the residents with urine ACR >3 mg/mmol (30 mg/g). This is in contrast to the prevalence of albuminuria in the USA of approximately 9%. When examining glomerular filtration rate, the Punjab had much higher average eGFR and a lower prevalence of eGFR <60 mL/min/1.73 m2 (2.0% vs 3.8%). Because of the high prevalence of albuminuria in the Punjab, almost half the population falls into the ‘moderately high risk’ CKD risk category per KDIGO risk stratification criteria. Even more striking is the fact that the between country differences in the prevalence estimates of albuminuria could not be explained by traditional risk factors for CKD, such as age, hypertension, and diabetes.
If true, these findings have enormous public health and resource implications for a low–middle income country such as India, specifically in the realm of CKD, cardiovascular disease and other NCDs. Currently, there are no definitive estimates of prevalence of CKD in India, as there is no ongoing national kidney registry/surveillance system. Recent publications have suggested that 220 000 patients are diagnosed with end stage renal disease (ESRD) every year.14 It is estimated that this will result in demand for an additional 34 million dialysis sessions in India each year. Besides the growing population of patients with kidneys disease, the country is faced with a shortage of nephrologists, late referral of patients, inadequate health awareness about preventive measures and a lack of more cost-effective alternatives like renal transplantation or PD.14 It has been estimated that 70% of those who start dialysis in India eventually give up dialysis due to financial constraints or death.15 The healthcare system, with most out-of-pocket expenditures borne by the households pose significant barriers to accessing health services with approximately 60 million households pushed below the poverty line in India as a result each year.16
We believe that our finding of the discordance observed in the prevalence of albuminuria versus lower eGFR between India and the USA could be in part due to the epidemiological transition that is occurring in countries such as India, where early evidence of kidney damage but lower prevalence of low eGFR defined kidney disease or end-stage kidney disease, may be the result of higher death rates among the younger population from premature cardiovascular disease, so while early kidney disease evidenced by albuminuria is more common, prevalence of later stages of kidney disease is lower (but potentially rising). Although not to the same degree, we reported similar findings in a recent study comparing CKD between China and the USA.17 China, another country which has gone through great economic and population growth in recent years, displayed a low prevalence of advanced kidney disease (eGFR <60 mL/min/1.73 m2), but a higher prevalence of albuminuria than the USA. The strength of association between traditional risk factors, such as hypertension and diabetes, were also weaker among the Chinese sample, although the association between age and CKD prevalence was much stronger.
Supportive evidence for a high rate of albuminuria in India have been reported from the western state of Gujarat.18 This study represents a voluntary sample of participants who were screened during a World Kidney Day Screening Camp. Even though the investigators excluded individuals at risk of albuminuria (participants with known diabetes, stone diseases, hypertension, kidney/liver/cardiac disease, hepatitis, HIV, transplant recipients, pregnant women and those <18 years of age), they estimated a 13.8% prevalence of albuminuria in their study. This is higher than in the US general population random sample in NHANES, which includes the individuals most likely to have albuminuria.
The high prevalence of albuminuria in Punjab could be related to the metabolic syndrome known to be associated with albuminuria.19 In this context, insulin resistance and visceral adiposity are common in developing nations and mechanistically linked with the metabolic syndrome through adipocytokines and inflammation.20 The high prevalence of premature cardiovascular disease and hypertension can be accompanied by albuminuria from vascular dysfunction or damage, leading to disruption of the glomerular filtration barrier. Furthermore, the evidence linking kidney disease to environmental factors continues to grow.21 Air pollution (highly prevalent in that part of the world), is associated with both endothelial dysfunction and low grade inflammation with resultant albuminuria. In the USA, PM 2.5 levels have been linked to the prevalence of CKD, risk of incident CKD, and its progression.22 23 This association is also being explored outside the USA with findings published from Taiwan and Korea showing similar results.24–26 India currently has some of the highest levels of air pollution in the world. It is estimated that 1.5 million people died from the effects of air pollution in 2012.27 28 While less studied, it is also plausible that kidney disease may be influenced by pollutants in both the water and soil as well, similar to the factors potentially underlying the epidemic of CKD of unknown aetiology, although this has not been reported from northern India, and albuminuria is not the hallmark of this latter condition.29
Unless actions are undertaken now to further investigate and reduce the high rate of albuminuria (although based on single cross-sectional estimates) reported in this study, the infrastructure and economy in India will be faced with a daunting task of needing to care for an increasing burden of those progressing to ESRD, in the not too distant future. Further, since albuminuria is also a marker of endothelial dysfunction and has been linked to cardiovascular outcomes, even at low levels, the higher risk of premature cardiovascular disease needs to be kept in mind in relation to albuminuria.30–32
To the best of our knowledge, this is the first study to estimate kidney disease prevalence at state level in India based on a random sample of the adult population living in a large, populous, northern Indian state. Further, it is also the first to compare prevalence of CKD between India and the USA (after adjusting for patient characteristics around the same time period in the two nations). However, it is not without limitations. Because the sample from India was only from one state, the Punjab, we cannot generalise our findings to all of India. Although this is a large state, the risk factor distribution and prevalence could be different in other areas of the country.33 In addition, the people, land and environment in India are diverse and of a highly variegated nature with significant urban–rural differences. It should also be acknowledged that the Punjab STEPS survey is cross sectional in design and while appropriately sampled to be representative of the state, may be limited by its sample size. The Punjab STEPS survey also employed commercially available point-of-care test strips, to assess albuminuria, whereas in the USA, this was assessed on the urine collected in a central laboratory. Lastly, both NHANES and the Punjab STEPS survey checked albuminuria and serum creatinine at a single point in time, whereas the KDIGO definition of CKD requires demonstration of persistence of these abnormalities. We believe, however, that repeat sampling of blood and urine in public health surveys, while highly desirable, is often difficult to achieve in the real world. Variations in albuminuria both within the same patient and across populations are possible; however, only single readings of albuminuria were available for each participant in this study.
Future research to confirm our findings using repeat sampling and similar studies in other states, and further examination of the association between environmental factors and kidney disease in India is urgently warranted. Such studies would benefit from having population samples from multiple states, preferably be longitudinal in nature, and have the potential to examine multiple environmental factors, while accounting for the traditional risk factors for kidney disease.
In summary, we report very high prevalence of albuminuria in a large state (the Punjab) in northern India. Albuminuria is considered an early sign of kidney damage as well as may reflect endothelial dysfunction, a harbinger of atherosclerosis-related cardiovascular disease. Progression of this early stage kidney and cardiovascular disease elevates the potential for an epidemic of ESRD and higher rates of cardiovascular disease in a country undergoing rapid epidemiological and economic transition. Urgent action and further research is needed to determine the underlying cause(s) of these findings, in the hopes of stemming the tide of rising rates of kidney failure and cardiovascular disease. India must clearly prepare for an inevitable increase in the need for renal replacement therapy in the coming years.
Supplementary Material
Acknowledgments
Punjab: We appreciate the support from Department of Health and Family welfare, Punjab especially Professor. KK Talwar, Advisor (Health), Government of Punjab, Ms. Vini Mahajan, IAS, Principal Secretary, Department of Health and Family Welfare and Mr. Hussan Lal, IAS, Secretary, Medical Education and Mission Director, National Health Mission, Punjab for their guidance and support for the project in conducting the study. Contributions by Biosense Pvt. Limited are acknowledged. We would like to thanks the members of Technical Advisory Committee and chairperson Prof. SK Jindal who provided technical oversight for the designing and implementation of survey. In addition inputs from advisors from University of Michigan were valuable. USA: The CDC CKD Surveillance System team has been led jointly by University of Michigan (Rajiv Saran (PI)); University of California, San Francisco (Neil Powe (PI)), since 2006 and funded and supported by Centers for Disease Control and Prevention (Nilka Ríos Burrows (Technical Advisor)).
Footnotes
Twitter: @drarnabpal, @rajiv_saran_1
Contributors: JB-G: data analysis and interpretation, manuscript writing, tables/figures creation. JST: study design (India), data collection, manuscript planning, manuscript review and editing. GJ: study design, data collection and programming, manuscript review and editing. SJ: study design, manuscript review and editing. AP: study design, manuscript review and editing. RP: study design, manuscript review and editing. SP: manuscript review and editing. RS: study concept, data interpretation, manuscript writing, manuscript review and editing.
Funding: Punjab: We sincerely acknowledge the financial support by National Health Mission (Punjab), Ministry of Health and Family Welfare, Government of India and technical support of WHO with special thanks to Dr Dhirendra N Sinha, Regional Advisor (NCD and Tobacco Surveillance), WHO-SEARO and Dr Lubna Bhatti, Epidemiologist, Prevention of Non-communicable Diseases (PND), World Health Organization, Geneva. Funding came from the National Health Mission, Punjab, India, JST. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. US: This work was supported in part by investigators working on the Supporting, Maintaining and Improving the Surveillance System for Chronic Kidney Disease in the U.S., Cooperative Agreement Number, U58 DP006254, funded by the Centers for Disease Control and Prevention. Its contents are solely the responsibility of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Department of Health and Human Services.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All US data from the National Health and Nutrition Examination Survey (NHANES) are publically available at https://www.cdc.gov/nchs/nhanes/index.htm. The Punjab data are available by request and approval through collaborative agreements with the sponsors. Professor JS Thakur is the Principal Investigator and can be contacted at jsthakur64@gmail.com.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Kundu M, Hazra S, Pal D, et al. . A review on noncommunicable diseases (NCDS) burden, its socio-economic impact and the strategies for prevention and control of NCDS in India. Indian J Public Health 2018;62:302–4. 10.4103/ijph.IJPH_324_16 [DOI] [PubMed] [Google Scholar]
- 2.Dandona L, Dandona R, Kumar GA, et al. . Nations within a nation: variations in epidemiological transition across the states of India, 1990–2016 in the global burden of disease study. The Lancet 2017;390 10.1016/S0140-6736(17)32804-0. [Epub ahead of print: 2017 Nov 14]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thakur JS, Jeet G, Pal A, et al. . Profile of risk factors for non-communicable diseases in Punjab, Northern India: results of a State-Wide steps survey. PLoS One 2016;11:e0157705. 10.1371/journal.pone.0157705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tripathy JP, Thakur JS, Jeet G, et al. . Alarmingly high prevalence of hypertension and pre-hypertension in North India-results from a large cross-sectional steps survey. PLoS One 2017;12:e0188619. 10.1371/journal.pone.0188619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathy JP, Thakur JS, Jeet G, et al. . Prevalence and risk factors of diabetes in a large community-based study in North India: results from a steps survey in Punjab, India. Diabetol Metab Syndr 2017;9:2017 10.1186/s13098-017-0207-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC), National center for health statistics (NCHS) . National health and nutrition examination survey data. Hyattsville, MD: U.S. department of health and human services, centers for disease control and prevention, 2013-2014. Available: https://wwwn.cdc.gov/nchs/nhanes/ContinuousNhanes/Default.aspx?BeginYear=2013
- 7.St John A, Tirimacco R, Badrick T, et al. . Internet support for point-of-care testing in primary care. Aust Fam Physician 2015;44:10–11. [PubMed] [Google Scholar]
- 8.Lim S, Yu H-J, Lee S, et al. . Evaluation of the URiSCAN 2 ACR strip to estimate the urine albumin/creatinine ratios. J Clin Lab Anal 2018;32:e22289. 10.1002/jcla.22289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavers BM, Simonson J, Michael AF. A solid phase fluorescent immunoassay for the measurement of human urinary albumin. Kidney Int 1984;25:576–8. 10.1038/ki.1984.57 [DOI] [PubMed] [Google Scholar]
- 10.Mulay AV, Gokhale SM. Comparison of serum creatinine-based estimating equations with gates protocol for predicting glomerular filtration rate in Indian population. Indian J Nephrol 2017;27:124–8. 10.4103/0971-4065.200515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.KDIGO: Kidney Disease: Improving Global Outcomes CKD Work Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013;3:1–150. [Google Scholar]
- 12.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 13.Zou GY, Donner A. Extension of the modified poisson regression model to prospective studies with correlated binary data. Stat Methods Med Res 2013;22:661–70. 10.1177/0962280211427759 [DOI] [PubMed] [Google Scholar]
- 14.Kaur G, Prinja S, Ramachandran R, et al. . Cost of hemodialysis in a public sector tertiary hospital of India. Clin Kidney J 2018;11:726–33. 10.1093/ckj/sfx152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kher V. End-Stage renal disease in developing countries. Kidney Int 2002;62:350–62. 10.1046/j.1523-1755.2002.00426.x [DOI] [PubMed] [Google Scholar]
- 16.Balarajan Y, Selvaraj S, Subramanian SV. Health care and equity in India. The Lancet 2011;377:505–15. 10.1016/S0140-6736(10)61894-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang F, He K, Wang J, et al. . Prevalence and risk factors for CKD: a comparison between the adult populations in China and the United States. Kidney Int Rep 2018;3:2018 Sep:1135–43. 10.1016/j.ekir.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trivedi H, Vanikar A, Patel H, et al. . High prevalence of chronic kidney disease in a semi-urban population of Western India. Clin Kidney J 2016;9:438–43. 10.1093/ckj/sfw009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rashidbeygi E, Safabakhsh M, Delshad Aghdam S, et al. . Metabolic syndrome and its components are related to a higher risk for albuminuria and proteinuria: evidence from a meta-analysis on 10,603,067 subjects from 57 studies. Diabetes Metab Syndr 2019;13:830–43. 10.1016/j.dsx.2018.12.006 [DOI] [PubMed] [Google Scholar]
- 20.Kumari R, Kumar S, Kant R. An update on metabolic syndrome: metabolic risk markers and adipokines in the development of metabolic syndrome. Diabetes Metab Syndr 2019;13:2409–17. 10.1016/j.dsx.2019.06.005 [DOI] [PubMed] [Google Scholar]
- 21.Xu X, Nie S, Ding H, et al. . Environmental pollution and kidney diseases. Nat Rev Nephrol 2018;14:313–24. 10.1038/nrneph.2018.11 [DOI] [PubMed] [Google Scholar]
- 22.Bragg-Gresham J, Morgenstern H, McClellan W, et al. . Centers for disease control and prevention CKD surveillance system. County-level air quality and the prevalence of diagnosed chronic kidney disease in the US Medicare population. PLoS One 2018;13:e0200612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowe B, Xie Y, Li T, et al. . Particulate matter air pollution and the risk of incident CKD and progression to ESRD. J Am Soc Nephrol 2018;29:218–30. 10.1681/ASN.2017030253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin S-Y, Hsu W-H, Lin C-L, et al. . Association of exposure to Fine-Particulate air pollution and acidic gases with incidence of nephrotic syndrome. Int J Environ Res Public Health 2018;15:E2860 10.3390/ijerph15122860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan T-C, Zhang Z, Lin B-C, et al. . Long-Term exposure to ambient fine particulate matter and chronic kidney disease: a cohort study. Environ Health Perspect 2018;126:107002. 10.1289/EHP3304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim H-J, Min J-Y, Seo Y-S, et al. . Association between exposure to ambient air pollution and renal function in Korean adults. Ann Occup Environ Med 2018;30:2018:14. 10.1186/s40557-018-0226-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma AK, Baliyan P, Kumar P. Air pollution and public health: the challenges for Delhi, India. Rev Environ Health 2018;33:77–86. 10.1515/reveh-2017-0032 [DOI] [PubMed] [Google Scholar]
- 28.Bulletin of the World Health Organization 2016;94:487-488. Available: 10.2471/BLT.16.020716 [DOI] [PMC free article] [PubMed]
- 29.Correa-Rotter R, Wesseling C, Johnson RJ. Ckd of unknown origin in central America: the case for a Mesoamerican nephropathy. Am J Kidney Dis 2014;63:506–20. 10.1053/j.ajkd.2013.10.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang M-J, Wei R-B, Zhao J, et al. . Albuminuria and endothelial dysfunction in patients with non-diabetic chronic kidney disease. Med Sci Monit 2017;23:4447–53. 10.12659/MSM.903660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmieder RE, Schrader J, Zidek W, et al. . Low-grade albuminuria and cardiovascular risk : what is the evidence? Clin Res Cardiol 2007;96:247–57. 10.1007/s00392-007-0510-3 [DOI] [PubMed] [Google Scholar]
- 32.Seliger SL, Salimi S, Pierre V, et al. . Microvascular endothelial dysfunction is associated with albuminuria and CKD in older adults. BMC Nephrol 2016;17:82. 10.1186/s12882-016-0303-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes M, Begum R, Sati P, et al. . Nationwide mortality studies to quantify causes of death: Relevant lessons from india’s million death study. Health Aff 2017;36:1887–95. 10.1377/hlthaff.2017.0635 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-040444supp001.pdf (63.1KB, pdf)