Abstract
Immune memory cells residing in previously infected, nonlymphoid tissues play a role in immune surveillance. In the event that circulating antibodies fail to prevent virus spread to the tissues in a secondary infection, these memory cells provide an essential defense against tissue reinfection. CNS tissues are isolated from circulating immune cells and antibodies by the blood–brain barrier, making the presence of tissue-resident immune memory cells particularly needed to combat recurrent infection by neurotropic viruses. Wild-type and laboratory-engineered rabies viruses are neurotropic, differ in pathogenicity, and have varying effects on BBB functions. These viruses have proven invaluable tools in demonstrating the importance of tissue-resident immune memory cells in the reinfection of CNS tissues. Only Type 1 immune memory is effective at therapeutically clearing a secondary infection with wild-type rabies viruses from the CNS and does so despite the maintenance of blood–brain barrier integrity.
Keywords: : antibody, brain immune memory, CNS, encephalitis, rabies, tissue-resident memory cells, tumor biology, Type 1 immunity
Lay abstract
The immune system is a complex network of cells and organs that help the body fight infection caused by the invasion of germs, such as bacteria and viruses. When battling such an attack, our immune system learns how to recognize the invader’s specific characteristics by generating immune memory cells. These cells can deal with the infectious agent more rapidly and effectively when it is reencountered. Immune memory cells have various properties depending on their localization in the body. Specialized immune memory cells that remain in organs, rather than traveling back into the bloodstream, are called tissue-resident memory cells and are the key responders to reinfection of the tissue where they reside. Here, we review the role of brain tissue-resident immune memory cells in fighting the deadly rabies virus.
Tissue-resident memory cells
The maintenance of long-term immunity and protection against reinfection is a hallmark of the adaptive immune response to most pathogens. This feature is due to the persistence of functionally and phenotypically distinct populations of antigen-specific memory cells (Table 1). These subsets include CD4 and CD8 T cells, central memory T cells (Tcm), effector memory T cells (Tem) and tissue-resident memory T cells (Trm) [1]. Memory B cells, long-lived plasma cells and Trm maintain antigen-specific B cell longevity [2]. Tcm and Tem generally recirculate through the blood and lymphatics or in secondary lymphoid organs. However, some do not recirculate and remain in the tissue involved in the initial response. These Trm play a key role in the rapid deployment of immune effectors in response to reinfection [3]. Evidence of Trm has been described for various tissues, including intestinal mucosa, skin, liver, lung and brain [4,5].
Table 1. . Memory immune cell subsets.
| Subset name | Location | Blood trafficking | Lymph node trafficking | Function |
|---|---|---|---|---|
| Tcm | Lymphoid organs | Yes | Yes | Enhance likelihood of specific T cell–APC encounters and magnitude of the secondary immune response |
| Tem | Nonlymphoid organs | Yes | No | Effector function in chronic and secondary reinfection |
| Trm | Nonlymphoid organs | No | No | Direct tissue immune response and immune surveillance |
| Bm | Germinal center lymphoid organs | Yes | Yes | Secondary humoral immune response |
This table summarizes the main subsets of immune memory cells with their localization and functions.
APC: Antigen-presenting cell; Bm: Memory B lymphocyte; Tcm: Central memory T cell; Tem: Effector memory T cell; Trm: Tissue-resident memory T cell.
Neuroimmunity & the blood–brain barrier
The neurovasculature has specialized tight junctions and other properties, collectively known as the blood–brain barrier (BBB), that restrict the infiltration of circulating cells and factors into vulnerable brain tissues [6]. As seen with rabies virus (RABV) infection, immune effectors’ inability to enter the CNS can have pathological repercussions. Naturally occurring RABV are highly pathogenic, causing lethal infection of the CNS in mammals, including humans. When inactivated or attenuated by a variety of approaches, some RABV strains can be used for vaccination. Inactivated RABV vaccines effectively prevent clinical rabies in exposed individuals when combined with the administration of virus-neutralizing antibodies (VNA). However, this post-exposure prophylaxis paradigm fails when wild-type RABV enters CNS tissues by retrograde axonal spread, leaving the BBB intact, and therefore, preventing the infiltration of immune effectors [7]. Despite using the same trans-axonal route to enter CNS tissues, certain attenuated RABV are efficiently cleared from the CNS [8,9]. Figure 1 illustrates the two extremes with respect to access of immune cells to CNS tissues infected with RABV. However, the immune response generated by a concomitant secondary infection (superinfection) with a live-attenuated RABV can clear wild-type RABV from the CNS [10]. This observation indicates that unlike live-attenuated RABV infection of the CNS, similar infection with wild-type RABV fails to induce processes leading to ‘opening’ the BBB, thereby preventing immune effector delivery into CNS tissue and evading immune clearance [11]. Clearance involves immune effector infiltration into CNS tissues through a BBB that has become permeable, but to a much lesser extent than that seen in a CNS inflammatory response [12]. The mechanism of BBB permeability induced by infection with attenuated RABV involves the production in the neurovasculature of IFN-gamma and radicals dependent upon the activity of peroxynitrite (ONOO-), a downstream product of nitric oxide and superoxide [13,14]. Wild-type RABV, such as the dog-derived (DRV4) or the bat-derived silver-haired bat rabies virus (SHBRV) viruses, are strictly neurotropic, infect neurons exclusively and do not open the BBB. However, it has been recently demonstrated that attenuated RABV can also infect astrocytes ([15], Bertoune et al., submitted) inducing astrocytes to produce anti-viral IFN-beta [16]. Moreover, virus particles can be detected in close vicinity to blood vessels (Unpublished Data). Given the crucial role of astrocytic end-feet in the maintenance of BBB homeostasis there is a strong possibility that the infection of astrocytes, unique to attenuated RABV, is responsible for induction of the processes in the neurovascular unit that lead to immune effector infiltration across the BBB and, ultimately, virus clearance from CNS tissues.
Figure 1. . Clearance of rabies virus from the CNS depends on blood–brain barrier permeability and immune cell infiltration.
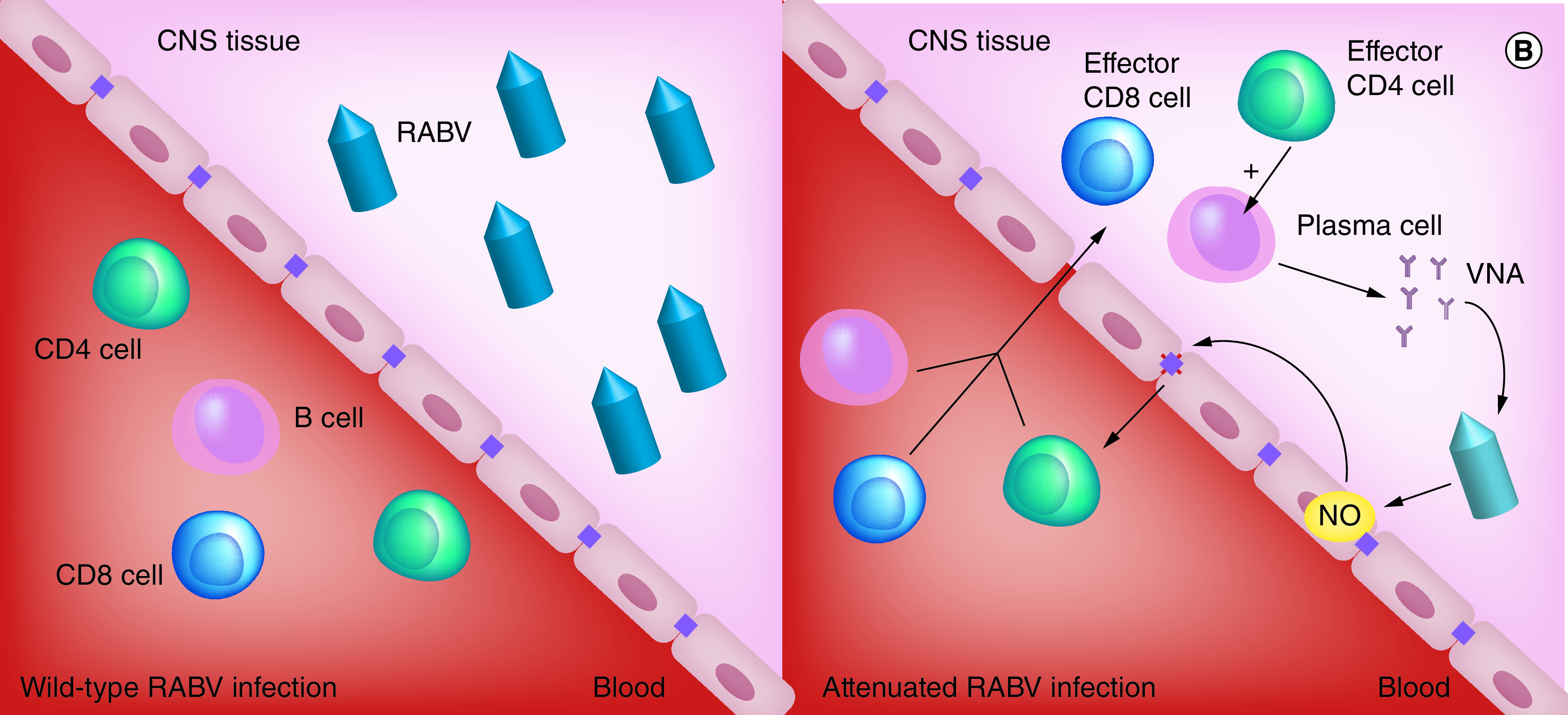
(A) Wild-type RABV travel by axonal retrograde transport from the infection site to the CNS, thereby leaving the BBB intact and preventing the infiltration of immune effectors. (B) Infection with an attenuated virus leads to the BBB’s permeabilization, allowing the recruitment of immune effectors to the CNS. The cooperation of CD4+ cells and B cells induces VNA and clearance of the virus.
BBB: Blood–brain barrier; EC: Endothelial cell; NO: Nitric oxide; RABV: Rabies virus; VNA: Virus neutralizing antibody.
Immune bias in the RABV response
There is a dichotomy between the immune mechanisms that clear RABV from the periphery or from the CNS. In both cases, the major effector of RABV clearance is VNA [17]. Vaccination with inactivated RABV is highly effective at inducing VNA production and preventing RABV spread from the periphery to the CNS. However, killed viruses, as a vaccine, offer inadequate protection against intranasal challenge with wild-type virus [18]. Thus, it is not surprising that post-exposure prophylaxis using inactivated RABV vaccine fails when a wild-type virus reaches the CNS. Inactivated vaccines are ineffective because they fail to induce RABV-specific immune mechanisms that are effective in CNS tissues including CD8 T cells. Indeed, the fact that mice lacking CD8 T cells exhibit a significant delay in clearing attenuated RABV from CNS tissues but eventually do support this hypothesis [19]. In our view, the evidence of a dichotomy between peripheral and CNS immunity has implications for the therapy of rabies patients. With respect to the active immune response, one requirement would be the use of a vaccine that engenders Type 1 immunity as Type 2 immunity is not as effective in CNS tissues. In this regard, VNA are central to the rapid control of virus spread yet not all VNA appear to be equivalent at blocking cell-to-cell transmission. Consequently, the correct VNA administered systemically and perhaps even intrathecally would likely be essential to provide time for adaptive immunity to develop. Then, there is the issue of delivering immune effectors into CNS tissue, which would require infiltration across the BBB. By therapeutically driving the appropriate immune effectors into CNS tissues intranasal administration of a live-attenuated RABV vaccine may prove suitable in this scenario.
Immune responses against infectious agents are generally biased toward either Type 1 or Type 2 responses. Type 1 immune responses involve cytolytic CD8 T cells, the production of IFN-gamma by CD4 T helper 1 (Th1) cells, and the production of IgG1 and IgG3 antibodies in humans or IgG2a antibodies in mice. Type 2 immune responses require secretion of IL-4 and IL-5 by CD4 Th2 cells and the production of IgG2 and IgG4 antibodies in humans or IgG1 antibodies in mice. Having identified IFN-gamma as a key player in the induction of BBB permeability, we wondered if immune bias may contribute to the difference in outcomes between vaccination with inactivated versus live attenuated RABV vaccines. Therefore, we examined RABV immunity in mice lacking the T-bet transcription factor (T-bet-/- mice), which is essential for the development of Th1 cells and the production of IFN-gamma [20]. In these animals, infiltrating CD8 T and NK cells produce the IFN-gamma detected in the CNS. Additionally, these mice show normal Th2 activity, produce copious amounts of RABV-specific IgG1 VNA in response to intranasal infection with attenuated RABV, and only have a minor deficit in clearing the virus from CNS tissues [21]. Except for CD4 T cells, immune cell infiltration into CNS tissues is comparable between T-bet-/- and control mice [21]. However, antibody production in CNS tissues of T-bet-/- animals is severely curtailed. T-bet-/- mice that have cleared attenuated RABV from their CNS tissues are poorly protected against subsequent intranasal challenge with wild-type RABV [21]. As illustrated in Figure 2, the deficit is evidently in the local production of VNA due to the inability of the CD4 Th2 cells that infiltrate CNS tissues to provide help for B cells. These data led us to conclude that a Type 1 immune response is required for long-term protection of CNS tissues from reinfection with RABV.
Figure 2. . Clearance of rabies virus from the CNS requires Type 1 immunity.
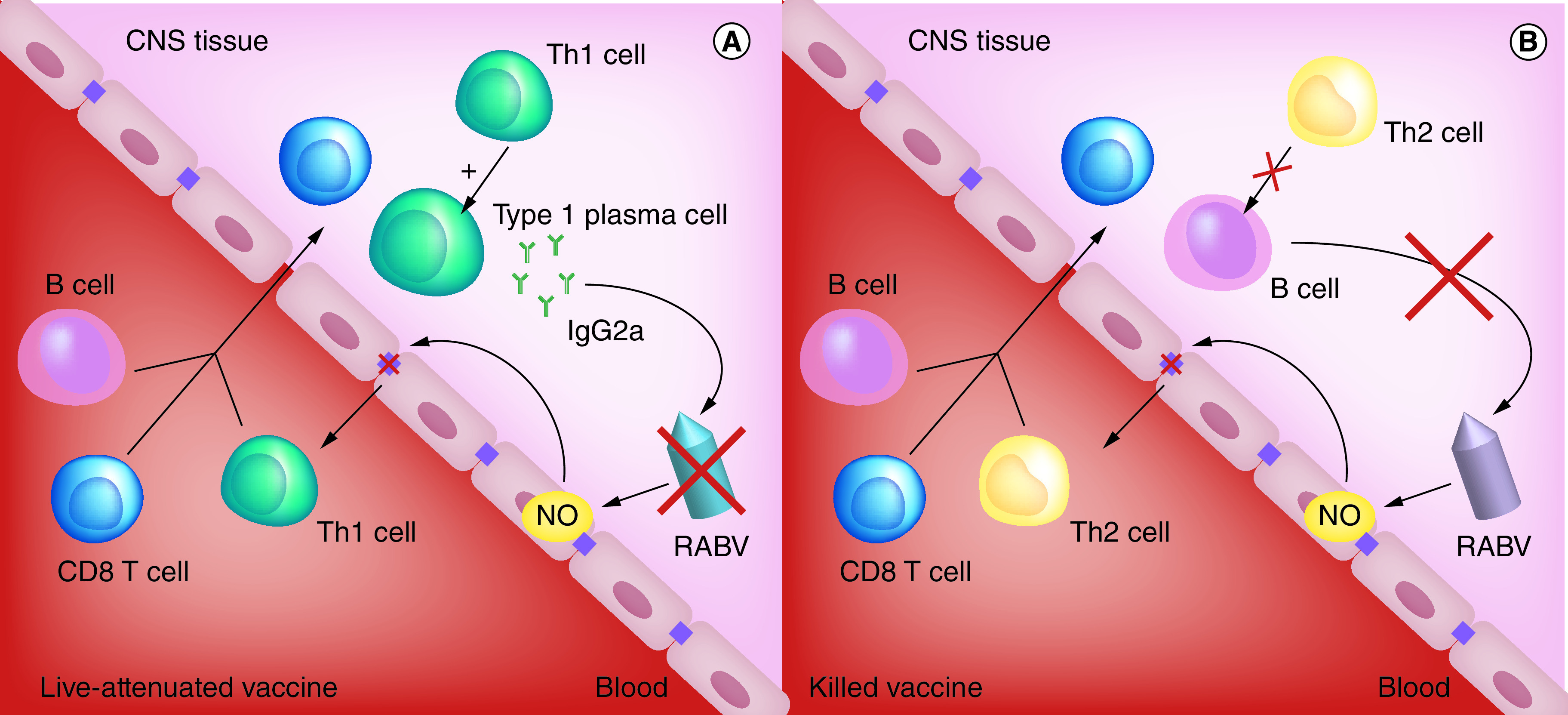
(A) Infection with a live-attenuated rabies vaccine induces the recruitment of Th1 cells in the brain and leads to the production of virus-killing IgG2a antibodies. (B) Infection with a killed vaccine induces the recruitment of Th2 cells. These cells are inactive in the CNS, severely curtailing the production of virus-killing antibodies.
EC: Endothelial cell; NO: Nitric oxide; RABV: Rabies virus; Th1: CD4+ T helper cell.
CNS immune memory
CD4, CD8 T and B cells expressing resident memory markers have been demonstrated in brain tissues following acute infection with vesicular stomatitis virus [22–24], West Nile virus [25–27], and attenuated RABV [5]. The importance of these cells in recurrent infections is questionable. In the case of RABV, recurrent infections of CNS tissues are unlikely since exposure to the virus is peripheral (leg or finger), where immune recall mechanisms would act to prevent spread locally. However, brain tissue-resident memory cells may play an essential role in persisting virus infections. Most studies of brain tissue-resident immune memory have focused on CD8 Trm, where cytolytic responses are the most effective [28]. Wild-type rabies viruses are somewhat different. By replicating in neurons that usually express low levels of MHC class I, VNA are considered the most critical immune effector [19,29]. Besides, there have been several cases of aerosol challenges resulting in serious CNS infections in previously immunized individuals [30,31]. These facts make RABV of interest for the study of brain tissue-resident memory cells. The availability of different classes of rabies vaccines and intranasal challenge with wild-type virus represent unique resources to do so.
Building on the understanding that only a Type 1 immune response offers long-term protection against intranasal challenge with wild-type RABV [18], we sought to determine whether resident memory cells could be detected in brain tissue following the clearance of attenuated RABV. This hypothesis proved to be the case [5]. However, this does not guarantee that the cells contribute to a recall response in CNS tissues. Peripheral memory cells, or even persisting VNA in the circulation, could conceivably be responsible for a secondary response in the CNS. To test this possibility, we took advantage of a highly attenuated RABV with limited capacity to spread to the CNS from the periphery. Groups of mice were immunized with this virus, TriGAS, in either the gastrocnemius or masseter muscles, with only the latter resulting in detectable virus replication in the CNS. Both routes of infection induced comparable RABV-specific Type 1 immunity as measured by serum VNA. Nevertheless, only the mice that had cleared the attenuated virus from CNS tissues were fully protected against an intranasal challenge with wild-type RABV [5]. These data strongly suggested that tissue-resident memory cells play an essential role in protecting the CNS against recurrent infection. We envisaged two possible scenarios in wild-type RABV clearance, as shown in Figure 3: 1/memory T cells promote BBB permeability, but immune effectors entering from the circulation are responsible for virus clearance, or 2/tissue-resident memory cells are primarily responsible for virus clearance from the CNS. Only in the latter case could the BBB remain intact. We could not detect any change in BBB integrity during a rapid increase in immune cell numbers and activity in CNS tissues as wild-type RABV was cleared from the CNS tissues of mice previously immunized with attenuated RABV [11]. Therefore, we concluded that Type 1 immune memory cells resident in CNS tissues after immunization are capable of clearing a secondary infection independently of the de novo delivery of immune effectors from the periphery.
Figure 3. . Hypothetical roles of brain tissue-resident immune memory cells in secondary infection with a wild-type rabies virus.
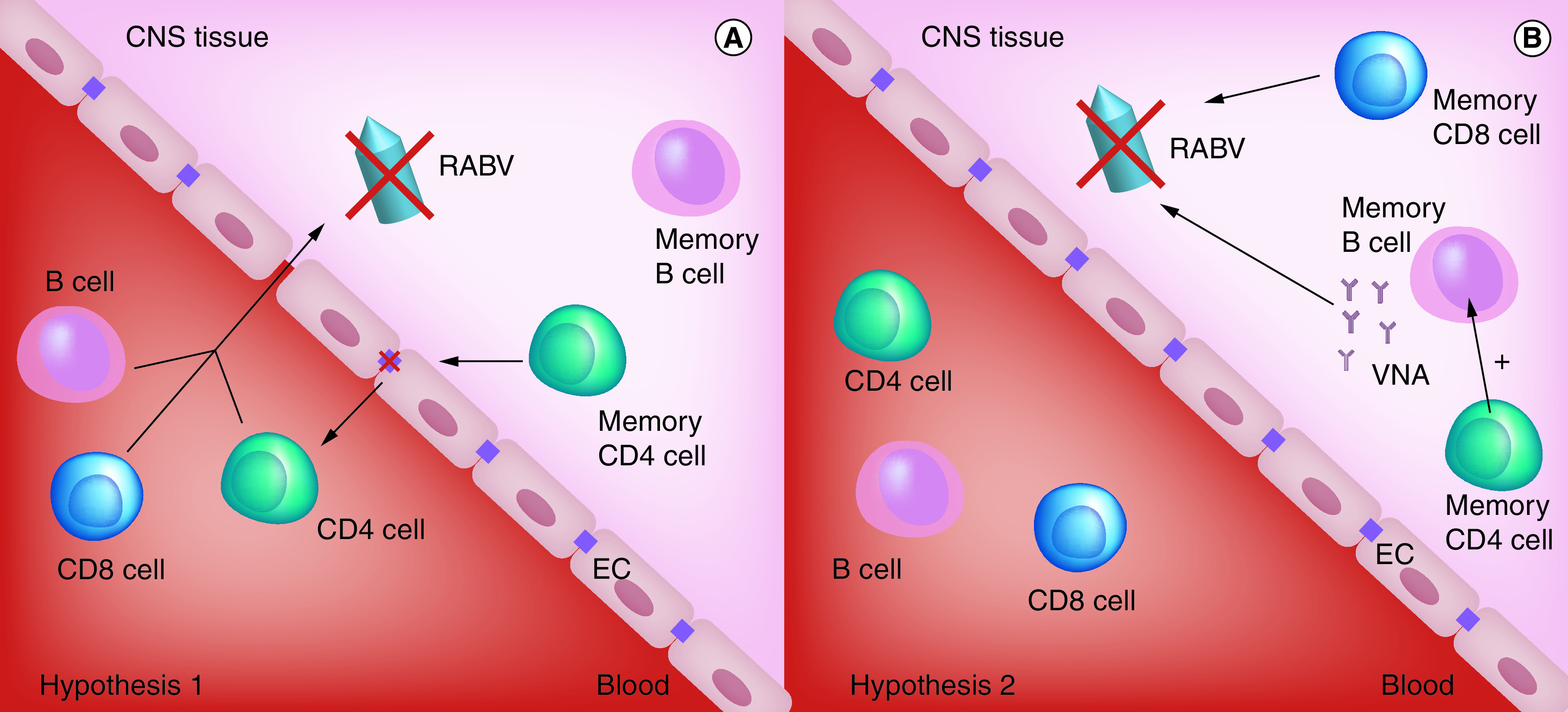
We envisaged two possible scenarios in wild-type RABV clearance: (A) memory T cells promote BBB permeability, but immune effectors entering the brain from the circulation are responsible for virus clearance, or (B) tissue-resident memory cells are primarily responsible for virus clearance from the CNS.
BBB: Blood–brain barrier; EC: Endothelial cell; RABV: Rabies virus; VNA: Virus neutralizing antibody.
Conclusion
Clearance of an infectious agent from tissues generally results in the local establishment of immune memory cells that mediate a rapid local response to reinfection of the tissues. Our studies demonstrate that for RABV brain tissues are no exception. When attenuated RABV are cleared from the CNS through the activity of immune cells that infiltrate across the BBB, immune memory cells become resident in brain tissues. This does not happen when an immune response to RABV is restricted to the periphery. In the absence of tissue-resident memory cells, secondary CNS infection with wildtype RABV, for example by the intranasal route, is lethal despite the presence of strong peripheral immunity. Infection with wild-type RABV fails to induce the changes in the BBB that promote immune cell infiltration from the periphery into CNS tissues. Under these circumstances the activities of tissue-resident immune memory cells are key to virus clearance and survival.
Future perspective
While rare occurrences, the fact that several individuals previously immunized against rabies still suffered serious secondary CNS infections with wild-type RABV, likely due to aerosol exposure, illustrates the importance of brain tissue-resident memory cells [30,31]. Due to the absence of viral antigen and immune stimulation in the CNS, vaccination with inactivated rabies vaccine would not result in the establishment of immune memory in CNS tissues. Moreover, the response triggered by conventional human rabies vaccination has a Type 2 immune bias, and our data indicate that Th2 cells are not active in brain tissues. Additionally, in such cases the infiltration of immune effectors into the CNS would likely be delayed due to the requirement to open the BBB. Brain tissue-resident memory cells are clearly capable of mediating the clearance of RABV in the absence of alterations in BBB integrity. Since exposures to RABV are generally through bites or scratches by an infected animal, the role of CNS resident immune memory in rabies is limited. Nevertheless, studies with RABV provide clear evidence that such cells may be major contributors to the immune control of other CNS disease states including persisting infections of the CNS and brain tumors. Immune effectors in the circulation must cross the BBB to infiltrate CNS tissues but the inflammatory mechanisms that lead to BBB permeability most often present a risk of significant pathology. CNS resident immune memory cells seeded during viral encephalitis would be capable of controlling recurrent infection without reopening the BBB. In the case of brain cancer, the establishment of CNS resident immune memory while the BBB is compromised during tumor progression or surgery may impede tumor recurrence.
The lack of BBB alterations allowing infiltration of immune effectors is a major obstacle for immunotherapy where, for instance, cytotoxic CD8 activity against tumor antigens is necessary. In this regard the unique effects of attenuated RABV on the BBB are also of importance. CNS infection with these viruses induces a Type 1 immune response that promotes immune cell entry in the absence of inflammatory damage to the BBB [32]. As an example, this strategy can be used to therapeutically modulate the brain tumor microenvironment [33]. A more advanced modality for CNS immunotherapy would likely result from a better understanding of the virus-induced changes in the cells composing the BBB and how the response to RABV avoids macrophage activation despite high-level IFN-gamma production. While other studies of CNS resident immune memory elicited by virus infection have proven the existence of CD8 Trm [28,34], our work with RABV clearly shows that CD4 and B memory cells persist in CNS tissues and evidently cooperate in the rapid production of antibody in response to recurrent infection. While VNA are clearly important in the inactivation or RABV and other viruses, studies in the mouse GL261 model suggest that antibodies are also critical for glioma immunity [33]. We should, therefore, be striving for the establishment of CD4, CD8 and B cell tissue-resident immune memory for long-lasting surveillance against viral reactivation and tumor recurrence in the CNS.
Executive summary.
Tissue-resident memory cells
In addition to CD8 tissue-resident memory cells, long-lived tissue-resident CD4 T and B memory cells can be established in brain tissue to locally mediate a secondary humoral response to challenge while blood–brain barrier integrity is maintained.
Brain immunity & the blood–brain barrier
The prior establishment of rabies virus (RABV)-specific CD4, CD8 and B memory cells in CNS tissues is required for the effective clearance of a secondary CNS infection with wild-type RABV. Comparable immune mechanisms restricted to the periphery are not sufficient.
Immune bias in the RABV response
CD4 Th1 cells are active in CNS tissues, but Th2 cells are not.
CNS immune memory
Strong peripheral immunity to RABV cannot prevent the inhaled virus from reaching CNS tissues.
Footnotes
Author contributions
DC Hooper conceived the hypothesis; A Lebrun and DC Hooper wrote the paper; A Lebrun created the figures and RB Kean proofread the manuscript.
Financial & competing interests disclosure
This work was supported by grants from the National Institutes of Health: R01 AI093369 – Virus clearance from the CNS and U01 AI083046 – Host–pathogen competition in IFN-mediated antiviral defense. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Masopust D, Soerens AG. Tissue-resident T cells and other resident leukocytes. Annu. Rev. Immunol. 37, 521–546 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This is an excellent review giving a good overview of tissue-resident memory cells.
- 2.Allie SR, Randall TD. Resident memory B cells. Viral Immunol. 33(4), 282–293 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosato PC, Beura LK, Masopust D. Tissue resident memory T cells and viral immunity. Curr. Opin. Virol. 22, 44–50 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bevan MJ. Memory T cells as an occupying force. Eur. J. Immunol. 41(5), 1192–1195 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia SA, Lebrun A, Kean RB, Craig Hooper D. Clearance of attenuated rabies virus from brain tissues is required for long-term protection against CNS challenge with a pathogenic variant. J. Neurovirol. 24(5), 606–615 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Contains data supporting the concept that brain tissue-resident immune memory cells are important in protecting CNS tissues against in rabies virus reinfection.
- 6.Obermeier B, Verma A, Ransohoff RM. The blood-brain barrier. Handb. Clin. Neurol. 133, 39–59 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Roy A, Hooper DC. Immune evasion by rabies viruses through the maintenance of blood–brain barrier integrity. J. Neurovirol. 14(5), 401–411 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Hooper DC, Roy A, Barkhouse DA, Li J, Kean RB. Rabies virus clearance from the central nervous system. Adv. Virus Res. 79, 55–71 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Hooper DC, Roy A, Kean RB, Phares TW, Barkhouse DA. Therapeutic immune clearance of rabies virus from the CNS. Future Virol. 6(3), 387–397 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy A, Hooper DC. Lethal silver-haired bat rabies virus infection can be prevented by opening the blood–brain barrier. J. Virol. 81(15), 7993–7998 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy A, Phares TW, Koprowski H, Hooper DC. Failure to open the blood–brain barrier and deliver immune effectors to central nervous system tissues leads to the lethal outcome of silver-haired bat rabies virus infection. J. Virol. 81(3), 1110–1118 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood–brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J. Immunol. 176(12), 7666–7675 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Hooper DC, Kean RB, Scott GS. et al. The central nervous system inflammatory response to neurotropic virus infection is peroxynitrite dependent. J. Immunol. 167(6), 3470–3477 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood–brain barrier permeability changes during a central nervous system inflammatory response: TNF-alpha is neither necessary nor sufficient. J. Immunol. 178(11), 7334–7343 (2007). [DOI] [PubMed] [Google Scholar]
- 15.Preuss MA, Faber ML, Tan GS. et al. Intravenous inoculation of a bat-associated rabies virus causes lethal encephalopathy in mice through invasion of the brain via neurosecretory hypothalamic fibers. PLoS Pathog. 5(6), e1000485 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfefferkorn C, Kallfass C, Lienenklaus S. et al. Abortively infected astrocytes appear to represent the main source of interferon beta in the virus-infected brain. J. Virol. 90(4), 2031–2038 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Presents the first evidence that rabies-infected astrocytes produce anti-viral cytokines.
- 17.Hooper DC, Phares TW, Fabis MJ, Roy A. The production of antibody by invading B cells is required for the clearance of rabies virus from the central nervous system. PLoS Negl. Trop. Dis. 3(10), e535 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lebrun A, Garcia S, Li J, Kean RB, Hooper DC. Protection against CNS-targeted rabies virus infection is dependent upon Type-1 immune mechanisms induced by live-attenuated rabies vaccines. Trop. Med. Infect. Dis. 2(3), 22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides data supporting the use of live-attenuated rabies virus stains rather than a killed virus for the induction of immune mechanisms capable of efficacy in CNS tissues.
- 19.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J. Virol. 72(5), 3711–3719 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100(6), 655–669 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Lebrun A, Portocarrero C, Kean RB, Barkhouse DA, Faber M, Hooper DC. T-bet is required for the rapid clearance of attenuated rabies virus from central nervous system tissue. J. Immunol. 195(9), 4358–4368 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Provides evidence of the key role of Th1 lymphocytes in the clearance of rabies virus from CNS tissues.
- 22.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J. Virol. 81(4), 2039–2046 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wakim LM, Woodward-Davis A, Liu R. et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J. Immunol. 189(7), 3462–3471 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zickovich JM, Meyer SI, Yagita H, Obar JJ. Agonistic anti-CD40 enhances the CD8+ T cell response during vesicular stomatitis virus infection. PLoS ONE 9(8), e106060 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo H, Winkelmann E, Xie G. et al. MAVS is essential for primary CD4(+) T cell immunity but not for recall T cell responses following an attenuated West Nile virus infection. J. Virol. 91(6), e02097–16 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parsons R, Lelic A, Hayes L. et al. The memory T cell response to West Nile virus in symptomatic humans following natural infection is not influenced by age and is dominated by a restricted set of CD8+ T cell epitopes. J. Immunol. 181(2), 1563–1572 (2008). [DOI] [PubMed] [Google Scholar]
- 27.Smith HL, Monath TP, Pazoles P. et al. Development of antigen-specific memory CD8+ T cells following live-attenuated chimeric West Nile virus vaccination. J. Infect. Dis. 203(4), 513–522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad S, Lokensgard JR. Brain-resident T Cells following viral infection. Viral Immunol. 32(1), 48–54 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gnanadurai CW, Zhou M, He W. et al. Presence of virus neutralizing antibodies in cerebral spinal fluid correlates with non-lethal rabies in dogs. PLoS Negl. Trop. Dis. 7(9), e2375 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibbons RV. Cryptogenic rabies, bats, and the question of aerosol transmission. Ann. Emerg. Med. 39(5), 528–536 (2002). [DOI] [PubMed] [Google Scholar]
- 31.Winkler WG, Fashinell TR, Leffingwell L, Howard P, Conomy P. Airborne rabies transmission in a laboratory worker. JAMA 226(10), 1219–1221 (1973). [PubMed] [Google Scholar]
- 32.Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. Blood–brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. PNAS 105(40), 15511–15516 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bongiorno EK, Garcia SA, Sauma S, Hooper DC. Type 1 immune mechanisms driven by the response to infection with attenuated rabies virus result in changes in the immune bias of the tumor microenvironment and necrosis of mouse GL261 brain tumors. J. Immunol. 198(11), 4513–4523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Supports the concept that the CNS immune response to attenuated rabies virus is Type 1 in reprogramming the Type 2 tumor microenvironment causing tumor cell death.
- 34.Wu X, Wu P, Shen Y, Jiang X, Xu F. CD8(+) resident memory T Cells and viral infection. Front. Immunol. 9, 2093 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


