Key Points
Question
Does mindfulness-based stress reduction (MBSR) improve migraine outcomes and affective/cognitive processes compared with headache education?
Findings
In this randomized clinical trial of 89 adults who experienced between 4 and 20 migraine days per month, standardized training in mindfulness and yoga through MBSR did not improve migraine frequency more than headache education about migraine, as both groups had similar decreases.
Meaning
Mindfulness meditation may help treat the total burden of migraine, although a larger, more definitive study is needed to further investigate these results to understand the association of mindfulness with migraine outcomes.
This randomized clinical trial examines whether mindfulness-based stress reduction and headache education improve migraine day frequency and improvements in disability, quality of life, depression scores, pain catastrophizing, and self-efficacy for people who experience migraines.
Abstract
Importance
Migraine is the second leading cause of disability worldwide. Most patients with migraine discontinue medications due to inefficacy or adverse effects. Mindfulness-based stress reduction (MBSR) may provide benefit.
Objective
To determine if MBSR improves migraine outcomes and affective/cognitive processes compared with headache education.
Design, Setting, and Participants
This randomized clinical trial of MBSR vs headache education included 89 adults who experienced between 4 and 20 migraine days per month. There was blinding of participants (to active vs comparator group assignments) and principal investigators/data analysts (to group assignment).
Interventions
Participants underwent MBSR (standardized training in mindfulness/yoga) or headache education (migraine information) delivered in groups that met for 2 hours each week for 8 weeks.
Main Outcomes and Measures
The primary outcome was change in migraine day frequency (baseline to 12 weeks). Secondary outcomes were changes in disability, quality of life, self-efficacy, pain catastrophizing, depression scores, and experimentally induced pain intensity and unpleasantness (baseline to 12, 24, and 36 weeks).
Results
Most participants were female (n = 82, 92%), with a mean (SD) age of 43.9 (13.0) years, and had a mean (SD) of 7.3 (2.7) migraine days per month and high disability (Headache Impact Test-6: 63.5 [5.7]), attended class (median attendance, 7 of 8 classes), and followed up through 36 weeks (33 of 45 [73%] of the MBSR group and 32 of 44 [73%] of the headache education group). Participants in both groups had fewer migraine days at 12 weeks (MBSR: −1.6 migraine days per month; 95% CI, −0.7 to −2.5; headache education: −2.0 migraine days per month; 95% CI, −1.1 to −2.9), without group differences (P = .50). Compared with those who participated in headache education, those who participated in MBSR had improvements from baseline at all follow-up time points (reported in terms of point estimates of effect differences between groups) on measures of disability (5.92; 95% CI, 2.8-9.0; P < .001), quality of life (5.1; 95% CI, 1.2-8.9; P = .01), self-efficacy (8.2; 95% CI, 0.3-16.1; P = .04), pain catastrophizing (5.8; 95% CI, 2.9-8.8; P < .001), depression scores (1.6; 95% CI, 0.4-2.7; P = .008), and decreased experimentally induced pain intensity and unpleasantness (MBSR group: 36.3% [95% CI, 12.3% to 60.3%] decrease in intensity and 30.4% [95% CI, 9.9% to 49.4%] decrease in unpleasantness; headache education group: 13.5% [95% CI, −9.9% to 36.8%] increase in intensity and an 11.2% [95% CI, −8.9% to 31.2%] increase in unpleasantness; P = .004 for intensity and .005 for unpleasantness, at 36 weeks). One reported adverse event was deemed unrelated to study protocol.
Conclusions and Relevance
Mindfulness-based stress reduction did not improve migraine frequency more than headache education, as both groups had similar decreases; however, MBSR improved disability, quality of life, self-efficacy, pain catastrophizing, and depression out to 36 weeks, with decreased experimentally induced pain suggesting a potential shift in pain appraisal. In conclusion, MBSR may help treat total migraine burden, but a larger, more definitive study is needed to further investigate these results.
Trial Registration
ClinicalTrials.gov Identifier: NCT02695498
Introduction
Migraine is the second leading cause of worldwide disability.1 Two-thirds of patients with migraine discontinue medications due to inefficacy or adverse effects,2 despite significant disability caused by migraine.3 Although the American Headache Society recommends against opioids, with risks of opioid use disorder and the development of the refractory condition of medication overuse headache, one-third of patients turn to opioids.4,5,6 A significant need exists for nonopioid, nondrug migraine treatments.7
Mindfulness-based stress reduction (MBSR), a standardized mind-body treatment that teaches momentary awareness with decreased sensory percept judgment, is associated with improvements in many chronic pain conditions.8,9,10,11 Mindfulness may be particularly helpful for migraine, as it diminishes affective responses to stress,12,13 the most common migraine trigger.14 Furthermore, mindfulness decreases affective (ie, pain unpleasantness) and sensory (ie, pain intensity) experimental pain by engaging brain regions important for cognitive and affective pain modulation.15,16 Affective/cognitive processes, such as depression, pain catastrophizing, and self-efficacy, can play a significant role in migraine and its associated disability.17,18 While patients with migraine commonly use mind-body treatments,19,20 and small studies demonstrate safety, feasibility, and preliminary benefits,21,22,23,24 standardized, rigorous approaches evaluating both clinical benefit and mechanisms are needed.22
We conducted a double-blinded, randomized clinical trial of MBSR vs headache education for adults with migraine. We hypothesized that, compared with headache education, MBSR would improve migraine frequency, disability, quality of life, and affective/cognitive processes (eg, depression, pain catastrophizing, and self-efficacy). We used quantitative sensory testing (QST) to evaluate pain perception, hypothesizing that MBSR would decrease experimentally induced affective pain (unpleasantness) more than sensory (intensity) pain.
Methods
Study Design, Setting, and Participants
Participants were recruited by targeting patients and health care professionals from widespread community advertising and a large tertiary care academic medical center in Winston-Salem, North Carolina (detailed recruiting efforts described in eMethods in Supplement 1). Enrollment occurred from August 26, 2016, through October 1, 2018, over 7 cohorts (cohort details in eTable 1 in Supplement 1). Eligibility was assessed with (1) phone screens (eMethods in Supplement 1); (2) in-person evaluation by a neurologist/United Council of Neurological Subspecialties–certified headache specialist including the Structured Diagnostic Interview for Headache25,26; and (3) 4-week baseline headache log (Figure 1). Inclusion criteria were diagnosis of migraine (International Classification of Headache Disorders-2 [ICHD-2], the edition in effect at the time the study began); between 4 and 20 migraine days per month; history of migraine for at least 1 year; at least 18 years old; and availability for 8 weekly classes. For each cohort, 1 day/time class option was available; if the participant was not available on that day/time, they were not eligible for that cohort but could be notified for future cohort eligibility. Exclusion criteria were regular mind-body practice; unstable medical or psychiatric illness; severe clinical depression (Patient Health Questionnaire, PHQ-9, > 20); nonmigraine chronic pain; medication overuse headache (MOH by ICHD-2); current or planned pregnancy; use of new migraine medication within 4 weeks; inability to maintain stable medications for study duration; incomplete baseline headache log; and absence of pain ratings to noxious (49 °C) stimuli. While the study targeted episodic migraine, headache frequency up to 20 per month was included given significant monthly headache variability27; the MOH exclusion limited chronic migraine (see eTable 2 in Supplement 1 for eligibility criteria justification). This study was conducted in accordance with the International Conference on Harmonization Good Clinical Practice guidelines, the Declaration of Helsinski,28 and migraine pharmacological and behavioral research guidelines.29,30 All participants provided written informed consent at the screening visit with a study team member. This study was approved by the Wake Forest Baptist Institutional Review Board (study protocol in Supplement 2) and followed Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. A National Institutes of Health certificate of confidentiality was obtained to protect research participant identity.
Figure 1. Overview of Study Design, Conducted for Each Cohort.
MBSR indicates mindfulness-based stress reduction.
aTrained study team members conducted the phone screens, including the principal investigator, master’s-level students, and undergraduate students.
bParticipants were initially required to be migraine-free for 48 hours prior to each study visit, but to complete the study, this was changed (after cohort 1’s 12-week study visit) to migraine-free the day of study visits (given that participants’ headache frequencies could be up to 20 days per month). See details in Supplement 2.
cQuestionnaires were completed in-person at the screening visit and in-person or remotely for follow-up visits (when headache-free).
dAll quantitative sensory testing (QST) assessments were in-person, with confirmation of no pain relieving medications taken within 12 hours.
eThis run-in period confirmed ability to maintain daily headache log and confirmed eligibility criteria (assessment of migraine frequency and exclusion for medication overuse headache).
fQualitative interview results will be reported elsewhere.
Masking and Randomization
To blind participants to active vs comparator group randomization, recruitment materials described the study as “classes to learn information that may help headaches without medications” without course content details. This avoided (1) group assignment dissatisfaction/dropouts; (2) group expectation differences; and (3) selection bias with only participants interested in mindfulness. The principal investigator, coinvestigators, data analysts, and QST administrators were blinded to group assignment.
Treatment assignments were generated with SAS PROC PLAN statement by a study biostatistician (who did not conduct data analyses) using permuted blocks with randomly varying block size and sealed in numbered, opaque envelopes and given to the research coordinator. After eligibility was confirmed, a research coordinator opened each sequentially numbered sealed envelope to inform participants of group assignment. Participants were randomized (1:1) to receive MBSR or headache education, stratified by baseline headache log frequency (4-9 per month, low, vs 10-20 per month, high).
Interventions
Participants could continue current acute and preventive migraine medications and were requested to maintain stable medications for study duration. The MBSR and headache education interventions were comparable in duration (2 hours/week for 8 weeks, with optional retreat day), format (group), and participants per group. The MBSR instructor followed the standardized curriculum31 to teach mindfulness meditation/yoga without migraine modifications. The headache education group received instruction on headaches, pathophysiology, triggers, stress, and treatment approaches (eTable 3 in Supplement 1 for course content). The MBSR participants received electronic audio files for home practice and were encouraged to practice at home 30 minutes per day.
Treatment Fidelity, Expectations, and Satisfaction
We implemented a detailed treatment fidelity plan according to the National Institutes of Health Behavior Change Consortium32,33 and Template for Intervention Description and Replication Checklist34 for mindfulness-based research35 (eTable 4 in Supplement 1). Participants were considered “completers” with attendance of at least 5 of 8 classes. The following instruments (and time points) were assessed: Credibility/Expectancy Questionnaire36 (at baseline, after second class, and at 36 weeks); patient-centered communication skills37 (PCC, 12 weeks); Working Alliance Inventory (WAI, after the second class, 12 weeks); and client satisfaction questionnaire (CSQ, 12 weeks).
Follow-up
Participants completed follow-up study visits at 12, 24, and 36 weeks. The QST assessments were in-person, while follow-up Research Electronic Data Capture (REDCap) surveys could be completed remotely.
Measures
National Institute of Neurological Disorders and Stroke Common Data Elements for Headache informed the sociodemographic information obtained at the screening visit (Table 1). Outcome data were captured using REDCap, hosted at Wake Forest School of Medicine.38
Table 1. Baseline Characteristics of Study Participants.
| Baseline characteristic | No. (%)a | |
|---|---|---|
| MBSR (n = 45) | Headache education (n = 44) | |
| Sociodemographics | ||
| Age, mean (SD), y | 44 (12) | 44 (14) |
| Sex | ||
| Female | 42 (93) | 40 (91) |
| Male | 3 (7) | 4 (9) |
| Race | ||
| White | 40 (89) | 39 (89) |
| Black or African American | 5 (11) | 5 (11) |
| Ethnicity | ||
| Hispanic or Latino | 2 (4) | 4 (9) |
| Not Hispanic or Latino | 43 (96) | 40 (91) |
| Primary health insurance | ||
| Private | 40 (89) | 33 (75) |
| Medicare/Medicaid/other public | 5 (11) | 9 (20) |
| None | 0 | 2 (5) |
| Marital status | ||
| Married/living with partner | 31 (69) | 26 (59) |
| Divorced/separated/widowed | 6 (13) | 8 (18) |
| Single, never married | 8 (18) | 10 (23) |
| Household incomeb | ||
| <$15 000 | 4 (9) | 4 (9) |
| $15 000-49 999 | 9 (20) | 13 (30) |
| $50 000-149 999 | 23 (51) | 22 (50) |
| >$150 000 | 9 (20) | 4 (9) |
| Current employment statusc | ||
| Employed/self-employed full time (>30 h/wk) | 30 (67) | 25 (57) |
| Employed part time | 4 (9) | 5 (11) |
| Student, homemaker, volunteer | 7 (16) | 3 (7) |
| Unemployed, retired | 2 (4) | 7 (16) |
| Education | ||
| ≤High school | 3 (7) | 2 (5) |
| College | 28 (62) | 30 (68) |
| Graduate degree | 14 (31) | 12 (27) |
| Recruitment source | ||
| Academic medical center/clinician referrald | 20 (44) | 23 (52) |
| Communitye | 25 (56) | 21 (48) |
| Baseline physiology, mean (SD)f | ||
| Body mass index | 27 (8) | 29 (7) |
| Systolic blood pressure, mm Hg | 120 (16.5) | 122 (12.9) |
| Diastolic blood pressure, mm Hg | 73 (11.4) | 73 (8.8) |
| Heart rate, beats/min | 73 (13) | 78 (13) |
| Headache features | ||
| Years with migraine, mean (SD) | 24 (13) | 24 (14) |
| Migraine with aura | 16 (36) | 18 (41) |
| Family history of headache | 31 (69) | 28 (64) |
| Headache days during 28-d baseline, mean (SD) | 9.5 (3.4) | 9.8 (3.6) |
| Migraine days during 28-d baseline, mean (SD) | 7.2 (2.5) | 7.4 (3.0) |
| Use of treatments | ||
| Current use of prophylactic treatmentg | 18 (40) | 31 (71) |
| Daily medication | 11 (24) | 22 (50) |
| No. of daily prophylactic medications, mean (SD) | 1.3 (0.6) | 1.5 (0.6) |
| Procedures (Botox/occipital nerve blocks) | 5 (11) | 5 (11) |
| Supplement | 10 (22) | 14 (32) |
| Current use of acute medicationg | 41 (91) | 36 (82) |
| Triptan | 25 (56) | 31 (70) |
| Nonsteroidal anti-inflammatory | 28 (62) | 19 (44) |
| Antinausea | 8 (18) | 7 (16) |
| No. of previously tried daily prophylactic medications, mean (SD) | 2.8 (1.7) | 3.2 (2.4) |
| No. of previously tried acute medications, mean (SD) | 4.9 (3.1) | 4.9 (3.2) |
| No. of previously tried integrative treatments, mean (SD)h | 3.6 (2.5) | 4.3 (2.9) |
| Experienced headache medication side effect | 25 (56) | 31 (71) |
| Of those with triggers, No. of triggers, mean (SD)i | 7.2 (2.7) | 6.4 (3.3) |
| Stress or let-down stress as a trigger | 35 (78) | 31 (71) |
| Comorbid conditions | ||
| Current or past diagnosis of depression | 19 (42) | 19 (43) |
| Current or past diagnosis of anxiety | 15 (33) | 19 (43) |
Abbreviation: MBSR, mindfulness-based stress reduction.
No. (%) reported unless otherwise specified.
Based on n = 43 for headache education (n = 1 [2%] of data missing).
Based on n = 43 for MBSR (n = 2 [4%] of data missing); based on n = 40 for headache education (n = 4 [9%] of data missing).
Clinician recruitment included direct referrals, referrals through electronic medical record, through the Wake Forest Be Involved clinical trial registry, through the electronic medical record, or from prior headache research recruitment. See Supplement 1 for further details.
Community recruitment included flyers, social media (Facebook/Twitter), email listservs from local organizations, television advertising, magazines, online advertisements, and friends/family referrals. See Supplement 1 for further details.
Blood pressure and heart rate measurements are from baseline visit.
Percentages do not add to 100 as individuals may be on more than 1 treatment. Prophylactic treatment options included daily migraine medication, regular onabotulinum toxin A or occipital nerve blocks, or daily use of a migraine supplement. Calcitonin gene-related peptide medications were not yet US Food and Drug Administration approved at study initiation; we screened out participants with medication overuse headache, excluding patients who may have been taking opioids.
Integrative treatments included acupuncture/acupressure, physical therapy, stress reduction, ice/cold compresses, yoga, meditation, deep breathing, massage, chiropractic, biofeedback, supplements (including magnesium, riboflavin, coenzyme Q10, feverfew, butterbur, melatonin), or other.
Triggers included menses, caffeine, weather changes, alcohol, too little sleep, too much sleep, hunger, missed meals, psychological stress, “let down” after stressful period, food additives, light glare, odors, altitude, exercise, certain food, sex, other.
Primary Outcome
The primary outcome was a change in monthly migraine day frequency from baseline to 12 weeks, defined as a calendar day with moderate to severe headache (6-10 on 0-10 scale) lasting more than 4 hours, or treated with acute medication. Participants maintained daily REDCap headache logs for study duration, capturing presence, duration, intensity, unpleasantness, symptoms, and medication use.
Secondary Outcomes
Headache day frequency, intensity, unpleasantness, and duration were also assessed. Reliable, well-validated survey instruments were completed at each study visit to assess headache-related disability, quality of life, and well-being measures. Headache-related disability was assessed with the Migraine Disability Assessment (MIDAS)-1 month39,40 and the Headache Impact Test-6 (HIT-6).41,42,43 Quality of life was assessed with the Migraine-Specific Quality of Life Questionnaire, version 2.1 (MSQv2.1),44,45 depression with the Patient Health Questionnaire-9 (PHQ-9),46 anxiety with the Generalized Anxiety Disorder-7 (GAD-7),47 pain catastrophizing with the Pain Catastrophizing Scale (PCS),48,49 self-efficacy with the Headache Management Self-Efficacy Scale,50 and trait mindfulness with the Five-Facet Mindfulness Questionnaire.51,52 Each outcome assessed changes from baseline to 12, 24, and 36 weeks. Additional assessments (eg, capturing hope, optimism, sleep) were completed and will be reported separately.
The QST assessments were only conducted when participants were migraine-free using a 16 × 16-mm thermal probe with the MEDOC TSA-II; all temperatures were lower than 50 °C to prevent tissue damage. Using a 15 cm sliding visual analogue scale,53,54 participants quantified intensity (from “no pain sensation” to “most intense imaginable”) and unpleasantness (from “not at all unpleasant” to “most unpleasant imaginable”). Participants were familiarized with 32 (or 16 at follow-up visits) 5-second stimuli (35-49 °C) on the left arm, away from increased migraine allodynia of head region.55,56 Thermal stimulation was then administered on the right calf, starting at 35 °C with a 6 °C rise/fall rate and 5-second plateau up to randomly administered temperatures of 43, 45, 47, and 49 °C; each temperature repeated 3 times; each series repeated twice, with intensity and unpleasantness rated after each temperature. To minimize sensitization, habituation, and hyperalgesia with repetitive site stimulation, all trials were separated by 30 seconds and systematically distributed over the calf.57
Adverse Events
Adverse events were systematically queried and tracked at each study visit. Quarterly reports were provided to the Wake Forest School of Medicine Data Safety and Monitoring Board, which recommended continuation without modification at every evaluation.
Sample Size and Statistical Analysis
All statistical analyses were performed using R Statistical Software,58 with packages mice for multiple imputation59 and lme4 for regression analyses.60 To model headache and migraine frequencies, headache log entries were grouped into 4-week phases. For each phase, we calculated the change in migraine days from baseline using a multivariable linear mixed model to determine change scores as a function of treatment group, phase, baseline migraine rate, years with migraine diagnosis, classes attended, and cohort, controlling for within-participant variation via random intercepts, with α = .05 significance based on treatment group and phase (time) interaction. All covariates were assessed at an α = .05 level of significance and reported with point estimates and 95% CIs. Using effect sizes from our pilot trial,21 and by analyzing the data with our mixed effects multivariable hierarchical regression models, we estimated a final sample of 44 participants per group (n = 88) would provide greater than 90% power with α = .05 to detect a difference of 1.3 migraine days per month between groups (using PASS statistical software). Accounting for potential 10% dropout, our recruitment aim was 98 participants.
We modeled secondary outcomes using a multivariable linear mixed model framework controlling for baseline value of the outcome of interest, treatment group, cohort, and within-participant variation via random effects, with significance based on the treatment group effect and phase interaction at .05 significance level. Assessments of secondary outcomes are exploratory for future research and did not control for multiple comparisons; significant results found are viewed to provide an indication of potential treatment effect for future research, not confirm one.
To assess QST results, a multivariable linear mixed model was used to model the percent change from baseline in perceived pain intensity and unpleasantness at each visit for each of 6 measures at 49 °C. Change scores were modeled as a function of treatment group and visit, controlling for baseline. To assess medication use over time, among days when a participant reported the presence of a headache, we modeled the probability of medication use (overall and for specific medication classes) using a generalized linear mixed model with logit link function, with headache log phase and treatment group as predictors. Treatment fidelity measures (PCC, WAI, CSQ, CEQ) between groups were compared using 2-sided t tests.
Missing Data and Sensitivity Analysis
Our primary analysis was based on a modified intention to treat group: participants who were randomized, attended at least 1 class, and recorded at least 1 headache log entry (n = 89). For frequency analyses, we calculated the aggregated number of headaches and migraines over each 28-day period for each participant. If fewer than 50% of log entries were missing during a phase, the daily migraine rate was calculated and converted to reflect that of a 28-day period; if more than 50% of log entries were missing in a phase (>14/28), the logs were assumed missing and multiple imputation was used for headache and migraine rates59 (missing data details in Supplement 2).
We conducted 3 sensitivity analyses of our primary analysis: (1) assuming missing data entries were simply days with no headache (modeling similar to primary analysis); (2) including only complete (nonimputed) data in a multivariable generalized linear mixed model to determine the probability of headache/migraine for a given day, then multiplying the estimated daily probability by 28 for a 28-day rate, which makes full use of the available headache data; and (3) given the baseline disproportionate use of prophylactic treatments across groups, creating an additional model adjusting for prophylactic use, including it as a covariate.
Results
Participant Characteristics
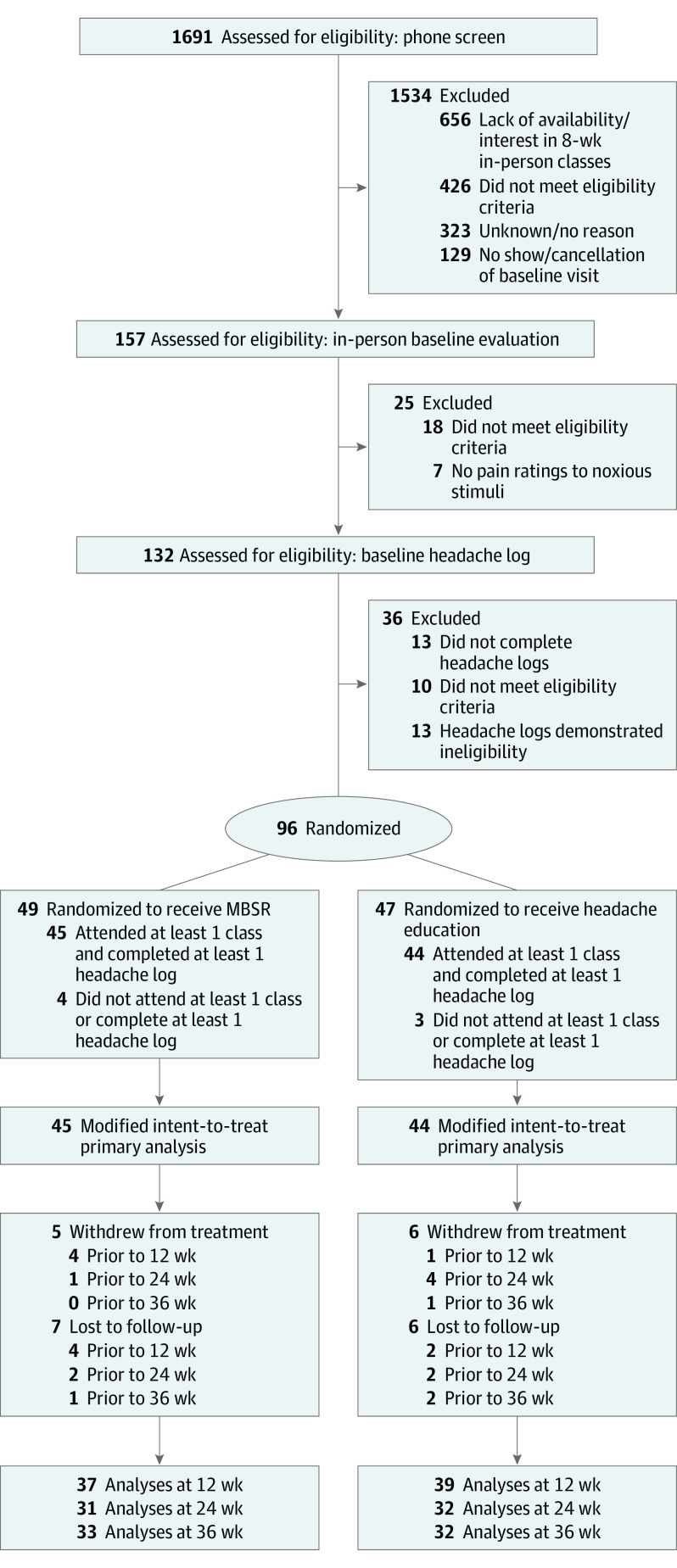
After 1691 phone screens and 157 in-person evaluations were completed, 96 participants were randomized and 89 participants attended at least 1 class and completed at least 1 headache log (MBSR, 45; headache education, 44) across 7 cohorts (mean [SD] size 12.7 [5.0]) (Figure 2 and eTable 5 and eTable 6 in Supplement 1). Baseline sociodemographic characteristics were balanced across groups, as most of the 89 participants were women (n = 82, 92%), White (n = 79, 89%), mean (SD) age was 43.9 (13.0) years, with college/graduate education (n = 84, 94%) (Table 1). Participants had a mean (SD) 7.3 (2.7) migraine days per month and 9.6 (3.5) headache days per month with high headache-related disability (mean [SD] Headache Impact Test-6 score: 63.5 [5.7]). While most participants in both groups were currently using acute medications (n = 77, 87%), 71% of headache education participants were using prophylactic treatments compared with only 40% of MBSR participants (P = .01). Current or prior history of depression (n = 38, 43%) and anxiety (n = 34, 38%) was common.
Figure 2. CONSORT Flow Diagram of Study Participation.
See eTable 5 and eTable 6 in Supplement 1 for details of reasons and time points for exclusion, withdrawal, and lost to follow-up.
Treatment Adherence, Fidelity, Satisfaction, and Credibility
Most participants in both MBSR and headache education groups attended at least 5 of 8 classes (84% and 82%, respectively, with median attendance 7 of 8 classes for both groups) and completed the study through 36 weeks (73% for both groups). During the treatment period, participants practiced MBSR skills at home a mean (SD) 4.2 (2.5) days per week for 32.6 (14.6) minutes per day; home practice persisted during posttreatment period (2.4 [2.8] days per week for 27.2 [11.5] minutes per day). Participants in both groups demonstrated similar therapeutic relationships with the instructors (PCC: MBSR: 19.4 [1.8], headache education: 19.8 [0.6], P = .26; WAI: MBSR: 73.0 [9.8], headache education: 69.6 [11.7], P = .18). Program satisfaction was high (CSQ >24) in both groups, although higher in the MBSR group (MBSR: 28.4 [3.3], headache education: 25.1 [5.1], P = .001). Intervention credibility and expectation were similar without group differences at baseline, after the second class, and at 36 weeks. About 50% of participants in both groups reported classes being better than expected.
Primary Outcome
Participants in both groups demonstrated a reduction of migraine days per month from baseline at 12 weeks, without statistical differences between groups (MBSR: −1.6; 95% CI, −0.7 to −2.5; headache education: −2.0; 95% CI, −1.1 to −2.9; group differences from baseline between headache education vs MBSR, −0.5; 95% CI, −0.9 to 1.7; P = .50). Sensitivity analyses did not yield different conclusions.
Secondary Outcomes
Headache frequency (days/month) decreased from baseline at 12 weeks without group differences (MBSR, −2.0; 95% CI, −0.9 to −3.0; headache education, −2.4; 95% CI, −1.4 to −3.4; P = .52). Both groups sustained reductions in frequency of migraine (MBSR, −2.2; 95% CI, −1.2 to −3.2; vs headache education, −2.7; 95% CI, −1.7, −3.8) and headache (MBSR, −3.2; 95% CI, −2.2 to −4.3; vs headache education, −3.9, 95% CI, −2.8 to −5.0]) out to 36 weeks without group differences (P = .49 and .45, respectively). There were no significant changes over time or group differences on headache pain unpleasantness, intensity, or duration.
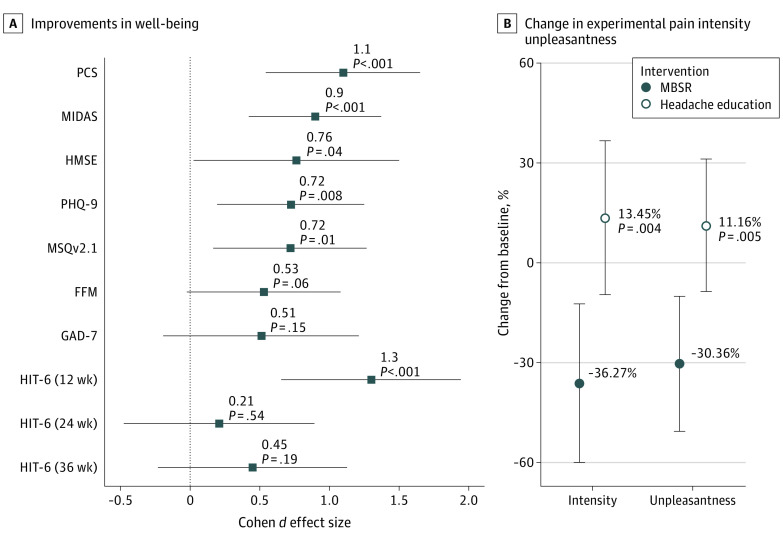
Compared to headache education, MBSR participants had statistically significant improvements from baseline at all follow-up time points in headache-related disability, quality of life, self-efficacy, pain catastrophizing, and depression scores (Table 261), with medium to large effect sizes (Figure 3A62,63). Similar improvements for anxiety and mindfulness were seen but were not statistically significant.
Table 2. Changes in Standardized Instruments Over Time in Mindfulness-Based Stress Reduction (MBSR) vs Headache Educationa.
| Instrument | Mean (95% CI) | Point estimate of effect difference between groups (95% CI)c | P value | |||
|---|---|---|---|---|---|---|
| Baseline | 12 weeksb | 24 weeksb | 36 weeksb | |||
| Migraine Disability Assessment-1 month (MIDAS)d | ||||||
| MBSR | 16.9 (12.3 to 21.5)e | 6.7 (4.1 to 9.2) | 6.4 (3.8 to 9.1) | 5.2 (2.6 to 7.8) | 5.9 (2.8 to 9.0) | <.001 |
| Headache education | 11.8 (9.5 to 14.4) | 12.6 (10.1 to 15.1) | 12.4 (9.8 to 15.0) | 11.1 (8.5 to 13.7) | ||
| Pain Catastrophizing Scale (PCS)f | ||||||
| MBSR | 18.5 (14.9 to 22.1) | 13.3 (10.9 to 15.6) | 13.0 (10.6 to 15.5) | 9.8 (7.4 to 12.1) | 5.8 (2.9 to 8.8) | <.001 |
| Headache education | 20.8 (16.9 to 24.6) | 19.1 (16.8 to 21.3) | 18.8 (16.5 to 21.2) | 15.6 (13.2 to 17.9) | ||
| Patient Health Questionnaire 9 Depression (PHQ-9)g | ||||||
| MBSR | 4.7 (3.3 to 6.1) | 3.9 (3.0 to 4.9) | 4.0 (3.0 to 4.9) | 3.6 (2.6 to 4.5) | 1.6 (0.4 to 2.7) | .008 |
| Headache education | 5.5 (4.2 to 6.8) | 5.5 (4.6 to 6.4) | 5.6 (4.6 to 6.5) | 5.1 (4.2 to 6.1) | ||
| Migraine Specific Quality of Life (MSQv2.1)h | ||||||
| MBSR | 44.9 (40.0 to 49.7) | 33.6 (30.5 to 36.6) | 29.9 (26.7 to 33.1) | 29.6 (26.5 to 32.8) | 5.1 (1.2 to 8.9) | .01 |
| Headache education | 43.5 (40.0 to 47.1) | 38.6 (35.6 to 41.6) | 35.0 (31.9 to 38.1) | 34.7 (31.6 to 37.8) | ||
| Headache Management Self-Efficacy (HMSE)i | ||||||
| MBSR | 110 (103 to 118) | 127 (121 to 133) | 128 (122 to 134) | 129 (123 to 135) | 8.2 (0.3 to 16.1) | .04 |
| Headache education | 114 (107 to 122) | 119 (113 to 125) | 120 (114 to 126) | 121 (115 to 127) | ||
| Generalized Anxiety Disorder (GAD-7)j | ||||||
| MBSR | 12.1 (10.8 to 13.5) | 11.0 (10.0 to 12.0) | 10.8 (9.8 to 11.8) | 10.9 (9.85 to 11.9) | 1.2 (−0.05 to 2.4) | .06 |
| Headache education | 12.7 (11.3 to 14.1) | 12.2 (11.2 to 13.1) | 12.0 (11.0 to 13.0) | 12.1 (11.06 to 13.0) | ||
| Five Facet Mindfulness (FFM)k | ||||||
| MBSR | 138 (132 to 144) | 140 (136 to 144) | 142 (138 to 147) | 143 (139 to 148) | 3.9 (−1.5 to 9.3) | .15 |
| Headache education | 134 (128 to 139) | 136 (132 to 140) | 138 (134 to 143) | 140 (135 to 144) | ||
| Headache Impact Test (HIT-6)l | ||||||
| MBSR | 63.0 (60.8 to 65.2) | 56.3 (54.4 to 58.2) | 57.9 (55.8 to 59.9) | 56.6 (54.6 to 58.6) | 5.3 (2.7 to 7.9)m; 0.9 (−1.9 to 3.6)n; 1.9 (−0.9 to 4.6)o | <.001m; .54n; .19o |
| Headache education | 63.0 (61.8 to 64.3) | 61.6 (59.8 to 63.4) | 58.7 (56.8 to 60.7) | 58.5 (56.5 to 60.4) | ||
Results represent n = 78 (participants with at least 1 follow-up visit).
Multivariable linear mixed regression model was used to assess instrument means by follow-up visit and treatment group, adjusted for baseline measures with random intercepts for each patient. For all but HIT-6, follow-up means are based on main-effects from the linear mixed regression model without an interaction effect between treatment group and time due to insignificant interaction effects. For HIT-6, means are based on results from a significant treatment – time interaction effect.
Treatment effect measures evaluated from baseline across all 3 follow-up time points. The effect difference is in terms of a positive clinical improvement in the MBSR group relative to the headache education group (eg, a greater reduction in MIDAS or increase in FFM). Statistically significant differences between treatment groups for each time point are the same as denoted in Figure 3 (represented in the figure with Cohen d effect sizes).
Instrument score ranges: Migraine Disability Assessment-one month (0-93), higher scores reflect greater disability, MIDAS is typically used as an average over 3 months; to facilitate interpretation of the MIDAS-1 month data presented, the mean estimate results (but not the confidence intervals) can be multiplied by 3 for conversion to the typical 3-month assessment61; score range for 3-month MIDAS: 0-5: little or no disability, 6-10 mild disability, 11-20 moderate disability, 21+ severe disability.
There was not a statistically significant difference in baseline measures for all instruments except MIDAS. Baseline difference between treatment groups in MIDAS is statistically significant (P = .033). There were 3 identified outlier patients in the MBSR group with baseline MIDAS scores >50 (for reference, the maximum baseline MIDAS in the Headache Education group was 36). With these outliers removed, the mean baseline MIDAS in the MBSR group is 14.44 (11.75, 17.14) and the baseline difference between treatment groups is no longer statistically significant (P = .20).
Pain Catastrophizing Scale (0-52), higher scores reflect greater pain catastrophizing.
Patient Health Questionnaire-9 Depression (0-27), higher scores reflect greater depression, score range: 1-4: minimal depression, 5-9: mild depression, 10-14: moderate depression, 15-19: moderately severe depression, 20-27: severe depression.
Migraine Specific Quality of Life (0-100), lower scores reflect greater quality of life.
Headache Management Self-Efficacy (0-175), higher scores reflect more self-efficacy.
Generalized Anxiety Disorder-7 (0-21), higher scores reflect greater anxiety, score range: 0-4: minimal anxiety, 5-9: mild anxiety, 10-14: moderate anxiety, 15-21 severe anxiety.
Five Facet Mindfulness (0-195), higher scores reflect greater mindfulness.
Headache Impact Test-6 (36-78), higher scores reflect greater headache impact, score range: <49: little to no impact, 50-55: some/moderate impact, 56-59: substantial impact, 60+ severe impact. HIT-6 point estimates of effect differences between groups are displayed at three time points due to a significant treatment-visit interaction. All other instrument treatment effect measures did not significantly differ across visits (P > .05).
12 weeks.
24 weeks.
36 weeks.
Figure 3. Changes in Well-Being and Experimental Pain Intensity and Unpleasantness Between Mindfulness-Based Stress Reduction (MBSR) and Headache Education.
FFM indicates Five Facet Mindfulness; GAD-7, Generalized Anxiety Disorder 7; HIT-6, Headache Impact Test; HMSE, Headache Management Self-Efficacy; MIDAS, Migraine Disability Assessment, one month; MSQv2.1, Migraine Specific Quality of Life; PCS, Pain Catastrophizing Scale; PHQ-9, Patient Health Questionnaire-9 Depression.
A, Point estimates of Cohen d effect size differences between treatment groups with 95% CIs, with positive directional effect indicating an improvement in MBSR relative to headache education for each measure. HIT-6 displayed at 3 time points due to a significant treatment-visit interaction. All other instrument treatment effect measures did not significantly differ across visits (P > .05). Cohen d effect sizes of 0.2 are considered small, 0.5 medium, 0.8 large, and 1.2 very large.62,63 B, Experimental pain measured with quantitative sensory testing, with visual analog scale range of 0-10.
Quantitative sensory testing results revealed that MBSR participants reported a greater decrease in percent change from baseline for perception of experimental pain unpleasantness and intensity, while the headache education group showed no significant change (Figure 3B). Based on the linear mixed model, the trend persisted and increased at all time points, such that by 36 weeks, the MBSR group demonstrated a 36.3% (95% CI, 12.3% to 60.3%) decrease in intensity and 30.4% (95% CI, 9.9% to 49.4%) reduction in unpleasantness while the headache education group demonstrated a 13.5% (95% CI, −9.9% to 36.8%) increase in intensity and an 11.2% (95% CI, −8.9% to 31.2%) increase in unpleasantness (between-group contrasts from the linear mixed model yielded P = .004 and .005 for intensity and unpleasantness, respectively). We found no statistically significant differences in medication use (headache specific or all medications) between treatment groups.
Adverse Events
One MBSR participant developed squamous cell carcinoma, deemed unrelated to the study protocol.
Discussion
In this study, MBSR was not associated with improved migraine frequency more than headache education, as both groups had decreases. Compared with headache education, MBSR participants had improvements in headache-related disability, quality of life, depression scores, self-efficacy, pain catastrophizing, and decreased experimentally induced pain intensity and unpleasantness out to 36 weeks.
Although we hypothesized that MBSR would decrease migraine frequency, we did not expect headache education would also decrease frequency, with both groups having clinically meaningful decreases.64 A recent randomized clinical trial found that both behavioral weight loss and headache education resulted in decreased migraine frequency (3 to 4 fewer migraines per month),65 demonstrating, consistent with the present study’s results, that headache education can have meaningful effect on migraine frequency. Selecting the appropriate control for behavioral research has always been inherently challenging.30 While headache education can serve as a time/attention control and may provide enough engagement to prevent differential group dropout, it does provide an active intervention, thus serving as a comparator group rather than a control group. The mechanisms underlying the improvements seen from this study’s headache education group likely differ from mindfulness. Migraine knowledge may provide empowerment and/or lead to behavioral changes that may be associated with change in migraine frequency without change in overall well-being.65 A recent meta-analysis of randomized clinical trials that assessed therapeutic patient education programs (where patient education was the active arm, although some programs also included active behavioral treatment strategies such as stress management, self-regulation skills, and/or relaxation) demonstrated strong to moderate evidence for improvement of headache frequency, without any evidence on self-efficacy or depression.66
We accurately hypothesized the association of MBSR with improved disability, quality of life, and cognitive/affective processes. Although we hypothesized that MBSR would have a greater effect on affective (pain unpleasantness) vs sensory (pain intensity), the improvements in both may help explain the mechanism driving the clinical improvements. Mindfulness may strengthen cognitive and affective regulation of nociceptive input by training individuals to reassess sensory percepts (including pain) in a nonjudgmental way by modifying their appraisal of, and “turning towards” pain, resulting in decreased nociception.67,68 The changed pain perception, coupled with clinically meaningful improvements in cognitive/affective processes, both out to 36 weeks, suggests that MBSR participants learned a new way of processing pain that may have significant effect on long-term health.
The present study is consistent with most recent studies that demonstrate the positive effect of mindfulness on migraine disability, without improvements in headache frequency,22,69,70 although 2 recent studies showed mindfulness impacting migraine frequency. A nonrandomized clinical trial in chronic migraine MOH71 demonstrated that mindfulness decreased headache frequency as much as pharmacological treatment. Enhanced MBSR vs stress management showed a similar headache frequency decrease at 20 weeks with MBSR as the present study saw at 36 weeks (−3.2 headache days per month), which was more than their control group.72 However, group differences seen at 20 weeks disappeared by 52 weeks when both groups had equivocal decreases, which was similar to results in the present study. Changes seen in their study over time (with respect to the standardized instruments) were consistent with prior findings from our pilot study.21
The positive direction of the many secondary outcomes is consistent with our pilot data21 and worthy of further investigation, especially given the MIDAS-1 month improvements of 5.9 fewer days of disability per month seen in this study are clinically significant and surpass typical pharmacological effects61,73,74 and the minimally important difference (for the 3-month MIDAS the minimally important difference is 3.7, suggesting the calculated minimally important difference for the 1-month MIDAS is 1.23).75 For a condition with recurrent, lifelong unexpected attacks, improving a patient’s pain perception and ability to function despite migraine has significant implications for overall long-term emotional and social health.69 As recommended by migraine clinical trial guidelines available at the time of study design,29,30 migraine frequency was chosen as the primary outcome. The additional studies evaluating mindfulness in migraine published since the present study was designed have consistently shown effect on headache-related disability,22 demonstrating the importance of disability as a primary outcome and highlighting the need for updated migraine behavioral clinical trial guidelines to reflect this change. Additional research is underway to further understand the effect of mindfulness on migraine,22,76 along with similar treatments such as acceptance and commitment therapy.77
Strengths and Limitations
The present study’s strengths include blinding participants to active vs comparator group assignment and eligibility assessment by a United Council for Neurologic Subspecialties–certified headache specialist. Community recruitment increased generalizability, as did participants’ ability to continue current medications, which also increases potential adoption, as mindfulness can be combined with traditional treatments.78 While the active comparator group is a strength, it was not an inactive control condition as the information provided may have led to meaningful behavior changes. While the 2 groups were matched on weekly class duration and frequency, daily home practices were only a part of the MBSR group. Additional limitations include the commitment and scheduling challenges for intervention participation, as only 1 day and time option was available per cohort, which limited availability and was a deterrent for many patients who were not available for or interested in 8 weekly in-person classes. This may have contributed to the lack of participant diversity, as the study was limited to those with time and availability. Given that most participants in this study were white, highly educated, and overall healthy, future studies assessing effects in more diverse populations are important to understand generalizability.
Conclusions
At a time when opioids are still being used for migraine, finding nondrug options to prevent such use is critical. Once learned, mindfulness can be practiced anywhere at any time, a practical life skill with potential long-term effects that may have broad applicability to managing many health problems and life challenges. Mindfulness may be especially useful in light of current events. With the tremendous stress and anxiety of the COVID-19 pandemic, patients with migraine may have worsening migraine attacks,79,80 and mindfulness may be particularly beneficial. In summary, mindfulness may help treat the total burden of migraine. A larger, more definitive study is needed to understand the impact of mindfulness on migraine.
eTable1: Dates of Study Participation by Cohort: Recruitment, Classes, and Study Visits
eTable2: Eligibility Criteria and Justification
eTable 3: Course Content of MBSR and Headache Education Interventions
eTable 4: Treatment Fidelity Plan & Delivery
eTable 5: Reasons and Time Points for Exclusion
eTable 6: Reasons and Time Points for Withdrawal and Lost to Follow-Up
eMethods 1: Recruitment Strategies
eMethods 2: Phone Screen
eMethods 3: MBSR Treatment Fidelity Review Assessment
eMethods 4: Instructor Curriculum Checklists
Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858. doi: 10.1016/S0140-6736(18)32279-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald N, Lipscomb J, McCrory DC. Resource Utilization and Costs of Care for Treatment of Chronic Headache. In: AHRQ Technical Reviews and Summaries. US Agency for Health Care Policy and Research; 1999. [PubMed] [Google Scholar]
- 3.Lipton RB, Hutchinson S, Ailani J, et al. Discontinuation of acute prescription medication for migraine: results from the Chronic Migraine Epidemiology and Outcomes (CaMEO) Study. Headache. 2019;59(10):1762-1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loder E, Weizenbaum E, Frishberg B, Silberstein S; American Headache Society Choosing Wisely Task Force . Choosing wisely in headache medicine: the American Headache Society’s list of five things physicians and patients should question. Headache. 2013;53(10):1651-1659. doi: 10.1111/head.12233 [DOI] [PubMed] [Google Scholar]
- 5.Burch RC, Buse DC, Lipton RB. Migraine: epidemiology, burden, and comorbidity. Neurol Clin. 2019;37(4):631-649. doi: 10.1016/j.ncl.2019.06.001 [DOI] [PubMed] [Google Scholar]
- 6.Lipton RB, Munjal S, Buse DC, et al. Unmet acute treatment needs from the 2017 Migraine in America Symptoms and Treatment Study. Headache. 2019;59(8):1310-1323. doi: 10.1111/head.13588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells RE, Loder E. Mind/body and behavioral treatments: the evidence and approach. Headache. 2012;52(suppl 2):70-75. doi: 10.1111/j.1526-4610.2012.02238.x [DOI] [PubMed] [Google Scholar]
- 8.Rosenzweig S, Greeson JM, Reibel DK, Green JS, Jasser SA, Beasley D. Mindfulness-based stress reduction for chronic pain conditions: variation in treatment outcomes and role of home meditation practice. J Psychosom Res. 2010;68(1):29-36. doi: 10.1016/j.jpsychores.2009.03.010 [DOI] [PubMed] [Google Scholar]
- 9.Cherkin DC, Sherman KJ, Balderson BH, et al. Effect of mindfulness-based stress reduction vs cognitive behavioral therapy or usual care on back pain and functional limitations in adults with chronic low back pain: a randomized clinical trial. JAMA. 2016;315(12):1240-1249. doi: 10.1001/jama.2016.2323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4(1):33-47. doi: 10.1016/0163-8343(82)90026-3 [DOI] [PubMed] [Google Scholar]
- 11.Morone NE, Greco CM, Moore CG, et al. A mind-body program for older adults with chronic low back pain: a randomized clinical trial. JAMA Intern Med. 2016;176(3):329-337. doi: 10.1001/jamainternmed.2015.8033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74(8):786-792. doi: 10.4088/JCP.12m08083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Creswell JD, Pacilio LE, Lindsay EK, Brown KW. Brief mindfulness meditation training alters psychological and neuroendocrine responses to social evaluative stress. Psychoneuroendocrinology. 2014;44:1-12. doi: 10.1016/j.psyneuen.2014.02.007 [DOI] [PubMed] [Google Scholar]
- 14.Peroutka SJ. What turns on a migraine? A systematic review of migraine precipitating factors. Curr Pain Headache Rep. 2014;18(10):454. doi: 10.1007/s11916-014-0454-z [DOI] [PubMed] [Google Scholar]
- 15.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540-5548. doi: 10.1523/JNEUROSCI.5791-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidan F, Baumgartner JN, Coghill RC. The neural mechanisms of mindfulness-based pain relief: a functional magnetic resonance imaging-based review and primer. Pain Rep. 2019;4(4):e759. doi: 10.1097/PR9.0000000000000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seng EK, Buse DC, Klepper JE, et al. Psychological factors associated with chronic migraine and severe migraine-related disability: an observational study in a tertiary headache center. Headache. 2017;57(4):593-604. doi: 10.1111/head.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buse DC, Reed ML, Fanning KM, et al. Comorbid and co-occurring conditions in migraine and associated risk of increasing headache pain intensity and headache frequency: results of the migraine in America symptoms and treatment (MAST) study. J Headache Pain. 2020;21(1):23. doi: 10.1186/s10194-020-1084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells RE, Bertisch SM, Buettner C, Phillips RS, McCarthy EP. Complementary and alternative medicine use among adults with migraines/severe headaches. Headache. 2011;51(7):1087-1097. doi: 10.1111/j.1526-4610.2011.01917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Dennis JA, Leach MJ, et al. Complementary and alternative medicine use among US adults with headache or migraine: results from the 2012 National Health Interview Survey. Headache. 2017;57(8):1228-1242. doi: 10.1111/head.13148 [DOI] [PubMed] [Google Scholar]
- 21.Wells RE, Burch R, Paulsen RH, Wayne PM, Houle TT, Loder E. Meditation for migraines: a pilot randomized controlled trial. Headache. 2014;54(9):1484-1495. doi: 10.1111/head.12420 [DOI] [PubMed] [Google Scholar]
- 22.Wells RE, Seng EK, Edwards RR, et al. Mindfulness in migraine: a narrative review. Expert Rev Neurother. 2020;20(3):207-225. doi: 10.1080/14737175.2020.1715212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day MA, Thorn BE, Ward LC, et al. Mindfulness-based cognitive therapy for the treatment of headache pain: a pilot study. Clin J Pain. 2014;30(2):152-161. [DOI] [PubMed] [Google Scholar]
- 24.Gu Q, Hou J-C, Fang X-M. Mindfulness meditation for primary headache pain: a meta-analysis. Chin Med J (Engl). 2018;131(7):829-838. doi: 10.4103/0366-6999.228242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrew ME, Penzien DB, Rains JC, Knowlton GE, McAnulty RD. Development of a computer application for headache diagnosis: the headache diagnostic system. Int J Biomed Comput. 1992;31(1):17-24. doi: 10.1016/0020-7101(92)90050-3 [DOI] [PubMed] [Google Scholar]
- 26.Smitherman TA, Penzien DB, Rains JC, Nicholson RA, Houle TT. Headache: Advances in Psychotherapy-Evidence-Based Practice. Boston, MA: Hogrefe Publishing; 2015. [Google Scholar]
- 27.Serrano D, Lipton RB, Scher AI, et al. Fluctuations in episodic and chronic migraine status over the course of 1 year: implications for diagnosis, treatment and clinical trial design. J Headache Pain. 2017;18(1):101. doi: 10.1186/s10194-017-0787-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 29.Tfelt-Hansen P, Pascual J, Ramadan N, et al. ; International Headache Society Clinical Trials Subcommittee . Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia. 2012;32(1):6-38. [DOI] [PubMed] [Google Scholar]
- 30.Penzien DB, Andrasik F, Freidenberg BM, et al. ; American Headache Society Behavioral Clinical Trials Workgroup . Guidelines for trials of behavioral treatments for recurrent headache, first edition: American Headache Society Behavioral Clinical Trials Workgroup. Headache. 2005;45(suppl 2):S110-S132. [DOI] [PubMed] [Google Scholar]
- 31.Santorelli S, Meleo-Meyer F, Koerbel L, Kabat-Zinn J.. Mindfulness-Based Stress Reduction (MBSR) Authorized Curriculum Guide. Worcester, MA: University of Massachusetts Medical School Center for Mindfulness in Medicine, Health Care, and Society;2017. [Google Scholar]
- 32.Bellg AJ, Borrelli B, Resnick B, et al. ; Treatment Fidelity Workgroup of the NIH Behavior Change Consortium . Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychol. 2004;23(5):443-451. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli B. The assessment, monitoring, and enhancement of treatment fidelity in public health clinical trials. J Public Health Dent. 2011;71(s1):S52-S63. doi: 10.1111/j.1752-7325.2011.00233.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 35.Crane RS, Hecht FM. Intervention integrity in mindfulness-based research. Mindfulness (N Y). 2018;9(5):1370-1380. doi: 10.1007/s12671-018-0886-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. doi: 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- 37.Pbert L, Adams A, Quirk M, Hebert JR, Ockene JK, Luippold RS. The patient exit interview as an assessment of physician-delivered smoking intervention: a validation study. Health Psychol. 1999;18(2):183-188. [DOI] [PubMed] [Google Scholar]
- 38.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the Migraine Disability Assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. 2001;56(6)(suppl 1):S20-S28. doi: 10.1212/WNL.56.suppl_1.S20 [DOI] [PubMed] [Google Scholar]
- 40.Stewart WF, Lipton RB, Whyte J, et al. An international study to assess reliability of the Migraine Disability Assessment (MIDAS) score. Neurology. 1999;53(5):988-994. doi: 10.1212/WNL.53.5.988 [DOI] [PubMed] [Google Scholar]
- 41.Kosinski M, Bayliss MS, Bjorner JB, et al. A six-item short-form survey for measuring headache impact: the HIT-6. Qual Life Res. 2003;12(8):963-974. doi: 10.1023/A:1026119331193 [DOI] [PubMed] [Google Scholar]
- 42.Kawata AK, Coeytaux RR, Devellis RF, Finkel AG, Mann JD, Kahn K. Psychometric properties of the HIT-6 among patients in a headache-specialty practice. Headache. 2005;45(6):638-643. doi: 10.1111/j.1526-4610.2005.05130.x [DOI] [PubMed] [Google Scholar]
- 43.Yang M, Rendas-Baum R, Varon SF, Kosinski M. Validation of the Headache Impact Test (HIT-6™) across episodic and chronic migraine. Cephalalgia. 2011;31(3):357-367. doi: 10.1177/0333102410379890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jhingran P, Davis SM, LaVange LM, Miller DW, Helms RW. MSQ: Migraine-Specific Quality-of-Life Questionnaire. Further investigation of the factor structure. Pharmacoeconomics. 1998;13(6):707-717. doi: 10.2165/00019053-199813060-00007 [DOI] [PubMed] [Google Scholar]
- 45.Martin BC, Pathak DS, Sharfman MI, et al. Validity and reliability of the migraine-specific quality of life questionnaire (MSQ Version 2.1). Headache. 2000;40(3):204-215. doi: 10.1046/j.1526-4610.2000.00030.x [DOI] [PubMed] [Google Scholar]
- 46.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097. doi: 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 48.Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524-532. doi: 10.1037/1040-3590.7.4.524 [DOI] [Google Scholar]
- 49.Osman A, Barrios FX, Kopper BA, Hauptmann W, Jones J, O’Neill E. Factor structure, reliability, and validity of the Pain Catastrophizing Scale. J Behav Med. 1997;20(6):589-605. doi: 10.1023/A:1025570508954 [DOI] [PubMed] [Google Scholar]
- 50.French DJ, Holroyd KA, Pinell C, Malinoski PT, O’Donnell F, Hill KR. Perceived self-efficacy and headache-related disability. Headache. 2000;40(8):647-656. doi: 10.1046/j.1526-4610.2000.040008647.x [DOI] [PubMed] [Google Scholar]
- 51.Baer RA, Smith GT, Hopkins J, Krietemeyer J, Toney L. Using self-report assessment methods to explore facets of mindfulness. Assessment. 2006;13(1):27-45. doi: 10.1177/1073191105283504 [DOI] [PubMed] [Google Scholar]
- 52.Baer RA, Smith GT, Lykins E, et al. Construct validity of the five facet mindfulness questionnaire in meditating and nonmeditating samples. Assessment. 2008;15(3):329-342. doi: 10.1177/1073191107313003 [DOI] [PubMed] [Google Scholar]
- 53.Rosier EM, Iadarola MJ, Coghill RC. Reproducibility of pain measurement and pain perception. Pain. 2002;98(1-2):205-216. doi: 10.1016/S0304-3959(02)00048-9 [DOI] [PubMed] [Google Scholar]
- 54.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numerical rating scales. Pain. 1994;56(2):217-226. doi: 10.1016/0304-3959(94)90097-3 [DOI] [PubMed] [Google Scholar]
- 55.Chaves TC, Dach F, Florencio LL, et al. Concomitant migraine and temporomandibular disorders are associated with higher heat pain hyperalgesia and cephalic cutaneous allodynia. Clin J Pain. 2016;32(10):882-888. doi: 10.1097/AJP.0000000000000369 [DOI] [PubMed] [Google Scholar]
- 56.Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47(5):614-624. doi: [DOI] [PubMed] [Google Scholar]
- 57.Koyama T, McHaffie JG, Laurienti PJ, Coghill RC. The subjective experience of pain: where expectations become reality. Proc Natl Acad Sci U S A. 2005;102(36):12950-12955. doi: 10.1073/pnas.0408576102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2018. [Google Scholar]
- 59.van Buuren S, Groothuis-Oudshoorn K.. mice: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45(3):1-67. doi: 10.18637/jss.v045.i03 [DOI] [Google Scholar]
- 60.Bates D, Maechler M, Bolker B, Walker S.. Fitting linear mixed-effects models using lme4. Journal of Statistical Software. 2015;67(1):1-48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 61.Buse DC, Lipton RB, Hallström Y, et al. Migraine-related disability, impact, and health-related quality of life among patients with episodic migraine receiving preventive treatment with erenumab. Cephalalgia. 2018;38(10):1622-1631. [DOI] [PubMed] [Google Scholar]
- 62.Cohen J. Statistical Power Analysis for the Behavioral Sciences. United States of America: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 63.Sawilowsky S. New effect size rules of thumb. Journal of Modern Applied Statistical Methods. 2009;8(2):467-474. doi: 10.22237/jmasm/1257035100 [DOI] [Google Scholar]
- 64.Dodick DW, Turkel CC, DeGryse RE, et al. Assessing clinically meaningful treatment effects in controlled trials: chronic migraine as an example. J Pain. 2015;16(2):164-175. doi: 10.1016/j.jpain.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 65.Bond DS, Thomas JG, Lipton RB, et al. Behavioral weight loss intervention for migraine: a randomized controlled trial. Obesity (Silver Spring). 2018;26(1):81-87. doi: 10.1002/oby.22069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kindelan-Calvo P, Gil-Martínez A, Paris-Alemany A, et al. Effectiveness of therapeutic patient education for adults with migraine. A systematic review and meta-analysis of randomized controlled trials. Pain Med. 2014;15(9):1619-1636. doi: 10.1111/pme.12505 [DOI] [PubMed] [Google Scholar]
- 67.Kabat-Zinn J, Lipworth L, Burney R. The clinical use of mindfulness meditation for the self-regulation of chronic pain. J Behav Med. 1985;8(2):163-190. doi: 10.1007/BF00845519 [DOI] [PubMed] [Google Scholar]
- 68.Kabat-Zinn J. Full Catastrophe Living: Using the Wisdom of Your Body and Mind to Face Stress, Pain, and Illness. New York: Random House; 1990. [Google Scholar]
- 69.Seng EK, Singer AB, Metts C, et al. Does mindfulness-based cognitive therapy for migraine reduce migraine-related disability in people with episodic and chronic migraine? a phase 2b pilot randomized clinical trial. Headache. 2019;59(9):1448-1467. doi: 10.1111/head.13657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anheyer D, Leach MJ, Klose P, Dobos G, Cramer H. Mindfulness-based stress reduction for treating chronic headache: a systematic review and meta-analysis. Cephalalgia. 2019;39(4):544-555. [DOI] [PubMed] [Google Scholar]
- 71.Grazzi L, Sansone E, Raggi A, et al. Mindfulness and pharmacological prophylaxis after withdrawal from medication overuse in patients with chronic migraine: an effectiveness trial with a one-year follow-up. J Headache Pain. 2017;18(1):15. doi: 10.1186/s10194-017-0728-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seminowicz DA, Burrowes SAB, Kearson A, et al. Enhanced mindfulness-based stress reduction in episodic migraine: a randomized clinical trial with magnetic resonance imaging outcomes. Pain. Published online March 13, 2020. doi: 10.1097/j.pain.0000000000001860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silberstein SD, Stauffer VL, Day KA, Lipsius S, Wilson MC. Galcanezumab in episodic migraine: subgroup analyses of efficacy by high versus low frequency of migraine headaches in phase 3 studies (EVOLVE-1 & EVOLVE-2). J Headache Pain. 2019;20(1):75. doi: 10.1186/s10194-019-1024-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dodick DW, Silberstein SD, Bigal ME, et al. Effect of fremanezumab compared with placebo for prevention of episodic migraine: a randomized clinical trial. JAMA. 2018;319(19):1999-2008. doi: 10.1001/jama.2018.4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lipton RB, Desai P, Sapra S, Buse DC, Fanning KM, Reed ML. How much change in headache-related disability is clinically meaningful? estimating minimally important difference (MID) or change in MIDAS using data from the AMPP study. Headache. 2017;57(53):165-166.27902848 [Google Scholar]
- 76.Pressman A, Law H, Stahl R, et al. Conducting a pilot randomized controlled trial of community-based mindfulness-based stress reduction versus usual care for moderate-to-severe migraine: protocol for the Mindfulness and Migraine Study (M&M). Trials. 2019;20(1):257. doi: 10.1186/s13063-019-3355-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Grazzi L, Bernstein C, Raggi A, et al. ACT for migraine: effect of acceptance and commitment therapy (ACT) for high-frequency episodic migraine without aura: preliminary data of a phase-II, multicentric, randomized, open-label study. Neurol Sci. 2019;40(suppl 1):191-192. [DOI] [PubMed] [Google Scholar]
- 78.Kuruvilla D, Wells RE. Evidence-based integrative treatments for headache. Headache. 2019;59(6):971-972. doi: 10.1111/head.13555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells RE, Strauss LD. The value of headache-specific recommendations during COVID-19. Headache. 2020;60(5):820-823. doi: 10.1111/head.13821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chowdhury D, Datta D. Managing migraine in the times of COVID-19 pandemic. Ann Indian Acad Neurol. 2020;23(suppl 1):S33-S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable1: Dates of Study Participation by Cohort: Recruitment, Classes, and Study Visits
eTable2: Eligibility Criteria and Justification
eTable 3: Course Content of MBSR and Headache Education Interventions
eTable 4: Treatment Fidelity Plan & Delivery
eTable 5: Reasons and Time Points for Exclusion
eTable 6: Reasons and Time Points for Withdrawal and Lost to Follow-Up
eMethods 1: Recruitment Strategies
eMethods 2: Phone Screen
eMethods 3: MBSR Treatment Fidelity Review Assessment
eMethods 4: Instructor Curriculum Checklists
Protocol and Statistical Analysis Plan
Data Sharing Statement