Abstract
The introduction of tumor necrosis factor (TNF) inhibitors has significantly changed the treatment landscape in Crohn’s disease (CD). The overall therapeutic achievements with TNF inhibitors such as infliximab, adalimumab, and certolizumab pegol paved the way to push the boundaries of treatment goals beyond symptomatic relief and toward cessation of objective signs of inflammation, including endoscopic remission. Even though these agents are widely used for the treatment of moderate to severe CD, heterogeneity still exists in translating evidence-based guidelines on the use of anti-TNF agents into actual treatment algorithms in CD. This might be due to several reasons including disparities in health expenditure policies; lack of harmonization between countries; and variations in assessment of disease severity, use of disease monitoring tools, or application of treatment targets by physicians. With the advent of biosimilars, patent-free versions of reference biologics are now available to minimize health inequalities in drug availability. In this context, this article aims to provide practical clinical guidance for the use of infliximab and adalimumab biosimilars in patients with moderate to severe CD by outlining different clinical scenarios that patients may encounter during their treatment journey.
Keywords: biomarkers, clinical trials, endoscopy
In this review, a group of European IBD experts provide a practical guide for physicians regarding the use of anti-TNF therapy, including biosimilars, for adult patients with Crohn’s disease based on a summary of clinical data and expert opinion.
INTRODUCTION
Crohn’s disease (CD) is a chronic, inflammatory, incurable disease with increasing incidence that can affect the entire gastrointestinal tract.1, 2 It comprises heterogeneous clinical and pathogenic conditions marked by a disturbed interface between gut microbiota and the mucosal immune system that elicits chronic inflammation progressing toward irreversible gastrointestinal damage, including development of a complicated disease behavior (ie, stricturing or penetrating CD and irreversible bowel damage).2–4 Symptoms of CD depend on the location and extent of inflammation, disease behavior, and previous surgical interventions, and may include episodic or chronic diarrhea, incontinence, abdominal pain, fever, and weight loss.5 Extraintestinal manifestations (EIMs), such as peripheral arthritis and spondyloarthritis, pyoderma gangrenosum, (epi)scleritis, or uveitis, can contribute to the complex clinical presentations of patients with CD. The disease burden is further afflicted by anxiety, depression, and reduced social activities and working ability, entailing a pronounced impact on the patient’s quality of life.6, 7
No curative therapy is available at present, with most patients facing lifelong treatment aimed at the resolution of the inflammatory process and ensuing symptoms as a means to diminish the long-term risk of surgery and loss of intestinal functions.8 Approved biologic treatments for moderate to severe CD include the anti-tumor necrosis factor (anti-TNF) agents infliximab, adalimumab, and certolizumab pegol; the anti-adhesion antibody vedolizumab directed against α4β7 integrin; as well as ustekinumab, which blocks the p40 subunit of interleukin-12 and interleukin-23.9–14 These therapies brought significant changes to the treatment landscape of CD, leading to improvements in disease control and quality of life. Specifically, the introduction of the first 2 biologics, infliximab (a chimeric monoclonal antibody; approved 1998 US, 1999 EU) and adalimumab (a fully human monoclonal antibody; approved 2007 US and EU), constituted a quantum leap in efficacy with up to double the clinical and endoscopic remission rates previously seen with conventional therapies, and a decreased need for corticosteroids.15–21 Certolizumab pegol, a pegylated Fab’ fragment of a humanized monoclonal anti-TNF antibody, was subsequently approved in 2008 for the treatment of adult patients with moderate to severe luminal CD in the US, Switzerland, and other countries, but not the EU.22–24
Currently, evidence-based treatment guidelines for CD, including those issued by the European Crohn’s and Colitis Organisation (ECCO) and the American College of Gastroenterology (ACG), position anti-TNF agents as standard treatment options in patients with moderate to severe disease.5, 11 However, despite widespread acceptance and frequent quotation of those guidelines, there is significant heterogeneity in the uptake of anti-TNF agents in inflammatory bowel disease (IBD).25–28 Some variation arises from clinical practice and therapeutic guidelines. For example, a survey in 10 European countries highlighted significant variation in the criteria applied prior to initiation of a biologic therapy in CD, including differences in disease activity thresholds and the type and number of non-biologic therapies that must be tried before a biologic can be prescribed.25 The extent and impact of non-clinical barriers, principally cost, also vary greatly across different health systems. In the United States, an analysis of health care utilization found that infliximab accounted for 35% of a total of $4.6 billion in medical claim charges in IBD, despite its use being restricted to 11% of patients.26 In Europe, the cost of medications influences physicians’ willingness to prescribe biologic therapy,27 and GDP per capita was strongly correlated with biologic uptake.25
Access to anti-TNF agents began to improve with the introduction of biosimilars for infliximab in 2013 and adalimumab in 2018.29–32 However, there are also striking variations in the clinical management of patients with CD with respect to the use of anti-TNF agents. For example, the proportion of anti-TNF-treated CD patients was reported to fluctuate between 31% and 60% across high-volume IBD centers in the United States, with no underlying variation in disease characteristics.33 Although the exact causes of this fluctuation were not analyzed, differences in the appreciation of disease severity and patient complexity, too much latitude in the interpretability of guidelines, or contrasting approaches on the involvement of patients in the decision process could play a role.6, 34, 35 Variations in disease-monitoring strategy, disease-monitoring tools, treatment optimization, and treatment targets could further add to the heterogeneity of the clinical management of CD patients with anti-TNF agents.
Evidence-based medicine and derived consensus are indispensable in the management of CD. However, patients enrolled in randomized controlled trials are not representative of the postulation seen in routine clinical practice.36 Therefore, clinical expertise should be incorporated into guidance documents on the management of CD, a disease known for the plentiful variety of clinical scenarios that cannot all be tackled by evidence alone.
Our article aims to merge evidence and personal experience gathered over recent years to provide expert guidance on the use of anti-TNF agents in adult patients with moderate to severe CD, with a focus on ubiquitously available infliximab and adalimumab. By outlining and discussing the different clinical scenarios that a patient may encounter during the treatment journey with these drugs, we strive to contribute to a greater standardization of care and aim to reduce the negative impact on patient outcomes caused by disparities between treatment settings and clinicians.
PATIENT SELECTION AND ASSESSMENT
Introduction
Both infliximab and adalimumab are approved for the treatment of moderately to severely active CD in patients with inadequate response or intolerance to conventional therapy, including corticosteroids and/or immunomodulators, such as thiopurines or methotrexate.37, 38 Improved outcomes have been described in patients with short disease duration, supportive of ‘hit hard and early’ strategies such as the early introduction of infliximab in combination with immunomodulators (due to its immunogenic profile) in patients failing systemic or topical steroids, or of less immunogenic adalimumab monotherapy escalated to combination with immunomodulators in case of insufficient response to the former. In addition, infliximab is approved for the treatment of active fistulizing CD lacking response to conventional treatment including antibiotics, immunomodulators, and surgical drainage.38
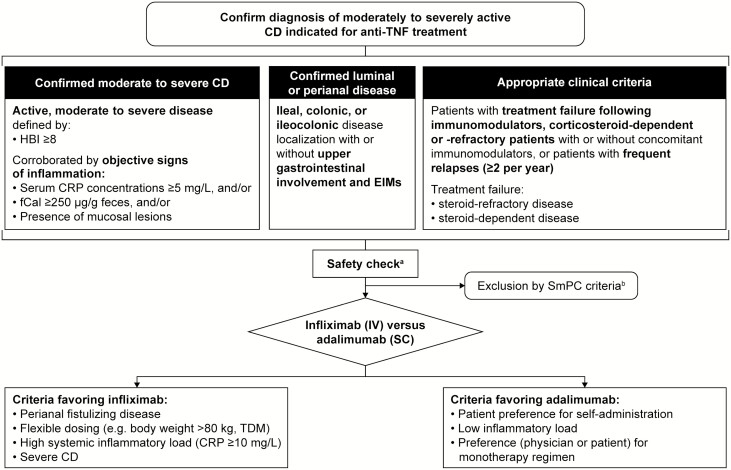
In routine clinical practice, identification of patients potentially indicated for anti-TNF treatment can be achieved according to the following criteria (Fig. 1):
FIGURE 1.
Selection of TNF inhibitor. aFor example, complete blood count, albumin, liver function; for safe application of biologics, see Miehsler et al. 2010; 39 for opportunistic infections and preventive live vaccinations, see Rahier et al. 2014.40bSee Infliximab Summary of Product Characteristics38, 41 and Adalimumab Summary of Product Characteristics.37, 42 Abbreviations: CD, Crohn’s disease; CRP, C-reactive protein; EIM, extraintestinal manifestation; fCal, fecal calprotectin; HBI, Harvey–Bradshaw Index; SmPC, summary of product characteristics; TDM, therapeutic drug monitoring; TNF, tumor necrosis factor.
Confirmed moderate to severe CD: active, moderate to severe disease defined by a Harvey–Bradshaw Index (HBI) ≥8 and serum C-reactive protein (CRP) concentrations ≥5 mg/L, and/or fecal calprotectin (fCal) ≥250 µg/g feces, and/or presence of mucosal lesions
Confirmed luminal or perianal disease: ileal, colonic, or ileocolonic disease localization with or without upper gastrointestinal involvement and EIMs
Appropriate clinical criteria: patients with treatment failure after immunomodulators, corticosteroid-dependent or -refractory patients with or without concomitant immunomodulators; or patients with frequent relapses (≥2 per year)
Patients presenting at a young age, with extensive small bowel disease, in need of immediate treatment with systemic steroids or with perianal disease are considered to have poor prognosis.11 A massive inflammatory burden—as suggested by indicators such as deep and extensive ulceration or diffuse bowel wall thickening in cross-sectional imaging/transabdominal ultrasonography—and excruciating EIMs such as arthritis, erythema nodosum, or pyoderma gangrenosum may likewise point to an aggressive course of disease and could also be considered as indicators for the early introduction of anti-TNF agents. There is less experience on the use of infliximab and adalimumab in patients with treatment failure after receiving biologics with other modes of action; however, it is clear that any biologic treatment failure reduces the likelihood of response to subsequent therapies.43, 44
Disease Activity Scoring
Before initiating therapy with an anti-TNF agent, it is mandatory to assess clinical disease activity. The pivotal clinical studies of anti-TNF agents in luminal CD applied the CD Activity Index (CDAI) for the assessment of disease activity, with a threshold of at least 220.45–47 As the CDAI has to be calculated over a period of 7 days, its practicality for routine clinical use is limited. An alternative assessment, the HBI, which involves collecting patient information on general well-being, abdominal pain, daily number of liquid or soft stools (stool frequency [SF]), abdominal mass, and complications on a single day, correlates well with the CDAI and is generally the preferred measurement tool in clinical practice (see Supplementary Table 1 in the online supplementary data).48, 49 Active CD can be defined by an HBI ≥5 and HBI >16 corresponds to severe CD. Recently, eligibility criteria for trials include patient-reported outcomes (PROs), such as average SF ≥4 and abdominal pain ≥2 over 1 week—values that appear transferable into clinical practice.50 However, it should be noted that any categorization of disease activity by particular HBI or PRO thresholds is arbitrary, and symptoms do not align well with the inflammatory burden of CD. Therefore, we strongly suggest that clinical activity scored by the HBI needs to be further corroborated by objective signs of inflammation before initiating an anti-TNF agent. Such signs may include the presence of mucosal lesions (confirmed by endoscopy, cross-sectional imaging, or transabdominal ultrasonography), fCal ≥250 µg/g feces, and/or elevated CRP ≥5 mg/L.
Inflammatory Biomarkers
The inflammatory biomarkers CRP and fCal provide an indication of inflammatory status in patients with CD.11, 51, 52 Elevation in CRP at baseline predicts response to anti-TNF agents, and, among responders to anti-TNF induction, normalization of CRP at week 14 is associated with maintenance of response to therapy.53–55 fCal positively correlates with endoscopic activity, and its decline and elevation may be associated with response to anti-TNF agents or a higher risk of relapse, respectively.56, 57 In the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) program, biomarker remission (CRP and fCal within normal range) was considered as an adjunctive target, but it is currently evaluated as one of the main proxies of endoscopic activity in clinical practice due to the positive experience from the CALM study.58 There is no clear optimal cut-off to define CRP elevation, and thus we suggest that the reference value cut-off of 5 mg/L may be used as applied in the CALM study.52, 58 Similar to CRP, a clear predictive threshold for fCal in CD has not been established. For clinical practice, we suggest using the same fCal threshold of ≥250 µg/g feces as in the CALM study as an indication of clinically relevant intestinal inflammation.58 However, it should be stressed that endoscopic examination/imaging should be performed before initiation of anti-TNF agents in naïve patients.
Endoscopy, Cross-sectional Imaging, and Transabdominal Ultrasonography
Ileocolonoscopy, cross-sectional imaging (computed tomography and magnetic resonance enterography [MRE]), and transabdominal ultrasonography are complementary procedures for establishing the diagnosis of CD and can be used to support or exclude the eligibility of a patient for anti-TNF therapy, by the detection and staging of inflammation and obstructive and penetrating complications.11
Currently, no strict definitions of the endoscopic criteria required to qualify for treatment with anti-TNF strategies are available. The Study of Biologic and Immunomodulator Naive Patients in Crohn’s Disease (SONIC) trial demonstrated that patients with endoscopic lesions at baseline derived the largest benefit from infliximab treatment (either alone or in combination with azathioprine) when compared with azathioprine monotherapy.21 In recent clinical trials that included patients with moderate to severe CD, endoscopic entry criteria were defined by the Simple Endoscopic Score of CD (SES-CD) ≥6, or ≥4 in the case of isolated terminal ileal disease.59 Those criteria have been arbitrarily selected in the absence of clear evidence of the predictive value of thresholds for total sum or individual item scores of the SES-CD for outcome, and as such, their validity is questionable. Even though the SES-CD is barely used in clinical practice due to its perceived complexity and associated workload, we believe that using the SES-CD score may currently be the best approach to standardize reports and ease communication on endoscopic disease activity between physicians. Therefore, we encourage using the SES-CD to describe baseline findings and any changes from baseline during treatment.
To qualify for treatment with infliximab or adalimumab, patients would be expected to present with the following endoscopic evidence of disease: either an ulcerated surface >10% or large ulcers with a diameter of 0.5–2 cm in at least 1 segment from the ileum to the rectum. Endoscopy is the most appropriate method to assess mucosal healing, and performing an endoscopic examination at baseline may therefore be useful when assessing response over time.
For MRE, a number of scoring systems have been developed with a focus on developing indices for quantification of active disease and its responsiveness to treatment. The Magnetic Resonance Index of Activity comprises several MRE features for each bowel segment, including bowel wall thickness, ulcers, edema, measurements of wall signal intensity before and after intravenous contrast administration, and relative contrast enhancement with a segmental score ≥7 marking active disease.60, 61 Score thresholds informing the use of anti-TNF treatment have not been explored and are not in use in clinical practice. Nevertheless, we believe that at least a semi-quantitative assessment of inflammatory disease activity by MRE can be helpful in the qualification for treatment with anti-TNF agents; this can be based on the magnitude of wall thickness (eg, >5 mm) and moderately increased intravenous contrast enhancement. Evidence on transabdominal ultrasonography similarly lacks validated thresholds of inflammatory disease activity with bowel wall thickness posing as the most prominent feature to rely on.
Disease Classification and Selection of Interventions
CD can be classified with the established Montreal classification by location (L1–L4; ileal, colonic, and ileocolonic disease with or without upper gastrointestinal tract involvement) and phenotype (B1–B3p; segregating inflammatory stricturing or penetrating disease with or without perianal involvement).62 Furthermore, ECCO advocates differentiation into localized and extensive disease, defined as intestinal CD affecting <30 cm and >100 cm in extent, respectively.11
The conventional indication for anti-TNF treatment in biologic-naïve, non-operated patients, according to the Montreal classification, is the B1 phenotype (ie, non-stricturing, non-penetrating disease), whereas for patients with the stricturing phenotype B2 or the penetrating phenotype B3, surgical options should be discussed first.62 However, in the subgroup of patients with symptomatic small bowel strictures (ie, those who have a prominent inflammatory component of the strictures), the recently published CREOLE study has shown that adalimumab may be successfully administered with long-term benefit, with 51% of the patients not requiring surgery during a 4-year follow-up.63 In the case of fistulizing disease, abscesses should be drained either radiologically or surgically before starting treatment because the presence of undrained sepsis is a contraindication to anti-TNF therapy. The management of internal fistulae is still controversial in terms of the need for resection of the fistula-deriving segment; 64 in general, patients with imminent risk for surgical intervention should not be treated with anti-TNFs due to potential for an increased risk of perioperative infections.11
In CD, symptoms can derive from various non-inflammatory causes and therefore inflammation needs to be confirmed as the cause of symptoms before treatment. Conversely, asymptomatic patients may have active inflammation that is appropriate for treatment. However, as those patients by definition fall outside the licensed indications for anti-TNF agents, treatment initiation should be considered after careful weighing of the individual benefit–risk balance. For this reason, an alternative holistic approach was proposed recently by Siegel and co-workers who developed the IBD index as an integrative tool to assess the long-term burden of the disease based on selected attributes that determine overall disease severity.65 These include the presence of mucosal lesions, fistulae or perianal abscesses, intestinal resection >40 cm, and high inflammatory load. Although this approach might be useful in future clinical practice to draw a picture that extends beyond acute clinical symptoms, this index has not yet been validated.
Preparing for Treatment Initiation
Before initiating treatment with anti-TNF agents, comprehensive safety checks should be performed as specified in the international and local guidelines, including testing for opportunistic infections and ensuring safe time intervals between preventive live vaccinations and exposure to anti-TNF agents (Fig. 1).39, 40 During this evaluation, attention should be paid to the contraindications for treatment with infliximab or adalimumab (active tuberculosis or other severe infections, such as sepsis and opportunistic infections; moderate to severe heart failure).37, 38, 41, 42 Patients with confirmed EIMs require a multidisciplinary team approach for effective management.66, 67
Before initiating therapy with an anti-TNF agent, it is good practice to set patient-specific treatment goals.57 A baseline patient questionnaire completed in the waiting room prior to the consultation can be used to document subjective symptoms and problems, facilitate the following consultation visit, and provide relevant information for ongoing monitoring and patient management (an example clinical follow-up questionnaire is included in the online supplementary data).
TREATMENT SELECTION
Infliximab and adalimumab have been studied extensively in patients with CD; however, prospective, double-blind, double-dummy studies directly comparing the 2 biologics are not available. Most of the available comparative evidence is derived from retrospective and some prospective observational cohort studies, which suggest no major differences in efficacy and safety between infliximab and adalimumab but a higher immunogenicity of the former (see Supplementary Table 2 in the online supplementary data and references therein).
Route and schedule of administration are likely to drive physician and patient preference (Fig. 1). Infliximab is administered intravenously and therefore must be administered over a significant time period in a clinical setting, whereas adalimumab is delivered subcutaneously, allowing for home-based treatment; the 2 therapies also differ regarding the administration schedule (see Therapeutic Scenarios).68 Treatment with infliximab can be easily tailored according to weight- and therapeutic drug monitoring (TDM)-based dosing adjustments, whereas dose optimization with adalimumab is limited by the 40 mg and 80 mg fixed-dose injections. This difference should be considered in patients with a weight >80 kg in whom the efficacy of the standard adalimumab dose of 40 mg once every 2 weeks seems to be less pronounced.11 A similar rationale applies to patients with presumably high drug clearance at baseline, in particular those with low serum albumin levels, high serum CRP concentrations, and obese patients, in whom more flexible dosing with infliximab may help achieve appropriate drug exposure.69 Physician preference may also be driven by perceptions of differing potency; a US survey indicated that infliximab may be regarded as more potent than adalimumab by both IBD experts and non-expert physicians.70
Combination therapy of the more immunogenic infliximab with azathioprine has been shown to be superior to either treatment alone in patients with CD and is the strategy of first choice for the use of infliximab.21 The efficacy of combining adalimumab with immunomodulators has been assessed in a meta-analysis of 18 studies that found that a combination therapy is potentially only marginally more effective than adalimumab monotherapy for the induction of clinical remission.71 Starting adalimumab as monotherapy escalated to combined immunosuppression is a strategy successfully explored in the CALM study.58 In general, we suggest considering stopping any concomitant immunomodulator during the first 6–12 months of treatment in patients achieving stable remission when corroborated by the absence of objective signs of inflammation, including normalization of inflammatory markers. However, it is important to consider that immunogenicity is a significant issue, particularly with infliximab,72 and so ongoing immunomodulatory therapy may be necessary to maintain the effectiveness of therapy. Based on vast experience, infliximab is approved and generally considered first choice in perianal fistulizing disease.73
The lack of availability of certolizumab pegol in Europe has resulted in limited experience with this agent. While recommended in European guidelines as an alternative to infliximab and adalimumab, data suggest that infliximab plus azathioprine and adalimumab have superior efficacy for induction of remission.74 A situation in which certolizumab pegol may be particularly useful is in pregnancy or during breastfeeding. Safety concerns associated with the use of anti-TNF during pregnancy have been extensively discussed.75–78 A 2016 consensus recommended to continue anti-TNF therapy in pregnant women with IBD who are on anti-TNF maintenance therapy (Statement 10A).79, 80 However, with certolizumab pegol, the FDA have granted a label update that includes pharmacokinetic data showing negligible to low transfer of the biologic through the placenta and minimal mother-to-infant transfer from breast milk.22, 78
Biosimilars
A biosimilar is a biologic medicine that is highly similar, in terms of clinical behavior (including efficacy, safety, immunogenicity, and pharmacokinetics) and product quality, to a previously approved existing biologic medicine (known as the reference medicine).81 Biosimilars are approved using a stringently controlled, specific regulatory pathway in which extensive preclinical demonstration of similarity at a physicochemical and functional level allows for an abbreviated, streamlined clinical trial program.82 Clinical trials comparing the biosimilar with the reference medicine are conducted in 1 or 2 indications known to be most sensitive to detect differences between the 2 molecules.82 Once similarity has been demonstrated in these indications, the totality of evidence for similarity between biosimilar and reference medicine from preclinical and clinical data allows approval in additional non-studied indications via extrapolation from the experience with the reference medicine.82
The rationale for the use of biosimilars in IBD, as in other conditions, is that the abbreviated pathway allows for a reduction in the overall costs of development, which can be passed on to health care systems in terms of more affordable prices compared with reference medicines.83 As such, the use of biosimilars in IBD, a disease with a high health economic and societal burden, may enable wider access to therapy as well as providing savings that may allow greater investment in additional medicines or technology.84
A large number of biosimilars of adalimumab and infliximab are currently approved in the EU for use in IBD85, 86 and several more are in the pipeline,87 in addition to biosimilars of newer biologics such as ustekinumab, certolizumab pegol and golimumab.88, 89 For the approved biosimilars of infliximab and adalimumab, pivotal trials used for approval were generally conducted in rheumatological diseases (rheumatoid arthritis [RA], ankylosing spondylitis, and psoriasis) rather than IBD.84, 89 The rationale for this decision was the extensive clinical experience with biosimilars in patients with rheumatological diseases, as well as increased sensitivity to detect treatment differences between biosimilar and reference medicine in these illnesses.
Following approval, a number of trials have been conducted for biosimilars in IBD. To date, these have focused mostly on infliximab, as the first infliximab biosimilars were approved in 2013,85 whereas adalimumab biosimilars have only been available since late 2018 in Europe, and are not yet available in the United States owing to patent issues.90 A meta-analysis of 11 observational studies conducted with an infliximab biosimilar demonstrated excellent efficacy and safety across both CD and ulcerative colitis patients.91 More recently, a large national observational study was conducted in over 3000 infliximab-naïve patients with ulcerative colitis in France.92 Patients received either reference infliximab or an infliximab biosimilar. The results showed that the biosimilar resulted in equivalent effectiveness on a composite endpoint (including death, ulcerative colitis-related surgery, all-cause hospitalization, and reimbursement for other biologics [other anti-TNF agents or vedolizumab]) and a potential improvement in the number of serious infections. These data suggest that biosimilars offer an effective and safe alternative to premium biologics in IBD.
Despite the stringent regulatory approval processes for biosimilars, which confirms extensive similarity with the reference medicine, one concern frequently raised with regard to biosimilars is the potential implications of a switch from reference medicine to biosimilar or vice-versa, or from biosimilar to biosimilar. Physicians, other health care professionals, and patients may anticipate differences in immunogenicity and safety, or even a loss of efficacy, following a switch between different versions of the same biologic.93 Several switching studies have been conducted in IBD, with the NOR-SWITCH study being perhaps the most noteworthy. NOR-SWITCH was conducted at 40 infusion centers in Norway in patients with rheumatological diseases who had received stable treatment with reference infliximab in a hospital setting for at least 6 months.94 Of 482 patients randomized into the full study, 155 (32%) had CD, and 93 (19%) had ulcerative colitis. Patients were randomized to continue to receive reference infliximab or switch to an infliximab biosimilar. Patients switched to biosimilar infliximab demonstrated non-inferiority to reference infliximab on a composite primary endpoint of disease worsening (assessed by the Harvey–Bradshaw Index and partial Mayo score). No significant differences were seen between groups on inflammatory markers, anti-drug antibodies, pharmacokinetics, safety, or number of patients in clinical remission at 1 year. Additional studies have confirmed these results. In a study in biologic-naïve patients with active CD,95 patients were randomized to reference or biosimilar infliximab for 30 weeks, then re-randomized to continue on their original assigned treatment or crossover to the other treatment group for a further 24 weeks. On the primary endpoint of clinical response (proportion of patients with a decrease of 70 points or more in CDAI) at week 6, biosimilar infliximab was non-inferior to reference infliximab; there were no significant differences in safety between the 2 groups. In a more recent study, 133 patients with IBD at a UK center were switched from one infliximab biosimilar (CT-P13) to a second one (SB2),96 a situation that is likely to become commonplace given the large numbers of infliximab biosimilars now available.97 In this study, no impact was observed on patient outcomes as assessed by disease activity, treatment-specific domains of IBD-Control (a validated patient-reported outcome measure in IBD), and drug persistence for at least 4 months. However, the results presented so far are preliminary in nature and further, longer-term data in multiple large cohorts are required to confirm the results and examine the impact of multiple switches between different biosimilars.96
A separate, though related, issue is the concept of interchangeability between different biologics—the ability for biologics to be substituted for one another without a prescriber intervention.98 The FDA defines interchangeability as the proven ability to produce the same clinical result as the reference medicine in all approved indications, which is evaluated based on similar criteria to those used for biosimilar approval.99 Although evidence from switching studies suggests that substitution in this manner should not result in any changes in efficacy, safety, or immunogenicity, no biologics have yet been approved by the FDA for interchangeable use, for any indication.100 In Europe, the EMA does not make recommendations on interchangeability of biosimilars and/or automatic substitution at the pharmacy level, instead devolving the decision process to individual member states.101 In practice, biologic choice often depends on formulary availability rather than physician or patient preference, with biosimilar use mandated on the basis of cost in some health systems.
In some cases, biosimilar manufacturers are driving innovation in therapy. For example, Celltrion has developed the first subcutaneously administered infliximab formulation, ahead of the developers of the reference medicine.102 After a trial in which patients with RA switched from intravenous to subcutaneous CT-P13, the drug has been approved in Europe for the treatment of RA and is under review by the FDA. A trial has also been conducted in IBD, which will form the basis for approval in Europe; in the United States, however, approval in IBD will follow the pathway for novel products rather than biosimilars.
TREATMENT INITIATION
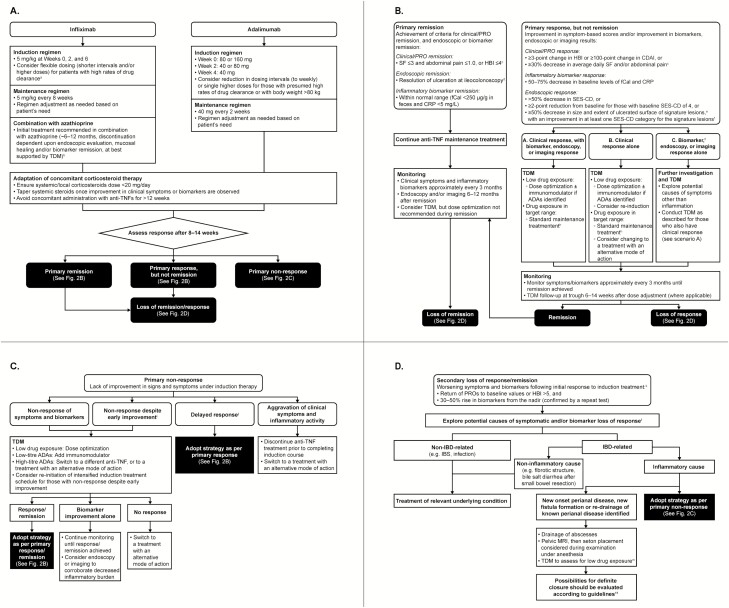
During the induction phase, infliximab is administered as an intravenous infusion at a fixed dose of 5 mg/kg of the patient’s body weight at weeks 0, 2, and 6 (Fig. 2A). However, clearance of infliximab varies between patients. Ideally, the dosing should be individually adjusted after the first dose to ensure similar subsequent exposures across patients and to reduce the risk of anti-drug antibody (ADA) formation and primary non-response due to rapid drug elimination. Strategies deploying Bayesian models to predict serum infliximab concentrations have been developed, but controlled data on the efficacy of those models during induction are still lacking. Nevertheless, patients with risk factors for high drug clearance at baseline, in particular obese patients and those with low serum albumin levels (<35 mg/L) or high serum CRP concentrations (>2 mg/dL), may benefit from flexible dosing of infliximab during induction, including shorter intervals and/or higher doses of up to 10 mg/kg.104–107 Response should be evaluated during and after the induction phase, before continuing with maintenance treatment. From week 14 onwards (maintenance phase), infliximab is administered every 8 weeks or at adjusted intervals according to the patient’s need.38, 41
FIGURE 2.
A, Initial treatment strategy. aSuch as obese patients and those with low serum albumin levels (<35 mg/L) or high serum CRP concentrations (>2 mg/dL). bDiscontinuation of azathioprine is recommended if CRP <5 mg/L and fCal <250 µg/g feces, serum trough levels are within therapeutic window and there is no evidence of immunogenicity. B, Treatment strategy for primary remission or response to anti-TNF therapy. cSF and abdominal pain scores should also be no greater than baseline values. dResolution of findings of inflammation on transabdominal ultra-sonography or cross-sectional imaging in patients who cannot be adequately assessed with ileocolonoscopy. eSignature lesions defined as the worst lesions identified. fFor example, very large ulcers (>2 cm) should decrease in size to large ulcers (>0.5–2 cm), large ulcers to small ulcers (≤0.5 cm), and small ulcers should heal completely. gInstances of discrepant results should be interpreted with caution, as biomarkers are not specific and may not reflect disease status. hFor those with objective signs of inflammation close to the ranges of remission, with a view to observe a potential late remission. C, Treatment strategy for primary non-response to anti-TNF therapy. iInitial brief improvement in symptoms and/or biomarkers, converting into a non-response during induction. jResponse after the induction treatment window; this scenario is often characterized by a disconnect between symptoms and biomarkers, where a reduction in symptoms during induction treatment precedes an improvement of biomarker levels. D, Treatment strategy for loss of remission/response to anti-TNF treatment during maintenance phase (secondary loss of response/remission). KIn unclear situations, endoscopy and/or cross-sectional imaging/transabdominal ultra-sonography may be needed. lThe diagnostic procedures to rule out other causes should be selected based on the appropriate guidelines and on the onset of symptoms, their dependency on food intake, the location of pain, associated complaints such as bloating or nausea, history of travel and exposure to infectious agents, among others. mPerianal disease reportedly requires higher target serum concentrations of infliximab.103
The induction phase for adalimumab spans a period of subcutaneous administrations of decreasing doses at week 0 (eg, 160 mg), week 2 (eg, 80 mg), and week 4 (eg, 40 mg), with the recommended induction doses varying between different regulatory agencies.37, 42, 108 The initial induction regimen is followed by injections every other week, and patients are likely to show clinical response with this regimen. A study exploring the efficacy of an optimized induction regimen with adalimumab in ulcerative colitis has finished recruitment,109 and the results are eagerly awaited. A clear definition of duration of induction with adalimumab is still disputed, and the approach recommended by ECCO guidelines is to start maintenance at week 6 with 40 mg administered every other week.11 However, evidence points to the possibility of induction response to adalimumab at later time points.110
The Personalising Anti-TNF Therapy in CD (PANTS) study, which evaluated predictors of anti-TNF treatment failure in anti-TNF-naïve patients with active luminal CD, showed in a multivariable analysis that the only factor independently associated with primary non-response to infliximab or adalimumab was low drug concentration at week 14 (both P < 0.0005). Optimal week 14 drug concentrations associated with both week 14 and week 54 remission were 7 mg/L for infliximab and 12 mg/L for adalimumab, and week 14 drug concentration was independently associated with non-remission at week 54 (P < 0.0001 for both).72 It remains unclear whether personalized induction regimens can be used to improve outcomes in patients treated with anti-TNF agents.
There are various opinions on how regularly response should be monitored during the induction phase. As a general approach, we recommend assessing induction response to adalimumab after 8–14 weeks of treatment. However, in patients with severe disease, additional visits can be planned immediately before infusions or injections at the start, middle, and end of induction (eg, at weeks 2, 6, and 14). Reduction of dosing intervals to weekly injections or single higher doses during the induction phase may be necessary to achieve response in patients with a presumed high drug clearance rate and patients with body weight >80 kg. With all anti-TNF therapies, TDM should be performed immediately after the induction phase to allow identification of subtherapeutic dosing levels and appropriate dose adjustments to correct drug levels.111
Upon initiation of anti-TNF administration, systemic or local corticosteroids should be kept below a dose of 20 mg per day, and concomitant administration should not exceed a treatment period of 12 weeks.11 We do not concur with the practice applied in clinical trials when concomitant systemic steroids need to be kept stable until the end of the induction treatment. We suggest starting to taper systemic steroids with the first signs of improvement of clinical symptoms and/or inflammatory biomarkers based on the evidence that additional immunosuppression increases the risk of infectious events, in particular in the setting of double or triple immunosuppression.112
Initiation of biosimilars may require a tailored approach beyond that used for reference biologics. Evidence suggests that patients may have significant concerns around the prescription of biosimilars; a survey conducted by the European Federation of Crohn’s and Ulcerative Colitis Associations reported that only 31% of respondents would be fully confident about receiving biosimilars, even if their prescription was made by their treating physician and was initiated with a full explanation.113 Furthermore, 20% of patients indicated that they would request to stop treatment with a biosimilar at the ‘first doubt, or alternative event.’ This lack of confidence in biosimilars can lead to the ‘nocebo effect’, in which a switch to a biosimilar version of a medicine can be linked to increased reporting of adverse events, subjective experience of disease worsening, and reduced treatment adherence.114, 115 Therefore, when initiating a biosimilar, it can be useful to employ positive framing around the biosimilar, as a patient’s expectation of a drug’s effect may influence efficacy. In addition, when transitioning from a reference medicine to a biosimilar, a structured communication strategy for the transition may increase adherence. For example, a study examining etanercept biosimilars employed a 3-part transition strategy. Patients were first informed by letter of an available option to switch from the reference medicine to a biosimilar. At their next prescription refill, they were asked by the pharmacy technician if they would be willing to switch to the biosimilar. If they agreed, they received the biosimilar, whereas if not, they were contacted by their treating physician to discuss the switch. Almost all of the patients eventually agreed to switch to the biosimilar, and adherence rates following the switch were comparable with historical cohorts, with no clinically relevant differences versus patients that continued to receive the reference medicine.116
TREATMENT GOALS
In the STRIDE program, the recommended treatment target for CD was clinical/PRO remission, defined as a combination of resolution of abdominal pain and diarrhea/altered bowel habit, and endoscopic remission, the latter described as resolution of ulceration at ileocolonoscopy or resolution of findings of inflammation on cross-sectional imaging in patients who cannot be adequately assessed with ileocolonoscopy.57 Overall, there is extensive literature on the impact of mucosal healing on long-term outcomes.117 Specifically, mucosal healing in response to anti-TNF therapy has been associated with improved outcomes of CD and is predictive of long-term remission rates.118 In contrast, there is currently no evidence that resolution of PROs can influence the long-term course of CD. As discussed above, inflammatory burden may not be directly correlated with symptoms in CD, which strengthens the notion that mucosal healing should be prioritized over resolution of symptoms in cases where they seem to be disconnected from each other.
THERAPY MONITORING
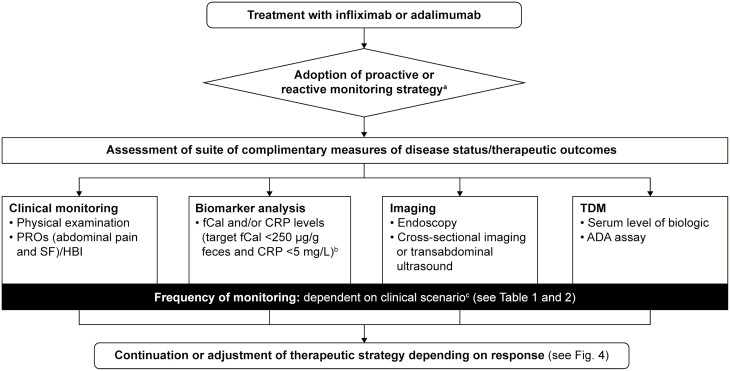
A large proportion of patients with CD who are treated with anti-TNF agents experience loss of response within a year; 119 therefore, monitoring and adjusting therapy may improve outcome. Patient monitoring can take 4 different forms: clinical monitoring, biomarker analysis, imaging, and TDM, each delivering complementary information on the disease status or the pharmacokinetics of the drug within the patient (Fig. 3). A report from the IBD Ahead Steering Committee, drafted following a structured literature search and a series of consensus meetings, recommended that disease activity should be monitored on a regular basis using objective parameters such as fCal and endoscopy.120 Demographic and clinical features can also be used to identify patients at a higher risk for complications, and additional monitoring should be tailored to meet individual patient needs.74, 120 The monitoring schedule should be set with the objectives of achieving the treatment goal of disease remission.57
FIGURE 3.
Therapy monitoring. aReactive: aimed at triggering treatment escalation in case of clinical deterioration/insufficient clinical response; proactive: aimed at making adjustments prompted by the lack of achievement of a non-symptom-based target or its proxy at defined stages of treatment, such as increased biomarker levels. bBased on the CALM study tight control criteria.58cFrequency of monitoring may be influenced by the clinical presentation and level of inflammatory disease activity, with shorter intervals in patients with high inflammatory burden at baseline and widened intervals in those achieving stable remission supported by objective signs of inflammation. Abbreviations: ADA, anti-drug antibody; CRP, C-reactive protein; fCal, fecal calprotectin; HBI, Harvey–Bradshaw index; PRO, patient-reported outcome; SF, stool frequency; TDM, therapeutic drug monitoring.
In routine clinical practice, the monitoring strategy depends on local availability, practice, and costs. Strategies can be either reactive (aimed at triggering treatment escalation in case of clinical deterioration/insufficient clinical response), or proactive (referring to adjustments prompted by the lack of achievement of a non-symptom-based target or its proxy at defined stages of treatment, such as increased biomarker levels, as used in the CALM study).58, 121
Clinical monitoring comprises physical examination and recording of PROs, including abdominal pain and SF documented by a standardized questionnaire and used to populate the HBI. The value of biomarkers in therapy monitoring is supported by the recently published results of the CALM study, where tight biomarker monitoring (based on thresholds of fCal ≥250 µg/g feces and/or elevated CRP ≥5 mg/L) was more beneficial to patients in terms of mucosal healing (primary endpoint) compared with decisions based on clinical symptoms alone.58
The frequency of clinical and biomarker monitoring may vary, but the schedule of 1 visit every 12 weeks applied in the CALM study may be feasible for the first year of treatment. Adjustments may be needed based on the clinical presentation and inflammatory disease activity, with shorter intervals in patients with high inflammatory burden at baseline and widened intervals in those achieving stable remission supported by objective signs of inflammation. It is debatable to what extent biomarker monitoring can replace endoscopy and cross-sectional imaging or transabdominal ultrasonography in patients with luminal, non-stricturing, non-penetrating (non-operated) CD. Nevertheless, the experience from the CALM study suggests that after the baseline assessment, imaging may be dispensable during the first years of follow-up in patients in whom no disease complications are suspected.
TDM considers 2 relevant issues: (1) the serum level of the biologic, which needs to be consistently within an assumed therapeutic window, and (2) the presence of ADAs, which have the potential to inhibit the activity of the biologic over protracted periods of time. Prospective studies on the clinical value of TDM of anti-TNF agents in CD are scarce and do not unequivocally support its use in clinical practice, although it should be noted that criticisms have been raised regarding potential bias in trials assessing TDM, including non-adherence to the protocol, imprecision and indirectness in randomized controlled trials; and retrospective design and imprecision in observational studies.122 Most convincing are associative studies that reveal insightful exposure–response relationships for infliximab and adalimumab during maintenance treatment.123, 124 Mucosal healing was found to be associated with levels of infliximab above 5 μg/mL and levels of adalimumab above 7.1 μg/mL.14 Based on these findings, adalimumab levels above 5 μg/mL and infliximab levels between 3 and 7 μg/mL are considered therapeutically relevant; however, recent studies suggest that in some patients an infliximab trough concentration above 7 μg/mL may provide better disease control.125 In patients with perianal fistulizing disease, higher infliximab trough concentrations of 10–20 μg/mL may be required to achieve healing.111
Note should also be made of the fact that these guidelines should be interpreted in the context of variability in inter-assay results. Fewer data are available for induction treatment.14 The presence of ADAs is clinically relevant due to their association with poor treatment outcomes, and ADA formation has been shown to be curbed by the use of concomitant immunomodulators.72, 126, 127
For biosimilars, drug levels before and after a switch from the reference medicine to a biosimilar display excellent agreement,128 suggesting that the same assays can be used for TDM regardless of which version of the biologic is administered. In addition, studies have shown that sera obtained from patients with ADAs to a reference medicine cross-react to the biosimilar, and correlation between ADA titres with reference medicine and biosimilar was excellent.100 These data have important implications for switching to biosimilars, in that patients experiencing a secondary loss of response to a certain biologic should not be prescribed another version of that same biologic, as the response will likely be limited and the patient may even be exposed to an increased risk of ADA-related adverse events such as anaphylaxis.100
THERAPEUTIC SCENARIOS
Scenarios of Anti-TNF Treatment Outcomes
Primary/continuous remission
According to the STRIDE program, remission is defined as a combination of resolution of clinical symptoms and endoscopic remission as outlined previously.57 In current clinical trials, clinical remission is defined by average daily threshold values calculated from outcomes assessed on a weekly basis (eg, an average daily SF ≤2.8 and average daily abdominal pain score ≤1.0, both also not greater than baseline). As weekly recording of PROs is not practiced in clinical routine, an SF ≤3 and an abdominal pain score ≤1.0 on the day prior to the visit, both also not greater than baseline, could be adopted as a more practical definition of clinical remission (Fig. 2B). An HBI score ≤4 is recommended as an alternative. As mentioned above, endoscopy is the standard procedure for evaluating decreases in ulcer size and surface from baseline during treatment, with transabdominal ultra-sonography and cross-sectional imaging being alternative and/or complementary procedures. The feasibility of imaging is often limited by cost, invasiveness, and operator dependency; therefore, in routine clinical practice, the presence of inflammatory biomarkers within normal range (ie, fCal <250 µg/g feces and CRP <5 mg/L) may be used as a substitute for mucosal healing after induction treatment.
In patients achieving remission during treatment with anti-TNF agents, clinical symptoms and inflammatory biomarkers should be monitored approximately every 3 months (Fig. 2B). To confirm continuous remission, endoscopy and/or imaging is proposed after 6–12 months, which may also be combined with TDM to check that the serum trough levels are therapeutic. However, the established serum concentration thresholds of infliximab and adalimumab do not exclude the possibility of mucosal healing below the cut-offs. Therefore, dose optimization is not recommended in patients who are in clinical and endoscopic remission or biomarker remission. However, loss of response and development of ADA is more likely in patients whose serum trough levels become subtherapeutic. Therefore, proactive TDM and dose optimization should be considered even in patients without evidence of active disease. Any increases in biomarker levels within the range of biomarker remission should ideally be reassessed after 1–3 months to ensure that remission is maintained. Loss of biomarker remission in patients with clinical remission should prompt TDM (if not already being carried out proactively), endoscopy, and/or imaging, followed by subsequent treatment optimization. Suitable optimizations include reduction of dose intervals for infliximab and adalimumab or dose increase for infliximab for asymptomatic patients. Observed improvements in serum concentrations of anti-TNF agents should be reassessed at trough 6–14 weeks after optimization.
Primary response, but not remission
Up to 70%–85% of patients respond at any time during induction with an anti-TNF treatment based on levels of symptoms and/or inflammatory biomarkers (see above for the discussion of time window for assessments of infliximab and adalimumab induction treatment).118 Response is usually defined by the change of a symptom-based score from treatment initiation to the end of induction period. A 3-point change in the HBI corresponds to a 100-point change in the CDAI, and both are considered clinically relevant improvements qualifying for the definition of clinical response.49 With the regulatory requirement to apply a PRO-based primary endpoint in clinical trials for CD, clinical response is defined as ≥30% decrease in average daily SF and/or ≥30% decrease in average daily abdominal pain score, both of which should not be worse than baseline.
Both HBI- and PRO-based clinical response criteria are applied in routine clinical practice but are only weakly associated with improvements in objective measures of inflammation.129, 130 Therefore, the authors suggest that assessment of clinical response should be accompanied by measuring inflammatory biomarkers, endoscopy, and/or transabdominal ultrasonography (Fig. 2B). Biomarker-based response criteria have not been established in prospective clinical trials, but an example of applying absolute thresholds can be derived from the CALM study (fCal ≥250 µg/g feces and/or elevated CRP ≥5 mg/L).58 It may, however, be difficult to achieve for patients starting with a high inflammatory burden (eg, fCal ≥1000 µg/g feces and/or elevated CRP ≥20 mg/L), in whom the relative change from baseline may be more appropriate. Response defined as a 50%–75% decrease from baseline in the levels of these biomarkers appears to be meaningful in our assessment.
In current clinical trials, endoscopic response is defined by a decrease in SES-CD >50% from baseline or at least a 2-point reduction from baseline for patients with baseline SES-CD of 4, such as patients with small bowel disease.59 An alternative approach for clinical practice could be to follow up on the worst lesions (ie, signature ulcerations), which should improve by at least 50% in size and extent of ulcerated surface. In keeping with the use of the SES-CD, signature lesions should improve by at least 1 category; for example, very large ulcers (>2 cm) should decrease in size to large ulcers (>0.5–2 cm), large ulcers to small ulcers (≤0.5 cm), and small ulcers should heal completely (see Supplementary Table 3 in the online supplementary data).131 The same approach could be applied to the lesions observed on cross-sectional imaging and transabdominal ultrasonography (eg, enhancement, bowel wall thickness).
If a patient achieves biomarker or endoscopic remission, but clinical symptoms are not resolved, alternative causes for the symptoms that are not related to inflammation should be explored.
With remission as the treatment target, response alone does not suffice as a long-term outcome. Therefore, if the patient is responding to induction treatment, but full remission is not achieved, the following measures can be considered (Fig. 2B).
First, in patients who respond clinically and on the level of biomarkers/endoscopy and/or imaging, TDM may inform dose optimization in cases of low drug exposure. Where ADA formation is identified, the addition of an immunomodulator in parallel with dose optimization is advised. In patients with improvements in objective signs of inflammation close to the ranges of remission and adequate drug exposure, standard maintenance treatment could be started to observe a potential late remission.
Second, in patients who respond clinically, but not on the level of biomarkers, endoscopy, or imaging, the same approach as above could be taken. Re-induction can also be considered in cases of low exposure. As a spurious improvement of symptoms cannot be excluded in this scenario, a change to a different mode of action can be considered if drug exposure is in the targeted range.
Third, in patients who are responding on the level of biomarkers, endoscopy, or imaging but not clinically, symptoms may result from causes other than inflammation and further investigation is warranted. As in the settings described in the first and second points, TDM may help inform dose optimization in case of low exposure, and addition of an immunomodulator to dose optimization could be considered in cases of ADA formation. For the interpretation of discrepant results, we note that inflammatory biomarker levels should be interpreted with caution as they are not specific to CD and therefore may not always reflect the disease status.
Symptoms and biomarkers should be monitored approximately every 3 months until remission is achieved, and TDM should be followed up at trough 6–14 weeks after dose adjustment. As recommended above, endoscopy and/or imaging can be performed 12 months after achieving remission, but primary monitoring via fecal biomarkers is preferred.
Primary non-response to therapy
. This is the most challenging situation for both the patient and the treating gastroenterologist, as it is associated with a reduced likelihood of a long-term benefit from anti-TNF agents. Although there is no consensus on its detailed definition, primary non-response constitutes a lack of improvement of signs and symptoms after induction therapy (ie, within 14 weeks).102 Non-response scenarios are detailed below (Fig. 2C).
Non-response of symptoms and biomarkers. If clinical symptoms and inflammatory biomarkers (CRP and fCal) both indicate non-response, low serum trough levels may be used to support dose escalation of the anti-TNF therapy, and in patients receiving infliximab or adalimumab monotherapy, low serum trough levels or low-titer ADAs support the addition of an immunomodulator. If symptoms do not resolve after dose adjustment and/or the addition of an immunomodulator but a decrease in inflammatory biomarkers occurs, continued monitoring is required until response/remission is achieved. Endoscopy or imaging may be performed to corroborate the decrease of inflammatory burden. In parallel, continued TDM may be relevant to confirm that the therapeutic window for the anti-TNF agent has been reached. Once the therapeutic window has been reached, if there is no response after treatment optimization, switching out of class should be considered. In patients who develop high-titer antibodies under induction therapy, a switch within class or to another mode of action should be considered.
Delayed response. Delayed response is defined as response after the induction treatment window (Fig. 2C). This scenario is often characterized by a disconnect between symptoms and biomarkers, where a reduction in symptoms during induction treatment is preceded by an improvement of biomarker levels. Delayed response requires the same therapeutic strategies and monitoring as primary response.
Aggravation of clinical symptoms and inflammatory activity. Some patients may deteriorate during induction treatment. Assuming that TDM reveals therapeutic dose levels, one option in this case would be to discontinue anti-TNF treatment before completing a full induction course, as the patient may have non-TNF driven inflammation.132 Options for these patients include initiation of an alternative drug with a different mechanism of action, or in severe cases, referral for surgical intervention. However, it must also be considered that TDM may be less useful in induction therapy. Because of this, an additional option is to escalate the dose of the initial drug and evaluate potential response.
Non-response despite early improvement. Some patients display an initial brief improvement in symptoms and/or biomarkers, which rapidly converts into a non-response during induction. These are typically patients with a high inflammatory burden in need of higher drug exposure. TDM and re-initiation of an intensified induction treatment schedule might be helpful, but early ADA formation should be excluded first. In those with high-titer ADAs, a switch to a different anti-TNF should be attempted first, as the initial response suggests potential for additional response with a different anti-TNF agent. If a continued lack of response is observed, therapy with an agent with alternative mode of action should be considered.
Loss of remission/response to anti-TNF treatment during maintenance phase (secondary loss of remission/response)
Loss of response to anti-TNF agents is estimated to occur in approximately 13%–40% of patients per year.118 It is defined as worsening of symptoms and biomarkers after initial response to the induction treatment. Various approaches have been proposed to identify loss of response. We suggest using symptomatic assessment (eg, increase of the PROs back to baseline values or HBI >2) substantiated by a 30%–50% rise in biomarkers from the nadir. In case of a rise in biomarkers alone, the increase should be confirmed by a repeated test. In unclear situations, endoscopy and/or cross-sectional imaging/transabdominal ultrasonography may be needed.
In patients with a symptomatic loss of response, the worsening of symptoms may not be directly related to the inflammatory component of CD (eg, fibrotic stricture or bile salt diarrhea after small bowel resection) or may have other non-CD causes (eg, irritable bowel syndrome). An infection can cause an increase in serum and fecal biomarker levels, in which case the peaks are often higher than during a CD flare. The diagnostic procedures to rule out other causes should be selected based on the appropriate guidelines and on the onset of symptoms, their dependency on food intake, the location of pain, associated complaints such as bloating or nausea, history of travel, and exposure to infectious agents, amongst others (Fig. 2D).11
In the situation of new onset perianal disease, new fistula formation, or increased activity of known perianal disease, abscesses should be drained and seton placement considered during examination under anesthesia after a pelvic MRI. Possibilities for definite closure of the fistula tract should be evaluated according to guidelines.11 TDM may be useful, as perianal disease reportedly requires higher target serum concentrations of infliximab.103
Patients with loss of response in symptoms and biomarkers should follow the same management strategy as described above for non-response.
In line with our recommendation during induction treatment, response alone is not sufficient and the ideal long-term outcome should be remission.
Therapy monitoring in different scenarios
Here, we summarize the schedule of therapy monitoring divided into induction and maintenance phases of treatment. In case of non-response or early loss of response during induction treatment, TDM is recommended to determine whether the drug serum concentration is below the therapeutic window (Table 1).
TABLE 1.
Patient Monitoring During Induction Phase (up to 14 weeks)
| Primary Response | Primary Non-response | Loss of Remission/Response | |
|---|---|---|---|
| HBI/PRO | X | X | X |
| Biomarker | X | X | X |
| Imaging | (X) | (X) | |
| TDM | (X)a | X | X |
aMay be useful in patients who respond to treatment but do not achieve full remission. (X) = optional.
Abbreviations: HBI, Harvey–Bradshaw Index; PRO, patient-reported outcome; TDM, therapeutic drug monitoring.
During maintenance treatment, a similar approach applies (Table 2). Over time, imaging will play a major role in disease monitoring, and endoscopy will be required to confirm the absence of mucosal lesions or ulcers.
TABLE 2.
Patient Monitoring During Maintenance Phase (>14 weeks)
| Remission | Response | Loss of Remission/Response | |
|---|---|---|---|
| HBI/PRO | X | X | X |
| Biomarker | X | X | X |
| Imaging | (X)a | X | X |
| TDM | (X) | X | X |
aTo confirm remission, mucosal healing needs to be evaluated by endoscopy. (X) = optional.
Abbreviations: HBI, Harvey–Bradshaw Index; PRO, patient-reported outcome; TDM, therapeutic drug monitoring.
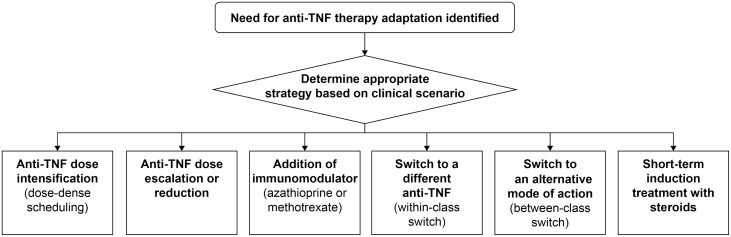
When therapy with an anti-TNF agent needs to be adapted, several options are available (Fig. 4): dose intensification by changes in dose scheduling; re-induction treatment; dose escalation or dose reduction; addition of immunomodulators (azathioprine, mercaptopurine, or methotrexate); switch within class; switch to an agent with a different mode of action (out of class); or short-term induction treatment with steroids.
FIGURE 4.
Adaptation of anti-TNF therapy. Abbreviation: TNF, tumor necrosis factor.
DISCUSSION AND CONCLUSIONS
Our manuscript aims to merge evidence and personal experience gathered over recent years on the optimization of treatment with reference to anti-TNF agents infliximab and adalimumab and their biosimilar versions in patients with CD. The potential clinical scenarios patients may encounter during treatment with these medicines are manifold and complex. Therefore, we intend to contribute to consistency and standardization in the treatment of patients across and within institutions to improve patient outcomes. Similar standardization measures have resulted in improved outcomes in other areas, including sepsis, migraine, and cholecystitis surgery.133–135
An accumulating body of literature has investigated various optimization strategies in patients with CD treated with anti-TNF agents. A clear benefit of combination treatment with infliximab and azathioprine over monotherapy was established in the SONIC study, a benefit of early combined immunosuppression was shown in the Randomised Evaluation of an Algorithm for Crohn’s Treatment (REACT) study, the value of a TDM-based monitoring strategy was suggested in the Trough Concentration Adapted Infliximab Treatment (TAXIT) study, and the CALM study displayed the superiority of a biomarker-based tight monitoring strategy over symptomatic control.21, 58, 136, 137 Many other studies that may provide further guidance on optimizing and personalizing treatment for patients with CD are underway. For example, the REACT2 study compares the step-up care algorithm that involves treatment escalation solely based on symptoms with an accelerated care algorithm that allows for early combined antimetabolite and adalimumab therapy and treatment intensification based on ileocolonoscopic findings.138 Expert opinion-based treatment algorithms are urgently needed to contextualize the key learnings of the available findings and transform them into evidence that can help guide treatment decisions.
The treatment algorithms described here provide practical clinical guidance on an optimized management of patients treated with anti-TNF agents in situations ranging from rapid remission to early non-response. The choice of therapy should be based on individual patient profiles and personalized benefit–risk assessment, with optimization throughout the course of treatment.
Supplementary Material
ACKNOWLEDGMENTS
Under the direction of the authors, Ben Caldwell, Paul Scutt, and Olga Ucar (of Spirit, a division of Spirit Medical Communications Group Ltd, Manchester, UK) provided assistance with medical writing and editorial support.
Supported by: This work was supported by Sandoz, a Novartis division. Medical writing support was funded by Sandoz, a Novartis division.
Conflicts of interest: SD has received consultancy fees from AbbVie, Allergan, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Ferring, Gilead, Hospira, Janssen, Johnson & Johnson, MSD, Mundipharma, Pfizer, Roche, Sandoz, Takeda, TiGenix, UCB, and Vifor Pharma. KG has received consultancy fees and/or speaker’s honoraria from AbbVie, Amgen, Biogen, Boehringer Ingelheim, Ferring, Hospira, Immunic Therapeutics, Janssen, MSD, Pfizer, Samsung Bioepis, Sandoz, Takeda, Tigenix, and Tillotts Pharma. JH has served as a speaker, a consultant, and/or an advisory board member for AbbVie, Celgene, Celltrion, Ferring, Hospira, Janssen, Medivir, MSD, Novartis, Olink Proteomics, Pfizer, Prometheus Laboratories Inc., Sandoz, Shire, Takeda, Thermo Fisher, Tillotts Pharma, Vifor Pharma, and UCB; and received research grants from Janssen, MSD, and Takeda. PI has served as a speaker, a consultant, and/or an advisory board member for AbbVie, Falk Pharma, Ferring, Genentech, Hospira, Johnson & Johnson, MSD, Pharmacosmos, Samsung Bioepis, Shire, Takeda, Topivert, Vifor Pharma, and Warner Chilcott; and has received research funding from MSD and Takeda. JJ has served as a speaker, consultant, or advisory board member for AbbVie, Astro Pharma, BMS, Boehringer Ingelheim, Celltrion, Ferring, Hikma, Janssen, Meda, MSD, Napp Pharma, Orion Pharma, Pfizer, Pharmacosmos, Roche, Sandoz, Takeda, and Tillotts Pharma. LP-B has received honoraria from AbbVie, Allergan, Alma, Amgen, Arena, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Enterome, Ferring, Genentech, Gilead, Hikma, Index Pharmaceuticals, Janssen, MSD, Nestle, Pfizer, Pharmacosmos, Roche, Samsung Bioepis, Sandoz, Sterna, Takeda, and Tillotts Pharma; has received grants from AbbVie, MSD, and Takeda; and holds stock options in CTMA. WR has served as a speaker for Abbott Laboratories, AbbVie, Aesca, Aptalis, Astellas, Celltrion, Centocor, Danone Austria, Elan, Falk Pharma GmbH, Ferring, Immundiagnostik, Mitsubishi Tanabe Pharma Corporation, MSD, Otsuka, PDL, Pharmacosmos, PLS Education, Schering-Plough, Shire, Takeda, Therakos, Vifor, and Yakult; served as a consultant for Abbott Laboratories, AbbVie, Aesca, Algernon, Amgen, AM Pharma, AMT, AOP Orphan, Arena Pharmaceuticals, Astellas, AstraZeneca, Avaxia, Roland Berger GmBH, Bioclinica, Biogen IDEC, BMS, Boehringer Ingelheim, Celgene, Cellerix, Celltrion, Centocor, Chemocentryx, Covance, Danone Austria, DSM, Elan, Eli Lilly, Ernst & Young, Falk Pharma GmbH, Ferring, Galapagos, Genentech, Gilead, Grünenthal, ICON, Index Pharma, Inova, Intrinsic Imaging, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, LivaNova, Mallinckrodt, Medahead, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nash Pharmaceuticals, Nestle, Nippon Kayaku, Novartis, Ocera, OMass, Otsuka, Parexel, PDL, Periconsulting, Pfizer, Pharmacosmos, Philip Morris Institute, Procter & Gamble, Prometheus, Protagonist, Provention, Robarts Clinical Trial, Sandoz, Schering-Plough, Second Genome, Seres Therapeutics, Setpointmedical, Sigmoid, Sublimity, Takeda, Therakos, Theravance, Tigenix, UCB, Vifor, Zealand, Zyngenia, and 4SC; served as an advisory board member for Abbott Laboratories, AbbVie, Aesca, Amgen, AM Pharma, Astellas, AstraZeneca, Avaxia, Biogen IDEC, Boehringer Ingelheim, BMS, Celgene, Cellerix, Celltrion, Centocor, Chemocentryx, Danone Austria, DSM, Elan, Ferring, Galapagos, Genentech, Grünenthal, Inova, Janssen, Johnson & Johnson, Kyowa Hakko Kirin Pharma, Lipid Therapeutics, MedImmune, Millenium, Mitsubishi Tanabe Pharma Corporation, MSD, Nestle, Novartis, Ocera, Otsuka, PDL, Pfizer, Pharmacosmos, Procter & Gamble, Prometheus, Schering-Plough, Second Genome, Setpointmedical, Takeda, Therakos, Tigenix, UCB, Zealand, Zyngenia, and 4SC; and has received research funding from Abbott Laboratories, AbbVie, Aesca, Centocor, Falk Pharma GmbH, Immundiagnostik, and MSD. GR has consulted for AbbVie, Augurix, BMS, Boehringer Ingelheim, Calypso, Celgene, Falk Pharma, Ferring, Fisher, Genentech, Gilead, Janssen, MSD, Novartis, Pfizer, Phadia, Roche, Takeda, Tillotts Pharma, UCB, Vifor Pharma, Vital Solutions, and Zeller; has received speaker’s honoraria from AbbVie, AstraZeneca, Falk Pharma, Janssen, MSD, Pfizer, Phadia, Tillotts Pharma, UCB, Vifor Pharma, and Zeller; and has received educational grants and research grants from AbbVie, Ardeypharm, Augurix, Calypso, Falk Pharma, Flamentera, MSD, Novartis, Pfizer, Roche, Takeda, Tillotts Pharma, UCB, and Zeller. SS has served as an advisory board member for AbbVie, Arena, Biogen, BMS, Celgene, Celltrion, Falk Pharma, Fresenius, Gilead, IMAB, Janssen, MSD, Mylan, Pfizer, Protagonist, Provention Bio, Takeda, and Theravance.
REFERENCES
- 1. Kaplan GG, Ng SC. Understanding and preventing the global increase of inflammatory bowel disease. Gastroenterology. 2017;152:313–321.e2. [DOI] [PubMed] [Google Scholar]
- 2. Baumgart DC, Sandborn WJ. Crohn’s disease. Lancet. 2012;380:1590–1605. [DOI] [PubMed] [Google Scholar]
- 3. Colombel JF, Narula N, Peyrin-Biroulet L. Management strategies to improve outcomes of patients with inflammatory bowel diseases. Gastroenterology. 2017;152:351–361.e5. [DOI] [PubMed] [Google Scholar]
- 4. Geremia A, Biancheri P, Allan P, et al. . Innate and adaptive immunity in inflammatory bowel disease. Autoimmun Rev. 2014;13:3–10. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology . Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483; quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 6. Román AL, Muñoz F. Comorbidity in inflammatory bowel disease. World J Gastroenterol. 2011;17:2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jones JL, Nguyen GC, Benchimol EI, et al. . The impact of inflammatory bowel disease in Canada 2018: quality of life. J Can Assoc Gastroenterol. 2019;2:S42–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vetter M, Neurath MF. Treatment perspectives in Crohn’s disease. Digestion. 2018;98:135–142. [DOI] [PubMed] [Google Scholar]
- 9. Deeks ED. Certolizumab pegol: a review in inflammatory autoimmune diseases. Biodrugs. 2016;30:607–617. [DOI] [PubMed] [Google Scholar]
- 10. Deepak P, Sandborn WJ. Ustekinumab and anti-interleukin-23 agents in Crohn’s disease. Gastroenterol Clin North Am. 2017;46:603–626. [DOI] [PubMed] [Google Scholar]
- 11. Gomollón F, Dignass A, Annese V, et al. ; ECCO . 3rd European Evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 12. Scribano ML. Vedolizumab for inflammatory bowel disease: from randomized controlled trials to real-life evidence. World J Gastroenterol. 2018;24:2457–2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stidham RW, Lee TC, Higgins PD, et al. . Systematic review with network meta-analysis: the efficacy of anti-TNF agents for the treatment of Crohn’s disease. Aliment Pharmacol Ther. 2014;39:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ungar B, Levy I, Yavne Y, et al. . Optimizing anti-TNF-α therapy: serum levels of infliximab and adalimumab are associated with mucosal healing in patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol. 2016;14:550–557.e2. [DOI] [PubMed] [Google Scholar]
- 15. Ananthakrishnan AN, Cagan A, Cai T, et al. . Comparative effectiveness of infliximab and adalimumab in Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2016;22:880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Asgharpour A, Cheng J, Bickston SJ. Adalimumab treatment in Crohn’s disease: an overview of long-term efficacy and safety in light of the EXTEND trial. Clin Exp Gastroenterol. 2013;6:153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Osterman MT, Haynes K, Delzell E, et al. . Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12:811–817.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rutgeerts PJ. Review article: efficacy of infliximab in Crohn’s disease–induction and maintenance of remission. Aliment Pharmacol Ther. 1999;13 Suppl 4:9–15; discussion 38. [DOI] [PubMed] [Google Scholar]
- 19. Singh S, Pardi DS. Update on anti-tumor necrosis factor agents in Crohn disease. Gastroenterol Clin North Am. 2014;43:457–478. [DOI] [PubMed] [Google Scholar]
- 20. Song YN, Zheng P, Xiao JH, et al. . Efficacy and safety of adalimumab for the Crohn’s disease: a systematic review and meta-analysis of published randomized placebo-controlled trials. Eur J Clin Pharmacol. 2014;70:907–914. [DOI] [PubMed] [Google Scholar]
- 21. Colombel JF, Sandborn WJ, Reinisch W, et al. ; SONIC Study Group . Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;362:1383–1395. [DOI] [PubMed] [Google Scholar]
- 22. FDA. Highlights of Prescribing Information: Cimzia. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125160s283lbl.pdf. Accessed March 18, 2018. [Google Scholar]
- 23. EMA. Questions and Answers on Recommendation for the Refusal of the Marketing Authorisation for Cimzia. https://www.ema.europa.eu/en/documents/medicine-qa/questions-answers-recommendation-refusal-marketing-authorisation-cimzia-international-non_en.pdf. Accessed August 2, 2008. [Google Scholar]
- 24. UCB. Cimzia - Crohn’s Disease. https://www.ucb.com/our-products/Products/cimzia%C2%AE-crohn-s-disease. Accessed August 2, 2018. [Google Scholar]
- 25. Rencz F, Péntek M, Bortlik M, et al. . Biological therapy in inflammatory bowel diseases: access in Central and Eastern Europe. World J Gastroenterol. 2015;21:1728–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Deen WK, van Oijen MG, Myers KD, et al. . A nationwide 2010-2012 analysis of U.S. health care utilization in inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20:1747–1753. [DOI] [PubMed] [Google Scholar]
- 27. Lakatos PDE, Kellner H, Marsal J, et al. . Access to biologics and biosimilars across 11 european union countries. J Crohns Colitis. 2016;10:S345. [Google Scholar]
- 28. Rogler G, Bernstein CN, Sood A, et al. . Role of biological therapy for inflammatory bowel disease in developing countries. Gut. 2012;61:706–712. [DOI] [PubMed] [Google Scholar]
- 29. Jahnsen J, Kaasen Jørgensen K. Experience with biosimilar infliximab (remsima) in Norway. Dig Dis. 2017;35:83–90. [DOI] [PubMed] [Google Scholar]
- 30. Kurti Z, Gonczi L, Lakatos PL. Progress with infliximab biosimilars for inflammatory bowel disease. Expert Opin Biol Ther. 2018;18:633–640. [DOI] [PubMed] [Google Scholar]
- 31. Van Assche G, Atreya R, Bouhnik Y, et al. . Anti-tumour necrosis factor in inflammatory bowel disease: Inventory and outlook. EMJ. 2018;3: 34–41. [Google Scholar]
- 32. GaBI Online. Adalimumab Biosimilars Amgevita and Imraldi Launched in Europe. http://www.gabionline.net/Biosimilars/News/Adalimumab-biosimilars-Amgevita-and-Imraldi-launched-in-Europe. Accessed August 2, 2018. [Google Scholar]
- 33. Ananthakrishnan AN, Kwon J, Raffals L, et al. . Variation in treatment of patients with inflammatory bowel diseases at major referral centers in the United States. Clin Gastroenterol Hepatol. 2015;13:1197–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lofland JH, Johnson PT, Ingham MP, et al. . Shared decision-making for biologic treatment of autoimmune disease: influence on adherence, persistence, satisfaction, and health care costs. Patient Prefer Adherence. 2017;11:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burisch J, Pedersen N, Cukovic-Cavka S, et al. ; EpiCom Group . Initial disease course and treatment in an inflammatory bowel disease inception cohort in Europe: the ECCO-EpiCom cohort. Inflamm Bowel Dis. 2014;20:36–46. [DOI] [PubMed] [Google Scholar]
- 36. Ha C, Ullman TA, Siegel CA, Kornbluth A. Patients enrolled in randomized controlled trials do not represent the inflammatory bowel disease patient population. Clin Gastroenterol Hepatol. 2012;10:1002–1007; quiz e78. [DOI] [PubMed] [Google Scholar]
- 37. EMA. Summary of Product Characteristics: Humira. https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. Accessed March 12, 2018. [Google Scholar]
- 38. EMA. Summary of Product Characteristics: Remicade. https://www.ema.europa.eu/en/documents/product-information/remicade-epar-product-information_en.pdf. Accessed March 18, 2018. [Google Scholar]
- 39. Miehsler W, Novacek G, Wenzl H, et al. ; Austrian Society of Gastroenterology and Hepatology . A decade of infliximab: the Austrian evidence based consensus on the safe use of infliximab in inflammatory bowel disease. J Crohns Colitis. 2010;4:221–256. [DOI] [PubMed] [Google Scholar]
- 40. Rahier JF, Magro F, Abreu C, et al. ; European Crohn’s and Colitis Organisation (ECCO) . Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443–468. [DOI] [PubMed] [Google Scholar]
- 41. EMA. Summary of Product Characteristics: Zessly. https://www.ema.europa.eu/en/documents/product-information/zessly-epar-product-information_en.pdf. Accessed August 13, 2018. [Google Scholar]
- 42. EMA. Summary of Product Characteristics: Hyrimoz. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/004320/WC500252822.pdf. Accessed August 13, 2018. [Google Scholar]
- 43. Barré A, Colombel JF, Ungaro R. Review article: predictors of response to vedolizumab and ustekinumab in inflammatory bowel disease. Aliment Pharmacol Ther. 2018;47:896–905. [DOI] [PubMed] [Google Scholar]
- 44. Gisbert JP, Marín AC, McNicholl AG, Chaparro M. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41:613–623. [DOI] [PubMed] [Google Scholar]
- 45. Hanauer SB, Sandborn WJ, Rutgeerts P, et al. . Human anti-tumor necrosis factor monoclonal antibody (adalimumab) in Crohn’s disease: the CLASSIC-I trial. Gastroenterology. 2006;130:323–333; quiz 591. [DOI] [PubMed] [Google Scholar]
- 46. Rutgeerts P, D’Haens G, Targan S, et al. . Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn’s disease. Gastroenterology. 1999;117:761–769. [DOI] [PubMed] [Google Scholar]
- 47. Targan SR, Hanauer SB, van Deventer SJ, et al. . A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035. [DOI] [PubMed] [Google Scholar]
- 48. Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1:514. [DOI] [PubMed] [Google Scholar]
- 49. Vermeire S, Schreiber S, Sandborn WJ, et al. . Correlation between the Crohn’s disease activity and Harvey-Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8:357–363. [DOI] [PubMed] [Google Scholar]
- 50. Bojic D, Bodger K, Travis S. Patient reported outcome measures (PROMs) in inflammatory bowel disease: new data. J Crohns Colitis. 2017;11:S576–S585. [DOI] [PubMed] [Google Scholar]
- 51. Miranda-García P, Chaparro M, Gisbert JP. Correlation between serological biomarkers and endoscopic activity in patients with inflammatory bowel disease. Gastroenterol Hepatol. 2016;39:508–515. [DOI] [PubMed] [Google Scholar]
- 52. Vilela EG, Torres HO, Martins FP, et al. . Evaluation of inflammatory activity in Crohn’s disease and ulcerative colitis. World J Gastroenterol. 2012;18:872–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Reinisch W, Wang Y, Oddens BJ, Link R. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post-hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568–576. [DOI] [PubMed] [Google Scholar]
- 54. Sandborn WJ, Rutgeerts P, Enns R, et al. . Adalimumab induction therapy for Crohn disease previously treated with infliximab: a randomized trial. Ann Intern Med. 2007;146:829–838. [DOI] [PubMed] [Google Scholar]
- 55. Schreiber S, Rutgeerts P, Fedorak RN, et al. ; CDP870 Crohn’s Disease Study Group . A randomized, placebo-controlled trial of certolizumab pegol (CDP870) for treatment of Crohn’s disease. Gastroenterology. 2005;129:807–818. [DOI] [PubMed] [Google Scholar]
- 56. D’Haens G, Ferrante M, Vermeire S, et al. . Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis. 2012;18:2218–2224. [DOI] [PubMed] [Google Scholar]
- 57. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. . Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 58. Colombel JF, Panaccione R, Bossuyt P, et al. . Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet. 2018;390:2779–2789. [DOI] [PubMed] [Google Scholar]
- 59. Khanna R, Nelson SA, Feagan BG, et al. . Endoscopic scoring indices for evaluation of disease activity in Crohn’s disease. Cochrane Database Syst Rev. 2016;(8): CD010642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rimola J, Ordás I, Rodriguez S, et al. . Magnetic resonance imaging for evaluation of Crohn’s disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis. 2011;17:1759–1768. [DOI] [PubMed] [Google Scholar]
- 61. Rimola J, Rodriguez S, García-Bosch O, et al. . Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn’s disease. Gut. 2009;58:1113–1120. [DOI] [PubMed] [Google Scholar]
- 62. Satsangi J, Silverberg MS, Vermeire S, et al. . The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55:749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bouhnik Y, Carbonnel F, Laharie D, et al. ; GETAID CREOLE Study Group . Efficacy of adalimumab in patients with Crohn’s disease and symptomatic small bowel stricture: a multicentre, prospective, observational cohort (CREOLE) study. Gut. 2018;67:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Gionchetti P, Dignass A, Danese S, et al. ; ECCO . 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 2: surgical management and special situations. J Crohns Colitis. 2017;11:135–149. [DOI] [PubMed] [Google Scholar]
- 65. Siegel CA, Whitman CB, Spiegel BMR, et al. . Development of an index to define overall disease severity in IBD. Gut. 2018;67:244–254. [DOI] [PubMed] [Google Scholar]
- 66. Levine JS, Burakoff R. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2011;7:235–241. [PMC free article] [PubMed] [Google Scholar]
- 67. Ghosh S. Multidisciplinary teams as standard of care in inflammatory bowel disease. Can J Gastroenterol. 2013;27:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirai F, Watanabe K, Matsumoto T, et al. . Patients’ assessment of adalimumab self-injection for Crohn’s disease: a multicenter questionnaire survey (The PEARL Survey). Hepatogastroenterology. 2014;61:1654–1660. [PubMed] [Google Scholar]
- 69. Lopetuso LR, Gerardi V, Papa V, et al. . Can we predict the efficacy of anti-tnf-alpha agents? Int J Mol Sci. 2017;18(9). pii: E1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Swaminath A, Lebwohl B, Capiak KM, et al. . Practice patterns in the use of anti-tumor necrosis factor alpha agents in the management of Crohn’s disease: a US national practice survey comparing experts and non-experts. Dig Dis Sci. 2011;56:1160–1164. [DOI] [PubMed] [Google Scholar]
- 71. Kopylov U, Al-Taweel T, Yaghoobi M, et al. . Adalimumab monotherapy versus combination therapy with immunomodulators in patients with Crohn’s disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1632–1641. [DOI] [PubMed] [Google Scholar]
- 72. Kennedy NA, Heap GA, Green HD, et al. ; UK Inflammatory Bowel Disease Pharmacogenetics Study Group . Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4:341–353. [DOI] [PubMed] [Google Scholar]
- 73. Gecse KB, Bemelman W, Kamm MA, et al. ; World Gastroenterology Organization, International Organisation for Inflammatory Bowel Diseases IOIBD, European Society of Coloproctology and Robarts Clinical Trials; World Gastroenterology Organization International Organisation for Inflammatory Bowel Diseases IOIBD European Society of Coloproctology and Robarts Clinical Trials . A global consensus on the classification, diagnosis and multidisciplinary treatment of perianal fistulising Crohn’s disease. Gut. 2014;63:1381–1392. [DOI] [PubMed] [Google Scholar]
- 74. Torres J, Caprioli F, Katsanos KH, et al. . Predicting outcomes to optimize disease management in inflammatory bowel diseases. J Crohns Colitis. 2016;10:1385–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lichtenstein GR, Feagan BG, Mahadevan U, et al. . Pregnancy outcomes reported during the 13-year TREAT registry: a descriptive report. Am J Gastroenterol. 2018;113:1678–1688. [DOI] [PubMed] [Google Scholar]
- 76. Luu M, Benzenine E, Doret M, et al. . Continuous anti-TNFα use throughout pregnancy: possible complications for the mother but not for the fetus. A retrospective cohort on the French national health insurance database (EVASION). Am J Gastroenterol. 2018;113:1669–1677. [DOI] [PubMed] [Google Scholar]
- 77. Wieringa JW, Driessen GJ, Van Der Woude CJ. Pregnant women with inflammatory bowel disease: the effects of biologicals on pregnancy, outcome of infants, and the developing immune system. Expert Rev Gastroenterol Hepatol. 2018;12:811–818. [DOI] [PubMed] [Google Scholar]
- 78. Mariette X, Förger F, Abraham B, et al. . Lack of placental transfer of certolizumab pegol during pregnancy: results from CRIB, a prospective, postmarketing, pharmacokinetic study. Ann Rheum Dis. 2018;77:228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Nguyen GC, Seow CH, Maxwell C, et al. ; IBD in Pregnancy Consensus Group; Canadian Association of Gastroenterology . The Toronto Consensus statements for the management of inflammatory bowel disease in pregnancy. Gastroenterology. 2016;150:734–757.e1. [DOI] [PubMed] [Google Scholar]
- 80. Mahadevan U, Robinson C, Bernasko N, et al. . Inflammatory bowel disease in pregnancy clinical care pathway: a report from the american gastroenterological association IBD parenthood project working group. Gastroenterology. 2019;156:1508–1524. [DOI] [PubMed] [Google Scholar]
- 81. FDA. Biosimilar and Interchangeable Products. https://www.fda.gov/drugs/biosimilars/biosimilar-and-interchangeable-products. Accessed February 6, 2017. [Google Scholar]
- 82. FDA. Biosimilar Development, Review, and Approval. https://www.fda.gov/drugs/biosimilars/biosimilar-development-review-and-approval. Accessed February 6, 2017. [Google Scholar]
- 83. Jurczak W, Cohen S, Illidge TM, et al. . Scientific rationale underpinning the development of biosimilar rituximab in hematological cancers and inflammatory diseases. Future Oncol. 2019;15:4223–4234. [DOI] [PubMed] [Google Scholar]
- 84. Gecse KB, Cumming F, D’Haens G. Biosimilars for inflammatory bowel disease: how can health care professionals help address patients’ concerns? Expert Rev Gastroenterol Hepatol. 2019;13:143–155. [DOI] [PubMed] [Google Scholar]
- 85. EMA. Medicines. Biosimilars. Infliximab. https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine/field_ema_med_status/authorised-36/ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_active_substance_and_inn_common_name/infliximab?sort=search_api_aggregation_sort_ema_active_substance_and_inn_common_name&order=asc. Accessed February 6, 2020. [Google Scholar]
- 86. EMA. Medicines. Biosimilars. Adalimumab. https://www.ema.europa.eu/en/medicines/field_ema_web_categories%253Aname_field/Human/ema_group_types/ema_medicine/field_ema_med_status/authorised-36/ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_medicine_types/field_ema_med_biosimilar/search_api_aggregation_ema_active_substance_and_inn_common_name/adalimumab?sort=search_api_aggregation_sort_ema_active_substance_and_inn_common_name&order=asc. February 6, 2020. [Google Scholar]
- 87. Berinstein JA, Steiner CA, Higgins PDR. Inflammatory bowel disease: A practical approach, series #106. The IBD therapeutic pipeline is primed to produce. Pract Gastro. 2019;18:24–62. [Google Scholar]
- 88. The Center for Biosimilars. Formycon Starts Phase 1 Trial for Proposed Ustekinumab Biosimilar. https://www.centerforbiosimilars.com/news/formycon-starts-phase-1-trial-for-proposed-ustekinumab-biosimilar. February 6, 2019. [Google Scholar]
- 89. Ben-Horin S, Vande Casteele N, Schreiber S, et al. Biosimilars in inflammatory bowel disease: facts and fears of extrapolation. Clin Gastroenterol Hepatol. 2016;14:1685–1696. [DOI] [PubMed] [Google Scholar]
- 90. Gellad WF, Good CB. Adalimumab and the challenges for biosimilars. JAMA. 2019. doi: 10.1001/jama.2019.16275. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 91. Komaki Y, Yamada A, Komaki F, et al. . Systematic review with meta-analysis: the efficacy and safety of CT-P13, a biosimilar of anti-tumour necrosis factor-α agent (infliximab), in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;45:1043–1057. [DOI] [PubMed] [Google Scholar]
- 92. Meyer A, Rudant J, Drouin J, et al. . The effectiveness and safety of infliximab compared with biosimilar CT-P13, in 3112 patients with ulcerative colitis. Aliment Pharmacol Ther. 2019;50:269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cohen HP, Blauvelt A, Rifkin RM, et al. . Switching reference medicines to biosimilars: a systematic literature review of clinical outcomes. Drugs. 2018;78:463–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Jørgensen KK, Olsen IC, Goll GL, et al. ; NOR-SWITCH study group . Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): a 52-week, randomised, double-blind, non-inferiority trial. Lancet. 2017;389:2304–2316. [DOI] [PubMed] [Google Scholar]
- 95. Ye BD, Pesegova M, Alexeeva O, et al. . Efficacy and safety of biosimilar CT-P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double-blind, phase 3 non-inferiority study. Lancet. 2019;393:1699–1707. [DOI] [PubMed] [Google Scholar]
- 96. Harris C, Harris R, Young D, et al. . Ibd biosimilar to biosimilar infliximab switching study: preliminary results. United European Gastroenterol J. 2019;7:361; P0419. [Google Scholar]
- 97. Márquez JR, Gomollón F. The role of biosimilars in inflammatory bowel disease: a reality in our country. Rev Colomb Gastroenterol. 2017;32:311–325. [Google Scholar]
- 98. Regulatory Affairs Professional Society. Regulatory Focus. Updated: Interchangeable Biosimilars: Fda Finalizes Guidance. https://www.raps.org/news-and-articles/news-articles/2019/5/interchangeable-biosimilars-fda-finalizes-guidanc. Accessed February 6, 2019. [Google Scholar]
- 99.Food and Drug Administration; Center for Drug Evaluation and Research (CDER); Center for Biologics Evaluation and Research (CBER). Considerations in Demonstrating Interchangeability With a Reference Product Guidance for Industry. FDA: Silver Spring, MD, USA, 2019. Available at: https://www.fda.gov/media/124907/download. Accessed April 22, 2020. [Google Scholar]
- 100. Rudrapatna VA, Velayos F. Biosimilars for the treatment of inflammatory bowel disease. Pract Gastroenterol. 2019;43:84–91. [PMC free article] [PubMed] [Google Scholar]
- 101. European Medicines Agency and European Commission. Biosimilars in the eu. Information Guide for Healthcare Professionals. https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf. Accessed February 6, 2019. [Google Scholar]
- 102. The Center for Biosimilars. Subcutaneous Formulation of Celltrion’s Biosimilar Infliximab Authorized by European Commission. https://www.centerforbiosimilars.com/news/subcutaneous-formulation-of-celltrions-biosimilar-infliximab-authorized-by-european-commission. Accessed February 23, 2019. [Google Scholar]
- 103. El-Matary W, Walters TD, Huynh HQ, et al. . Higher postinduction infliximab serum trough levels are associated with healing of fistulizing perianal Crohn’s disease in children. Inflamm Bowel Dis. 2019;25:150–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Doherty G, Katsanos KH, Burisch J, et al. . European Crohn’s and colitis organisation topical review on treatment withdrawal [’Exit Strategies’] in inflammatory bowel disease. J Crohns Colitis. 2018;12:17–31. [DOI] [PubMed] [Google Scholar]
- 105. Hendler SA, Cohen BL, Colombel JF, et al. . High-dose infliximab therapy in Crohn’s disease: clinical experience, safety, and efficacy. J Crohns Colitis. 2015;9:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Scaldaferri F, D’Ambrosio D, Holleran G, et al. . Body mass index influences infliximab post-infusion levels and correlates with prospective loss of response to the drug in a cohort of inflammatory bowel disease patients under maintenance therapy with Infliximab. Plos One. 2017;12:e0186575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Fasanmade AA, Adedokun OJ, Olson A, et al. . Serum albumin concentration: a predictive factor of infliximab pharmacokinetics and clinical response in patients with ulcerative colitis. Int J Clin Pharmacol Ther. 2010;48:297–308. [DOI] [PubMed] [Google Scholar]
- 108. Abbvie Inc. Prescribing Information: Humira (adalimumab) Injection, for Subcutaneous Use. http://www.rxabbvie.com/pdf/humira.pdf. Accessed March 1, 2019. [Google Scholar]
- 109. ClinicalTrials.gov. Nct02065622. Study to Evaluate the Safety and Efficacy of Two Drug Regimens in Subjects With Moderate to Severe Ulcerative Colitis. https://clinicaltrials.gov/ct2/show/NCT02065622. Accessed July 19, 2019. [Google Scholar]
- 110. Panaccione R, Löfberg R, Rutgeerts P, et al. . Efficacy and safety of adalimumab by disease duration: analysis of pooled data from Crohn’s disease studies. J Crohns Colitis. 2019;13:725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mitrev N, Vande Casteele N, Seow CH, et al. ; IBD Sydney Organisation and the Australian Inflammatory Bowel Diseases Consensus Working Group . Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment Pharmacol Ther. 2017;46:1037–1053. [DOI] [PubMed] [Google Scholar]
- 112. Toruner M, Loftus EV Jr, Harmsen WS, et al. . Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134:929–936. [DOI] [PubMed] [Google Scholar]
- 113. Peyrin-Biroulet L, Lönnfors S, Roblin X, et al. . Patient perspectives on biosimilars: a survey by the european federation of Crohn’s and ulcerative colitis associations. J Crohns Colitis. 2017;11:128–133. [DOI] [PubMed] [Google Scholar]
- 114. Pouillon L, Danese S, Hart A, et al. . Consensus report: clinical recommendations for the prevention and management of the nocebo effect in biosimilar-treated IBD patients. Aliment Pharmacol Ther. 2019;49:1181–1187. [DOI] [PubMed] [Google Scholar]
- 115. Rezk MF, Pieper B. Treatment outcomes with biosimilars: be aware of the nocebo effect. Rheumatol Ther. 2017;4:209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Tweehuysen L, Huiskes VJB, van den Bemt BJF, et al. . Open-label, non-mandatory transitioning from originator etanercept to biosimilar SB4: six-month results from a controlled cohort study. Arthritis Rheumatol. 2018;70:1408–1418. [DOI] [PubMed] [Google Scholar]
- 117. Reinink AR, Lee TC, Higgins PD. Endoscopic mucosal healing predicts favorable clinical outcomes in inflammatory bowel disease: a meta-analysis. Inflamm Bowel Dis. 2016;22:1859–1869. [DOI] [PubMed] [Google Scholar]
- 118. Ding NS, Hart A, De Cruz P. Systematic review: predicting and optimising response to anti-TNF therapy in Crohn’s disease - algorithm for practical management. Aliment Pharmacol Ther. 2016;43:30–51. [DOI] [PubMed] [Google Scholar]
- 119. Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. 2011;33:987–995. [DOI] [PubMed] [Google Scholar]
- 120. Sauter B, Beglinger C, Girardin M, et al. . Monitoring disease activity and progression in Crohn’s disease. A Swiss perspective on the IBD ahead ‘optimised monitoring’ recommendations. Digestion. 2014;89:299–309. [DOI] [PubMed] [Google Scholar]
- 121. Papamichael K, Cheifetz AS. Use of anti-TNF drug levels to optimise patient management. Frontline Gastroenterol. 2016;7:289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Feuerstein JD, Nguyen GC, Kupfer SS, et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee . American Gastroenterological Association Institute guideline on therapeutic drug monitoring in inflammatory bowel disease. Gastroenterology. 2017;153:827–834. [DOI] [PubMed] [Google Scholar]
- 123. Bodini G, Giannini EG, Savarino V, et al. . Adalimumab trough serum levels and anti-adalimumab antibodies in the long-term clinical outcome of patients with Crohn’s disease. Scand J Gastroenterol. 2016;51:1081–1086. [DOI] [PubMed] [Google Scholar]
- 124. Roblin X, Boschetti G, Duru G, et al. . Distinct thresholds of infliximab trough level are associated with different therapeutic outcomes in patients with inflammatory bowel disease: a prospective observational study. Inflamm Bowel Dis. 2017;23:2048–2053. [DOI] [PubMed] [Google Scholar]
- 125. Drobne D, Kurent T, Golob S, et al. . Success and safety of high infliximab trough levels in inflammatory bowel disease. Scand J Gastroenterol. 2018;53:940–946. [DOI] [PubMed] [Google Scholar]
- 126. Jani M, Barton A, Warren RB, et al. . The role of DMARDs in reducing the immunogenicity of TNF inhibitors in chronic inflammatory diseases. Rheumatology (Oxford). 2014;53:213–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Prado MS, Bendtzen K, Andrade LEC. Biological anti-TNF drugs: immunogenicity underlying treatment failure and adverse events. Expert Opin Drug Metab Toxicol. 2017;13:985–995. [DOI] [PubMed] [Google Scholar]
- 128. Lukas M, Pesinova V, Bortlik M, et al. . Impact of the switch from original adalimumab to biosimilar adalimumab sb5 on serum drug trough levels, clinical and biological disease activity in patients with ibd. United European Gastroenterol J. 2019;7:361; P1806. [Google Scholar]
- 129. de Jong MJ, Huibregtse R, Masclee AAM, et al. . Patient-reported outcome measures for use in clinical trials and clinical practice in inflammatory bowel diseases: a systematic review. Clin Gastroenterol Hepatol. 2018;16:648–663.e3. [DOI] [PubMed] [Google Scholar]
- 130. Ricanek P, Brackmann S, Perminow G, et al. ; IBSEN II Study Group . Evaluation of disease activity in IBD at the time of diagnosis by the use of clinical, biochemical, and fecal markers. Scand J Gastroenterol. 2011;46:1081–1091. [DOI] [PubMed] [Google Scholar]
- 131. Daperno M, D’Haens G, Van Assche G, et al. . Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: the SES-CD. Gastrointest Endosc. 2004;60:505–512. [DOI] [PubMed] [Google Scholar]
- 132. Papamichael K, Vande Casteele N, Ferrante M, et al. . Therapeutic drug monitoring during induction of anti-tumor necrosis factor therapy in inflammatory bowel disease: defining a therapeutic drug window. Inflamm Bowel Dis. 2017;23:1510–1515. [DOI] [PubMed] [Google Scholar]
- 133. Greenwald JA, McMullen HF, Coppa GF, et al. . Standardization of surgeon-controlled variables: impact on outcome in patients with acute cholecystitis. Ann Surg. 2000;231:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Leung S, Bulloch B, Young C, et al. . Effectiveness of standardized combination therapy for migraine treatment in the pediatric emergency department. Headache. 2013;53:491–197. [DOI] [PubMed] [Google Scholar]
- 135. Micek ST, Roubinian N, Heuring T, et al. . Before-after study of a standardized hospital order set for the management of septic shock. Crit Care Med. 2006;34:2707–2713. [DOI] [PubMed] [Google Scholar]
- 136. Khanna R, Bressler B, Levesque BG, et al. ; REACT Study Investigators . Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet. 2015;386:1825–1834. [DOI] [PubMed] [Google Scholar]
- 137. Vande Casteele N, Ferrante M, Van Assche G, et al. . Trough concentrations of infliximab guide dosing for patients with inflammatory bowel disease. Gastroenterology. 2015;148:1320–9.e3. [DOI] [PubMed] [Google Scholar]
- 138. D’Haens GR, Sartor RB, Silverberg MS, et al. . Future directions in inflammatory bowel disease management. J Crohns Colitis. 2014;8:726–734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.