Abstract
Background & Aims
Malnutrition with the accumulation of fat tissue and nonalcoholic fatty liver disease (NAFLD) are conditions associated with inflammatory bowel disease (IBD). Visceral fat and NAFLD-related liver dysfunction can both worsen intestinal inflammation. Because the Mediterranean diet (Md) has been shown to ameliorate both obesity and NAFLD, the aim of this study was to analyze the impact of Md on the nutritional state, liver steatosis, clinical disease activity, and quality of life (QoL) in IBD patients.
Methods
Patients with IBD, both Crohn’s disease (CD) and ulcerative colitis (UC), followed Md for 6 months. Their body mass index (BMI), body tissue composition, liver steatosis and function, serum lipid profile, clinical disease activity, and inflammatory biomarkers (C-reactive protein and fecal calprotectin) were collected at baseline (T0) and compared with those obtained after 6 months (T180) to evaluate the impact of Md.
Results
One hundred forty-two IBD patients, 84 UC and 58 CD, followed Md for 6 months. At T180, diet-adherent CD and UC improved BMI (UC −0.42, P = 0.002; CD −0.48, P = 0.032) and waist circumference (UC −1.25 cm, P = 0.037; CD −1.37 cm, P = 0.041). Additionally, the number of patients affected by liver steatosis of any grade was significantly reduced in both groups (UC T0 31 of 84 [36.9%] vs T180 18 of 84 [21.4%], P = 0.0016; CD T0 27 of 58 [46.6%] vs T180 18 of 58 [31.0%], P < 0.001) after dietary intervention. Finally, after 6 months of the diet, fewer UC and CD patients with stable therapy had active disease (UC T0 14 of 59 [23.7%] vs T180 4 of 59 [6.8%], P = 0.004; CD T0 9 of 51 [17.6%] vs T180 2 of 51 [3.0%], P = 0.011) and elevated inflammatory biomarkers. Mediterranean diet improved QoL in both UC and CD, but neither serum lipid profile nor liver function were modified by the diet.
Conclusions
A significant reduction of malnutrition-related parameters and liver steatosis was observed in both CD and UC patients after short-term dietary intervention based on the adoption of Md, and this was associated with a spontaneous improvement of disease activity and inflammatory markers.
Keywords: Mediterranean diet, inflammatory bowel disease, quality of life, liver steatosis
In this prospective study, 6 months of Mediterranean diet in IBD patients was associated with improvement of several metabolic syndrome–associated parameters and response to therapies. The Mediterranean diet might be considered a “background therapy” in the therapeutic algorithm of IBD.
INTRODUCTION
Inflammatory bowel diseases (IBD), whose main forms are ulcerative colitis (UC) and Crohn’s disease (CD), are characterized by chronically relapsing-remitting inflammation of the gastrointestinal (GI) tract.1, 2 Despite the increasing number of new drugs available for the treatment of IBD, clinical remission is reached in less than half of the patients, and loss of response in initially responsive patients is observed over time with negative impact on disease progression and quality of life (QoL).3 In the context of IBD clinical management, the role of the diet is probably underestimated. Diet represents one of the main determinants of human gut microbiota, and a misbalanced diet can contribute to establish a condition of dysbiosis with multiple effects on the host homeostasis. Dysbiosis has been shown to induce and sustain intestinal inflammation directly and indirectly promoting the development of adipose tissue, which represents an important source of pro-inflammatory cytokines.4, 5 Moreover, dysbiosis and obesity sustain the accumulation of fat in the liver and the increase of intestinal permeability.6 In turn, the influx of bacterial components such as lipopolysaccharide (LPS) have been shown to promote liver inflammation and fibrosis in animal models of liver steatosis.7 Inflammation-induced liver dysfunction and the alteration of bile acid conjugation might also sustain intestinal inflammation through the induction of dysbiosis.8 Overall, these evidences suggest that the systemic disorder involving the adipose tissue, the liver, and more recently the gut microbiota might be factors of a self-sustaining pro-inflammatory mechanism operating in IBD.
According to this hypothesis, obesity and its related comorbidities have been observed in IBD, previously believed to be a condition characterized by undernourishment and low body mass index (BMI). Increasing evidences show that IBD does not strictly imply hyponutrition but frequently leads to malnutrition, with an increased risk for IBD patients, both children and adults, to be overweight or obese.9 In a recent study, Lomer et al found in a cohort of 390 IBD patients an overweight and obesity prevalence of 29% and 18%, respectively with only 16 patients (4%) underweight.10 Moreover, visceral adipose tissue (VAT) has been associated with complicated disease, higher rate of postoperative recurrence in CD ,and more severe disease course, thus implying that adipose tissue might worsen inflammation in IBD.11 Finally, obesity has been associated with poor response to conventional and biological therapies in both CD and UC.11 Strictly related to obesity and malnutrition is the development of nonalcholic fatty liver disease (NAFLD). The prevalence of NAFLD in IBD has been shown to be around 30%–40%, thus suggesting that IBD patients might be at risk of NAFLD and NAFLD-related complications.12, 13
Despite the increased prevalence of overweight and obesity in the IBD population, the impact of nutritional intervention on disease activity, response to therapies, obesity-related comorbidities such as NAFLD, and QoL is poorly defined.
The Mediterranean diet (Md)—characterized by a high consumption of vegetables, fruits, cereals, nuts, legumes, unsaturated fat such as olive oil, a medium intake of fish, dairy products, wine, a low consumption of saturated fat, meat, and sweets—was associated to reduced inflammation and was shown to be protective in some diseases such as colorectal cancer.14 The primary endpoint of this study was to evaluate the impact of Md on IBD patients’s nutritional status, presence and severity of liver steatosis, response to therapy, and QoL.
MATERIALS AND METHODS
Study Design and Population
This is a prospective, interventional study aiming at evaluating the impact of Md on disease activity, obesity, obesity-related complications, and QoL in IBD patients. To this end, between January 2018 and December 2018, patients in active follow-up at the Gastroenterology Unit of the University Hospital Monserrato, University of Cagliari, Italy, were prospectively examined for inclusion and exclusion criteria. At baseline (T0), each patient underwent clinical and disease activity evaluation. Blood tests exploring liver function and fat metabolism were performed. Fecal calprotectin was also tested at baseline and at T180. Abdominal ultrasound was performed in all of the enrolled patients to establish the presence and severity of NAFLD. Nutritional status was evaluated and the following anthropometric parameters recorded: weight, BMI, visceral fat, lean body mass, fat body mass, waist circumference using Body Composition Monitor (Omron Healthcare Co., Ltd, Kyoto, Japan). Patients were also asked to fill out the Inflammatory Bowel Disease Questionnaire (IBD-Q) to estimate perception of their own QoL. Dietary habits were examined through 24 hours’ recall as previously reported.15 Anthropometric measurements and each 24 hours’ dietary recall were recorded into the Dieto System Terapia Alimentare (DS Medica S.r.l., Milano, Italy) software for nutrients.
Nutritional counseling was performed by a nutritionist searching for incorrect dietary habits and giving patients advice with the objective of improving adherence to Md and redistributing nutrients through 3 principal meals and 2 light snacks during the day. Hypocaloric diet was given in the presence of obesity. In all the other cases, an isocaloric diet was prescribed. After 6 months (T180), adherence to diet was assessed by the nutritionist in all patients enrolled in the study; and disease activity, anthropometric measurements, abdominal ultrasound, and QoL were reevaluated after dietetic intervention. We also collected data on patients’ therapy at baseline and T180 to exclude therapy changes or dose escalations as confounders.
The research project was approved by the Ethics Board (Prot. PG/2018/15554). The study was conducted according to the Helsinki Declaration.
Outcomes of the Study
The primary outcome of the study was to evaluate the impact of short-term dietary intervention based on the adoption of the Md on anthropometric parameters (ie, BMI, waist circumference, lean and fat body mass, and visceral fat), serum lipid profile, liver function and steatosis, and intestinal disease activity in an unselected population of IBD patients.
Inclusion and Exclusion Criteria
Patients age 18 years or older with diagnosis of IBD for at least 6 months in active follow-up were enrolled.
Exclusion criteria were inability to provide a valid informed consent, pregnancy, significant alcohol consumption (≥20 and ≥30 g per day in females and males, respectively), and any other causes of chronic liver disease.
Diagnostic Criteria
Inflammatory bowel disease diagnosis was performed by combination of clinical examination, endoscopy, radiological signs, and chemical and histologic analyses. The disease activity was calculated by Crohn’s disease activity index (CDAI) and partial Mayo score (PMS) for CD and UC, respectively. Disease extension was evaluated by endoscopy and/or CT- or MRI-enteroclysis according to national and international guidelines.1, 2
An expert GI radiologist performed abdominal ultrasound to estimate the presence of NAFLD. Typical findings such as bright liver echo pattern, higher than renal cortex, and loss of resolution of intrahepatic structures were evaluated. Hepatic steatosis was graded as follows: grade 0, normal echogenicity; grade 1, slight and diffuse increase of fine parenchymal echoes, normal visualization of diaphragm, and intrahepatic vessel borders; grade 2, moderate, diffuse increase in fine echoes with slightly impaired visualization of intrahepatic vessels and diaphragm; grade 3, marked increase in fine echoes with poor or nonvisualization of the intrahepatic vessel borders, diaphragm, and posterior right lobe of the liver.16 To be defined on stable therapy, patients had to have no therapy changes during the observation period (ie, neither change of current drug dosage or way of administration nor introduction of new drugs) and had to be on biologics and/or thiopurines for at least 6 months before enrolment.
Dietary Assessment and Counseling
All patients underwent nutritional evaluation. Anthropometric measurement (eg, BMI, waist circumference, visceral fat, body fat, lean body mass) were assessed using Body Composition Monitor (Omron Healthcare) to evaluate the presence and grade of obesity. A 24-hour dietary recall was performed to estimate the usual dietary intake. The nutritionist entered the anthropometric measurements and each 24-hour dietary recall into the Dieto System Terapia Alimentare software for nutrients. Based on nutritional analysis, specific indications were given to correct excessive intake of proteins, lipids, and sweets. Moreover, according to the current literature,17 the consumption of vegetables (≥2 servings every meal), fruits (1–2 servings every meal), breads and cereals (1–2 servings every meal), olive oil every meal, legumes (≥2 servings weekly), fish/seafood (≥2 servings weekly), eggs (2–4 servings weekly), poultry (2 servings weekly), dairy foods (2 servings daily), and low consumption of red meat (<2 servings weekly) and sweets (<2 serves weekly) were recommended. Patients’ adherence to diet was evaluated during the nutritional interview at T180.
Statistical Analysis
Continuous variables were reported as mean (±SD), and categorical variables were reported as number of cases and percentages. Different tests were performed to compare variables at baseline (T0) and after 6 months (T180). Paired t test for continuous variables, Wilcoxon matched-pairs signed-ranks test for qualitative ordinal variables, and McNemar Test for dichotomous variables. P < 0.05 was considered significant for all tests. Analyses were performed using STATA/SE, version 14, software (StataCorp LP, College Station, TX, USA).
RESULTS
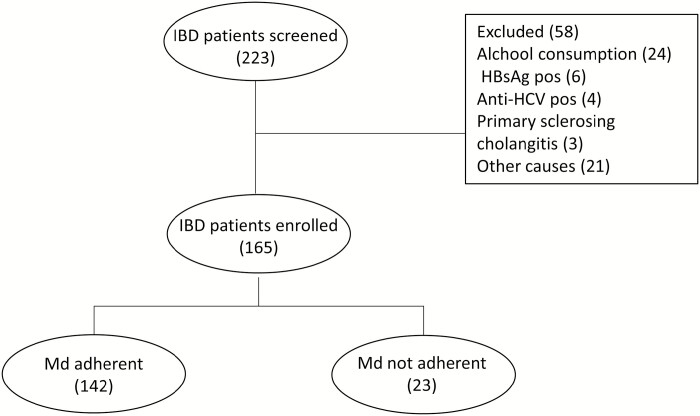
From January 2018 to December 2018, a total of 223 IBD patients in active follow-up at the University Hospital Monserrato, University of Cagliari, Italy, were screened. Fifty-eight patients (38%) were excluded because they did not match the inclusion criteria. Of the 165 patients enrolled in the study at baseline, 142 (86.1%) resulted adherent to the nutritional prescription; however in the remaining patients, adherence was considered insufficient, and they were discarded from the analysis (Fig. 1). Among the adherent patients, 84 were diagnosed with UC (59.2%) and 58 CD (40.8%). The median age was 52 (iterquartile range [IQR] 44.5–61.3) and 48 (IQR 34.8–57.5) years, the median disease duration was 10 (IQR 3.5–19.5) and 7 (IQR 5.0–15.0) years, and the median BMI was 24.8 (IQR 22.6–27.8) and 25 (IQR21.9–28.2) in UC and CD, respectively (Table 1). At baseline, liver steatosis was present in 31 (36.9%) UC and 27 (46%) CD patients. Forty-three (51.2%) UC and 30 (51.7%) CD patients were classified as being overweight or obese, and the disease was active in 26 (31.0%) and 9 (15.5%) of UC and CD patients, respectively. Twenty-five (29.8%) UC and 52 (89.7%) CD patients had biologic experience, whereas 17 (20.2%) UC and 6 (10.3%) CD had received immunosuppressants (ISS) in the past.
FIGURE 1.
Study flow chart. Abbreviations: HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus.
TABLE 1.
Patients’ Characteristics
| UC N = 84 | CD N = 58 | |||
|---|---|---|---|---|
| Median | (IQR) | Median | (IQR) | |
| Age (mean) | 52 | (44.5–61.3) | 48.0 | (34.8–57.5) |
| Disease duration (yrs) | 10 | (3.5–19.5) | 7.0 | (5.0–15.0) |
| BMI | 24.8 | (22.6–27.8) | 25.0 | (21.9–28.2) |
| N | (%) | N | (%) | |
| Sex (F) | 44 | (52.4) | 22 | (37.9) |
| Smokers | 7 | (8.3) | 17 | (29.3) |
| Extent (E) | ||||
| E1 | 8 | (9.5) | — | — |
| E2 | 40 | (47.6) | — | — |
| E3 | 36 | (42.9) | — | — |
| Localization (L) | ||||
| Ileal (L1) | — | — | 25 | (43.1) |
| Colonic (L2) | — | — | 8 | (13.8) |
| Ileo-colonic (L3) | — | — | 23 | (39.7) |
| pMAYO score | ||||
| Remission | 58 | (69.0) | — | — |
| Mild | 14 | (16.7) | — | — |
| Moderate | 8 | (9.5) | — | — |
| Severe | 4 | (4.8) | — | — |
| CDAI | ||||
| Remission | — | — | 49 | (84.5) |
| Mild | — | — | 8 | (13.8) |
| Moderate | — | — | 1 | (1.7) |
| Severe | — | — | 0 | (0.0) |
| Liver steatosis | ||||
| Absent | 53 | (63.1) | 31 | (53.4) |
| Mild | 11 | (13.1) | 6 | (10.3) |
| Moderate | 13 | (15.5) | 10 | (17.2) |
| Severe | 7 | (8.3) | 11 | (19.0) |
| BMI class | ||||
| Underweight | 1 | (1.2) | 3 | (5.2) |
| Normal | 40 | (47.6) | 25 | (43.1) |
| Overweight | 30 | (35.7) | 22 | (37.9) |
| Obese Class I | 9 | (10.7) | 7 | (12.1) |
| Obese Class II | 4 | (4.8) | 0 | (0.0) |
| Obese Class III | 0 | (0.0) | 1 | (1.7) |
| Previous biologics | 25 | (29.8) | 52 | (89.7) |
| Previous ISS | 17 | (20.2) | 6 | (10.3) |
Ulcerative Colitis Patients
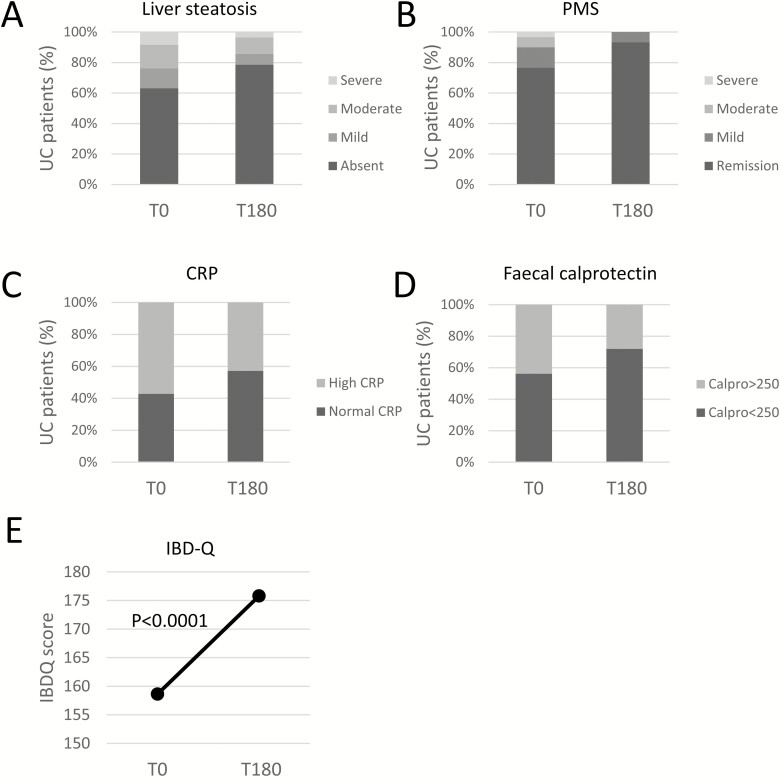
Among UC patients after 6 months of diet, BMI decreased by 0.42 ± 1.22 points (P = 0.002), and waist circumference decreased by 1.25 cm ± 5.39 (P = 0.037; Table 2). Fat body mass decreased by 1.27% ± 7.76, and lean body mass increased by 1% ± 4.89, but these differences did not reach the statistical significance. However, the amount of visceral fat remained unchanged, and the serum lipid profile was not affected by the dietary intervention (total cholesterol 203.0 ± 38.2 mg/dL vs 201.2 ± 38.9mg/dL; and low density lipoproteins 118.9 ± 30.7 mg/dL vs 125.2 ± 29.6 mg/dL at T0 and T180, respectively). A statistically significant reduction of alanine aminotransferase (T0 20.3 ± 5.3 international unit [IU]/l vs T 180 18.4 ± 6.7 IU/l; P = 0.029), and gamma-glutamyl transferase (T0 23.2 ± 15.9 IU/l vs T180 21.3 ± 16.7 IU/l; P = 0.028) was observed, but this occurred within the reference range. At baseline, 7 patients (8.33%) showed high-grade, 13 (15.48%) moderate-grade, and 11 (13.09%) mild-grade liver steatosis. After 6 months, patients on diet showed a significant improvement of liver steatosis, being high grade in 3 patients (3.57%), moderate in 9 (10.71%), and mild in 6 (7.14%; P = 0.0016; Fig. 2A). During the 6 months of diet, therapy changes were recorded in 16 of 84 (19.0%) patients: 8 patients received systemic steroids, and 13 patients received topical steroids. Considering patients with stable therapy (n = 68) and complete (ie, T0 and T180) clinical (n = 59 of 68 [86.8%]) and inflammatory biomarker data (n = 56 of 68 [82.4%]), 14 patients (23.7%) had active disease at baseline by PMS (mild in 8 [13.6%], moderate in 4 [6.8%], and severe in 2 [3.4%] patients; Fig. 2B). After 6 months, only 4 patients (6.8%) still showed mild disease (P = 0.004). Accordingly, the number of patients with high C-reactive protein (CRP) decreased from 28 of 56 (50%) to 21 of 56 (37.5%; P = 0.013), and the number of patients with fecal calprotectin above 250 mg/kg also decreased from 25 of 57 (43.8%) to 16 of 57 (28.1%; P = 0.049; Figs. 2C, D). Quality of life also improved in UC patients adherent to diet, as shown by the increased of IBD-Q mean by 17.17 ± 26.48 (P < 0.001; Fig. 2E).
TABLE 2.
Comparison Between Means of Continuous Variables in UC Patients
| UC | ||||
|---|---|---|---|---|
| T0 (±SD) | T180 (±SD) | Mean diff | P | |
| BMI | 25.7 (±9.0) | 25.3 (±4.2) | −0.42 | 0.002 |
| Waist circumference (cm) | 90.2 (±11.4) | 89.0 (±11.2) | −1.25 | 0.037 |
| Fat mass (%) | 30.2 (±10.6) | 28.9 (±9.1) | −1.27 | 0.136 |
| Lean mass(%) | 29.7 (±6.7) | 30.7 (±6.4) | 1.00 | 0.064 |
| Visceral fat (%) | 9.0 (±4.6) | 8.7 (±4.5) | −0.24 | 0.244 |
| AST (UI/L) | 20.0 (±5.4) | 19.0 (±4.8) | −0.94 | 0.127 |
| ALT (UI/L) | 20.3 (±8.2) | 18.4 (±6.7) | −1.94 | 0.029 |
| GammaGT (UI/L) | 23.2 (±15.9) | 21.3 (±16.7) | −1.85 | 0.028 |
| Total cholesterol (mg/dL) | 203.0 (±38.2) | 201.2 (±38.9) | −1.82 | 0.853 |
| LDL cholesterol (mg/dL) | 118.9 (±30.7) | 125.2 (±29.6) | 6.29 | 0.098 |
| HDL cholesterol (mg/dL) | 61.0 (±19.3) | 63.2 (±20.6) | 2.28 | 0.312 |
| Triglycerides (mg/dL) | 89.0 (±37.4) | 93.4 (±11.9) | 4.40 | 0.273 |
FIGURE 2.
A, Liver steatosis before, (B) disease activity by partial Mayo score (PMS: remission 0–1; mild 2–4; moderate 5–6; severe 7–9), (C) C-reactive protein, (D) fecal calprotectin (high ≥250; normal <250), and (E) IBDQ at (T0) and after (T180) diet intervention in UC patients.
Crohn’s Disease Patients
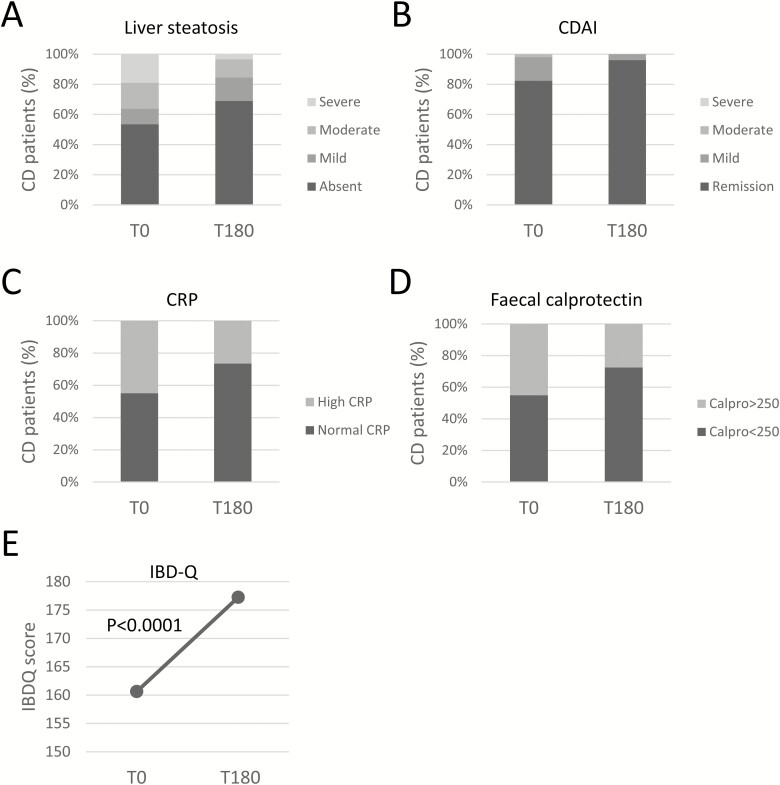
Body mass index and waist circumference decreased by 0.48 ± 1.57 points (P = 0.023) and 1.4 cm ± 5.03 (P = 0.04), respectively, among diet-adherent CD patients after 6 months of diet (Table 3). A trend in the reduction of the fat body mass (−2.75% ± 12.16; P = 0.09) was observed. No other changes in the biochemical and serum lipid profile was observed. At baseline, 31 patients (53.45%) had no signs of steatosis; 6 (10.34%) had mild, 10 (17.24%) moderate, and 11 (18.97%) high-grade liver steatosis (Fig. 3A). After 6 months, there was a significant improvement of liver steatosis in CD patients who followed the dietetic prescription. Forty patients (68.97%) had no evidence of steatosis, with 10 (17.24%) having mild, 6 (10.34%) moderate, and 2 (3.45%) high grade steatosis (P < 0.001). During the 6 months of diet, 2 of 58 (3.5%) CD patients needed therapy changes: both patients required systemic steroid course. Of the remaining 56 patients with stable therapy, 53 (91.4%) and 51 (87.9%) had complete clinical activity and CRP data, respectively. At baseline, 9 (17.0%) CD patients had active disease: 8 (15.1%) mild and 1 (1.9%) moderate disease activity by CDAI (Fig. 3B). After 6 months on diet, only 2 patients (3.8%) showed mild disease (P = 0.011). Accordingly, there were 22 of 49 patients with elevated CRP at baseline (44.9%), and this number was reduced to 13 of 49 (26.5%) after dietary intervention (P = 0.035, Fig. 3C). In these patients, fecal calprotectin also decreased from 18 of 40 (45.0%) to 11 of 40 (27.5%; P = 0.035; Fig. 3D). After 6 months of diet, IBD-Q mean increased by 16.61 ± 26.25 points in CD patients adherent to Mediterranean diet (P < 0.001; Fig. 3E).
TABLE 3.
Comparison Between Means of Continuous Variables in CD Patients
| CD | ||||
|---|---|---|---|---|
| T0 (±SD) | T180 (±SD) | Mean diff | P | |
| BMI | 25.1 (±4.0) | 24.6 (±4.1) | −0.48 | 0.032 |
| Waist circumference (cm) | 89.5 (±11.2) | 88.2 (±11.5) | −1.37 | 0.041 |
| Fat mass (%) | 29.4 (±14.2) | 26.6 (±9.6) | −2.75 | 0.097 |
| Lean mass(%) | 32.0 (±6.9) | 31.9 (±6.8) | −0.09 | 0.895 |
| Visceral fat (%) | 8.4 (±4.8) | 8.1 (±4.3) | −0.27 | 0.293 |
| AST (UI/L) | 21.8 (±7.3) | 21.4 (±8.6) | −0.42 | 0.739 |
| ALT (UI/L) | 20.9 (±10.2) | 20.3 (±9.7) | −0.61 | 0.955 |
| GammaGT (UI/L) | 24.7 (±8.3) | 24.4 (±28.8) | −0.25 | 0.854 |
| Total cholesterol (mg/dL) | 176.8 (±8.9) | 173.8 (±39.6) | −2.98 | 0.853 |
| LDL cholesterol (mg/dL) | 108.4 (±8.9) | 101.7 (±32.0) | −6.68 | 0.098 |
| HDL cholesterol (mg/dL) | 52.4 (±8.8) | 54.8 (32.0) | 2.41 | 0.312 |
| Triglycerides (mg/dL) | 105.8 (±8.8) | 90.6 (±17.7) | −15.12 | 0.273 |
FIGURE 3.
A, Liver steatosis before, (B) disease activity by partial Crohn’s disease activity index (CDAI: remission <150, mild 150–219, moderate 220–400, severe >400), (C) C-reactive protein, (D) fecal calprotectin (high ≥250; normal <250), and (E) IBDQ at (T0) and after (T180) diet intervention in CD patients.
DISCUSSION
In this study, we assessed the effect of short-term Mediterranean diet on the IBD clinical course. In diet-adherent patients, we observed the improvement of anthropometric variables associated with the development of metabolic syndrome, the reduction of liver steatosis, and disease activity indexes in both CD and UC patients.
The role of nutrition in the management of IBD has been so far underestimated. However, the Western diet characterized by low fiber and high fat intake might worsen intestinal inflammation acting on different pathways. Inflammatory bowel disease patients are characterized by a misbalance between the lean and fat mass, and overweight and obesity conditions are frequently observed in these patients.9 The visceral adipose tissue has been shown to be an important source of pro-inflammatory cytokines (eg, interleukin [IL]-6, IL-8, tumor necrosis factor-α) and to sustain inflammation in different organs including the gut.18, 19 High-fat content in the diet has also been shown to increase intestinal permeability, and the excessive influx of bacteria is believed to induce the loss of tolerance toward microbiota-derived antigens and sustain gut inflammation in IBD patients.20, 21 Moreover, chronic inflammation and the increased gut permeability are inducers of dysbiosis, a condition characterized by an altered microbiota composition, which independently sustains intestinal inflammation.22
Patients with IBD have been shown to have an increased risk to develop NAFLD, a high-fat diet-related condition.23 In the presence of increased intestinal permeability, the translocation of bacteria and their constituents like LPS in the portal vein can cause low-grade inflammation of the liver and the rapid progression of NAFLD to liver fibrosis and hepatocellular carcinoma.24 Therefore, the progression to fibrosis and fibrosis-related complications might potentially have devastating effects on the management of IBD patients. Finally, NAFLD might have important consequences on intestinal immune homeostasis. Indeed, the NAFLD- and fibrosis-related liver dysfunction can induce modifications of the bile acid composition, further contributing to intestinal dysbiosis and inflammation.25
In contrast to the negative effect of the Western diet, the Mediterranean diet, characterized by the consumption of fruits, vegetables, whole grains, olive oil, red wine, and yogurt, might have positive outcomes in IBD. Experimental evidences suggest that Md could contribute to prevent the onset of dysbiosis by sustaining the presence of anti-inflammatory bacterial species. In 153 individuals, Md was associated with an increased level of fecal short-chain fatty acids that are known to induce anti-inflammatory regulatory T cells and the expansion of Lacnospira and L-Ruminococcus species, which are reduced in IBD.26–28 Accordingly, in a small cohort of CD patients, 6 weeks on Md showed a trend in the “normalization” of the gut microbiota.29 Mediterranean diet has also been shown to rebalance the nutritional state and to reduce the pro-inflammatory visceral fat if associated to energy restriction.30, 31 Finally, data from cross-sectional studies have shown an inverse correlation between Md adherence and the prevalence and severity of NAFLD.32–34 In a small randomized control trial involving patients affected by biopsy-diagnosed NAFLD, 6 weeks of Md reduced liver steatosis by 38% as compared with isoenergetic, low fat and high carbohydrate diet.35
Based on the pro-inflammatory role of visceral fat, dysbiosis, intestinal permeability, liver steatosis, and the positive effects of Md on each of these factors, it is tempting to speculate that Md could reduce the systemic inflammatory burden acting at the same time on different targets, thus improving the efficacy of therapies and the control of the disease.
According to this hypothesis, our data indicate that the adherence to short-term Md not only improved obesity-related parameters and liver steatosis in both CD and UC but also significantly reduced disease activity and inflammation-related biomarkers. It is noteworthy that the effect of Md on disease activity was observed in patients with stable therapy, where patients with even minor changes in the concomitant therapy (eg, short-term 5-ASA enemas course in addition to maintenance therapy) were excluded from the analysis to prevent the effect of potential confounders.
In our cohort, Mediterranean diet also improved QoL, as assessed by IBD-Q score in both UC and CD patients. Our results highlight the role of dietary habits on well-being perception. In addition to the improved disease activity observed in diet-adherent patients, other possible mechanisms might account for this observation. As reported by Henríquez et al, there is a direct linear association between the adherence to a Mediterranean diet and some aspects of self-perceived physical and mental QoL.36 Additionally, the reduction of BMI related to a healthy lifestyle could explain the improvement in the QoL of these patients.37, 38
Several studies emphasize the importance of dietary habits in IBD patients,39 although few of them investigate the role of Md in this condition. In a cohort of 41 clinically active CD patients, Papada et al demonstrated the effect of Md in improving QoL. Moreover, Md was negatively associated with disease activity.40 Our research confirms these observations in a larger cohort of patients. Additionally, a recently published prospective cohort study conducted in more than 80,000 individuals demonstrates that the adherence to the Md significantly reduces the risk to develop late-onset CD.41 Overall, these data underscore the key role of the Md in the control of the intestinal immune system in IBD patients.
This study has potential limitations. First, the absence of a control group does not rule out that clinical improvement would have occurred in some patients independently of dietary intervention. However, by excluding from the efficacy analysis those patients undergoing any change of therapy during the observation period and those who started biologics and/or thiopurines less than 6 months before enrolment, we minimized the possible effect of therapy changes and the carryover effect of therapies started before dietary intervention. Second, we focused on IBD outpatients, and most of them were in clinical remission or affected by mild disease. This might lead to overestimate the effect of the dietary intervention on the disease activity and QoL. However, all patients initially affected by moderate to severe disease were in remission or showed mild disease activity after 6 months of the Mediterranean diet. The improvement of disease activity was further confirmed by the significant higher number of patients who normalized CRP and fecal calprotectin after dietary intervention. We did not use any specific score to quantify adherence to diet, and this was mainly based on patients’ dietary recall. However, since the period of observation was relatively short, it is unlikely that the assessment of adherence to the prescribed diet was affected by recall bias. Finally, although statistically significant, BMI and waist circumference reductions were small. This was probably due to the relatively short duration of dietary intervention, and it is plausible that a longer period on diet would have had a much bigger impact on anthropometric parameters, along with the improved response to concomitant therapies. The point of strengths of our work is the prospective design evaluating a considerable number of IBD patients representing a general outpatient population.
In conclusion, our study emphasizes the importance of a multidimensional approach in the management of IBD, not limited to the treatment of luminal inflammation but extended to the correction of the nutritional status and liver steatosis. The relevance of this approach seems to be remarkable on disease control with a possible drug-sparing effect, on patients’ wellness, and in reducing the risk of other life-threatening conditions, one of which is liver steatosis and its complications.
Our data support the role of nutritional counseling in the multidisciplinary management of IBD. The adoption of a proper alimentary habit based on Md and the achievement of an adequate compliance might be pivotal in the clinical management of these patients. However, more studies with larger cohorts of patients are needed to improve our knowledge on the relationship between diet and IBD.
ACKNOWLEDGMENTS
The authors are grateful to the patients for participating in this study.
Author Contribution: FC contributed to patient recruitment, data collection, study design, data analysis, and writing the manuscript. SM contributed to patient recruitment, data collection, data analysis, and writing the manuscript. AC contributed to data collection, data analysis, and writing the manuscript; DP, MP, FT, and FZ contributed to patient recruitment and data collection. AM contributed to data collection and study design. LC contributed to data analysis. MCF revised the manuscript. PU contributed to development of study concept and design and supervised the study. All authors approved the final version of the manuscript including the authorship list. All authors have read and approved the final manuscript.
REFERENCES
- 1. Gomollón F, Dignass A, Annese V, et al. ; ECCO . 3rd European Evidence-based Consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11:3–25. [DOI] [PubMed] [Google Scholar]
- 2. Magro F, Gionchetti P, Eliakim R, et al. ; European Crohn’s and Colitis Organisation [ECCO] . Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11:649–670. [DOI] [PubMed] [Google Scholar]
- 3. Becker HM, Grigat D, Ghosh S, et al. . Living with inflammatory bowel disease: a Crohn’s and Colitis Canada survey. Can J Gastroenterol Hepatol. 2015;29:77–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agarwal AK. Spice up your life: adipose tissue and inflammation. J Lipids. 2014;2014:182575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. [DOI] [PubMed] [Google Scholar]
- 6. Mouries J, Brescia P, Silvestri A, et al. . Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol. 2019;71:1216–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gäbele E, Dostert K, Hofmann C, et al. . DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55:1391–1399. [DOI] [PubMed] [Google Scholar]
- 8. Wahlström A, Sayin SI, Marschall HU, et al. . Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. [DOI] [PubMed] [Google Scholar]
- 9. Long MD, Crandall WV, Leibowitz IH, et al. ; ImproveCareNow Collaborative for Pediatric IBD . Prevalence and epidemiology of overweight and obesity in children with inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:2162–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lomer MCE, Cahill O, Baschali A, et al. . A multicentre study of nutrition risk assessment in adult patients with inflammatory bowel disease attending outpatient clinics. Ann Nutr Metab. 2019;74:18–23. [DOI] [PubMed] [Google Scholar]
- 11. Singh S, Dulai PS, Zarrinpar A, et al. . Obesity in IBD: epidemiology, pathogenesis, disease course and treatment outcomes. Nat Rev Gastroenterol Hepatol. 2017;14:110–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Magrì S, Paduano D, Chicco F, et al. . Nonalcoholic fatty liver disease in patients with inflammatory bowel disease: beyond the natural history. World J Gastroenterol. 2019;25:5676–5686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Saroli Palumbo C, Restellini S, Chao CY, et al. . Screening for nonalcoholic fatty liver disease in inflammatory bowel diseases: a cohort study using transient elastography. Inflamm Bowel Dis. 2019;25:124–133. [DOI] [PubMed] [Google Scholar]
- 14. Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148:1244–60.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ananthakrishnan AN, Khalili H, Song M, et al. . High school diet and risk of Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis. 2015;21:2311–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saadeh S, Younossi ZM, Remer EM, et al. . The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 17. Bach-Faig A, Berry EM, Lairon D, et al. ; Mediterranean Diet Foundation Expert Group . Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011;14:2274–2284. [DOI] [PubMed] [Google Scholar]
- 18. Zhou YJ, Zhou H, Li Y, et al. . NOD1 activation induces innate immune responses and insulin resistance in human adipocytes. Diabetes Metab. 2012;38:538–543. [DOI] [PubMed] [Google Scholar]
- 19. Zulian A, Cancello R, Micheletto G, et al. . Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut. 2012;61:86–94. [DOI] [PubMed] [Google Scholar]
- 20. Landers CJ, Cohavy O, Misra R, et al. . Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–699. [DOI] [PubMed] [Google Scholar]
- 21. Vetrano S, Rescigno M, Cera MR, et al. . Unique role of junctional adhesion molecule-a in maintaining mucosal homeostasis in inflammatory bowel disease. Gastroenterology. 2008;135:173–184. [DOI] [PubMed] [Google Scholar]
- 22. Miranda-Ribera A, Ennamorati M, Serena G, et al. . Exploiting the zonulin mouse model to establish the role of primary impaired gut barrier function on microbiota composition and immune profiles. Front Immunol. 2019;10:2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McHenry S, Sharma Y, Tirath A, et al. . Crohn’s disease is associated with an increased prevalence of nonalcoholic fatty liver disease: a cross-sectional study using magnetic resonance proton density fat fraction mapping. Clin Gastroenterol Hepatol. 2019;17:2816–2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kolodziejczyk AA, Zheng D, Shibolet O, et al. . The role of the microbiome in NAFLD and NASH. EMBO Mol Med. 2019;11:e9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang C, Zhu C, Shao L, et al. . Role of bile acids in dysbiosis and treatment of nonalcoholic fatty liver disease. Mediators Inflamm. 2019;2019:7659509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arpaia N, Campbell C, Fan X, et al. . Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. De Filippis F, Pellegrini N, Vannini L, et al. . High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–1821. [DOI] [PubMed] [Google Scholar]
- 28. Walker AW, Sanderson JD, Churcher C, et al. . High-throughput clone library analysis of the mucosa-associated microbiota reveals dysbiosis and differences between inflamed and non-inflamed regions of the intestine in inflammatory bowel disease. BMC Microbiol. 2011;11:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marlow G, Ellett S, Ferguson IR, et al. . Transcriptomics to study the effect of a Mediterranean-inspired diet on inflammation in Crohn’s disease patients. Hum Genomics. 2013;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mayr HL, Itsiopoulos C, Tierney AC, et al. . Ad libitum Mediterranean diet reduces subcutaneous but not visceral fat in patients with coronary heart disease: a randomised controlled pilot study. Clin Nutr ESPEN. 2019;32:61–69. [DOI] [PubMed] [Google Scholar]
- 31. Panizza CE, Lim U, Yonemori KM, et al. . Effects of intermittent energy restriction combined with a mediterranean diet on reducing visceral adiposity: a randomized active comparator pilot study. Nutrients. 2019;11:1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baratta F, Pastori D, Polimeni L, et al. . Adherence to mediterranean diet and non-alcoholic fatty liver disease: effect on insulin resistance. Am J Gastroenterol. 2017;112:1832–1839. [DOI] [PubMed] [Google Scholar]
- 33. Chan R, Wong VW, Chu WC, et al. . Diet-quality scores and prevalence of nonalcoholic fatty liver disease: a population study using proton-magnetic resonance spectroscopy. PLoS One. 2015;10:e0139310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kontogianni MD, Tileli N, Margariti A, et al. . Adherence to the Mediterranean diet is associated with the severity of non-alcoholic fatty liver disease. Clin Nutr. 2014;33:678–683. [DOI] [PubMed] [Google Scholar]
- 35. Ryan MC, Itsiopoulos C, Thodis T, et al. . The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138–143. [DOI] [PubMed] [Google Scholar]
- 36. Henríquez Sánchez P, Ruano C, de Irala J, et al. . Adherence to the Mediterranean diet and quality of life in the SUN Project. Eur J Clin Nutr. 2012;66:360–368. [DOI] [PubMed] [Google Scholar]
- 37. Katz DA, McHorney CA, Atkinson RL. Impact of obesity on health-related quality of life in patients with chronic illness. J Gen Intern Med. 2000;15:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lemstra ME, Rogers MR. Improving health-related quality of life through an evidence-based obesity reduction program: the Healthy Weights Initiative. J Multidiscip Healthc. 2016;9:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Halmos EP, Gibson PR. Dietary management of IBD–insights and advice. Nat Rev Gastroenterol Hepatol. 2015;12:133–146. [DOI] [PubMed] [Google Scholar]
- 40. Papada E, Amerikanou C, Forbes A, et al. . Adherence to mediterranean diet in Crohn’s disease. Eur J Nutr. 2020;59:1115–1121. [DOI] [PubMed] [Google Scholar]
- 41. Khalili H, Hakansson N, Chan SS, et al. . Adherence to a Mediterranean diet is associated with a lower risk of later-onset Crohn’s disease: results from two large prospective cohort studies. Gut. 2020. [DOI] [PubMed] [Google Scholar]