Abstract
We report the third presentation of an intermixed arteriovenous malformation and hemangioblastoma. The rare occurrence of the diagnostic histologic features of both a neoplasm and vascular malformation in a single lesion is more common in gliomas, as angioglioma, and is termed an ‘intermixed’ lesion. We review the literature concerning the developmental biology of each lesion, and potential interplay in the formation of an intermixed vascular neoplasm and vascular malformation. The roles of cellular origin, genetic susceptibility, favourable microenvironment, altered local gene expression and key regulatory pathways are reviewed. Our review supports angiography and genetic profiling in intermixed lesions to inform management strategies. Consideration should be given to multimodality therapeutic interventions as required, including microsurgical resection, stereotactic radiosurgery and further research to exploit emerging molecular targets.
Keywords: : arteriovenous malformation, hemangioblastoma, intermixed
Arteriovenous malformation (AVM) and capillary hemangioblastoma (HB) are distinct lesions, constituting vascular malformations and vascular neoplasms of the CNS, respectively [1].
As AVM and HB are distinct lesions with respect to classification, developmental biology and management, their copresentation – spatially, temporally or as intermixed lesions – is a rare occurrence. We report the third presentation of an ‘intermixed’ AVM and HB.
‘Intermixed/intermingled/coexistent’ is the pathological finding of diagnostic pathological features of two lesions present within the same lesion. For example, in glioma, the term angioglioma is used to describe such intermixed lesions [2,3]. Only two such intermixed AVM-HB lesions have been previously described [4,5]. The paucity of this occurrence raises questions about the (co)development, of such lesions.
In addition, we review the literature regarding such lesions in isolation and mechanisms of co-existence in the context of emerging developmental biology. Finally, we explore management options if such a rare lesion is suspected.
Case report
History
A 46-year-old man presented with a 2-month history of worsening headache, vomiting, weight loss and tinnitus. On examination, the patient had a broad-based ataxic gait, profound lateral nystagmus, past-pointing on the right and oropharyngeal dysphagia. Past medical and family histories were noncontributory. Neuroimaging (Figure 1) revealed a large 3.5 × 2.8 cm mixed cystic and solid posterior fossa lesion with moderate obstructive hydrocephalus, suggestive of a HB. Computed tomography thorax abdomen and pelvis and spinal MRI were unremarkable. The patient underwent a posterior fossa craniectomy and total resection of a craniocervical junction lesion (Figure 2). Intraoperatively, this was a highly vascular lesion, extending from the vermis to involve the posterior spinal cord, which hemorrhaged at high pressure resulting in 1500 ml estimated blood loss.
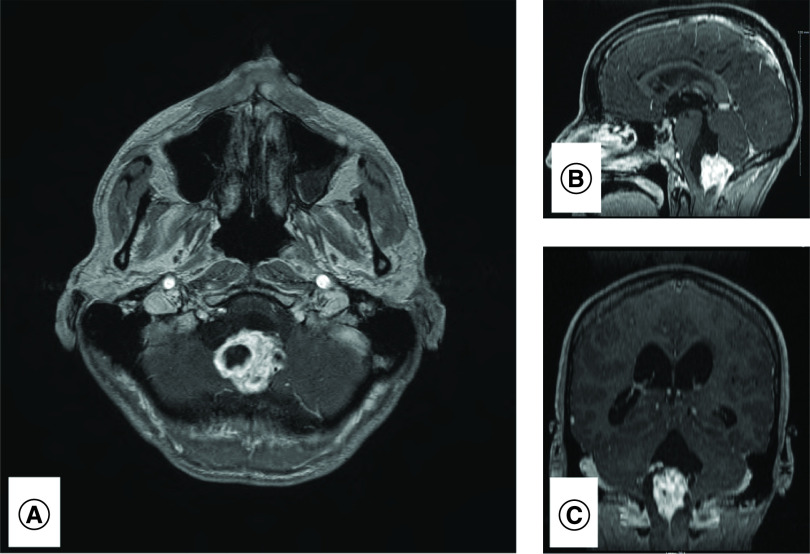
Figure 1. . Radiology pre-resection.
MRI demonstrates obstructive enhancing mixed solid-cystic lesion in axial (A), saggital (B) and coronal (C) planes.
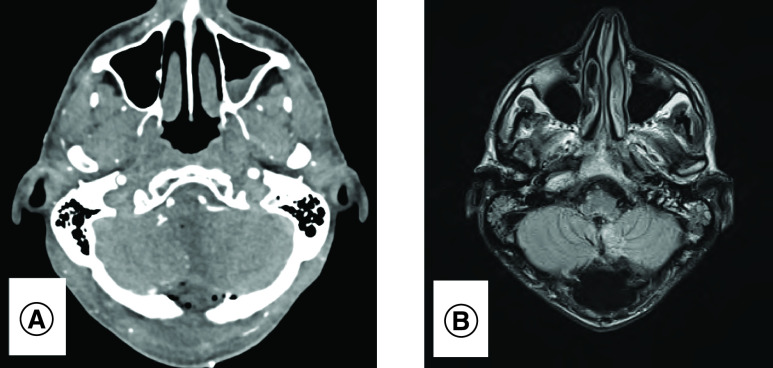
Figure 2. . Radiology post-resection.
Total resection of lesion on postoperative computed tomography angiogram (A) and MRI brain (B).
Pathological findings
Microscopy found the lesion to be composed of thick- and thin-walled dilated blood vessels which were partly separated from each other by gliotic neuropil in which there was ‘neovascularization’. Within the gliotic neuropil there were numerous Rosenthal fibers. Amorphous nonrefractile eosinophilic material was located within some of the vascular channels. Arteries, veins and arterialized veins were all present. Evidence of prior perilesional hemorrhage in the form of hemosiderin granules was noted. Fragments of ‘surgicel’ were included. In isolation the appearances were those of an AVM (Figure 3 & Figure 4).
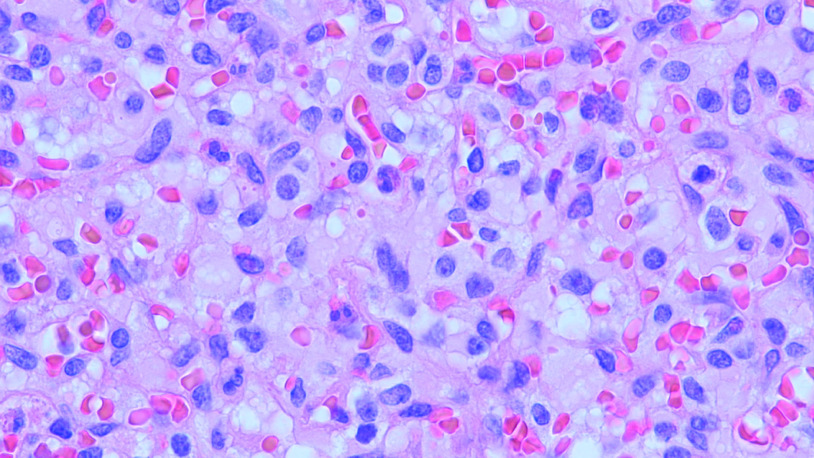
Figure 3. . Haemangioblastoma.
Representative image of hemangioblastoma characterized by vacuolated stromal cells separated by capillary vessels filled with red blood cells.
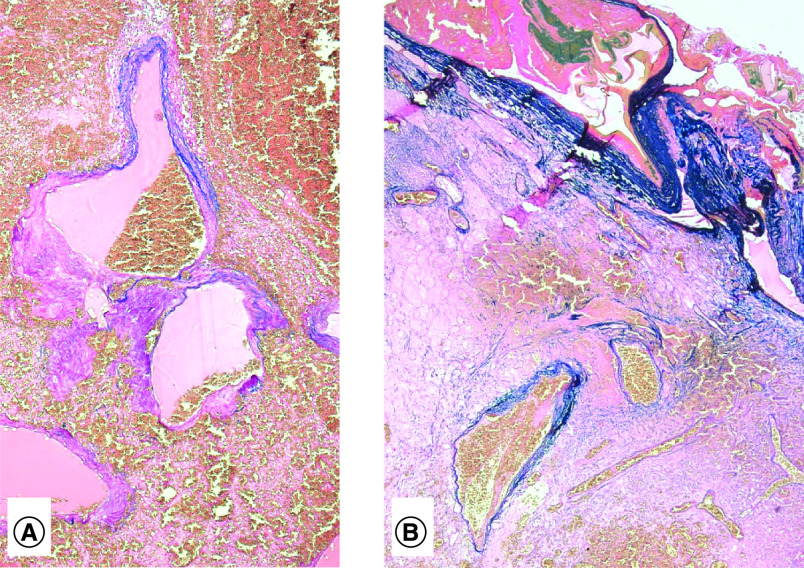
Figure 4. . Arteriovenous malformation.
(A) Large thin-walled vessels located in center of tumor and (B) extending up on to meningeal surface of brain.
Additionally, however, the degree of ‘neovascularization’ in the form of proliferating capillary vessels was intense and was accompanied by large vacuolated lipid filled cells. Mitoses were not present. Necrosis was not present. Taken in isolation, these appearances were those of a capillary HB. However, the overall appearances were of a capillary HB, WHO grade I with an AVM, together with evidence of prior perilesional hemorrhage.
Discussion
Intermixed lesions
We report the third presentation (Table 1) of an intermixed AVM and HB, in other words, the finding of diagnostic pathological features of two classified lesions present within the same lesion [4,5].
Table 1. . Intermixed arteriovenous malformation and hemangioblastoma, reported cases and characteristics.
| Study (year) | Age (years), sex | Type of tumor/ malformation | Location of lesion | Vascular anatomy | Size | Presentation | Management | Outcome | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Raynor & Kingman (1965) | 19, M | HB/AVM | Cerebellar – vermian | Large midline vascular lesion, suggestive of AVM | 5.5 × 4 × 3 cm | Hemorrhage of lesion (confirmed on lumbar puncture) | Initial posterior fossa exploration without resection owing to lesion complexity; subsequent resection for interval growth on angiography | Mortality, day 12 post-operatively | [5] |
| Medvedev et al. (1991) | 38, M | HB/AVM | Cerebellar – mesobasal aspect of left cerebellar hemisphere | Left AICA and PICA feeder vessels rapidly shunting to the tentorial sinuses | 4 × 4 × 4 cm | Symptomatic hydrocephalus and cerebellar signs | CSF diversion (ventricular catheter); posterior fossa exploration and total excision | Survival | [4] |
| Healy et al. (2020) | 46, M | HB/AVM | Cerebellar and spinal – vermian and including posterior cervical cord | No angiographic phase pre-operatively | 3.5 × 2.8 cm | Symptomatic hydrocephalus and cerebellar signs | Posterior fossa exploration and total resection | Survival, some lower cranial nerve dysfunction | Current article |
AICA: Anterior inferior cerebellar artery; AVM: Arteriovenous malformation; CSF: Cerebrospinal fluid; HB: Hemangioblastoma; PICA: Posterior inferior cerebellar artery.
AVM and HB may be considered distinct lesions with regard to morphology, classification and physiology. AVMs are high-flow vascular malformations, involving aberrant arteriovenous shunting between dysplastic arteries and veins, with the characteristic absence of an intervening capillary bed [6]. There is no intervening neural parenchyma in the nidus [7,8]. In contrast, HB are well-circumscribed WHO grade I vascular neoplasms, which may be solid or solid with a peritumoral cystic component, and histologically comprise numerous capillary channels separated by neoplastic stromal cells [1,9,10].
Notably, in response to the case of Raynor and Kingman [5], Stehbens [8] suggests that the AVM described may be the hypervascularization of an HB, rather than intermixed lesions. This observation is noted by Medvedev et al., and refuted as an explanation for their intermixed lesions. Medvedev et al. reference the ‘interspersed and distinct nature of the thick-walled blood vessels, without evidence of neoplastic involvement’ as most supportive of two distinct pathological processes [4].
Developmental biology
It suggested that intermixed AVM and HB features may be attributable to a common cellular origin [4]. AVMs are agreed to originate from mesoderm-derived angioblasts during the formation of the vascular plexuses between week 3–8 of embryogenesis [5,11]. However, there is controversy as to the tumor initiating cell of HB. Most authors favor the mesoderm-derived, embryologically arrested hemangioblast [6,12–14]. These cells may remain arrested, with subsequent reactivation of the hematopoietic or endothelial differentiation potential under various environmental stimuli, thus forming HB [13,15]. This is supported by analysis of protein expression shared by hemangioblast progenitors and stromal cells (the neoplastic cell in mature HB) [15–17]. It is the neoplastic stromal cells which further differentiate into the ‘vasoformative elements’ (endothelial cells and pericytes) [10]. Controversy exists however, with Ma et al. favoring the HB tumor-initiating cell to originate from neoplastic transformation of neuroectodermal-derived neural stem cells/progenitors (via SSEA-1 expression analysis) with multiprogenitor ability suggested, dependent on a HB niche microenvironment [18]. In the context of a disputed cell of origin for HB, it is not possible to definitively attribute the pathological findings within an intermixed lesion to a shared cellular origin.
Second, the interplay of genetics and environment must be considered in an intermixed lesion. It is possible that one initiating lesion may constitute the appropriate microenvironment to provoke the development of the second pathology. This may be in the context of genetic susceptibility, or in the maturation of a pre-existing structural vascular lesion. There is precedence in the multiple cases reports of glial neoplasms in close association with AVMs. This refutes classical thinking, which suggested AVM and HB to be formed at different time points – approximately preweek 8 (i.e., congenital) for AVM and after week 12 for HB [5].
It is now accepted that both genetic and environmental roles contribute to the development of clinically significant AVMs and HB. In HB, this is supported by arrest-reactivation theories of hemangioblast development, as above [13,15]. In AVM, emerging research finds a large subpopulation of AVMs to be an acquired pathology. Morales-Valero supports this by the rarity of neonatal AVM diagnoses despite high-resolution imaging, in addition to multiple case reports of de novo AVMs, despite previously negative neuroaxis imaging [24].
Under the Morales-Valero model, the ‘acquired’ AVM formation is the result of genetic susceptibility – in other words, appropriate single nucleotide polymorphisms (SNiPs) – in the context of appropriate microenvironmental triggers or ‘second hits’ – such as cerebral infarction, inflammation, trauma or in association with neoplasia [2,19,20]. For example, the genetic ‘first hit’ of gap-junction protein Connexin40 deficiency-forming transient arteriovenous shunts, which may develop into clinically significant AVMs in the appropriate microenvironmental ‘second hit’ [21,22]. This is in keeping with Ramey et al.’s hierarchical model, whereby following embryogenic vasculogenesis, a structural vascular dysgenesis is believed to form aberrant arteriovenous shunts without intervening capillary networks. Ramey et al. propose such arteriovenous fistulae constitute ‘AVM precursors’, which mature via an environmental ‘second hit’ into a clinically significant AVM [19,23,24]. A physico-epigenetic component is suggested by Thomas et al., whereby altered local hemodynamics affect cellular metabolism, triggering epigenetic factors that modulate AVM-associated genes to direct aberrant vessel phenotypes [25]. Thus, one lesion may constitute a favorable microenvironment for the development of a second lesion, in the context of genetic susceptibility.
Third, in both HB and AVM, there is a significant genetic component to development, which overlaps between lesions. This overlap, such as must be appreciated in an intermixed lesion, continues to be elucidated as the individual processes of neovascularization in HB and AVM continues to be researched, including the differential role of component cell types, and their variable activation of angiogenic pathways [26,27].
HB tumorigenesis strongly associates with von Hippel–Lindau (VHL)-silencing, in all familial HB and >78% sporadic HB [16,28–30]. However, familial and sporadic may be distinguished by markers of glial, neuroepithelial and neuronal differentiation [17]. VHL tumor suppressor gene inactivation results in less pVHL-mediated degradation of hypoxia-inducible factor 1 alpha (HIF-1α). The normoxic VHL cell therefore behaves as if in a hypoxic state, with accumulating HIF-1α associating with HIF-1β to exert transcriptional roles on target genes to produce multiple synergistic angiogenic and growth factors [28,31,32]. These include primarily VEGF/VEGFR2, but also: Notch/Dll4, EphB4/EphrinB2 and SDF1α/CXCR4 pathways [33]. HB neovascularization results from VHL-silencing associated mechanisms which include the classically appreciated VEGF-mediated angiogenesis in addition to VEGF-independent angiogenesis and vasculogenesis [12,26,34,35]. VEGF-independent mechanisms of angiogenesis also exist, increasingly discovered following disappointing clinical trials focused on VEGF-mediated angiogenesis [36,37]. Endothelial VHL-silencing associated Twist1 accumulation is a VEGF-independent mechanism of common angiogenesis that is only recently reported [26,37].
There is some overlap between HB and the key regulatory pathways associated with sporadic AVM development, including: VEGF, Notch/Dll4, Eph/Ephrin ligand-receptor, TGF-β, hedgehog pathways and others [38]. Inherited AVM syndromes, such as hereditary hemorrhagic telangiectasia (HHT), associate with the TGF-β superfamily members such as ALK1/Avcrl1, ENG/Endoglin or Smad4 mutations [39]. SMAD4 induces both endothelial cell proliferation and hypertrophy, in addition to exhibiting altered mural cell coverage and distorted artery-vein gene expression, including decreased VEGFR2 expression. This SMAD4 murine model thus links the TGF-β superfamily and VEGF signaling pathways – one example of the overlap and potential synergism between HB and AVM [40].
Finally, in terms of location, all reported cases of intermixed AVM-HB lesions were cerebellar. Classically, HBs favor the cerebellum (76%), while AVMs exhibit an anatomic predilection for cerebral hemispheres [16]. Lindau and Bailey originally suggested the predilection of HB for the cerebellum occurs due to the coincident development of the cerebellum and vascular mesenchyme on the posterior medullary velum in the third month. This vascular primordium is situated at the cerebello-pontine angle, and becomes a rich capillary network, which comes to form the choroid plexus of the fourth ventricle [5].
Management considerations
AVMs are dynamic lesions, exhibiting vascular remodeling, progressive growth and aberrant hemodynamics – frequently progressing from low-flow juvenile lesions to medium-to-high flow, high pressure lesions over time [5,7]. These features contribute to the pathologic characteristics of presentation, including mass effect, mural instability and hemorrhagic propensity [7,23].
Posterior fossa AVMs exhibit a more aggressive natural history than supratentorial AVMs, with annual rupture rates as high as 11.6%, supporting more aggressive treatment [41,42]. While the modified Spetzler-Martin AVM grading system and three-tiered management system informs treatment, posterior fossa location must be considered in intermixed lesions, if pre-operative angiographic imaging is suggestive and individualized treatment favored.
In HB, the need for treatment is dependent on both symptoms and location. It is clear from case series in VHL disease patients that both size and rate of growth correlate with symptoms. While almost all tumors exhibited measurable growth, many tumors never produce symptoms and do not require treatment [43]. Neurological symptoms and morbidity frequently result from peritumoral cysts. Cyst development was associated with germline partial deletion of the VHL gene [9]. In VHL disease with multiple lesions, radiological progression alone is deemed not an indication for treatment [43].
Genetic profiling may be insightful, in that genetic differences differentiating syndromic versus sporadic AVM significantly determine clinical presentation. In HHT, AVMs are typically smaller, multiple, of lower Spetzler-Martin grades, less frequently temporal and may exhibit a lower hemorrhagic risk than sporadic lesions [44–48]. Tissue from intermixed lesions could be considered for genetic profiling to elucidate a causative genetic basis.
If intermixed lesions are suspected, from vascularity or nidus, pre-operative digital subtraction angiography and discussion at neurovascular multidisciplinary meeting may be indicated. Consideration must be given to classical features of AVM classification and resectability and clinical symptoms.
Therapeutic strategies
In AVM, treatment strategies are multiple and include: (micro)surgical resection, stereotactic radiosurgery (SRS), endovascular embolization and combined multimodality management. Analyses report very good outcomes for all modalities; however, high-quality comparative data to individualize treatment is limited [49–59]. Medical management alone may constitute appropriate and effective treatment as per the ARUBA trial, noting, however, that long-term follow-up may favor other treatment modalities – resection, SRS, etc. [60–63].
HB may be approached by resection, SRS or surveillance only. However, independent predictors of overall survival include surgical management, younger age and Caucasian race, while radiation therapy outcomes were not validated (possibly owing to short follow-up and HB growth patterns) in a SEER-based analysis [64]. A 2018 paper supports gross total resection as the gold standard, and suggests SRS has comparable outcomes to subtotal resection only [65].
In mixed lesions, individualized treatment including multidisciplinary team discussion is favored.
In terms of emerging molecular targets in AVM, radiosurgery is seen to both induce thrombotic occlusion of nidus vessels in addition to inhibiting Notch1 and Notch4 signaling in endothelial cells [66]. Both increased and decreased Notch activity induce AVM formation in sporadic AVM and syndromic-type Alk1 knockout models, respectively [21,67,68]. Of note, Notch normalization induces structural regression of arteriovenous shunts, and may be normalized as part of the response to radiation in SRS treatment [69].
Genetic profiling of lesions is important, to replicate successes such as endoglin and ALK1 now offering promising therapeutic targets in HHT AVMs. These genes induce disease states by haploinsufficiency, which may be amenable to therapeutic approaches to enhance protein expression/function [39,70]. For example, the administration of an exogenous endoglin isoform, soluble endoglin, in murine models partly restores normal protein expression patterns of endothelial cells, decreasing AVM size and incidence. However, the soluble endoglin effect appears to depend on endogenous expression of endoglin [71]. Bazedoxifene, a selective estrogen receptor modulator, has been demonstrated in vivo to compensate for ENG and ALK1 haploinsufficiency, increasing ENG and ALK1 mRNA levels and reducing HHT lesion-associated hemorrhage [72].
For HB, the clinical translation of anti-angiogenic therapeutics has been disappointing [73]. In HB, this is attributed, at least in part, to the lack of knowledge in terms of the cytological origin, evolutionary processes and neovascularization in this lesion [37]. A 2009 Dartmouth trial (NCT01015300) of anti-VEGF bevacizumab (Avastin) in VHL disease-associated unresectable/recurrent HB was terminated in 2012 due to low accrual, while a case report of favorable outcome exists [74]. EGFR expression suggests tyrosine kinase activity may represent the signal transduction initiator [13,36]. In terms of tyrosine kinase inhibitors, a single trial and multiple case reports exist in the use of multityrosine kinase inhibitor pozopanib for VHL disease associated HB, with moderate success [75–78]. Other multityrosine kinase inhibitor trials for HB were disappointing, with the dovitinib trial discontinued owing to toxicity, while erlotinib and sunitinib were found to be of limited benefit [79–82]. Emerging research into HB neovascularization mechanisms – such as Twist1 signaling, described above – may provide additional anti-angiogenic therapeutic targets [26].
In the context of the above, intermixed lesions are of limited precedence, and must therefore be approached on an individualized basis. Multimodality interventions, or combinations thereof, may be of value in such complex lesions. The emerging role of molecular therapeutics is promising.
Conclusion
In summary, we contribute a case of intermixed AVM and HB, and review the literature concerning the developmental biology of each lesion, and potential interplay in the formation of an intermixed vascular neoplasm and vascular malformation. The roles of cellular origin, genetic susceptibility, favourable microenvironment, altered local gene expression and key regulatory pathways are reviewed. Significant potential for overlap and synergism is appreciated, and continues to be elucidated as the individual processes of neovascularization in HB and AVM continues to be researched, including the differential role of component cell types, and their variable activation of angiogenic pathways.
Clinically, our review supports pre- and post-operative angiography in suspected highly vascular lesions to inform management strategies. Consideration should be given to intermixed lesions as a differential diagnosis. Genetic profiling should be considered in atypical cases, in that genetic differences differentiating syndromic versus sporadic arteriovenous malformations and hemangioblastomas may significantly determine lesion anatomopathology and clinical presentation, which may inform management. Multidisciplinary discussion should inform individualised treatment, that appreciates management considerations of both neoplastic lesions and vascular malformations. Consideration should be given to multimodality therapeutic interventions as required, including surgery, stereotactic radiosurgery, endovascular embolisation and further research to exploit emerging molecular targets.
Executive summary.
Angiographic imaging should be considered pre- and post-operatively in suspected highly vascular lesions, with consideration given to intermixed lesions as a differential.
Genetic profiling should be considered, in that genetic differences differentiating syndromic versus sporadic arteriovenous malformations and hemangioblastomas may significantly determine lesion anatomopathology and clinical presentation, which may inform management.
Individualized treatment, informed by multidisciplinary discussion, should be considered that appreciates management considerations of both neoplastic lesions and vascular malformations.
Management strategies should consider multimodality treatment, including combinations of microsurgical resection, stereotactic radiosurgery, endovascular embolization and/or further research to exploit emerging molecular therapeutics.
Surveillance alone, as a management practice in isolated hemangioblastomas, may not apply in the presence of an intermixed vascular malformation, and particularly should appreciate the higher rate of rupture of infratentorial arteriovenous malformations.
Consideration of consented submission of specimens to appropriate biobanks or research bodies may be considered as per local policy.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Informed consent disclosure
The authors state that they have obtained verbal and written informed consent from the patient for the inclusion of their medical and treatment history within this case report.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sepulveda A, Buchanan EP. Vascular tumors. Semin. Plast. Surg. 28(2), 49–57 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yano H, Nakayama N, Ohe N, Takagi T, Shinoda J, Iwama T. Surgical strategy in case with co-existence of malignant oligodendroglioma and arteriovenous malformation: a case report. Case Reports Clin. Med. 2(08), 473–478 (2013). [Google Scholar]
- 3.Licata C, Pasqualin A, Freschini A, Barone G, Da Pian R. Management of associated primary cerebral neoplasms and vascular malformations: 2. Intracranial arterio-venous malformations. Acta Neurochir. (Wien) 83(1–2), 38–46 (1986). [DOI] [PubMed] [Google Scholar]
- 4.Medvedev YA, Matsko DE, Zubkov YN, Pak VA, Alexander LF. Coexistent hemangioblastoma and arteriovenous malformation of the cerebellum. J. Neurosurg. 75(1), 121–125 (1991). [DOI] [PubMed] [Google Scholar]; • An existing case report of an intermixed arteriovenous malformations (AVMs) and hemangioblastoma.
- 5.Raynor RB, Kingman AF. Hemangioblastoma and vascular malformations as one lesion. Arch. Neurol. 12, 39–48 (1965). [DOI] [PubMed] [Google Scholar]; • An existing case report of an intermixed AVMs and hemangioblastoma.
- 6.Shively SB, Falke EA, Li J. et al. Developmentally arrested structures preceding cerebellar tumors in von Hippel–Lindau disease. Mod. Pathol. 24(8), 1023–1030 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenberg MS. Handbook of Neurosurgery (8th Edition). Thieme, NY, USA: (2016). [DOI] [PubMed] [Google Scholar]
- 8.Stehbens W. Pathology of the Cerebral Blood Vessels (2nd Edition). CV Mosby, MO, USA: (1972). [Google Scholar]
- 9.Huntoon K, Wu T, Elder JB. et al. Biological and clinical impact of hemangioblastoma-associated peritumoral cysts in von Hippel–Lindau disease. J. Neurosurg. 124(4), 971–976 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhry AP, Montes M, Cohn GA. Ultrastructure of cerebellar hemangioblastoma. Cancer 42(4), 1834–1850 (1978). [DOI] [PubMed] [Google Scholar]; • Excellent contextual understanding to the histopathology and developmental biology of vascular lesions.
- 11.Sabin FR. Preliminary note on the differentiation of angioblasts and the method by which they produce blood-vessels, blood-plasma and red blood-cells as seen in the living chick. J. Hematother. Stem Cell Res. 11(1), 5–7 (2002). [DOI] [PubMed] [Google Scholar]; •• Excellent contextual understanding to the histopathology and developmental biology of vascular lesions, with particular insight from the original observations of Sabin.
- 12.Zhuang Z, Frerich JM, Huntoon K. et al. Tumor derived vasculogenesis in von Hippel–Lindau disease-associated tumors. Sci. Rep. 4, 4102 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park DM, Zhuang Z, Chen L. et al. von Hippel–Lindau disease-associated hemangioblastomas are derived from embryologic multipotent cells. PLoS Med. 4(2), e60 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vortmeyer AO, Frank S, Jeong S-Y. et al. Developmental arrest of angioblastic lineage initiates tumorigenesis in von Hippel–Lindau disease. Cancer Res. 63(21), 7051–7055 (2003). [PubMed] [Google Scholar]
- 15.Gläsker S, Li J, Xia JB. et al. Hemangioblastomas share protein expression with embryonal hemangioblast progenitor cell. Cancer Res. 66(8), 4167–4172 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Hussein MR. Central nervous system capillary haemangioblastoma: the pathologist’s viewpoint. Int. J. Exp. Pathol. 88(5), 311–324 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Epari S, Bhatkar R, Moyaidi A. et al. Histomorphological spectrum and immunohistochemical characterization of hemangioblastomas: an entity of unclear histogenesis. Indian J. Pathol. Microbiol. 57(4), 542–548 (2014). [DOI] [PubMed] [Google Scholar]
- 18.Ma D, Zhang M, Chen L. et al. Hemangioblastomas might derive from neoplastic transformation of neural stem cells/progenitors in the specific niche. Carcinogenesis 32(1), 102–109 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Pabaney AH, Rammo RA, Tahir RA, Seyfried D. Development of de novo arteriovenous malformation following ischemic stroke: case report and review of current literature. World Neurosurg. 96, 608.e5–608.e12 (2016). [DOI] [PubMed] [Google Scholar]
- 20.Mouchtouris N, Jabbour PM, Starke RM. et al. Biology of cerebral arteriovenous malformations with a focus on inflammation. J. Cereb. Blood Flow Metab. 35(2), 167–175 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peacock HM, Caolo V, Jones EAV. Arteriovenous malformations in hereditary haemorrhagic telangiectasia: looking beyond ALK1-NOTCH interactions. Cardiovasc. Res. 109(2), 196–203 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Gkatzis K, Thalgott J, Dos-Santos-Luis D. et al. Interaction between ALK1 signaling and Connexin40 in the development of arteriovenous malformations. Arterioscler. Thromb. Vasc. Biol. 36(4), 707–717 (2016). [DOI] [PubMed] [Google Scholar]
- 23.Ramey WL, Martirosyan NL, Zabramski JM, Spetzler RF, Kalani MYS. A hierarchical model for the development of cerebral arteriovenous malformations. Clin. Neurol. Neurosurg. 126, 126–129 (2014). [DOI] [PubMed] [Google Scholar]; • Excellent discussion on the developmental biology of AVMs.
- 24.Morales-Valero SF, Bortolotti C, Sturiale C, Lanzino G, Lanzino G. Are parenchymal AVMs congenital lesions? Neurosurg. Focus 37(3), E2 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Thomas JM, Surendran S, Abraham M, Rajavelu A, Kartha CC. Genetic and epigenetic mechanisms in the development of arteriovenous malformations in the brain. Clin. Epigenetics 8, 78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent discussion on the developmental biology of AVMs.
- 26.Wang Y, Chen D-Q, Chen M-Y, Ji K-Y, Ma D-X, Zhou L-F. Endothelial cells by inactivation of VHL gene direct angiogenesis, not vasculogenesis via Twist1 accumulation associated with hemangioblastoma neovascularization. Sci. Rep. 7, 5463 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jinesh GG, Kamat AM. RalBP1 and p19-VHL play an oncogenic role, and p30-VHL plays a tumor suppressor role during the blebbishield emergency program. Cell Death Discov. 3, 17023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratmann R, Krieg M, Haas R, Plate KH. Putative control of angiogenesis in hemangioblastomas by the von Hippel–Lindau tumor suppressor gene. J. Neuropathol. Exp. Neurol. 56(11), 1242–1252 (1997). [DOI] [PubMed] [Google Scholar]
- 29.Lee JY, Dong SM, Park WS. et al. Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 58(3), 504–508 (1998). [PubMed] [Google Scholar]
- 30.Shankar GM, Taylor-Weiner A, Lelic N. et al. Sporadic hemangioblastomas are characterized by cryptic VHL inactivation. Acta Neuropathol. Commun. 2, 167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ziyad S, Iruela-Arispe ML. Molecular mechanisms of tumor angiogenesis. Genes Cancer 2(12), 1085–1096 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiao Y-H. The von Hippel–Lindau gene and protein in tumorigenesis and angiogenesis: a potential target for therapeutic designs. Curr. Med. Chem. 10(22), 2461–2470 (2003). [DOI] [PubMed] [Google Scholar]
- 33.Pierscianek D, Wolf S, Keyvani K. et al. Study of angiogenic signaling pathways in hemangioblastoma. Neuropathology 37(1), 3–11 (2017). [DOI] [PubMed] [Google Scholar]; • Informative frameworks to approach vasculogenesis and angiogenesis.
- 34.Patan S. Vasculogenesis and angiogenesis. Cancer Treat. Res. 117, 3–32 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Gläsker S, Smith J, Raffeld M, Li J, Oldfield EH, Vortmeyer AO. VHL-deficient vasculogenesis in hemangioblastoma. Exp. Mol. Pathol. 96(2), 162–167 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Informative frameworks to approach vasculogenesis and angiogenesis.
- 36.Chen GJ, Karajannis MA, Newcomb EW, Zagzag D. Overexpression and activation of epidermal growth factor receptor in hemangioblastomas. J. Neurooncol. 99(2), 195–200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Y, Chen D, Chen M, Ji K, Ma D, Zhou L. A comprehensive procedure to evaluate the in vitro performance of the putative hemangioblastoma neovascularization using the spheroid sprouting assay. J. Vis. Exp. 134, e57183 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walcott BP, Winkler EA, Rouleau GA, Lawton MT. Molecular, cellular, and genetic determinants of sporadic brain arteriovenous malformations. Clin. Neurosurg. 63(Suppl. 1), 37–42 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; • Excellent discussion on the developmental biology of AVMs.
- 39.Ruiz-Llorente L, Gallardo-Vara E, Rossi E, Smadja DM, Botella LM, Bernabeu C. Endoglin and alk1 as therapeutic targets for hereditary hemorrhagic telangiectasia. Expert Opin. Ther. Targets 21(10), 933–947 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Crist AM, Lee AR, Patel NR, Westhoff DE, Meadows SM. Vascular deficiency of Smad4 causes arteriovenous malformations: a mouse model of hereditary hemorrhagic telangiectasia. Angiogenesis 21(2), 363–380 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spetzler RF, Ponce FA. A 3-tier classification of cerebral arteriovenous malformations. J. Neurosurg. 114(3), 842–849 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Arnaout OM, Gross BA, Eddleman CS, Bendok BR, Getch CC, Batjer HH. Posterior fossa arteriovenous malformations. Neurosurg. Focus 26(5), E12 (2009). [DOI] [PubMed] [Google Scholar]
- 43.Ammerman JM, Lonser RR, Dambrosia J, Butman JA, Oldfield EH. Long-term natural history of hemangioblastomas in patients with von Hippel–Lindau disease: implications for treatment. J. Neurosurg. 105(2), 248–255 (2006). [DOI] [PubMed] [Google Scholar]
- 44.Burrows P. Angioarchitecture of hereditary arteriovenous malformations. Semin. Intervent. Radiol. 34(3), 250–257 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brinjikji W, Iyer VN, Wood CP, Lanzino G. Prevalence and characteristics of brain arteriovenous malformations in hereditary hemorrhagic telangiectasia: a systematic review and meta-analysis. J. Neurosurg. 127(2), 302–310 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Gamboa NT, Joyce EJ, Eli I. et al. Clinical presentation and treatment paradigms of brain arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia. J. Clin. Neurosci. 51, 22–28 (2018). [DOI] [PubMed] [Google Scholar]
- 47.Yang W, Liu A, Hung AL. et al. Lower risk of intracranial arteriovenous malformation hemorrhage in patients with hereditary hemorrhagic telangiectasia. Neurosurgery 78(5), 684–693 (2016). [DOI] [PubMed] [Google Scholar]
- 48.Meybodi AT, Kim H, Nelson J. et al. Surgical treatment vs nonsurgical treatment for brain arteriovenous malformations in patients with hereditary hemorrhagic telangiectasia: a retrospective multicenter consortium study. Neurosurgery 82(1), 35–47 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elsenousi A, Aletich VA, Alaraj A. Neurological outcomes and cure rates of embolization of brain arteriovenous malformations with n-butyl cyanoacrylate or Onyx: a meta-analysis. J. Neurointerv. Surg. 8(3), 265–272 (2016). [DOI] [PubMed] [Google Scholar]
- 50.Shtaya A, Millar J, Sparrow O. Multimodality management and outcomes of brain arterio-venous malformations (AVMs) in children: personal experience and review of the literature, with specific emphasis on age at first AVM bleed. Childs Nerv. Syst. 33(4), 573–581 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Conger JR, Ding D, Raper DM. et al. Preoperative embolization of cerebral arteriovenous malformations with silk suture and particles: technical considerations and outcomes. J. Cerebrovasc. Endovasc. Neurosurg. 18(2), 90–99 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierot L, Cognard C, Herbreteau D. et al. Endovascular treatment of brain arteriovenous malformations using a liquid embolic agent: results of a prospective, multicentre study (BRAVO). Eur. Radiol. 23(10), 2838–2845 (2013). [DOI] [PubMed] [Google Scholar]
- 53.Crowley RW, Ducruet AF, Kalani MYS, Kim LJ, Albuquerque FC, McDougall CG. Neurological morbidity and mortality associated with the endovascular treatment of cerebral arteriovenous malformations before and during the Onyx era. J. Neurosurg. 122(6), 1492–1497 (2015). [DOI] [PubMed] [Google Scholar]
- 54.Derdeyn CP, Zipfel GJ, Albuquerque FC. et al. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48(8), e200–e224 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Mendes GAC, Kalani MYS, Iosif C. et al. Transvenous curative embolization of cerebral arteriovenous malformations: a prospective cohort study. Neurosurgery 83(5), 957–964 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Raboud M, Tuleasca C, Maeder P. et al. Gamma knife radiosurgery for arteriovenous malformations: general principles and preliminary results in a Swiss cohort. Swiss Med. Wkly. 148(13–14), w14602 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Russell D, Peck T, Ding D. et al. Stereotactic radiosurgery alone or combined with embolization for brain arteriovenous malformations: a systematic review and meta-analysis. J. Neurosurg. 128(5), 1338–1348 (2018). [DOI] [PubMed] [Google Scholar]
- 58.Ilyas A, Chen C-J, Ding D. et al. Volume-staged versus dose-staged stereotactic radiosurgery outcomes for large brain arteriovenous malformations: a systematic review. J. Neurosurg. 128(1), 154–164 (2018). [DOI] [PubMed] [Google Scholar]
- 59.Sackey FNA, Pinsker NR, Baako BN. Highlights on cerebral arteriovenous malformation treatment using combined embolization and stereotactic radiosurgery: why outcomes are controversial? Cureus 9(5), e1266 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohr JP, Parides MK, Stapf C. et al. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non-blinded, randomised trial. Lancet 383(9917), 614–621 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong CS, Peterson EC, Ding D. et al. Intervention for a randomized trial of unruptured brain arteriovenous malformations (ARUBA) — eligible patients: an evidence-based review. Clin. Neurol. Neurosurg. 150, 133–138 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Wong J, Slomovic A, Ibrahim G, Radovanovic I, Tymianski M. Microsurgery for ARUBA trial (a randomized trial of unruptured brain arteriovenous malformation)–eligible unruptured brain arteriovenous malformations. Stroke 48(1), 136–144 (2017). [DOI] [PubMed] [Google Scholar]
- 63.Ding D, Starke RM, Kano H. et al. Radiosurgery for cerebral arteriovenous malformations in a randomized trial of unruptured brain arteriovenous malformations (ARUBA)-eligible patients. Stroke 47(2), 342–349 (2016). [DOI] [PubMed] [Google Scholar]
- 64.Nguyen HS, Doan NB, Gelsomino M. et al. Intracranial hemangioblastoma: a SEER-based analysis 2004–2013. Oncotarget 9(46), 28009–28015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang Y, Chan L, Bai HX. et al. Assessment of care pattern and outcome in hemangioblastoma. Sci. Rep. 8, 11144 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tu J, Li Y, Hu Z, Chen Z. Radiosurgery inhibition of the Notch signaling pathway in a rat model of arteriovenous malformations. J. Neurosurg. 120(6), 1385–1396 (2014). [DOI] [PubMed] [Google Scholar]
- 67.Roman BL, Hinck AP. ALK1 signaling in development and disease: new paradigms. Cell. Mol. Life Sci. 74(24), 4539–4560 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Murphy PA, Kim TN, Huang L. et al. Constitutively active Notch4 receptor elicits brain arteriovenous malformations through enlargement of capillary-like vessels. Proc. Natl Acad. Sci. USA 111(50), 18007–18012 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murphy PA, Kim TN, Lu G, Bollen AW, Schaffer CB, Wang RA. Notch4 normalization reduces blood vessel size in arteriovenous malformations. Sci. Transl. Med. 4(117), 117ra8–117ra8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardelean DS, Letarte M. Anti-angiogenic therapeutic strategies in hereditary hemorrhagic telangiectasia. Front. Genet. 6, 35 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallardo-Vara E, Tual-Chalot S, Botella LM, Arthur HM, Bernabeu C. Soluble endoglin regulates expression of angiogenesis-related proteins and induction of arteriovenous malformations in a mouse model of hereditary hemorrhagic telangiectasia. Dis. Model. Mech. 11(9), dmm034397 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zarrabeitia R, Ojeda-Fernandez L, Recio L. et al. Bazedoxifene, a new orphan drug for the treatment of bleeding in hereditary haemorrhagic telangiectasia. Thromb. Haemost. 115(6), 1167–1177 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr. Drug Targets 11(8), 1000–1017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Omar AI. Bevacizumab for the treatment of surgically unresectable cervical cord hemangioblastoma: a case report. J. Med. Case Rep. 6, 238 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jonasch E, McCutcheon IE, Gombos DS. et al. Pazopanib in patients with von Hippel–Lindau disease: a single-arm, single-centre, Phase II trial. Lancet Oncol. 19(10), 1351–1359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim BYS, Jonasch E, McCutcheon IE. Pazopanib therapy for cerebellar hemangioblastomas in von Hippel–Lindau disease: case report. Target Oncol. 7(2), 145–149 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Migliorini D, Haller S, Merkler D. et al. Recurrent multiple CNS hemangioblastomas with VHL disease treated with pazopanib: a case report and literature review. CNS Oncol. 4(6), 387–392 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Taylor DG, Ilyas A, Mehta GU. et al. Variable response of CNS hemangioblastomas to pazopanib in a single patient with von Hippel–Lindau disease: case report. J. Clin. Neurosci. 50, 154–156 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Oudard S, Elaidi R, Brizard M. et al. Sunitinib for the treatment of benign and malignant neoplasms from von Hippel–Lindau disease: a single-arm, prospective Phase II clinical study from the PREDIR group. Oncotarget 7(51), 85306–85317 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jonasch E, McCutcheon IE, Waguespack SG. et al. Pilot trial of sunitinib therapy in patients with von Hippel–Lindau disease. Ann. Oncol. 22(12), 2661–2666 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pilié P, Hasanov E, Matin SF. et al. Pilot study of dovitinib in patients with von Hippel–Lindau disease. Oncotarget 9(34), 23390–23395 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rogers LR, LoRusso P, Nadler P, Malik G, Shields A, Kaelin W. Erlotinib therapy for central nervous system hemangioblastomatosis associated with von Hippel–Lindau disease: a case report. J. Neurooncol. 101(2), 307–310 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]