Abstract
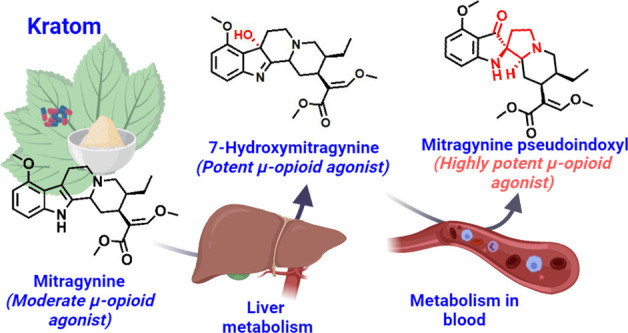
Kratom is widely consumed in the United States for self-treatment of pain and opioid withdrawal symptoms. Mitragynine is the most abundant alkaloid in kratom and is a μ-opioid receptor agonist. 7-Hydroxymitragynine (7-HMG) is a mitragynine metabolite that is a more potent and efficacious opioid than its parent mitragynine. 7-HMG contributes to mitragynine’s antinociceptive effects in mice, but evidence suggests it may also have a higher abuse potential. This in vitro study demonstrates that 7-HMG is stable in rodent and monkey plasma but is unstable in human plasma. Surprisingly, in human plasma 7-HMG is converted to mitragynine pseudoindoxyl, an opioid that is even more potent than either mitragynine or 7-HMG. This novel metabolite is formed in human plasma to a much greater extent than in the preclinical species tested (mouse, rat, dog, and cynomolgus monkey) and due to its μ-opioid potency may substantially contribute to the pharmacology of kratom in humans to a greater extent than in other tested species.
Keywords: mitragynine, 7-hydroxymitragynine, Mitragyna speciosa, mitragynine pseudoindoxyl, plasma stability, metabolism
Mitragynine is the most abundant and pharmacologically well-characterized alkaloid (∼ 66% of total alkaloidal content)1,2 contained in the psychoactive substance kratom (Mitragyna speciosa Korth.). Mitragynine interacts with several receptors3 including weak activity as a μ-opioid receptor agonist.4 7-Hydroxymitragynine (7-HMG) is a major mitragynine metabolite that in rodent models significantly contributes to mitragynine’s analgesic and behavioral pharmacology.5 7-HMG is 22-fold more potent than mitragynine as a μ-opioid receptor agonist,6 and in the electrically stimulated guinea pig ileum twitch assay, it is 13- fold more potent than morphine (46-fold greater than mitragynine).7 In mice, mitragynine’s antinociceptive properties can mostly be largely accounted for by its conversion to 7-HMG,8 and while rats do not self-administer mitragynine, they will self-administer 7-HMG.9 If rodent and human mitragynine metabolism is similar, then these data would suggest that 7-HMG may have a significantly higher abuse potential than that of mitragynine. However, different species often show significant quantitative and/or qualitative differences in drug metabolism, so following mitragynine/kratom consumption human exposure to 7-HMG may differ from that of rodents. One example of the differences between species is how rodents predominantly metabolize morphine to its inactive morphine 3-O-glucuronide, while humans also form morphine 6-O-glucuronide, a metabolite that is a more potent opioid agonist than its parent morphine.10
The current study aims to increase understanding of human systemic exposure to opioidergic mitragynine metabolites by comparing the plasma stability of 7-HMG in human and model animal species and elucidating the structures and activity resulting from 7-HMG metabolism.
Result and Discussion
Plasma Stability of 7-HMG
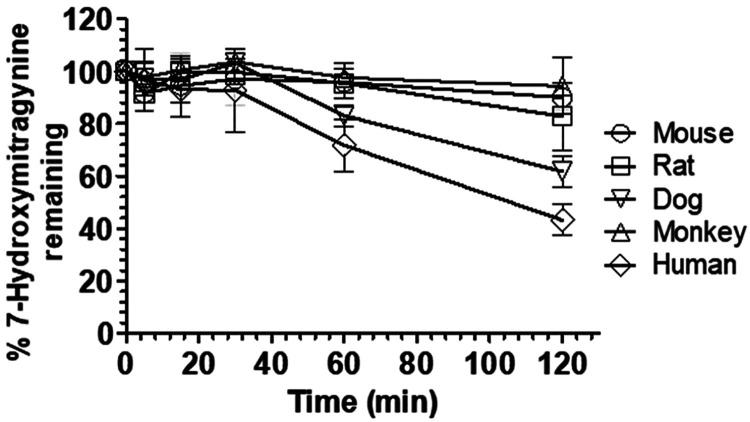
To determine which nonhuman animal model best predicts human exposure to 7-HMG, we evaluated the stability of 7-HMG in pooled mouse, rat, dog, monkey, and human plasma (Figure 1) under standard in vitro conditions (incubation of 1 μM 7-HMG in respective species plasma for 120 min at 37 °C). 7-HMG stability varied markedly across species, with high stability observed in mouse, rat, and monkey plasma (>80% 7-HMG remaining after 120 min incubation) and intermediate stability in dog plasma (>61% 7-HMG remaining after 120 min). 7-HMG was the least stable in human plasma (∼40% 7-HMG remaining after 120 min) with a half-life (T1/2) of 98.7 min as shown in Figure 1.
Figure 1.
Stability of 7-HMG in mouse, rat, dog, cynomolgus monkey, and human plasma. The data represented as a percentage of 7-HMG remaining versus time in cross-species plasma). All values are plotted as mean ± SD (n = 3).
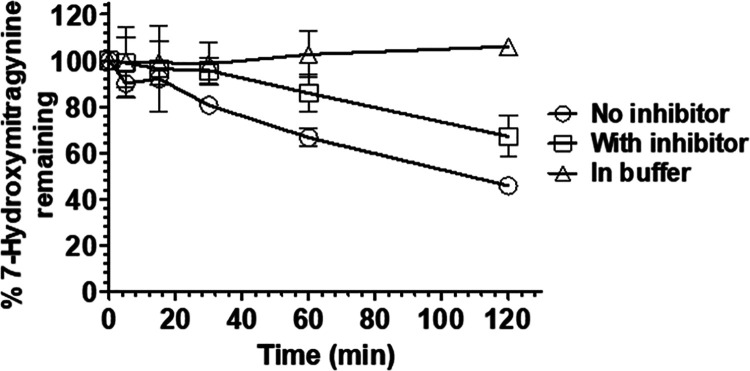
Plasma is known to catalyze numerous hydrolytic drug metabolizing reactions, particularly ester cleavage, such as the β-methoxyacrylate moiety found in 7-HMG. Therefore, we next evaluated the stability of 7-HMG in human plasma in the presence and absence of a protease inhibitor cocktail comprised of 4-(2-aminoethyl)benzenesulfonyl fluoride hydrochloride, aprotinin, bestatin, E-64, EDTA, leupeptin, and pepstatin A. Together this cocktail is sufficient to inhibit most serine proteases, cysteine proteases, metalloproteases, and calpain proteases. The chemical degradation of 7-HMG was also assessed in a 50 mM phosphate buffer (pH 7.4) as a control. In phosphate buffer alone, 7-HMG was stable, and in human plasma, the protease inhibitors somewhat reduced the rate of 7-HMG degradation (68% remaining at 120 min vs 46% in untreated human plasma; Figure 2). These data suggested that 7-HMG was being enzymatically degraded. However, the liquid chromatography high-resolution mass spectrometry (LC-HRMS) analysis failed to identify a plasma metabolite that corresponds to a 7-HMG hydrolysis product with an expected m/z of 401.2071 (Figure S1).
Figure 2.
Stability of 7-HMG in human plasma in the presence and absence of protease cocktail inhibitor and 50 mM phosphate buffer pH 7.4 control. The data are represented as a percentage of 7-HMG remaining versus time in either human plasma with or without protease cocktail inhibitor treatment and blank phosphate buffer pH 7.4. All values are plotted as mean ± SD (n = 3).
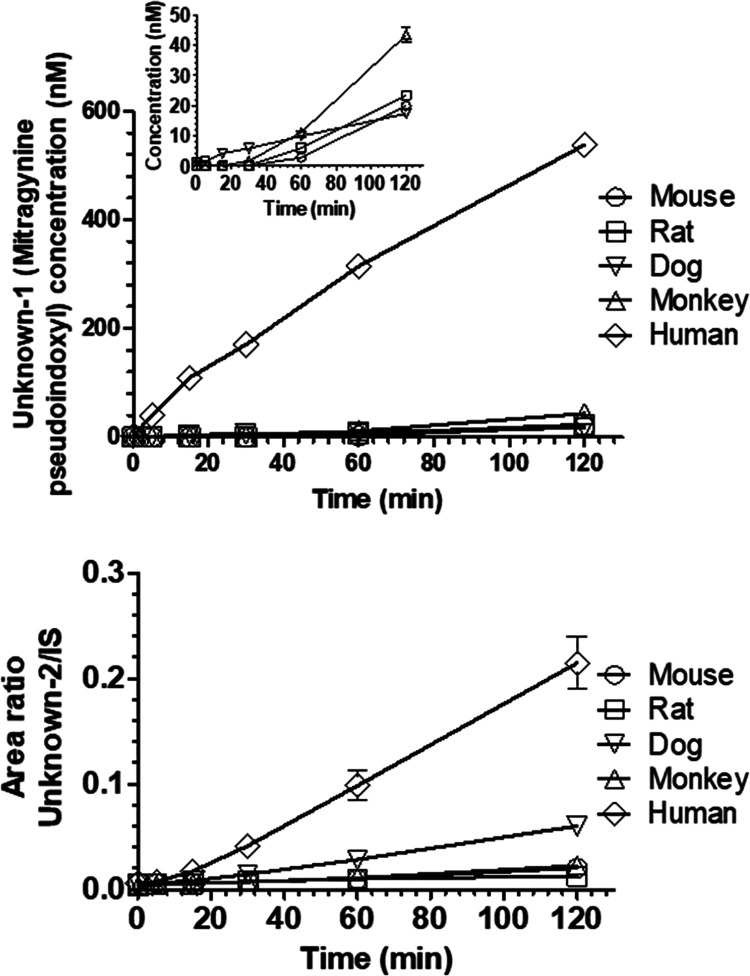
Following incubation of 7-HMG with human plasma, two unknown peaks (unknown-1 and -2) with the same nominal mass (m/z 415.2227) and same daughter ions peaks (m/z 190.0863 and 226.1438) were observed. The abundance of these metabolites increased in a manner proportional to the incubation time of 7-HMG in human plasma (Figure 3). Relative to the conversion in human plasma, the percent conversion values of 7-HMG to unknown-1 in mouse, rat, dog, and monkey plasma were 3.7 ± 0.0, 4.4 ± 0.3, 3.2 ± 0.0, and 8 ± 0.0% (mean ± SD), respectively.
Figure 3.
Unknown-1 (mitragynine pseudoindoxyl) and unknown-2 formation upon 7-HMG incubation with the mouse, rat, dog, cynomolgus monkey, and human plasma. Two unknown peaks (Unknown-1 and -2) were detected having mass transitions similar to that of 7-HMG. These are represented as the concentration of unknown-1 (mitragynine pseudoindoxyl, top) and ratio of unknown-2 to internal standard (IS) peak area (bottom) versus time. The inset in the top panel represents the concentration of unknown-1 (mitragynine pseudoindoxyl) in species other than human. All values are plotted as mean ± SD (n = 3).
Similarly, relative to the conversion in human plasma, the percent conversion values of 7-HMG to unknown-2 in mouse, rat, and monkey plasma were 9.5 ± 0.7, 5.9 ± 0.3, and 10.6 ± 1%, respectively, and 28.0 ± 2.1% in dog plasma. These data demonstrate that human plasma predominantly metabolized 7-HMG to unknown-1. The degradation of 7-HMG in dog plasma to unknown-1 and -2 explains somewhat the contribution toward the total degradation, while other degradation products in dog plasma remain to be identified. Likewise, whether the conversion of 7-HMG in human plasma was exclusively to unknown-1 and -2 remains to be identified. A similar MS2 fragmentation pattern (Figure S2) was observed for unknowns-1 and -2 and 7-HMG suggesting the same molecular structure, i.e., unknown--1 and -2 are structural isomers of 7-HMG.
Isolation, Purification, and Structure Elucidation of Unknown-1 Metabolite
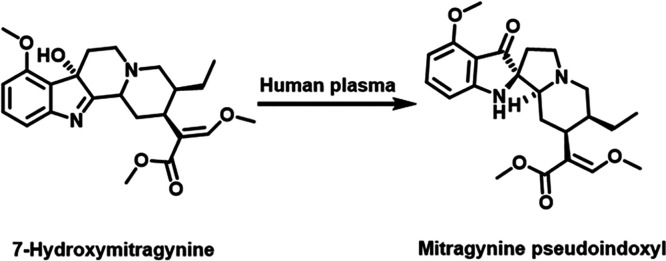
Multiple incubations of 7-HMG with human plasma were necessary to obtain a sufficient amount of unknown-1 for analysis using fractionation, preparative thin-layer chromatography (TLC), and high-performance liquid chromatography (HPLC) techniques. The relative abundance of unknown-2 was almost 10-fold lower than that of unknown-1 in human plasma, so it was not further evaluated due to the challenges in obtaining enough compound for further characterization. The 1H NMR spectrum of unknown-1 (Figure S3) showed an unusual aromatic first-order (large chemical shift separations and clear coupling pattern) spin-system AMX, where the aromatic protons at δH 6.42 ppm (d, J = 8.1 Hz, 1H) and 6.15 ppm (d, J = 8.0 Hz, 1H) were low-shifted in comparison with those of 7-HMG at δH 7.20 ppm (d, J = 7.8 Hz, 1H), and 6.73 ppm (d, J = 7.8 Hz, 1H). Additional 1H NMR signals of unknown-1 revealed the presence of one aromatic methoxy substituent, the typical signals for the β-methoxy acrylate group, and aliphatic protons, similar to those for 7-HMG (Figure S4), suggesting that the structural changes are located in the ring B of the corynanthe skeleton (Figure S4). The main differences between unknown-1 and 7-HMG were the absence of a quaternary carbon on 7-HMG at δC 81.0 ppm (Figure S5) and the presence of the highly deshielded carbon on unknown-1 at δC 199.5 ppm (Figure S6), suggesting conversion of the quaternary hydroxylated carbon to a carbonyl carbon. The presence of the quaternary carbon at δC 75.0 ppm is consistent with a ring B rearrangement, resulting in a spiro linkage between rings B and C of the corynanthe core (Figure S4). Analysis of the 1H–1H correlation spectroscopy (COSY), heteronuclear single quantum coherence spectroscopy (HSQC), heteronuclear multiple bond coherence (HMBC), and selective nuclear Overhauser effect (NOE) data (Figures S7–S10) confirmed the difference between ring B of unknown-1 and 7-HMG. Taken together, these data suggest a semipinacol–pinacolone rearrangement, similar to the biosynthetic pathway for oxindoles on corynanthe skeletons.11 By comparing the NMR data for unknown-1 with those of reported corynanthe alkaloids, we concluded that unknown-1 was likely to be mitragynine pseudoindoxyl. We then synthesized a mitragynine pseudoindoxyl standard using the Varadi et al. procedure12 and demonstrated that the standard and unknown-1 exhibited identical NMR spectra, UPLC (retention times), and HRMS data (Figures S11–S13). Therefore, we conclude that unknown-1 is mitragynine pseudoindoxyl (Figure 4). Furthermore, we used the synthetic standard of mitragynine pseudoindoxyl to quantify the percentage of mitragynine pseudoindoxyl formed via the plasma incubation of 7-HMG. The percentages of mitragynine pseudoindoxyl formed at the end of 120 min of incubation of 7-HMG in mouse, rat, dog, monkey, and human plasma were 2.0 ± 0.0, 2.4 ± 0.2, 1.7 ± 0.0, 4.3 ± 0.2, and 53.8 ± 1.6%, respectively. Also, we evaluated the stability of mitragynine pseudoindoxyl in the plasma of each tested species using experimental conditions similar to those used for 7-HMG. Mitragynine pseudoindoxyl was found to be stable in the plasma of each species, confirming that the conversion of 7-HMG to mitragynine pseudoindoxyl is irreversible (Figure S14).
Figure 4.
Novel metabolic conversion of 7-HMG to mitragynine pseudoindoxyl in human plasma. The incubation of 7-HMG in human plasma at 37 °C in 5% CO2 incubator at 100 rpm for 2 h resulted in the metabolic conversion of 7-HMG to mitragynine pseudoindoxyl.
Plasma enzymes are known to catalyze hydrolytic and chiral inversion reactions, but to the best of our knowledge semipinacol rearrangement reactions such as those observed here have not been previously described to occur in mammalian plasma. Furthermore, mitragynine pseudoindoxyl biosynthesis has not even been reported in Mitragyna speciosa,1 although Zarembo et al. have reported that some fungi can catalyze the semipinacol rearrangement of mitragynine into mitragynine pseudoindoxyl.13
The discovery that 7-HMG is converted in mitragynine pseudoindoxyl in human but not rodent plasma not only is unexpected but also may significantly alter our conclusions about the translatability of mitragynine findings using rodent models to the human condition.8,9,14 While 7-HMG is more potent and efficacious than mitragynine in its ability to activate μ-opioid receptors, mitragynine pseudoindoxyl is yet more potent and at least as efficacious as 7-HMG. In GTPγS functional assays of μ-opioid activation, mitragynine pseudoindoxyl has been reported to be 31-fold more potent than 7-HMG and 119-fold greater than mitragynine (EC50 1.7 ± 0.1 nM, Emax 84 ± 5%).12 In the electrically stimulated contraction of guinea pig ileum model, mitragynine pseudoindoxyl is 20-fold more potent than morphine at inhibiting contractions and 100-fold more potent than mitragynine.15 Previously, questions have been raised about whether the observed pharmacological activity in humans following kratom administration is due to mitragynine or mediated by the metabolite, 7-HMG. The systemic exposure of 7-HMG when calculated as percentage ratio of area under the curve of 7-HMG to mitragynine following mitragynine oral dosing of female beagle dogs16 was found to be 12.6 ± 1.6%. This suggests that a reasonable amount of 7-HMG is present in systemic circulation which could be further metabolized to mitragynine pseudoindoxyl in plasma; however, the absolute conversion rate of 7-HMG to mitragynine pseudoindoxyl is unknown. These findings raise the possibility that opioid-like effects may be mediated by a combination of mitragynine, 7-HMG, and mitragynine pseudoindoxyl. The abuse potential of mitragynine pseudoindoxyl has not been well characterized. However, 7-HMG has been reported to have high abuse potential, and given that mitragynine pseudoindoxyl is an even more potent opioid agonist, it is likely to also be highly abusable.9
The metabolism of mitragynine to 7-HMG in humans is known to be catalyzed by the enzyme CYP3A4,2,8 but the mechanism of this newly discovered conversion into mitragynine pseudoindoxyl in plasma is unknown. Additionally, microsomal metabolism of mitragynine did not result in the formation of mitragynine pseudoindoxyl in mouse, rat, dog, monkey, or human liver microsomes. It is possible that this plasma metabolism may be catalyzed by a genetically polymorphic enzyme or enzymes, which would potentially lead to substantial variability in an individual’s relative exposure to 7-HMG and mitragynine pseudoindoxyl following administration of a defined kratom dose. Given the difference in potency between these two metabolites, such differential exposure could result in population variability in the pharmacological and adverse effects of kratom consumption. In the present study, conversion of 7-HMG to mitragynine pseudoindoxyl has been identified, but the extent of systemic exposure will depend on the rates of 7-HMG biosynthesis and its further conversion to mitragynine pseudoindoxyl, which remain to be defined. Assuming similar rates of 7-HMG formation across species, our in vitro plasma stability data suggest that systemic human exposure to 7-HMG in humans may be lower than that in nonhuman species tested.
7-HMG is substantially metabolized by rat and monkey liver microsomes (T1/2 = 15 and 26 min, respectively, Figure S15), but metabolism in mouse, dog, and human liver microsomes was much slower (T1/2 > 60 min, Figure S15). These systems only establish the phase I metabolism of 7-HMG, but the data suggest that liver metabolism of 7-HMG in rats and monkeys is much greater than that in plasma. Furthermore, comparing the 7-HMG rate of metabolism in humans (plasma and liver microsomes), the plasma metabolism of 7-HMG could form substantial amounts of mitragynine pseudoindoxyl. Studies comparing the intrinsic clearance of 7-HMG in plasma and hepatocytes (phases I and II metabolic systems present) would help us to gauge the relevance of plasma metabolism of 7-HMG in the respective species and could be used to identify species having a metabolic profile similar to humans for further translational characterization.
Conclusion
This study has demonstrated the potential for human plasma to form mitragynine pseudoindoxyl. Further research is required to elucidate whether the same mechanisms also reside in other tissue types, potentially resulting in significant local exposure to this highly potent opioid.
Experimental Section
Plasma Stability of 7-HMG
The plasma stability of 7-HMG (synthesized in-house from mitragynine) was performed using heparinized pooled non-Swiss albino mouse (Innovative Research, Novi, MI), Sprague–Dawley rat (GeneTex, Irvine, CA), beagle dog (BioIVT, Hicksville, NY), cynomolgus monkey (BioIVT, Hicksville, NY), and human plasma (with sodium citrate as an anticoagulant; Innovative Research, Novi, MI). 7-HMG was spiked in a respective species preincubated plasma sample (n = 3) at a final concentration of 1 μM and kept in a 5% CO2 incubator shaker (100 rpm) maintained at 37 °C. The concentration of organic solvents was kept below 0.5% (v/v).17,18 The aliquots (25 μL) were withdrawn at 0, 5, 15, 30, 60, and 120 min. The reaction was quenched by mixing the aliquots with 4 volumes acetonitrile containing 10 ng/mL phenacetin as IS. The quenched samples were vortex-mixed for 5 min, and these samples were then filtered through 0.45 μm pore size 96-well filtration plate. The filtrate was then subjected to ultraperformance liquid chromatography tandem mass spectrometry analysis (UPLC-MS/MS). The sample analysis was performed using a Waters Acquity UPLC system coupled with Waters Xevo TQ-S micro mass spectrometer (Waters, Milford, MA) using MassLynx V4.1 software (Waters, Milford, MA). Chromatographic separations were performed on a UPLC column Acquity, BEH, C18, 1.7 μm, 2.1 × 50 mm; maintained at 50 °C temperature. The mobile phase consisted of solvent A (10 mM ammonium acetate with 1% acetic acid in water) and solvent B (acetonitrile) delivered at a flow rate of 0.35 mL/min with a linear gradient as follows: 25% B until 3 min, 25–40% B over 3.9 min, 40–70% B from 3.9 to 4 min, decreased to 25% B from 4 to 4.6 min, and maintained at 25% B until 5.5 min. The MS was operated in the positive electrospray mode at a capillary temperature of 450 °C, source temperature of 150 °C, capillary voltage of 0.5 kV, desolvation gas flow of 900 L/h, and cone gas flow of 50 L/h. The analysis was conducted in multiple reaction monitoring modes (MRM, m/z 415.2 > 190.1). Enalapril for rodents and procaine or disulfiram for nonrodent plasma were used as positive controls to assess the enzyme activity. The positive control results were comparable to published reports.19,20
Human Plasma Stability of 7-HMG with or without Protease Inhibitors
7-HMG at 1 μM was incubated with or without a protease inhibitor cocktail treatment and in 50 mM phosphate buffer pH 7.4 for 120 min at 37 °C in a CO2 incubator. Aliquots (25 μL) were withdrawn at 0, 5, 15, 30, 60, and 120 min and mixed with 4 volumes of acetonitrile containing IS. The quenched reaction mixtures were vortex-mixed for 5 min and then filtered through a 0.45 μm 96-well filtration plate. The filtrate was subjected to UPLC-MS/MS analysis as described above. Procaine was used as a positive control to assess esterases enzyme activity. The percentage of parent compound remaining was calculated as the percentage ratio of analyte peak to the IS peak area ratio at time t to that at 0 min.
Isolation, Purification, and Structure Elucidation of Unknown-1 Metabolite
To identify the chemical structure of the unknown-1 metabolite, multiple incubations of 7-HMG (1 mM) with undiluted human plasma (1 mL) for 24 h at 37 °C were performed to obtain enough amount of metabolite unknown-1. These incubation reactions were mixed and quenched with 4 volumes of acetonitrile. The metabolite unknown-1 was then isolated, purified using several chromatographic techniques, and chemical structure elucidation was performed by 1D and 2D NMR analyses. The details of isolation, purification, and structural elucidation of unknown-1 (mitragynine pseudoindoxyl) and its chemical synthesis are described in the Supporting Information.
Acknowledgments
The views and opinions expressed in this manuscript are those of the authors only and do not necessarily represent the views, official policy or position of the U.S. Department of Health and Human Services or any of its affiliated institutions or agencies. Dr. Hampson was substantially involved in UG3DA048353, consistent with his role as Scientific Officer. He had no substantial involvement in the other cited grants. C.L.-L. thanks the Program of young talent and regional impact-2018, MinCiencias-Colombia, for sponsoring her abroad stage.
Glossary
Abbreviations
- 7-HMG
7-hydroxymitragynine
- T1/2
half-life
- LC-HRMS
liquid chromatography high-resolution mass spectrometry
- TLC
thin-layer chromatography
- HPLC
high-performance liquid chromatography
- CYP3A4
cytochrome P450 3A4
- NMR
nuclear magnetic resonance
- MRM
multiple reaction monitoring mode.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00075.
Experimental procedures and HPLC, NMR, and HRMS characterization data for mitragynine pseudoindoxyl (PDF)
This study was supported by UG3 DA048353 and R01 DA047855 grants from the National Institute on Drug Abuse and the University of Florida Clinical and Translational Science Institute, which is supported in part by the NIH National Center for Advancing Translational Sciences under award number UL1TR001427.
The authors declare no competing financial interest.
Supplementary Material
References
- Hassan Z.; Muzaimi M.; Navaratnam V.; Yusoff N. H.; Suhaimi F. W.; Vadivelu R.; Vicknasingam B. K.; Amato D.; von Horsten S.; Ismail N. I.; et al. (2013) From Kratom to mitragynine and its derivatives: physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 37 (2), 138. 10.1016/j.neubiorev.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Kamble S. H.; Sharma A.; King T. I.; Leon F.; McCurdy C. R.; Avery B. A. (2019) Metabolite profiling and identification of enzymes responsible for the metabolism of mitragynine, the major alkaloid of Mitragyna speciosa (kratom). Xenobiotica 49 (11), 1279. 10.1080/00498254.2018.1552819. [DOI] [PubMed] [Google Scholar]
- Boyer E. W.; Babu K. M.; Adkins J. E.; McCurdy C. R.; Halpern J. H. (2008) Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction 103 (6), 1048. 10.1111/j.1360-0443.2008.02209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel A. C.; Gassaway M. M.; Kapoor A.; Varadi A.; Majumdar S.; Filizola M.; Javitch J. A.; Sames D. (2016) Synthetic and Receptor Signaling Explorations of the Mitragyna Alkaloids: Mitragynine as an Atypical Molecular Framework for Opioid Receptor Modulators. J. Am. Chem. Soc. 138 (21), 6754. 10.1021/jacs.6b00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck W. C.; Jivan J. K.; Andurkar S. V. (2012) Pharmacology of kratom: an emerging botanical agent with stimulant, analgesic and opioid-like effects. J. Am. Osteopath. Assoc. 112 (12), 792–799. [PubMed] [Google Scholar]
- Obeng S.; Kamble S. H.; Reeves M. E.; Restrepo L. F.; Patel A.; Behnke M.; Chear N. J.; Ramanathan S.; Sharma A.; Leon F.; et al. (2020) Investigation of the Adrenergic and Opioid Binding Affinities, Metabolic Stability, Plasma Protein Binding Properties, and Functional Effects of Selected Indole-Based Kratom Alkaloids. J. Med. Chem. 63 (1), 433. 10.1021/acs.jmedchem.9b01465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto K.; Horie S.; Ishikawa H.; Takayama H.; Aimi N.; Ponglux D.; Watanabe K. (2004) Antinociceptive effect of 7-hydroxymitragynine in mice: Discovery of an orally active opioid analgesic from the Thai medicinal herb Mitragyna speciosa. Life Sci. 74 (17), 2143. 10.1016/j.lfs.2003.09.054. [DOI] [PubMed] [Google Scholar]
- Kruegel A. C.; Uprety R.; Grinnell S. G.; Langreck C.; Pekarskaya E. A.; Le Rouzic V.; Ansonoff M.; Gassaway M. M.; Pintar J. E.; Pasternak G. W.; et al. (2019) 7-Hydroxymitragynine is an active metabolite of mitragynine and a key mediator of its analgesic effects. ACS Cent. Sci. 5 (6), 992. 10.1021/acscentsci.9b00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemby S. E.; McIntosh S.; Leon F.; Cutler S. J.; McCurdy C. R. (2019) Abuse liability and therapeutic potential of the Mitragyna speciosa (kratom) alkaloids mitragynine and 7-hydroxymitragynine. Addict Biol. 24 (5), 874. 10.1111/adb.12639. [DOI] [PubMed] [Google Scholar]
- Fura A. (2006) Role of pharmacologically active metabolites in drug discovery and development. Drug Discovery Today 11 (3–4), 133. 10.1016/S1359-6446(05)03681-0. [DOI] [PubMed] [Google Scholar]
- Lopes A. A.; Chioca B.; Musquiari B.; Crevelin E. J.; Franca S. C.; Fernandes da Silva M.; Pereira A. M. S. (2019) Unnatural spirocyclic oxindole alkaloids biosynthesis in Uncaria guianensis. Sci. Rep. 9 (1), 11349. 10.1038/s41598-019-47706-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varadi A.; Marrone G. F.; Palmer T. C.; Narayan A.; Szabo M. R.; Le Rouzic V.; Grinnell S. G.; Subrath J. J.; Warner E.; Kalra S.; et al. (2016) Mitragynine/corynantheidine pseudoindoxyls as opioid analgesics with mu agonism and delta antagonism, which do not recruit beta-arrestin-2. J. Med. Chem. 59 (18), 8381. 10.1021/acs.jmedchem.6b00748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarembo J. E.; Douglas B.; Valenta J.; Weisbach J. A. (1974) Metabolites of mitragynine. J. Pharm. Sci. 63 (9), 1407. 10.1002/jps.2600630916. [DOI] [PubMed] [Google Scholar]
- Yue K.; Kopajtic T. A.; Katz J. L. (2018) Abuse liability of mitragynine assessed with a self-administration procedure in rats. Psychopharmacology (Berl) 235 (10), 2823. 10.1007/s00213-018-4974-9. [DOI] [PubMed] [Google Scholar]
- Yamamoto L. T.; Horie S.; Takayama H.; Aimi N.; Sakai S.; Yano S.; Shan J.; Pang P. K.; Ponglux D.; Watanabe K. (1999) Opioid receptor agonistic characteristics of mitragynine pseudoindoxyl in comparison with mitragynine derived from Thai medicinal plant Mitragyna speciosa. Gen. Pharmacol. 33 (1), 73. 10.1016/S0306-3623(98)00265-1. [DOI] [PubMed] [Google Scholar]
- Maxwell E. A.; King T. I.; Kamble S. H.; Raju K. S. R.; Berthold E. C.; Leon F.; Avery B. A.; McMahon L. R.; McCurdy C. R.; Sharma A. (2020) Pharmacokinetics and safety of mitragynine in beagle dogs. Planta Med. 5475. 10.1055/a-1212-5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil P. G.; Kamble S. H.; Shah T. S.; Iyer K. R. (2015) Effect of Water Miscible Organic Solvents on p-Nitrophenol hydroxylase (CYP2E1) activity in rat liver microsomes. Indian J. Pharm. Sci. 77 (3), 283. 10.4103/0250-474X.159613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah T. S.; Kambl S. H.; Patil P. G.; Iyer K. R. (2015) Effect of water-miscible organic solvents on cyp450-mediated metoprolol and imipramine metabolism in rat liver microsomes. Indian J. Pharm. Sci. 77 (4), 382. 10.4103/0250-474X.164783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad N.; Samiulla D. S.; Teh B. P.; Zainol M.; Zolkifli N. A.; Muhammad A.; Matom E.; Zulkapli A.; Abdullah N. R.; Ismail Z. (2018) Bioavailability of eurycomanone in its pure form and in a standardised eurycoma longifolia water extract. Pharmaceutics 10 (3), 90. 10.3390/pharmaceutics10030090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman D. A. (2003) Determination of the stability of drugs in plasma. Curr. Protoc Pharmacol 19, 7.6.1. 10.1002/0471141755.ph0706s19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.