Almost 70,000 adult lung transplant procedures have been reported to the International Society for Heart and Lung Transplantation (ISHLT) International Thoracic Organ Transplant (TTX) Registry since its inception, each one the result of the selfless kindness of a grieving donor family. With each year’s report, we provide more detailed analyses on a particular focus theme important to recipient outcomes. Since 2013, these have been donor and recipient age, retransplantation, early graft failure, indication for transplant, allograft ischemic time, multiorgan transplantation, and donor and recipient size matching.1–7 The goal of this year’s report is to focus entirely on changes in donor factors over the past 3 decades and to identify important donor characteristics and transplant processes that may influence post-transplant outcomes. Because of small numbers, heart–lung donor characteristics and transplant outcomes have not been included. This 37th annual adult lung transplant report is based on data submitted to the ISHLT TTX Registry on 67,493 adult recipients of deceased donor transplants between January 1, 1992 and June 30, 2018. In response to a changing regulatory environment, the ISHLT TTX Registry is undergoing an update in data acquisition, and the patient cohort examined in this report is therefore derived from the same data source or datasets as that examined in the 2019 annual reports.2,8–10 We refer the reader to the 2019 report for the detailed description of the baseline characteristics of the cohort and additional core analyses not directly related to the focus explored in this year’s report.
Data collection, conventions, and statistical methods
National and multinational transplant collectives and individual transplant centers submit data to the ISHLT TTX Registry. Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 centers that perform combined heart–lung transplants have reported data to the ISHLT TTX Registry.2,8–10 It is estimated that data submitted represents approximately three quarters of the worldwide thoracic transplant activity. This report references specific online e-slides when particular data are discussed but not shown because of space limitations; e-slide numbers refer to the online adult lung transplant slides, shortened to L(a), and available at https://ishltregistries.org/registries/slides.asp. The ISHLT web site also contains slide sets for previous annual reports.
The TTX Registry website (https://ishlt.org/research-data/registries/ttx-registry) provides detailed spreadsheets of the data elements collected in the Registry. The Registry requires submission of core donor, recipient, and transplant procedure variables at baseline (i.e., around the time of transplantation) and at annual follow-up, and these variables therefore have low rates of missingness. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that depend on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytic conventions
Unless otherwise specified, analyses of lung transplants do not include combined heart–lung transplant data. The Registry does not capture the exact occurrence date for most secondary outcomes (e.g., bronchiolitis obliterans syndrome [BOS]), but it does capture the window of occurrence (e.g., the event occurred between the first and the second year annual follow-up visits). For the report’s analyses, we use the midpoint between the annual follow-ups as a surrogate for the event date. Because deceased subjects no longer contribute to the secondary outcomes, to reduce the potential of underestimating event rates or other outcomes, we restrict some analyses to include only surviving recipients. For time-to-event analyses, we censor the follow-up of recipients who have not yet experienced the event at the most recent annual follow-up or the time of retransplantation. We truncate time-to-event graphs (e.g., survival graphs) when the number of individuals at risk becomes <10. Previous Registry report themes provide more details regarding specific donor and recipient characteristics and outcomes.1–7
Focus theme: Deceased donor characteristics
The ongoing disparity between the demand for transplantation for potential recipients with end-stage lung disease and the supply of donor organs has, over time, altered perception of what constitutes an acceptable organ and, hence, the characteristics of the accepted donor. However, aside from a more aggressive stance on organ acceptance, other factors such as demographic changes (aging population and declining smoking rates) have also impacted donor characteristics over the past 30 years. Against this background, we examine donor characteristics by year of transplantation.
Adult lung transplant donor characteristics stratified by era (1992–2000, 2001–2009, and 2010–2018) are presented in Table 1. The number of transplants performed has gradually increased over time, with the proportion of procedures performed in North America falling relative to the rest of the world. Notably, overall median donor age has steadily increased from 30 to 40 years and in Europe is now 51 years (Figure 1a, eSlide L(a) 6). The aging of the donor population over time likely reflects an increased willingness to accept older donor lungs in the setting of a limited donor pool, general population aging, and changes in the mode of death prompting consideration of organ donation, as discussed in more detail hereafter. In line with increasing donor age, the prevalence of cytomegalovirus and Epstein-Barr virus seropositivity have both increased slightly over time, from 55.3% to 61.6% and 91.6% to 93.7%, respectively (Table 1). The proportion of lung donors who are female has increased from 38.1% to 43.8% (Table 1, eSlide L(a) 7), and median donor height has fallen slightly in line with these changing demographics. Median lung donor body mass index has increased in all geographic regions, with a global increase from 23.3 kg/m2 in the 1992–2000 era to 25.1 kg/m2 in the 2010–2018 era (Figure 1b, eSlide L(a) 6), reflecting the overall rising prevalence of obesity in the general population.11
Table 1.
Adult Lung Transplant Donor Characteristics by Era (Transplants: January 1992–June 2018)
| Characteristic | Jan 1992–Dec 2000 (N = 11,796) |
Jan 2001–Dec 2009 (N = 21,806) |
Jan 2010–June 2018 (N = 33,891) |
p-value |
|---|---|---|---|---|
| Geographic location | <0.0001 | |||
| Europe | 3,751 (31.8)% | 7,818 (35.9)% | 12,414 (36.6)% | |
| North America | 7,324 (62.1)% | 12,545 (57.5)% | 18,594 (54.9)% | |
| Other | 721 (6.1)% | 1,443 (6.6)% | 2,883 (8.5)% | |
| Age (years) | 30 (15–54) | 37 (16–60) | 40 (17–65) | <0.0001 |
| Male | 61.9% | 56.9% | 56.2% | <0.0001 |
| Height (cm) | 173.0 (157.0–188.0) | 172.7 (157.0–188.0) | 172.0 (155.0–188.0) | <0.0001 |
| BMI (kg/m2) | 23.3 (18.2–31.0)a | 24.2 (18.8–33.1) | 25.1 (19.2–35.5) | <0.0001 |
| Blood type | 0.1946 | |||
| A | 39.1% | 38.8% | 38.5% | |
| AB | 2.3% | 2.4% | 2.3% | |
| B | 9.9% | 10.0% | 10.6% | |
| O | 48.6% | 48.7% | 48.6% | |
| Cause of death | ||||
| Anoxia | 3.5% | 7.9% | 19.8% | <0.0001 |
| CVA/stroke | 38.4% | 44.7% | 41.2% | |
| Head trauma | 49.7% | 44.5% | 36.0% | |
| Other | 8.3% | 2.9% | 3.0% | |
| CMV antibody positive | 55.3% | 60.4% | 61.6% | <0.0001 |
| EBV antibody positive | — | 91.6%b | 93.7% | <0.0001 |
| Hep B antibody positive | 2.4% | 2.5% | 2.6% | 0.6759 |
| Hep C antibody positive | 0.6% | 0.1% | 0.6% | <0.0001 |
| Smoking history | 31.7% | 20.7% | 12.1% | <0.0001 |
| Alcohol use | — | 12.8%b | 14.6% | 0.0001 |
| Cocaine use | — | 10.8% | 14.3% | <0.0001 |
| Other drugs use | — | 28.1% | 40.7% | <0.0001 |
| Diabetes | 2.3%a | 4.6% | 7.2% | <0.0001 |
| Hypertension | 12.4%a | 18.4% | 24.0% | <0.0001 |
| PO2 (mm Hg) | — | 424.0 (115.0–578.4)c | 409.0 (109.0–563.0) | <0.0001 |
Abbreviations: BMI, body mass index; CVA, cerebrovascular accident; CMV, cytomegalovirus; EBV, Epstein-Barr virus; Hep B, hepatitis B; Hep C, hepatitis C; PO2, partial pressure of oxygen.
Summary statistics excluded transplants with missing data.
Continuous factors are expressed as median (5th–95th percentiles).
Comparisons for categorical variables were made using the chi-square statistic.
Comparisons for continuous variables were made using the Wilcoxon test.
Based on April 1994–December 2000 transplants.
Based on April 2006–December 2009 transplants.
Based on July 2004–December 2009 transplants.
Figure 1.
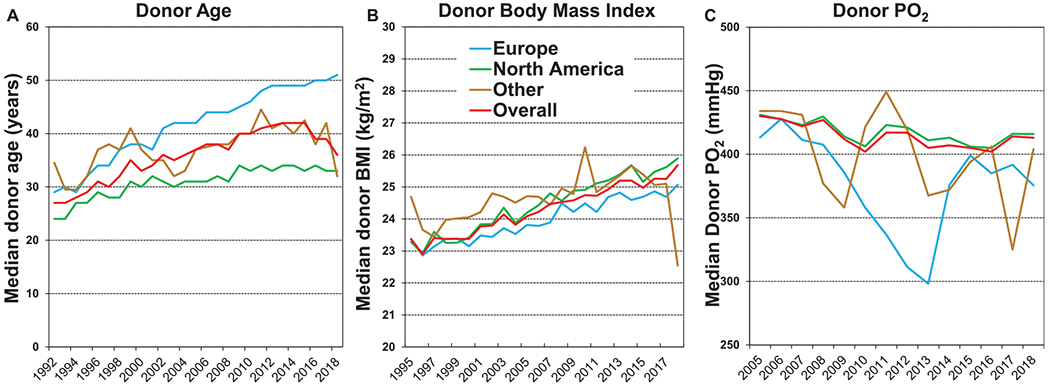
Donor age, body mass index, and PO2 by year and region (transplants from eras as shown). (a) Age. (b) Body mass index. (c) PO2.
The median donor arterial partial pressure of oxygen (PO2) has declined slightly over the past 2 decades (data is not available for the 1992–2000 era) from 424 to 409 mm Hg (p < 0.0001, Table 1 and Figure 1c). This trend probably reflects both an increased appetite for perceived risk when accepting donor organs in the face of a limited donor pool and an increasing recognition that donor PO2 does not have a significant impact on post-transplant outcomes, at least within the range where organ acceptance is common (≥250 mm Hg) (see survival analyses hereafter). Notably, in previous ISHLT TTX Registry Reports, donor PO2 has not appeared in the list of variables that are independently associated with either short or long-term survival.2,3
The distribution of donors by blood type has been quite stable, with approximately 39% of donors being blood type A, 49% blood type O, 10% blood type B, and the remaining 2% blood type AB (Table 1). Donor cause of death varies by geographic region and has changed significantly over the past 3 decades (Figure 2), as also noted in the 2020 adult heart report.12 In Europe, donors have predominantly died from cerebrovascular accident/stroke, whereas the proportion of donors who have died of head trauma has declined. These observations likely reflect fewer overall deaths related to motor vehicle accidents and gun violence. At the same time, the aging donor population seen particularly in Europe is more likely to succumb because of neurological events. In contrast, in North America, head trauma remains the most common cause of death (Figure 2, eSlide L(a) 8), whereas deaths owing to anoxia have increased in number and proportion, likely because of the opioid epidemic and drug overdose deaths.
Figure 2.
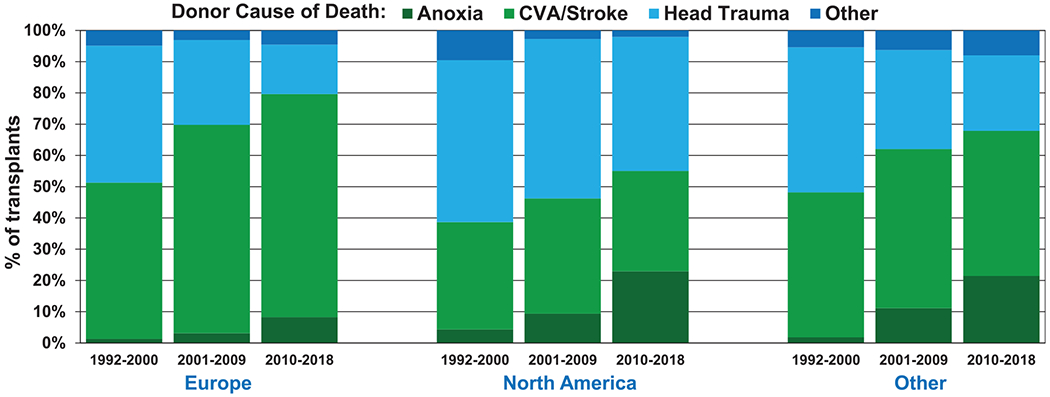
Donor cause of death by era and region (transplants: January 1992–June 2018). CVA, cerebrovascular accident.
Interesting trends are apparent on examining changes in donor substance use over time (Table 1). The proportion of donors with a >20 pack year history of cigarette smoking has declined dramatically from almost 35% to 10% (Figure 3a, eSlide L(a) 9) since the 1990s. The proportion of adult lung donors with a history of alcohol use (≥2 standard alcoholic drinks per day) has increased slightly (Figure 3b). The proportion of donors with a history of cocaine use has doubled from under 10% to over 20% (Figure 3c), and there has been a dramatic and troubling increase in the proportion of donors who have ever abused or been dependent upon non-intravenous street drugs (crack cocaine; marijuana; and prescription narcotics, sedatives, hypnotics, and stimulants) from 20% to almost 50% (Figure 3d). Unfortunately, it is not possible to examine changes in donor substance use by geographic region, because of constraints in data collection and reporting.
Figure 3.
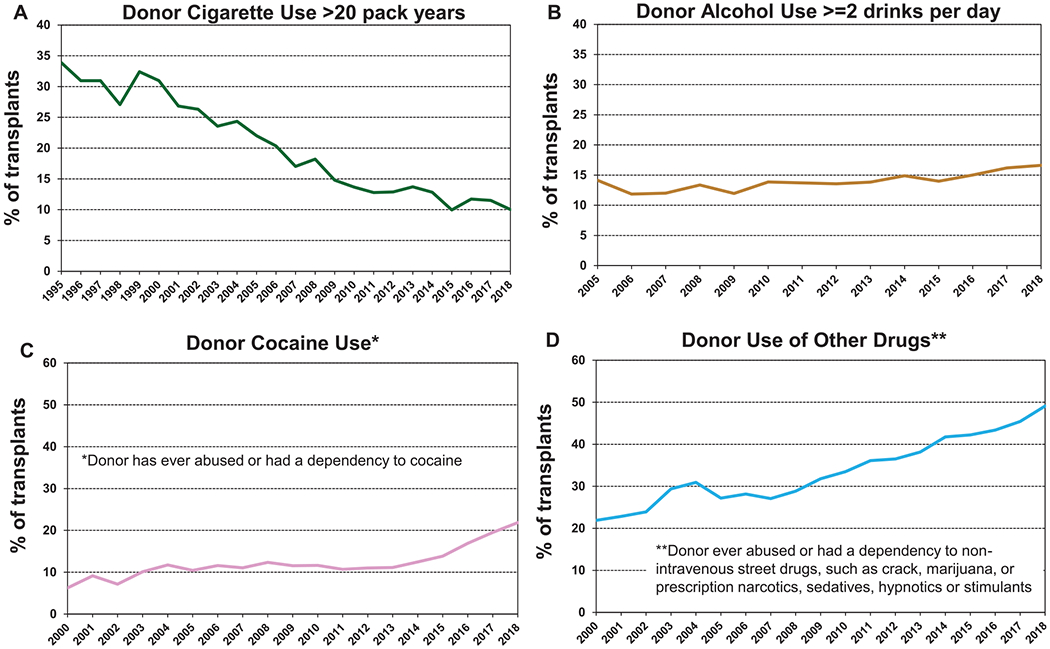
Donor substance use by year (transplants from eras as shown). (a) Smoking. (b) Alcohol. (c) Cocaine. (d) Other.
Important changes in adult lung donor comorbidities (Table 1) have occurred over the past 3 decades, with the potential for significant, and perhaps unexpected, impact on post-transplant outcomes (see survival analyses hereafter). The proportion of adult lung donors with a history of diabetes has tripled from 2% to 3% to almost 10% (Figure 4a), and the proportion with a history of systemic hypertension has increased from 10% to over 25% (Figure 4b). Multiple factors likely underlie these changes, including the aging donor pool and rising obesity rates.
Figure 4.

Donor diabetes and hypertension by year (transplants: January 1995–June 2018). (a) Diabetes. (b) Hypertension.
Survival analyses
We next examined associations between donor risk factors and post-transplant survival. We present a number of stratified unadjusted analyses. Multivariable analyses were not performed; hence, it should be kept in mind that these analyses have not been adjusted for potential confounders that may account for some of the reported differences. All 5-year survival analyses are conditional on survival to 1 year. The impact of ischemic time on outcomes was the focus of the 2017 Report3 and so has not received heavy focus in this Report.
Donor age
Older donor age was weakly associated with lower 12-month post-transplant survival, as has been previously noted.2,4 In unadjusted Kaplan–Meier analyses, survival was lower in recipients of organs from donors aged ≥50 years at 12 months (Figure 5a, eSlide L(a) 13). However, when survival at 5 years conditional on survival to 1 year was considered, there was no difference (Figure 5b, eSlide L(a) 26), suggesting that the effect of donor age on post-transplant survival is transient. The impact of older donor age on survival to 12 months post-transplant was consistent across geographic regions (eSlide L(a) 15) and a range of recipient ages (Figure 6, eSlide L(a) 14), although the differences did not reach significance in recipients aged 18 to 39 years. Ischemic time did not modulate the effect of older donor age on survival at 12 months (eSlide L(a) 21). This interaction was examined in depth in the 2017 Report.3 In summary, this more focused analysis is consistent with findings from previous reports suggesting a relatively weak and short-lived impact of donor age on recipient survival.
Figure 5.

Kaplan–Meier survival for adult lung transplant recipients by donor age (transplants: January 2000–June 2017 and January 2000–June 2013, respectively). (a) 12 months. (b) 5 years conditional on survival to 1 year. NS, not significant.
Figure 6.
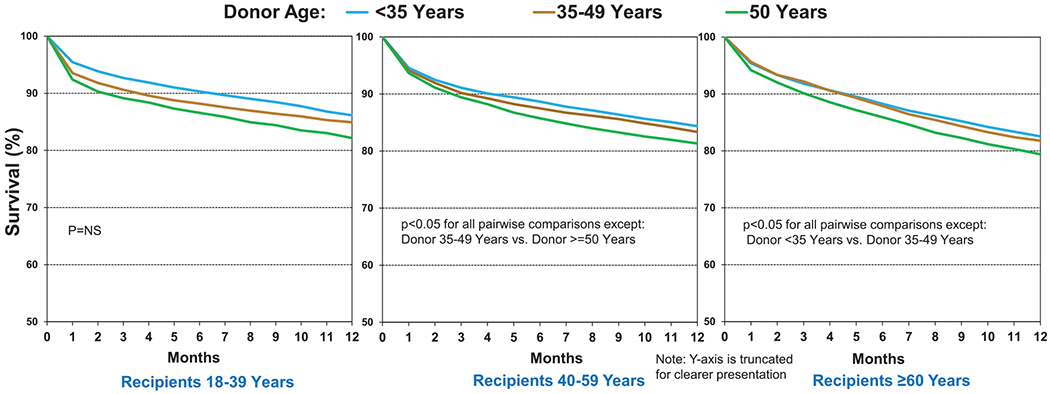
Kaplan–Meier survival within 12 months for adult lung transplant recipients by donor and recipient age (transplants: January 2000–June 2017). NS, not significant.
Donor cause of death and PO2
Donor cause of death is not a core data element in the ISHLT Registry and so is variably reported by contributing transplant centers. Although this limitation must be kept in mind, broad trends in the events leading to death and subsequent lung donation are evident from the available data. Although transplant recipients who received organs from donors with a history of cerebrovascular accident experienced the lowest 12-month survival, and those with a history of donor anoxia or trauma the highest, none of these differences were statistically significant (Figure 7a, eSlide L(a) 16). Any trends in survival may well relate to the older age of donors who died of cerebrovascular accident. Furthermore, there was no clinically meaningful impact of donor cause of death on 5-year survival, conditional on survival to 1 year (Figure 7b, eSlide L(a) 29). One of the most important pieces of information that is acquired during the process of donor assessment is the PO2 measured while 100% oxygen is administered to the potential lung donor. Despite its perceived importance, the donor PO2 was not associated with transplant survival at 1 and 5 years (Figure 8a and b, eSlides L(a) 16 and 29), as noted in previous reports,1,2 and as also noted in the 2020 pediatric lung report.13 Notably, the number of transplants where the donor PO2 was reported to the Registry as <250 mm Hg was 6,133, approximately 25% of the total number for whom PO2 was available. This large proportion suggests that some of the PO2 values reported were not obtained on 100% fraction of inspired oxygen. Nevertheless, there was no clinically significant association between donor PO2 and post-transplant survival, even if the donors with PO2 < 250 mm Hg were to be disregarded.
Figure 7.
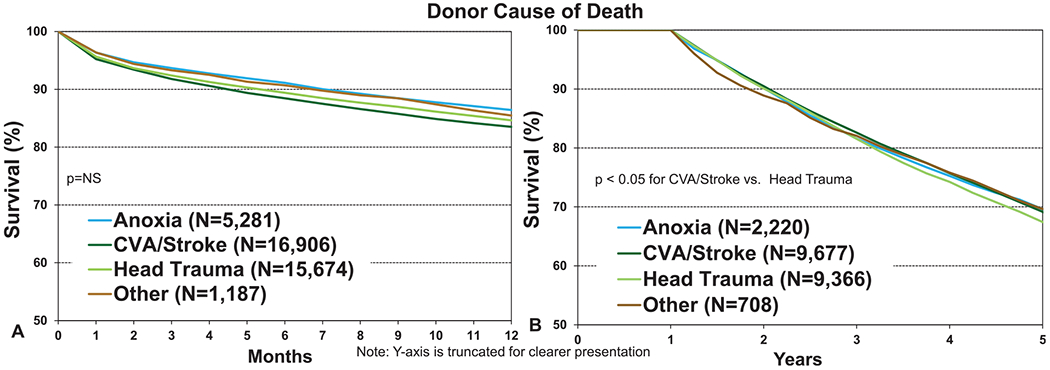
Kaplan–Meier survival for adult lung transplant recipients by donor cause of death (transplants: January 2000–June 2017 and January 2000–June 2013, respectively). (a) 12 months. (b) 5 years conditional on survival to 1 year. CVA, cerebrovascular accident; NS, not significant.
Figure 8.
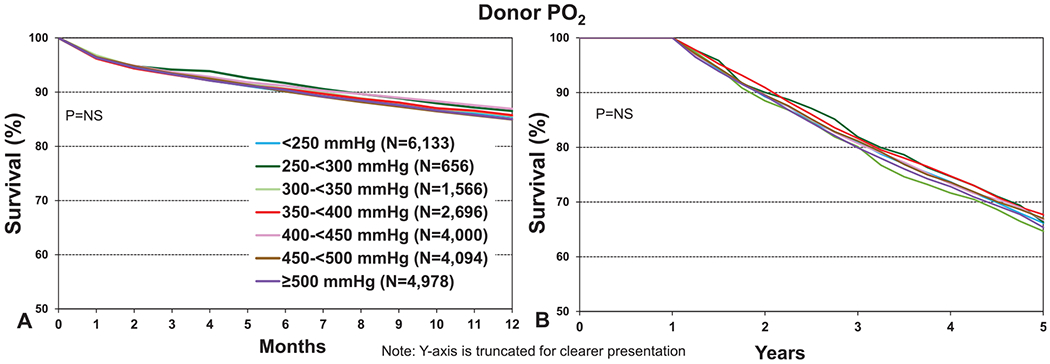
Kaplan–Meier survival for adult lung transplant recipients by donor PO2 (transplants: January 2005–June 2017 and January 2005–June 2013, respectively). (a) 12 months. (b) 5 years conditional on survival to 1 year. NS, not significant.
Donor substance abuse
We next examined recipient survival by donor substance abuse, with 12-month survival data shown in Figure 9 and 5-year survival conditional on survival to 1 year in Figure 10. As shown in Figure 9a (eSlide L(a) 17), receipt of lungs from a donor with a history of smoking (more than 20 pack years) was associated with 3% lower 12-month survival and a 1.5% lower 5-year conditional survival (Figure 10a, eSlide L(a) 30). A history of donor alcohol use (defined as 2 or more alcoholic drinks per day) was not associated with survival at 12 months (Figure 9b, eSlide L(a) 17) or at 5 years (Figure 10b, eSlide L(a) 30). Donor cocaine use was associated with slightly lower 12-month (Figure 9c, eSlide L(a) 18) and 5-year (Figure 10c, eSlide L(a) 31) survival, although the absolute differences were very small (85% vs 85.2% and 57.5% vs 59% at 1 and 5 years, respectively). Use of other drugs (non-intravenous street drugs such as crack; marijuana; or prescription narcotics, sedatives, hypnotics, or stimulants) was not associated with a difference in survival at 12 months (Figure 9d, eSlide L(a)18); however, an impact on longer-term survival was apparent. Five-year survival, conditional on survival to 1 year, was significantly different between recipients who received an organ from a donor with a history of such substance abuse compared with those without such history (57.3% vs 59.7% respectively, p = 0.0002, Figure 10d, eSlide L(a) 31), although again the absolute differences were very small. This differential impact on short- vs longer-term survival may have several explanations. For example, if there is a genuine detrimental impact on transplant outcome where there is a history of this kind of substance abuse in the donor, the impact on early outcomes may be ameliorated by the younger age and lack of other medical comorbidities of donors with this social history.
Figure 9.
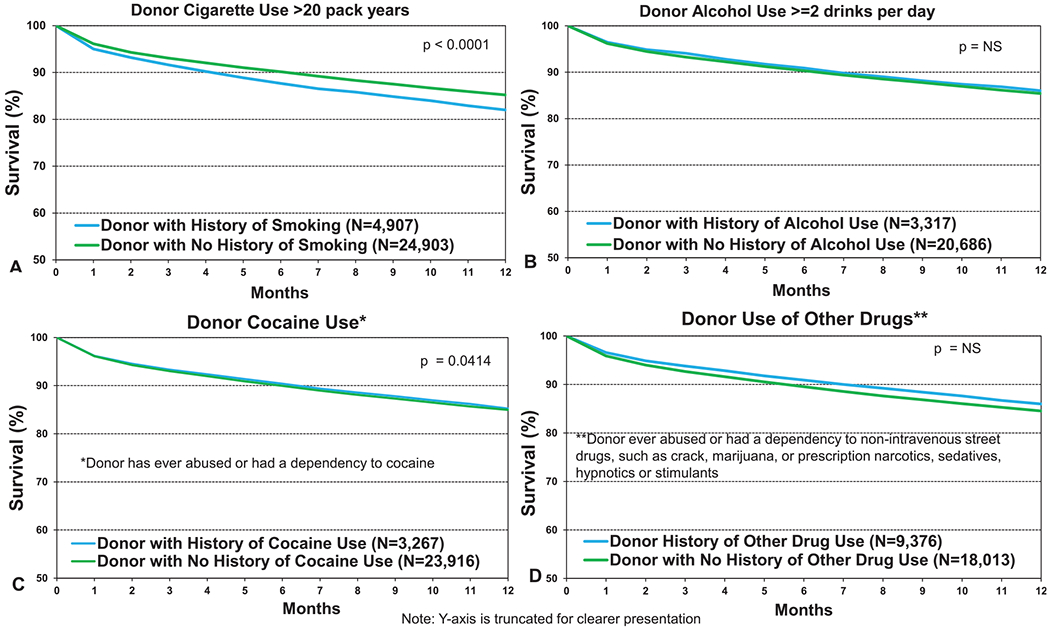
Kaplan–Meier survival within 12 months for adult lung transplant recipients by donor substance use (transplants from eras as shown). (a) Smoking. (b) Alcohol. (c) Cocaine. (d) Other. NS, not significant.
Figure 10.
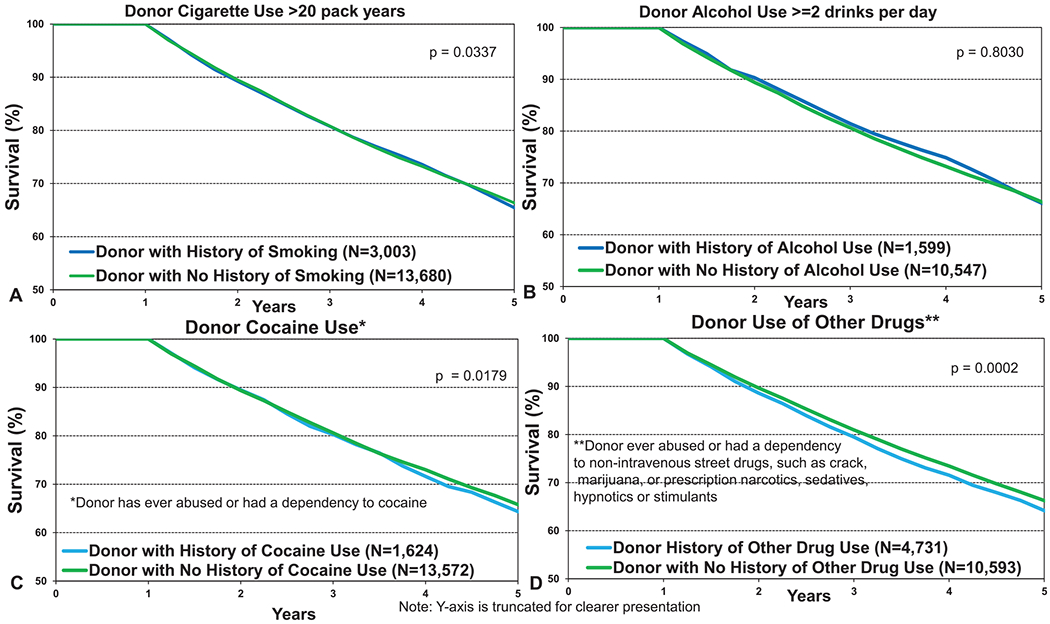
Kaplan–Meier survival within 5 years conditional on survival to 1 year for adult lung transplant recipients by donor substance use (transplants from eras as shown). (a) Smoking. (b) Alcohol. (c) Cocaine. (d) Other.
Donor diabetes and hypertension
Some of the most interesting findings were seen in recipients of organs from donors with a history of systemic hypertension and diabetes. Although these donor factors are known to impact cardiac transplant survival,12 an effect on lung transplant survival is not well recognized and may not be anticipated. Recipients of organs from a donor with a history of diabetes had significantly lower 12-month and 5-year conditional survival (Figure 11a and b, eSlides L(a) 19 and 32). The numerical differences (81.3% vs 85%, p < 0.0001 at 12-month and 63% vs 67%, p = 0.0014 at 5 years) were as large as the differences noted for donors with a history of smoking and older donors at 12 months, mandating further investigation. Although multivariable analyses were not performed for this report, and further analyses of the impact of donor diabetes were limited by the relatively small number of procedures (eSlides L(a) 24 and 37), this association has been previously noted in adjusted analyses of post-transplant survival in adult lung transplant recipients.1,2 This suggests that the impact on post-transplant survival is independent of other factors recorded in the Registry. The adverse effect of donor hypertension was also significant at both 1 and 5 years; however, the differences were not as large (Figure 12a and b, eSlides L(a) 19 and 32). Donor hypertension was also found to be independently associated with post-transplant survival in the multivariable analyses included in previous reports.2 Together, these data suggest that these 2 donor comorbidities, both part of the metabolic syndrome, have a long-lasting impact on survival in adult lung transplant recipients. The biological mechanism(s) underlying what appears to be a robust association are unclear, but the implications are intriguing. Although diabetes and hypertension are very well established risk factors for cardiac disease, their impact on lung disease and by inference the cells (pulmonary epithelium, stroma, and endothelium) that would be transferred enduringly to the recipient during transplantation has not been well studied. However, recent studies have identified an association between diabetes and restrictive lung function impairment14 as well as activation of profibrotic signaling pathways.15 These findings reinforce the associations noted in previous reports and highlight a rich area for further investigation.
Figure 11.
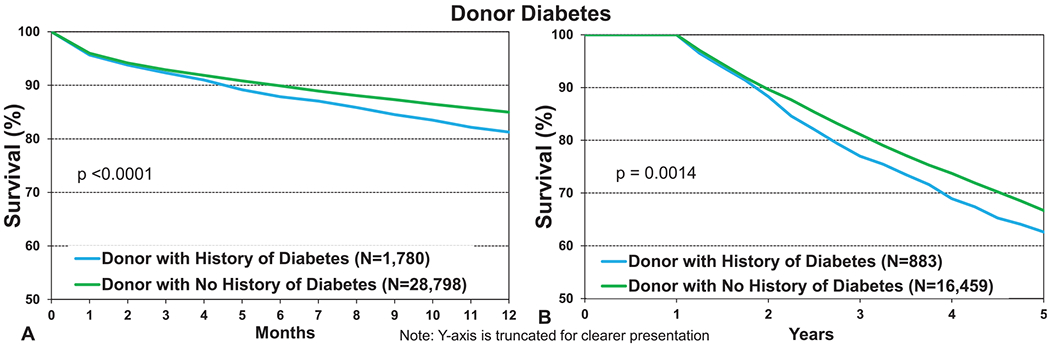
Kaplan–Meier survival for adult lung transplant recipients by donor diabetes (transplants from eras as shown). (a) 12 months. (b) 5 years conditional on survival to 1 year.
Figure 12.
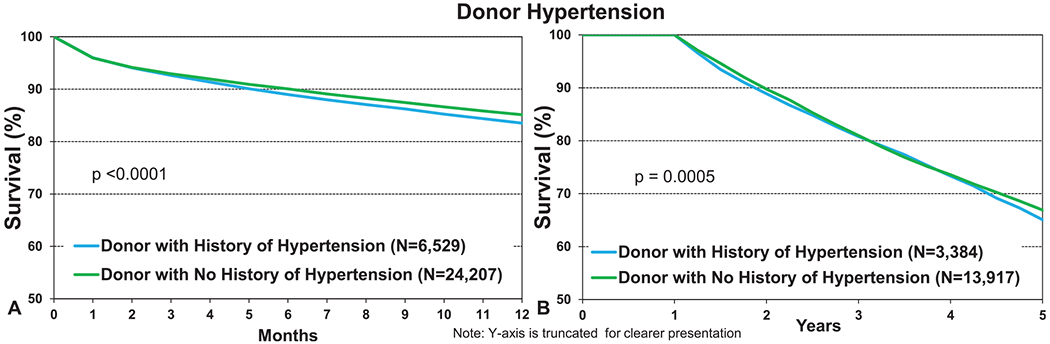
Kaplan–Meier survival for adult lung transplant recipients by donor hypertension (transplants: January 2000–June 2017 and January 2000–June 2013, respectively). (a) 12 months. (b) 5 years conditional on survival to 1 year.
Ischemic time
The impact of ischemic time on adult lung transplant outcomes was the focus of the 2017 Report and so is not examined in depth in this Report. Nevertheless, the equivalent, and possibly even superior, outcomes seen in recipients of organs with longer ischemic times are again apparent (eSlides L(a) 20 and L(a) 33) and are also noted in the pediatric lung report.13 This association is one with longevity, with differences in survival becoming more apparent over time, such that 5-year post-transplant survival is 70% in recipients where the ischemic time is ≥4 hours, compared with 65% in recipients of organs with shorter ischemic times (p < 0.0001, eSlide L(a) 33). Recipients with ischemic time ≥4 hours and donor age ≥50 years had lower 12-month survival than those with donor age <35 years, but there was no impact on long-term survival (eSlides L(a) 21 and 34). We next examined the effect of donor hypertension, in combination with ischemic time, on recipient survival. Although not all pairwise comparisons were significant, the highest survival at both 1 and 5 years was seen in recipients of donor organs where there was no history of hypertension and the ischemic time was ≥4 hours (eSlide L(a) 22 and Figure 13, eSlide L(a) 35).
Figure 13.
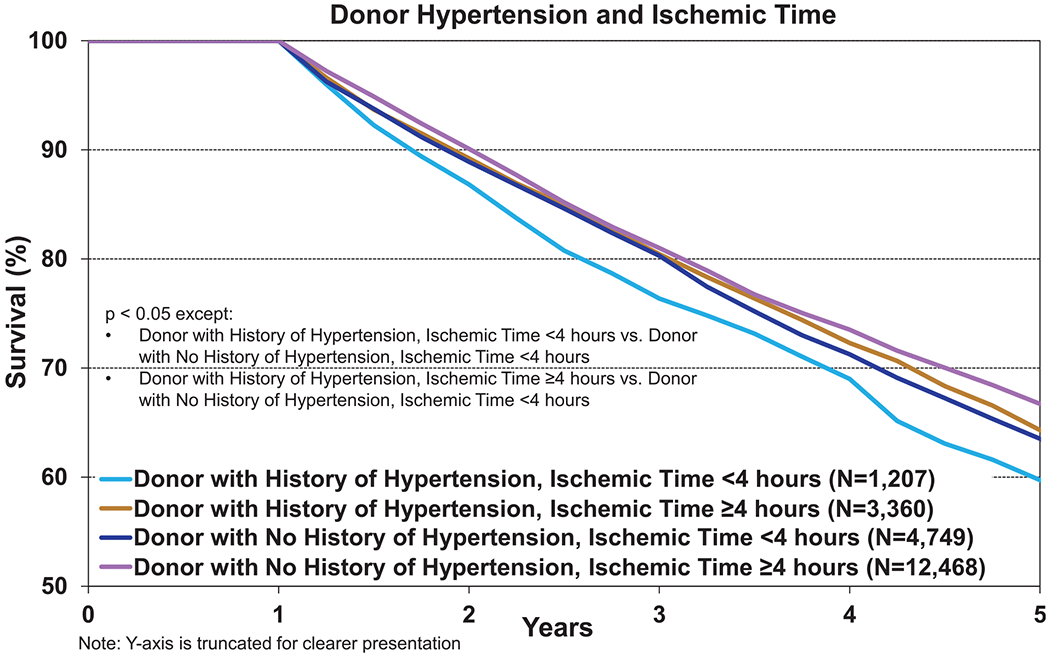
Kaplan–Meier survival within 5 years conditional on survival to 1 year for adult lung transplant recipients by donor history of hypertension and ischemic time (transplants: January 2005–June 2013).
Freedom from BOS conditional on survival to discharge
BOS is the leading cause of long-term graft dysfunction and graft loss after lung transplantation.16 Unfortunately, despite decades of research, management remains largely empirical with few evidence-based treatment options. Large epidemiological analyses, including previous Registry Reports3 incorporating donor and recipient factors, have provided important insight into risk factors for the subsequent development of BOS. These reports highlight what may be the most pertinent pathogenic mechanisms and thus provide a potential focus for future mechanistic studies. We therefore next examined associations between donor risk factors and BOS development in this focus theme report.
We first explored freedom from BOS by donor age. As seen in Figure 14 (eSlide L(a) 39), there was an association between donor age and freedom from BOS, but this difference was small and only significant in the oldest vs youngest donor age groups. The proportion of recipients free from BOS at 10 years post-transplant was 24% for recipients of organs from donors <35 years, 21% for donors age 35 to 49 years, and 20.6% for donors aged ≥50 years (Figure 14, eSlide L(a) 39). Older donors will have different causes of death, more comorbidities, and potentially a more extensive history of substance use and of environmental exposures, so we next investigated the impact of these factors on freedom from BOS. We found no association between donor cause of death or donor PO2 and freedom from BOS (eSlide L(a) 40). There was a comparatively strong association between ischemic time and freedom from BOS (eSlide L(a) 41), consistent with the findings of the 2017 Report with longer ischemic times associated with enduring freedom from BOS.3 At 10 years post-transplant, 18.9% of recipients where the ischemic time was <4 hours are free from BOS, compared with 24.6% of those where the ischemic time was ≥4 hours (eSlide L(a) 41), reinforcing the paradoxical effect of ischemic time on one of the most critically important outcomes in the field of adult lung transplantation.
Figure 14.
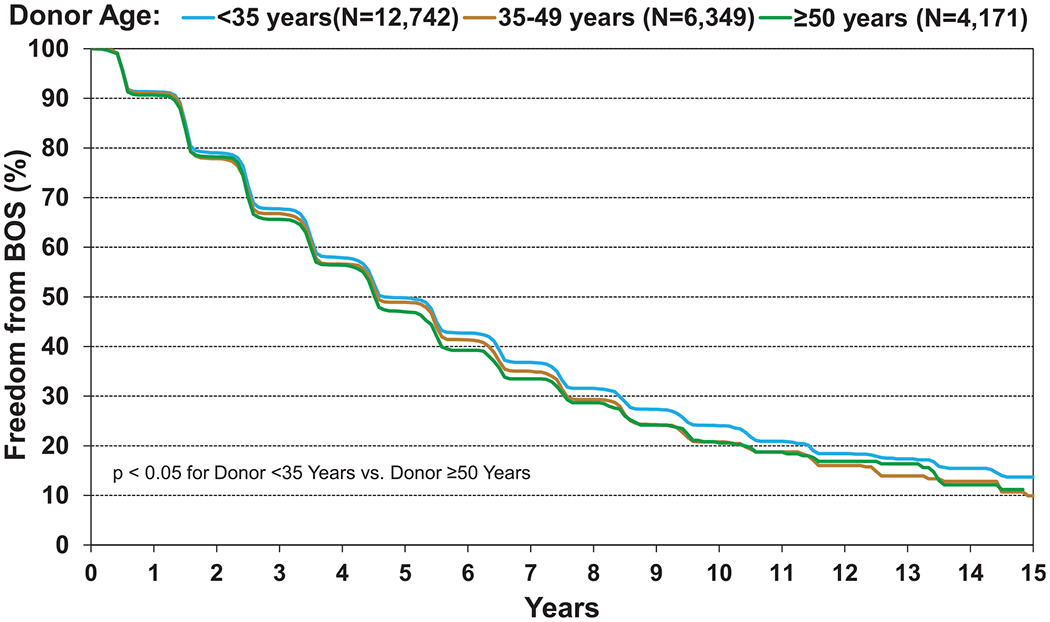
Kaplan–Meier freedom from BOS conditional on survival to discharge for adult lung transplant recipients by donor age (transplants: January 2000–June 2017). BOS, bronchiolitis obliterans syndrome.
Although we did not observe an association between donor smoking and freedom from BOS (Figure 15a, eSlide L(a) 42), we did note an association between donor alcohol use and freedom from BOS (Figure 15b, eSlide L(a) 42). Recipients of organs from donors with a history of consuming 2 or more alcoholic drinks per day experienced lower freedom from BOS; however, the numerical differences were small (Figure 15b, eSlide L(a) 42). There was no association between donor cocaine or other drug use and freedom from BOS (Figure 15c and d, eSlide L(a) 43).
Figure 15.

Kaplan–Meier freedom from BOS conditional on survival to discharge for adult lung transplant recipients by donor substance use (transplants from eras as shown). (a) Smoking. (b) Alcohol. (c) Cocaine. (d) Other. BOS, bronchiolitis obliterans syndrome; NS, not significant.
Finally, given the significant associations seen between donor diabetes and hypertension and post-transplant survival, we examined associations between these donor comorbidities and freedom from BOS. There was no association between either donor diabetes (Figure 16a, eSlide L(a) 44) or donor hypertension (Figure 16b, eSlide L(a) 44) and freedom from BOS, suggesting perhaps that the lower survival seen in recipients of organs with these donor characteristics may be related to complications other than BOS.
Figure 16.
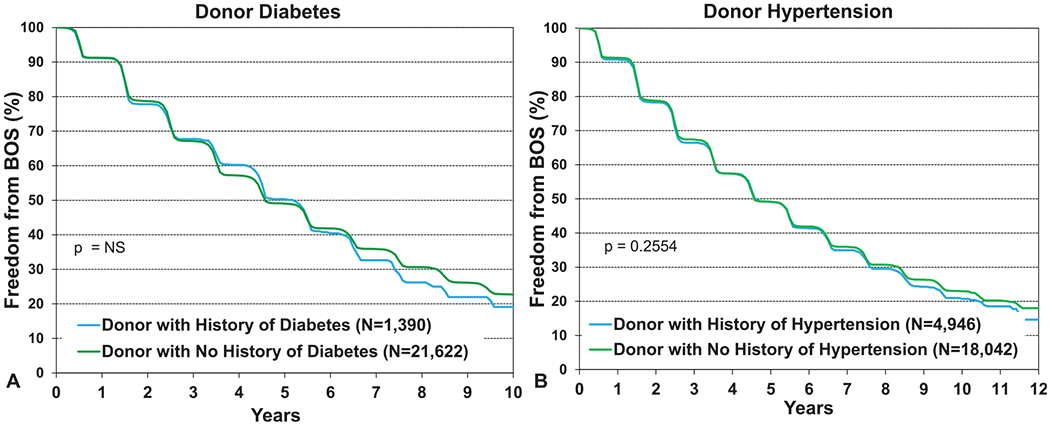
Kaplan–Meier freedom from BOS conditional on survival to discharge for adult lung transplant recipients by donor diabetes and hypertension (transplants from January 2000 to June 2017). (a) Diabetes. (b) Hypertension. BOS, bronchiolitis obliterans syndrome; NS, not significant.
Conclusions
In this 2020 ISHLT adult lung transplant report, we focused on deceased donor trends over time. We observed many important changes over the past 30 years, reflecting changing practice and demographics worldwide. We noted a gradual aging of the donor pool; a decline in smoking rates; and an increase in metabolic syndrome comorbidities, including diabetes and hypertension. The cause of death leading to lung donation is also changing, with a decline in traumatic deaths and increases in cerebrovascular accidents and anoxia. In univariate analyses, we identified significant associations between donor factors and recipient outcomes. We confirmed the findings of previous reports where factors that receive the most attention at the time of donor assessment (PO2, age, ischemic time, and donor smoking) have either no association with post-transplant outcomes, less of an effect than may be anticipated, or a paradoxical effect. We also found that factors that are often overlooked at the time of an organ offer, in particular donor diabetes, may be more important than previously recognized. We hope that this information will assist those who are making organ acceptance decisions to select donor lungs based on the best available evidence, with choices made by carefully quantifying and balancing a suite of donor and recipient factors against the risk of wait-list mortality. Furthermore, we hope these analyses will stimulate further research focused on refining donor selection algorithms and safely expanding the donor pool, as well as more fundamental research exploring ways to improve allograft and recipient longevity.
Acknowledgments
Disclosure statement
Kiran Khush is supported by National Institutes of Health/National Heart, Lung, and Blood Institute award R01HL125303 to study evidence-based strategies for donor heart evaluation and serves as scientific adviser and speaker for CareDx, Inc. Luciano Potena serves as a speaker for Thermo Fisher, Sandoz, Abbott, and Novartis. Josef Stehlik is supported by American Heart Association grant 16SFRN31890003 to study patient health status in disease transitions and serves as consultant for Medtronic and Abbott. Daniel Chambers received research funding from Astellas and Boeringher-Ingelheim. Andreas Zuckermann serves on the speakers bureau of Paragonix, Mallinckrodt, and Franz Kohler Chemie. Eileen Hsich is supported by National Institutes of Health/National Heart, Lung, and Blood Institute award R01HL141892 to study disparities in survival among heart transplant candidates and recipients. The remaining authors have no conflicts of interest to disclose.
References
- 1.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1169–83. [DOI] [PubMed] [Google Scholar]
- 2.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chambers DC, Yusen RD, Cherikh WS, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-fourth adult lung and heart-lung transplantation report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1047–59. [DOI] [PubMed] [Google Scholar]
- 4.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report—2013; focus theme: age. J Heart Lung Transplant 2013;32:965–78. [DOI] [PubMed] [Google Scholar]
- 5.Yusen RD, Edwards LB, Dipchand AI, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-third adult lung and heart-lung transplant report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1170–84. [DOI] [PubMed] [Google Scholar]
- 6.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report—2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009–24. [DOI] [PubMed] [Google Scholar]
- 7.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: thirty-second official adult lung and heart-lung transplantation report–2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1264–77. [DOI] [PubMed] [Google Scholar]
- 8.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossano JW, Singh TP, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-second pediatric heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes D Jr, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-second pediatric lung and heart-lung transplantation report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999-2010. JAMA 2012;307:491–7. [DOI] [PubMed] [Google Scholar]
- 12.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-seventh adult heart transplantation report - 2020; focus theme: focus on changing donor characteristics [e-pub ahead of print]. J Heart Lung Transplant. doi: XXX, accessed XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes D Jr, Harhay MO, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: twenty-third pediatric lung transplantation report — 2020; focus on deceased donor characteristics [e-pub ahead of print]. J Heart Lung Transplant. doi: 10.1016/j.healun.2020.07.007, accessed August 6, 2020. [DOI] [PMC free article] [PubMed]
- 14.Sonoda N, Morimoto A, Tatsumi Y, et al. A prospective study of the impact of diabetes mellitus on restrictive and obstructive lung function impairment: the Saku study. Metabolism 2018;82:58–64. [DOI] [PubMed] [Google Scholar]
- 15.Talakatta G, Sarikhani M, Muhamed J, et al. Diabetes induces fibrotic changes in the lung through the activation of TGF-β signaling pathways. Sci Rep 2018;8:11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: an International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant 2019;38:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]


