Abstract
The development of medical countermeasures against acute and delayed multi-organ injury requires animal models predictive of the human response to radiation and its treatment. Late chronic injury is a well-known feature of radiation nephropathy, but acute kidney injury has not been reported in an appropriate animal model. We have established a single-fraction partial-body irradiation model with minimal marrow sparing in non-human primates. Subject-based medical management was used including parenteral fluids according to prospective morbidity criteria. We show herein that 10 or 11 Gy exposures caused both acute and chronic kidney injury. Acute and chronic kidney injury appear to be dose-independent between 10 and 11 Gy. Acute kidney injury was identified during the first 50 days postirradiation and appeared to resolve before the occurrence of chronic kidney injury, which was progressively more severe up to 180 days postirradiation, which was the end of the study. These findings show that mitigation of the acute radiation syndrome by medical management will unmask delayed late effects that occur months after partial-body irradiation. They further emphasize that both acute and chronic changes in kidney function must be taken into account in the use and timing of mitigators and medical management for acute radiation syndrome and delayed effects of acute radiation exposure (DEARE).
INTRODUCTION
Late effects of total- or partial-body irradiation include chronic kidney injury (CKI), involving the renal vasculature, glomeruli and tubulointerstitium, which may progress to fibrotic renal failure (1). This is well-documented in human clinical experience and experimental models. Although acute kidney injury (AKI) occurs after accidental radiation exposures to humans (2), it has not been reported in experimental models of radiation nephropathy except after high-dose 30 Gy or higher exposures (3). The modern threat of accidental or intentional radiation exposures makes it imperative to identify suitable animal models at relevant doses that will guide treatment and future mitigation. The occurrence of acute kidney injury after total- and partial-body irradiation is highly relevant to planning for medical management of exposed subjects. The occurrence of acute and chronic kidney injury in a large animal model is an essential step before testing mitigators that would be effective in irradiated humans. There are no FDA-approved medical countermeasures against either acute or chronic kidney injury.
The latency, incidence, severity and progression of the hematopoietic, gastrointestinal and pulmonary syndromes were recently documented in a non-human primate (NHP) model of partial-body irradiation (PBI) (4, 5). Acute and chronic weight loss are important additional features (6). Kidney injury has not been reported in this model. We report herein the incidence, time course, severity and progression of AKI and CKI in a relevant NHP model of PBI with approximately 5% bone marrow sparing (PBI/BM5). Renal injury in non-human primate irradiation models has not been extensively reported, which this report will address. We analyzed further the effect of supportive care that included supplemental enteral and parenteral fluids.
METHODS
Animals
Male rhesus macaques (n = 36), Macaca mulatta [5.5–11.3 kg body weight (bw)], were exposed to PBI in doses of 10.0 or 11.0 Gy and sparing the tibiae, ankles and feet, which have approximately 5% of the NHP bone marrow (PBI/BM5). Fifteen NHP underwent 10 Gy and 21 animals underwent 11 Gy PBI/BM5 exposures. An additional six were nonirradiated controls. All irradiated NHP were observed for up to 180 days postirradiation, or until they were euthanized according to published criteria (4, 5).
All NHP were in good health at the start of study, were free of simian immunodeficiency virus, simian T-cell leukemia virus type 1, malaria, herpes B virus and tuberculosis. Animals were acclimated to a supine restraint device prior to irradiation. All animal procedures were performed according to an approved Institutional Animal Care and Use Committee (IACUC) protocol.
Housing, Care, Food and Water
Animal housing and care was performed in accordance with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals. Irradiated animals were single-housed in stainless steel cages at the University of Maryland Association for Assessment and Accreditation of Laboratory Animal Care (AAA-LAC)-accredited animal facility. Animals were provided primate chow ad libitum that was supplemented with fresh fruit and primate treats. After irradiation, citrus was eliminated from their diet. The animals had unlimited access to water that had been deionized, filtered through carbon and sand and disinfected with UV light.
Anesthesia
Ketamine (Ketaset, Fort Dodge, IA) (10 ± 5 mg kg−1), either alone or in combination with xylazine (AnaSed, Fort Dodge, IA) (1 ± 0.5 mg kg−1) was given intramuscularly (IM) to sedate animals before procedures. Yohimbine (Yobine, Shenandoah, IA) [(0.2 ± 0.1 mg kg−1, IM or intravenously (IV)] was given to reverse xylazine sedation, if required.
RADIATION EXPOSURE AND DOSIMETRY
Approximately 18 h before exposure, food was removed from each animal’s cage to minimize the occurrence of radiation-induced emesis. On the day of irradiation, NHP were given an antiemetic, Zofran (GlaxoSmithKline, Research Triangle Park, Durham, NC) or Ondansetron, (Hospira, Inc., Lake Forest, IL) (1–2 mg kg−1 IV or IM), 45–90 min before exposure. Anesthetized NHP were placed in a plexiglas supine restraint device, and then transported from their housing area to the linear accelerator (LINAC) facility at the University of Maryland, Department of Radiation Oncology.
The NHP were given xylazine to ensure proper radiation-field placement would be maintained, and then exposed to 10 or 11 Gy PBI/BM5 with 6 megavolt (MV) LINAC-derived photons. Calibration and dosimetry were done as previously reported (4). PBI was delivered at an approximate dose rate of 0.80 Gy min−1. Animals were positioned such that their tibiae were outside of the beam field. Animals were observed via in-room cameras throughout the exposure procedure. After PBI, NHP were anesthetized, transported back to the NHP housing area, given lactated Ringer’s Solution (LRS) (10–15 mL kg−1 IV) and a second dose of Zofran or Ondansetron (1–2 mg kg−1 IM or IV) and returned to their home cage.
Medical Management
Cage-side observations.
Nonsedated NHPs were observed twice daily or more often by trained staff. Activity, posture, stool consistency, presence of blood in stool, emesis, hemorrhage, respirations, presence of seizures and alopecia were graded and recorded.
Clinical observations.
NHPs were anesthetized daily from day 0–21 to assess body weight, body temperature, hydration status, presence of mouth ulcers and a complete blood count (CBC) (Beckman Coulter Ac·T diff, Beckman Coulter, Inc., Miami, FL). After day 21, clinical observations and supportive measures were performed weekly through day 60 and biweekly thereafter unless the animal’s health status required more frequent intervention.
Analgesics.
Buprenorphine HCl (Buprenex, Hospira, Lake Forest, IL) [0.01 mg kg−1 to 0.02 mg kg−1 IM twice a day (BID)] was given from day 5 to 35 postirradiation and when mouth ulcers or bloody stool were observed. Doses were increased for mouth ulcers and bloody stool. Mouth ulcers were cleansed with hydrogen peroxide or Nolvasan solution and rinsed with saline. Bupivacaine gel, a mixture of 0.1 mL of 25% Bupivacaine HCl (Marcaine, Hospira, Lake Forest, IL) with a dab of surgical lubricant (Surgilube, Fougera, Melville, NY), was applied to the area with a cotton-tipped applicator.
Anti-emetic.
Zofran [0.1–0.2 mg kg−1 IV or IM, once a day (QD) or BID] was given when emesis was observed.
Anti-ulcerative.
Sucralfate (Carafate, Axcan Scandipharm Inc., Birmingham, AL and Nostrum Laboratories, Inc., New Brunswick, NJ) was given [0.5 g per os (PO) or by oral gavage (OG) BID] from day 5 to 35 postirradiation and when mouth ulcers or bloody stool were observed.
Anti-diarrheals.
Antidiarrheal treatment was given according to a three-tier grading of stool consistency, where grade 0 was formed stool, grade 1 was soft stool and grade 2 was diarrhea. Following the observation of diarrhea, loperamide hydrochloride (Imodium, McNeil Consumer Healthcare, Fort Washington, PA) (0.1–0.2 mg kg−1 PO or by OG BID) was given. If diarrhea persisted after three successive days of loperamide treatment, or if watery stool was observed, diphenoxylate hydrochloride (Lomotil, Pfizer Inc., New York, NY) (0.1 mg kg−1 PO or by OG BID) was given. If diarrhea persisted after three successive days of Lomotil treatment, Imodium treatment was repeated. If diarrhea persisted after another three successive days of Imodium treatment, Lomotil treatment was repeated. Antidiarrheal treatment was stopped when stool became formed.
Antibiotics.
Antibiotics were started when the absolute neutrophil count (ANC) was <500 μL−1 and continued until the ANC was >500 μL−1 for 48 h. The primary antibiotic given was enrofloxacin (Baytril, Bayer HealthCare LLC, Shawnee Mission, KS) (10 mg kg−1 IM or IV, QD). Additionally, gentamicin sulfate (GentaMax, Phenoix Scientific, Inc., St. Joseph, MO) (5 mg kg−1 IM or IV, QD) was given in combination with enrofloxacin when the body temperature was ≥103.0°F and was continued for 24–48 h. Ceftriaxone (Roche Laboratories Inc., Nutley, NJ) (50 mg kg−1 IM, QD) or ertapenem (Merck & Co Inc., Whitehouse Station, NJ) (10 mg kg−1 IM, BID) was given when there was microbial resistance to enrofloxacin or gentamicin, via positive blood culture sensitivity results or persistent fever.
Anti-inflammatory (corticosteroids).
Dexamethasone (Butler Schein, Dublin, OH) was given to animals in respiratory distress (>80 bpm) during daily cage-side observations. The dose, frequency and duration of treatment were guided by the consulting veterinarian. Generally, NHP were treated with a planned taper as follows: 1 mg kg−1 IM, BID on the first day of treatment, 0.5 mg kg−1 BID for three days, 0.5 mg kg−1 QD for three days and 0.5 mg kg−1 every other day (QOD) for three doses.
Antipyretic.
Carprofen (Rimadyl, Pizer Inc., New York, NY) (2.2 mg kg−1 BID or 4.4 mg kg−1 IM or IV, QD) was given when the body temperature rose to ≥104.0°F. It was continued for 48 h after the first day the temperature was <104.0°F.
Diuretic.
Furosemide (FuroJect, Butler Schein, Dublin, OH) was given when edema was observed. The dose, frequency and duration of treatment were guided by the consulting veterinarian. Generally, NHP were treated with a planned taper as follows: 1.0 mg kg−1 QD on the first day of treatment, 0.5 mg kg−1 QD for three days and 0.25 mg kg−1 QD for three days.
Nutritional support.
On all days postirradiation, animals received fresh fruit, soft food and bottles containing diluted fruit juice or oral rehydration solution (Prange™, Bio-Serv, Frenchtown, NJ). Animals that lost 10% of their preirradiated body weight were given BIO-SERV certified Rhesus Liquidiets (15 ml kg−1 by OG). The volume of liquid nutrition was reduced to 7 ml kg−1 if the animal was also receiving treated water by OG for hydration.
Blood product support.
Transfusions of whole blood were given at 7–14 ml kg−1, IV through an 18 μ blood filter (Hemo Nate® Filter, Utah Medical Products, Inc., Midvale, UT), for any of the following: platelet count (Plt) <2,000 μL−1; Plt ≤20,000 μL−1 with hemoglobin count (Hgb) <6.7 g dL−1 or hematocrit (Hct) <20%; a decrease of ≥5% in Hct within a 24 h time period that resulted in Hct ≤25%; a decrease of ≥7% in Hct within a 24 h time period; or uncontrolled hemorrhage. Whole blood, anti-coagulated with 10% citrate-phosphate-dextrose-adenine (CPD-A) (Sigma-Aldrich, St. Louis, MO), was obtained from healthy NHPs that weighed ≥7.0 kg.
Hydration status and fluid support.
Hydration status was graded according to a four-tier system. Animals were graded 0 if they had normal hydration. Animals were considered mildly dehydrated, grade 1, if they exhibited any of the following: tacky mucous membranes, capillary refill time (CRT) or skin tent time (STT) ≥2 but <3 s. Mildly dehydrated animals were given a bolus of lactate ringer’s saline (LRS) (10–15 mL kg−1 over 15–20 min) and reverse osmosis (RO) purified water (10–15 mL kg−1 by OG). Animals were considered moderately dehydrated, grade 2, if they exhibited any of the mild symptoms plus any of the following symptoms: dry mucous membranes, >3% increase in Hct from the previous day (not related to a previous blood transfusion), sunken eyes, or CRT or STT ≥3 s. Moderately-dehydrated animals were given a bolus of LRS (20–30 mL kg−1) over 15–20 min and RO water (7–10 mL kg−1 by OG). Animals were considered severely dehydrated, grade 3, if they exhibited any of the mild or moderate symptoms plus any of the following symptoms: pale mucous membranes, >5% increase in Hct from the previous day (not related to a previous blood transfusion), rapid or weak pulse, cold extremities, lethargy or rapid breathing. Severely-dehydrated animals were given fluids as described for moderate dehydration, with the addition of an IV infusion of LRS (10–20 mL kg−1 h−1) given over a period of 2–4 h. Animals were placed in a restraint device after these assessments and treatments and allowed to awaken. Midazolam HCl (Bedford Laboratories, Bedford, OH) (0.2 mg kg−1 IM or IV) was given as needed to calm the NHP while in the restraint.
Euthanasia
Animals were euthanized for cause based on criteria previously reported (4, 5), and all surviving animals were euthanized at 180 ± 10 days postirradiation. This was in compliance with the American Veterinary Medical Association Guidelines for the Euthanasia of Animals and our IACUC.
Blood Chemistry
Blood was taken by venipuncture of saphenous vein under ketamine anesthesia just before euthanasia. BUN and serum creatinine were done using commercial kits (Alfa Wasserman ACE Clinical Chemistry System, West Caldwell, NJ). Normal ranges were obtained from age-matched naive NHP.
Renal Histology
Organs procured at necropsy were processed for histology. All samples were immersed in 10% neutral-buffered formalin for at least 48 h, embedded in paraffin, cut in 5 micron sections and stained with Masson Trichrome. Slides were scanned using a Hamamatsu Nanozoomer HT slide scanner and viewed using the Aperio ImageScope software (Leica Biosystems, version 12.3.0.5056).
Acute kidney injury.
AKI was scored for specimens obtained during the first 50 days after PBI/BM5. The acute injury score was for tubular injury (none, scattered and diffuse) glomerular thromboses (none, scattered and diffuse) and medullary congestion (none, scattered and diffuse), each grade being 0, 1 and 2 for a maximum score of 6. There were 11 NHP in this day 0–50 group, of which one had no scanned tissue sections. Four had undergone 10 Gy PBI/BM5 and seven had undergone 11 Gy PBI/BM5. Six additional NHP were not irradiated and served as controls.
Chronic kidney injury.
CKI was scored for specimens obtained from days 50–180 after PBI/BM5. This was for cysts (none, micro- and macroscopic), interstitial fibrosis (none, scattered and diffuse) casts (none, scattered and diffuse) glomerular thrombosis (none, few and most glomeruli of 20 examined), glomerulosclerosis (none, 1–2, 3–4, >4 of 20) and mesangiolysis (none, variable, most and all glomeruli of 20 examined), for a maximum score of 14. There were 25 NHP in the day 51–180 group, of which two had no scanned tissue sections. Eleven had undergone 10 Gy PBI/BM5, and 14 had undergone 11 Gy PBI/BM5. Six NHP were not irradiated and served as controls.
Experimental Endpoints
The primary endpoints were renal function, as azotemia and the histological injury score.
Statistical Methods
Continuous data are shown in Figs. 1–11. These are compared between groups using nonparametric statistics. Dichotomous variables were compared by Fisher exact tests. Survival was calculated by the Kaplan-Meier method and compared by log-rank testing. Correlations were tested by Spearman tests. P values of equal or <0.05 were deemed significant (Prism5, GraphPad Software, La Jolla, CA).
FIG. 1.
Survival of non-human primates that received 10 or 11 Gy partial-body irradiation with 5% bone marrow shielding. Kaplan-Meier survival curves showing time- and dose-dependent survival outcome. The survival after 11 Gy PBI is worse than that after 10 Gy PBI but this difference is just below the level of statistical significance (P = 0.06, log-rank test).
FIG. 11.
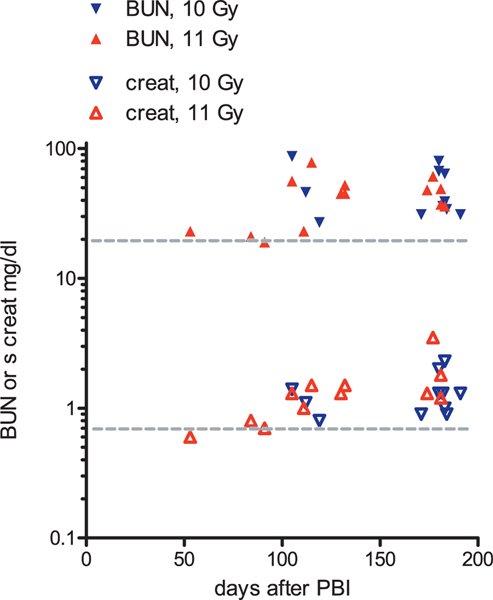
Correlation of chronic injury score to azotemia after 10 or 11 Gy PBI/BM5. There is a direct correlation of both BUN (panel a) and serum creatinine (panel b) to the chronic injury score, and both correlations are statistically significant (P = 0.0001 and 0.0005, respectively). The BUN and serum creatinine are shown on a log scale because of their nonlinear correlation with renal function. The normal values for the score and for the BUN and serum creatinine are indicated with gray dotted lines.
RESULTS
Survival
The NHP exposed to 11 Gy PBI had worse survival than the NHP exposed to 10 Gy, with median survival times of 171 and 105 days, respectively, but this was just below statistical significance (P = 0.06, log-rank test). Forty-six percent of the NHP that underwent 10 Gy PBI/BM5 survived to 180 days, and 14% of the NHP that underwent 11 Gy PBI/BM5 survived to that time point (Fig. 1).
Occurrence of an Acute and a Chronic Injury Phase
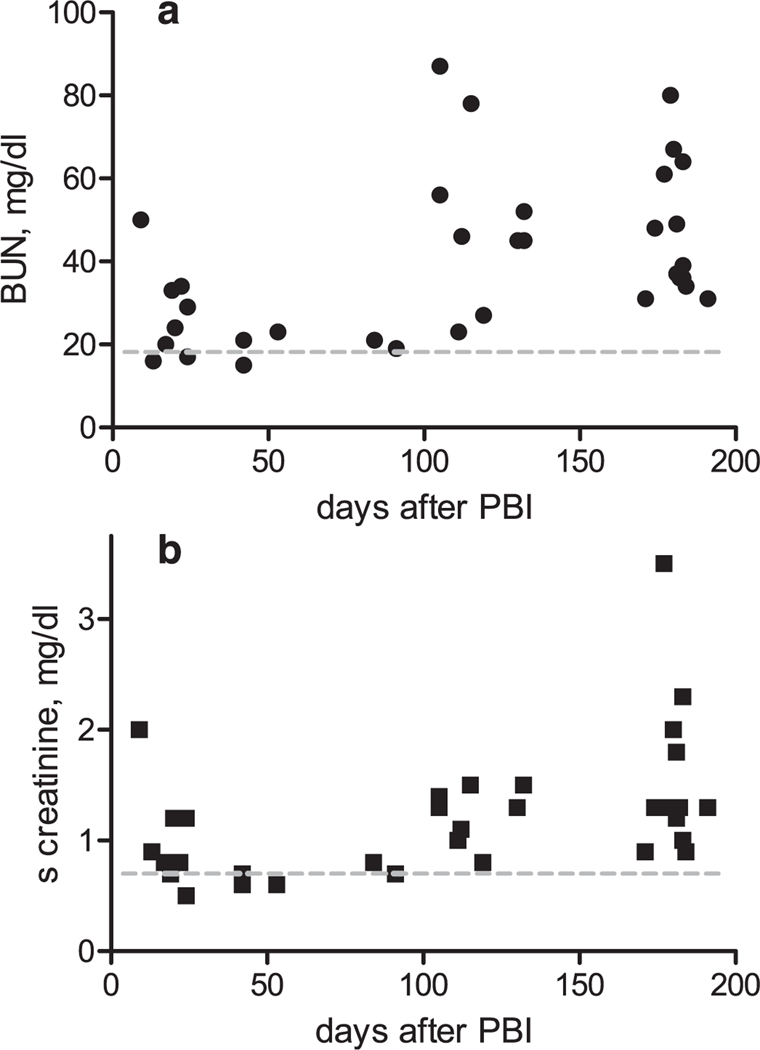
BUN and serum creatinine concentrations were used as indices of renal functional injury. The BUN or serum creatinine concentrations were increased after either 10 or 11 Gy PBI/BM5 in a bi-phasic pattern (Fig. 2a and b). There was an early phase, within the first 50 days after PBI and a later phase after that to the end of the studies at 180 days. The 50-day time point was based on the evolution of BUN and serum creatinine for the combined 10 and 11 Gy dose cohorts; this showed azotemia within ten days after PBI that subsequently declined by day 50 and azotemia that rose progressively after day 100.
FIG. 2.
Evolution of the BUN (panel a) and the serum creatinine (panel b) after 10 or 11 Gy PBI/BM5. There is an early acute increase in BUN or serum creatinine, then a period during which there was little or no azotemia at euthanasia, followed by a chronic phase of progressively higher azotemia that was greatest at the end of the 180-day study. The gray dotted lines indicate the normal BUN or serum creatinine concentrations.
Supportive Care Potential Variables in the Development of Kidney Injury for NHP That Underwent 10 or 11 Gy PBI/BM5
Dehydration.
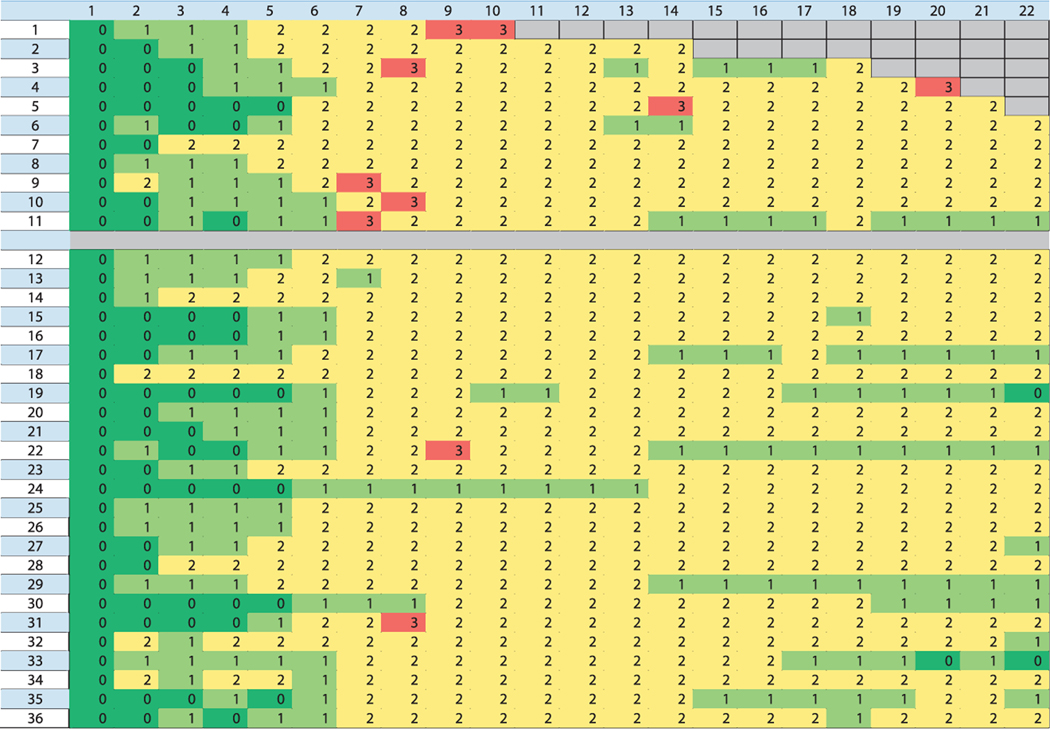
Mild, moderate and/or severe dehydration occurred as shown on Fig. 3. Severe dehydration was a significant feature of the animals that were euthanized within 50 days postirradiation. It was found in 7 of the 11 animals that were euthanized in the first 50 days and in only two of the 25 that lived beyond that time (P = 0.001, Fisher’s exact test). Four of the 15 NHP exposed to 10 Gy had severe dehydration (27%) and five of the 21 exposed to 11 Gy had severe dehydration (24%); these rates did not differ (P = 1, Fisher’s exact test). The occurrence and intensity of dehydration was greatest during the first 21 days after PBI and diminished thereafter.
FIG. 3.
Diagram of the severity grade of dehydration for each animal as a function of time after PBI/BM5. The animal numbers are shown on the far-left column and the day after PBI is shown on the top row. The color intensity indicates the severity of dehydration. No dehydration is the green color, mild is light green, moderate is yellow and severe is red. Severe dehydration did not occur after 22 days. None of the first 11 animals survived beyond 50 days and seven of these had at least one day of severe dehydration. Only two of the 25 animals surviving beyond 50 days had severe dehydration. This difference is statistically significant (P = 0.001, Fisher’s exact test).
Anti-microbial, nonsteroidal and steroid anti-inflammatories.
Gentamicin was given to 3 of 11 (27%) NHP in the acute phase (first 50 days) and 4 of 25 (16%) in the chronic phase (51–180 days). Carprofen was given to 2 of 11 (18%) NHP during the acute phase and 10 of 25 NHP (40%) in the chronic phase. Dexamethasone was given to none of the NHP that were euthanized in the acute phase before day 50 and to 14 of the 25 NHP that were euthanized thereafter during the 180-day time course.
Acute Kidney Injury in NHP That Underwent 10 or 11 Gy PBI/BM5
Clinical chemistry.
Eleven NHP were in the group that were euthanized up to 50 days postirradiation. Of these, ten had peripheral blood analyses done at time of euthanasia. BUN or serum creatinine concentrations at euthanasia were above normal in seven of ten NHP during the first 50 days after PBI as shown in Fig. 4. There did not appear to be a major difference between the severity of AKI for those NHP that underwent 10 Gy compared to 11 Gy PBI/BM5.
FIG. 4.
Evolution of BUN or serum creatinine during the first 50 days after PBI/BM5. Ten NHP in this group have a BUN and corresponding serum creatinine value. There is an early increase in both BUN and serum creatinine in this model, then a subsequent decline. The normal values for BUN and serum creatinine are indicated with the gray dotted lines.
Tissue injury.
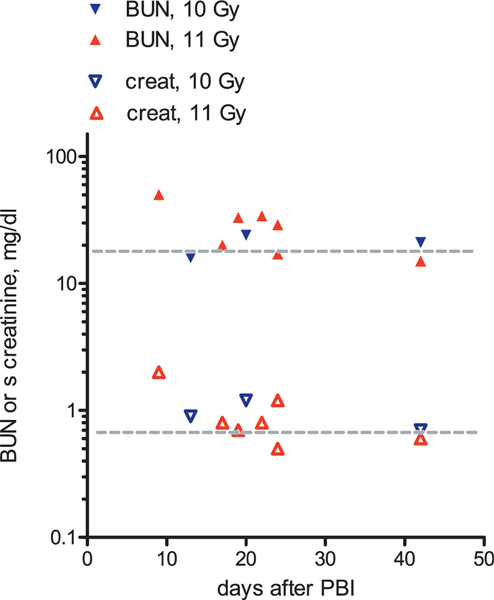
Ten animals of this group had good quality tissue sections. Figure 5 shows representative photomicrographs with tubular injury. There was substantial acute histological injury in seven of ten irradiated NHP (Fig. 6). Acute injury scores appeared worse after 10 Gy compared to 11 Gy PBI/BM5, but the numbers of NHP at each dose are low, and the times of euthanasia differ, which prevents conclusive comparison of acute injury scores according to dose.
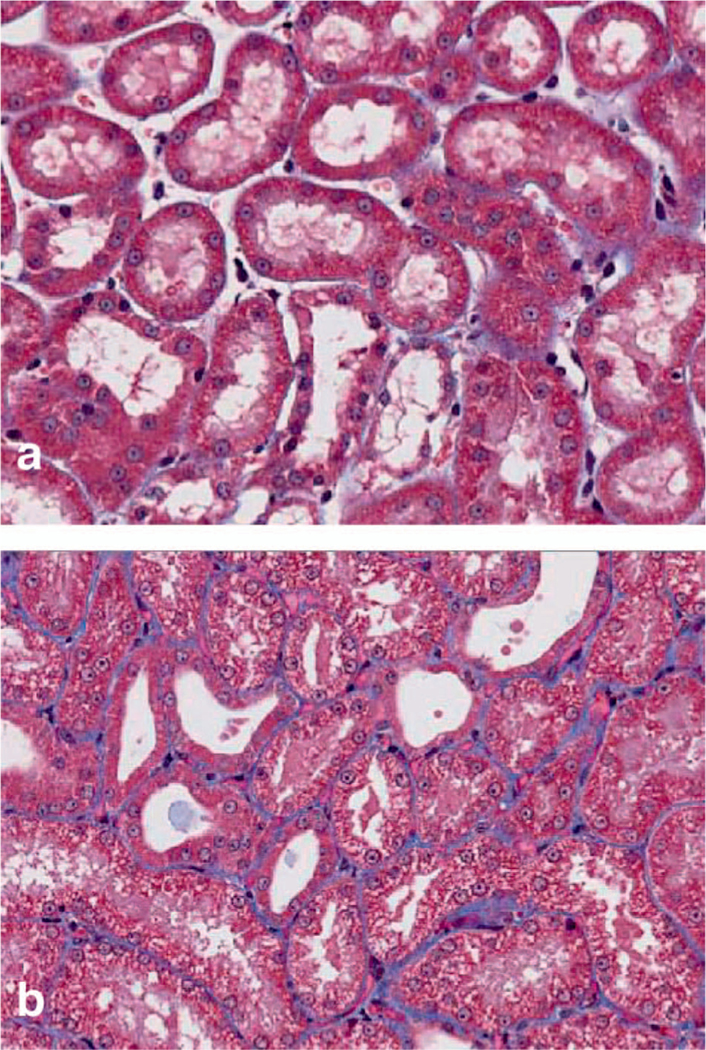
FIG. 5.
Photomicrographs of acute kidney injury in this NHP model. Panel a shows normal kidney from a nonirradiated animal, with normal tubules. Panel b shows tubular injury, with vacuole formation, epithelial flattening, and tubular dilation, which are all features of acute kidney injury. Masson trichrome stain, 200× magnification.
FIG. 6.
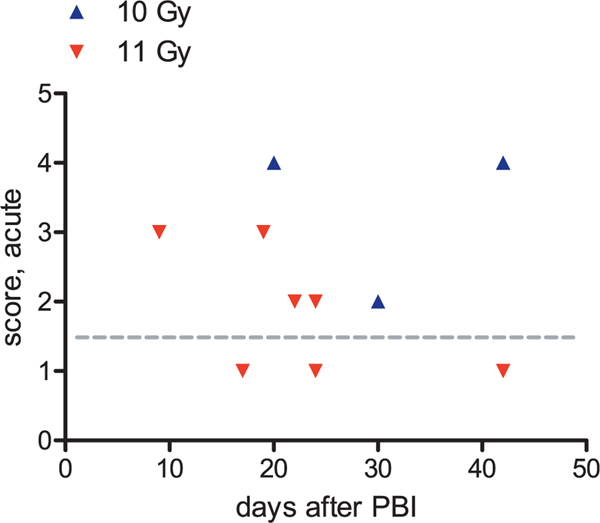
Occurrence of histological acute kidney injury after 10 or 11 Gy PBI/BM5. Seven of ten NHP had higher than normal injury scores. Histological injury appeared worse after 10 Gy (blue triangles) than after 11 Gy (red triangles) exposures, but time of euthanasia differed for these two dose groups, which prevents conclusive dose comparison. The average acute injury score for nonirradiated NHP is indicated with the gray dotted line.
There was a trend for a direct correlation of BUN with the acute injury score (r = 0.5, P = 0.08), as shown (Fig. 7a). There was also a trend for a direct correlation of serum creatinine with the acute injury score (r = 0.4, P = 0.1) (Fig. 7b).
FIG. 7.
Correlation of acute injury score to azotemia after 10 or 11 Gy PBI/BM5. There is a direct correlation of both BUN (panel a) and serum creatinine (panel b) to the acute injury score, but neither trend attained statistical significance (P = 0.08 and 0.1, respectively). The BUN and serum creatinine are shown on a log scale because of their nonlinear correlation with renal function. The normal values for the score and for the BUN and serum creatinine are indicated with gray dotted lines.
Chronic Kidney Injury for NHP That Underwent 10 or 11 Gy PBI/BM5
Clinical chemistry.
There were 25 NHP in the chronic phase, of which two did not have serum creatinine data. The chronic phase showed an elevated BUN or serum creatinine from 100 days onward in all NHP, consistent with chronic renal failure (Fig. 8).
FIG. 8.
Evolution of BUN or serum creatinine from 50 to 180 days after 10 or 11 Gy PBI/BM5. The 25 NHP in this group have a BUN and 23 have a serum creatinine value. Each serum creatinine value has a corresponding BUN value for that day. This phase shows a steady increase in both BUN and serum creatinine concentration, and all NHP have an above-normal BUN and serum creatinine by day 180. The normal values for BUN and serum creatinine are indicated with gray dotted lines.
Tissue injury.
Twenty-four NHP had good quality tissue sections. A representative photomicrograph is shown in Fig. 9. Interstitial fibrosis was seen in all sections, appeared to start in the medullary rays and was more severe with increasing time after irradiation. Tissue cellular inflammation was not a prominent feature. There was progressive elevation in the chronic kidney injury score, which exceeded that of nonirradiated NHP in all but two cases (Fig. 10). There was no difference between the chronic injury scores of the NHP that underwent 10 Gy compared to those that underwent 11 Gy PBI/BM5.
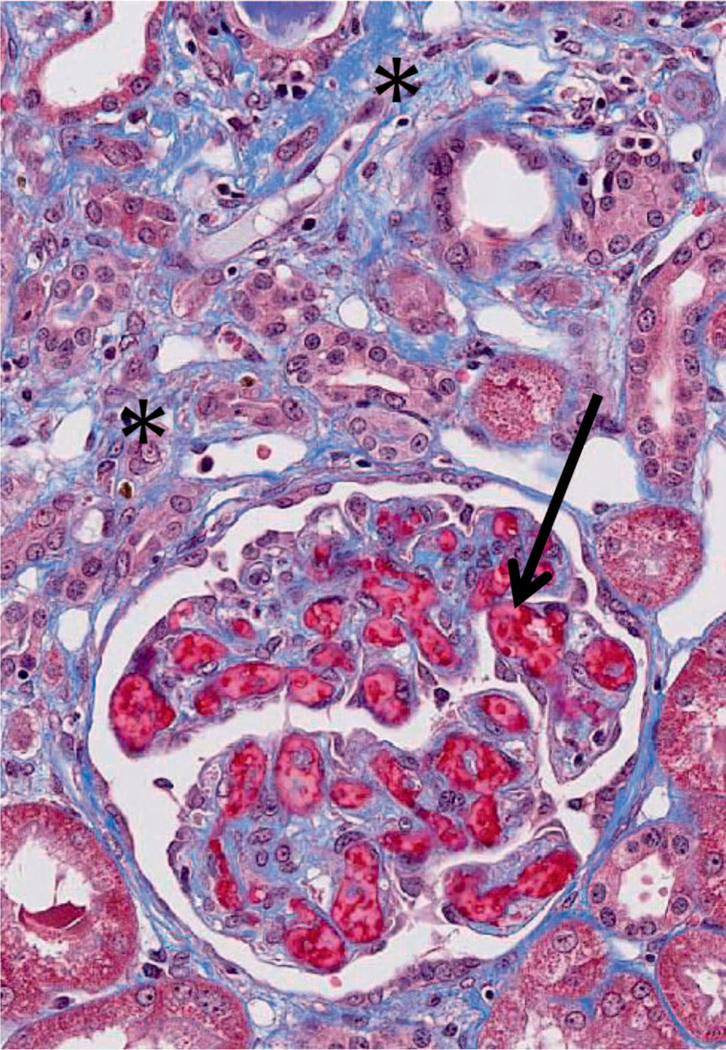
FIG. 9.
Photomicrograph of chronic kidney injury in this NHP model. There is accumulating blue-staining collagenous fibrosis (*), which separates the tubules from each other. There is glomerular capillary thrombosis (arrow). This animal had undergone 11 Gy PBI/BM5 177 days before. Masson trichrome stain, 400×.
FIG. 10.
Evolution of histological chronic kidney injury after 10 or 11 Gy PBI/BM5. There is progressively more severe injury with time and all NHP have definite chronic kidney injury by day 180. The injury scores for the 10 Gy animals (blue triangles) do not appear to differ from those of the 11 Gy animals (red triangles), but time of euthanasia differed for these two dose groups, which prevents conclusive dose comparison. The average chronic injury score for nonirradiated NHP is indicated with the gray dotted line.
There was a direct correlation of the BUN and the serum creatinine to the chronic injury score (r = 0.7, P < 0.0001 and r = 0.6, P = 0.0005, respectively) (Fig. 11a and b).
DISCUSSION
Chronic kidney injury is a well-known sequel of total- or partial-body irradiation in rodent models (1, 7). It also occurs in humans after total-body irradiation used before hematopoietic stem cell transplantation (8). Acute kidney injury has been reported in an animal model after 50 or 100 Gy single-fraction irradiation (3) but not after lower doses relevant to accidental or intentional exposures. But AKI can complicate accidental radiation exposures in humans (2, 9). The current data are thus highly relevant to accidental or belligerent radiation exposures.
Acute kidney injury in this model is evident by blood chemistries and histological injury within the first 50 days postirradiation, which coincides with the known acute gastrointestinal and hematopoietic acute radiation syndromes (GI-, H-ARS) (4). The GI-ARS will cause extracellular volume depletion, bacterial translocation, and sepsis, which are potent causes for AKI from acute tubular necrosis (ATN). Rapid weight loss in this model, within the first 30 days after 10 or 11 Gy PBI/BM5 (6). This is a loss of 10–15% of the baseline weight and in 80% of the NHP this acute weight loss is accompanied by significant signs of volume depletion including poor skin turgor and or dry mucous membranes (5). The latter clinical features are apt to coincide with AKI when they occur in humans (10). By protocol in these studies, affected NHP are given parenteral crystalloid fluids. This will tend to correct volume depletion, and, in NHP that survive the GI-ARS, lead to less-severe AKI in animals euthanized at later time points. The BUN and serum creatinine are normal at or about 50 days after PBI/BM5. This is consistent with the time course for resolution of human clinical ATN that results from volume depletion. The severity of AKI in these studies did not differ between the NHP exposed to 10 or 11 Gy. This is consistent with it being an indirect effect of irradiation, via volume depletion, rather than a direct radiotoxicity to kidneys. It may also be explained because the NHP survival times were not the same for each dose.
Chronic kidney injury became apparent by day 100 after PBI/BM5, which is consistent with the timing of its occurrence in rodent models that undergo similar irradiation protocols. All animals surviving to 180 days had CKI at time of euthanasia. Herein, we use the term CKI rather than chronic kidney disease because we lack data on other features of chronic kidney disease such as proteinuria or hypertension. CKI is thus a more accurate term. The severity of CKI in these studies did not differ between the NHP that were exposed to 10 or 11 Gy. This may be because the survival times were not the same for each dose cohort.
We assessed kidney function using the blood urea nitrogen (BUN) and also the serum creatinine. The serum creatinine value was missing from 2 animals that had BUN values. Both the serum creatinine and the BUN are commonly used as measures of kidney function and the serum creatinine is an essential component of the acute kidney injury network (AKIN) and risk, injury, failure, loss and end-stage renal disease (RIFLE) classifications for AKI. Other biomarkers for acute or chronic renal injury are reported, such as kidney injury molecule-1, interleukin-18 and cystatin C. They may have increased sensitivity for renal injury; one can speculate that these might show renal injury at lesser irradiation doses. At the doses used in the current study, the BUN, serum creatinine and renal histology clearly show the occurrence of acute and chronic kidney injury. Renal clearance studies might be better than blood metabolite levels to assess kidney function, but clearance studies are very difficult to do in NHP and formula-based estimates of glomerular-filtration rate do not exist for NHP. The BUN is a key indicator of the severity of renal failure, acute or chronic. A BUN near or over 100 mg/dl is often used as a biochemical indicator of the need for dialysis in humans with either acute or chronic renal failure. In a rat model of radiation nephropathy, BUN is very highly correlated with survival (11). BUN is therefore a good indicator of renal function.
We assessed histological injury after a qualitative survey of the tissue sections. The histology of AKI includes tubular injury and medullary vascular congestion, and those features were thus scored. There were clear-cut glomerular thromboses in the tissue sections from NHP euthanized during the first 50 days after PBI/BM5 and this has been reported in other NHP models of renal radiation injury (12). Therefore we added glomerular thrombosis to the acute injury score. The histology of CKI includes glomerular scarring, interstitial fibrosis, intratubular cast formation and cyst formation. Cyst formation is also a reported feature of radiation nephropathy in NHP (13). Glomerular thromboses was seen in the NHP euthanized at most times after PBI/BM5, so this feature was added to the chronic injury score.
The finding of glomerular thromboses is consistent with the evidence that glomerular endothelial injury is an early and lasting feature of radiation nephropathy. Glomerular endothelial injury occurs within a month after similar radiation doses in a porcine and also in a murine model of radiation nephropathy (12, 14), and it is a prominent feature in human radiation nephropathy (15, 16).
Neither serum creatinine nor BUN correlated well with the acute tissue injury, which mimics the human situation. Indeed, humans with acute renal failure from any cause may often have an increased BUN and serum creatinine but only scant or no tissue injury on renal biopsy (17).
There was a good correlation of the chronic injury score to the BUN and the serum creatinine. This is consistent with the good correlation of renal function with the injury score in a rodent model of radiation nephropathy (18). Neither BUN nor serum creatinine were elevated until the chronic injury score was 3 or more. This is consistent with renal adaptation to lesser degrees of histologic injury, including hyperfiltration of still-functioning nephrons (19). The latter can compensate for tissue destruction up to a point, then the BUN and serum creatinine will start to rise because the compensation limit has been exceeded.
Cause of Acute Kidney Injury
Acute kidney injury occurring within days of exposure is not a typical feature of radiation nephropathy, either clinically or in animal models (1, 20). But AKI does happen after human accidental radiation exposures. A major feature of this NHP model is substantial GI- and H-ARS despite the use of partial marrow shielding (5). This will lead to depletion of bodily fluids and weight loss, as documented in this model (6). The AKI of this model correlates well with the severe dehydration scores in the acute phase. The histological features are consistent with that interpretation, including the tubular injury and medullary congestion. The superimposed glomerular thromboses could be the result of direct endothelial injury from irradiation and or disseminated intravascular coagulation caused by sepsis (21). But AKI caused by endotoxin given to rhesus macaques is associated with accumulation of leukocytes in the peritubular capillaries (22), which was not seen in these animals.
Medical Management Considerations
Supportive care included dexamethasone, which was given to treat radiation pneumonitis and could by its catabolic effect cause an increase in the BUN. But no animal in this cohort received dexamethasone until 68 days or more postirradiation, so that an effect of dexamethasone to increase the BUN in the first 50 days after PBI is excluded.
Gentamicin is not likely to be a general cause of the AKI, because it was given to only 3 animals of the 11 in the acute phase group. Carprofen was given to 2 animals in this group, so is also not a general cause of the AKI. Only one animal was given furosemide during the first 50 days.
Implications of Acute Kidney Injury
The mortality during the first 50 days corresponds to the AKI phase, which links AKI to mortality in this model. Mortality in critically ill humans that do not get dialysis treatment worsens in direct relationship to their degree of renal insufficiency (23). AKI leads to volume overload and lethal electrolyte disturbances such as hyperkalemia. It is compounded by alterations in pharmacokinetics of medications. AKI will also have adverse effects on other organs, including brain and lung (24). AKI in the current studies would have been worse if individualized support had not been provided. Finally, radiation injury mitigators that could adversely impact renal function cannot be used during this acute phase. Their start time will have to wait until after the AKI has resolved.
Acute kidney injury may be a risk factor for CKI (25). This could imply that mitigation of AKI would reduce the occurrence and or severity of CKI. We cannot conclude this from the current data. Nonetheless, the early supportive care that was provided is likely to have enabled the survival of NHP through the first 50 days, thus “unmasking” the later development of CKI.
Cause of Chronic Kidney Injury
The timing and histology of the chronic injury are likely to be related to irradiation itself. But CKI was not worse in the 11 Gy cohort compared to the 10 Gy cohort. The expected dose-response relationship is perhaps not evident because NHP in this study were not euthanized at the same time, and the 180-day study duration was probably not long enough to show clear-cut differences. The chronic injury morphology in the current study is very similar to those seen in other NHP radiation nephropathy models. Moreover, we saw glomerulo-tubular neck stenosis as a striking feature of chronic injury, which we have previously reported as a quantitative fibrotic feature of experimental radiation nephropathy (26, 27).
Medical Management Considerations
Gentamicin is not likely to be a general cause of the CKI, because it was given to only 4 animals of the 25 in the chronic-phase group. Carprofen was given to 10 animals in this group, and in 7 of those was given at twenty or more days before euthanasia so is also not a general cause of the CKI. Nine animals were given furosemide during the chronic phase and in 5 of these was given at 20 or more days before euthanasia. Furosemide can cause elevation of the BUN by extracellular volume depletion (“dehydration”). The median dehydration score was zero just before euthanasia in the animals that got furosemide. Furosemide could also cause allergic interstitial nephritis but that was not seen in these studies.
Dexamethasone is also reported to exert a mitigating effect on radiation-induced nephropathy in rats (28). In the current NHP studies, dexamethasone was given parenterally for a median time of eleven days at a time, whereas in the rat studies it was given orally for months. Dexamethasone could cause an increase in the BUN by its protein catabolic effect. But dexamethasone would not have an independent effect to elevate the serum creatinine. We doubt a confounding influence of dexamethasone on kidney function in these studies.
Evolution of Chronic Kidney Injury
Chronic kidney injury shows progressive worsening of injury and function in this model. This is consistent with the rat TBI model in which CKI is progressive after either 10 or 11 Gy, with severe uremia by four to six months after TBI. The possible mechanisms of chronic progressive renal failure include hypertension, proteinuria, oxidative stress, profibrotic cytokines and even fibrosis itself (29). Hypertension and proteinuria are probably present in this NHP model as they are in human radiation-induced nephropathy, but those measurements were not made. Chronic persistent oxidative stress has not been found in radiation nephropathy (30). Expression of transforming growth factor beta 1 has been implicated in a porcine model of radiation nephropathy (31) but previous studies in this model do not show a correlation of connective tissue growth factor (CTGF) expression to the renal fibrosis (32) and fibrosis appears to depend on the combined action of TGFbeta1 and CTGF (33). Further investigations are needed.
Implications of Chronic Kidney Injury
The mortality from 100 days onward corresponds to the CKI phase, which links CKI to mortality in this model. Morbidity and mortality in humans increase in direct relationship to increasing azotemia of any cause (34). In the current studies, the NHP underwent euthanasia for prospective criteria that did not include kidney disease. But the approximate doubling of BUN and serum creatinine in these NHP by the end of the study corresponds to a more than 50% reduction of the glomerular filtration rate, which would be chronic kidney disease stage 3 in a human. This CKI affects all of the NHP that survived to day 180 in this study, which makes it a major delayed effect of acute radiation exposure (DEARE). Subjects with CKI have increased cardiovascular mortality and are at risk to evolve to end-stage renal failure. They will have abnormalities of bone and mineral metabolism such as secondary hyperparathyroidism, and they may develop metabolic acidosis. They will also have altered pharmacokinetics of any medication that depends on kidneys for metabolism and excretion. This must be taken into account when developing mitigators of DEARE.
SUMMARY
This report documents the occurrence of acute and chronic kidney injury in a non-human primate model of partial-body irradiation with 5% bone marrow sparing. Reduced renal function by itself will adversely impact morbidity and mortality, and must also be taken into account when developing or using medical mitigators of radiation injury. These data apply directly to accidental or intentional human radiation exposures.
ACKNOWLEDGMENTS
This project has been funded in whole or in part with Federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services, under Contracts No. HHSN272201500013I and HHSN277201000046C (Thomas J. MacVittie, PI), and in part by resources and facilities at the Baltimore VAMC.
REFERENCES
- 1.Cohen EP, Robbins ME. Radiation nephropathy. Semin Nephrol 2003; 23:486–499. [DOI] [PubMed] [Google Scholar]
- 2.Fliedner TM, D Dorr H, Meineke V. Multi-organ involvement as a pathogenetic principle of the radiation syndromes: a study involving 110 case histories documented in SEARCH and classified as the bases of haematopoietic indicators of effect. BJR Suppl 2005; 27:1–8. [Google Scholar]
- 3.Madrazo A, Suzuki Y, Churg J. Radiation nephritis: acute changes following high dose of radiation. Am J Pathol 1969. March; 54:507–27. [PMC free article] [PubMed] [Google Scholar]
- 4.MacVittie TJ, Farese AM, Bennett A, Gelfond D, Shea-Donohue T, Tudor G, et al. The acute gastrointestinal subsyndrome of the acute radiation syndrome: a rhesus macaque model. Health Phys 2012; 103:411–26. [DOI] [PubMed] [Google Scholar]
- 5.MacVittie TJ, Bennett A, Booth C, Garofalo M, Tudor G, Ward A, et al. The prolonged gastrointestinal syndrome in rhesus macaques: the relationship between gastrointestinal, hematopoietic, and delayed multi-organ sequelae following acute, potentially lethal, partial-body irradiation. Health Phys 2012; 103:427–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cui W, Bennett AW, Zhang P, Barrow KR, Kearney SR, Hankey KG, et al. A non-human primate model of radiation-induced cachexia. Sci Rep 2016; 6:23612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moulder JE, Fish BL. Late toxicity of total body irradiation with bone marrow transplantation in a rat model. Int J Radiat Oncol Biol Phys 1989; 16:1501–09. [DOI] [PubMed] [Google Scholar]
- 8.Antignac C, Gubler MC, Leverger G, Broyer M, Habib R. Delayed renal failure with extensive mesangiolysis following bone marrow transplantation. Kidney Int 1989; 35:1336–44. [DOI] [PubMed] [Google Scholar]
- 9.Moulder JE, Cohen EP. Radiation-induced multi-organ involvement and failure: the contribution of radiation effects on the renal system. BJR Suppl 2005; 27:82–8. [Google Scholar]
- 10.Acute renal failure In: Maxwell MH, Kleeman CR, editor. Clinical disorders of fluid and electrolyte metabolism. Second ed.: McGraw-Hill; 1972. p. 727–66. [Google Scholar]
- 11.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res 2011; 175:29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stephens LC, Robbins ME, Johnston DA, Thames HD, Price RE, Peters LJ, et al. Radiation nephropathy in the rhesus monkey: morphometric analysis of glomerular and tubular alterations. Int J Radiat Oncol Biol Phys 1995; 31:865–73. [DOI] [PubMed] [Google Scholar]
- 13.Niemer-Tucker MM, Sluysmans MM, Bakker B, Davelaar J, Zurcher C, Broerse JJ. Long-term consequences of high-dose total-body irradiation on hepatic and renal function in primates. Int J Radiat Biol 199; 68:83–96. [DOI] [PubMed] [Google Scholar]
- 14.Fajardo LF, Brown JM, Glatstein E. Glomerular and juxta-glomerular lesions in radiation nephropathy. Radiat Res 1976; 68:177–83. [PubMed] [Google Scholar]
- 15.Cogan MG, Arieff AI. Radiation nephritis and intravascular coagulation. Clin Nephrol 1978; 10:74–78. [PubMed] [Google Scholar]
- 16.Cohen EP. Radiation nephropathy after bone marrow transplantation. Kidney Int 2000; 58:903–18. [DOI] [PubMed] [Google Scholar]
- 17.Finckh ES, Jeremy D, Whyte HM. Structural renal damage and its relation to clinical features in acute oliguric renal failure. Q J Med 1962; 31:429–46. [PubMed] [Google Scholar]
- 18.Moulder JE, Fish BL, Cohen EP. Treatment of radiation nephropathy with ACE inhibitors. Int J Radiat Oncol Biol Phys 1993; 27:93–9. [DOI] [PubMed] [Google Scholar]
- 19.Brenner BM. Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 1983; 23:647–55. [DOI] [PubMed] [Google Scholar]
- 20.Luxton RW. Radiation nephritis. A long-term study of 54 patients. Lancet 1961; 2(7214):1221–24. [DOI] [PubMed] [Google Scholar]
- 21.Krigsfeld GS, Savage AR, Billings PC, Lin L, Kennedy AR. Evidence for radiation-induced disseminated intravascular coagulation as a major cause of radiation-induced death in ferrets. Int J Radiat Oncol Biol Phys 2014; 88:940–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richman AV, Gerber LI, Balis JU. Peritubular capillaries. A major target site of endotoxin-induced vascular injury in the primate kidney. Lab Invest 1980; 43:327–32. [PubMed] [Google Scholar]
- 23.Masewu A, Makulo JR, Lepira F, Amisi EB, Sumaili EK, Bukabau J, et al. Acute kidney injury is a powerful independent predictor of mortality in critically ill patients: a multicenter prospective cohort study from Kinshasa, the Democratic Republic of Congo. BMC Nephrol 2016; 17:118–016–0333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int 2016; 89:555–64. [DOI] [PubMed] [Google Scholar]
- 25.Leung KC, Tonelli M, James MT. Chronic kidney disease following acute kidney injury-risk and outcomes. Nat Rev Nephrol 2013:77–85. [DOI] [PubMed] [Google Scholar]
- 26.Cohen EP, Robbins ME, Whitehouse E, Hopewell JW. Stenosis of the tubular neck: a possible mechanism for progressive renal failure. J Lab Clin Med 1997; 129:567–73. [DOI] [PubMed] [Google Scholar]
- 27.Cohen EP, Regner K, Fish BL, Moulder JE. Stenotic glomer-ulotubular necks in radiation nephropathy. J Pathol 2000; 190:484–88. [DOI] [PubMed] [Google Scholar]
- 28.Geraci JP, Mariano MS, Jackson KL, Taylor DA, Still ER. Effects of dexamethasone on late radiation injury following partial-body and local organ exposures. Radiat Res 1992; 129:61–70. [PubMed] [Google Scholar]
- 29.Cohen EP. Fibrosis causes progressive kidney failure. Med Hypotheses 1995; 45:459–62. [DOI] [PubMed] [Google Scholar]
- 30.Cohen SR, Cohen EP. Chronic oxidative stress after irradiation: An unproven hypothesis. Med Hypotheses 2013; 80:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen EP, Bonsib SA, Whitehouse E, Hopewell JW, Robbins ME. Mediators and mechanisms of radiation nephropathy. Proc Soc Exp Biol Med 2000; 223:218–25. [DOI] [PubMed] [Google Scholar]
- 32.Zhang P, Cui W, Hankey KG, Gibbs AM, Smith CP, Taylor-Howell C, et al. Increased expression of connective tissue growth factor (CTGF) in multiple organs after exposure of non-human primates (NHP) to lethal doses of radiation. Health Phys 2015; 109:374–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Usinger W, Nichols B, Gray J, Xu L, Seeley TW, et al. Cooperative interaction of CTGF and TGF-beta in animal models of fibrotic disease. Fibrogenesis Tissue Repair 2011; 4:4–1536–4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]