Abstract
Purpose of review:
Alpha-gal syndrome encompasses a constellation of symptoms associated with immune-mediated hypersensitivity responses to galactose-alpha-1,3-galactose (alpha-gal). The purpose of this review is to discuss our current understanding of the etiology, clinical symptoms, natural history, epidemiology, and management of alpha-gal syndrome.
Recent findings:
Sensitization to alpha-gal is associated with bites from ectoparasites like the lone star tick Amblyomma americanum. Allergic reactions in alpha-gal syndrome are often delayed and inconsistent. The magnitude of the allergic response depends on co-factors like exercise and alcohol consumption and the amount of alpha-gal and fat present in the food. Assaying alpha-gal specific IgE in the serum is the primary diagnostic test used to confirm the allergy. Long-term management of the condition involves avoidance of both mammalian food products and tick bites.
Summary:
Alpha-gal syndrome disrupts the current paradigm for understanding food allergy. Exposure to an ectoparasite is critical for the development of specific IgE antibodies underlying sensitization, and allergic reactions depend on the activation of mast cells and basophils sensitized with IgE against a carbohydrate rather than a protein. Research in this field may lead to the development of improved diagnostic and therapeutic tools that can revolutionize the management of patients with alpha-gal syndrome.
Keywords: alpha-gal syndrome, red meat allergy, mammalian meat, tick, glycolipid
Introduction : What is Alpha-gal syndrome
Alpha-gal syndrome encompasses a constellation of symptoms associated with immune-mediated hypersensitivity responses to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal). Galactose-alpha-1,3-galactose moieties are present in several glycoproteins and glycolipids found in non-primate mammals (cow, pig, goat, sheep, etc). Alpha-gal syndrome includes both drug allergy, characterized by immediate hypersensitivity responses to injected pharmaceutical products containing alpha-gal, and food allergy, characterized by delayed reactions after ingestion of mammal-derived foods (meat, innards, organs, and dairy). Allergic reactions to alpha-gal were first described by Chung et al (1). Their report grew from observations that cancer patients living in the Southeastern US experienced significantly more anaphylactic reactions than patients in New England, California, or Europe following initial intravenous doses of cetuximab, a monoclonal antibody and cancer chemotherapeutic agent (2). Chung and colleagues found that IgE antibodies specific for galactose-alpha-1,3-galactose glycosylating the monoclonal antibody were critical for the development of these immediate hypersensitivity responses (1). Shortly afterward, Commins et al (3) and Van Nunen et al (4) published case series with scores of patients from the Southeastern US and Australia, respectively, describing allergic symptoms including pruritus, urticaria, angioedema, and abdominal discomfort hours after eating mammalian meat. Commins et al identified the presence of circulating alpha-gal-specific IgE in these patients (3). Both groups also reported that history of tick bite was common to all these patients.
As a food allergy, alpha-gal syndrome challenges the current paradigm for food allergy. Whereas in conventional food allergies, subjects develop allergic symptoms typically within minutes of eating the food, in alpha-gal syndrome, allergic symptoms frequently begin greater than 2 hours after ingesting mammalian products (5–7). Moreover, the IgE antibodies driving allergic responses in alpha-gal syndrome form against the carbohydrate alpha-gal, rather than a protein antigen (7). Typically, sensitization, or the development of IgE antibodies to food proteins, occur after a primary exposure to that food via an epithelial barrier like the oral mucosa, gastrointestinal epithelium, or the skin (8). With alpha-gal syndrome, sensitization is associated with exposure to ectoparasites; in the US, the culprit ectoparasite is the lone star tick (Amblyomma americanum) (9, 10).
Clinical Presentation
In alpha-gal syndrome, allergic reactions are frequently delayed and may not occur with each ingestion. The variability in the magnitude of the allergic response depends on co-factors including exercise and alcohol, which can also impact speed and severity of conventional allergic responses to food proteins (7, 11). The dose and form of alpha-gal consumed also seems to influence the magnitude and speed of the allergic response. Allergic symptoms may appear rapidly in alpha-gal allergic individuals who consume organ meats like pork kidney, which contains high concentrations of alpha-gal (12, 13). By contrast, consuming fatty mammalian products delays allergic reactions (14). This may be related to differences in how alpha-gal allergic and non-allergic patients metabolize proteins and lipids (15). Prior to the first episode of allergic symptoms, patients with alpha-gal syndrome often report a large local reaction to a tick or arthropod bite, with this bite frequently serving as the herald of the development of alpha-gal syndrome (7).
Alpha-gal syndrome in children highlights different clinical phenotypes
Although alpha-gal syndrome was first described in adult patients who had safely consumed meat for years, the condition has also been reported in children (5, 6, 16–18). Kennedy and colleagues published the first case series of 51 children (ages 4–17) reporting delayed urticaria, angioedema, and/or anaphylaxis after eating mammalian meat. They detected alpha-gal specific IgE in nearly 90% of these children (16). Using electronic health records of pediatric patients in southwest Missouri, another study found that the average age of diagnosis among pediatric patients was 9.95 years with most presenting with urticaria and angioedema (99%) followed by anaphylaxis (29%) and gastrointestinal symptoms (17%) (17).
Studies involving children in the US, Spain, and South Africa have led to increasing recognition of different clinical phenotypes for alpha-gal syndrome (5, 16–18). Multiple groups have reported that cutaneous symptoms characterized by urticaria, angioedema, and pruritus are the presenting symptoms for 80–90% of individuals with alpha-gal syndrome (5, 6), followed by anaphylaxis (60%) (6). A gastrointestinal-predominant form of alpha-gal syndrome has also emerged, affecting approximately 20% of patients; patients describe severe, persistent abdominal cramping and diarrhea (5, 17). Moreover, gastrointestinal reflux can be an early symptom, occurring within minutes to 1 hour after ingestion of mammalian products (7).
Alpha-gal syndrome may be the underlying etiology for many cases of idiopathic anaphylaxis. A 2018 study from the University of Tennessee reported that alpha-gal syndrome accounted for 33% of anaphylaxis cases with known cause (19). Moreover, the ability to diagnose alpha-gal syndrome led to a noticeable drop in the number of idiopathic anaphylaxis cases reported in this center, from 59% in 2006 to 35% in 2018 (19). A study from the National Institutes of Health, found 9% of subjects diagnosed with idiopathic anaphylaxis had circulating, detectable alpha-gal-specific IgE (≥0.35 kU/L), reported a tick bite history, and resided within the Southeastern and Mid-Atlantic US where lone star ticks also live (20).
Sensitization to alpha-gal and cardiovascular disease
Alpha-gal specific IgE has also been linked to two cases of premature bioprosthetic heart valve degeneration in patients who developed alpha-gal syndrome years after their aortic valve replacements (21). Clinical symptoms, including chest pain and dyspnea, in conjunction with prosthetic valvular insufficiency and degeneration, developed 1–2 years after diagnosis with alpha-gal syndrome, requiring replacement with mechanical prosthetic valves (21). By contrast, another case series showed that porcine or bovine valve replacement in patients with pre-existing alpha-gal syndrome was associated with peri- and postoperative anaphylaxis attributed to the rapid release of residual alpha-gal antigen in bioprosthetic valves, but longer-term tolerance of their valve replacements (22). Immediate allergic reactions were managed with epinephrine and adjunctive therapies like H1 and H2 blockers and corticosteroids. At 6 to 12 months of followup, they were tolerating their bioprosthetic valves, possibly due to a significant reduction in alpha-gal antigen release from the valve over time (22). Longitudinal observational studies are clearly needed to determine definitively if alpha-gal specific IgE is associated with untimely bioprosthetic valve degeneration.
Circulating alpha-gal specific IgE has also been associated with an increased atherosclerotic burden and unstable plaques in the setting of coronary artery disease, although the association is not causal. This has led some to hypothesize that in alpha-gal sensitized subjects, allergic effector cells activated by alpha-gal promote the production of inflammatory chemicals that contribute to vessel wall damage (23, 24). Further investigation into the potential relationship between alpha-gal sensitization and atherosclerotic disease would enhance our understanding of alpha-gal avoidance and its impact on the natural history and management of atherosclerotic disease.
Epidemiology
There are currently no estimates of the global prevalence of alpha-gal syndrome. Yet, cases of alpha-gal syndrome, both self-reported and physician-diagnosed have been described across the world, including North, Central and South America, Europe, Southern and Western Africa, East and South Asia, Australia and New Zealand (10, 25). A global heat map of Google searches for the term “Alpha-gal allergy” over a 15 year period from 2004–2019 showed the highest search volumes in Sweden, the United States, Canada, Australia, and South Africa (26). There is significant variation in the prevalence of sensitization to alpha-gal, depending on the region of the world and the patient population studied. Importantly, sensitization to alpha-gal does not always reflect the presence of symptomatic disease. For example, 35% of a rural population of German forest service employees and hunters were sensitized to alpha-gal, but only 8.6% of those with detectable circulating alpha-gal specific IgE ≥ 0.35 kU/L were symptomatic (27). An Italian study found that rural populations had sensitization frequencies 20 times higher than urban populations (28), likely due to increased tick exposure and the strong association of alpha-gal specific IgE with tick bite history.
The prevalence of alpha-gal syndrome in the US remains unknown. Estimated rates of sensitization in the Southeastern US range from 15–25% based on small studies of 40 to 250 patients (2, 29, 30). In our institution, the University of North Carolina –Chapel Hill, at least 2500 cases have been identified since 2014 (7). Over 34,000 cases of alpha-gal syndrome have been identified nationwide using 105,000 unique patient samples tested for circulating alpha-gal-specific IgE by Viracor (Armstrong P, Commins SP, personal communication). The high rate (32%) identified among Viracor samples likely reflects the fact that, unlike patient samples in the studies from Europe and the Southeastern US, Viracor’s patient samples are enriched for samples from symptomatic individuals with high pre-test probabilities for having alpha-gal syndrome.
There continues to be a critical need for large studies to determine the prevalence of both alpha-gal sensitization and alpha-gal syndrome itself. Some have used regional and temporal patterns of web-based search activity for alpha-gal allergy as a surrogate for monitoring disease emergence. Using online search traffic on Google, looking at searches for “Alpha-gal allergy” and “lone star tick,” from 2004–2019, Iglesia et al found that these terms were topics of interest in 47 out of 50 states with the top 5 states (Arkansas, Kentucky, Tennessee, Virginia, and North Carolina) located in the Southeastern US (26). They also found that the search volume for alpha-gal allergy queries increased over time with search query peaks coinciding with popular news media coverage of the condition (26).
Similarly, publically available maps show that the geographic distribution of the lone star tick is expanding (https://www.cdc.gov/ticks/geographic_distribution.html). When juxtaposed with maps from crowd-sourced, web-based applications like Zee Maps, which allows individuals to share their locations, it becomes evident that the geographic distribution of the lone star tick mirrors the geographic distribution of self-reported alpha-gal syndrome cases (https://www.zeemaps.com/map?group=555038#).
Blood type also appears to influence the risk of developing alpha-gal syndrome. Rispens et al and Apostolovic et al found that alpha-gal specific IgE antibodies were significantly less abundant in individuals with blood group B or AB (31, 32). Hamsten et al showed that 80% of healthy blood donors and Lyme disease patients sensitized to alpha-gal were B negative (33). A meta-analysis that pooled data from four different cohorts from different countries, Japan, Spain, Sweden, and the US, found that the observed frequency of blood type B and AB among those with red (mammalian) meat allergy was five times lower than expected (34). By contrast, Fischer and colleagues were not able to associate any relationship between susceptibility to the development of alpha-gal-specific IgE antibodies in their patient cohort and blood type B (27). This discrepancy may result from different techniques used to determine blood types, or may be a finding unique to the patient cohort studied. All the studies demonstrate that even if the association is lower, the risk of developing sensitization or allergy to alpha-gal remains, even in those with blood type B.
The working hypothesis to explain the effect of blood type on alpha-gal sensitization and allergy proposes that subjects with blood type B make low quantities of alpha-gal IgE with possibly low binding affinity for alpha-gal. Alpha-gal is closer in structure to blood type B than to A and O; individuals with blood type B may be less likely to create an antibody against a foreign carbohydrate (alpha-gal) very similar in structure to a self-carbohydrate (group B antigen) (35).
Pathophysiology
The process driving sensitization to alpha-gal and the delayed allergic reaction following oral consumption of alpha-gal is not well understood. Sensitization has been linked to bites from ectoparasites, i.e. adult and nymphal stage seed ticks (10, 36, 37). Mouse models have also been used to show that live tick infestation (38) or injection of tick components, including whole body tick extract (39); tick salivary gland extract (TSGE) and tick saliva (40) can generate alpha-gal specific IgE responses in galactosyltransferase deficient (AGKO) mice that lack the ability to make alpha-gal. Moreover, the kinetics of rising alpha-gal specific IgE levels in AGKO mice after TSGE injection mirror the kinetics of alpha-gal specific IgE rise after exposure to ticks like A. americanum (40).
How a tick bite promotes the generation of alpha-gal specific IgE, however, remains unclear. Humans make high titers of IgG, IgM, and IgA antibodies against alpha-gal, likely driven by chronic antigen stimulation by glycans from the commensal microbiota (41, 42). Several tick species, including species linked to alpha-gal syndrome, contain alpha-gal epitopes in the gastrointestinal tract (33) and in the saliva and salivary glands (43, 44). One possibility is that culprit tick species introduce alpha-gal antigen into the host through the skin while simultaneously injecting adjuvant compounds that skew the immune response to alpha-gal to a type 2 (allergic) response that promotes alpha-gal specific antibody class switching from IgG to IgE (39). Other contributing factors could include type 2 allergic immune deviation in the setting of trauma to the skin barrier or alteration of the host skin microbiome. Such insults may drive epithelial cell production of cytokines like TSLP, IL-33, and IL-25, known to promote allergic responses through their influence on dendritic cells, basophils, and innate lymphocytes (8).
The B cell population that produces alpha-gal-specific IgE has not been fully characterized, and may include plasmablasts and short lived plasma cells, perhaps explaining why alpha-gal IgE levels wane over time. Mouse models suggest that alpha-gal specific B cell function may depend in part on the presence of functional CD4+ T cells (39). However, innate T-lymphocyte populations like gamma delta T cells or invariant natural killer T cells that recognize glycolipids could also be involved in the sensitization phase in alpha-gal allergy, and a possible role for these cell populations in alpha-gal allergy is under active investigation (45).
The effector phase of the allergic response to alpha-gal, especially the delay in the development of allergic symptoms following oral ingestion of alpha-gal are also under active study. During experimental mammalian meat challenges, Commins et al found that basophils from alpha-gal allergic subjects are activated 4 hours after alpha-gal ingestion (46). The delay in basophil activation does not seem to be attributable to the alpha-gal antigen itself, because alpha-gal glycoproteins and glycolipids can induce allergic effector cell activation in standard basophil activation tests within 30–60 minutes (13, 14, 47). Interestingly, significant differences in lipid and fatty acid metabolism pathways between individuals with and without alpha-gal syndrome have been reported (15). Thus, delayed allergic reactions in alpha-gal allergy may reflect the hours required for lipid absorption and metabolism (Figure 1).
Figure 1:
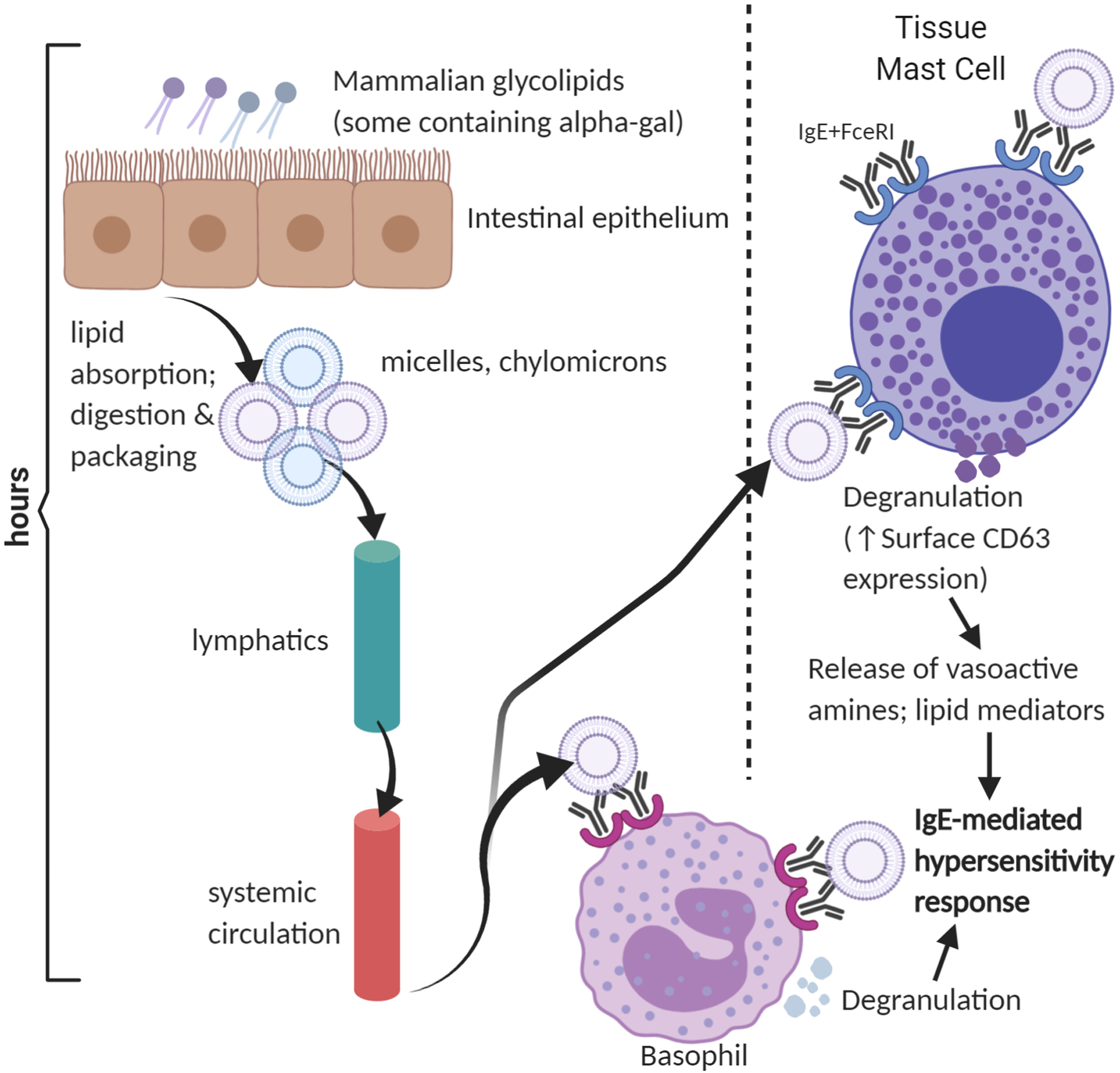
Mammalian glycolipids containing alpha-gal are absorbed, entering the hepatic (not shown) and systemic circulation. Alpha-gal glycolipids, packaged in micelles or chylomicrons or incorporated into lipid bilayers, crosslink alpha-gal specific IgE-FcεRI complexes on basophils and mast cells. The hours required for absorption, processing, and delivery of alpha-gal glycolipids to allergic effector cells may explain the delay in symptoms in alpha-gal syndrome.
Diagnosis
The standard of care to confirm a diagnosis of alpha-gal syndrome is to use serologic testing for alpha-gal specific IgE. The sensitivity of skin prick testing for alpha-gal using commercially available mammalian meat extracts is too low to confirm alpha-gal syndrome, with skin prick tests often negative or borderline positive (3). Intradermal testing with commercially available food extracts is more reliably positive (9), but is outside the standard of care due to concerns of inducing anaphylaxis during testing. Some centers perform prick-to-prick testing with cooked meats, or skin prick and/or intradermal testing with cetuximab or gelatin. These produce large, consistent wheals (≥5 mm) in alpha-gal allergic subjects (48–50).
Regarding serologic testing, the source of alpha-gal differs depending on the testing center. The University of Virginia, which published the first cases of alpha-gal syndrome in the US, uses the alpha-gal-rich cetuximab, estimated to have 2040 microgram (mcg) alpha-gal content per gram cetuximab (51), as an alpha-gal glycoprotein source. Commercially available testing conducted by Viracor in the US and Thermofisher / Phadia in Europe relies on beef thyroglobulin (5.6 mcg alpha-gal / gram protein, (51)) as the source of alpha-gal. Surprisingly, despite the 350-fold difference in alpha-gal content between cetuximab and beef thyroglobulin, there is a strong correlation between alpha-gal specific IgE levels detected using each source (52).
With a convincing clinical history, alpha-gal IgE levels ≥ 0.1 kU/L confirm the diagnosis of alpha-gal syndrome (7). With a murky clinical history, some have argued that an alpha-gal IgE ≥ 2 kU/L or ≥ 2% of the total IgE should be used to confirm the diagnosis of alpha-gal syndrome (50). Some test sites include alpha-gal specific IgE testing within an “alpha-gal panel” which also includes beef, mutton/lamb, pork, and cow’s milk specific IgE testing because patients allergic to alpha-gal frequently have specific IgE that binds to multiple mammal-derived allergens (3), presumably binding to alpha-gal glycans rather than peptide epitopes. This testing, in conjunction with testing for IgE to cat and aeroallergens, serves as a crude surrogate to confirm alpha-gal syndrome if alpha-gal specific IgE testing is not readily available. However, the alpha-gal specific IgE and total IgE levels are the most useful serologic tests for confirming the diagnosis of alpha-gal syndrome.
We also check basal serum tryptase levels, as a surrogate for mast cell burden, particularly in those who present with anaphylaxis. Just as anaphylaxis to stinging insect venom can unmask an underlying systemic mastocytosis (53), individuals with alpha-gal syndrome have also been subsequently diagnosed with indolent systemic mastocytosis (ISM). One study showed that individuals with concurrent alpha-gal syndrome and systemic mastocytosis had more severe clinical reactions after alpha-gal ingestion; elevated serum tryptase levels that were 4-fold higher; and alpha-gal specific IgE levels that were 3-fold lower than alpha-gal allergic subjects without ISM (20).
Management
As with all food or drug allergies, acute management of alpha-gal-induced anaphylaxis requires recognition and immediate treatment with an epinephrine autoinjector. We counsel our patients that they should treat any sign of respiratory or cardiovascular distress following known or suspected exposure to alpha-gal OR physical signs involving two or more organ systems with epinephrine and activate emergency medical services (54). When patients present to the emergency department or urgent care setting, it is also helpful to obtain total serum tryptase levels, especially if patients present within 4 hours of the event. If the tryptase obtained acutely is elevated compared to basal tryptase levels, this confirms anaphylaxis. Results can take several days to return, however, and are not always elevated in the setting of food-induced anaphylaxis (55). If both acutely-obtained and basal tryptase levels are above normal, this suggests an elevated mast cell burden and possible mastocytosis (20).
Currently, as with all food and drug allergies, long term management of alpha-gal allergic patients centers on avoidance of alpha-gal-containing foods and drugs. We suggest 3 different tiers of alpha-gal avoidance (Figure 2). “Tier 1” avoidance involves elimination of dietary beef, pork, lamb, venison and other mammalian meats and innards as well as intravenous medications with high alpha-gal content including cetuximab, gelatin-based colloid agents, and -- after weighing risks and benefits -- anti-venom therapies. We also remind our patients to watch for “hidden sources” of alpha-gal including beef broth in soups, animal fat drippings in gravy, pork encasings for chicken or turkey sausages, or fatback or bacon in vegetable dishes and salads. Unless the patient reports an adverse reaction following the ingestion of dairy products, we encourage patients to incorporate moderate amounts of dairy in their diets. In our clinical experience, hard cheeses like cheddar or parmesan, skim (0%), low-fat (1–2%), or whole (4%) milk are typically well tolerated. Heavy cream (18–36% milkfat), soft cheeses like brie or mozzarella, and full-fat ice cream are more likely to induce adverse responses. Symptoms resolve in ~80% of alpha-gal allergic patients with Tier 1 avoidance.
Figure 2:
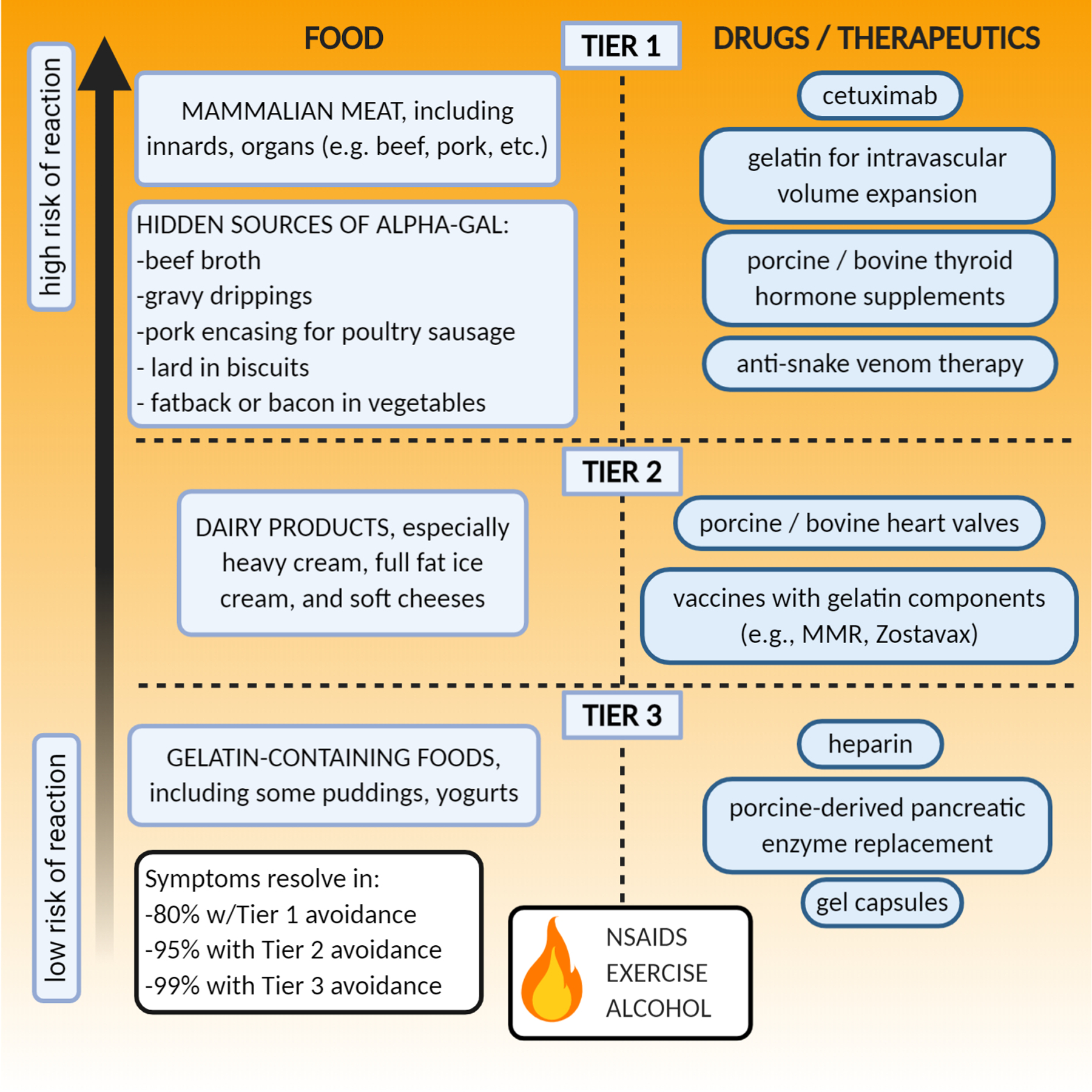
Long-term management of alpha-gal syndrome involves avoidance of alpha-gal containing foods and medications, including hidden sources of alpha-gal, and counseling on the accelerant effects of co-factors like exercise and alcohol on allergic responses to alpha-gal.
“Tier 2” avoidance includes Tier 1 avoidance plus the elimination of dietary dairy products; avoiding heart valve replacements with bovine or porcine products; and avoiding (if possible), gelatin-containing vaccines (e.g. Zostavax; measles, mumps, and rubella (MMR)). Symptoms resolve in 95% percent of alpha-gal allergic patients with Tier 2 avoidance. “Tier 3” avoidance involves not only Tier 1 and 2 avoidance, but also elimination of gelatin-containing foods (some puddings or yogurt), gelcaps, heparin, and pancreatic enzyme replacement that might contain trace amounts of alpha-gal (7, 50).
Importantly, the vast majority of patients with alpha-gal syndrome tolerate most orally delivered pharmaceutical products, even those with trace amounts of alpha-gal. If a medication that may contain alpha-gal is critical for the good health of an alpha-gal allergic patient and there are no suitable alternatives, we recommend referral to an allergist for an oral challenge with this medication (56), even in patients with confirmed alpha-gal syndrome and positive skin testing to the drug (57). For those patients who remain symptomatic, even after removing all known sources of alpha-gal from the diet and medication regimen, we recommend as needed use of H1 antihistamines to block allergic mediators produced by mast cells and basophils, and cromolyn to stabilize mast cells, especially in the gut.
Finally, we counsel patients to avoid bites from both adult and nymphal stage ticks, (sometimes referred to as seed ticks or “chiggers” in the Southeastern US (58)) since these bites have been associated with acute increases in both total and alpha-gal specific IgE levels (58).
Natural history and reintroducing mammalian meat
“Will I ever be able to eat steak again, Doc?”
No studies currently address whether individuals can “outgrow” alpha-gal allergy and reintroduce dietary mammalian meat. Kim et al have shown that alpha-gal allergic individuals who avoid tick bites experience a decline in alpha-gal specific IgE levels (59). Therefore, we check alpha-gal specific IgE and total IgE levels in our alpha-gal allergic patients at least once yearly. No tools currently exist to predict the likelihood or rate of decline for alpha-gal specific IgE levels. In our patients practicing Tier 1 avoidance, if after at least 12 months of monitoring, alpha-gal specific IgE levels have dropped to ≤0.35 kU/L or ≤2% of the total IgE, with no adverse reactions to accidental mammalian meat ingestion or known dairy ingestion, we discuss re-introducing mammalian products back into the diet. For most patients, the risk of anaphylaxis outweighs any benefit of reintroducing mammal meat. We counsel those who elect to add back mammalian meat to their diet on when and how to use their epinephrine autoinjectors and seek emergency help. The process is done slowly and cautiously, starting with very small portions of low-fat, lean meats in the presence of a friend or family member.
At our center, between 1 and 10% of alpha-gal allergic patients each year have reintroduced mammalian meat successfully into their diets. Patients whose symptoms are controlled with Tier 1 avoidance (i.e. they tolerate dairy), who avoid tick bites and have significant drops in alpha-gal specific IgE are more likely to successfully re-incorporate mammalian food products into their diet if desired (7).
Re-introduction of mammalian meat into an alpha-gal allergic person’s diet was also described in a case report about the successful desensitization of a child with alpha-gal syndrome to beef, an integral part of his family’s diet that he could not avoid (60). Similar to oral immunotherapy for food-protein allergy, this subject must eat a serving of beef daily or risk re-developing adverse symptoms to mammalian meat (60).
Conclusion
With its delayed symptom onset driven by IgE antibodies to a glycan rather than a protein, alpha-gal syndrome challenges conventional conceptions of food allergy. Research on this allergic condition continues to expand. Probing how bites from certain tick species drive sensitization to alpha-gal, and exploring host characteristics that predispose to developing alpha-gal syndrome, be they immune, genetic, metabolic, and beyond, will inform future diagnostic and therapeutic interventions critical for the management of individuals with this condition.
Key points:
Alpha-gal syndrome is an allergic condition with global reach.
Alpha-gal syndrome refers to the symptoms associated with IgE-mediated hypersensitivity responses to the carbohydrate galactose-alpha-1,3-galactose.
Alpha-gal syndrome manifests as both immediate hypersensitivity responses to intravenously administered pharmaceuticals containing alpha-gal and delayed hypersensitivity responses following the ingestion of mammalian food products, especially meat, innards, and organs.
Sensitization to alpha-gal is linked to bites from ectoparasites, including the lone star tick Amblyomma americanum.
Delayed allergic reactions following the ingestion of alpha-gal may reflect the hours required for alpha-gal glycolipid absorption, metabolism, and trafficking to sensitized allergic effector cells in the blood and tissues.
Acknowledgements
We thank Dr. Scott Commins and Claire Amelio, RN for their helpful discussions. We thank Drs. Claire Van Eenwyk and Kathleen Wang for critical review of this manuscript. Figures were created with BioRender.com.
Disclosure of Funding:
Dr. Iweala is supported by NIH grant K08AI141691, a 2020 AAAAI Foundation Career Development Award, and a 2019 Thurston Arthritis Center Pilot Award. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of interest. Dr. Patel reports no relevant disclosures. Dr. Iweala is a consultant for Matzellen Bio and Blueprint Medicines.
References and Recommended Reading
(*) Special interest
(**) Outstanding interest
- 1.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358(11):1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neil BH, Allen R, Spigel DR, Stinchcombe TE, Moore DT, Berlin JD, et al. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25(24):3644–8. [DOI] [PubMed] [Google Scholar]
- 3.Commins SP, Satinover SM, Hosen J, Mozena J, Borish L, Lewis BD, et al. Delayed anaphylaxis, angioedema, or urticaria after consumption of red meat in patients with IgE antibodies specific for galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2009;123(2):426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Nunen SA, O’Connor KS, Clarke LR, Boyle RX, Fernando SL. An association between tick bite reactions and red meat allergy in humans. Med J Aust. 2009;190(9):510–1. [DOI] [PubMed] [Google Scholar]
- 5.Mabelane T, Basera W, Botha M, Thomas HF, Ramjith J, Levin ME. Predictive values of alpha-gal IgE levels and alpha-gal IgE: Total IgE ratio and oral food challenge-proven meat allergy in a population with a high prevalence of reported red meat allergy. Pediatr Allergy Immunol. 2018. [DOI] [PubMed] [Google Scholar]; *This is the first report to formally describe the gastrointestinal-predominant alpha-gal syndrome phenotype. This is also the only report to date describing a cohort of Black Africans with alpha-gal syndrome.
- 6.Wilson JM, Schuyler AJ, Workman L, Gupta, James HR, Posthumus J, et al. Investigation into the alpha-Gal Syndrome: Characteristics of 261 Children and Adults Reporting Red Meat Allergy. J Allergy Clin Immunol Pract. 2019;7(7):2348–58 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This observational study follows over 250 children and adults with red meat allergy and reports the most common etiologies for it (alpha-gal syndrome) the most common clinical presentations, and relationships between alpha-gal syndrome and blood type in this cohortof patients from the Southeastern US.
- 7.Commins SP. Diagnosis & management of Alpha-gal Syndrome: Lessons from 2,500 patients. Expert Rev Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; **A Comprehensive review of how to diagnose and manage Alpha-gal syndrome drawing on the literature and the clinical experience of the author who published the seminal paper in the field of alpha-gal syndrome.
- 8.Iweala OI, Nagler CR. The Microbiome and Food Allergy. Annu Rev Immunol. 2019;37:377–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Commins SP, James HR, Kelly LA, Pochan SL, Workman LJ, Perzanowski MS, et al. The relevance of tick bites to the production of IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2011;127(5):1286–93 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cabezas-Cruz A, Hodzic A, Roman-Carrasco P, Mateos-Hernandez L, Duscher GG, Sinha DK, et al. Environmental and Molecular Drivers of the alpha-Gal Syndrome. Front Immunol. 2019;10:1210. [DOI] [PMC free article] [PubMed] [Google Scholar]; **A comprehensive review of the tick species associated with the development of alpha-gal syndrome, and proposed mechanisms to explain why tick bites lead to development of alpha-gal specific IgE.
- 11.Rutkowski K, Wagner A, Rutkowski R, Sowa P, Pancewicz S, Moniuszko-Malinowska A. Alpha-gal syndrome: An emerging cause of food and drug allergy. Clin Exp Allergy. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Hilger C, Fischer J, Swiontek K, Hentges F, Lehners C, Eberlein B, et al. Two galactose-alpha-1,3-galactose carrying peptidases from pork kidney mediate anaphylactogenic responses in delayed meat allergy. Allergy. 2016;71(5):711–9. [DOI] [PubMed] [Google Scholar]
- 13.Mehlich J, Fischer J, Hilger C, Swiontek K, Morisset M, Codreanu-Morel F, et al. The basophil activation test differentiates between patients with alpha-gal syndrome and asymptomatic alpha-gal sensitization. J Allergy Clin Immunol. 2019;143(1):182–9. [DOI] [PubMed] [Google Scholar]
- 14.Iweala OI, Choudhary SK, Addison CT, Batty CJ, Kapita CM, Amelio C, et al. Glycolipid-mediated basophil activation in alpha-gal allergy. J Allergy Clin Immunol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]; *One of the first reports to suggest a role for glycolipids in the effector phase of IgE-mediated food allergy by demonstrating that alpha-gal glycolipids can activate basophils sensitized with plasma from alpha-gal allergic subjects in an IgE-dependent fashion.
- 15.Steinke JW, Pochan SL, James HR, Platts-Mills TAE, Commins SP. Altered metabolic profile in patients with IgE to galactose-alpha-1,3-galactose following in vivo food challenge. J Allergy Clin Immunol. 2016;138(5):1465–7 e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kennedy JL, Stallings AP, Platts-Mills TA, Oliveira WM, Workman L, James HR, et al. Galactose-alpha-1,3-galactose and delayed anaphylaxis, angioedema, and urticaria in children. Pediatrics. 2013;131(5):e1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donaldson B, Le MN. The clinical presentation of alpha-gal allergy among pediatric patients with food allergy in southwest Missouri. Ann Allergy Asthma Immunol. 2019;123(5):524–5. [DOI] [PubMed] [Google Scholar]; *One of the few studies describing the epidemiology and clinical features of alpha-gal syndrome in a pediatric population.
- 18.Martin-Lazaro J, Nunez-Orjales R, Gonzalez-Guzman LA, Gonzalez MT, Boquete M, Carballada F. Galactose-alpha-1,3-galactose (alpha-gal) allergy: first pediatric case in a series of patients in Spain. Allergol Immunopathol (Madr). 2020;48(3):251–8. [DOI] [PubMed] [Google Scholar]
- 19.Pattanaik D, Lieberman P, Lieberman J, Pongdee T, Keene AT. The changing face of anaphylaxis in adults and adolescents. Ann Allergy Asthma Immunol. 2018;121(5):594–7. [DOI] [PubMed] [Google Scholar]
- 20.Carter MC, Ruiz-Esteves KN, Workman L, Lieberman P, Platts-Mills TAE, Metcalfe DD. Identification of alpha-gal sensitivity in patients with a diagnosis of idiopathic anaphylaxis. Allergy. 2018;73(5):1131–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hawkins RB, Frischtak HL, Kron IL, Ghanta RK. Premature Bioprosthetic Aortic Valve Degeneration Associated with Allergy to Galactose-Alpha-1,3-Galactose. J Card Surg. 2016;31(7):446–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mozzicato SM, Tripathi A, Posthumus JB, Platts-Mills TAE, Commins SP. Porcine or bovine valve replacement in 3 patients with IgE antibodies to the mammalian oligosaccharide galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2014;2(5):637–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson JM, Nguyen AT, Schuyler AJ, Commins SP, Taylor AM, Platts-Mills TAE, et al. IgE to the Mammalian Oligosaccharide Galactose-alpha-1,3-Galactose Is Associated With Increased Atheroma Volume and Plaques With Unstable Characteristics-Brief Report. Arterioscler Thromb Vasc Biol. 2018;38(7):1665–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wilson JM, McNamara CA, Platts-Mills TAE. IgE, alpha-Gal and atherosclerosis. Aging (Albany NY). 2019;11(7):1900–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin M, Apostolovic D, Biedermann T, Commins SP, Iweala OI, Platts-Mills TAE, et al. Galactose alpha-1,3-galactose phenotypes: Lessons from various patient populations. Ann Allergy Asthma Immunol. 2019;122(6):598–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iglesia EGA, Stone CA Jr., Flaherty MG, Commins SP. Regional and temporal awareness of alpha-gal allergy: An infodemiological analysis using Google Trends. J Allergy Clin Immunol Pract. 2020;8(5):1725–7 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer J, Lupberger E, Hebsaker J, Blumenstock G, Aichinger E, Yazdi AS, et al. Prevalence of type I sensitization to alpha-gal in forest service employees and hunters. Allergy. 2017;72(10):1540–7. [DOI] [PubMed] [Google Scholar]
- 28.Villalta D, Pantarotto L, Da Re M, Conte M, Sjolander S, Borres MP, et al. High prevalence of sIgE to Galactose-alpha-1,3-galactose in rural pre-Alps area: a cross-sectional study. Clin Exp Allergy. 2016;46(2):377–80. [DOI] [PubMed] [Google Scholar]
- 29.Burk CM, Beitia R, Lund PK, Dellon ES. High rate of galactose-alpha-1,3-galactose sensitization in both eosinophilic esophagitis and patients undergoing upper endoscopy. Dis Esophagus. 2016;29(6):558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keating K, Walko C, Stephenson B, O’Neil BH, Weiss J. Incidence of cetuximab-related infusion reactions in oncology patients treated at the University of North Carolina Cancer Hospital. J Oncol Pharm Pract. 2014;20(6):409–16. [DOI] [PubMed] [Google Scholar]
- 31.Rispens T, Derksen NI, Commins SP, Platts-Mills TA, Aalberse RC. IgE production to alpha-gal is accompanied by elevated levels of specific IgG1 antibodies and low amounts of IgE to blood group B. PLoS One. 2013;8(2):e55566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apostolovic D, Rodrigues R, Thomas P, Starkhammar M, Hamsten C, van Hage M. Immunoprofile of alpha-Gal- and B-antigen-specific responses differentiates red meat-allergic patients from healthy individuals. Allergy. 2018;73(7):1525–31. [DOI] [PubMed] [Google Scholar]
- 33.Hamsten C, Starkhammar M, Tran TA, Johansson M, Bengtsson U, Ahlen G, et al. Identification of galactose-alpha-1,3-galactose in the gastrointestinal tract of the tick Ixodes ricinus; possible relationship with red meat allergy. Allergy. 2013;68(4):549–52. [DOI] [PubMed] [Google Scholar]
- 34.Brestoff JR, Tesfazghi, Zaydman, Jackups R Jr., Kim BS, Scott MG, et al. The B antigen protects against the development of red meat allergy. J Allergy Clin Immunol Pract. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A meta-analysis showing that in patients with alpha-gal syndrome / red meat allergy express lower levels of the B blood group antigen or beef specific IgE than patients without B-antigen.
- 35.Bircher AJ, Hofmeier KS, Link S, Heijnen I. Food allergy to the carbohydrate galactose-alpha-1,3-galactose (alpha-gal): four case reports and a review. Eur J Dermatol. 2017;27(1):3–9. [DOI] [PubMed] [Google Scholar]
- 36.Platts-Mills TAE, Commins SP, Biedermann T, van Hage M, Levin M, Beck LA, et al. On the cause and consequences of IgE to galactose-alpha-1,3-galactose: A report from the National Institute of Allergy and Infectious Diseases Workshop on Understanding IgE-Mediated Mammalian Meat Allergy. J Allergy Clin Immunol. 2020;145(4):1061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hashizume H, Fujiyama T, Umayahara T, Kageyama R, Walls AF, Satoh T. Repeated Amblyomma testudinarium tick bites are associated with increased galactose-alpha-1,3-galactose carbohydrate IgE antibody levels: A retrospective cohort study in a single institution. J Am Acad Dermatol. 2018;78(6):1135–41 e3. [DOI] [PubMed] [Google Scholar]
- 38.Commins SP, Karim S. Development of a novel murine model of alpha-gal meat allergy. J Allergy Clin Immunol. 2017;139:AB193. [Google Scholar]
- 39.Chandrasekhar JL, Cox KM, Loo WM, Qiao H, Tung KS, Erickson LD. Cutaneous Exposure to Clinically Relevant Lone Star Ticks Promotes IgE Production and Hypersensitivity through CD4(+) T Cell- and MyD88-Dependent Pathways in Mice. J Immunol. 2019;203(4):813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhary S, Iweala OI, Addison CT, Commins SP. Tick salivary extract induces alpha-gal allergy in alpha-gal deficient mice. J Allergy Clin Immunol. 2019;143:AB252. [Google Scholar]
- 41.Galili U Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roman-Carrasco P, Hemmer W, Klug C, Friedrich A, Stoll P, Focke-Tejkl M, et al. Individuals with IgE antibodies to alpha-Gal and CCD show specific IgG subclass responses different from subjects non-sensitized to oligosaccharides. Clin Exp Allergy. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araujo RN, Franco PF, Rodrigues H, Santos LCB, McKay CS, Sanhueza CA, et al. Amblyomma sculptum tick saliva: alpha-Gal identification, antibody response and possible association with red meat allergy in Brazil. Int J Parasitol. 2016;46(3):213–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crispell G, Commins SP, Archer-Hartman SA, Choudhary S, Dharmarajan G, Azadi P, et al. Discovery of Alpha-Gal-Containing Antigens in North American Tick Species Believed to Induce Red Meat Allergy. Front Immunol. 2019;10:1056. [DOI] [PMC free article] [PubMed] [Google Scholar]; *This study reported the presence of detectable alpha-gal in 2 common American tick species and localized the alpha-gal moieties to the salivary glands of the ticks.
- 45.Iweala OI, Savage PB, Commins SP. A Role for CD1d-restricted Invariant Natural Killer T cells and Glycolipids in Alpha-Gal Allergy. J Allergy Clin Immunol. 2018;141(2, Supplement):AB288. [Google Scholar]
- 46.Commins SP, James HR, Stevens W, Pochan SL, Land MH, King C, et al. Delayed clinical and ex vivo response to mammalian meat in patients with IgE to galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2014;134(1):108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roman-Carrasco P, Lieder B, Somoza V, Ponce M, Szepfalusi Z, Martin D, et al. Only alpha-Gal bound to lipids, but not to proteins, is transported across enterocytes as an IgE-reactive molecule that can induce effector cell activation. Allergy. 2019;74(10):1956–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fischer J, Hebsaker J, Caponetto P, Platts-Mills TA, Biedermann T. Galactose-alpha-1,3-galactose sensitization is a prerequisite for pork-kidney allergy and cofactor-related mammalian meat anaphylaxis. J Allergy Clin Immunol. 2014;134(3):755–9 e1. [DOI] [PubMed] [Google Scholar]
- 49.Caponetto P, Fischer J, Biedermann T. Gelatin-containing sweets can elicit anaphylaxis in a patient with sensitization to galactose-alpha-1,3-galactose. J Allergy Clin Immunol Pract. 2013;1(3):302–3. [DOI] [PubMed] [Google Scholar]
- 50.Platts-Mills TAE, Li RC, Keshavarz B, Smith AR, Wilson JM. Diagnosis and Management of Patients with the alpha-Gal Syndrome. J Allergy Clin Immunol Pract. 2020;8(1):15–23 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]; **This review by a reknowned center for alpha-gal syndrome research provides practical advice on the diagnosis and management of patients with alpha-gal syndrome with helpful schematics and tables.
- 51.Mullins RJ, James H, Platts-Mills TA, Commins S. Relationship between red meat allergy and sensitization to gelatin and galactose-alpha-1,3-galactose. J Allergy Clin Immunol. 2012;129(5):1334–42 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jappe U, Minge S, Kreft B, Ludwig A, Przybilla B, Walker A, et al. Meat allergy associated with galactosyl-alpha-(1,3)-galactose (alpha-Gal)-Closing diagnostic gaps by anti-alpha-Gal IgE immune profiling. Allergy. 2018;73(1):93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Golden DB, Demain J, Freeman T, Graft D, Tankersley M, Tracy J, et al. Stinging insect hypersensitivity: A practice parameter update 2016. Ann Allergy Asthma Immunol. 2017;118(1):28–54. [DOI] [PubMed] [Google Scholar]
- 54.LoVerde D, Iweala OI, Eginli A, Krishnaswamy G. Anaphylaxis. Chest. 2018;153(2):528–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dua S, Dowey J, Foley L, Islam S, King Y, Ewan P, et al. Diagnostic Value of Tryptase in Food Allergic Reactions: A Prospective Study of 160 Adult Peanut Challenges. J Allergy Clin Immunol Pract. 2018;6(5):1692–8 e1. [DOI] [PubMed] [Google Scholar]
- 56.Eberlein B, Mehlich J, Reidenbach K, Pilz C, Hilger C, Darsow U, et al. Negative oral provocation test with porcine pancreatic enzyme plus cofactors despite confirmed alpha-Gal syndrome. J Investig Allergol Clin Immunol. 2020:0. [DOI] [PubMed] [Google Scholar]
- 57.Stone CA Jr., Choudhary S, Patterson MF, Rukasin CRF, Coleman DT, Phillips EJ, et al. Tolerance of porcine pancreatic enzymes despite positive skin testing in alpha-gal allergy. J Allergy Clin Immunol Pract. 2020;8(5):1728–32 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stoltz LP, Cristiano LM, Dowling APG, Wilson JM, Platts-Mills TAE, Traister RS. Could chiggers be contributing to the prevalence of galactose-alpha-1,3-galactose sensitization and mammalian meat allergy? J Allergy Clin Immunol Pract. 2019;7(2):664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim MS, Straesser MD, Keshavarz B, Workman L, McGowan EC, Platts-Mills TAE, et al. IgE to galactose-alpha-1,3-galactose wanes over time in patients who avoid tick bites. J Allergy Clin Immunol Pract. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yucel E, Sipahi Cimen S, Varol S, Suleyman A, Ozdemir C, Tamay ZU. Red meat desensitization in a child with delayed anaphylaxis due to alpha-Gal allergy. Pediatr Allergy Immunol. 2019;30(7):771–3. [DOI] [PubMed] [Google Scholar]


