Abstract
Amantadine has recently been shown to improve patients with COVID-19. In addition to its known mechanism of actions, we performed docking prediction of this drug on the receptor-binding domain of severe acute respiratory syndrome coronavirus 2, SARS-CoV-2. We hypothesize that such interaction may possibly have contributed a role in the clinical improvements reported.
Keywords: SARS-CoV-2, COVID-19, Wuhan coronavirus outbreak, viral pandemics, SARS virus, MERS virus, bat virus, zoonotic infections, biological agents, BSL 4. vaccine and antibody against SARS-CoV-2
A. Amantadine
A1. Chemistry
Amantadine, originally marketed as an antiviral agent for influenza A, is Adamantan-1-amine. 1-Adamantylamine is a synthetic tricyclic amine, and it is a member of the class of adamantanes.
A2. Pharmacology of Amantadine
Amantadine, originally marketed as an antiviral agent for influenza A, is an alternative medication for antipsychotic-induced parkinsonism and avoids the adverse central nervous system and peripheral effects of anticholinergic medications.1 Its mechanism of action is unclear but appears to involve presynaptic dopamine (DA) reuptake blockade, facilitation of DA release, postsynaptic DA agonism, and receptor modulation.2 Originally, amantadine was used in the Influenza A virus (strain H2N2) and its antiviral activity is considered to involve its binding to the influenza A virus M2 proton-selective ion channel.1,2 Amantadine given orally is well absorbed from the gastrointestinal tract and is given normally in a dose of 100 mg, twice a day for at least 14 days in adults. Amantadine has a mild diuretic action and is mostly excreted unchanged in the urine. The lowest reported acute lethal dose was noted to be 2 g in adults.2
B. Receptor-Binding Domain of the SARS-CoV-2 Spike Protein
The SARS-CoV-2 expresses a cell surface spike (S) protein, a specific segment of which is the receptor-binding domain (RBD), which plays a vital role in infecting the host cell by binding to a cell surface protein angiotensin-converting enzyme 2 (ACE2).3−5 A sequence of events follows the initial binding of RBD of SARS-CoV-2 to the ACE2, involving TMPRSS2, Furin, and Cathepsin L results in the fusion of cell membranes of the SARS-CoV-2 with the host cell, with the ultimate entry of the RNA of the SARS-CoV-2 into the cells.5 Subsequently, the SARS-CoV-2 hijacks the host cellular protein synthesis machinery, and the cascade of events that follows results in the budding of the virus from the host cell and eventual cell death. Of the several proteins that can be potential druggable targets in SARS-CoV-2, understandably the RBD of the S-protein is one of the most desired molecular target because of its cardinal role in host cell binding. We got involved in the SARS-CoV-2 related research due to our forte in use of bioinformatics computational tools in drug target discovery that were expected to identify vulnerabilities in the RBD of SARS-CoV-2 that can be exploited for therapeutic gains.5 With the interest in drug discovery by the repurposing of already existing drugs, we planned on molecular docking of FDA approved drugs that could show an induced-fit docking prediction by interacting with the RBD of SARS-CoV-2. It was hypothesized that drugs that had the potential to engage with the RBD with strong bonds could neutralize or interfere with the potential of the SARS-CoV-2 binding and therefore its subsequent entry into the host cells. A list of FDA approved drugs and their structural analogues were inferred to be valuable compounds that could be used for molecular docking and subsequent in vitro testing in SARS-CoV-2. With the unavailability of clinical isolates at present, instead of a direct in vitro testing on clinical isolates of SARS-CoV-2, we first planned to strengthen our rationale of the potential of FDA approved drugs to dock over the RBD by performing molecular dockings of the drugs on the RBD of SARS-CoV-2. In-depth analysis of the constellation of amino acids in the RBD of SARS-CoV-2 that interact with human ACE2, and hinted by the chemistry of the ligand-binding attributes of the RBD of SARS-CoV-2, we selected amantadine and its analogues for molecular dockings predictions.
B1. Docking Predictions of Amantadine over the RBD of SARS-CoV-2
Three-dimensional chemical structure of selected compounds (ligands) were retrieved from PubChem. The structure of the RBD of the spike glycoprotein of SARS-CoV-2 uncovered in recent publications with PDB IDs 6vsb.1, 6m0j, and 6vw1(3−4) were downloaded from Protein Data Bank (PDB). The RBD structures were first analyzed using a Discovery Studio (DS) modeling visualizer. The RBD structure was processed with AutoDock Vina and the constellation of amino acids that interact with the human ACE2 receptors was identified. The amino acids that were identified were further compared with the amantadine binding sites within the RBD of SARS-CoV-2. Amino acids on the RBD that are directly involved in interacting with the ACE2 receptor and the amantadine in 3D format were visualized to ascertain the interaction of amino acids of the RBD with the amantadine. After identification of the binding site, a grid was formed on the structure of RBD. With the maximum number of binding modes set to 10, the top docking results were obtained. Grid values were set in AutoDock and the 3D structure of RBD and amantadine were saved in PDBQT format which is readable by AutoDock Vina. Docking results scores were ranked according to the virtual docking binding free energy that sets up an affinity binding score between ligand and target protein receptors. Ligands with considerable binding scores were further analyzed using a DS visualizer. We identified the structure of binding atoms and interactions between bonding atoms of ligand and receptor on the binding site as well.
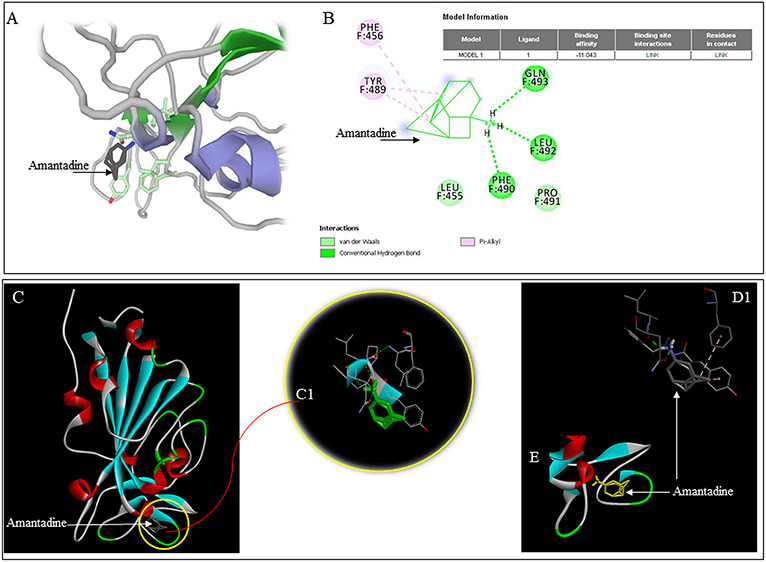
B2. Docking of Amantadine on RBD of SARS-CoV-2
The AutoDock docking results were visualized using a DS visualizer. The amantadine docking site on RBD is shown (Figure 1A) with the contact area magnified (Figure 1B). Interaction of amantadine without receptor surface (Figure 1C) and with receptor surface demarcations (Figure 1 D) shows the interaction sites of amantadine within the binding pocket.
Figure 1.
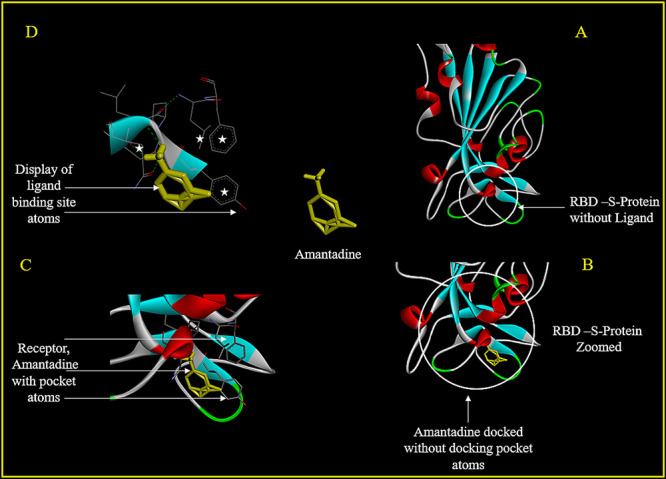
Drug docking details for amantadine to the RBD of SARS-CoV-2. The RBD of SARS-CoV-2 is shown (A-circle), with the amantadine (yellow) docked and zoomed (B-circle and arrow) without the atoms in the docking pocket. Amantadine (yellow) docked onto the RBD zoomed along with interacting atoms (C-arrow) and magnified further (D). The receptor surface on the RBD is shown with binding atoms in the docking pocket (D, stars).
B3. Attributes of the Docking Prediction of Amantadine on RBD of SARS-CoV-2
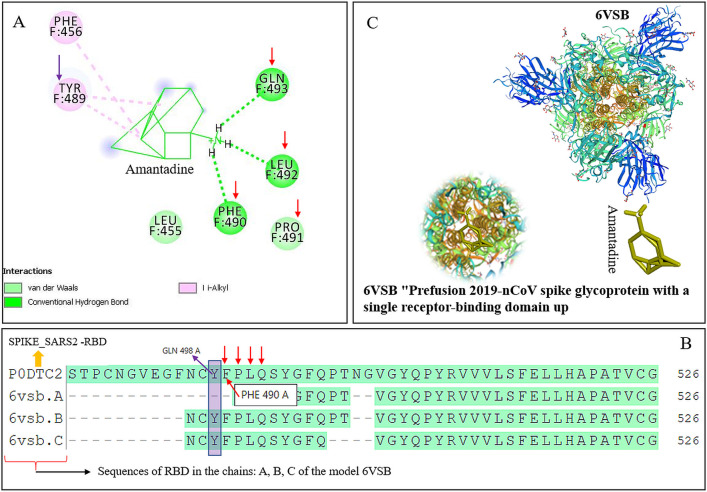
Docking results showed that amantadine has the potential to bind with all the models of RBD selected mentioned above. The binding energy value for the receptor–ligand interaction obtained for 6vsb.1 was −11.043 and similar values that were obtained for 6m0j and 6vw1 were noted to be exceptionally better as compared to other ligands docked onto the RBD, reported recently. Amino acids that were predicted to be involved in ligand–receptor interactions are Tyr489, Phe456, Gln493, Leu 492, Phe490, Pro491, and Leu455. Of the above amino acids that interact with the RBD, there were two categories found, those with direct binding to amantadine in no-covalent interactions while others served as supporting moieties. Interestingly, the docking prediction results show (Figure 2A) that amantadine interacts with the amino acids, within the RBD that comes in direct contact with amino acids of ACE25 (Figure 2B,C) that enables the enzymatic cascade needed for SARS-CoV-2 to enter the host cell. Two target amino acids Tyr489 and Phe456 (comes in direct contact with amino acids of ACE2 during host cell binding) that are within the pocket of the RBD showed to be involved in bonding π–alkyl interactions between them and amantadine (Figure 2A).
Figure 2.
Nature of the bonds and amino acids engaged in the binding of amantadine to the RBD of SARS-CoV-2. (A) The amantadine ligand plot details show the amino acids that the RBD engaged and the nature of the bonding of amantadine. (B) The amino acid sequences in the RBD chains (6vsb.1A, B, C) are shown in boxes with arrows that can be matched with the constellation shown in (A). Model of 6vsb.1 (C) with the amantadine docked on the RBD is shown (circular zoomed area).
C. Discussion
The causative agent SARS-CoV-2 of the ongoing pandemic COVID-19 has proven to be a notorious and die-hard virus that has speeded the research to discover vaccines, monoclonal antibodies,5 and drugs to treat affected patients. Many drugs such as famotidine and hydroxyquinoline, and antiviral agents such as remdesivir, have surfaced and recently have been given to patients with COVID-19 with mixed results. The repurposing of already existing drugs that are approved by the FDA is a strength that can speed up our fight against COVID-19. Amantadine was originally launched as an antiviral drug1,2 for influenza A infections. There have been recommendations for its use in COVID-19, and its use recently has been reported to be efficacious in an observational study,6 but the interaction of amantadine with the RBD of the SARS-CoV-2 has not been reported yet. We for the first time hypothesized that, in addition to its known mechanism of actions mentioned above, there was a possibility that the interaction of amantadine with the amino acids of the RBD that interact with human ACE2 would have played a role in the results reported recently with amantadine.5 The exact nature and the effects of the binding of amantadine to the RBD of the SARS-CoV-2 are not known yet, but we infer that the drug binding could act as a physical impediment and therefore could weaken the interaction of the RDB with the ACE2 receptor.
Future Directions
Future investigations are expected to highlight the in-depth nature and effects of the interaction of amantadine with the amino acids shown in this study. We implore researchers to further investigate the finding we have reported here so that this drug can be repurposed for use against COVID-19. Also, important is to speed up the synthesis of the analogues of amantadine to improve interaction with the remaining amino acids of the RBD that interact with the ACE2 receptor.
Acknowledgments
The author would like to thank the AKU and the department of BBS for their support. The authors have no association with the local or global manufacturers of amantadine.
The authors declare no competing financial interest.
This paper was published ASAP on November 3, 2020 with incorrect Figures and associated captions and textual references. The corrected version was posted November 19, 2020.
References
- Goodman L. S., Brunton L. L., Chabner B., and Knollmann B. C. (2011) Goodman & Gilman’s pharmacological basis of therapeutics, McGraw-Hill, New York. [Google Scholar]
- Law V.; Knox C.; Djoumbou Y.; Jewison T.; Guo A. C.; Liu Y.; Maciejewski A.; Arndt D.; Wilson M.; Neveu V.; Tang A.; Gabriel G.; Ly C.; Adamjee S.; Dame Z. T.; Han B.; Zhou Y.; Wishart D. S. (2014) Drug Bank 4.0: shedding new light on drug metabolism. Nucleic Acids Res. 42 (1), D1091–7. 2014 Jan 1 PubMed ID: 24203711 10.1093/nar/gkt1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan R.; Zhang Y.; Li Y.; Xia L.; Guo Y.; Zhou Q. (2020) Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 367 (6485), 1444–1448. 10.1126/science.abb2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan J.; Ge J.; Yu J.; Shan S.; Zhou H.; Fan S.; Zhang Q.; Shi X.; Wang Q.; Zhang L.; Wang X. (2020) Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581 (7807), 215–220. 2020 May Epub 2020 Mar 30. PMID: 32225176 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- Shang J.; Ye G.; Shi K.; Wan Y.; Luo C.; Aihara H.; Geng Q.; Auerbach A.; Li F. (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581 (7807), 221–224. 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig A. M.; Khaleeq A; Syeda H. (2020) Elucidation of cellular targets and exploitation of the receptor-binding domain of SARS-CoV-2 for vaccine and monoclonal antibody synthesis. J. Med. Virol 26212 10.1002/jmv.26212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda-Abreu G. E., Aranda-Martínez J. D., Araújo R, Hernández-Aguilar M. E., Herrera-Covarrubias D, and Rojas-Durán F. (2020). Observational study of people infected with SARS-Cov-2, treated with amantadine. Pharmacol Rep. 2020 Oct 10. 10.1007/s43440-020-00168-1. Epub ahead of print. PMID: 33040252. [DOI] [PMC free article] [PubMed] [Google Scholar]