Abstract
Bevacizumab is a monoclonal antibody which targets vascular endothelial growth factor A (VEGF-A) and is used to treat various cancers and recently COVID-19. The dosage recommendations for bevacizumab are determined on the basis of body weight, and the drug is administered after defined time intervals, when it is presumed to still be above its minimum effective serum concentration. Interindividual and disease-stage-related variations in bevacizumab catabolism, however, can affect the proper dosing of patients, resulting in plasma concentrations which may not be within the optimal therapeutic window for the drug. Therapeutic drug monitoring (TDM) enables the assessment of patients’ serum concentrations and allows personalized dosing which has the potential to improve efficacy and reduce side effects. While TMD is often performed using ligand-based assays, mass spectrometry (MS)-based TDM offers improved specificity. Here, we present a robust multiple reaction monitoring (MRM)-MS-based TDM method for the precise quantification of bevacizumab plasma concentrations, based on the controlled oxidation of the methionine-containing peptide, STAYLQMNSLR. The assay shows good linearity (r2 = 0.9951), robustness, and precision (CVs < 20%) for the quantification of bevacizumab, with a lower limit of quantification (S/N > 10) of 1.8 μg/mL of plasma, without the need for enrichment and requiring less than 1 μL of plasma and less than 6 h from sampling to result.
Keywords: therapeutic drug monitoring, drug dosage, targeted therapies, assays, precision medicine
Bevacizumab is a recombinant humanized monoclonal antibody (mAb) that binds to vascular endothelial growth factor A (VEGF-A) and inhibits binding to the VEGFR1 and VEGFR2 receptors.1,2 VEGF-A is a potent angiogenic factor that is up-regulated in many tumors, contributing to tumor growth.3−6 Bevacizumab was the first antiangiogenetic treatment approved by the US Food and Drug Administration (FDA) as first line treatment of metastatic colorectal cancer,7 and it remains an important option for patients with advanced cancer.8 So far, bevacizumab has been FDA-approved for the treatment of cervical cancer, metastatic colorectal cancer, glioblastoma, nonsquamous nonsmall cell lung cancer, ovarian, fallopian tube or primary peritoneal cancer, and metastatic renal cell carcinoma,9 and it has been commercialized under different brand names by different companies. Recently, bevacizumab has been used in clinical trials of patients with COVID-19.10,11 Because VEGF is a potent vascular permeability inducer, it is a therapeutic target in patients who exhibit severe symptoms such as acute lung injury (ALI) and acute respiratory distress syndrome (ARDS). Bevacizumab is typically administered to patients in doses of 3–15 mg per kg of body weight, every 2 or 3 weeks, depending on (i) the type of cancer, (ii) its stage, and (iii) treatment with concomitant drugs.1,12,13 Bevacizumab’s mean clearance, defined as the volume of blood that gets cleared from the drug per day, has been reported as 0.21 L/day.14 Clearance can be divided into specific and nonspecific clearance: specific clearance depends on the interaction of the mAb with its target and is a function of the internalization rate, the antigen density, the binding affinity, and the turnover kinetics of the antigen, while nonspecific clearance relates to target-independent cellular uptake of mAbs via pinocytosis/proteolysis and removal from circulation.15 Finally, the formation of antidrug antibodies (ADAs) can also contribute to accelerated clearance.16 The rate of clearance is an important determinant of effective dosing and treatment. The “trough concentration”, i.e., the serum concentration of the mAb at the end of the dosing interval, should be slightly above the minimum effective concentration to ensure that a patient is well within the optimal therapeutic range of the drug, but interpatient variation in antibody clearance can lead to a considerably variability in the trough concentration. It has been reported for advanced gastric cancer that bevacizumab clearance was higher in patients with less-advanced disease,17 while low mAb trough concentrations were associated with poor prognosis in a range of solid and hematologic tumors.16 Clearance of mAb has generally been found to depend on multiple parameters, including (i) body weight, (ii) gender, (iii) tumor burden and inflammatory activity, (iv) expression level of the target, (v) the levels of covariates of mAb-clearance in blood, and (vi) immunogenicity leading to the formation of ADAs.16 For example, it has been reported that bevacizumab clearance is 19% faster in patients having low serum albumin levels, while being 23% faster in patients with high alkaline phosphatase levels.14
Therapeutic drug monitoring (TDM), i.e., the measurement of a drug’s concentration in a patient’s blood, can assist in adjusting the dose based on the pharmacokinetics of an individual patient. This improved dosing has been associated with increased therapeutic efficacy while reducing toxicity and concurrently avoiding unnecessary costs for the healthcare system, as mAbs are high-cost drugs.18−20 TDM is routinely done using enzyme-linked immunosorbent assays (ELISA), but significant imprecision has been reported for this method.21 Consequently, mass spectrometry (MS)-based TDM assays are increasingly being developed and used.19,22,23 In particular, multiple reaction monitoring (MRM) MS, combined with the use of stable isotope-labeled internal standard (SIS) peptides, enables the highly precise and sensitive determination of protein concentrations within samples over a wide dynamic range and has been shown to provide comparable results across different platforms and laboratories.24,25 Compared to ELISA, MS (i) offers more precise quantitation, (ii) offers a higher multiplexing capacity, and (iii) is based on a more specific readout than antibody-based detection methods.19,26−31 A variety of MS-based methods to quantify mAbs have been introduced, most of which require some type of enrichment of the drug, for example, protein G enrichment,19,32−34 resin enrichment in nanosurface and molecular orientation limited (nSMOL) proteolysis,35−37 or enrichment by using biotinylated target protein and mass spectrometry immunoassay (MSIA).38
Our goal was to develop a liquid chromatography-MRM (LC-MRM) TDM method for the quantification of bevacizumab directly in plasma, i.e., without the need for enrichment or fractionation of samples. To determine the best proteotypic peptides for quantifying bevacizumab, we performed a data-dependent acquisition (DDA) LC-MS/MS analysis of the drug after tryptic digestion and observed that the methionine (Met)-containing peptide STAYLQMNSLR from within the variable part of the heavy chain, yielded the highest number of peptide-spectrum-matches (PSMs) and the highest signal intensities of all of the unique peptides identified. Because of oxidation, however, Met-containing peptides often appear as two chromatographically separated peaks in LC-MS, with the more hydrophilic oxidized variant eluting earlier in reversed-phase chromatography.39 Because Met oxidation in biological samples (endogenous as well as induced during sample preparation) as well as in the spiked-in SIS peptides, cannot be avoided and the extent of this oxidation cannot be predicted, Met-containing peptides cannot be quantified with high precision using conventional strategies. Moreover, in-source oxidation during LC-MS can lead to a second oxidized methionine peak that co-elutes with the nonoxidized peptide,39,40 further complicating the quantitation of Met-containing peptides.
Because of the nonideal presence of a Met residue in the sequence of the most sensitive bevacizumab peptide, we had initially examined two other unique sequences from the bevacizumab protein. These target peptides, however, had poor properties for synthesis and purification. The strategy described in this paper was developed to address the problem of quantifying a target peptide that contains Met, and resulted in a highly sensitive and precise assay for bevacizumab.
Materials and Methods
Reagents used, selection of proteotypic peptides for MRM, MRM assay development, proteolytic digestion of bevacizumab in plasma using S-TrapTM mini-columns, and MRM assay validation are described in the Supporting Information.
Results and Discussion
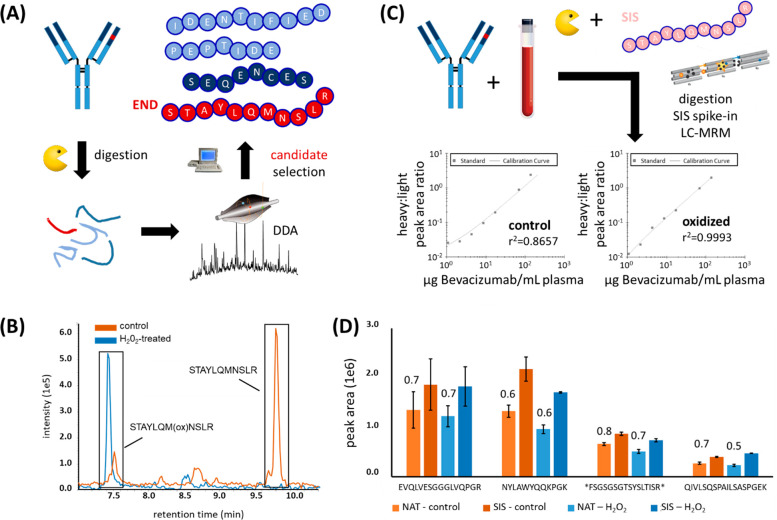
Analysis of the tryptic digests of bevacizumab by DDA resulted in several proteotypic peptide candidates for use in a targeted assay (Figure 1A). The potential target peptides VLIYFTSSLHSGVPSR and GLEWVGWINTYTGEPTYAADFK, however, were excluded for LC-MRM-based TDM of bevacizumab due to problems during peptide synthesis and purification, so the Met-containing peptide STAYLQMNSLR remained as the best option for the quantitation of bevacizumab. However, due to the typical problems encountered with Met-containing peptides, as mentioned above, the peptide STAYLQMNSLR was always detected in both the oxidized and the nonoxidized variants (Figure 1 B, orange trace).
Figure 1.
Bevacizumab quantitation using the Met-containing peptide STAYLQMNSLR. (A) Bevacizumab was digested using trypsin and analyzed by data-dependent acquisition (DDA) to identify proteotypic peptide candidates that can be used for an MRM-based assay. The Met-containing peptide STAYLQMNSLR showed the highest response during LC-MS. (B) STAYLQMNSLR either treated with (blue) or without H2O2 (orange), analyzed on a Sciex TripleTOF 6600 (AB Sciex). Extracted ion chromatograms of the oxidized (∼7.5 min) and nonoxidized (∼9.7 min) forms are shown, indicating the presence of both variants in the nontreated sample, while the nonoxidized variant was completely absent upon treatment with H2O2. (C) Bevacizumab was spiked at different concentrations into human plasma, followed by proteolytic digestion, addition of SIS peptide as normalizer, and analysis by LC-MRM (left: no oxidation, right: oxidation with H2O2). (D) Non-Met-containing control peptides are not significantly affected by H2O2 treatment. Light (NAT) peptides representing various mAbs were spiked into plasma and treated with (blue) or without H2O2 (orange), followed by addition of SIS peptides and LC-MRM analysis. n = 3. H2O2 oxidation did not considerably alter precision, nor did it considerably change the peak area ratios of corresponding NAT/SIS pairs. These ratios are indicated above the respective NAT peptide peak area bars. Error bars represent standard deviations. * = peak area × 10.
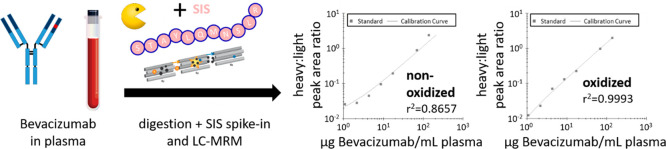
Interestingly, we did not observe in-source oxidation, which would have led to the coelution of the oxidized variant at the retention time of the nonoxidized peptide. STAYLQMNSLR, however, did not meet our criteria for a quantitative assay because the spiking of increasing amounts of bevacizumab into human plasma, followed by tryptic digestion, addition of the SIS peptide, and analysis by LC-MRM showed poor linearity (r2 = 0.8657; y = 1.1216 × 10−2x + 1.0082 × 10−2) and poor robustness (CV of 28%) (Figure 1c, left). This can be attributed to varying extents of oxidation in both the peptide from the mAb and the SIS peptide. Oxidation with H2O2, however, resulted in a complete shift of the STAYLQMNSLR peptide to its oxidized form STAYLQM(ox)NSLR (+16 Da), without the presence of artifacts (Figure 1b, blue trace). Spiking bevacizumab into plasma under the same conditions as above, but including a 30 min H2O2 oxidation step immediately before LC-MRM, showed considerably improved linearity (r2 = 0.9993; y = 1.4123 × 10−2x − 3.6383 × 10−3) and precision (Figure 1c, right).
To evaluate the general impact of H2O2-based oxidation on peptide quantitation, we monitored four additional synthetic peptides that represent other mAbs (adalimumab, rituximab, and trastuzumab) and which do not contain Met, but do contain other residues potentially prone to oxidation such as tryptophane (Trp) or tyrosine (Tyr). Notably, none of these peptides showed a considerable change in signal intensity upon H2O2 oxidation, e.g., NAT control versus NAT H2O2, and NAT/SIS ratios were effectively unchanged (Figure 1d), indicating that the oxidation step did not affect these peptides. We observed the largest deviation between NAT/SIS ratios with (0.5) and without H2O2 oxidation (0.7) for the peptide QIVLSQSPAILSASPGEK, corresponding to a relative standard deviation of 23%. This is in the range of the expected error, considering that each ratio is based on the LC-MRM measurement of two peptides in experimental triplicates.
While we excluded matrix interference for our MRM assay by measuring plasma samples from different donors that have not received the drug, hypothetically other mAbs used to treat cancer patients may interfere with our LC-MRM assay as well. We, therefore, performed in silico digestions of seven other drugs that may be used to treat colorectal cancer (aflibercept, cetuximab, ramucirumab, pembrolizumab, nivolumab, ipilimumab, and panitumumab) using the MS-Digest online tool,41 allowing up to 1 missed tryptic cleavage site, and considering oxidation of methionine as a variable and carbamidomethylation of cysteine as a fixed modification. None of these seven drugs contained our targeted peptide sequence; in addition, only one aflibercept peptide (VTSPNITVTLKK, 1 missed cleavage site; m/z 650.902+) was within ±2.5 m/z units of the precursor ion m/z (m/z 650.32) which we used for Q1-selection in our bevacizumab quantitation. To validate that this peptide, nevertheless, does not represent a potential source of interference for our LC-MRM assay, we also performed in silico fragmentation using the online tool MS-Product42 and confirmed that none of the theoretically generated fragment ions, including internal fragments, were within ±9.0 m/z units of the quantifier ion m/z (m/z 877.45) which we used for Q3-selection in bevacizumab quantitation. We can, therefore exclude the possibility of interference from these drugs with our bevacizumab assay.
We next generated a calibration curve by spiking the STAYLQMNSLR NAT peptide in increasing concentrations into human plasma at a constant level of SIS (normalizer), followed by H2O2-oxidation. The calibration curve was done in 5 independently prepared replicates, and showed linearity in plasma from 14–1400 fmol of NAT on-column (r2 = 0.9951; y = 2.7965 × 10–3x + 2.2519 × 10–3) with coefficients of variation between 4.0 and 18.5% for all points of the curve, except for 14.6 fmol, the highest data point, where the CV was 20.8% (Figure 2, Table 1).
Figure 2.
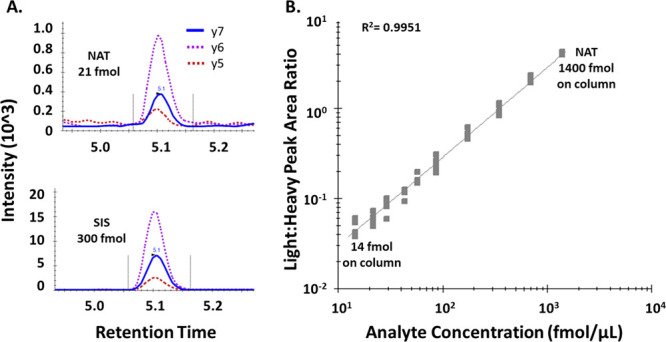
Validation of bevacizumab LC-MRM assay. (A) Two transitions of the peptide STAYLQM(ox)NSLR ((M + 2H)2+ → y6+, (M + 2H)2+ → y5+) were used as qualifiers and one transition ((M+2H)2+ → y7+) was used as the quantifier. (B) Full STAYLQM(ox)NSLR calibration curve. NAT peptide was spiked at different concentrations into digested plasma, using SIS as constant normalizer, followed by H2O2 oxidation and LC-MRM. The linear range was from 14 to 1400 fmol NAT on-column. The curve was conducted in 5 independent replicates, yielding coefficients of variations <15.6% for all points except 14.6 fmol with a CV of 20.8% (Table 1).
Table 1. Bevacizumab Calibration Curvea.
| analyte on column (fmol) | accuracy | CV |
|---|---|---|
| 1400 | 101.5% | 4.0% |
| 700 | 106.1% | 6.7% |
| 350 | 102.1% | 12.8% |
| 175 | 104.2% | 12.9% |
| 87.5 | 97.8% | 18.5% |
| 58.3 | 96.9% | 13.0% |
| 43.75 | 91.6% | 11.1% |
| 29.2 | 105.7% | 9.0% |
| 21.9b | 93.1%b | 15.6%b |
| 14.6 | 108.9% | 20.8% |
Accuracies and % CVs are given.
The LLOQ was determined to be 21.9 fmol.
After H2O2 oxidation, the lower limit of quantitation (LLOQ), i.e., the amount on-column showing an S/N of >10 by MRM, was 21.9 fmol when injecting 1.5 μL of plasma digest on-column (corresponding to approximately 100 μg of total protein; Table 1), which represents 1.8 μg of bevacizumab per mL of plasma. Considering an initial dosage of 10 mg bevacizumab per kg of body weight, an average adult with a weight of 70 kg, and a blood volume of 5 L, this LLOQ would allow the quantification of bevacizumab even after 98.7% had been cleared from circulation, without the need for prior enrichment as in other approaches.
To verify the recovery of bevacizumab in our assay, we spiked 70 μg of bevacizumab into 1 mL of human plasma, followed by S-Trap sample preparation, as described above. Samples were processed, including H2O2 oxidation, and analyzed by LC-MRM. When comparing the drug-derived STAYLQM(ox)NSLR signal to a calibration curve done with NAT peptides and 300 fmol SIS spiked in as a normalizer, the average recovery rates from triplicates were 99, 100, and 116%.
Finally, to test our novel assay on authentic samples, we processed samples from three patients with colorectal cancer that were undergoing treatment with bevacizumab. Samples were received from the Jewish General Hospital Central Biobank (ethics approval 2021–2431 received by the Ethics Committee of the Jewish General Hospital, Montreal). This biobank is affiliated to the Réseau de recherche sur le cancer (RRCancer) of the FRQS and to the Canadian Tumor Repository Network (CTRNet). A 4.5 μL sample of plasma from each patient was processed in triplicate, using the S-Trap-based digestion followed by H2O2 oxidation and LC-MRM, injecting 0.75 μL of plasma digest on-column (corresponding to approximately 100 μg of total protein). The bevacizumab concentrations in blood from all three patients were well above the LLOQ of 1.8 μg/mL and could be determined with high precision, as 110 ± 10, 90 ± 3, and 51 ± 6 μg/mL, with the % CVs of the technical replicates being between 3 and 11%.
Conclusion
We have developed a robust and sensitive LC-MRM based assay to quantify bevacizumab from plasma samples. Our assay does not require any type of prior enrichment of the drug from blood, which might be accompanied by potential losses of sample that are difficult to control and which could negatively affect the quantitative precision. The workflow is straightforward and requires only a 30 min incubation with H2O2 to completely shift the best proteotypic peptide STAYLQMNSLR to its Met-oxidized form (+16 Da). The complete procedure, from sample to result, requires only 6 h per sample. The sample preparation can be parallelized using 96-well Strap columns, and the LC-MRM method requires only 12 min for measurement. Thus, our assay allows same-day determination of bevacizumab concentrations in patient plasma in order to rapidly determine whether a patient is in need of another dose of the drug. While we applied external calibration in this study, we envision that the bevacizumab assay presented here can be combined with our recently introduced 2-PIC calibration strategy, where two different SIS isotopologues of different molecular mass are added at different concentrations for internal calibration.43 As we have recently demonstrated, this strategy allows quantification of target proteins over a wide dynamic range and with high precision while avoiding the need for cumbersome external calibration, which is associated with additional laboratory and measurement time and increased cost.
Using our here described LC-MRM method, bevacizumab could be quantified with high sensitivity (LLOQ of 1.8 μg per mL of plasma), specificity (interference free quantifier transition), and robustness (CVs < 18.5% except for the LLOD with 20.8%). We also demonstrated that the H2O2 oxidation step does not considerably affect the signal intensities of other peptides that contain amino acids that are potentially prone to oxidation, such as Trp or Try. Thus, this workflow should be applicable to multiplexed TDM approaches, targeting several mAb drugs, without compromising precision and accuracy. This assay allows the accurate quantification of bevacizumab in plasma, even after most of the drug has been cleared from circulation, traces of the mAb could still be measured in a simple, reliable, and cost-efficient manner.
Acknowledgments
We are grateful to Genome Canada for financial support through the Genomics Technology Platform (GTP: 264PRO). We are also grateful for financial support from the Terry Fox Research Institute. C.H.B. is also grateful for support from the Segal McGill Chair in Molecular Oncology at McGill University (Montreal, Quebec, Canada), and for support from the Warren Y. Soper Charitable Trust and the Alvin Segal Family Foundation to the Jewish General Hospital (Montreal, Quebec, Canada). This study was also supported by the MegaGrant of the Ministry of Science and Higher Education of the Russian Federation (Agreement with Skolkovo Institute of Science and Technology, No. 075-10-2019-083).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.0c00134.
Experimental procedures (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Yang J. C.; Haworth L.; Sherry R. M.; Hwu P.; Schwartzentruber D. J.; Topalian S. L.; Steinberg S. M.; Chen H. X.; Rosenberg S. A. (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N. Engl. J. Med. 349, 427–434. 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presta L. G.; Chen H.; O’Connor S. J.; Chisholm V.; Meng Y. G.; Krummen L.; Winkler M.; Ferrara N. (1997) Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 57, 4593–4599. [PubMed] [Google Scholar]
- Duffy A. M., Bouchier-Hayes D. J., and Harmey J. H. (2013) Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF, in Madame Curie Bioscience Database [Internet], Landes Bioscience, https://www.ncbi.nlm.nih.gov/books/NBK6482/.
- Chen C.-H.; Lai J.-M.; Chou T.-Y.; Chen C.-Y.; Su L.-J.; Lee Y.-C.; Cheng T.-S.; Hong Y.-R.; Chou C.-K.; Whang-Peng J. (2009) VEGFA upregulates FLJ10540 and modulates migration and invasion of lung cancer via PI3K/AKT pathway. PLoS One 4, e5052. 10.1371/journal.pone.0005052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallas N. A.; Fan F.; Gray M. J.; Van Buren G.; Lim S. J.; Xia L.; Ellis L. M. (2007) Functional significance of vascular endothelial growth factor receptors on gastrointestinal cancer cells. Cancer Metastasis Rev. 26, 433. 10.1007/s10555-007-9070-2. [DOI] [PubMed] [Google Scholar]
- Capp C.; Wajner S. M.; Siqueira D. R.; Brasil B. A.; Meurer L.; Maia A. L. (2010) Increased expression of vascular endothelial growth factor and its receptors, VEGFR-1 and VEGFR-2, in medullary thyroid carcinoma. Thyroid 20, 863–871. 10.1089/thy.2009.0417. [DOI] [PubMed] [Google Scholar]
- Ranieri G.; Patruno R.; Ruggieri E.; Montemurro S.; Valerio P.; Ribatti D. (2006) Vascular endothelial growth factor (VEGF) as a target of bevacizumab in cancer: from the biology to the clinic. Curr. Med. Chem. 13, 1845–1857. 10.2174/092986706777585059. [DOI] [PubMed] [Google Scholar]
- Keating G. M. (2014) Bevacizumab: a review of its use in advanced cancer. Drugs 74, 1891–1925. 10.1007/s40265-014-0302-9. [DOI] [PubMed] [Google Scholar]
- Gerriets V., and Kasi A. (2020) Bevacizumab, in Stat Pearls [Internet], StatPearls Publishing, https://www.ncbi.nlm.nih.gov/books/NBK482126/. [PubMed] [Google Scholar]
- Teuwen L.-A.; Geldhof V.; Pasut A.; Carmeliet P. (2020) COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 20, 389. 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein-Khannazer N.; Shokoohian B.; Shpichka A.; Aghdaei H. A.; Timashev P.; Vosough M. (2020) Novel therapeutic approaches for treatment of COVID-19. J. Mol. Med. (Heidelberg, Ger.) 98, 789. 10.1007/s00109-020-01927-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannistra S. A.; Matulonis U. A.; Penson R. T.; Hambleton J.; Dupont J.; Mackey H.; Douglas J.; Burger R. A.; Armstrong D.; Wenham R.; McGuire W. (2007) Phase II Study of Bevacizumab in Patients With Platinum-Resistant Ovarian Cancer or Peritoneal Serous Cancer. J. Clin. Oncol. 25, 5180–5186. 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- Genentech, Inc. (2020) Avastin (bevacizumab) prescribing information, https://www.gene.com/download/pdf/avastin_prescribing.pdf. [Google Scholar]
- Lu J.-F.; Bruno R.; Eppler S.; Novotny W.; Lum B.; Gaudreault J. (2008) Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother. Pharmacol. 62, 779–786. 10.1007/s00280-007-0664-8. [DOI] [PubMed] [Google Scholar]
- Ovacik M.; Lin K. (2018) Tutorial on monoclonal antibody pharmacokinetics and its considerations in early development. Clinical and translational science 11, 540–552. 10.1111/cts.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oude Munnink T.; Henstra M.; Segerink L. I.; Movig K.; Brummelhuis-Visser P. (2016) Therapeutic drug monitoring of monoclonal antibodies in inflammatory and malignant disease: Translating TNF-α experience to oncology. Clin. Pharmacol. Ther. 99, 419–431. 10.1002/cpt.211. [DOI] [PubMed] [Google Scholar]
- Han K.; Jin J.; Maia M.; Lowe J.; Sersch M. A.; Allison D. E. (2014) Lower exposure and faster clearance of bevacizumab in gastric cancer and the impact of patient variables: analysis of individual data from AVAGAST phase III trial. AAPS J. 16, 1056–1063. 10.1208/s12248-014-9631-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura C. K. (2019) Therapeutic drug monitoring of monoclonal antibodies: Applicability based on their pharmacokinetic properties. Drug Metab. Pharmacokinet. 34, 14–18. 10.1016/j.dmpk.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Conti M.; Cavedagna T. M.; Ramazzotti E.; Mancini R.; Calza L.; Rinaldi M.; Badia L.; Guardigni V.; Viale P.; Verucchi G. (2018) Multiplexed therapeutic drug monitoring (TDM) of antiviral drugs by LC-MS/MS. Clinical Mass Spectrometry 7, 6–17. 10.1016/j.clinms.2017.12.002. [DOI] [Google Scholar]
- Mitrev N.; Vande Casteele N.; Seow C.; Andrews J.; Connor S.; Moore G.; Barclay M.; Begun J.; Bryant R.; Chan W. (2017) Review article: consensus statements on therapeutic drug monitoring of anti-tumour necrosis factor therapy in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 46, 1037–1053. 10.1111/apt.14368. [DOI] [PubMed] [Google Scholar]
- Schmitz E. M.; van de Kerkhof D.; Hamann D.; van Dongen J. L.; Kuijper P. H.; Brunsveld L.; Scharnhorst V.; Broeren M. A. (2016) Therapeutic drug monitoring of infliximab: performance evaluation of three commercial ELISA kits. Clin. Chem. Lab. Med. 54, 1211–1219. 10.1515/cclm-2015-0987. [DOI] [PubMed] [Google Scholar]
- Adaway J. E.; Keevil B. G. (2012) Therapeutic drug monitoring and LC-MS/MS. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 883, 33–49. 10.1016/j.jchromb.2011.09.041. [DOI] [PubMed] [Google Scholar]
- Shipkova M.; Svinarov D. (2016) LC-MS/MS as a tool for TDM services: where are we?. Clin. Biochem. 49, 1009–1023. 10.1016/j.clinbiochem.2016.05.001. [DOI] [PubMed] [Google Scholar]
- Addona T. A.; Abbatiello S. E.; Schilling B.; Skates S. J.; Mani D.; Bunk D. M.; Spiegelman C. H.; Zimmerman L. J.; Ham A.-J. L.; Keshishian H.; et al. (2009) Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nat. Biotechnol. 27, 633–641. 10.1038/nbt.1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy A. J.; Tamura-Wells J.; Albar J. P.; Aloria K.; Amirkhani A.; Araujo G. D. T.; Arizmendi J. M.; Blanco F. J.; Canals F.; Cho J.-Y.; Colomé-Calls N.; Corrales F. J.; Domont G.; Espadas G.; Fernandez-Puente P.; Gil C.; Haynes P. A.; Hernáez M. L.; Kim J. Y.; Kopylov A.; Marcilla M.; McKay M. J.; Mirzaei M.; Molloy M. P.; Ohlund L. B.; Paik Y.-K.; Paradela A.; Raftery M.; Sabidó E.; Sleno L.; Wilffert D.; Wolters J. C.; Yoo J. S.; Zgoda V.; Parker C. E.; Borchers C. H. (2015) Inter-laboratory evaluation of instrument platforms and experimental workflows for quantitative accuracy and reproducibility assessment. EuPa Open Proteomics 8, 6–15. 10.1016/j.euprot.2015.06.001. [DOI] [Google Scholar]
- Mistri H. N.; Jangid A. G.; Pudage A.; Gomes N.; Sanyal M.; Shrivastav P. (2007) High throughput LC-MS/MS method for simultaneous quantification of lamivudine, stavudine and nevirapine in human plasma. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 853, 320–332. 10.1016/j.jchromb.2007.03.047. [DOI] [PubMed] [Google Scholar]
- Rust K. Y.; Wilkens H.; Kaiser R.; Bregel D.; Wilske J.; Kraemer T. (2012) Detection and Validated Quantification of the Phosphodiesterase Type 5 Inhibitors Sildenafil, Vardenafil, Tadalafil, and 2 of Their Metabolites in Human Blood Plasma by LC-MS/MS–Application to Forensic and Therapeutic Drug Monitoring Cases. Ther. Drug Monit. 34, 729–735. 10.1097/FTD.0b013e31827318b8. [DOI] [PubMed] [Google Scholar]
- Koster R. A.; Dijkers E. C.; Uges D. R. (2009) Robust, high-throughput LC-MS/MS method for therapeutic drug monitoring of cyclosporine, tacrolimus, everolimus, and sirolimus in whole blood. Ther. Drug Monit. 31, 116–125. 10.1097/FTD.0b013e318192304c. [DOI] [PubMed] [Google Scholar]
- Rauh M. (2012) LC-MS/MS for protein and peptide quantification in clinical chemistry. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 883, 59–67. 10.1016/j.jchromb.2011.09.030. [DOI] [PubMed] [Google Scholar]
- Popp R.; Basik M.; Spatz A.; Batist G.; Zahedi R.; Borchers C. (2018) How iMALDI can improve clinical diagnostics. Analyst 143, 2197–2203. 10.1039/C8AN00094H. [DOI] [PubMed] [Google Scholar]
- Sobsey C. A.; Ibrahim S.; Richard V. R.; Gaspar V.; Mitsa G.; Lacasse V.; Zahedi R. P.; Batist G.; Borchers C. H. (2020) Targeted and Untargeted Proteomics Approaches in Biomarker Development. Proteomics 20, 1900029. 10.1002/pmic.201900029. [DOI] [PubMed] [Google Scholar]
- Mills J. R.; Cornec D.; Dasari S.; Ladwig P. M.; Hummel A. M.; Cheu M.; Murray D. L.; Willrich M. A.; Snyder M. R.; Hoffman G. S. (2016) Using mass spectrometry to quantify rituximab and perform individualized immunoglobulin phenotyping in ANCA-associated vasculitis. Anal. Chem. 88, 6317–6325. 10.1021/acs.analchem.6b00544. [DOI] [PubMed] [Google Scholar]
- Willeman T.; Jourdil J.-F.; Gautier-Veyret E.; Bonaz B.; Stanke-Labesque F. (2019) A multiplex liquid chromatography tandem mass spectrometry method for the quantification of seven therapeutic monoclonal antibodies: Application for adalimumab therapeutic drug monitoring in patients with Crohn’s disease. Anal. Chim. Acta 1067, 63–70. 10.1016/j.aca.2019.03.033. [DOI] [PubMed] [Google Scholar]
- Chiu H.-H.; Tsai I.-L.; Lu Y.-S.; Lin C.-H.; Kuo C.-H. (2017) Development of an LC-MS/MS method with protein G purification strategy for quantifying bevacizumab in human plasma. Anal. Bioanal. Chem. 409, 6583–6593. 10.1007/s00216-017-0607-0. [DOI] [PubMed] [Google Scholar]
- Iwamoto N.; Takanashi M.; Shimada T.; Sasaki J.; Hamada A. (2019) Comparison of Bevacizumab Quantification Results in Plasma of Non-small Cell Lung Cancer Patients Using Bioanalytical Techniques Between LC-MS/MS, ELISA, and Microfluidic-based Immunoassay. AAPS J. 21, 101. 10.1208/s12248-019-0369-z. [DOI] [PubMed] [Google Scholar]
- Iwamoto N.; Takanashi M.; Yokoyama K.; Yonezawa A.; Denda M.; Hashimoto M.; Tanaka M.; Ito H.; Matsuura M.; Yamamoto S.; et al. (2019) Multiplexed monitoring of therapeutic antibodies for inflammatory diseases using Fab-selective proteolysis nSMOL coupled with LC-MS. J. Immunol. Methods 472, 44–54. 10.1016/j.jim.2019.06.014. [DOI] [PubMed] [Google Scholar]
- Iwamoto N.; Umino Y.; Aoki C.; Yamane N.; Hamada A.; Shimada T. (2016) Fully validated LCMS bioanalysis of Bevacizumab in human plasma using nano-surface and molecular-orientation limited (nSMOL) proteolysis. Drug Metab. Pharmacokinet. 31, 46–50. 10.1016/j.dmpk.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Jourdil J.-F.; Némoz B.; Gautier-Veyret E.; Romero C.; Stanke-Labesque F. (2018) Simultaneous Quantification of Adalimumab and Infliximab in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry. Ther. Drug Monit. 40, 417–424. 10.1097/FTD.0000000000000514. [DOI] [PubMed] [Google Scholar]
- Lao Y. W.; Gungormusler-Yilmaz M.; Shuvo S.; Verbeke T.; Spicer V.; Krokhin O. V. (2015) Chromatographic behavior of peptides containing oxidized methionine residues in proteomic LC-MS experiments: Complex tale of a simple modification. J. Proteomics 125, 131–139. 10.1016/j.jprot.2015.05.018. [DOI] [PubMed] [Google Scholar]
- Lengqvist J.; Eriksson H.; Gry M.; Uhlén K.; Björklund C.; Bjellqvist B.; Jakobsson P.-J.; Lehtiö J. (2011) Observed peptide pI and retention time shifts as a result of post-translational modifications in multidimensional separations using narrow-range IPG-IEF. Amino Acids 40, 697–711. 10.1007/s00726-010-0704-2. [DOI] [PubMed] [Google Scholar]
- University of California, San Francisco . (2020) MS-Digest, http://prospector.ucsf.edu/prospector/cgi-bin/msform.cgi?form=msdigest (accessed October 15, 2020).
- University of California, San Francisco . (2020) Description, Instructions, and Tips for MS-Product, http://prospector.ucsf.edu/prospector/html/instruct/prodman.htm (accessed October 15, 2020).
- Ibrahim S.; Froehlich B.; Aguilar-Mahecha A.; Aloyz R.; Poetz O.; Basik M.; Batist G.; Zahedi R. P.; Borchers C. H. (2020) Using two peptide isotopologues as internal standards for the streamlined quantification of low-abundance proteins by immuno-MRM and immuno-MALDI. Anal. Chem. 92, 12407–12414. 10.1021/acs.analchem.0c02157. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Duffy A. M., Bouchier-Hayes D. J., and Harmey J. H. (2013) Vascular endothelial growth factor (VEGF) and its role in non-endothelial cells: autocrine signalling by VEGF, in Madame Curie Bioscience Database [Internet], Landes Bioscience, https://www.ncbi.nlm.nih.gov/books/NBK6482/.