Abstract
The eye and eyesight are exquistly designed and are precious, and yet we often take them for granted. Good vision is critical for our long-term survival and for humanity’s enduring progress. Unfortunately, since ocular diseases do not culminate in life-and-death scenarios, awareness of the plight of millions of people suffering from such eye ailments is not publicized as other diseases. However, losing eyesight or falling victim to visual impairment is a frightening outlook for most people. Glaucoma, a collection of chronic optic neuropathies, of which the most prevalent form, primary open-angle glaucoma (POAG), is the second leading cause of irreversible blindness. POAG currently afflicts >70 million people worldwide and is an insidious, progressive, silent thief of sight that is asymptomatic. On the other hand, allergic conjunctivitis (AC), and the associated rhinitis (“hay-fever”), frequently victimizes a huge number of people worldwide, especially during seasonal changes. While not life-threatening, sufferers of AC soon learn the value of drugs to treat their signs and symptoms of AC as they desire rapid relief to overcome the ocular itching/pain, redness, and tearing AC causes. Herein, I will describe the collective efforts of many researchers whose industrious, diligent, and dedicated team work resulted in the discovery, biochemical/pharmacological characterization, development and eventual launch of drugs to treat AC (e.g., olopatadine [Patanol/Pataday/Pazeo] and emedastine [Emedine]), and for treating ocular hypertension and POAG (e.g., travoprost [Travatan ] and Simbrinza). This represents a personal perspective.
Keywords: allergic conjunctivitis, olopatadine, Patanol, Pataday, ocular hypertension, glaucoma, travoprost, Travatan
It was a humbling but exalting feeling to be invited to chart the journey of my colleagues and I in the processes of establishing, validating, and implementing a few drug discovery/development platforms and projects that eventually yielded drugs to treat two different types of eye diseases. The first of these was allergic conjunctivitis (AC), and in particular seasonal AC (SAC), a bothersome ocular disorder, the hallmark signs and symptoms of which are undeniable (intense ocular itching, hyperemia (redness), tearing and swelling of the eyelids with possible pain that people of all ages suffer from every few months, some even more frequently.1−3 The second disease was ocular hypertension (OHT)/primary open-angle glaucoma (POAG),4−9 that involves a slowly progressing, symptomless but unrelenting demise of retinal ganglion cells (RGCs) and their axons that connect the eye to the brain. The net result of OHT/POAG is loss of peripheral vision that eventually leads to pan-visual impairment culminating in blindness if the patient remains undiagnosed and untreated. While SAC is an acutely debilitating eye disorder, OHT/POAG is achronic disease that mainly affect older individuals across our planet. As can be imagined, the research strategies, tactics, molecule design/syntheses, screening paradigms, go/no-go criteria stage-gates, in vitro assays, and in vivo animal models were totally different for each target disease, including the target product profiles for each drug.6−8 Needless to say, both drug classes required a high therapeutic index for patient tolerability, acceptance, and eventual introduction into clinical practice following health authority approvals in different geographic jurisdictions.
Prior to discussing the etiologies of the ocular diseases of interest and our drug discovery strategies, it is worth elaborating on the overall incidence, impact on patient quality of life (QoL), and the economic burden associated with management of ophthalmic diseases in order to provide a contextual perspective. Based on the data provided by the World Health Organization (WHO, 2018)9 and other key organizations such as National Eye Institute (US), globally greater than 2 billion people are dealing with visual impairment or blindness. Sadly, up to 1 billion of these impairments were preventable.9 Currently, there are 196 million patients suffering from age-related macular degeneration (AMD), greater than 76 million with glaucoma, 146 million with diabetic retinopathy, and several billion people who have myopia and near-sight problems (presbyopia) around the world. With an aging world population, the number of people afflicted with AMD is projected to rise to 243 million, and for glaucoma is expected to increase to greater than 95 million by 2030. Others estimate that by 2040, there may be as many as 112 million patients with glaucoma, with the highest prevalence in Asia and Africa.5,9,10 As for allergic conjunctivitis (AC), as much as 40% of the population is affected by some symptoms of AC, with the majority of the cases (up to 95%) ascribed to SAC or perennial AC (PAC).1−3,11,12 Therefore, ophthalmic disorders and diseases represent a significant healthcare issue since our society, communities, education systems, economies, sports, media, and every facet of our waking lives are incredibly reliant on the ability to see. Sight is important from infant to mother bonding, to continued learning/educational achievements over the course of life, development of social skills/personality and character, and is critical for physical development and health, and for mental health and self-esteem development and maintenance. Hence, eyesight is critical for survival and life progression, and undoubtedly people with visual impairment are frightened of losing their sight. Economically, the annual cost to society from ophthalmic diseases runs into billions of dollars when considering the lost productivity, decrease in QoL, disability, and morbidity associated with them. For instance, it is estimated that visual impairment (mild to severe) in the US costs greater than $16 billion, while the global financial burden associated with uncorrected myopia and short-sight vision impairment alone is $244 billion and $25 billion, respectively (WHO, 2018).9 The various eye diseases mentioned above have recently been discussed in the literature.6,8,13,14
The Eye and Some Major Ocular Diseases
Since the ocular drug discovery to be described in what follows depends on having at least a basic understanding of the structural and functional components of the visual system, I believe a short introduction to the eye anatomy and physiology would be helpful to the readers. Because of their physical location on the face, the eyes are susceptible to injury, and during waking hours are exposed to light and other radiation, and all kinds of pollutants in the air around us. Preserving and protecting visual function is therefore a major challenge and requires attention.
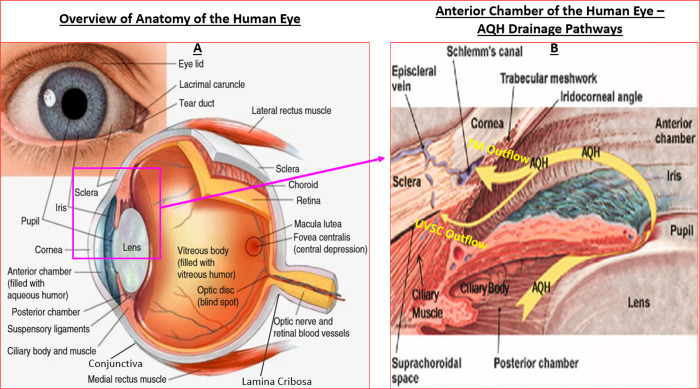
The human eye is made up of three layers of which the outermost layer, known as the fibrous tunic, is composed of the cornea and sclera which give the eye its shape and which provide support for the deeper structural elements (Figure 1A). The thin membranous tissue that stretches on from the cornea, covers part of the sclera and then forms the underside lining of the eyelids is the conjunctiva, formed by highly vascularized tissues. The middle layer, known as the uvea or vascular tunic, encompasses the choroid, ciliary body, pigmented epithelium, and the iris. Lastly, the innermost layer is represented by the retina, which is composed of several layers of highly specialized cells that receive their nutrients and oxygen from the choroidal circulation at the back of the eye, while other retinal vessels supply the anterior parts of the retina. The clear fluid in the anterior segment of the eye (aqueous humor (AQH), residing between the cornea and lens) and the jelly like substance (vitreous humor [made up of water and numerous classes of proteins], located behind the lens) that fills the posterior segment of the eye also help maintain the eyeball shape in addition to the scleral outer covering of the eyeball (Figure 1A/1B). Since the anterior segment of the eye is avascular, the cells lining the anterior chamber receive their nutrition and oxygen from the circulating AQH made by the ciliary processes (nonpigmented ciliary epithelium [NPCE] cells) within the ciliary body (composed of the ciliary processes and ciliary muscle [CM]) (Figure 1B). The lens is connected to the ciliary body by hundreds of fine transparent fibers (suspensory ligaments) which help change the shape of the lens through muscular forces to aid image focusing (accommodation). Under normal circumstances the VH turns over very slowly, whereas the AQH is constantly produced, flows through the anterior chamber, delivers nutrients and removes toxic waste, and exits the latter area through the trabecular meshwork (TM)/Schlemm’s canal (SC) (Figure 1B). Again, in the normal situation the light enters the eye via the cornea, passes through the pupil, is focused by the lens and is projected onto the retina. At a macrolevel, complex biochemical reactions in the photoreceptors convert the received information into signals that the retinal ganglion cells (RGCs) then convert into electric impulses which are transmitted down the RGC axons, that are bundled together to form the optic nerve, to the thalamic region of the brain (superior colliculus/lateral geniculate nucleus) from where they are relayed to the visual cortex in the brain. The visual cortex decodes the information to form the visual images the person sees.
Figure 1.
Basic anatomy and structural elements of the human eye to illustrate key features discussed in this article. Panel A reproduced and modified with permission from ref (8). Copyright 2020 Springer Publishing Company. Panel B reproduced and updated with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
Briefly, ocular surface disorders comprise allergic conjunctivitis, dry eye, corneal perforation, and corneal and conjunctival pain.8 Within the anterior chamber (ANC), dysfunctional corneal endothelial cells cause corneal dystrophies, while AQH drainage disorders due to blockage of the TM raise intraocular pressure (IOP) to cause ocular hypertension (OHT) that is frequently linked to glaucoma.5,6,10 Aggregation of proteins in the lens, due to excessive exposure to sunlight or due to smoking and diabetes, results in cataract formation.8 When the iris repeatedly brushes up against the lens, cellular debris and iridial pigment are shed into the AQH. Eventually these materials arrive at the TM and they obstruct the latter tissue causing exfoliation/pigmentary glaucoma due to elevation of IOP. Even though VH acts as a cushion to the surrounding tissues and vascular elements in the back of the eye (Figure 1A/1B), many retinal diseases exist or develop due to defects in the cellular machinery of the many specialized cells within the retina-choroid. These include dry and wet age-related macular degeneration (AMD), retinitis pigmentosa, diabetic retinopathy, glaucomatous optic neuropathy [GON], that require specific treatment modalities.5−10 Since I will be focusing on glaucoma and glaucomatous optic neuropathy (GON), the features of this disease and its treatment will be discussed further ahead and have recently been reviewed.6−8,13,14
Challenges and Strategies to Discover Novel Drugs to Treat Ocular Allergic Diseases
Up to 30% of the population is affected by allergic hypersensitivity, due to a hereditary component, and are prone to experience various atopic conditions including eczema, asthma, and allergic rhinitis. Atopic eye disorders encompass, SAC, PAC, keratoconjunctivitis, giant papillary conjunctivitis, and vernal conjunctivitis. SAC and PAC account for 80–98% of all cases of eye allergies, with 20–30% of the population succumbing to their symptoms throughout the year.1−3,11,12,15−17 While SAC and PAC are somewhat acute self-limiting disorders, the other forms of conjunctivitis mentioned above can be chronic sight-threatening diseases. Most people would attest that one of the most annoying and malaise-causing problems they frequently experience is “allergies”. Itchy, watery, swollen red eyes coupled with sneezing and runny nose and light-sensitivity herald the signs and symptoms of AC and “hay fever” (rhinitis), respectively.12,15,16 Episodic misery from these ailments easily incapacitates us all many times in our lives for which we seek immediate relief.17,18 The unbearable urge to rub the eyes due to itching is almost uncontrollable.18−21 Herein, I will deal with the etiology, diagnosis, and treatment of AC, and more specifically SAC, since that was the target malady we deemed of highest importance when we began our drug discovery campaign in 1992/1993 at Alcon Laboratories Inc. (Fort Worth, TX), which later became Alcon Research, Ltd.
During the day we experience eye strain from computer/mobile device-related work, and in addition our eyes are constantly being assaulted by airborne allergens, pathogens, and other irritants. Blinking, use of artificial tears, and gently rubbing the eyes provide some relief and get rid of some of the offending substances. But this relief is short-lived. When the seasons change or at times of sustained high humidity the air quality sharply declines as the air gets filled with high levels of new allergens such as pollen (from grasses, trees, flowers, weeds), and with fungal spores and other pollutants. Furthermore, some people are highly sensitive to dust mites, pet dander, and dust that accumulates in the house. The irritants elicit the classic allergic/inflammatory cascade in the cornea/conjunctiva but also within the nasopharynx as we breathe in these allergens.
The pathophysiology of SAC unfolds mainly in the conjunctival epithelium underneath the eyelids which contains a large number of dendritic cells and macrophages along with a rich supply of blood vessels.2,20,21 The latter cells are responsible for the innate and adaptive immunity of the conjunctiva. As an allergen (antigen) such as pollen binds to a B-lymphocyte and is cross-linked to the immunoglobulin-E (IgE) in a sensitized individual, the latter binds to the high-affinity IgE receptor on the mast cells in the conjunctiva and triggers the mast cell to immediately empty its content of preformed mediators such as histamine, bradykinin (BK), platelet activating factor (PAF), serotonin, cathepsin G, and tryptase onto the surrounding tissues.1,22−25 The immediate actions of histamine (and probably that of BK and PAF) are to cause vasodilation of conjunctival blood vessels and to enhance vascular permeability.1−3,22−25 This acute early phase response begins to subside but the damage has been initiated and the cascade of other deleterious events ensues. Over the next few minutes to hours, the mast cells release newly generated prostaglandins (PGs; mainly PGD2), leukotrienes and cytokines (e.g., interleukin-3 [IL-3], IL-6, IL-8, and tumor neurosis factor-α [TNF-α]) as part of a delayed late-phase secondary response to the allergen.2,3,21,24 The cytokines in turn induce IgE synthesis/release by B-cells and cause inflammatory white blood cells (e.g., eosinophils) to infiltrate the conjunctiva, and to cause leukocyte adhesion, migration, and activation, thereby amplifying and exacerbating the situation.2,23−25 By now the clinical manifestation of the allergic inflammation in the eye is readily observable and the patient feels the swollen eyelids that are itchy, red, and increasingly becoming painful and further irritated.1−3,20−25 Since the human conjunctival epithelial (HCE),26−32 human corneal epithelial (HCEPI),33−38 and human corneal fibroblast (HCF)26,27,29,30 cells express functionally active receptors for histamine (H1-type), for BK (B2-type, and perhaps the B1-type which is induced under pathological conditions), and for PAF, synergistic activation of these cell-types could potentiate the allergic response in the cornea and conjunctiva due to the release of various cytokines by the latter epithelial cells,39−43 perhaps in a yet to be defined coordinated or uncoordinated manner. In fact, HCEPI and HCF cells would be expected to respond immediately to the mast cells mediators such as histamine, BK, and PAF,22 and promote an early phase of cytokine release upon the ocular surface since these receptors react very quickly to their cognate ligands to generate intracellular inositol phosphates (IPs) and mobilize intracellular Ca2+ [Ca2+]i over a few seconds that leads to the final biological response.28,33,36
Clearly, the multiplicity of mediators involved in the onset and progression of ocular allergic disease is overwhelming and the interconnecting pathways and mechanisms very complex and complicated. For these reasons it was difficult to decide which mediator(s) and which cell-type(s) to target with respect to finding suitable new drugs to treat SAC/PAC around the 1992/1993 time-period. Historical data concerning the skin had shown histamine to be a potent itch-causing (pruritic) agent with BK inducing a milder and transient response but causing pain.44 Similarly, serotonin was significantly weaker than histamine in provoking dermal itching, and since various prostaglandins while not very pruritogenic by themselves appeared to synergize with serotonin and histamine (PGE2 actually promoted histamine release),45 various neuropeptides, including BK, apparently produced itching by releasing histamine.46 On the basis of these findings and the fact that only peptide antagonists for BK receptors were available at the time, and the complexity of trying to unravel and identify the specific type(s) of receptors involved in AC within each class of potential target(s), we ruled out BK (at least two receptor subtypes), serotonin (at least seven major receptors with several subtypes within each class) and PG (at least five major receptors with many subtypes) receptor antagonists as targets for our drug discovery program for AC. Added to this complexity was the uncertainty of translating results obtained from one organ to the next, and the known significant heterogeneity among mast cells and epithelial cells,22,47 and of the many species differences in disease pathology and known differences in compound affinities and potencies across mammalian species.1−3,47 Since PAF exhibited potent chemotactic and chemokinetic activity for eosinophils and PAF receptor antagonists were showing promise as antiasthmatic drugs,48 and a dual PAF/histamine receptor antagonist (SCH-37370) had recently been reported,49 our interest in PAF began to grow. However, since SCH-37370 exhibited a relatively low antagonist affinity/potency at PAF and H1-histamine receptors (IC50 = 0.6 and 1.2 μM, respectively), we decided not to pursue PAF antagonists for AC.38
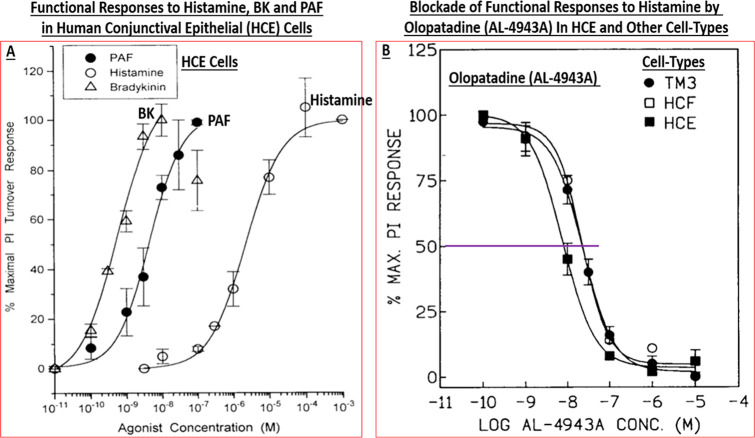
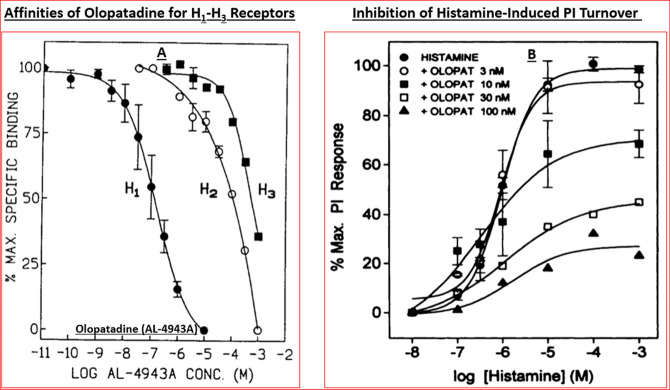
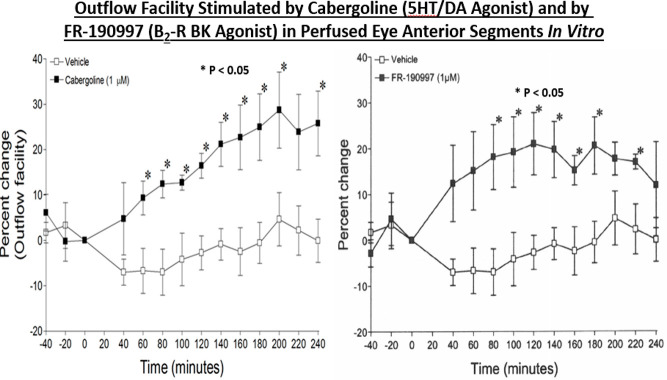
Importantly, Berdy et al.50 had reported that H1-receptor antagonists were effective at significantly reducing histamine-induced ocular itch, ocular congestion, and redness of the eye in healthy human volunteers.50 The major problem seemed to be that existing antihistamines and other antiallergic agents at that time were not fast-acting and their efficacy was not durable, therefore requiring multiple topical ocular (t.o.) dosing regimens.1−3 Collectively, it was decided that we would endeavor to find the next generation of histamine receptor antagonists with a high affinity, a greater receptor-selectivity, higher potency, perhaps having multiple mechanisms of actions, and having a superior in vivo efficacy and a longer duration of action than the current medications in the early 1990s in order to mitigate the signs and symptoms associated with AC. This necessitated a better understanding of the human ocular cells and tissues involved in, or implicated in, the pathology of AC using a fundamental pharmacological approach. Accordingly, the team established appropriate radioligand-based receptor binding assays, second messenger-based functional assays, and rendered them into a high throughput screening (HTS) platform. Likewise, other team members started a program to isolate, cultivate, and propagate various resident cells in post-mortem human conjunctiva and cornea that were deemed important for compound profiling. The use of human ocular cells was critical in order to remain focused on target tissues and the target population. Such primary cells included human conjunctival mast cells (HCMCs),39,51−58 human conjunctival epithelial cells (HCE) cells,26−32,58−60 human conjunctival fibroblasts (HCF),26,27,29,30 human corneal epithelial (HCEPI) cells,33−38 and also other cells obtained from human ocular tissues such as trabecular meshwork (TM) cells. The ready availability of sufficiently large quantities of these early passage primary cells allowed us to first perform a small survey of guanine protein-coupled receptors (GPCRs) present on these cell-types. It was discovered that primary target HCE cells expressed functionally active major mast cell mediator and neurotransmitter receptors including β2-adrenergic, prostaglandin EP4, vasoactive intestinal peptide, and 5-HT receptors positively coupled to adenylate cyclase, the activation of which resulted in cAMP generation.32 Of the phospholipase C-coupled receptors present on HCE cells, BK, PAF, and histamine (and leukotrienes) robustly stimulated phosphoinositide (PI) turnover by generating [3H]-IPs (e.g., Figure 2A)31,32 and rapidly enhanced [Ca2+]i mobilization.31,32
Figure 2.
Panel A shows the concentration-dependent stimulation of PI turnover by three key mast cell mediators in HCE cells. The antagonism of histamine-induced responses in three different human cell-types by olopatadine (AL-4943A) are displayed in panel B. Reproduced and updated with permission from refs (29 and 32). Copyright 1996 and 1997 Mary Ann Liebert Publishing Inc.
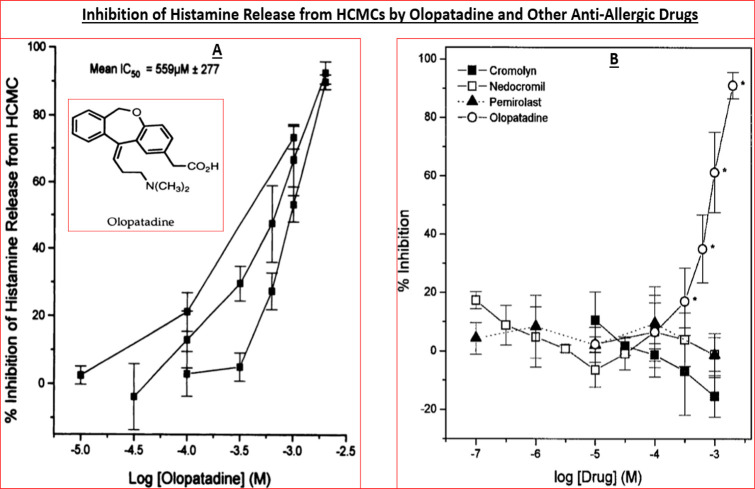
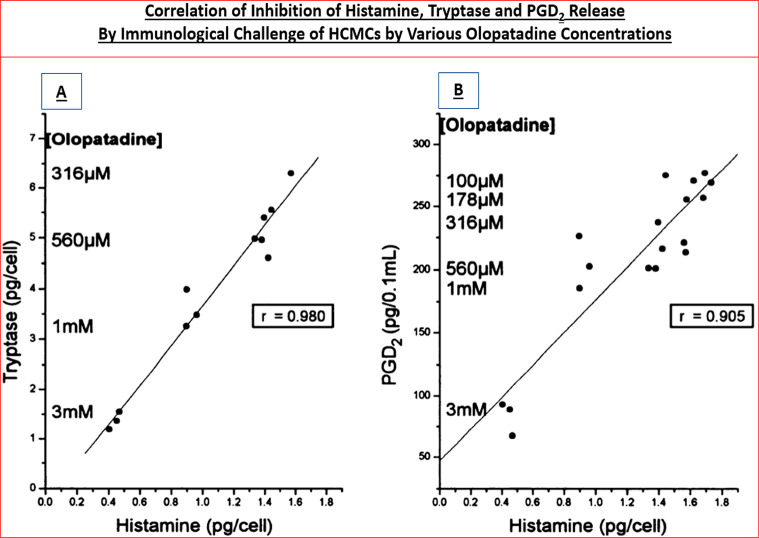
Interestingly, primary (and immortalized) HCEPI cells also expressed BK, PAF, and histamine-1 (H1) receptors that were functionally responsive to various agonists and antagonists of these receptor classes.33−38 Furthermore, stimulation of the latter receptors initiated release of various pro-inflammatory cytokines such as interleukin-6 (IL-6) and IL-8 from HCE cells58−60 and also from HCEPI cells33−38in vitro, indicating that the machinery for creating, propagating, and sustaining the cellular signaling mechanisms involved in the allergic inflammation on the ocular surface all existed within these key ocular cell-types. We deemed it necessary to examine the effects of mast cell mediators, especially histamine, on HCEPI cells since they are also directly exposed to the allergens on the ocular surface and are also recipients and potential responders to HCMCs/HCE cell-secreted mediators, and because clusters of corneal epithelial cells apparently co-reside in the conjunctival epithelium.43 HCMCs were isolated and interrogated for mediator release characteristics since they are the major and primary cells involved in the AC pathology. In the absence of the multiplexed screening tools of today where potentially several dozen mediators can be detected and quantified simultaneously, it was encouraging to observe that exposure of isolated HCMCs to human IgE and other provocative stimuli resulted in degranulation and release of histamine, tryptase, leukotrienes, and PGD2 (e.g., Figures 3 and 4)51,57 and numerous cytokines and adhesion molecules.40,53,54,61
Figure 3.
Histamine release from isolated human conjunctival mast cells (HCMCs) exposed to an immunological challenge and the ability of olopatadine and other antiallergic drugs to inhibit the release is depicted. Figure reproduced with permission from ref (30). Copyright 1996 American Society for Pharmacology and Experimental Therapeutics.
Figure 4.
Correlations of immunologic-challenge-induced secretion of histamine, tryptase (A) and PGD2 (B), and their inhibition by different concentrations of olopatadine, from human conjunctival mast cells is shown. Figure reproduced with permission from ref (30). Copyright 1996 American Society for Pharmacology and Experimental Therapeutics.
Using these encouraging data, we embarked on our drug discovery campaign to find high affinity, high potency, and highly selective, more efficacious, and fast-acting antihistamines for the topical treatment of AC. At the time the commercially available H1-antagonists included antazoline, brompheniramine, chlorpheniramine, clemastine, diphenhydramine, ketotifen, pheniramine, and pyrilamine.50 Additional compounds in this class were obtained as generous gifts from other companies and tested in parallel (e.g., Emedastine from Kanebo Ltd., Osaka, Japan; Levocabastine from Janssen Pharmaceuticals, Beerse, Belgium). All these drugs were quickly profiled for their relative affinities and selectivities at the guinea pig brain histamine receptor subtypes (Tables 1 and 2), tested for their ability to prevent histamine-induced [3H]-inositol phosphates ([3H]-IPs) production and [Ca2+]i mobilization in HCE cells, and to reduce guinea pig conjunctival vascular permeability in vivo. To our delight emedastine was found to be a high affinity (Ki = 1.2 nM; Table 1) and a high potency H1-receptor antagonist preventing PI turnover in HCE cells (KB = 0.88 nM)26,27 and at blocking histamine-induced IL-6, IL-8, and granulocyte macrophage-colony stimulating factor (GM-CSF) secretion from HCE cells (IC50 = 1.5–3.4 nM);60 Weimer et al., 1998), and the most H1-receptor selective (>12 000–33 000 fold) among all the antihistamines tested (Tables 1 and 2).26,27 Emedastine was also found to be the most potent/efficacious drug at inhibiting histamine-induced conjunctival vascular leakage in guinea pig eyes,62 being 3–17-times more potent than ketotifen, pheniramine, and antazoline, and equipotent with pyrilamine. Moreover, emedastine was 7, 10, 10, 100, 3333, 357, and 5813 times more potent than brompheniramine, chlorpheniramine, clemastine, levocabastine, diphenhydramine, pheniramine, and antazoline, respectively, in this animal model.62
Table 1. Competition by Selected Histamine Antagonists for Specific Radioligand Binding to H1–H3 Receptorsa.
| equilibrium
inhibition constants of drugs at guinea pig brain H1–H3 receptors sub-types (Ki, nM ± SEM) |
|||
|---|---|---|---|
| test drugs | H1 receptors | H2 receptors | H3 receptors |
| clemastine | 0.23 ± 0.1 | 143 ± 33 | 4 015 ± 1 617 |
| pyrilamineb | 0.7 ± 0.1 | 8 612 ± 1 275 | 9 820 ± 1 098 |
| emedastine | 1.2 ± 0.1 | 39 860 ± 7 453 | 14 498 ± 2 257 |
| ketotifenb | 1.2 ± 0.1 | 1 122 ± 127 | 2 458 ± 203 |
| chlorpheniramine | 1.4 ± 0.3 | 7 980 ± 649 | 3 103 ± 198 |
| brompheniramine | 9.9 ± 0.9 | 5 350 ± 247 | 5 750 ± 1994 |
| diphenhydramine | 11.9 ± 2.9 | 1 595 ± 141 | 31 480 ± 12 020 |
| pheniramineb | 32.3 ± 2.8 | 14 475 ± 939 | 10 190 ± 1 190 |
| olopatadine | 36.0 ± 5.7 | 153 983 ± 94 313 | 137 980 ± 28 603 |
| antazolineb | 39.3 ± 3.4 | 40 850 ± 3 794 | 35 295 ± 8 380 |
| levocabastineb | 52.6 ± 9.9 | 27 075 ± 4 996 | 9 506 ± 5 825 |
Table 2. Relative Selectivities of Key Compounds for Histamine Receptor Sub-Types (H1–H3)a.
| relative
selectivities for guinea pig brain histamine receptor sub-types |
|||
|---|---|---|---|
| test drug | H1 relative to H2 | H1 relative to H3 | H3 relative to H2 |
| emedastine | 33 217 | 12 082 | 3 |
| pyrilamineb | 12 303 | 14 028 | <1 |
| chlorpheniramine | 5 700 | 2 216 | 3 |
| olopatadine | 4 277 | 3 833 | <1 |
| antazolineb | 1 039 | 898 | 1 |
| ketotifenb | 935 | 2 048 | <1 |
| clemastine | 621 | 17 456 | <1 |
| brompheniramine | 540 | 580 | <1 |
| levocabastineb | 515 | 181 | 3 |
| pheniramineb | 448 | 315 | 1 |
| diphenhydramine | 134 | 2 645 | <1 |
The Table has been arranged to reflect high to low relative selectivity with focus on the H1-receptor since that was predominantly involved in mediating the majority of the proinflammatory effects of histamine in AC by enhancing conjunctival vascular permeability and causing itching.
In clinical studies, emedastine (0.05%; twice daily topically applied) was compared with levocabastine (0.05%; twice daily topically applied) in one main study involving 222 patients with SAC aged four years and older.63 The main end point for effectiveness was the reduction in itching and redness, measured on a nine-point scale over and up to 6 weeks. Emedastine was as effective as levocabastine in reducing symptoms of seasonal conjunctivitis. In both groups of patients, itching scores fell from around 5.1 at the start of the study, to around 3.8 after five minutes and around 2.7 after two hours. Similar reductions in redness scores were seen, falling from 4.5 to 3.7 after five minutes and 2.7 after two hours.63 In the long term, the itching scores fell from an average of around 3.9 on the first day, falling to 0.8 for emedastine and 2.0 for levocabastine after 6 weeks. For redness, the scores fell from around 2.7 to 0.5 for emedastine and to 1.1 for levocabastine.63 Similar results were obtained for emedastine in earlier studies in which a conjunctival allergen-challenge (CAC) model was utilized to compare the efficacy of 0.05% emedastine with 0.5% ketorolac64 and with 0.05% levocabastine.65 On the basis of all these collective data, the FDA approved emedastine 0.05% (Emadine) in December 1997, and the European Medicines Agency (EMA) approved emedastine 0.05% (Emadine) in January 1999 for use in treating SAC. While emedastine exhibited superior pharmacological properties to both levocabastine and ketotifen in terms of a higher or equivalent H1-receptor affinity, greater in vitro potency, greater H1-receptor selectivity26,27 and efficacy in the animal models of AC,62 the 5–8 h duration of action in the CAC model66 and twice-daily topical ocular dosing requirement to control the signs and symptoms of SAC were considered less than ideal.62−66 Therefore, Emadine was strategically only marketed in the EU while we continued our search for a better ocularly suited antihistamine with superior characteristics to Emadine.
During the ongoing research described above, we had also profiled an antiallergic drug, olopatadine, from another Japanese company (Kyowa Hakko Kogyo, Tokyo, Japan). Olopatadine was originally synthesized and reported by the team of Ohshima et al.67 and it was shown to be effective at inhibiting histamine-induced skin weal. The Alcon team had obtained olopatadine and profiled it in several in vitro assays and in vivo models of AC. Although olopatadine exhibited a lower H1-receptor affinity (Ki = 36 nM) as compared with emedastine (Ki = 1.2 nM) (Tables 1,2), it possessed a greater H1-receptor selectivity than antazoline, ketotifen, and levocabastine vs H2- and H3-receptors of the guinea pig brain preparation (Table 2; Figure 5A).29 It was interesting to find later on that olopatadine had a higher affinity (Ki = 2.5 nM) for the human H1-receptor68 than for the guinea pig H1-receptor.29 Olopatadine potently antagonized histamine-induced PI turnover in isolated HCE, HCF, and HTM cells (IC50s = 10–40 nM; Figure 2B) and potently inhibited cytokine secretion from HCE cells.60,69 In isolated HCMCs, olopatadine concentration-dependently inhibited anti-IgG-stimulated histamine secretion (IC50 = 559 ± 277 μM; Figure 3), but unlike ketotifen which promoted histamine release (also PGD2 and tryptase release) from HCMCs at high concentrations, olopatadine did not exhibit such toxicity effects even up to 2 mM.57−59
Figure 5.
This montage depicts the relative affinity and selectivity of olopatadine for H1–H3 receptors subtypes (A), and the ability of histamine to increase production of [3H]-IPs in isolated h-TM cells and the noncompetitive antagonism of these responses by different concentrations of olopatadine (B). Panel A reproduced with permission from ref (29). Copyright 1996 Mary Ann Liebert Publishing Inc. Panel B reproduced with permission from ref (30). Copyright 1996 American Society for Pharmacology and Experimental Therapeutics.
The specific way olopatadine and epinastine interact with cell membranes appears to stabilize and perhaps strengthen the latter70 thereby preventing HMC degranulation in response to the pollen-induced immune reaction in the conjunctiva. Such characteristics and additional specific binding of olopatadine may also explain why this drug was 10-fold more potent at inhibiting histamine-stimulated cytokine release from HCE cells59,60,69 than its H1-receptor binding affinity using guinea pig brain cell membranes.26,27 Olopatadine, levocabastine, and emedastine were significantly more potent antagonists than antazoline and pheniramine in the histamine-mediated cytokine secretion assays.58−60 These mast cell stabilizing and antihistaminergic activities of olopatadine translated well to the in vivo models of AC. Thus, topical ocular application of olopatadine effectively blocked antigen- and histamine-stimulated conjunctivitis in guinea pigs.56,58 Passive anaphylaxis in guinea pig conjunctiva was also attenuated by olopatadine applied 30 min prior to intravenous or topical ocular antigen challenge (ED50 values 0.0067% and 0.017%, w/v, respectively).30,56 Likewise, olopatadine applied topical ocularly (t.o.) from 5 min to 24 h prior to a histamine challenge effectively and concentration-dependently attenuated the vascular permeability response.30,56 These data strongly indicated that olopatadine had an acceptable onset of action, and a durable therapeutic effect. Such preclinical results helped elevate olopatadine for clinical testing in the CAC and SAC models of AC after suitable Investigation New Drug (IND)-enabling studies were conducted to ensure requisite safety of the drug, and eventual effectiveness in human subjects. Results from an environmental study demonstrated that Patanol was effective in the treatment of the signs and symptoms of allergic conjunctivitis when dosed twice daily for up to 6 weeks. Results from conjunctival antigen challenge studies demonstrated that Patanol, when subjects were challenged with antigen both initially and up to 8 h after dosing, was significantly more effective than its vehicle in preventing ocular itching associated with allergic conjunctivitis. Such clinical evaluations of olopatadine (0.01–0.15%) in the CAC model of AC demonstrated optimal efficacy at 0.1% with a duration of action up to 8 h using a twice-daily dosing paradigm relative to placebo.71−76 Olopatadine 0.1% (Patanol), in a relatively simple formulation, was approved by the FDA and marketed in 1996 for treating SAC-related ocular itching. Subsequently, Patanol was shown to be more efficacious than oral loratadine (Claritin),73,74 and more effective than topical ocular azelastine75 and nedocromil.76 Hence, the use of a dual pharmacophoric compound (antihistaminic and mast cell stabilizer) for the treatment of SAC and PAC became the standard of care during the mid-1990s.25,54,58
Even though the team and the company were delighted to make this ground-breaking contribution, the relatively short duration of action and twice-daily dosing regimen remained a concern. These challenges were overcome by finding a solubilization formulation (that contained povidone and edentate disodium) that permitted generation of olopatadine 0.2% that possessed a greater efficacy and was compatible with a once-daily dosing with up to 16 h of effectiveness. Thus, results from clinical studies of up to 12 weeks duration demonstrated that olopatadine 0.2% solution when dosed once a day is effective in the treatment of ocular itching associated with allergic conjunctivitis. This became Pataday and was FDA-approved and marketed in 2004 to treat the itching due to SAC (Table 3),77−81 and which is now available over the counter. With further refinement of the formulation for olopatadine, a higher concentration became possible a few years later when the formulation was augmented with viscosity enhancing excipients such as hydroxypropyl-gamma-cyclodextrin, polyethylene glycol 400, and hypromellose, and with a slightly higher concentration of the preservative benzalkonium chloride (0.015% vs 0.01%). Patients were evaluated with an ocular itching severity score ranging from 0 (no itching) to 4 (incapacitating itch) at several time points after CAC administration. Table 3 displays the mean ocular itching severity scores after ocular administration of a specific antigen using the CAC model in Studies 1 and 2, respectively. A one-unit difference compared to vehicle is considered a clinically meaningful change in the ocular itching severity score. Olopatadine 0.77% demonstrated statistically significantly improved relief of ocular itching compared to vehicle at 30–34 min, 16 h, and 24 h after study treatment. Olopatadine 0.77% provided statistically significantly improved relief of ocular itching compared to Pataday at 24 h after study treatment, but not at 30–34 min after study treatment. Olopatadine 0.77% demonstrated once-daily dosing efficacy and a 24-h duration of action to reduce ocular pruritis in pollen-sensitive patients in the CAC model of AC (e.g., Table 3).82−84 Olopatadine 0.77% became Pazeo and was FDA-approved and marketed for clinical introduction for SAC and PAC in 2015 (Table 3).82−84 While all the marketed antihistamines and mast cell stabilizer drugs for AC treatment are safe and effective (to varying degrees with differences in their onset and duration of action), all have side-effects as can be found in the package inserts of these drugs. Thus, for Pazeo the most commonly reported adverse reactions occurred in 2–5% of patients and included blurred vision, dry eye, superficial punctate keratitis, dysgeusia (bad taste) and abnormal sensation in eye.84−86
Table 3. Reduction of Itching Scores by Pataday and Pazeo in Human Subjectsa.
| Pazeo (olopatadine, 0.7%) | Pataday
(olopatadine, 0.2%) |
Vehicle |
||||
|---|---|---|---|---|---|---|
| time point | mean | mean | difference (95% CI) | mean | difference (95% CI) | |
| Study 1 | N = 66 | (N = 68) | (N = 68) | |||
| onset | 3 min | 0.36 | 0.39 | –0.02 (−0.31 0.26) | 1.90 | –1.54 (−1.82 −1.25) |
| 5 min | 0.53 | 0.61 | –0.08 (−0.39 0.22) | 2.06 | –1.53 (−1.84 −1.22) | |
| 7 min | 0.48 | 0.61 | –0.13 (−0.44 0.17) | 1.97 | –1.49 (−1.80 −1.18) | |
| 16 h | 3 min | 0.70 | 0.87 | –0.17 (−0.44 0–11) | 2.20 | –1.50 (−1.77 −1.23) |
| 5 min | 0.79 | 1.04 | –0.24 (−0.55 0.07) | 2.27 | –1.4S (−1.79 −1.16) | |
| 7 min | 0.75 | 0.98 | –0.23 (−0.54 0.08) | 2.13 | –1.38 (−1.69 −1.07) | |
| 24 h | 3 min | 0.93 | 1.41 | –0.48 (−0.76 −0.20) | 2.54 | –1.61 (−1.88 −1.33) |
| 5 min | 1.10 | 1.52 | –0.42 (−0.72 −0.12) | 2.62 | –1.51 (−1.81 −1.21) | |
| 7 min | 1.09 | 1.50 | –0.41 (−0.72 −0.10) | 2.50 | –1.41 (−1.72 −1.11) | |
| Study 2 | (N = 98) | (N = 99) | (N = 49) | |||
| onset | 3 min | 0.38 | 0.47 | –0.09 (−0.28 0.09) | 1.91 | –1.53 (−1.76 −1.30) |
| 5 min | 0.53 | 0.61 | –0.08 (−0.29 0.12) | 1.99 | –1.46 (−1.71 −1.22) | |
| 7 min | 0.65 | 0.61 | 0.04 (−0.18 0.26) | 1.82 | –1.17 (−1.45 −0.90) | |
| 24 h | 3 min | 1.01 | 1.33 | –0.31 (−0.57 −0.06) | 2.30 | –1.29 (−1.60 −0.97) |
| 5 min | 1.22 | 1.48 | –0.26 (−0.51 -0.01) | 2.37 | –1.15 (−1.46 -0.84) | |
| 7 min | 1.25 | 1.41 | –0.16 (−0.42 0.11) | 2.14 | –0.89 (−1.22 −0.57) | |
Mean score estimates, treatment differences, and corresponding 95% confidence intervals (CIs) were based on analysis of repeated measures using a mined model with itching scores frm each eye (left or right) as the dependent variable and fixed effect terms for investigator, treatment, eye-type (left or right), time, and treatment-by-time interaction. The ocular itching score range is 0–4, where 0 is none and 4 is incapacitating itch. The comparative clinical data shown above are from the package insert of Pazeo available from the FDA Web site.
In closing out this section, it is worth mentioning that since the FDA approvals of Patanol and Pataday and since our research began on emedastine and olopatadine, there has been progress made in identifying additional mast cell mediators/mast cell chemoattractants including a host of chemokine ligands (e.g., CCL2, CCL3, CCL5-CCL11) and adhesion molecules (ICAM-1 and VCAM-1). CCL7, for example, is a potent chemoattractant for monocytes, memory T-lymphocytes, eosinophils, basophils, dendritic cells, and natural killer cells, all of which are heavily implicated in the secondary phase of AC following the increased vascular permeability induced by histamine and other inflammatory mediators during the early/acute phase of AC discussed above. The cloning of a fourth histamine receptor and its localization on animal and human conjunctiva and eosinophils87 led to the finding that only H1-and H4-receptors are involved in mediating the itching sensation.88 Furthermore, the involvement of serotonin-1 and 2-receptors along with protease-activated receptor-2 in propagating the itch response involves transient receptor potential-ion-channel mediated signaling pathways,18 thereby laying the foundation for potential future therapeutic intervention for AC using these targets. Alcaftadine appears to be the only ocularly utilized antihistamine to-date that possesses a somewhat weak micromolar affinity (IC50 = 4.4 μM) and micromolar antagonist potency at the H4-receptor.85,89 However, since a bona fide H4-receptor antagonist of nanomolar affinity and high potency/selectivity, JNJ7777120, induced histamine release on the rat conjunctiva,90 and H4-receptors may not be fully operational on human eosinophils,91 more research is warranted to clarify the relative contribution of H4-receptors in mediating the ocular inflammatory actions of mast cell-derived histamine in AC. Because H4-receptors are expressed by mast cells, leukocytes, and CD4+ cells, there is the potential for drugs with H4-antagonist activity to inhibit recruitment of eosinophils and thus reduce the severity of the late-phase of allergic phenomenon, in particular the ocular itching.18,88,92
Lastly, since the advent of the first mast cell stabilizer with potent H1-receptor antagonists activity, olopatadine, and the approval of Patanol (1996), Pataday (2004), and Pazeo (2015), some other dual action drugs approved for SAC treatment have surfaced. These include: ketotifen (Zaditor, approved 1999), azelastine (Optivar, approved 2000), epinastine (Elastat, approved 2003), bepotastine (Bepreve, approved 2009), alcaftadine (Lastacaft, approved 2010) and cetirizine (Zerviate, approved 2017). In the early clinical trials for suppressing ocular itching (and hyperemia) in the CAC model of AC, ketotifen, azelastine, and epinastine performed poorly against olopatadine 0.1–0.2%. Only alcaftadine (0.25%) exhibited a greater efficacy than olopatadine 0.2% in preventing ocular itching at 3 min and up to 16 h postchallenge/instillation.92 However, it would be interesting to see how alcaftadine (0.25%) would compare with olopatadine 0.77% (Pazeo) in a future clinical trial for AC treatment. Regardless, it would appear that olopatadine and alcaftadine may remain the gold standards for treating SAC and PAC until more superior drugs are discovered, developed, and approved by health authorities. It is hoped and anticipated that novel medicines for treatment of SAC may come from the many areas of active research involving synthetic organic drugs, immunomodulators, and antibodies directed to integrins, adhesion molecules, leukotriene, and Toll-like receptors, among other modalities.12,85,92−96
Taken together, this three-generational product-line featuring olopatadine for the treatment of SAC/PAC earned the major contributors involved in the olopatadine research and development for AC research at Alcon (Dr. Najam Sharif, Dr. John Yanni, and Mr. Steve Miller, and Mr. Shouxi Xu) the “Sir James Black Award for contributions to drug discovery” from the British Pharmacological Society in December 2017.
Discovery, Development, and Approval of Travatan for Treatment of Ocular Hypertension (OHT) and Primary Open-Angle Glaucoma (POAG)
The neurodegenerative eye disease “glaucoma” comprises several different multifactorial optic neuropathies, the cardinal features of which encompass slow but progressive destruction of the optic nerve that connects the retinal ganglion cells via their axons in the anterior retina to the brain. The loss of such connectivity can result in vision deterioration and ultimately blindness in the absence of suitable treatment(s). Around 80 million people worldwide are currently suffering from Primary Open-Angle Glaucoma (POAG), the predominant form of glaucoma, which is the second leading cause of blindness around the world. Epidemiological surveys project this number to increase to over 112 million victims of POAG by 2040,10 with resultant poor quality of life and high economic and social burdens. Risk factor analyses have indicated that elevated intraocular pressure (IOP) is highly correlated with the onset and progression of POAG,5,6,10 but increasing age, comorbidities such as diabetes, retinal vascular abnormalities,97 and lower than normal intracranial fluid pressure (ICFP)98 all exacerbate the condition and may under certain circumstances be more responsible causative factors than high IOP.6,14,97 Thus, some people with fairly normal IOPs (∼16–21 mmHg) still experience progressive visual impairment and blindness, suggesting that factors other than IOP are involved in the pathology and progression of “normotensive glaucoma” (NTG).99,100 Research over the years has yielded some clues including the concept that RGCs and their axons in NTG patients have a lower threshold for damage due to even relative low IOPs, perhaps they are more sensitive and susceptible to IOP fluctuations, ischemia/hypoxia, and to metabolic and oxidative stress than POAG patients.99,100
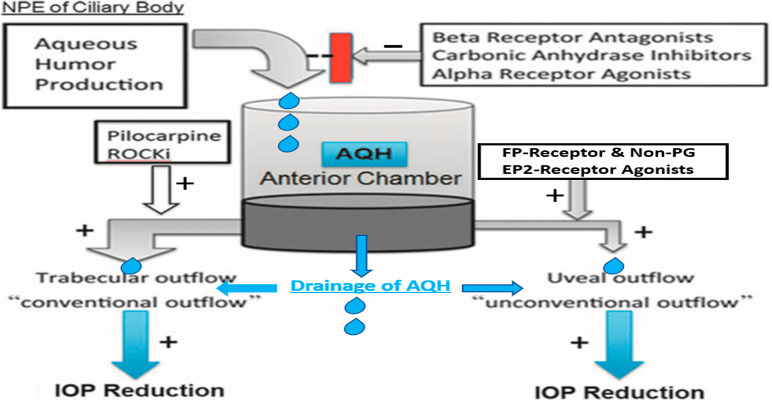
Homeostatic control of IOP is maintained due to a balance between aqueous humor (AQH) production by the ciliary processes (nonpigmented ciliary epithelium [NPCE] cells) and its drainage from the anterior chamber (ANC) of the eye through two different pathways, the major conventional trabecular meshwork (TM) outflow and the minor uveoscleral outflow (UVSC) pathway (Figure 1A/B; Figure 6). In most POAG patients, AQH does not egress or the drainage is extremely slow due to severe blockage of the trabecular meshwork (TM) and Schlemm’s canal (SC) (Figure 1A/B) resulting from accumulation of cellular debris and excessive extracellular matrix (ECM).5,6 The IOP rises and is propagated throughout the eyeball with a major impact on the rear of the globe. This process starts damaging the delicate fenestrated tissue at the back of the eye in the optic nerve head (ONH) region, the lamina cribosa (LC),100−104 which supports the million RGC axons as they pass through to form the optic nerve. The stress and strain of the high IOP initiates local release of inflammatory substances and matrix metalloproteinases (MMPs) that degrade the ECM of the LC, and its structural integrity declines and the optic nerve and associated blood vessels bend and constrict,101−104 causing ischemia.105,106 This aberrant tissue remodeling103,107 adversely affects the RGC axons, and their tensile strength decreases. The ensuing ischemia/hypoxia causes further inflammatory factors to be released, and the vicious cycle continues. During this time, the axonal transport of mitochondria and neurotrophic factors from the brain to the RGC somas and dendrites via the axons in the optic nerve is retarded,108−111 and the axonal injury is increased to the point where their terminals in the brain thalamic nuclei begin to atrophy.112 RGC axons, followed by the RGCs themselves, are depleted of energy109,110,113,114 and growth factors,111,112 and apoptotic death of the RGCs follows. While these are slow processes and their detrimental effects take several years to manifest as visual disturbances, the cascade of deleterious events and factors continues to spiral out of control unless there is therapeutic intervention. Also, because POAG is a “silent thief of sight” the patient is usually unaware of their disease until quite late into the progression phase. It appears that in the early phase of glaucoma development the brain compensates for the loss of contrast sensitivity,115 and due to the asymptomatic nature of this insidious disease, the patient finally notices visual impairment when ∼40% of the RGCs have been destroyed and peripheral vision has significantly deteriorated. It is now critical to quickly diagnose and begin treatment to lower the elevated IOP,4−6 the only modifiable end point that has thus far shown to alleviate the damage caused to the RGCs and their axons during the pathogenesis and progression of POAG and NTG.4−6,97,99 AQH production can be slowed and/or its drainage stimulated using pharmaceutical or surgical means to lower the IOP and to save the sight of these patients (Figure 6; Table 4).5−7,116
Figure 6.
Diagram showing the generation of AQH and its inhibition by certain drugs, and drainage of AQH from the anterior chamber of the eye via two different outflow pathways as promoted by different drug classes. Reproduced and updated with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
Table 4. Classes of Clinically Utilized Drugs for Treating Ocular Hypertension/POAG/NTGa.
| pharmacological class of drug | general name of approved drugs (brand name) | mode(s) of action | pertinent comments |
|---|---|---|---|
| Conventional AQH Outflow Promoting Drugs | |||
| cholinergic muscarinic receptor agonists | pilocarpine (Isopto Carpine); carbachol (Miostat) | enhancement of AQH via conventional outflow pathway | oldest drug therapy known for glaucoma; use limited by 4× daily topical ocular [t.o.] dosing and brow ache and meiosis |
| rho kinase (ROCK) inhibitors | ripasudil (Galanatec); netarsudil (Rhopressa) | increase conventional outflow of AQH (perhaps also enhancing episcleral venous outflow) | relatively efficacious IOP-lowering; increased propensity for hyperemia induction |
| AQH Production (Inflow) Inhibitor Drugs | |||
| carbonic anhydrase inhibitors | dorzolamide (Trusopt); brinzolamide (Azopt) | AQH Inflow inhibition at ciliary processes | oral acetazolamide and methazolamide were used in the past; currently used for acute IOP control instead of chronic therapy; 2x-t.o. daily dosing |
| beta-adrenergic receptor antagonists (“beta blockers”) | timolol (Timoptic); betaxolol (Betoptic); levobunolol (Betagan) | AQH Inflow inhibition at ciliary processes | widely utilized; 2×-t.o. daily dosing; can induce bradycardia; asthmatics treated very cautiously. |
| alpha2-adrenergic receptor agonists | brimonidine (Alphagan); apraclonidine (Iopidine) | AQH Inflow suppression at ciliary processes and enhancement of uveoscleral outflow of AQH | epinephrine and dipivefrin used historically; brimonidine widely used nowadays; 2×- daily t.o. dosing but propensity to cause ocular allergic reaction |
| Uveoscleral Outflow Promoting Drugs | |||
| prostaglandin analogs (FP-receptor agonists), a novel non-PG EP2-receptor agonist (OMDI)) | latanoprost (Xalatan); travoprost (Travatan); bimatoprost (Lumigan); tafluprost (Zioptan). omidenepag isopropyl (OMDI) (Eybelis) | enhancement of uveoscleral and also conventional outflow of AQH Enhancement of uveoscleral outflow of AQH | FP-receptor agonists are the most widely used most potent and most efficacious drug class enabling 1x-t.o. dosing; cosmetic side-effects in and around eyes (iridial color change; deepening of eyelid sulcus). OMDI approved in Japan does not have the aforementioned side-effects. |
| Multiple Modes of Action Drugs | |||
| prostaglandin conjugates | latanoprostene bunod (latanoprost conjugated to an nitric oxide [NO] donor) (Vyzulta) | increase uveoscleral and also conventional outflow of AQH | efficacious IOP-lowering using dual mechanisms of action; 1×-t.o. dosing; propensity for greater hyperemia induction due to NO |
| combination products | examples include: dorzolamide + timolol (Cosopt); brimonidine + brinzolamide (Simbrinza); travoprost + timolol (DuoTrav); latanoprost + netarsudil (Roclatan) | enhancement of outflow and suppression of inflow of AQH | efficacious IOP-lowering using dual modes of action; 1×-t.o. dosing; Patients who are refractory or poor responders to standards of care usually require combination products. |
While t.o. drugs are the mainstay treatment for OHT/POAG/NTG, some patients are recalcitrant to pharmaceutical agents. Thus, use of the above-mentioned drugs is often secondarily supplemented with implantation of AQH microshunts or surgeries to reduce the IOP down to or below the normal range in order to help preserve vision in these patients.5−7,13,99,116
In the early 1990s, at the time our research into drug discovery for treating OHT/POAG started, only some old drugs such as pilocarpine, timolol, brimonidine, dorzolamide, brinzolamide, and trabeculoplasty were available to the clinicians (see Table 4). Even though these drugs offered IOP-lowering efficacy, their low potencies necessitated 2–4-times daily ocular dosing, and their side-effect profiles were not ideal.5−7,116 Early stage academic research (and later, work at Pharmacia Inc.) had begun to show that various classes of prostaglandins (PGs) possessed ocular hypotensive activity in various animals.117−123 However, the natural PGs in their free acid forms (and later as esters) caused corneal/conjunctival vasodilation and thus hyperemia.117−123 In some cases, inflammation, foreign body sensation, and localized hemorrhages on the ocular surface were also observed when t.o. dosed. The FP-receptor agonist class became the preferred target when researchers demonstrated that upon esterification of the free acid and modification of the lower chain of PGF2α, the IOP-lowering efficacy could be enhanced and the side-effects significantly reduced due to improved receptor selectivity.13,119,121,124−126 Pharmacia Inc., which later became part of Pfizer, had numerous compounds they were trying to optimize for their clinical trials. Our internal research review of such a program revealed that Alcon could compete in this area of ocular discovery research and bolster the portfolio beyond betaxolol (beta-blocker) and brinzolamide (carbonic anhydrase inhibitor) (Table 4).
With senior management’s support, a number of existing biologists were reassigned to my newly created Molecular Pharmacology Unit, and I rapidly hired several new scientist biologists and began establishing and validating numerous specific PG receptor binding and functional assays and rendered them into the HTS platform. Simultaneously, our expert medicinal chemists had begun synthesizing key reference and novel PG molecules. Together, we launched a multidimensional drug discovery program, initially focusing on FP-receptor agonists but then also spreading the net wider to capture novel compounds that may have IOP-lowering potential by engaging other PG receptor types and/or subtypes. At first, progress was slow due to the novelty of the new drug discovery paradigm being implemented. However, as the team members and other associates sharpened their focus, gelled together scientifically and personality-wise, and some good reproducible data began to emerge, the team got the necessary motivational boost, and productivity and innovation accelerated. The team established compound screening funnels with stringent criteria for Go/No Go decisions to be made. A pharmacological mindset was also a huge catalyst that yielded dividends! Full concentration–inhibition and concentration–response in vitro studies allowed the team to rank order compounds and select leads for animal safety and efficacy studies based on receptor affinity and agonist potency (+ relative intrinsic activity). Thankfully, the full range of PG receptor binding and functional assays had been established and validated with suitable agonists and antagonists, and thus we began to also generate relative PG-receptor selectivity data for our key compounds of interest. This was critical since the published literature on various PGs was incomplete or somewhat inaccurate. In certain cases, the literature data could not be reproduced, and thus our internal database became our guide that gave everyone much more confidence on results from our internal screening efforts.
The HTS paradigm and system permitted automatic transfer of raw data from receptor binding and second messenger assay readout machines, automatic curve-fitting, and data archival.7,29,31 Thus, data sharing became routinely automated and the biologists and medicinal chemists in various departments utilized the information to render further design modifications to the compounds, and the triaged in vitro-active compounds meeting defined criteria were flagged and prioritized for in vivo testing for ocular safety (topical ocular testing at defined concentration(s) in preagreed standardized formulation in rabbits).7 Those compounds that met the “Go-Criteria” were scheduled and tested for effectiveness in the cat pupil diameter measurement model and ocular hypertensive cynomolgus monkey eyes model for IOP-lowering activity.7,127 Typically, efficacy results from a single standardized t.o.-dose study and any side-effects were reviewed by the team before a dose–response study was conducted. This stage-gate screening paradigm ensured speed without impacting data quality, ensured data integrity due to internal data access restrictions, reduced burden, ensured animal safety and health and minimized animal usage and associated cost, especially those connected with tertiary animal models such as the OHT monkeys.7 The iterative molecular design of new compounds helped develop the structure–activity-relationship (SAR), and novelty and patentability was thereby assured. The team built various correlation plots of in vitro receptor binding and functional assay data, and the latter compared with in vivo data from different animals and models. It was gratifying to find good correlations between these various parameters,7 and the discovery program continued to experience growth and continued internal funding support.
With sufficient novel data we also began laying the foundation for quality publications that pharmacologically validated various in vitro techniques such as RT-PCR128 and receptor autoradiography129 and assays involving receptor binding,130−136 cell-based functional assays,135−158 and in vivo animal models,127,155−157 and which permitted intellectual property protection via strategic patent filings before public presentations and/or publication of data. The screening and profiling of natural PGs along with new synthetic compounds revealed that indeed the endogenous PGs were on the whole not that selective for their cognate receptor types. Thus, for instance PGF2α exhibited appreciable affinity for EP3, FP, EP4, and EP1 receptors (Table 5), while R/S-fluprostenol and its S-enantiomer (travoprost acid; AL-5858) were significantly more FP-receptor-selective than other synthetic PGs tested (Figure 7; Table 5).158
Table 5. Relative Affinities and Selectivities of Synthetic Prostaglandins for PG Receptor Sub-Typesa.
| PG receptor
binding inhibition constants (Ki, nM) and FP receptor selectivity (x) |
||||||||
|---|---|---|---|---|---|---|---|---|
| PG analogue | DP | EP1 | EP2 | EP3 | EP4 | FP | IP | TP |
| travoprost free acid ((S)-fluprostenol) | 52 000 ± 7 200 (x 1 486) | 9 540 ± 1 240 (x 273) | nd | 3 501 ± 461 (x 100) | 41 000 ± 2 590 (x 1 171) | 35 ± 5 | ≥90 000 (x 2 571) | ≥121 000 (x 3 457) |
| (R/S)-fluprostenol free acid | >50 000 (x 510) | 12 300 ± 1 240 (x 126) | >100 000 (x 1020) | 4 533 ± 597 (x 46) | 14 400 ± 1 550 (x 147) | 98 ± 9 | >60 500 (x 617) | 121 063 ± 20 714 (x 1 235) |
| bimatoprost free acid (17-phenyl-PGF2α) | >90 000 (x 1 084) | 95 ± 27 (x 1) | nd | 387 ± 126 (x 5) | 25 700 ± 2 060 (x 310) | 83 ± 2 | >100 000 (x 1 205) | >77 000 (x 928) |
| latanoprost free acid (PHXA85) | ≥20 000 (x 204) | 2 060 ± 688 (x 21) | 39 667 ± 5 589 (x 405) | 7 519 ± 879 (x 77) | 75 000 ± 2 830 (x 765) | 98 ± 11 | ≥90 000 (x 918) | ≥60 000 (x 612) |
| bimatoprost (Amide) | >90 000 (x 14) | 19 100 ± 1 450 (x 3) | nd | >100 000 (x 16) | >100 000 (x 16) | 6 310 ± 1 650 | >100 000 (x 16) | >100 000 (x 16) |
| unoprostone (free acid) | >43 000 (x 7) | 11 700 ± 2 710 (x 2) | nd | ≥22 000 (x 4) | 15 200 ± 3 500 (x 3) | 5 900 ± 710 | >30 000 (x 5) | >30 000 (x 5) |
| natural endogenous PG ligand PGF2α | 18 000 ± 6 460 (x 138) | ±12 (x 5) | 964 ± 64 # | 24 ± 8 (x 0.2) | ±25 (x 3) | 130 ± 6 | ≥50 000 (x 385) | ≥190 000 (x 1 462) |
Data are mean ± SEMs from >3 experiments for each compound in each assay. The values in parentheses are the relative FP-receptor selectivities of the compounds. Note that the naturally occurring PGF2α lacks selectivity but the synthetic compounds such as travoprost free acid and latanoprost free acid exhibit significant FP-receptor selectivity.158
Figure 7.
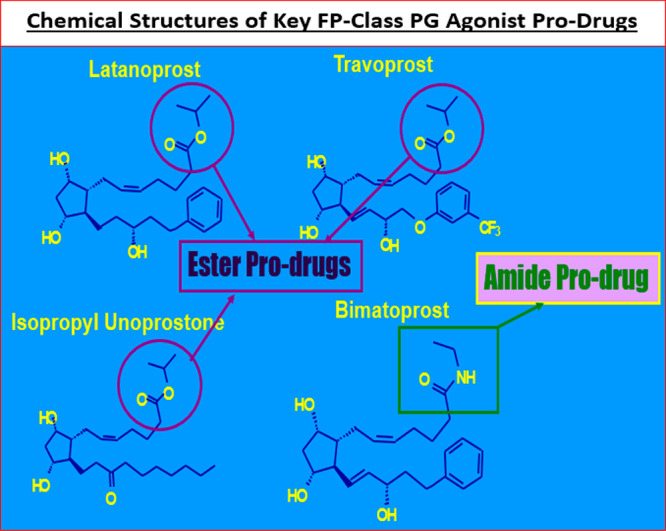
Structures of the key PG pro-drugs discussed in this article are shown in this figure.
Furthermore, second messenger-based functional assays confirmed that the natural PGs were quite promiscuous and nonselective with respect to which PG receptors they activated (Table 6). These findings supported the ocular side-effect observed with the natural PGs when they were instilled to the animal eyes,117,120,122 and hence the need for more potent and PG-receptor-selective agents.158 Even though some of the synthetic PGs such as bimatoprost acid, latanoprost acid, and cloprostenol exhibited relatively high potency at the their cognate FP-receptor, they also stimulated additional PG receptors at fairly low concentrations thereby rendering them to be also not so FP-selective (Table 6). Fortuitously, the most FP-receptor-selective and potent new PG turned out to be travoprost acid as shown by the bolded potency values in Table 6 below.155,158 These types of receptor affinity, potency, and receptor-selectivity data helped the team to frame S-fluprostenol as a viable drug candidate, and this compound progressed to detailed side-effect profiling, rabbit/guinea pig hyperemia, cat eye meiosis, and conscious OHT monkey eyes IOP studies (see ahead).
Table 6. Relative Agonist Potencies of Natural and Synthetic Prostaglandins for PG Receptor Subtypesa.
| agonist
potency (EC50\; nM) at various prostaglandin receptors
and subtypes |
||||||||
|---|---|---|---|---|---|---|---|---|
| compound | DP-receptor (↑ cAMP) | EP1-receptor (↑ PI turnover; or other response) | EP2-receptor (↑ cAMP; or other response | EP3-receptor (↓ cAMP various functional responses) | EP4-receptor (↑ cAMP) | FP-receptor (↑ PI turnover at human cloned FP-receptor; or other responses) | IP-receptor (↑ cAMP or other response) | TP-receptor (↑ PI turnover; or other response) |
| PGD2 | 74 | 3190 | 58 000 | nd | >10 000 | >100; 222 | >10 000 | >10 000 |
| PGI2 | >10 000 | 319 | >10 000 | 3 019 | >10 000 | >5 000 | 7 | >10 000 |
| PGE2 | >1 000 | 2.9 | 67 | 19.9; 45; 4.5 | 40 | >2 500 | 3 310 | >10 000 |
| PGF2α | >10 000 | 29 | >10 000 | 691; 2 000 | >10 000 | 29 ± 2 | 3 000 | >10 000 |
| bimatoprost free acid | >10 000 | 2.7 | >10 000 | nd | >10 000 | 3.3 ± 0.7 | >10 000 | >10 000 |
| travoprost free acid | >10 000 | nd | >10 000 | >10 000 | >10 000 | 2.4 ± 0.3 | >10 000 | >10 000 |
| latanoprost free acid (PHXA85) | >10 000 | 119 | 20 000 | 12 000 | >10 000 | 45.7 ± 8.4 | >10 000 | >10 000 |
| cloprostenol | >10 000 | 93 | >10 000 | 228 | >10 000 | 0.73 ± 0.1 | >10 000 | >10 000 |
| unoprostone (free acid; UF-021) | >10 000 | >30 000 | >10 000 | >10 000 | >10 000 | 3 220 ± 358 | >10 000 | >10 000 |
Data are from various sources using different methodologies and functional readouts. Note that the endogenously produced PGs exhibit poor receptor selectivity in isolated cell/tissue preparations. Receptor selectivity by the natural PGs may be achieved at the site of action in vivo depending on the local PG concentration. nd = not determined.158
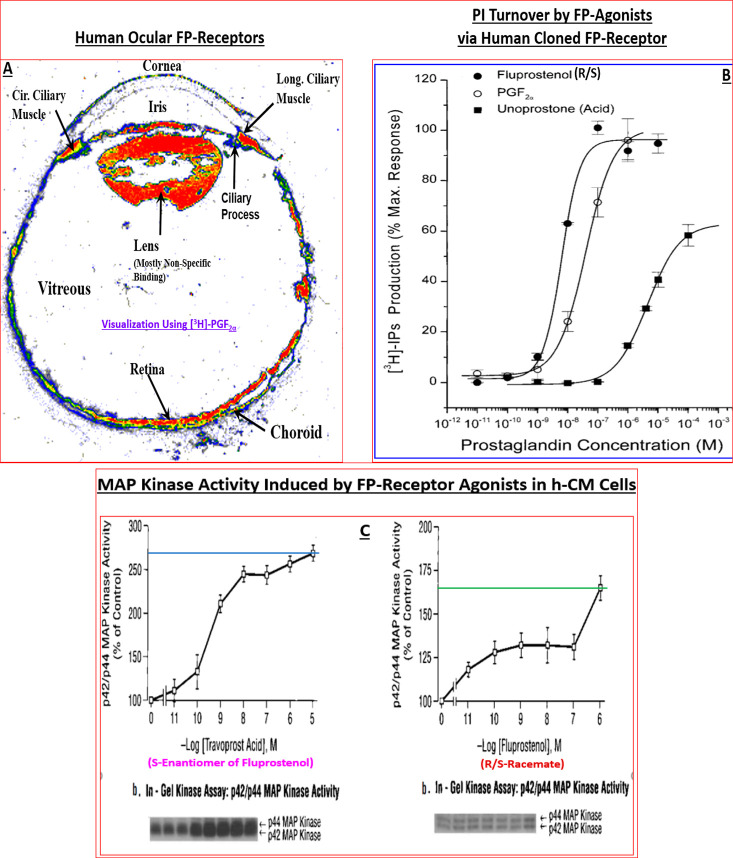
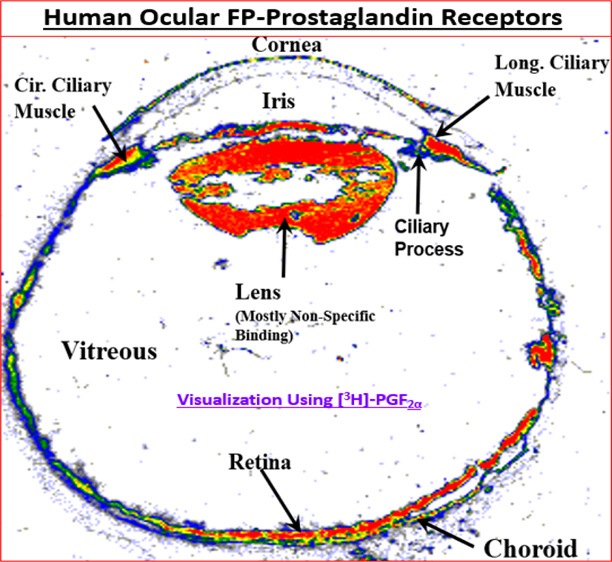
Along the way, target localization studies were conducted to verify the presence of the key PG receptors of interest in post-mortem human eye sections using quantitative autoradiography technology.129,136,159−161 Indeed, FP-receptors visualized with [3H]-PGF2α129,153 and then later with [3H]-AL-5848 (S-fluprostenol acid)129,136 demonstrated a relatively high density of receptors in the longitudinal and circular ciliary muscle (CM) (e.g., Figure 8A) whose functional activity was confirmed by measuring PI turnover, [Ca2+]i mobilization, MAP-kinase studies in isolated human CM (h-CM) cells (Figure 8B,C).158,162 These results were supported by similar findings using isolated and propagated human TM (h-TM) cells,163 and human ciliary body cloned FP-receptor expressed in host HEK-293 cells.164,165
Figure 8.
(A) Autoradiographically visualized FP-receptors in a section of human eye in vitro are shown (total binding of [3H]-PGF2α). The black/white radioautograph was pseudocolor coded to illustrate the relative density of the FP-receptors. Red indicates the highest density, followed by orange, yellow, green, and blue. (B) PI turnover and accumulation of intracellular [3H]-IPs following stimulation by fluprostenol (R/S; racemate), PGF2α, and unoprostone free acid in HEK-943 cells expressing human cloned ciliary body FP-receptor. Reproduced with permission from ref (164). Copyright 2002 Mary Ann Liebert Publishing Inc. (C) MAP kinase activity stimulated by travoprost acid (S-enantiomer of fluprostenol) and fluprostenol (R/S; racemate) in isolated and cultivated hCM cells. Reproduced with permission from ref (162). Copyright 2003 Mary Ann Liebert Publishing Inc.
Since CM and TM cells are responsible for mediating the AQH outflow enhancing ability of FP-receptor agonists to lower IOP (Figure 1B), the presence of functionally responsive FP-receptors in these key cells helped explain the relatively high potency and efficacy of FP-agonists, in particular travoprost acid (AL-5848; S-fluprostenol) in efficaciously lowering IOP in the OHT monkey eyes.155,156 These data helped further support our research discovery program. Additional data were obtained for certain compounds such as extensive receptor profiling (on- and off-target) and using Alcon sponsored research with academic collaborators162,164,166 and contract research facilities155,167 to round-off the SAR development. Compounds of interest were tested at multiple doses for their ability to lower and control IOPs in the relevant animal models including the OHT cynomolgus monkeys with 8–12 animals per group (e.g., Figure 9).155,156 Such IOP data were also confirmed in different colonies of OHT monkeys in-house and also at external collaborator facilities to confirm the efficacy of lead compounds, thereby confirming internal data and enhancing the overall confidence in the testing paradigms.
Figure 9.
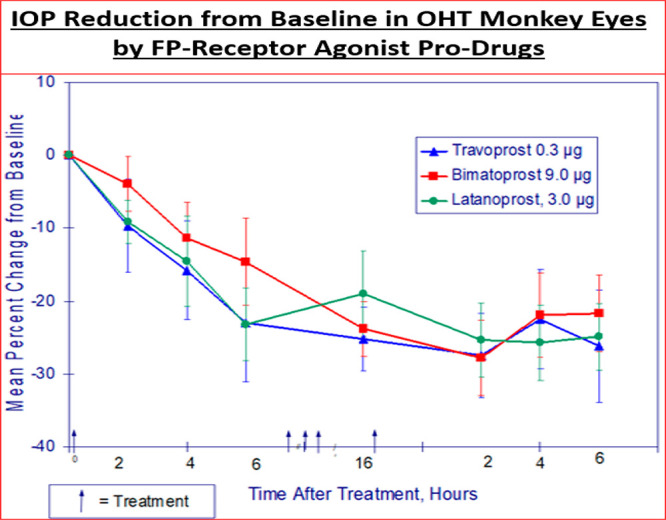
IOP reduction by three different PG pro-drug compounds tested t.o. at different doses in the OHT eyes of conscious cynomolgus monkeys.
Additionally, the latter collaborations permitted studies to delineate the mechanisms of action of the lead compounds in terms of their effects on Ca2+-mediated (Figure 10A)162−165 secretion of matrix metalloproteinases (MMPs) from h-CM cells (e.g., Figure 10B), and AQH outflow via the conventional TM164 pathway and via the uveoscleral pathway (which involves egress of AQH through expanded spaces in the CM and sclera)162 to lower IOP (Figure 9). It became apparent that the new FP-receptor agonist class of PGs (Figure 7) primarily activated phospholipase C to generate inositol phosphates (IPs; Figure 8)162 and mobilized [Ca2+]i (Figures 10A),162−165 with similar potencies in a variety of human ocular and animal cells (Table 7),158 that induced MMPs secretions (e.g., Figure 10B) which then digested the ECM in CM and TM to create/expand UVSC outflow pathway (and to some degree the TM-pathway) to lower IOP (Figure 9) following a single drop t.o. instillation of the FP-receptor agonist drug.
Figure 10.
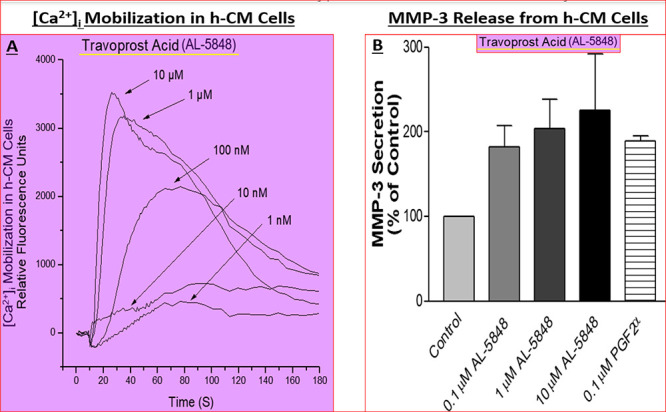
(A,B) Concentration-dependent mobilization of [Ca2+]i (A) and MMP-3 secretion from h-CM cells (B) in response to travoprost acid (AL-5848). Reproduced and updated with permission from ref (162). Coopyright 2003 Mary Ann Liebert Publishing Inc.
Table 7. Agonist Potencies of Synthetic Prostaglandins for FP-Receptors Expressed in Various Cell-Typesa.
| stimulation
of PI turnover and production of IPs (functional response) in different
cell types (agonist potency, EC50 [nM]) |
|||||
|---|---|---|---|---|---|
| compound | human ciliary muscle (h-CM) cells | human trabecular meshwork (h-TM) cells | human cells (HEK-293) expressing cloned human ocular FP receptor | mouse Swiss 3T3 fibroblast cells | rat A7r5 vascular smooth muscle cells |
| travoprost free acid ((S)-fluprostenol) | 1.4 ± 0.2 | 3.6 ± 1.3 | 2.4 ± 0.3 | 2.6 ± 0.2 | 2.6 ± 0.5 |
| bimatoprost free acid (17-phenyl-PGF2α) | 3.8 ± 0.9 | 28 ± 18 | 3.3 ± 0.7 | 2.8 ± 0.2 | 2.8 ± 0.6 |
| (R/S)-fluprostenol | 4.3 ± 1.3 | 11 ± 2 | 4.6 ± 0.4 | 3.7 ± 0.4 | 4.4 ± 0.2 |
| PGF2α | 104 ± 19 | 62 ± 16 | 29 ± 2 | 26 ± 3 | 31 ± 3 |
| travoprost (isopropyl ester) | 123 ± 65 | 103 ± 27 | 40.2 ± 8.3 | 81 ± 18 | 46 ± 6 |
| latanoprost free acid (PHXA85) | 124 ± 47 | 35 ± 2 | 45.7 ± 8.4 | 32 ± 4 | 35 ± 8 |
| latanoprost (isopropyl ester) | 313 ± 90 | 564 ± 168 | 173 ± 58 | 142 ± 24 | 110 ± 19 |
| unoprostone (UF-021; free acid) | 3 503 ± 1 107 | 3 306 ± 1 700 | 3 220 ± 358 | 617 ± 99 | 878 ± 473 |
| unoprostone isopropyl ester | 8 420 ± 912 | 2 310 ± 1 240 | 9 100 ± 2 870 | 560 ± 200 | 458 ± 85 |
| bimatoprost (amide) | 9 600 ± 1 100 | 3 245 ± 980 | 681 ± 165 | 12 100 ± 1 200 | 6 850 ± 1 590 |
Data taken from ref (158).
Such multiyear in vitro and in vivo research resulted in the identification and nomination of clinically viable lead compounds. Following IND-enabling studies and having met all stage-gate “Go Criteria”, some of the leads entered clinical trials for human ocular safety, efficacy, and durability of the IOP-lowering effect based on the classic Phase 1–3 studies paradigm. These proof-of-concept and formal clinical investigations culminated in the approval of Travatan (0.004% travoprost isopropyl ester) by the US FDA in 2001 and by EMA for the treatment of OHT associated with POAG (Table 8). Specifically, Travatan (travoprost 0.004%) and a slightly lower concentration FDA-approved drug (Izba; travoprost 0.003%) lowered IOP by 7.1–8.4 mmHg from baseline and maintained this reduced IOP at various time points during the day after a single topical ocularly administered drop of either drug at night in up to 442 OHT/POAG patients (Table 8).
Table 8. Mean IOP (mmHg) by Treatment Group and Treatment Difference in Mean IOP in Response to Travatan and Izbaa.
| IOP
change from baseline (mmHg) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| IZBA |
TRAVATAN |
||||||||
| visit | N | 8 AM | 10 AM | 4 PM | N | 8 AM | 10 AM | 4 PM | |
| week 2 | mean | 442 | –8.0 | –7.3 | –7.1 | 416 | –8.1 | –7.5 | –7.1 |
| 95% CI | (−8.3, −7.7) | (−7.6, −7.0) | (−7.4, −6.8) | (−8.4, −7.8) | (−7.8, −7.2) | (−7.4, −6.8) | |||
| week 6 | mean | 440* | –8.1 | –7.4 | –7.2 | 413 | –8.3 | –7.5 | –7.2 |
| 95% CI | (−8.4, −7.9) | (−7.6, −7.1) | (−7.5, −6.9) | (−8.7, −8.0) | (−7.9, −7.2) | (−7.5, −6.9) | |||
| month 3 | mean | 432* | –8.2 | –7.5 | –7.1 | 408 | –8 4 | –7.6 | –7.3 |
| 95% CI | (−8.6, −7.9) | (−7.9, −7.2) | (−7.4, −6.8) | (−8.7, –8.1) | (−7.9, −7 2) | (−7.7, −7.0) | |||
| visit/time point | IZBA (Travoprost 0.003%) | TRAVATAN (Travaprost 0.004%) | Difference |
|---|---|---|---|
| mean (SE) | mean (SE) | mean (95% CI) * | |
| baseline | (N = 442) | (N = 418) | |
| 8 AM | 26.9 (0.12) | 27.1 (0.14) | –0.2 (−0.5 0.2) |
| 10 AM | 25.4 (0.13) | 25.6 (0.15) | –0.2 (−0.6 0.2) |
| 4 PM | 24.6 (0.14) | 24.8 (0.16) | –0.2 (−0.6 0.2) |
| week 2 | (N = 442) | (N = 416) | |
| 8 AM | 19.4 (0.16) | 19.5 (0.17) | –0.1 (−0.5 0.3) |
| 10 AM | 18.6 (0.16) | 18.6 (0.16) | –0.0 (−0.4 0.4) |
| 4 PM | 18.0 (0.16) | 18.3 (0.16) | –0.3 (−0.7 0.1) |
| week 6 | (N = 440**) | (N = 413) | |
| 8 AM | 19.3 (0.16) | 19.3 (0.17) | –0.0 (−0.4 0.4) |
| 10 AM | 18.5 (0.16) | 18.6 (0.17) | –0.1 (−0.5 0.3) |
| 4 PM | 18.0 (0.16) | 18.1 (0.17) | –0.2 (−0.6 0.2) |
| month 3 | (N = 432**) | (N = 408) | |
| 8 AM | 19.2 (0.17) | 19.3 (0.18) | –0.1 (−0.5 0.3) |
| 10 AM | 18.3 (0.17) | 18.6 (0.18) | –0.3 (−0.7 0.1) |
| 4 PM | 18.0 (0.16) | 18.0 (0.17) | 0.0 (−0.4 0.4) |
Data are from the package insert of Izba that compares the IOP-lowering efficacy with Travatan. The asterisks (∗) indicate statistical significance.
These and additional IOP-lowering clinical data for Travatan compared well with those published for latanoprost (0.005%; Xalatan) in terms of efficacy and duration of action over a 24-h period studied during several months (recently reviewed, ref (168).) Travoprost isopropyl ester’s active moiety, travoprost free acid, was found to be a potent and efficacious FP-receptor full agonist (e.g., Figure 8B, 8C),155,158,162−166 and the parent drug suitable for once-daily t.o. dosing at night. Such FP-class-PG-directed research also helped identify and characterize other useful FP-receptor class PG analogues that met in vitro and in vivo potency/efficacy parameters and were qualified as clinical candidate ocular hypotensive drugs. These included compounds such as AL-12128169,170 and several other novel FP-receptor agonists that were potent and efficacious ocular hypotensive agents as demonstrated in the OHT eyes of conscious cynomolgus monkeys in multiple studies.126,171,172
Even though FP-class PGs (latanoprost 0.005, travoprost 0.004, bimatoprost 0.03%, tafluprost 0.0015%) became first-line therapeutic drugs for treating OHT/POAG in the late 1990s and early 2000s, it is important to balance their excellent IOP-lowering activities with several ocular side-effects which are well documented.119,121,156,168,168a,173 By example, the most common ocular adverse event observed in controlled clinical studies with Travatan 0.004% was ocular hyperemia which was reported in 35 to 50% of patients. Approximately 3% of patients discontinued therapy due to conjunctival hyperemia. Ocular adverse events reported at an incidence of 5 to 10% included decreased visual acuity, eye discomfort, foreign body sensation, pain, and pruritus. Ocular adverse events reported at an incidence of 1 to 4% included, abnormal vision, blepharitis, blurred vision, cataract, cells, conjunctivitis, dry eye, eye disorder, flare, iris discoloration, keratitis, lid margin crusting, photophobia, subconjunctival hemorrhage, and tearing. Nonocular adverse events reported at a rate of of 1 to 5% were accidental injury, angina pectoris, anxiety, arthritis, back pain, bradycardia, bronchitis, chest pain, cold syndrome, depression, dyspepsia, gastrointestinal disorder, headache, hypercholesterolemia, hypertension, hypotension, infection, pain, prostate disorder, sinusitis, urinary incontinence, and urinary tract infection. The latter are reported in the package insert for this ocular hypotensive drug. It is evident from all the reported studies that all FP-class PG analogues, including bimatoprost and its free acid, share the same common side-effects described above.173
During the aforementioned research, the Alcon medicinal chemistry team also synthesized and tested many analogues of PGD2, both free acids and various esters, and successfully identified safe and efficacious drugs such as AL-6598 that entered clinical trials.156,161 Although initial studies identified the free acid of AL-6598 as a DP-receptor agonist,156 more detailed investigations of AL-6556 led to the discovery that it possessed dual pharmacophoric activity being a full agonist at the DP receptor but a partial agonist at the EP2 receptor.161 AL-6598 was a potent and efficacious ocular hypotensive in OHT monkey and human eyes.156 However, the relatively high degree of hyperemia associated with t.o. dosing of AL-6598 precluded its future development, even though we tried to lower the risk and intensity of this side-effect by utilizing a low dose of a vasoconstrictor α2-agonist, brimonidine, that did not interfere with the IOP-lowering efficacy of AL-6598.156
Some years later, the antiglaucoma team also successfully characterized147,157,174 and obtained effective lowering of IOP by the S-enantiomer of betaxolol (Betaxon) in animal models of OHT157 and in OHT/POAG patients.175 However, for strategic marketing purposes, even though Betaxon was approved by the FDA, Betaxon was not marketed in the US. Instead, the antiglaucoma team focused on generating sufficient data to secure FDA approval and then successfully launched a few combination products such as DuoTrav (travoprost + timolol; see ref (13) for review), and Simbrinza (brinzolamide + brimonidine) (Table 9), the latter being the first combination product devoid of a β-blocker, to help those patients who were low-responders to individual ocular hypotensive drugs or those who were refractory to other pharmaceutical treatments to lower their IOPs.
Table 9. IOP Lowering Data for Simbrinza in OHT/POAG Patientsa (Data Are from the Package Insert for Simbrinza).
| Simbrinza | brinzolamide | brimonidine | |||
|---|---|---|---|---|---|
| mean | mean | difference (95% CI)b | mean | difference (95% CI)b | |
| Study 1 | (N = 209) | (N = 224) | (N = 216) | ||
| week 2 | |||||
| 8 AM | 20.4 | 22.0 | –1.6 (−2.3, −0.9) | 22.4 | –2.0 (−2.7, −1.3) |
| 10 AM | 17.1 | 20.5 | –3.4 (−4.1, −2.7) | 19.4 | –2.3 (−3.0, −1.6) |
| 3 PM | 18.4 | 20.4 | –1.9 (−2.6, −1.3) | 20.6 | –2.2 (−2.9, −1.5) |
| 5 PM | 16.6 | 19.7 | –3.2 (−3.9, −2.5) | 18.4 | –1.9 (−2.6, −1.2) |
| week 6 | |||||
| 8 AM | 20.4 | 21.9 | –1.5 (−2.2, −0.8) | 22.6 | –2.3 (−3.0, −1.6) |
| 10 AM | 17.5 | 20.2 | –2.7 (−3.4, −2.0) | 19.5 | –2.0 {-2.7, −1.3) |
| 3 PM | 18.9 | 20.2 | –1.2 (−1.9, −0.5) | 21.1 | –2.1 (−2.8, −1.4) |
| 5 PM | 17.0 | 19.7 | –2.6 (−3.3, −1.9) | 18.6 | –1.5 (−2.2, −0.8) |
| month 3 | |||||
| 8 AM | 20.5 | 21.6 | –1.1 (−1.8, −0.4) | 23.3 | –2.8 (−3.5 −2.1) |
| 10 AM | 17.2 | 20.4 | –3.2 (−3.9, −2.5) | 19.7 | –2.5 (−3.2, −l.8) |
| 3 PM | 18.7 | 20.4 | –1.8 (−2.5, −1.1) | 21.3 | –2.6 (−3.3, −1.9) |
| 5 PM | 17.0 | 20.0 | –3.0 (−3.7, −2.3) | 18.8 | –1.8 (−2.5, −1.1) |
| Study 2 | (N = 218) | (N = 229) | (N = 232) | ||
| week 2 | |||||
| 8 AM | 20.5 | 22.2 | -1.7 (−2.4, −1.0) | 22.8 | –2.4 (−3.1, −1.7) |
| 10 AM | 17.4 | 20.7 | –3.3 (−4.0, −2.6) | 19.2 | -1.8 (−2.5, −1.2) |
| 3 PM | 18.7 | 20.5 | -1.7 (−2.4, −1.1) | 21.1 | –2.3 (−3.0, −1.6) |
| 5 PM | 16.5 | 20.1 | –3.6 (−4.3, −2.9) | 18.3 | -1.8 (−2.4, −1.1) |
| week 6 | |||||
| 8 AM | 20.7 | 21.9 | -1.2 (−1.9, −0.5) | 23.2 | –2.5 (−3.2, –l.8) |
| 10 AM | 17.4 | 20.5 | –3.1 (−3.8, −2.4) | 19.7 | –2.3 (−3.0, −1.6) |
| 3 PM | 19.3 | 20.2 | –0.8 (−1.5, −0.2) | 21.2 | –1.9 (−2.6, −1.2) |
| 5 PM | 16.9 | 19.9 | –3.0 (−3.7, −2.3) | 18.5 | –1.7 (−2.4, −1.0) |
| month 3 | |||||
| 8 AM | 21.1 | 22.0 | –1.0 (−1.7, −0.3) | 23.2 | –2.2 (−2.9, −1.5) |
| 10 AM | 18.0 | 20.8 | –2.8 (−3.5, −2.1) | 19.9 | –1.9 (−2.6, −1.2) |
| 3 PM | 19.5 | 20.7 | –1.2 (−1.9, −0.5) | 21.5 | –2.0 (−2.7, −1.3) |
| 5 PM | 17.2 | 20.4 | –3.2 (−3.9, −2.5) | 18.9 | –1.7 (−2.4, −1.0) |
Based on the Intent-to-Treat Population defined as all patients who received study drug and completed at least 1 on-therapy study visit.
The estimates are based on least-squares means derived from a linear mixed model that accounts for correlated IOP measurements within patient; Treatment difference is Simbrinza minus individual component. CI = 95% confidence interval.
Similarly, for expansion of the Alcon antiglaucoma drug treatment portfolio and franchise, additional drug targets were investigated, and several drug discovery projects were launched in the ensuing years after global approval and marketing of Travatan. Thus, we also found some relatively potent and efficacious ocular hypotensive rho kinase (ROCK) inhibitors175−179 that matched the in vitro and in vivo properties of ripasudil and netarsudil, and that showed the classic actomyosin-relaxing activity observed with other well-known literature reference ROCK inhibitors such as Y-27632 and Y-39983 (e.g., Figure 11).179,180
Figure 11.
Morphological changes induced by ROCK inhibitors in h-TM cells. Reproduced with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
In the intervening months we decided to launch a totally diverse pioneering drug discovery program that involved finding suitable ocular hypotensive drugs from the serotonergic (5-hydroxy tryptamine; 5-HT) receptor field, even though this was an extremely difficult task. The literature and our early studies in this field had shown that 5-HT was an important transmitter in the eye.181−184 What confounded the issue was the diversity of conflicting reports connected with IOP-lowering actions of various 5-HT receptor agonists and antagonists (see refs (185 and 186) for reviews). Initial studies in-house centered around routinely and methodically testing commercially available serotonergic compounds in the rabbit and monkey models of OHT in order to delineate involvement of specific 5-HT receptors in the process of IOP-lowering and to generate a database upon which to build the discovery program. The team successfully ruled out several receptor classes and zoned in on the 5-HT2 receptor family.184 We patented certain novel serotonergic compounds that were potent and efficacious ocular hypotensives with IOP-lowering up to 30% or greater in the OHT eyes of conscious Cynomolgus monkeys187−190 and published several patents and papers181−204 describing our efforts to lead the field and inspire other researchers to join the hunt for novel and efficacious drugs to treat OHT/POAG. Lessons learnt from the PG drug discovery programs helped us stay focused and we successfully established in-house receptor binding, cell-based functional assays and deployed the in vivo models to rapidly screen compounds that medicinal chemists synthesized. We mapped 5-HT receptor mRNAs in human ocular tissues,193 and localized the 5-HT2 receptors in human eye sections by autoradiography198 to ensure our target receptors were indeed accessible and engageable with compounds of interest. Again, as with PG receptor studies, we demonstrated that functional 5-HT2 receptors were present on isolated and propagated h-CM198 and h-TM,199 and in NPCE,193 the key cells/tissues that modulate AQH dynamics in the ANC of the eye. This research project finally yielded a novel class of 5-HT2 receptor agonists with high affinity and selectivity for the 5-HT2A receptor subtype and which potently and effectively reduced IOP in OHT monkey eyes,187,189,190,192,195,200,201 and some of which (AL-34662; AL-37807)188,189,200 exhibited efficacy in OHT/POAG patients. Agents with dual receptor activity such as cabergoline201 were found to activate 5-HT2 and dopamine receptors and to enhance outflow facility via the TM pathway in porcine eyes in vitro (Figure 12A) and 5-HT2A-receptor-selective compounds such as 1-(4-iodo-2,5-dimethoxyphenyl)-2-aminopropane (DOI) to lower IOP in the OHT eyes of monkeys (e.g., Figure 13).201
Figure 12.
Ability of two different classes of compounds to stimulate conventional outflow facility in isolated anterior chambers of porcine eyes. Reproduced with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
Figure 13.
Ability of different t.o. doses of the two enantiomers of a potent and selective 5-HT2A receptor agonist (DOI) to lower and control IOP in conscious OHT cynomolgus eyes. Reproduced with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
Serendipity (or creative luck?) played a big part in the final ocular hypotensive drug discovery program that I was involved in as a project leader. Because of my early interest in bradykinin (BK) as an edema-causing algesic endogenous inflammatory peptide that also has numerous other functions in the body,205−208 I was intrigued by the potential role that this peptide may play in ocular physiology and pathology. The discovery and characterization of excitable B2-subtype of BK receptors on immortalized h-TM cells206 was also a catalyst that inspired me to patent certain nonpeptide B2-receptor antagonists (e.g., WIN-64338) as potential IOP-lowering drugs.207 At that time, I reasoned that compounds such as WIN-64338, being organic stable structure molecules would likely penetrate the cornea upon t.o. dosing, whereas the natural nonapeptide would not cross the barriers on the ocular surface and may actually cause too much inflammation, a lesson from the AC story relayed above.33−35 What happened next was a total but a delightful surprise. Since we had a very successful program of material transfer agreements with other pharma companies with subsequent in-licensing and marketing of drugs such as for AC treatment (see above discourse), I requested a sample of FR-199097, a recently reported nonpeptide B2-receptor BK agonist208 from Fugisawa Pharmaceutical Co Ltd. (Tokyo, Japan) because I wished to compare its activity with the B2-Antagonist (WIN-64338) and to see whether an agonist or an antagonist may be better suited for lowering IOP. After months of waiting, the compound arrived and was tested for ocular safety followed by ocular hypotensive activity as per our standard protocol. To my amazement, FR-190997 exhibited very little ocular side-effects but potently and efficaciously reduced OHT monkey IOP at low microgram doses of the compounds, akin to the PGs our team had tested in the past! These data were subsequently reproduced in the same original monkey colony and the compound was also effective in another colony of OHT cynomolgus monkeys. Dose–response studies revealed that the effect was specific and not a random unphysiological response. Indeed, later we showed that FR-190997’s IOP-lowering effect could be totally blocked by t.o. dosing with a B2-receptor-selective BK antagonist (FR-165649), making the response totally pharmacologically relevant and validated. These compelling data, and with management’s full support, helped me launch and lead this discovery program in an effort to find better and more effective ocular hypotensive nonpeptide B2-receptor agonist drugs.
A robust array of receptor binding and functional assays using prior knowledge of the kininergic system209−211 were setup to begin the screening process for analogues of FR-190997 that our medicinal chemists designed and synthesized and that we patented.212−214 B2-receptors were mapped using immunohistochemical techniques,215,216 and functional responses were observed in isolated h-CM,215 h-TM,217 and h-NPCE216 cells that involved BK- and FR-190997-induced PI hydrolysis, IPs accumulation, [Ca2+]i mobilization, PGE2 release, MMP secretion, h-TM cell volume reduction, stimulation of TM-outflow in isolated/perfused porcine eyes (Figure 12B), and of course IOP reduction in vivo in numerous OHT cynomolgus monkeys that involved mostly enhancement of UVSC outflow of AQH (Table 10).218−220 These studies involved much in-house and external collaborative effort with multiple academic colleagues218,219 who were coauthors on the publications who had tested our coded compounds in a masked manner. As the project moved forward, another important nonpeptide B2-receptor agonist (BK2A78)221 was identified and fully characterized in biochemical, pharmacological assays and in the in vivo models and which also demonstrated potent and effective ocular hypotensive efficacy and minimal ocular and systemic side-effects. Last but not least, the apparent contradictory results of the first generation B2-receptor antagonist, WIN-64338, causing pronounced IOP reduction in rabbits after ivt injection of the compound222 was due to the multiplicity of receptors of different classes being activated by this molecule (a good example of polypharmacology in action).222 This conclusion was supported by the fact that other more selective and potent nonpeptidic B1- (LF23-1591) and B2-receptor antagonists (FR-165649; FR-173657) failed to lower IOP when injected into the rabbit eye vitreous.222 Taken together, as with the “so-called” inflammatory PGs (see above), which were chemically modified to render them into suitable non-inflammatory drugs to lower and control IOP in OHT/POAG/NTG patients,119,121,155,156,161 the possibility of using non-peptide B2-kinin receptor partial agonists such as FR-190997 as future ocular hypotensive drugs provides an intriguing novel target to pursue for future drug discovery to help glaucoma patients.205,218,219,221
Table 10. FR-190997 (a Nonpeptide BK Agonist) Promotes UVSC Outflow of AQH from OHT Eyes of Ketamine-Sedated Cynomolgus Monkeysa.
| baseline |
T.O.
treatment with FR-190997 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| normotensive eye OD | n | hypertensive eye OS | n | stats. p-values | hypertensive eye OS | n | .stats. p-values | ||
| Fa | 1.63 ± 0.54 | 12 | 1.54 ± 0.80 | 12 | 0.50 | Fa | 1.48 ± 0.53 | 12 | 0.79 |
| Cfl | 0.42 ± 0.21 | 9 | 0.16 ± 0.17** | 12 | 0.00** | Cfl | 0.18 ± 0.16 | 9 | 0.47 |
| Cton | 0.22 ± 0.14 | 12 | 0.15 ± 0.09 | 10 | 0.21 | Cton | 0.17 ± 0.11 | 9 | 0.59 |
| Fufl | 0.16 ± 0.51 | 7 | 0.47 ± 0.57 | 11 | 0.14 | Fufl | 1.23 ± 0.91 | 9 | 0.00** |
| Futon | 0.48 ± 0.99 | 12 | 0.37 ± 1.04 | 9 | 0.46 | Futon | 1.45 ± 0.45 | 10 | 0.03* |
| ACvol | 76.0 ± 11.3 | 12 | 79.9 ± 9.12 | 12 | 0.16 | ACvol | 79.8 ± 9.2 | 12 | 0.74 |
| CCT | 0.48 ± 0.03 | 12 | 0.48 ± 0.03 | 12 | 0.36 | CCT | 0.48 ± 0.03 | 12 | 0.74 |
Note that only UVSC outflow of AQH (determined by 2-methods) is enhanced by FR-190997 in this monkey model of OHT. However, this drug also promoted TM-mediated conventional outflow of AQH as demonstrated in ex-vivo perfused porcine eyes (ref (218)). Data taken from ref (7). The asterisks (∗) indicate statistical significance.
However, during all the above-mentioned antiglaucoma ocular research, the team had also considered generating dual pharmacophoric drug conjugate drugs, as opposed to using fixed-dose combination products, for example Simbrinza and Duotrav. Conceptually it was thought that travoprost could be linked to other ocular hypotensive drugs to maximally lower IOP by engaging different mechanisms to modulate AQH dynamics, and patented that idea.223 Sticking with my keen interest in therapeutics for glaucoma treatment, lately I noticed the rather rapid onset of action and the extraordinary magnitude of IOP-reduction in OHT monkey eyes by a novel non-PG EP2-receptor agonist drug (omidenepag isopropyl [OMDI]; Eybelis).224 The profound IOP-lowering by OMDI (−46% at 1.5–2 h, −54% at 3–4 h, and −56% at 6–8 h post t.o. dosing of OHT monkey eyes) was remarkably greater than other EP2-receptor agonists reported in the literature. Therefore, I postulated that OMDI may be of value in emergency treatment of rapidly rising IOP and/or for treating angle-closure and uveitic glaucoma and quickly filed appropriate patent applications in Japan and USA followed by discussions of the novel pharmacological attributes of OMDI in the public forum.225−227 To support this hypothesis, the historic quantitative autoradiographic distribution of [3H]-PGE2-labeled receptor sites in human eye sections became useful.161 The relatively high density of specific [3H]-PGE2-labeled receptor binding to both longitudinal and circular ciliary muscle161 provides some basis of the action of OMDI lowering IOP in OHT/POAG patients by stimulating both uveoscleral and TM pathways.149
Discovery of Novel Pharmacological Tools for Ocular and Nonocular Research
The research conducted during the 1990s to find, characterize, develop, and finally launch Travatan (AL-6221, travoprost isopropyl ester; AL-5848 being the free acid, S-fluprostenol) to treat OHT/POAG as described above, yielded a number of other benefits. One such example worthy of mention is our unexpected discovery of a low intrinsic activity (Emax = 19–23% vs cloprostenol [100% Emax]) FP-receptor partial agonist (AL-8810). As described above, our team was focused on finding novel agonists to match the profile of travoprost acid. Thus, the team members were not so interested in AL-8810 since it exhibited a low potency (EC50 = 186–260 nM) for stimulating PI turnover in Swiss 3T3 fibroblasts and in A7r5 rat aortic smooth muscle cells, and its efficacy (in vitro intrinsic activity) was so low (Figure 14A).152,228−231 However, remembering my pharmacological training, I immediately recognized the potential value of AL-8810—low intrinsic activity agonists behave as antagonists in cells/tissues where the receptor reserve is in the low-to-moderate range. AL-8810 was quickly profiled for its PG receptor activity and it indeed behaved as a fairly selective and competitive antagonist (Figure 14B,C)152 at the FP-receptor in multiple assay systems. This was indeed fortuitous since no bona fide antagonist existed for the FP-receptor at that time. AL-8810 was further characterized,152,232 and along with close analogues (e.g., AL-3138),232 patented228−232 and out-licensed to commercial companies for other researchers to use.
Figure 14.
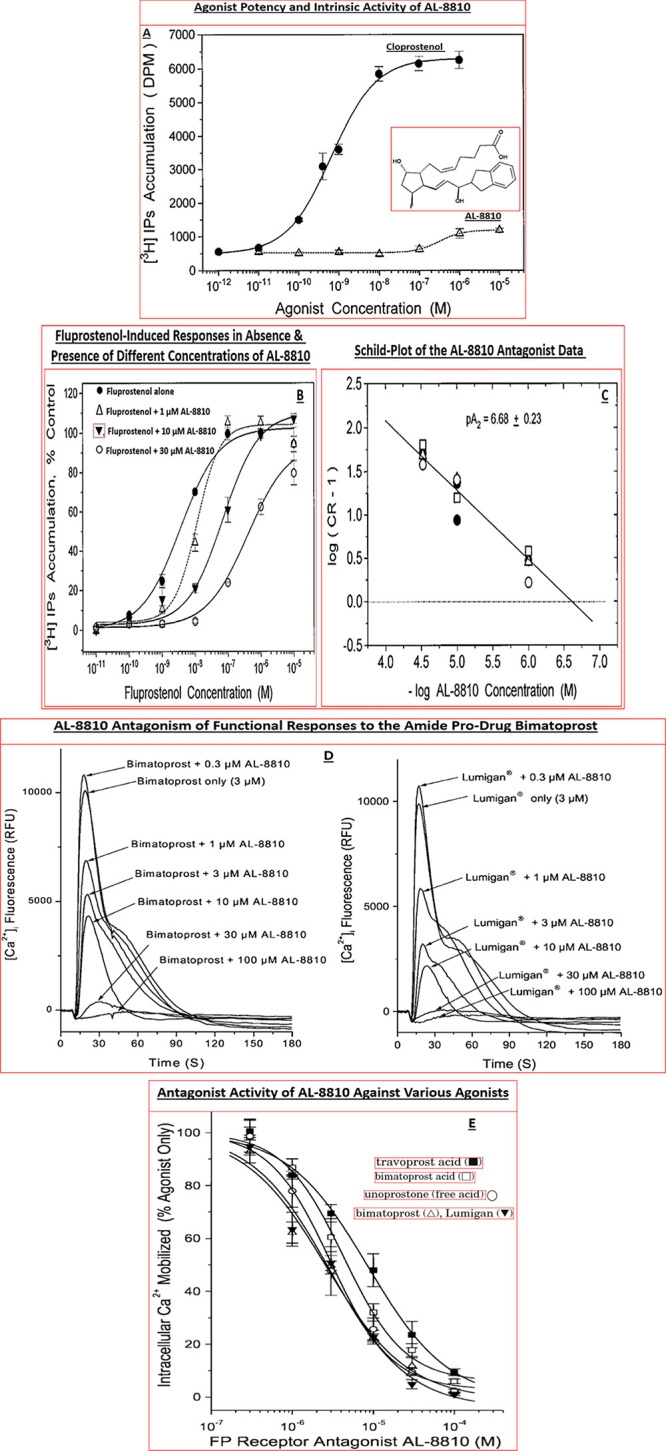
(A) Agonist potency and intrinsic activity of AL-8810 vs the full-agonist cloprostenol. (B,C) Schild analysis of the antagonist potency of AL-8810 vs fluprostenol-induced [3H]-IPs accumulation in A7r5 rat aortic cells in vitro. (D) AL-8810 concentration-dependently antagonized the functional activity of bimatoprost within a few seconds. (E) AL-8810 antagonized the functional responses to various FP-receptor agonists in a concentration-dependent manner. Overall, the antagonist potency of AL-8810 against a range of FP-agonists in various assay systems was 734 ± 228 nM. While not highly potent, it has proven very useful in clarifying the role of FP-receptors and/or endogenous and exogenous FP-receptor agonist in numerous systems in vitro and in vivo. Panels A–E are reproduced/modified with permission from refs (152 and 233). Copyright 1999 American Society for Pharmacology and Experimental Therapeutics and 2019 Wiley.
In the intervening years, AL-8810 has been successfully utilized by numerous researchers to probe the involvement of FP-receptors in normal and pathological conditions. AL-8810 has proven a valuable tool in ocular research in vitro(234−241,163−165,170) and in vivo.(241) Likewise, despite being a relatively low potency FP-antagonist (IC50/Ki = 734 ± 228 nM in numerous systems), AL-8810 has shown robust efficacy in abrogating parasitic infections,242 reducing structural and functional damage from experimental traumatic brain injury,243 significantly decreasing demyelination and motor dysfunction,244 reducing ischemic brain damage,245,246 and attenuating bacterial-toxin-induced inflammation.247 A full description of the discovery, characterization and possible uses of AL-8810 as a therapeutic agent has been recently reviewed.233
One significant element of AL-8810 pharmacology pertains to its use to address the possible mechanism of action at the receptor level of a new compound, Lumigan (bimatoprost; 17-phenyl-PGF2α-amide), launched in 2001 by a competitor company for the treatment of OHT/POAG. Authors of the controversial publication248 claimed, without any tangible and reproducible proof, that bimatoprost lowered IOP by interacting with a postulated “prostamide receptor” rather than being hydrolyzed to its free acid form, 17-phenyl-PGF2α, which is a potent FP-receptor agonist with nanomolar potency in many cells and tissues.158 Additionally, those authors claimed that the intact amide was the active moiety and that it directly activated the “prostamide receptor” without interacting with the FP-receptor, and thus was a novel lipid that was different from all the other PG antiglaucoma drugs. Many investigators voiced skepticism about this “labeling” of bimatoprost, and this was deemed an unacceptable way to promote Lumigan. The curiosity and controversy centered around the aforementioned proposed mechanism of action of bimatoprost prompted many arguments and studies. Several investigators independently showed that amidases present in animal and human cornea were able to convert bimatoprost into its free acid249−255 as hypothesized by many researchers. Therefore, it was likely that in vivo this process would be expected to liberate bimatoprost free acid into the AQH and this would activate the FP-receptors in the CM and TM like the free acids of other FP-class PG pro-drugs (latanoprost, travoprost, and tafluprost) to lower IOP. Indeed, a number of studies in cataract patients demonstrated that t.o. dosing with Lumigan resulted in detection of 3.2 nM, 11 nM, 16 nM, and 10 nM of bimatoprost free acid (observed at 0.5, 1, 3, 5 h postdose).251,252,255 These in vivo concentrations of bimatoprost free acid were at or several fold above the concentration required to achieve half-maximal activation of the FP-receptors in h-CM cells,162 human TM cells,163 at the human cloned ciliary body FP-receptor164,165 in mouse 3T3 cells,135,150,165 and in rat uterus167 and cat iris166 contraction assays. Hence, bimatoprost was no different from the other PG drugs approved for OHT/POAG treatment. Additionally, bimatoprost and its marketed version (Lumigan) were shown to directly interact with the FP-receptor since both “powder” and clinical solution forms of the compounds displaced [3H]-PGF2α binding, and since both compounds stimulated rapid [Ca2+]i mobilization in numerous cell-types including mouse 3T3 fibroblasts, A7r5 cells, h-CM and h-TM cells, and via the human cloned FP-receptor expressed in HEK-293 cells (e.g., Figure 14D; see above), and which also contracted rat uterine and cat iris strips like many FP-receptor agonists (see above and ref (256) for review). Furthermore, these actions of bimatoprost were concentration-dependently blocked by AL-8810 (e.g., Figure 14D,E), the FP-receptor antagonist described earlier. The collective data reported by a multitude of researchers therefore cast doubt on the existence of the “prostamide receptor”, and most scientists agreed that bimatoprost was indeed a pro-drug in the form of an amide, whereas the other FP-receptor agonist pro-drugs were isopropyl esters (Figure 7). Also, that all these drugs were metabolized to their respective free acids which were in fact the active moieties responsible for reducing IOP in animals and human subjects.256 The aforementioned controversy, resulting in heated debates at numerous conferences about the proposed mechanism(s) of action of the marketed PG analogues for glaucoma treatment, in particular for bimatoprost, was captured in an article titled “The Prostaglandin Wars”257 and was discussed in more detail in a review article.256
Another useful outcome of our detailed pharmacological studies in human ocular cells, in particular h-TM cells, relates to the mechanism of action of the FP-class PG agonists. The majority of the AQH dynamic studies conducted in OHT monkey eyes and OHT/POAG patients had concluded that drugs such as Xalatan, Travatan, Lumigan, Taflutan, and unoprostone isopropyl (Rescula) lowered IOP258−264 by releasing MMPs through activation of FP-receptors in the CM234,265,266 (Figure 8C), and through CM and scleral tissue remodeling, enhanced UVSC egress of AQH.267,268 However, when we demonstrated and fully characterized the presence of functionally active FP-receptors in h-TM cells163 clinical investigators became more aware and hence noticed and reported a significant enhancement of trabecular outflow of AQH induced by bimatoprost,269,270 latanoprost,270,271 travoprost,262,268,270 and by unoprostone isopropyl261 in ocular normotensive and OHT/POAG patients, in addition to the elevated UVSC outflow induced by these drugs. The TM conventional outflow facility enhancement by bimatoprost, for instance, was 23% of total in ocular normotensive human subjects, and by latanoprost in perfused human anterior eye segments TM outflow facility was increased by up to 67% of total AQH outflow.268 Interestingly, travoprost, tafluprost, and 15-keto-latanoprost did not appear to influence TM outflow in cynomolgus monkey eyes (see ref (268) for review). However, latanoprost was shown to increase murine outflow of AQH via the conventional TM pathway by 39% of total outflow after a single t.o. dose and within 2 h postdose.272 This acute ocular hypotensive activity correlated with up-regulation of MMP-2234 to enhance TM outflow facility, whereas the long-term protracted efficacy of the FP-PG agonists due to UVSC outflow was mediated through release of MMP-1, MMP-3, and MMP-9 which were synthesized and secreted over many hours postdosing (reviewed in ref (266)). These collective studies helped identify important species differences in how these drugs mediate their biological effects and how the FP-receptors located in the CM and TM are coresponsible for increasing AQH drainage to lower IOP.
Other helpful tools that resulted from our drug discovery campaigns for treating OHT/POAG included generation of more potent and more receptor-selective radioligands to study the pharmacological properties and autoradiographic visualization of FP-receptors using [3H]-travoprost acid ([3H]-AL-5848),136 and DP-receptors using [3H]-BWA868C (Figure 15A–C).138 The use of the latter radioligands coupled with quantitative phosphor-imaging technology129,136,138,153 allowed us to determine equilibrium dissociation constants (Kis) for a range of FP- and DP-class drugs on thin sections of post-mortem human eyes, an unparalleled accomplishment thus far as far as we know (Figure 15C, Table 11).153,273
Figure 15.
(A) Autoradiographic localization and quantification of DP-receptors in post-mortem human eye sections determined in vitro using the highly selective DP-receptor antagonist radioligand, [3H]-BWA868C. (B) The concentration-dependent displacement of [3H]-BWA868C from DP-receptors in human eye sections in vitro by a potent and selective DP-receptor agonist (BW245C), and (C) graphic representation of such data for a number of DP-receptor agonists. NSB = nonspecific binding. Reproduced with permission from ref (273). Copyright 2005 Mary Ann Liebert Publishing Inc.
Table 11. Relative Affinities of DP-Receptor Agonists for Tissues in Human Eye Sections Determined by Quantitative Autoradiographic Techniques and Using [3H]-BWA868C as the Radioliganda.
| DP-Class
prostaglandin inhibition constant (Ki, nM; mean ± SEMs) in human ocular tissues determined
by quantitative autoradiography |
||||
|---|---|---|---|---|
| compound | ciliary epithelium/processes | longitudinal ciliary muscle | circular ciliary muscle | iris |
| BW-245C (free acid) | 8.0 ± 1.8 | 7.0 ± 1.0 | 5.7 ± 0.8 | 4.4 ± 0.7 |
| SQ-27986 (free acid) | 9.0 ± 1.9 | 6.1 ± 0.8 | 6.4 ± 1.3 | 8.5; 6.6 |
| ZK-118182 (free acid) | 32.9 ± 7.4 | ±2.9 | ±2.6 | 7.3; 15.4 |
| AL-6556 (free acid of AL-6598) | ±1310 | ±160 | ±413 | nd |
| AL-6598 (isopropyl ester) | ±3000 | ±665 | ±825 | nd |
Note: the smaller is the Ki value, the higher is the affinity of the compound for the DP-receptors. nd = not determined. Data reproduced and updated from ref (273).
In a similar vein, even though we obtained useful functional data for FP-receptor directed compounds using Swiss 3T3 mouse fibroblasts135,150 and rat aortic cells,151,152 the latter findings being confirmed in isolated h-TM163 and h-CM cells,158,162 it was deemed imperative that we also demonstrate activity of our compounds at a human cloned FP-receptor directly without the issues of cross-activity through other receptor systems found on native cells and tissues. Accordingly, through an Alcon-funded collaboration, the human ciliary body FP-receptor was cloned and expressed in HEK-293 cells that were devoid of endogenous FP-receptors.164 Using this cloned receptor system and AL-8810 as the tools, we then verified and cross-correlated our data previously obtained from the cells and tissues expressing natural FP-receptors (see above). We were of course delighted to find excellent correlations between radioligand binding, PI turnover, [Ca2+]i mobilization, mitogen-activated protein kinase (MAPK) activity, tissue contractions and IOP-lowering for a range of FP-receptor agonists and the FP-receptor antagonist, AL-8810.13,127,167,233
Lastly, despite decades of research since the original discovery and clinical adoption of FP-class PG analogues as first-line therapeutics to treat OHT/POAG/NTG in the early 2000s, lowering and controlling IOP remains the single most validated treatment for this collection of ocular diseases.4−6 Therefore, new drugs and treatment options were still being sought to mitigate the damage caused to the optic nerve, RGCs, and their axons by elevated IOP. Only recently have new drugs to reduce IOP been added to the clinicians’ toolbox encompassing a conjugate of latanoprost and a nitric oxide donor (Latanoprostene Bunod),274 two ROCK inhibitors, one approved in Japan (Ripasudil)275 and the other in the US (Netarsudil),276 and a novel non-PG EP2-receptor agonist (Omidenepag Isopropyl).224−227 Sadly POAG/OHT patients who are recalcitrant to pharmaceutical drugs, and in some cases NTG patients, often require invasive ocular surgeries to reduce their IOPs to preserve their sight.4−7 The advent and introduction of microshunts to extrude AQH from the ANC of the eye are less invasive277 but still require significant surgical procedures, and will often be given fixed-dose combination278 ocular hypotensive drugs to maintain their lowered IOPs. Consequently, research has been directed toward finding ways to directly protect the RGCs and their axons from the ravages of IOP-induced visual impairment. Much effort has been expended in delineating the sequence of events that lead to optic nerve damage. It would appear that loss of energy at the level of mitochondrial ATP synthesis109,110,113,114,279,280 is at the heart of the problem in causing GON, and this is now a well accepted theory with some compelling evidence from recent animal and human clinical studies.281,282 We recognized this aspect during early years of our research in the late 1990s. Using nuclear magnetic resonance technology we showed that human and rat retinas subjected to hypoxic conditions, thereby simulating what may happen in vivo in GON, leads to a sharp decline in ATP and which could be recovered to a large extent using a Ca2+-channel blocker (diltiazem) or using a blocker of the N-methyl-d-aspartate receptor-coupled-channel (MK-801) (Figure 16; Table 12).113 Likewise we showed the presence of specific polyamine binding sites in human and rabbit retinas130,131 that may endogenously serve a neuroprotective function,283,284 and where reduction of glutamate-induced retinal toxicity can also protect the RGCs and their dendrites.285 Nevertheless, it is also a fact that the best treatment paradigm for the good eyesight and preservation of vision in glaucoma patients is to reduce and maintain the lowest possible IOP since that has repeatedly shown efficacy in reducing RGC injury and reversing their cellular dysfunctions.286
Figure 16.
ATP energy depletion and restoration after hypoxia/reperfusion of human (panel A) and rat retinas (panel B) in the absence and presence of MK-801 NMDA-receptor-channel blocker as determined by 31P NMR spectroscopy. Reproduced with permission from ref (7). Copyright 2018 Mary Ann Liebert Publishing Inc.
Table 12. Nuclear Magnetic Resonance (31P-NMR)-Derived ATP Levels in Rat and Human Retinas under Normal and Hypoxic Conditions and Restoration of ATP with Two Different Drugsa.
| experiments/treatments | ATP
and its phosphorus metabolites (% of control levels) |
||
|---|---|---|---|
| rat retinas | ATP | PCr | Pi |
| normal controls | 98.4 ± 1.15 | 85.7 ± 2.3 | 95.0 ± 2.3 |
| after 2.0 h hypoxia | 48.1 ± 2.1*** | nd | nd |
| after 1.0 h hypoxia | 69.5 ± 3.46*** | 56.0 ± 4.6*** | 75.0 ± 8.6 |
| after 1 h with MK-801 at 50 μM | 97.4 ± 4.6*** | 102.0 ± 4.5*** | 89.3 ± 4.04 |
| after 2 h with MK-801 at 50 μM | 72.6 ± 1.4** | nd | nd |
| after 1 h with MK-801 at 5 nM | 92.0 ± 1.4*** | 101.0 ± 5.7*** | 76.8 ± 3.57 |
| human retinas | % of control ATP |
|---|---|
| normal controls | 98 ± 12 |
| after 2.0 h hypoxia | 52 ± 8** |
| after 2 h with MK-801 at 50 μM | 74 ± 6* |
| after 2 h with diltiazem at 50 μM | 67 ± 4 |
Effect on tissue ATP and its metabolites after hypoxia/reperfusion of rat and human retinas in the absence and presence of MK-801 (NMDA-receptor-channel blocker) and diltiazem (Ca2+-channel blocker) as determined by 31P NMR spectroscopy. PCr = phosphocreatinin; Pi = inorganic phosphate; nd = not determined; *P < 0.05; **P < 0.02; ***P < 0.01. Data from ref (7).
Clearly much more needs to be accomplished to help preserve the sight of glaucoma patients, and it is hoped that the research described above may contribute in some small way in this endeavor and lead to novel means to arrest vision loss. In the quest for such mitigation strategies, the early diagnosis of OHT/POAG is tantamount to early intervention. It is hoped that the near-term availability and therapeutic utility of novel diagnostic and prognostic reagents, and rapid clinical introduction of safe and effective neuroprotective drugs, gene-, and cell-therapies, and innovative devices (including prostheses) to combat GON will positively impact patients losing their sight.
Acknowledgments
Sincere thanks and gratitude are extended to team members, colleagues, mentors, and supervisors at Alcon. The intention of this article has been to describe the collective efforts of many talented and dedicated scientists, managers, and leaders to bring suitable products to the market place and to introduce them into medical treatments of certain ophthalmic diseases that ail millions worldwide. As such, I declare that there is no conflict of interest in providing this treatise to help future drug hunters and students gain an insight into the drug discovery research processes, to learn about the history of the discovery/characterization of some now well-known drugs, and to review some of the diverse kinds of data gathered during such studies. Views in this work are those of the author and do not express the views of anyone else.
Author Present Address
Vice President, Global Alliances & External Research, Ophthalmology Innovation Center, Santen Incorporated USA, 6401 Hollis St., (Suite 125) Emeryville, CA 94608.
The author declares no competing financial interest.
References
- Allansmith M. R.; Ross R. N. (1988) Ocular allergy. Clin. Exp. Allergy 18, 1–13. 10.1111/j.1365-2222.1988.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Schaefer K. (1993) Conjunctivitis of allergic origin: immunologic mechanisms and current approaches to therapy. Surv. Ophthalmol. 38, 115–132. 10.1016/0039-6257(93)90036-7. [DOI] [PubMed] [Google Scholar]
- Schröder K.; Finis D.; Meller S.; et al. (2017) Seasonal allergic conjunctivitis. Ophthalmologe 114, 1053–1065. 10.1007/s00347-017-0580-1. [DOI] [PubMed] [Google Scholar]
- Schuster A. K.; Erb C.; Hoffmann E. M.; et al. (2020) The diagnosis and treatment of glaucoma. Dtsch Arztebl Int. 117, 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas J. B.; Aung T.; Bourne R. R.; et al. (2017) Glaucoma. Lancet 390, 2183–2193. 10.1016/S0140-6736(17)31469-1. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2017) Ocular hypertension and glaucoma: a review and current perspectives. Int. J. Ophthalmol. Vis. Sci. 2, 22–36. [Google Scholar]
- Sharif N. A. (2018) iDrugs and iDevices discovery and development - preclinical assays, techniques and animal model studies for ocular hypotensives and neuroprotectants. J. Ocul. Pharmacol. Ther. 34, 7–39. 10.1089/jop.2017.0125. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2020) Pharmacodynamic Evaluation: Ocular Pharmacology, in Drug Discovery and Evaluation: Methods in Clinical Pharmacology (Hock F. J., and Gralinski M. R., Eds.) Chapter 54, 46 pages, Springer Publishing Company; ( 10.1007/978-3-319-56637-5_54-1). [DOI] [Google Scholar]
- WHO (2018) Blindness and vision impairment-News. Fact sheets. WHO Priority eye diseases https://www.who.int/blindness/causes/priority/. [Google Scholar]
- Tham Y.-C.; Li X.; Wong T. Y.; et al. (2014) Global prevalence of glaucoma and projections of glaucoma burden through 2040. Ophthalmology 121, 2081–2090. 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- Berger W. E.; Granet D. B.; Kabat A. G. (2017) Diagnosis and management of allergic conjunctivitis in pediatric patients. Allergy Asthma Proc. 38, 16–27. 10.2500/aap.2017.38.4003. [DOI] [PubMed] [Google Scholar]
- Bielory L.; Delgado L.; Katelaris C. H.; et al. (2020) Diagnosis and management of allergic conjunctivitis. Ann. Allergy, Asthma, Immunol. 124, 118–134. 10.1016/j.anai.2019.11.014. [DOI] [PubMed] [Google Scholar]
- Klimko P.; Sharif N. A. (2019) Discovery, characterization and clinical utility of prostaglandin agonists for treatment of glaucoma. Br. J. Pharmacol. 176, 1051–1058. 10.1111/bph.14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A. (2018) Glaucomatous optic neuropathy treatment options: the promise of novel therapeutics, techniques and tools to help preserve vision. Neural Regener. Res. 13, 1145–1150. 10.4103/1673-5374.235017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti M.; Abicca I.; Bruscolini A.; et al. (2018) Allergic conjunctivitis: current concepts on pathogenesis and management. J. Biol. Regul Homeost Agents 32 (1 Suppl. 1), 49–60. [PubMed] [Google Scholar]
- Mishra G. P.; Tamboli V.; Jwala J.; Mitra A. K. (2011) Recent patents and emerging therapeutics in the treatment of allergic conjunctivitis. Recent Pat. Inflammation Allergy Drug Discovery 5, 26–36. 10.2174/187221311794474883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulati S.; Jain S. (2016) Ocular pharmacology of tear film, dry eye, and allergic conjunctivitis. Handb. Exp. Pharmacol. 242, 97–118. 10.1007/164_2016_73. [DOI] [PubMed] [Google Scholar]
- Mishra S.; Hoon M. (2013) The cells and circuitary for itch responses in mice. Science 340, 968–971. 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr W.; Schaeffer J.; Donnenfeld E. (2016) Treating allergic conjunctivitis: A once-daily medication that provides 24-h symptom relief. Allergy Rhinol. 7, e107–e11. 10.2500/ar.2016.7.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azari A. A.; Barney N. P. (2013) Conjunctivitis: a systematic review of diagnosis and treatment. J. Amer Med. Assoc. 310, 1721–1729. 10.1001/jama.2013.280318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban D. R.; Calder V.; Kuo C. H.; et al. (2013) New twists to an old story: novel concepts in the pathogenesis of allergic eye disease. Curr. Eye Res. 38, 317–330. 10.3109/02713683.2012.747617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proud D.; Sweet J.; Stein P.; et al. (1990) Inflammatory mediator release on conjunctival provocation of allergic subjects with allergen. J. Allergy Clin. Immunol. 85, 896–905. 10.1016/0091-6749(90)90075-F. [DOI] [PubMed] [Google Scholar]
- Moon T. C.; Befus A. D.; Kulka M. (2014) Mast cell mediators: their differential release and the secretory pathways involved. Front. Immunol. 5 (569), 1–18. 10.3389/fimmu.2014.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano F. M.; Stahl J. L.; Cook E. B.; Barney N. P. (2001) Conjunctival mast cells in ocular allergic disease. Allergy Asthma Proc. 22, 121–126. 10.2500/108854101778148782. [DOI] [PubMed] [Google Scholar]
- Church M. K.; McGill J. I. (2002) Human ocular mast cells. Curr. Opin Allergy Clin Immunol. 2, 419–422. 10.1097/00130832-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S.; Yanni J. M. (1994) Histamine receptor subtype affinities, selectivities and potencies of emedastine, a novel H1 selective antagonist, and other ocularly employed antihistamines. Drug Dev. Res. 33 (448), 453. 10.1002/ddr.430330408. [DOI] [Google Scholar]
- Sharif N. A.; Xu S.; Yanni J. M. (1994) Emedastine: a potent, high affinity histamine H1 selective antagonist for ocular use. receptor binding and second messenger studies. J. Ocul. Pharmacol. Ther. 10, 653–664. 10.1089/jop.1994.10.653. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S.; Magnino P.; Pang I. H. (1996) Human conjunctival epithelial cells express histamine-1 receptors coupled to phosphoinositide turnover and intracellular calcium mobilization: role in ocular allergic & inflammatory diseases. Exp. Eye Res. 3, 169–178. 10.1006/exer.1996.0105. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S.; Yanni J. M. (1996) Olopatadine (AL 4943A): Ligand binding and functional studies on a novel, long acting H1 selective histamine antagonist for use in allergic conjunctivitis. J. Ocul. Pharmacol. Ther. 12, 401–407. 10.1089/jop.1996.12.401. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S. X.; Miller S. T.; et al. (1996) Characterization of the ocular anti allergic and anti histaminic effects of olopatadine (AL 4943A), a novel drug for treating ocular allergic diseases. J. Pharmacol. Expt Ther. 278, 1251–1260. [PubMed] [Google Scholar]
- Sharif N. A., Hellberg M. H., and Yanni J. M. (1997) Anti histamines, topical ocular, in Burger’s Medicinal Chemistry & Drug Discovery (Wolff M. F., Ed.) 5th ed., Chapter 64, Vol. 5, pp 255–279, John Wiley & Sons, NY. [Google Scholar]
- Sharif N. A.; Crider J. Y.; Griffin B.; et al. (1997) Pharmacological analysis of mast cell mediator and neurotransmitter receptors coupled to adenylate cyclase and phospholipase C on immunocytochemically-defined human conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 13, 321–336. 10.1089/jop.1997.13.321. [DOI] [PubMed] [Google Scholar]
- Wiernas T. K.; Griffin B. W.; Sharif N. A. (1997) The expression of functionally-coupled bradykinin receptors in human corneal epithelial cells and their pharmacological characterization with agonists and antagonists. Br. J. Pharmacol. 121, 649–656. 10.1038/sj.bjp.0701168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiernas T. K.; Davis T. L.; Griffin B. W.; Sharif N. A. (1998) Effects of bradykinin on signal transduction, cell proliferation, and cytokine, prostaglandin E2 and collagenase-1 release from human corneal epithelial cells. Br. J. Pharmacol. 123, 1127–1137. 10.1038/sj.bjp.0701700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A.; Wiernas T. K.; Howe W. L.; et al. (1998) Human corneal epithelial cell functional responses to inflammatory agents and their antagonists. Invest Ophthalmol Vis Sci. 39, 2562–257. [PubMed] [Google Scholar]
- Sharif N. A.; Wiernas T. K.; Griffin B. W.; Davis T. L. (1998) Pharmacology of [3H]-pyrilamine binding and the histamine-induced phosphoinositide turnover, Ca2+-mobilization and cytokine release from human corneal epithelial cells. Br. J. Pharmacol. 125, 1336–1344. 10.1038/sj.bjp.0702194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord E.; Sharif N. A.; Mace K.; et al. (1999) Immortalized human corneal epithelial cells for ocular toxicity and inflammation studies. Invest Ophthalmol Vis Sci. 40, 1091–1101. [PubMed] [Google Scholar]
- Sharif N. A.; Wiernas T. K. (2010) Platelet-activating factor-induced intracellular signaling and release of cytokines and prostaglandin E2 in immortalized human corneal epithelial cells. J. Ocul. Pharmacol. Ther. 26, 21–29. 10.1089/jop.2009.0102. [DOI] [PubMed] [Google Scholar]
- Cook E. B.; Stahl J. L.; Miller S. T.; et al. (1998) Isolation of human conjunctival mast cells and epithelial cells: tumor necrosis factor-alpha from mast cells affects intercellular adhesion molecule-1 expression on epithelial cells. Invest Ophthalmol Vis Sci. 39, 336–343. [PubMed] [Google Scholar]
- Stahl J. L.; Cook E. B.; Graziano F. M.; Barney N. P. (2003) Differential and cooperative effects of TNFalpha, IL-1beta, and IFNgamma on human conjunctival epithelial cell receptor expression and chemokine release. Invest. Ophthalmol. Visual Sci. 44, 2010–2015. 10.1167/iovs.02-0721. [DOI] [PubMed] [Google Scholar]
- Hingorani M.; Calder V. L.; Buckley R. J.; Lightman S. L. (1998) The role of conjunctival epithelial cells in chronic ocular allergic disease. Exp. Eye Res. 67, 491–500. 10.1006/exer.1998.0528. [DOI] [PubMed] [Google Scholar]
- Irkeç M.; Bozkurt B. (2003) Epithelial cells in ocular allergy. Curr. Allergy Asthma Rep. 3, 352–357. 10.1007/s11882-003-0098-2. [DOI] [PubMed] [Google Scholar]
- Kawasaki S.; Tanioka H.; Yamasaki K.; et al. (2006) Clusters of corneal epithelial cells reside ectopically in human conjunctival epithelium. Invest. Ophthalmol. Visual Sci. 47, 1359–1367. 10.1167/iovs.05-1084. [DOI] [PubMed] [Google Scholar]
- Cormia F. E.; Dougherty J. W. (1960) Proteolytic activity in development of pain and itching. Cutaneous reactions to bradykinin and kallikrein. J. Invest. Dermatol. 35, 21–26. 10.1038/jid.1960.78. [DOI] [PubMed] [Google Scholar]
- Hägermark O.; Strandberg K. (1977) Pruritogenic activity of prostaglandin E2. Acta Derm Venereol. 57, 37. [PubMed] [Google Scholar]
- Hägermark O. (2004) Peripheral and central mediators of itch. Skin Pharmacol. 5, 1–8. 10.1159/000211009. [DOI] [PubMed] [Google Scholar]
- Irani A. M.; Schwartz L. B. (1994) Human mast cell heterogeneity. Allergy Asthma Proc. 15, 303–308. 10.2500/108854194778816472. [DOI] [PubMed] [Google Scholar]
- Hosford D.; Mencia-Huerta J. M.; Braquet P. (1990) Platelet-activating factor (PAF) and PAF antagonists in asthma. Crit. Rev. Ther. Drug Carrier Syst. 7, 261–273. [PubMed] [Google Scholar]
- Billah M. M.; Chapman R. W.; Watnick A. S.; et al. (1991) SCH-37370: a new drug combining antagonism of platelet-activating factor (PAF) with antagonism of histamine. Agents Actions Suppl. 34, 313–321. [PubMed] [Google Scholar]
- Berdy G. J.; Abelson M. B.; George M. A.; et al. (1991) Allergic conjunctivitis: a survey of new antihistamines. J. Ocul. Pharmacol. Ther. 7, 313–324. 10.1089/jop.1991.7.313. [DOI] [PubMed] [Google Scholar]
- Miller S.; Cook E.; Graziano F.; et al. (1996) Human conjunctival mast cell responses in vitro to various secretagogues. Ocul. Immunol. Inflammation 4, 39–50. 10.3109/09273949609069126. [DOI] [PubMed] [Google Scholar]
- Cook E. B.; Stahl J. L.; Barney N. P.; Graziano F. M. (2000) Olopatadine inhibits TNFalpha release from human conjunctival mast cells. Ann. Allergy, Asthma, Immunol. 84, 504–508. 10.1016/S1081-1206(10)62513-6. [DOI] [PubMed] [Google Scholar]
- Cook E. B.; Stahl J. L.; Barney N. P.; Graziano F. M. (2001) Olopatadine inhibits anti-immunoglobulin E-stimulated conjunctival mast cell upregulation of ICAM-1 expression on conjunctival epithelial cells. Ann. Allergy, Asthma, Immunol. 87, 424–429. 10.1016/S1081-1206(10)62926-2. [DOI] [PubMed] [Google Scholar]
- Cook E. B.; Stahl J. L.; Barney N. P.; Graziano F. M. (2002) Mechanisms of antihistamines and mast cell stabilizers in ocular allergic inflammation. Curr. Drug Targets: Inflammation Allergy 1, 167–180. 10.2174/1568010023344733. [DOI] [PubMed] [Google Scholar]
- Cook E. B.; Stahl J. L.; Sedgwick J. B.; et al. (2004) The promotion of eosinophil degranulation and adhesion to conjunctival epithelial cells by IgE-activated conjunctival mast cells. Ann. Allergy, Asthma, Immunol. 92, 65–72. 10.1016/S1081-1206(10)61712-7. [DOI] [PubMed] [Google Scholar]
- Yanni J. M.; Stephens D. J.; Miller S. T.; et al. (1996) The in vitro and in vivo ocular pharmacology of olopatadine (AL-4943A), an effective anti-allergic/antihistaminic agent. J. Ocul. Pharmacol. Ther. 12, 389–400. 10.1089/jop.1996.12.389. [DOI] [PubMed] [Google Scholar]
- Yanni J. M.; Miller S. T.; Gamache D. A.; et al. (1997) Comparative effects of topical ocular anti-allergy drugs on human conjunctival mast cells. Ann. Allergy, Asthma, Immunol. 79, 541–545. 10.1016/S1081-1206(10)63063-3. [DOI] [PubMed] [Google Scholar]
- Yanni J. M.; Sharif N. A.; Gamache D. A.; et al. (1999) A current appreciation of sites for pharmacological intervention in allergic conjunctivitis: effects of new topical ocular drugs. Acta Ophthalmol. Scand. 77, 33–37. 10.1111/j.1600-0420.1999.tb01171.x. [DOI] [PubMed] [Google Scholar]
- Yanni J. M.; Weimer L.; Sharif N. A.; et al. (1999) Inhibition of histamine-induced human conjunctival epithelial cell responses by ocular allergy drugs. Arch. Ophthalmol. 117, 643–647. 10.1001/archopht.117.5.643. [DOI] [PubMed] [Google Scholar]
- Weimer L. K.; Gamache D. A.; Yanni J. M. (1998) Histamine-stimulated cytokine secretion from human conjunctival epithelial cells: inhibition by the histamine H1 antagonist emedastine. Int. Arch. Allergy Immunol. 115, 288–293. 10.1159/000069459. [DOI] [PubMed] [Google Scholar]
- Macleod J. D.; Anderson D. F.; Baddeley S. M.; et al. (1997) Immunolocalization of cytokines to mast cells in normal and allergic conjunctiva. Clin. Exp. Allergy 27, 1328–1334. 10.1046/j.1365-2222.1997.1600959.x. [DOI] [PubMed] [Google Scholar]
- Yanni J. M.; Stephens D. J.; Parnell D. W.; Spellman J. M. (1994) Preclinical efficacy of emedastine, a potent, selective histamine H1 antagonist for topical ocular use. J. Ocul. Pharmacol. Ther. 10, 665–675. 10.1089/jop.1994.10.665. [DOI] [PubMed] [Google Scholar]
- Verin P.; Easty D. L.; Secchi A.; et al. (2001) Clinical evaluation of twice-daily emedastine 0.05% eye drops (Emadine eye drops) versus levocabastine 0.05% eye drops in patients with allergic conjunctivitis. Am. J. Ophthalmol. 131, 691–698. 10.1016/S0002-9394(00)00947-8. [DOI] [PubMed] [Google Scholar]
- Discepola M.; Deschenes J.; Abelson M. (1999) Comparison of the topical ocular antiallergic efficacy of emedastine 0.05% ophthalmic solution to ketorolac 0.5% ophthalmic solution in a clinical model of allergic conjunctivitis. Acta Ophthalmol. Scand. 228, 43–46. 10.1111/j.1600-0420.1999.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Netland P. A.; Leahy C.; Krenzer K. L. (2000) Emedastine ophthalmic solution 0.05% versus levocabastine ophthalmic suspension 0.05% in the treatment of allergic conjunctivitis using the conjunctival allergen challenge model. Am. J. Ophthalmol. 130, 717–723. 10.1016/S0002-9394(00)00689-9. [DOI] [PubMed] [Google Scholar]
- Horak F.; Stübner P.; Zieglmayer R.; et al. (2003) Onset and duration of action of ketotifen 0.025% and emedastine 0.05% in seasonal allergic conjunctivitis: efficacy after repeated pollen challenges in the Vienna challenge chamber. Clin. Drug Invest. 23, 329–337. 10.2165/00044011-200323050-00003. [DOI] [PubMed] [Google Scholar]
- Ohshima E.; Sato H.; Obase H.; Uchimura T.; et al. (1992) Synthesis of a dibenz[b,e]oxepin- bovine serum albumin conjugate for radioimmunoassay of KW-4679 ((Z)-11-[3-(dimethylamino)propylidene]-6,11-dihydrodibenz[b,e]oxepin-2-acetic acid hydrochloride). Chem. Pharm. Bull. 40, 2552–2554. 10.1248/cpb.40.2552. [DOI] [PubMed] [Google Scholar]
- Nonaka H.; Otaki S.; Ohshima E.; et al. (1998) Unique binding pocket for KW-4679 in the histamine H1 receptor. Eur. J. Pharmacol. 345, 111–117. 10.1016/S0014-2999(97)01620-8. [DOI] [PubMed] [Google Scholar]
- Gamache D. A.; Dimitrijevich S. D.; Weimer L. K.; et al. (1997) Secretion of proinflammatory cytokines by human conjunctival epithelial cells. Ocul. Immunol. Inflammation 5, 117–128. 10.3109/09273949709085060. [DOI] [PubMed] [Google Scholar]
- Brockman H. L.; Momsen M. M.; Knudtson J. R.; et al. (2003) Interactions of olopatadine and selected antihistamines with model and natural membranes. Ocul. Immunol. Inflammation 11, 247–268. 10.1076/ocii.11.4.247.18261. [DOI] [PubMed] [Google Scholar]
- Abelson M. B. (1998) Evaluation of olopatadine, a new ophthalmic antiallergic agent with dual activity, using the conjunctival allergen challenge model. Ann. Allergy, Asthma, Immunol. 81, 211–218. 10.1016/S1081-1206(10)62814-1. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Spitalny L. (1998) Combined analysis of two studies using the conjunctival allergen challenge model to evaluate olopatadine hydrochloride, a new ophthalmic antiallergic agent with dual activity. Am. J. Ophthalmol. 125, 797–804. 10.1016/S0002-9394(98)00044-0. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Lanier R. Q. (1999) The added benefit of local Patanol therapy when combined with systemic Claritin for the inhibition of ocular itching in the conjunctival antigen challenge model. Acta Ophthalmol. Scand. 77, 53–56. 10.1111/j.1600-0420.1999.tb01175.x. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Welch D. L. (2000) An evaluation of onset and duration of action of patanol (olopatadine hydrochloride ophthalmic solution 0.1%) compared to Claritin (loratadine 10 mg) tablets in acute allergic conjunctivitis in the conjunctival allergen challenge model. Acta Ophthalmol. Scand. 78, 60–63. 10.1034/j.1600-0420.2000.078s230060.x. [DOI] [PubMed] [Google Scholar]
- Spangler D. L.; Bensch G.; Berdy G. J. (2001) Evaluation of the efficacy of olopatadine hydrochloride 0.1% ophthalmic solution and azelastine hydrochloride 0.05% ophthalmic solution in the conjunctival allergen challenge model. Clin. Ther. 23, 1272–1280. 10.1016/S0149-2918(01)80106-5. [DOI] [PubMed] [Google Scholar]
- Butrus S.; Greiner J. V.; Discepola M.; Finegold I. (2000) Comparison of the clinical efficacy and comfort of olopatadine hydrochloride 0.1% ophthalmic solution and nedocromil sodium 2% ophthalmic solution in the human conjunctival allergen challenge model. Clin. Ther. 22, 1462–1472. 10.1016/S0149-2918(00)83044-1. [DOI] [PubMed] [Google Scholar]
- Vogelson C.; Abelson M. B.; Pasquine T.; et al. (2004) Preclinical and clinical antiallergic effect of olopatadine 0.2% solution 24 h after topical ocular administration. Allergy Asthma Proc. 25, 69–75. [PubMed] [Google Scholar]
- Abelson M. B.; Gomes P. J. (2008) Olopatadine 0.2% ophthalmic solution: the first ophthalmic antiallergy agent with once-daily dosing. Expert Opin. Drug Metab. Toxicol. 4, 453–461. 10.1517/17425255.4.4.453. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Gomes P. J.; Vogelson C. T.; et al. (2004) Clinical efficacy of olopatadine hydrochloride ophthalmic solution 0.2% compared with placebo in patients with allergic conjunctivitis or rhinoconjunctivitis: a randomized, double-masked environmental study. Clin. Ther. 26, 1237–1248. 10.1016/S0149-2918(04)80065-1. [DOI] [PubMed] [Google Scholar]
- Abelson M. B.; Shetty S.; Korchak M.; et al. (2015) Advances in pharmacotherapy for allergic conjunctivitis. Expert Opin. Pharmacother. 16, 1219–31. 10.1517/14656566.2015.1040760. [DOI] [PubMed] [Google Scholar]
- Galatowicz G.; Ajayi Y.; Stern M. E.; Calder V. L. (2007) Ocular anti-allergic compounds selectively inhibit human mast cell cytokines in vitro and conjunctival cell infiltration in vivo. Clin. Exp. Allergy 37, 1648–1656. 10.1111/j.1365-2222.2007.02782.x. [DOI] [PubMed] [Google Scholar]
- McLaurin E.; Narvekar A.; Gomes P.; et al. (2015) Phase 3 Randomized double-masked study of efficacy and safety of once-daily 0.77% olopatadine hydrochloride ophthalmic solution in subjects with allergic conjunctivitis using the conjunctival allergen challenge model. Cornea 34, 1245–1251. 10.1097/ICO.0000000000000562. [DOI] [PubMed] [Google Scholar]
- McLaurin E.; Bergmann M.; Narvekar A.; Adewale A.; Gomes P. J; Torkildsen G. (2017) Pooled analysis of two studies evaluating efficacy and safety of olopatadine hydrochloride 0.77% in patients with allergic conjunctivitis. Clin. Ophthalmol. 11, 1089–1097. 10.2147/OPTH.S131830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torkildsen G. L.; Ousler G. W.; Gomes P. (2008) Ocular comfort and drying effects of three topical antihistamine/mast cell stabilizers in adults with allergic conjunctivitis: a randomized, double-masked crossover study. Clin. Ther. 30, 1264–1271. 10.1016/S0149-2918(08)80050-1. [DOI] [PubMed] [Google Scholar]
- Wade L.; Bielory L.; Rudner S. (2012) Ophthalmic antihistamines and H1-H4 receptors. Curr. Opin Allergy Clin Immunol. 12, 510–516. 10.1097/ACI.0b013e328357d3ba. [DOI] [PubMed] [Google Scholar]
- Patel D.; Sarala N.; Datti N. P. (2018) Topical olopatadine hydrochloride versus ketotifen fumarate for allergic conjunctivitis. J. Ophthalmic Vis Res. 13, 119–123. 10.4103/jovr.jovr_85_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallois-Bernos Ac; Thurmond R. L. (2012) Alcaftadine, a new antihistamine with combined antagonist activity at histamine H1, H2, and H4 receptors. J. Recept., Ligand Channel Res. 5, 9–20. 10.2147/JRLCR.S39369. [DOI] [Google Scholar]
- Rossbach K.; Nassenstein C.; Gschwandtner M.; et al. (2011) Histamine H1, H3 and H4 receptors are involved in pruritus. Neuroscience 190, 89–102. 10.1016/j.neuroscience.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Bohets H.; McGowan C.; Mannens G.; et al. (2011) Clinical pharmacology of alcaftadine, a novel antihistamine for the prevention of allergic conjunctivitis. J. Ocul. Pharmacol. Ther. 27, 187–195. 10.1089/jop.2010.0153. [DOI] [PubMed] [Google Scholar]
- Zampeli E.; Thurmond R. L.; Tiligada E. (2009) The histamine H4 receptor antagonist JNJ7777120 induces increases in the histamine content of the rat conjunctiva. Inflammation Res. 58, 285–291. 10.1007/s00011-009-8245-4. [DOI] [PubMed] [Google Scholar]
- Reher T. M.; Neumann D.; Buschauer A.; Seifert R. (2012) Incomplete activation of human eosinophils via the histamine H4-receptor: evidence for ligand-specific receptor conformations. Biochem. Pharmacol. 84, 192–203. 10.1016/j.bcp.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Ackerman S.; Smith L. M.; Gomes P. J. (2016) Ocular itch associated with allergic conjunctivitis: latest evidence and clinical management. Ther. Adv. Chronic Dis. 7, 52–67. 10.1177/2040622315612745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacharn P.; Vichyanond P. (2013) Immunomodulators for conjunctivitis. Curr. Opin Allergy Clin Immunol. 13, 550–557. 10.1097/ACI.0b013e328364d86a. [DOI] [PubMed] [Google Scholar]
- Komi D.; Rambasek T.; Bielory L. (2018) Clinical implications of mast cell involvement in allergic conjunctivitis. Allergy 73, 528–539. 10.1111/all.13334. [DOI] [PubMed] [Google Scholar]
- Dattoli S. D.; Baiula M.; De Marco R.; et al. (2018) DS-70, a novel and potent α (4) integrin antagonist, is an effective treatment for experimental allergic conjunctivitis in guinea pigs. Br. J. Pharmacol. 175, 3891–3910. 10.1111/bph.14458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatarkiewicz J.; Rzodkiewicz P.ła.; Zochowska M.łg.; Staniszewska A.; Bujalska-Zadrozny M. (2019) New antihistamines – perspectives in the treatment of some allergic and inflammatory disorders. Arch. Med. Sci. 2, 537–553. 10.5114/aoms.2017.68534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G.; Weinreb R. N.; Leung C. K. (2014) Optic nerve head deformation in glaucoma: the temporal relationship between optic nerve head surface depression and retinal nerve fiber layer thinning. Ophthalmology 121, 2362–2370. 10.1016/j.ophtha.2014.06.035. [DOI] [PubMed] [Google Scholar]
- Berdahl J. P.; Allingham R. R.; Johnson D. H. (2008) Cerebrospinal fluid pressure is decreased in primary open-angle glaucoma. Ophthalmology 115, 763–768. 10.1016/j.ophtha.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Mallick J.; Devi L.; Malik P. K.; Mallick J. (2016) Update on normal tension glaucoma. J. Ophthalmic Vis Res. 11, 204–208. 10.4103/2008-322X.183914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E. J.; Kim T.-W.; Weinreb R. N.; Kim H. (2013) Reversal of lamina cribrosa displacement after intraocular pressure reduction in open-angle glaucoma. Ophthalmology 120, 553–559. 10.1016/j.ophtha.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Burgoyne C. F.; Crawford Downs J.; Bellezza A. J.; Francis Suh J.-K.; Hart R. T.; et al. (2005) The optic nerve head as a biomechanical structure; a new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retinal Eye Res. 24, 39–73. 10.1016/j.preteyeres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Daguman I. J.; Delfin M. S. (2019) Correlation of lamina cribosa and standard automated perimeter findings in glaucoma and non-glaucoma patients. J. Ophthal. Studies 2, 1–5. 10.16966/2639-152X.110. [DOI] [Google Scholar]
- Downs J. C.; Roberts M. D.; Sigal I. A. (2011) Glaucomatous cupping of the lamina cribrosa: a review of the evidence for active progressive remodeling as a mechanism. Exp. Eye Res. 93, 133–140. 10.1016/j.exer.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander H.; Makarov F.; Stefani F. H.; Stone J. (1995) Evidence of constriction of optic axons at the lamina cribrosa in the normotensive eye in humans and other mammals. Ophthalmic Res. 27, 296–309. 10.1159/000267739. [DOI] [PubMed] [Google Scholar]
- Flammer J.; Orgul S. (1998) Optic nerve blood-flow abnormalities in glaucoma. Prog. Retinal Eye Res. 17, 267–289. 10.1016/S1350-9462(97)00006-2. [DOI] [PubMed] [Google Scholar]
- Cull G.; Told R.; Burgoyne C. F.; Thompson S.; et al. (2015) Compromized optic nerve blood flow and autoregulation secondary to neural degeneration. Invest. Ophthalmol. Visual Sci. 56, 7286–7292. 10.1167/iovs.15-17879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez M. R.; Luo X. X.; Andrzejewska W.; Neufeld A. H. (1989) Age-related changes in the extracellular matrix of the human optic nerve head. Am. J. Ophthalmol. 107, 476–484. 10.1016/0002-9394(89)90491-1. [DOI] [PubMed] [Google Scholar]
- McElnea E. M.; Quill B.; Docherty N. G.; et al. (2011) Oxidative stress, mitochondrial dysfunction and calcium overload in human lamina cribrosa cells from glaucoma donors. Mol. Vis. 17, 1182–1189. [PMC free article] [PubMed] [Google Scholar]
- Osborne N. N. (2010) Mitochondria: Their role in ganglion cell death and survival in primary open angle glaucoma. Exp. Eye Res. 90, 750–757. 10.1016/j.exer.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Osborne N. N.; Nunez-Alvarez C.; Joglar B.; Del Olmo-Aguado S. (2016) Glaucoma: focus on mitochondria in relation to pathogenesis and neuroprotection. Eur. J. Pharmacol. 787, 127–133. 10.1016/j.ejphar.2016.04.032. [DOI] [PubMed] [Google Scholar]
- Pease M. E.; McKinnon S. J.; Quigley H. A.; et al. (2000) Obstructed axonal transport of BDNF and its receptor TrkB in experimental glaucoma. Invest Ophthalmol Vis Sci. 41, 764–774. [PubMed] [Google Scholar]
- Calkins D. J.; Horner P. J. (2012) The cell and molecular biology of glaucoma: axonopathy and the brain. Invest. Ophthalmol. Visual Sci. 53, 2482–2484. 10.1167/iovs.12-9483i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D.; Papadopoulo O.; Doshi R.; et al. (2000) Retinal ATP and phosphorus metabolites: reduction by hypoxia and recovery with MK-801 and diltiazem. Med. Sci. Res. 28, 87–91. [Google Scholar]
- Eells J. T. (2019) Mitochondrial dysfunction in the aging retina. Biology 8, 31. 10.3390/biology8020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bham H. A.; Dewsbery S. D.; Denniss J. (2020) Unaltered perception of suprathreshold contrast in early glaucoma despite sensitivity loss. Invest. Ophthalmol. Visual Sci. 61, 23. 10.1167/iovs.61.8.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyng P. F.; van Beek L. M. (2000) Pharmacological therapy for glaucoma: a review. Drugs 59, 411–434. 10.2165/00003495-200059030-00003. [DOI] [PubMed] [Google Scholar]
- Bito L. Z.; Camras C. B.; Gum G. G.; Resul B. (1989) The ocular hypotensive effects and side effects of prostaglandins on the eyes of experimental animals. Prog. Clin Biol. Res. 312, 349–368. [PubMed] [Google Scholar]
- Bito L. Z.; Miranda O. C.; Tendler M. R.; Resul B. (1990) Eicosanoids as a new class of ocular hypotensive agents. 3. Prostaglandin A2–1-isopropyl ester is the most potent reported hypotensive agent on feline eyes. Exp. Eye Res. 50, 419–428. 10.1016/0014-4835(90)90143-I. [DOI] [PubMed] [Google Scholar]
- Bito L. Z. (2001) A new approach to the medical management of glaucoma, from the bench to the clinic, and beyond: The Proctor Lecture. Invest Ophthalmol Vis Sci. 42, 1126–1133. [PubMed] [Google Scholar]
- Stjernschantz J.; Bito L. Z. (1989) The ocular effects of eicosanoids and other autacoids: historic background and the need for a broader perspective. Prog. Clin Biol. Res. 312, 1–13. [PubMed] [Google Scholar]
- Stjernschantz J. W. (2001) From PGF(2alpha)-isopropyl ester to latanoprost: a review of the development of xalatan: the Proctor Lecture. Invest Ophthalmol Vis Sci. 42, 1134–1145. [PubMed] [Google Scholar]
- Stjernschantz J. (2004) Studies on ocular inflammation and development of a prostaglandin analogue for glaucoma treatment. Exp. Eye Res. 78, 759–766. 10.1016/j.exer.2003.11.016. [DOI] [PubMed] [Google Scholar]
- Wang R. F.; Gagliuso D. J.; Mittag T. W.; et al. (2007) Effect of 15-keto latanoprost on intraocular pressure and aqueous humor dynamics in monkey eyes. Invest. Ophthalmol. Visual Sci. 48, 4143–4147. 10.1167/iovs.07-0035. [DOI] [PubMed] [Google Scholar]
- Resul B.; Stjernschantz J.; No K.; et al. (1993) Phenyl-substituted prostaglandins: potent and selective antiglaucoma agents. J. Med. Chem. 36, 243–248. 10.1021/jm00054a008. [DOI] [PubMed] [Google Scholar]
- Resul B.; Stjernschantz J.; Selén G.; Bito L. (1997) Structure-activity relationships and receptor profiles of some ocular hypotensive prostanoids. Surv. Ophthalmol. 41 (Suppl 2), S47–S52. 10.1016/S0039-6257(97)80007-0. [DOI] [PubMed] [Google Scholar]
- Klimko P. G.; Griffin B. G.; Davis T. L.; Sharif N. A. (2000) Synthesis and biological activity of a novel 11α-homo-(cyclohexyl)-prostaglandin. J. Med. Chem. 43, 3400–3407. 10.1021/jm990587w. [DOI] [PubMed] [Google Scholar]
- Sallee V.; McLaughlin M.; Griffin B. W.; Sharif N. A. (1998) Correlation of results from preclinical experimental models used for evaluation of FP prostaglandin agonists for therapy of glaucoma. Invest. Ophthalmol. Vis. Sci. 39, abs. #4274. [Google Scholar]
- Senchyna M.; Kyveris A.; May C.; Sharif N. A. (2000) RT-PCR analysis of prostanoid FP receptor mRNA in human pigmented ocular tissues: methodological considerations and results. Assoc. Res. Vision Opthalmol. S511, Abst # 2720-B966. [Google Scholar]
- Sharif N. A.; Davis T. L. (1999) Autoradiographic visualization and characterization of FP-prostaglandin receptors in human eyes using [3H]-PGF2α and [3H]AL-5848, the acid form of travoprost. Invest. Ophthalmol. Vis. Sci. 40 (# 4; Suppl), Abst. # 914. [Google Scholar]
- Sharif N. A.; Xu S. X. (1999) Pharmacological characterization of [3H]-ifenprodil binding to polyamine binding sites on rabbit and rat retinal homogenates: role in neuroprotection?. J. Ocul. Pharmacol. Ther. 15, 271–281. 10.1089/jop.1999.15.271. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S. X. (1999) Human retina contains polyamine-sensitive [3H]-ifenprodil binding sites: implications for neuroprotection?. Br. J. Ophthalmol. 83, 236–240. 10.1136/bjo.83.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T. L.; Sharif N. A. (2000) Pharmacological characterization of [3H]-prostaglandin E2 binding to the cloned human EP4 prostanoid receptor. Br. J. Pharmacol. 130 (2000), 1919–1926. 10.1038/sj.bjp.0703525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A.; Davis T. L. (2002) Cloned human EP1 prostanoid receptor pharmacology characterized using radioligand binding techniques. J. Pharm. Pharmacol. 54, 539–547. 10.1211/0022357021778655. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S. X. (2004) Pharmacological characterization and identification of EP3 prostanoid receptor binding sites in hamster uterus homogenates. J. Pharm. Pharmacol. 56, 197–203. 10.1211/0022357022557. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Williams G. W.; Xu S. X.; et al. (1998) Pharmacological analysis of [3H]PGE1/[3H]PGE2 and [3H]PGF2α binding in bovine corpus luteum: identification of EP3 and FP prostaglandin receptors and correlation with functional data. J. Pharmacol Exp Ther. 286, 1094–1102. [PubMed] [Google Scholar]
- Sharif N. A.; Davis T. L.; Williams G. W. (1999) [3H]AL-5848 (9-β-[+]fluprostenol): carboxylic acid of travoprost (AL-6221), a novel FP-prostaglandin to study the pharmacology and autoradiographic localization of the FP receptor. J. Pharm. Pharmacol. 51, 685–594. 10.1211/0022357991772989. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Crider J. Y.; Xu S. X.; Williams G. W. (2000) Affinities, selectivities, potencies and intrinsic activities of natural and synthetic prostanoids using endogenous receptors: focus on DP class prostanoids. J. Pharmacol Exp Ther. 293, 321–328. [PubMed] [Google Scholar]
- Sharif N. A.; Williams G. W.; Davis T. L. (2000) Pharmacology and autoradiography of human DP prostanoid receptors using [3H]-BWA868C, a DP receptor-selective antagonist radioligand. Br. J. Pharmacol. 131, 1025–1038. 10.1038/sj.bjp.0703686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A.; Crider J. Y.; Davis T. L. (2000) AL-3138 antagonizes FP prostanoid receptor-mediated inositol phosphates generation: comparison with some purported FP antagonists. J. Pharm. Pharmacol. 52, 1529–1539. 10.1211/0022357001777586. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Senchyna M.; Xu S. X. (2002) Pharmacological and molecular biological (RT-PCR) characterization of functional TP prostanoid receptors in immortalized human non-pigmented ciliary epithelial cells. J. Ocul. Pharmacol. Ther. 18, 141–162. 10.1089/108076802317373905. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kelly C. R.; Williams G. W. (2003) Bimatoprost (Lumigan) is an agonist at the cloned human ocular FP receptor: real-time FLIPR-based intracellular Ca2+ mobilization studies. Prostaglandins, Leukotrienes Essent. Fatty Acids 68, 27–33. 10.1016/S0952-3278(02)00232-6. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Xu S. X.; Griffin B. W.; Sharif N. A. (1998a) Use of a semi-automated, robotic radioimmunoassay to measure cAMP generated by activation of DP-, EP2- and IP-prostaglandin receptors in human ocular and other cell-types. Prostaglandins, Leukotri Essential Fatty Acids 59, 77–82. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Griffin B. W.; Sharif N. A. (1998) Prostaglandin-stimulated adenylyl cyclase activity via a pharmacologically-defined EP2 receptor in human NPE cells. J. Ocul. Pharmacol. Ther. 14, 293–304. 10.1089/jop.1998.14.293. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Griffin B. W.; Sharif N. A. (1999) Prostaglandin DP receptors positively coupled to adenylyl cyclase in embryonic bovine tracheal (EbTr) cells: pharmacological characterization using agonists and antagonists. Br. J. Pharmacol. 127, 204–210. 10.1038/sj.bjp.0702490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crider J. Y.; Griffin B. W.; Sharif N. A. (2000) Endogenous EP4 prostaglandin receptors coupled positively to adenylyl cyclase in Chinese hamster ovary cells: pharmacological characterization. Prostaglandins, Leukotrienes Essent. Fatty Acids 62, 21–26. 10.1054/plef.1999.0120. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Xu S. X.; Sharif N. A. (2001) Pharmacology of functional endogenous IP prostanoid receptors in NCB-20 cells: comparison with binding data from human platelets. Prostaglandins, Leukotrienes Essent. Fatty Acids 65, 253–258. 10.1054/plef.2001.0322. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Sharif N. A. (2002) Adenylyl cyclase activity mediated by β2-adrenoceptors in immortalized human trabecular meshwork and non-pigmented ciliary epithelial cells. J. Ocul. Pharmacol. Ther. 18, 221–230. 10.1089/108076802760116142. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Williams G. W.; Drace C. D.; et al. (2003) Pharmacological characterization of a serotonin receptor (5HT7) stimulating cAMP production in human corneal epithelial cells. Invest. Ophthalmol. Visual Sci. 44, 4837–4844. 10.1167/iovs.02-1292. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Sharif N. A. (2001) Functional pharmacological evidence for EP2 and EP4 prostanoid receptors in immortalized human trabecular meshwork and non-pigmented ciliary epithelial cells. J. Ocul. Pharmacol. Ther. 17, 35–46. 10.1089/108076801750125658. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Xu S. X.; Griffin B. W.; Sharif N. A. (1998a) Use of a semi-automated, robotic radioimmunoassay to measure cAMP generated by activation of DP-, EP2- and IP-prostaglandin receptors in human ocular and other cell-types. Prostaglandins, Leukotri Essential Fatty Acids 59, 77–82. [DOI] [PubMed] [Google Scholar]
- Crider J. Y.; Xu S. X.; Griffin B. W.; Sharif N. A. (1998) Use of a semi-automated, robotic radioimmunoassay to measure cAMP generated by activation of DP-, EP2- and IP-prostaglandin receptors in human ocular and other cell-types. Prostaglandins, Leukotrienes Essent. Fatty Acids 59, 77–82. 10.1016/S0952-3278(98)90055-2. [DOI] [PubMed] [Google Scholar]
- Griffin B. W.; Klimko P.; Crider J. Y.; Sharif N. A. (1999) AL-8810: a novel PGF2α analog with selective antagonist effects at the FP prostaglandin receptor. J. Pharmacol Exp Ther. 290, 1278–1284. [PubMed] [Google Scholar]
- Davis T. L.; Sharif N. A. (1999) Quantitative autoradiographic visualization and pharmacology of FP-prostaglandin receptors in human eyes using the novel phosphor-imaging technology. J. Ocul. Pharmacol. Ther. 15, 323–336. 10.1089/jop.1999.15.323. [DOI] [PubMed] [Google Scholar]
- Davis T. L.; Sharif N. A. (2000) Pharmacological characterization of [3H]-prostaglandin E2 binding to the cloned human EP4 prostanoid receptor. Br. J. Pharmacol. 130, 1919–1926. 10.1038/sj.bjp.0703525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg M. R.; Sallee V.; McLaughlin M.; Sharif N. A.; et al. (2001) Preclinical efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist. J. Ocul. Pharmacol. Ther. 17, 421–432. 10.1089/108076801753266802. [DOI] [PubMed] [Google Scholar]
- Hellberg M. R.; McLaughlin M. A.; Sharif N. A.; DeSantis L.; Dean T. R.; Kyba E. P.; Bishop J. E.; Klimko P. G.; Zinke P. W.; Selliah R. D.; Barnes G.; DeFaller J.; Kothe A.; Landry T.; Sullivan E.K.; Andrew R.; Davis A. A.; Silver L.; Bergamini M. V.W.; Robertson S.; Weiner A. L.; Sallee V. L.; et al. (2002) Identification and characterization of the ocular hypotensive efficacy of Travoprost, a potent and selective FP prostaglandin receptor agonist, and AL-6598, a DP prostaglandin receptor agonist. Surv. Ophthalmol. 47, S13–S33. 10.1016/S0039-6257(02)00293-X. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S. X.; Crider J. Y.; et al. (2001) Levobetaxolol (Betaxon) and other β–adrenergic antagonists: preclinical pharmacology, IOP-lowering activity and sites of action in human eyes. J. Ocul. Pharmacol. Ther. 17, 305–317. 10.1089/108076801753162726. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kelly C. R.; Crider J. Y.; et al. (2003) Ocular hypotensive FP prostaglandin (PG) analogs: PG receptor subtype binding affinities and selectivities, and agonist potencies at FP and other PG receptors in cultured cells. J. Ocul. Pharmacol. Ther. 19, 501–515. 10.1089/108076803322660422. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (1989) Quantitative autoradiography of TRH receptors in discrete brain regions of different mammalian species. Ann. N. Y. Acad. Sci. 553, 147–175. 10.1111/j.1749-6632.1989.tb46638.x. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Hughes J. (1989) Discrete mapping of brain mu and delta opioid receptors using selective peptides: quantitative autoradiography, species differences and comparison with kappa receptors. Peptides 10, 499–522. 10.1016/0196-9781(89)90135-6. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Williams G. W.; Crider J. Y.; et al. (2004) Molecular pharmacology of the ocular hypotensive DP/EP2 class prostaglandin AL-6598 and localization of DP and EP2 receptor sites in human eyes. J. Ocul. Pharmacol. Ther. 20, 489–508. 10.1089/jop.2004.20.489. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Crider J. Y.; Husain S.; et al. (2003) Human ciliary muscle responses to FP-class prostaglandin analogs: phosphoinositide hydrolysis, intracellular Ca2+-mobilization and MAP kinase activation. J. Ocul. Pharmacol. Ther. 19, 437–455. 10.1089/108076803322473006. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kelly C. R.; Crider J. Y. (2003) Human trabecular meshwork cell responses induced by bimatoprost, travoprost, unoprostone, and other FP prostaglandin receptor agonist analogues. Invest. Ophthalmol. Visual Sci. 44, 715–721. 10.1167/iovs.02-0323. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kelly C. R.; Crider J. Y. (2002) Agonist activity of bimatoprost, travoprost, latanoprost, unoprostone isopropyl ester and other prostaglandin analogs at the cloned human ciliary body FP prostaglandin receptor. J. Ocul. Pharmacol. Ther. 18, 313–324. 10.1089/10807680260218489. [DOI] [PubMed] [Google Scholar]
- Kelly C. R.; Williams G. W.; Sharif N. A. (2003) Real-time intracellular Ca2+-mobilization by travoprost acid, bimatoprost, unoprostone and other analogs via endogenous mouse, rat and cloned human FP prostaglandin receptors. J. Pharmacol. Exp. Ther. 304, 238–245. 10.1124/jpet.102.042556. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kaddour-Djebbar I.; Abdel-Latif A. A. (2008) Cat iris sphincter smooth muscle contraction: comparison of FP-class prostaglandin analog agonist activities. J. Ocul. Pharmacol. Ther. 24, 152–163. 10.1089/jop.2007.0076. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2008) Synthetic FP-class prostaglandin-induced contraction of rat uterus smooth muscle in vitro. Prostaglandins, Leukotrienes Essent. Fatty Acids 78, 199–207. 10.1016/j.plefa.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Tang W.; Zhang F.; Liu K.; Duan X. (2019) Efficacy and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: A meta-analysis. Medicine (Philadelphia, PA, U. S.) 98 (30), e16597. 10.1097/MD.0000000000016597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellberg M. R.; Ke T.-L.; Haggard K.; et al. (2003) The hydrolysis of the prostaglandin analog prodrug bimatoprost to 17-phenyl-trinor PGF2α by human and rabbit ocular tissues. J. Ocul. Pharmacol. Ther. 19, 97–103. 10.1089/108076803321637627. [DOI] [PubMed] [Google Scholar]
- Selliah R. D.; Hellberg M. R.; Sharif N. A.; et al. (2004) AL-12182, a novel 11-oxa prostaglandin analog with topical ocular hypotensive activity in the monkey. Bioorg. Med. Chem. Lett. 14, 4525–4528. 10.1016/j.bmcl.2004.06.037. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; McLaughlin M. A.; Kelly C. R.; et al. (2006) Preclinical pharmacology of AL-12182, a new ocular hypotensive 11-oxa-prostaglandin analog. J. Ocul. Pharmacol. Ther. 22, 291–309. 10.1089/jop.2006.22.291. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Hellberg M. R.; Sharif N. A.; et al. (2009) Discovery of 13-oxa prostaglandin analogs as novel antiglaucoma agents: synthesis and biological activity. Bioorg. Med. Chem. 17, 576–584. 10.1016/j.bmc.2008.11.070. [DOI] [PubMed] [Google Scholar]
- Klimko P.; Hellberg M. R.; McLaughlin M. A.; et al. (2004) 15-Fluoro prostaglandin FP agonists: a new class of topical ocular hypotensives. Bioorg. Med. Chem. 12, 3451–3469. 10.1016/j.bmc.2004.04.034. [DOI] [PubMed] [Google Scholar]
- Holló G. (2007) The side effects of the prostaglandin analogues. Expert Opin. Drug Saf. 6, 45–52. 10.1517/14740338.6.1.45. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Xu S. X. (2004) Binding affinities of ocular hypotensive β-blockers levobetaxolol, levobunolol and timolol at endogenous guinea pig β – adrenoceptors. J. Ocul. Pharmacol. Ther. 20, 93–99. 10.1089/108076804773710759. [DOI] [PubMed] [Google Scholar]
- Quaranta L.; Turano R.; Pizzolante T. (2007) Levobetaxolol hydrochloride: a review of its pharmacology and use in the treatment of chronic open-angle glaucoma and ocular hypertension. Clin Ophthalmol. 1, 93–99. [PMC free article] [PubMed] [Google Scholar]
- Henderson A. J.; Hadden M.; Guo C.; et al. (2010) 2,3-Diaminopyrines as rho kinase inhibitors. Bioorg. Med. Chem. Lett. 20, 1137–1140. 10.1016/j.bmcl.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Hellberg M. R., Sharif N. A., and May J. A.. et al. (2010) (Indazol-5-yl)-pyrazines and (1,3-hihydro-indol-2-one)-pyrazines for treating glaucoma and controlling intraocular pressure. US Patent US7655662.
- Chen H.-H., Sharif N. A., and Hellberg M. R. (2011) Hydroxyamino- and amino-substituted pyridine analogs for treating rho kinase-mediated diseases and conditions. US Patent US7867999.
- Chen H.-H.; Namil A.; Severns B.; et al. (2014) In vivo optimization of 2,3-diaminopyrazine Rho kinase inhibitors. Bioorg. Med. Chem. Lett. 24, 1875–1879. 10.1016/j.bmcl.2014.03.017. [DOI] [PubMed] [Google Scholar]
- Ramachandran C.; Patil R. V.; Combrink K.; et al. (2011) Rho-Rho kinase pathway in the actomyosin contraction and cell-matrix adhesion in immortalized human trabecular meshwork cells. Mol. Vision 17, 1877–1890. [PMC free article] [PubMed] [Google Scholar]
- Chidlow G.; DeSantis L. M.; Sharif N. A.; Osborne N. N. (1995) The characteristics of [3H]-5-hydroxytrytamine binding to iris ciliary body of the rabbit. Invest Ophthalmol Vis Sci. 36 (2238), 2245. [PubMed] [Google Scholar]
- Harris L. C.; Awe S. O.; Opere C. A.; et al. (2001) [3H]-Serotonin release from bovine iris-ciliary body: pharmacology of pre-junctional serotonin (5HT7) auto-receptors. Exp. Eye Res. 73, 59–67. 10.1006/exer.2001.1012. [DOI] [PubMed] [Google Scholar]
- Harris L. C.; Awe S. O.; Opere C. A.; et al. (2002) Pharmacology of serotonin receptors modulating electrically-induced [3H]-norepinephrine release from isolated mammalian iris-ciliary bodies. J. Ocul. Pharmacol. Ther. 18, 339–348. 10.1089/10807680260218506. [DOI] [PubMed] [Google Scholar]
- May J. M.; McLaughlin M. A.; Sharif N. A.; et al. (2003) Evaluation of the ocular hypotensive response of serotonin 5HT1A and 5HT2 receptor ligands in conscious ocular hypertensive cynomolgus monkeys. J. Pharmacol. Exp. Ther. 306, 301–309. 10.1124/jpet.103.049528. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2010) Serotonin-2 receptor agonists as novel ocular hypotensive agents and their cellular mechanisms of action: novel drug targets for glaucoma treatment. Curr. Drug Targets 11, 978–993. 10.2174/138945010791591278. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; May J. A. (2011) Potential for serotonergic agents to treat elevated intraocular pressure and glaucoma: focus on 5HT2 receptor agonists. Expert Rev. Ophthalmol. 6, 105–120. 10.1586/eop.10.69. [DOI] [Google Scholar]
- May J. A., Dean T. R., Sharif N. A., and Hellberg M. R. (2003) Serotonergic 5HT2 agonists for treating glaucoma. US Patent US6664286.
- May J. M.; Chen H.-H.; Rusinko A.; et al. (2003) A novel and selective 5HT2 receptor agonist with ocular hypotensive activity: (S)-(+)-1—(2-aminopropyl)-8,9-dihydropyrano-[3,2-e]indole. J. Med. Chem. 46, 4188–4195. 10.1021/jm030205t. [DOI] [PubMed] [Google Scholar]
- May J. A.; Dantanarayana A. P.; Zinke P. W.; et al. (2006) 1-((S)-2-Aminopropyl)-1H-indazol-6-ol: a potent peripherally acting 5HT2 receptor agonist with ocular hypotensive activity. J. Med. Chem. 49, 318–328. 10.1021/jm050663x. [DOI] [PubMed] [Google Scholar]
- May J. A.; Sharif N. A.; Chen H.-H.; et al. (2009) Pharmacological properties and discriminative stimulus effects of a novel and selective 5HT2 receptor agonist, AL-38022A [(S)-2- (8,9- dihydro-7H- pyrano[2,3g]indazol-1-yl)-1-methylethylamine]. Pharmacol., Biochem. Behav. 91, 307–314. 10.1016/j.pbb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangisetty J.; Dukat M.; Dowd C.; et al. (2001) 1-[2-methoxy-5-(3-phenylpropyl)]-2-aminopropane unexpectedly shows 5HT2A serotonin receptor affinity and antagonist character. J. Med. Chem. 44, 3283–3291. 10.1021/jm0100739. [DOI] [PubMed] [Google Scholar]
- Glennon R. A.; Bondarev M. L.; Khorana N.; Young R.; May J. A.; Hellberg M. R.; McLaughlin M. A.; Sharif N. A. (2004) β-Oxygenated analogues of the 5HT2A serotonin receptor agonist 1-(4-bromo-2,5-dimethoxyphenyl)-2-aminopropane. J. Med. Chem. 47, 6034–6041. 10.1021/jm040082s. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Senchyna M. (2006) Serotonin receptor subtype mRNA expression in human ocular tissues determined by RT-PCR. Mol. Vis. 12, 1040–1047. [PubMed] [Google Scholar]
- Kelly C. R.; Sharif N. A. (2006) Pharmacological evidence for a functional serotonin-2B receptor subtype in a human uterine smooth muscle cell line. J. Pharmacol. Exp. Ther. 317, 1254–1261. 10.1124/jpet.105.100172. [DOI] [PubMed] [Google Scholar]
- Feng Z.; Mohapatra S.; Klimko P. G.; et al. (2007) Novel benzodifuran analogs as potent 5HT2A receptor agonists with ocular hypotensive activity. Bioorg. Med. Chem. Lett. 17, 2998–3002. 10.1016/j.bmcl.2007.03.073. [DOI] [PubMed] [Google Scholar]
- Ohia S. E.; Njie-Mbye Y. F.; Robinson J.; et al. (2018) Serotonin-2B/2C receptors-mediate bovine ciliary muscle contraction: role in IOP regulation. J. Ocul. Pharmacol. Ther. 34, 70–75. 10.1089/jop.2017.0123. [DOI] [PubMed] [Google Scholar]
- Njie-Mbye Y. F.; Robinson J.; Mitchell-Bush L.; Mckoy M.; Opere C. A.; Ohia S. E.; Sharif N. (2018) Pharmacology of serotonin receptors causing contraction of bovine isolated posterior ciliary arteries: role in ocular blood-flow modulation. J. Ocul. Pharmacol. Ther. 34, 134–140. 10.1089/jop.2017.0124. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Crider J. Y.; Kelly C. R.; Davis T. L. (2006) Serotonin-2 (5HT2) receptor-mediated signal transduction in human ciliary muscle cells: role in ocular hypotension. J. Ocul. Pharmacol. Ther. 22, 389–401. 10.1089/jop.2006.22.389. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Kelly C. R.; McLaughlin M. A. (2006) Human trabecular meshwork cells express functional serotonin-2 (5HT2) receptors: role in IOP reduction. Invest. Ophthalmol. Visual Sci. 47, 4001–4010. 10.1167/iovs.06-0062. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; McLaughlin M. A.; Kelly C. R. (2007) AL-34662: a potent, selective, and efficacious ocular hypotensive serotonin-2 receptor agonist. J. Ocul. Pharmacol. Ther. 23, 1–13. 10.1089/jop.2006.0093. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; McLaughlin M. A.; Kelly C. R.; et al. (2009) Cabergoline: pharmacology, ocular hypotensive studies in multiple species, and aqueous humor dynamic modulation in cynomolgus monkey eyes. Exp. Eye Res. 88, 386–397. 10.1016/j.exer.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Drace C. D.; Williams G. W.; Crider J. Y. (2004) Cloned human 5HT1A receptor pharmacology determined using agonist binding and measurement of cAMP accumulation. J. Pharm. Pharmacol. 56, 1267–1274. 10.1211/0022357044346. [DOI] [PubMed] [Google Scholar]
- May J. A., Dean T. R., Sharif N. A., and Chen H.-H. (2006) Serotonergic 5HT7 receptor compounds for treating ocular and CNS disorders. US Patent US7060704.
- May J. A., Dean T. R., Sharif N. A., and Chen H.-H. (2007) Serotonergic 5HT7 receptor compounds for treating ocular and CNS disorders. US Patent US7285553.
- Sharif N. A. (2017) Prospects of treating ocular hypertension and glaucoma with peptidic and non-peptide kinin mimetic drugs. JOJ. Ophthalmol 3 (1), 555601. 10.19080/JOJO.2017.03.555601. [DOI] [Google Scholar]
- Sharif N. A.; Xu S. X. (1996) Pharmacological characterization of bradykinin receptors coupled to phosphoinositide turnover in SV40 immortalized human trabecular meshwork cells. Exp. Eye Res. 63, 631–637. 10.1006/exer.1996.0157. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2002). Non-peptide bradykinin antagonists for use in controlling intraocular pressure and treating glaucoma. US Patent US6500831.
- Asano M.; Hatori C.; Sawai H.; Johki S.; Inamura N.; Kayakiri H.; Satoh S.; Abe Y.; Inoue T.; Sawada Y.; Mizutani T.; Oku T.; Nakahara K. (1998) Pharmacological characterization of a nonpeptide bradykinin B2 receptor antagonist, FR165649, and agonist. Br. J. Pharmacol. 124, FR190997. 10.1038/sj.bjp.0701813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A.; Hunter J. C.; Hill R. G.; Hughes J. (1988) Bradykinin induced accumulation of [3H]inositol 1 phosphate in human embryonic pituitary tumor cells by activation of a B2 receptor. Neurosci. Lett. 86, 279–283. 10.1016/0304-3940(88)90496-X. [DOI] [PubMed] [Google Scholar]
- Ransom J.; Cherwinski H. M.; Dunne J. F.; Sharif N. A. (1991) Flow cytometric analysis of internal calcium mobilization via a B2 bradykinin receptor in a subclone of PC12 cells. J. Neurochem. 56, 983. 10.1111/j.1471-4159.1991.tb02018.x. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Whiting R. L. (1993) The neuropeptide bradykinin stimulates phosphoinositide turnover in HSDM1C1 cells: B2 antagonist sensitive responses and receptor binding studies. Neurochem. Res. 12, 1313. 10.1007/BF00975053. [DOI] [PubMed] [Google Scholar]
- Sharif N. A. (2010) Use of bradykinin and related B2R agonists to treat ocular hypertension and glaucoma. US Patent US7807629.
- Sharif N. A. (2012) Use of non-peptidic bradykinin receptor agonists to treat ocular hypertension and glaucoma. US Patent US8173668.
- Combrink K., Mohapatra S., and Hellberg M. R.. et al. (2012) Bradykinin receptor agonists and uses thereof to treat ocular hypertension and glaucoma. US Patent US8252793.
- Sharif N. A.; Xu S.; Li L.; et al. (2013) Protein expression, biochemical pharmacology of signal transduction, and relation to IOP modulation by bradykinin B2-receptors in ciliary muscle. Mol. Vision 19, 1356–1370. [PMC free article] [PubMed] [Google Scholar]
- Sharif N. A.; Wang Y.; Katoli P.; et al. (2014) Human non-pigmented ciliary epithelium bradykinin B2-receptors: receptor localization, pharmacological characterization of intracellular Ca2+ mobilization, and prostaglandin secretion. Curr. Eye Res. 39, 378–389. 10.3109/02713683.2013.816324. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Katoli P.; Kelly C. R.; et al. (2014) Trabecular meshwork bradykinin receptors: mRNA levels, immunohistochemical visualization, signaling processes pharmacology and linkage to IOP changes. J. Ocul. Pharmacol. Ther. 30, 21–34. 10.1089/jop.2013.0105. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Katoli P.; Scott D.; et al. (2014) FR-190997, a non-peptide bradykinin B2-receptor partial agonist, is a potent and efficacious intraocular pressure lowering agent in ocular hypertensive cynomolgus monkeys. Drug Dev. Res. 75, 211–223. 10.1002/ddr.21174. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Li L.; Katoli P.; et al. (2014) Preclinical pharmacology, ocular tolerability and ocular hypotensive efficacy of a novel non-peptide bradykinin mimetic small molecule. Exp. Eye Res. 128, 170–180. 10.1016/j.exer.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Patil R.; Li L.; Husain S. (2016) Human ciliary muscle cell responses to kinins: activation of ERK1/2 and pro-matrix metalloproteinases secretion. World J. Ophthalmol. 6, 20–27. 10.5318/wjo.v6.i3.20. [DOI] [Google Scholar]
- Prasanna G.; Sharif N. A.; Li B.; et al. (2014) BK2A78: a novel non-peptide bradykinin B2 agonist lowers intraocular pressure (IOP) in ocular hypertensive Cynomolgus monkeys. ARVO Abst. # 2883. [Google Scholar]
- Sharif N. A.; Klekar L.; Li L.; Xu S. (2015) Ocular hypotensive activity of a non-peptide bradykinin B2-receptor antagonist (WIN-64338) in Dutch-Belt rabbits: a case of poly-pharmacology in action. Int. J. Ophthalmol Clin Res. 2, 3. 10.23937/2378-346X/1410031. [DOI] [Google Scholar]
- Ellis D., Scheibler L., and Sharif N. A. (2017) Prostaglandin conjugates and derivatives for treating glaucoma and ocular hypertension. US Patent US9604949 B2.
- Kirihara T.; Taniguchi T.; Yamamura K.; et al. (2018) Pharmacologic characterization of omidenepag isopropyl, a novel selective EP2 receptor agonist, as an ocular hypotensive agent. Invest. Ophthalmol. Visual Sci. 59, 145–153. 10.1167/iovs.17-22745. [DOI] [PubMed] [Google Scholar]
- Kirihara T., Shimizaki A., and Sharif N. A. (2015) Prophylactic and/or therapeutic agent containing pyridylamino acetic acid compound. Japanese Patent; submitted; PCT filed 7th July 2016.
- Kirihara T., Shimizaki A., and Sharif N. A. (2018) Prophylactic and/or therapeutic agent containing pyridylamino acetic acid compound. US Patent Application US2018/ 0200239 A1.
- Sharif N. A.; Kirihara T.; Iwamura R.; Yoneda K.; Lu H.; Odani-Kawabata N.; Shams N. (2020) A novel non-prostaglandin EP2-receptor agonist for glaucoma treatment: Omidenepag Isopropyl (DE-117). FASEB J. 34, 1. 10.1096/fasebj.2020.34.s1.08817. [DOI] [Google Scholar]
- Sharif N. A., and Griffin B. W. (2002) 11β-fluoro-15β-hydroxy-PGF2α analogs as FP receptor antagonists. US Patent US6441033.
- Sharif N. A., and Griffin B. W. (2003) 11-Deoxy-16-fluoro-PGF2α and 11β-fluoro-15-β-hydroxy-PGF2α analogs as FP receptor antagonists. US Patent US6649655.
- Sharif N. A., and Griffin B. W. (2002) Treatment of FP receptor activation-related disorders, e.g. ocular hyperemia, involves use of 11-deoxy-16-fluoro-PGF2α analogs. US Patent US6492417.
- Sharif N. A., and Griffin B. W. (2003) 11β-fluoro-15 β-hydroxy-PGF2α analogs as FP receptor antagonists. US Patent US6649655.
- Sharif N. A.; Crider J. Y.; Davis T. L. (2000) AL-3138 antagonizes FP prostanoid receptor-mediated inositol phosphates generation: comparison with some purported FP antagonists. J. Pharm. Pharmacol. 52, 1529–1539. 10.1211/0022357001777586. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Klimko P. (2019) Prostaglandin FP receptor antagonists: discovery, pharmacological characterization and therapeutic utility. Br. J. Pharmacol. 176, 1059–1078. 10.1111/bph.14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain S.; Jafri F.; Crosson C. E. (2005) Acute effects of PGF2α on MMP-2 secretion from human ciliary muscle cells: a PKC- and ERK-dependent process. Invest. Ophthalmol. Visual Sci. 46, 1706–1713. 10.1167/iovs.04-0993. [DOI] [PubMed] [Google Scholar]
- Vysniauskiene I.; Allemann R.; Flammer J.; Haefliger I. O. (2006) Vasoactive responses of U46619, PGF2alpha, latanoprost, and travoprost in isolated porcine ciliary arteries. Invest Ophthalmol Vis Sci. 47, 295–298. 10.1167/iovs.05-0760. [DOI] [PubMed] [Google Scholar]
- Hinz B.; Rösch S.; Ramer R.; et al. (2005) Latanoprost induces matrix metalloproteinase-1 expression in human nonpigmented ciliary epithelial cells through a cyclooxygenase-2-dependent mechanism. FASEB J. 19, 1929–1931. 10.1096/fj.04-3626fje. [DOI] [PubMed] [Google Scholar]
- Thieme H.; Schimmat C.; Münzer G.; Boxberger M.; et al. (2006) Endothelin antagonism: effects of FP receptor agonists prostaglandin F2alpha and fluprostenol on trabecular meshwork contractility. Invest. Ophthalmol. Visual Sci. 47, 938–945. 10.1167/iovs.05-0527. [DOI] [PubMed] [Google Scholar]
- Romano M. R.; Lograno M. D. (2007) Evidence for the involvement of cannabinoid CB1 receptors in the bimatoprost-induced contractions on the human isolated ciliary muscle. Invest. Ophthalmol. Visual Sci. 48, 3677–3682. 10.1167/iovs.06-0896. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Williams G. W.; Kelly C. R. (2001) Bimatoprost and its free acid are prostaglandin FP receptor agonists. Eur. J. Pharmacol. 432, 211–213. 10.1016/S0014-2999(01)01486-8. [DOI] [PubMed] [Google Scholar]
- Kalouche G.; Beguier F.; Bakria M.; et al. (2016) Activation of prostaglandin FP and EP2 receptors differently modulates myofibroblast transition in a model of adult primary human trabecular meshwork cells. Invest. Ophthalmol. Visual Sci. 57, 1816–1825. 10.1167/iovs.15-17693. [DOI] [PubMed] [Google Scholar]
- Roddy G. W.; Rinkoski T. A.; Monson K. J.; et al. (2020) Stanniocalcin-1 (STC-1), a downstream effector molecule in latanoprost signaling, acts independent of the FP receptor for intraocular pressure reduction. PLoS One 15, e0232591. 10.1371/journal.pone.0232591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo-Santos T.; Rodriguez N. E.; Moura-Pontes S.; Dixt U. G.; Abanades D. R.; Bozza P. T.; Wilson M. E.; Borges V. M. (2014) Role of prostaglandin F2α production in lipid bodies from Leishmania infantum chagasi: insights on virulence. J. Infect. Dis. 210, 1951–1961. 10.1093/infdis/jiu299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glushakov A. V.; Robbins S. W.; Bracy C. L.; et al. (2013) Prostaglandin F2α FP receptor antagonist improves outcomes after experimental traumatic brain injury. J. Neuroinflammation 10, 132. 10.1186/1742-2094-10-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K.; Yamamoto S.; Takahashi M.; et al. (2014) Prostaglandin F2α FP receptor inhibitor reduces demyelination and motor dysfunction in a cuprizone-induced multiple sclerosis mouse model. Prostaglandins, Leukotrienes Essent. Fatty Acids 91, 175–82. 10.1016/j.plefa.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Kim Y. T.; Moon S. K.; Maruyama T.; Narumiya S.; Dore S. (2012) Prostaglandin FP receptor inhibitor reduces ischemic brain damage and neurotoxicity. Neurobiol Dis. 8, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga D. P.; Szabó Í; Varga VÉ; et al. (2020) The antagonism of prostaglandin FP receptors inhibits the evolution of spreading depolarization in an experimental model of global forebrain ischemia. Neurobiol. Dis. 137, 104780. 10.1016/j.nbd.2020.104780. [DOI] [PubMed] [Google Scholar]
- Maehara T.; Higashitarumi F.; Kondo R.; Fujimori K. (2020) Prostaglandin F2α receptor antagonist attenuates LPS-induced systemic inflammatory response in mice. FASEB J. 10.1096/fj.202001481R. [DOI] [PubMed] [Google Scholar]
- Woodward D. F.; Krauss A H-P; Chen J.; et al. (2001) The pharmacology of bimatoprost (Lumigan). Surv. Ophthalmol. 45 (Suppl. 4), S337–S345. 10.1016/S0039-6257(01)00224-7. [DOI] [PubMed] [Google Scholar]
- Maxey K. M.; Johnson J. L.; LaBrecque J. (2002) The hydrolysis of bimatoprost in corneal tissue generates a potent prostanoid FP receptor agonist. Surv. Ophthalmol. 47 (suppl 1), S34–S40. 10.1016/S0039-6257(02)00323-5. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Ke T-L; Haggard K. (2002) Bimatoprost hydrolysis to 17-phenyl-PGF2α by human and rabbit ocular tissues and agonist activity of bimatoprost and 17-phenyl-PGF2α. Assoc. Res. Vis. Ophthalmol. (ARVO) Abst. # 4080. [Google Scholar]
- Camras C. B.; Toris C. B.; Sjoquist B.; et al. (2004) Detection of the free acid of bimatoprost in aqueous humor samples from human eyes treated with bimatoprost before cataract surgery. Ophthalmology 111, 2193–2198. 10.1016/j.ophtha.2004.06.028. [DOI] [PubMed] [Google Scholar]
- Camras C. B.; Sharif N. A.; Wax M. B.; Stjernshantz J. (2008) Bimatoprost, the prodrug of a prostaglandin analogue. Br J. Ophthalmol. 92, 862–863. [PubMed] [Google Scholar]
- Davies S. S.; Ju W.-K.; Neufeld A. H.; et al. (2003) Hydrolysis of bimatoprost (Lumigan) to its free acid by ocular tissue in vitro. J. Ocul. Pharmacol. Ther. 19, 45–54. 10.1089/108076803762718105. [DOI] [PubMed] [Google Scholar]
- Kriatchko A.; Zhan G.; Cheruvu N. (2003) In vitro hydrolysis of bimatoprost in bovine cornea. Assoc. Res. Vis. Ophthalmol. (ARVO) Abst. # 4422. [Google Scholar]
- Faulkner R.; Sharif N. A.; Orr S.; et al. (2010) Aqueous humor concentrations of bimatoprost free acid, bimatoprost and travoprost free acid in cataract surgical patients administered multiple topical ocular doses of LUMIGAN or TRAVATAN. J. Ocul. Pharmacol. Ther. 26, 147–156. 10.1089/jop.2009.0098. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Klimko P. (2009) Update and commentary on the prodrug bimatoprost and a putative prostamide receptor. Expert Rev. Ophthalmol. 4, 477–489. 10.1586/eop.09.40. [DOI] [Google Scholar]
- Kaufman P. L. (2003) The prostaglandin wars. Am. J. Ophthalmol. 136, 727–728. 10.1016/S0002-9394(03)00658-5. [DOI] [PubMed] [Google Scholar]
- Nilsson S. F.; Samuelsson M.; Bill A.; Stjernschantz J. (1989) Increased uveoscleral outflow as a possible mechanism of ocular hypotension caused by prostaglandin F2 alpha-1-isopropyl ester in the cynomolgus monkey. Exp. Eye Res. 48, 707–716. 10.1016/0014-4835(89)90011-0. [DOI] [PubMed] [Google Scholar]
- Sherwood M.; Brandt J. (2001) Bimatoprost Study Groups 1 and 2. Six-month comparison of bimatoprost once-daily and twice-daily with timolol twice-daily in patients with elevated intraocular pressure. Surv. Ophthalmol. 45 (Suppl 4), S361–S368. 10.1016/S0039-6257(01)00219-3. [DOI] [PubMed] [Google Scholar]
- Toris C. B.; Zhan G. L.; Zhao J.; et al. (2001) Potential mechanism for the additivity of pilocarpine and latanoprost. Am. J. Ophthalmol. 131, 722–728. 10.1016/S0002-9394(01)00831-5. [DOI] [PubMed] [Google Scholar]
- Toris C. B.; Zhan G.; Camras C. B. (2004) Increase in outflow facility with unoprostone treatment in ocular hypertensive patients. Arch. Ophthalmol. 122, 1782–1787. 10.1001/archopht.122.12.1782. [DOI] [PubMed] [Google Scholar]
- Toris C. B.; Zhan G.; Fan S.; et al. (2007) Effects of travoprost on aqueous humor dynamics in patients with elevated intraocular pressure. J. Glaucoma 16, 189–195. 10.1097/IJG.0b013e31802fc6d3. [DOI] [PubMed] [Google Scholar]
- Sit A. J.; Weinreb R. N.; Crowston J. G.; et al. (2006) Sustained effect of travoprost on diurnal and nocturnal intraocular pressure. Am. J. Ophthalmol. 141, 1131–1133. 10.1016/j.ajo.2006.01.049. [DOI] [PubMed] [Google Scholar]
- Suh M. H.; Park K. H.; Kim D. M.; et al. (2009) Effect of travoprost on intraocular pressure during 12 months of treatment for normal-tension glaucoma. Jpn. J. Ophthalmol. 53, 18–23. 10.1007/s10384-008-0617-8. [DOI] [PubMed] [Google Scholar]
- Ussa F.; Fernandez I.; Brion M.; et al. (2015) Association between SNPs of metalloproteinases and prostaglandin F2α receptor genes and latanoprost response in open-angle glaucoma. Ophthalmology 122, 1040–1048. 10.1016/j.ophtha.2014.12.038. [DOI] [PubMed] [Google Scholar]
- Weinreb R. N.; Robinson M. R.; Dibas M.; Stamer W. D. (2020) Matrix metalloproteinases and glaucoma treatment. J. Ocul. Pharmacol. Ther. 36, 208–228. 10.1089/jop.2019.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahler C. K.; Howell K. G.; Hann C. R.; et al. (2008) Prostaglandins increase trabecular meshwork outflow facility in cultured human anterior segments. Am. J. Ophthalmol. 145, 114–119. 10.1016/j.ajo.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toris C. B.; Gabelt B. T.; Kaufman P. L. (2008) Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv. Ophthalmol. 53 (Suppl1), S107–120. 10.1016/j.survophthal.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G. A.; Nau C. B.; McLaren J. W.; et al. (2004) Mechanism of ocular hypotensive action of bimatoprost (Lumigan) in patients with ocular hypertension or glaucoma. Ophthalmology 111, 1658–1662. 10.1016/j.ophtha.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Lim K. S.; Nau C. B.; O’Byrne M. M.; et al. (2008) Mechanism of action of bimatoprost, latanoprost, and travoprost in healthy subjects. A crossover study. Ophthalmology 115, 790–795. 10.1016/j.ophtha.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinslage S.; Hueber A.; Diestelhorst M.; et al. (2004) The influence of Latanoprost 0.005% on aqueous humor flow and outflow facility in glaucoma patients: a double-masked placebo-controlled clinical study. Graefe's Arch. Clin. Exp. Ophthalmol. 242, 654–660. 10.1007/s00417-003-0835-1. [DOI] [PubMed] [Google Scholar]
- Crowston J. G.; Aihara M.; Lindsey J. D.; et al. (2004) Effect of latanoprost on outflow facility in the mouse. Invest. Ophthalmol. Visual Sci. 45, 2240–2245. 10.1167/iovs.03-0990. [DOI] [PubMed] [Google Scholar]
- Sharif N. A.; Davis T. L.; Williams G. W. (2005) Ocular hypotensive DP-class prostaglandin receptor affinities determined by quantitative autoradiography on human eye sections. J. Ocul. Pharmacol. Ther. 21, 121–132. 10.1089/jop.2005.21.121. [DOI] [PubMed] [Google Scholar]
- Cavet M. E.; Vittitow J. L.; Impagnatiello F.; et al. (2014) Nitric oxide (NO): an emerging target for the treatment of glaucoma. Invest. Ophthalmol. Visual Sci. 55, 5005–5015. 10.1167/iovs.14-14515. [DOI] [PubMed] [Google Scholar]
- Tanihara H.; Inoue T.; Yamamoto T.; et al. (2015) Additive intraocular pressure-lowering effects of the rho kinase inhibitor ripasudil (K-115) combined with timolol or latanoprost: a report of 2 randomized clinical trials. JAMA Ophthalmol. 133, 755–761. 10.1001/jamaophthalmol.2015.0525. [DOI] [PubMed] [Google Scholar]
- Lin C. W.; Sherman B.; Moore L. A.; et al. (2018) Discovery and Preclinical Development of Netarsudil, a Novel Ocular Hypotensive Agent for the Treatment of Glaucoma. J. Ocul. Pharmacol. Ther. 34, 40–51. 10.1089/jop.2017.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadruddin O.; Pinchuk L.; Angeles R.; Palmberg P. (2019) Ab externo implantation of the MicroShunt, a poly (styrene-block-isobutylene-blockstyrene) surgical device for the treatment of primary open-angle glaucoma: a review. Eye Vision 6, 36. 10.1186/s40662-019-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollo G.; Topouzis F.; Fechtner R. D. (2014) Fixed-combination intraocular pressure-lowering therapy for glaucoma and ocular hypertension: advantages in clinical practice. Expert Opin. Pharmacother. 15, 1737–1747. 10.1517/14656566.2014.936850. [DOI] [PubMed] [Google Scholar]
- Baltan S.; Inman D. M.; Danilov C. A.; et al. (2010) Metabolic vulnerability disposes retinal ganglion cell axons to dysfunction in a model of glaucomatous degeneration. J. Neurosci. 30, 5644–5652. 10.1523/JNEUROSCI.5956-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.; Li D.; Ying X.; Khaw P. T.; Raisman G. (2015) An energy theory of glaucoma. Glia 63, 1537–1552. 10.1002/glia.22825. [DOI] [PubMed] [Google Scholar]
- Williams P. A.; Harder J. M.; Foxworth N. E.; et al. (2017) Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 355, 756–760. 10.1126/science.aal0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui F.; Tang J.; Williams P. A.; McGuinness M. B.; Hadoux X.; Casson R. J.; Coote M.; Trounce I. A.; Martin K. R.; Wijngaarden P.; Crowston J. G. (2020) Improvement in inner retinal function in glaucoma with nicotinamide (vitamin B3) supplementation: A crossover randomized clinical trial. Clin Experiment Ophthalmol. 2020 Jul 48, 28. 10.1111/ceo.13818. [DOI] [PubMed] [Google Scholar]
- Madeo F.; Eisenberg T.; Pietrocola F.; Kroemer G. (2018) Spermidine in health and disease. Science 359, eaan2788. 10.1126/science.aan2788. [DOI] [PubMed] [Google Scholar]
- Xu W.; Gao L.; Li T.; et al. (2018) Neuroprotective role of agmatine in neurological diseases. Curr. Neuropharmacol. 16, 1296–1305. 10.2174/1570159X15666170808120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opere C. A.; Heruye S.; Njie-Mbye Y. F.; et al. (2018) Regulation of excitatory amino acid transmission in the retina: studies on neuroprotection. J. Ocul. Pharmacol. Ther. 34, 107–118. 10.1089/jop.2017.0085. [DOI] [PubMed] [Google Scholar]
- Sehi M.; Grewal D. S.; Goodkin M. L.; Greenfield D. S. (2010) Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology 117, 2329–2336. 10.1016/j.ophtha.2010.08.049. [DOI] [PubMed] [Google Scholar]