Abstract
BACKGROUND:
Previous reports from the Management of Myelomeningocele Study demonstrated that prenatal repair of myelomeningocele reduces hindbrain herniation and the need for cerebrospinal fluid shunting, and improves motor function in children with myelomeningocele. The trial was stopped for efficacy after 183 patients were randomized, but 30-month outcomes were only available at the time of initial publication in 134 mother-child dyads. Data from the complete cohort for the 30-month outcomes are presented here. Maternal and 12-month neurodevelopmental outcomes for the full cohort were reported previously.
OBJECTIVE:
The purpose of this study is to report the 30-month outcomes for the full cohort of patients randomized to either prenatal or postnatal repair of myelomeningocele in the original Management of Myelomeningocele Study.
STUDY DESIGN:
Eligible women were randomly assigned to undergo standard postnatal repair or prenatal repair <26 weeks gestation. We evaluated a composite of mental development and motor function outcome at 30 months for all enrolled patients as well as independent ambulation and the Bayley Scales of Infant Development, Second Edition. We assessed whether there was a differential effect of prenatal surgery in subgroups defined by: fetal leg movements, ventricle size, presence of hindbrain herniation, gender, and location of the myelomeningocele lesion. Within the prenatal surgery group only, we evaluated these and other baseline parameters as predictors of 30-month motor and cognitive outcomes. We evaluated whether presence or absence of a shunt at 1 year was associated with 30-month motor outcomes.
RESULTS:
The data for the full cohort of 183 patients corroborate the original findings of Management of Myelomeningocele Study, confirming that prenatal repair improves the primary outcome composite score of mental development and motor function (199.4 ± 80.5 vs 166.7 ± 76.7, P=.004). Prenatal surgery also resulted in improvement in the secondary outcomes of independent ambulation (44.8% vs 23.9%, P = .004), WeeFIM self-care score (20.8 vs 19.0, P = .006), functional level at least 2 better than anatomic level (26.4% vs 11.4%, P = .02), and mean Bayley Scales of Infant Development, Second Edition, psychomotor development index (17.3% vs 15.1%, P = .03), but does not affect cognitive development at 30 months. On subgroup analysis, there was a nominally significant interaction between gender and surgery, with boys demonstrating better improvement in functional level and psychomotor development index. For patients receiving prenatal surgery, the presence of in utero ankle, knee, and hip movement, absence of a sac over the lesion and a myelomeningocele lesion of ≤L3 were significantly associated with independent ambulation. Postnatal motor function showed no correlation with either prenatal ventricular size or postnatal shunt placement.
CONCLUSION:
The full cohort data of 30-month cognitive development and motor function outcomes validate in utero surgical repair as an effective treatment for fetuses with myelomeningocele. Current data suggest that outcomes related to the need for shunting should be counseled separately from the outcomes related to distal neurologic functioning.
Keywords: ankle, knee, and hip movement, fetal surgery, long-term follow-up, Management of Myelomeningocele Study, motor outcomes, myelomeningocele, postnatal motor function, shunt, ventricular size, ventriculomegaly
Introduction
Myelomeningocele (MMC) is a life-altering birth defect resulting from incomplete closure of the neural tube during the fourth week of gestation. The exposed spinal cord sustains intrauterine trauma, leaving children with lifelong paralysis, incontinence, and cognitive disabilities. MMC is a devastating disease for patients and families, not only physically and psychologically, but also financially: MMC health costs are 13 times greater than those of unaffected children.1,2
With the improvement of prenatal diagnostics and prenatal surgical techniques, surgeons began to repair the lesion before birth with the hope of preventing in utero spinal cord trauma. Preliminary studies indicated that prenatal intervention resulted in more desirable outcomes than postnatal repair.3–7
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Management of Myelomeningocele Study (MOMS) compared prenatal closure of the MMC defect with postnatal repair in a multicenter randomized trial. MOMS was stopped for efficacy after recruitment of 183 patients from a planned sample size of 200. The original article reported 30-month neurodevelopmental, self-care, and mobility outcomes from 134 of those patients.8 Initial publication demonstrated that prenatal repair of the MMC defect decreased hindbrain herniation, decreased the need for cerebrospinal fluid (CSF) shunting, and improved distal neurologic function.8 The full cohort data on maternal outcomes and the reduced need for CSF shunting have been previously pub-lished.9,10 Urologic outcomes at 30 months have also since been reported. The primary outcome in the urologic subgroup was the need for clean intermittent catheterization with secondary outcomes focusing on bladder and kidney abnormalities as defined by radiographic and urodynamic testing. Prenatal surgery did not significantly reduce the need for clean intermittent catheterization by 30 months of age but was associated with less bladder trabe-culation, vesicoureteral reflux, and open bladder neck.11 Since publication of MOMS, in utero repair has rapidly changed the treatment paradigm of MMC and prenatal therapy has become a standard of care choice for those mothers who meet the prenatal surgery selection criteria. There is a role for identifying fetuses unlikely to benefit from prenatal intervention to reduce maternal morbidity. Within the prenatal surgery group, we also sought to identify predictors of neurodevelopmental and motor outcomes.
Materials and Methods
MOMS was conducted by established centers at the University of California–San Francisco, Vanderbilt University, and the Children’s Hospital of Philadelphia; an independent data-coordinating center at the George Washington University Biostatistics Center; and the NICHD. The detailed trial design and procedures have been previously published (ClinicalTrials.gov ID NCT00060606).8 Briefly, eligible patients were women carrying a fetus diagnosed with MMC between 19–25 weeks’ gestation. All women received a prerandomization ultrasound and magnetic resonance imaging (MRI) to verify eligibility and record fetal measurements and status. Patients randomized to prenatal surgery underwent hysterotomy and MMC repair, and stayed at the center for monitoring until delivery (Figure). The postnatal surgery patients went home and returned to the center at 37 weeks for delivery and repair of the MMC defect. Children returned to the centers at 12 and 30 months of age for physical and neurological examinations and developmental testing. Patients unable to return to the center received a home visit.
FIGURE: Flow diagram for participants of the Management of Myelomeningocele Study (MOMS).
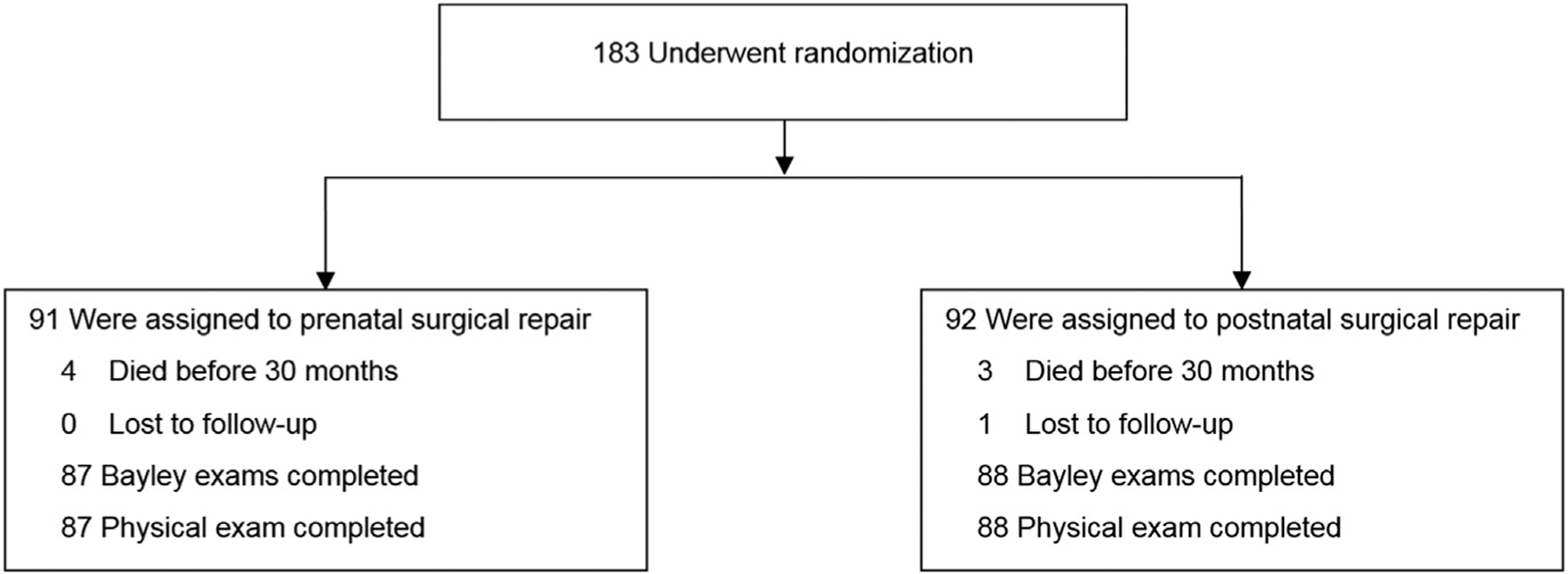
Consolidated Standards of Reporting Trials diagram showing flow of participants for randomized Management of Myelomeningocele Study.
Bayley, Bayley Scales of Infant Development, Second Edition.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
The first primary outcome, death or the need for a CSF shunt, for the full cohort data at 12 months of age was significantly reduced and has been reported previously.8,10 The second primary outcome, reported here, was a rank score derived from the Bayley Scales of Infant Development, Second Edition (BSID II) mental development index (MDI) and the difference between the anatomical and functional levels of the lesion evaluated at 30 months. The anatomic level of the lesion was determined by a panel of independent radiologists. Independent pediatricians blinded to treatment group determined the functional level of the lesion and this was confirmed by independent expert review of video footage. The difference between the functional level and anatomical level in vertebral segments was calculated. The composite score for each infant consisted of the sum of the 2 ranks.
The ultrasounds and MRIs were reviewed locally before randomization and then reviewed centrally by a team of independent expert radiologists. Childhood secondary measures included: walking independently for ≥10 steps, the psychomotor development index (PDI) and the MDI of BSID II, scores on the Peabody Developmental Motor Scales, walking status (no assistance vs orthotics and/or devices), and the degree of disability as measured by the WeeFIM instrument.1
Statistical analysis
In univariable analysis, categorical variables were compared by means of the χ2 test with relative risks and 95% confidence intervals (CI) calculated. Exact methods were used where appropriate. Continuous variables were compared with the Wilcoxon rank-sum test. Analysis was by intent to treat.
Ad hoc subgroup analyses were performed based on: (1) the superior level of the MMC lesion (T1-L2 vs L3-S1); (2) fetal gender; (3) ventricular size (<10, 10-< 15, ≥15 mm); and (4) degree of hindbrain herniation (mild/moderate vs severe) for each of 4 outcomes: (1) independent walking; (2) difference between functional and anatomic levels of the lesion (defined as a functional level ≥2 levels better than expected by the anatomic level); (3) PDI of at least 70; and (4) MDI of at least 70 (both representing no more than 2 SD below the mean). We tested for interaction using the Breslow-Day test.
Within the prenatal surgery group, we evaluated the following characteristics for association with the 4 outcomes above: (1) parameters from the prerandomization ultrasound including amniotic fluid index; biparietal diameter; head circumference; observed hip, ankle, or knee movement during the ultrasound; presence of clubfoot; third ventricle dilation; lesion level; ventricle measurements; and cerebellar measurement below the foramen magnum; and (2) parameters from the prerandomization MRI including degree of hindbrain herniation, structural abnormalities of the brain, degree of cerebellar herniation, and presence of a sac over the lesion. All parameters from the ultrasound were obtained from the local reading with the exception of third ventricle dilation, which was defined as present or absent by the central team of radiologists, who also assessed the MRIs.
Characteristics with data points too infrequent to be analyzed (eg, heterotopias and absence of corpus callosum) are presented in the Appendix.
Characteristics associated with an outcome were modeled with multivariable regression, which included the significant characteristics, to identify which, if any, were independently associated with the outcome after adjustment for the other factors. The four 30-month outcomes were also analyzed for association with presence of a shunt at 1 year in univariable analysis.
For secondary outcomes and interactions, a nominal P value of <.05, without adjustment for multiple comparisons, was considered to indicate statistical significance.
Results
From February 2003 through December 2010, 183 eligible women were recruited and underwent randomization. One child in the postnatal surgery group was lost to follow-up, so that a total of 182 children were included in the 30-month primary outcome, 91 in each surgery group, representing an additional 48 patients since the original report.
Baseline characteristics
For all randomized patients, baseline characteristics shown in Table 1 were similar between the groups, except that in the prenatal surgery group, the lesion level was higher on the spine (P=.02) and there were fewer female fetuses (P = .03).
TABLE 1:
Baseline characteristics
| Prenatal surgery N = 91 | Postnatal surgery N = 92 | |
|---|---|---|
| Fetal sex female | 42 (46.2) | 57 (62.0) |
| Gestational age at randomization, wk | 23.7 ± 1.4 | 23.9 ±1.3 |
| Maternal age at screening, wk | 29.2 ± 5.2 | 28.7 ± 4.8 |
| Race/ethnicity | ||
| White non-Hispanic | 85 (93.4) | 86 (93.5) |
| Black non-Hispanic | 1 (1.1) | 1 (1.1) |
| Hispanic | 3 (3.3) | 4 (4.3) |
| Other | 2 (2.2) | 1 (1.1) |
| Married | 84 (92.3) | 86 (93.5) |
| Schooling, y | 14.9 ± 1.7 | 14.9 ±1.7 |
| Body mass index at screening | 26.3 ± 3.7 | 26.3 ± 3.9 |
| Current smoker | 6 (6.6) | 5 (5.4) |
| Nullipara | 37 (40.7) | 37 (40.2) |
| Previous uterine surgeries, including cesarean | 12(13.2) | 11 (12.0) |
| Cervical length, transvaginal, mm | 39.5 ± 7.6 | 39.4 ± 5.9 |
| Anterior placenta | 43 (47.3) | 39 (42.4) |
| Lesion level, ultrasound | ||
| Thoracic | 4 (4.4) | 3 (3.3) |
| L1-L2 | 25 (27.5) | 13(14.1) |
| L3-L4 | 37 (40.7) | 54 (58.7) |
| L5-S1 | 25 (27.5) | 22 (23.9) |
| Lesion level <L3, ultrasound | 62 (68.1) | 76 (82.6) |
| Clubfoot, ultrasound | 24 (26.4) | 19(20.7) |
| Severe hindbrain herniation | 27 (29.7) | 23 (25.0) |
Data presented as no. of patients (%) or mean ± SD unless otherwise stated.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
30-Month outcomes
The original finding that the composite score (primary outcome) was significantly better in the prenatal surgery group than in the postnatal surgery group was validated in the full cohort (P = .004) (Table 2). There were 3 deaths in each group <30 months reported previously4 (1 fetal, 1 neonatal, and 1 at 28 months in the prenatal surgery group; 2 neonatal and 1 at 14 months in the postnatal surgery group). There was 1 additional death <30 months due to hepatic hemangioma at 13 months in the prenatal surgery group. Overall, there was no difference in mortality rates between the groups (P = .72).
TABLE 2:
Outcomes at 30 months of age
| Prenatal surgery | Postnatal surgery | Relative risk (95% Cl) | Pvalue | |
|---|---|---|---|---|
| Composite primary outcome scorea | 199.4 ±80.5 | 166.6 ±76.7 | .004 | |
| Died <30 mo | 4 (4.4) | 3 (3.3) | 1.34(0.31–5.85) | .72 |
| BSID II mental development index | 89.5 ±15.0 | 86.2 ±18.1 | .22 | |
| Difference between motor function and anatomic levels | −0.80 ± 5.5 | −1.56 ±4.7 | .002 | |
| BSID II mental development index | ||||
| ≥50 Cutoff | 83 (95.4) | 77 (87.5) | 1.09(1.00–1.19) | .06 |
| ≥70 Cutoff | 76 (87.4) | 73 (83.0) | 1.05(0.93–1.19) | .41 |
| ≥85 Cutoff | 65 (74.7) | 55 (62.5) | 1.20(0.98–1.46) | .08 |
| Difference between motor function and anatomic levels | .02 | |||
| ≥2 Levels better | 23 (26.4) | 10(11.4) | ||
| 1 Level better | 10(11.5) | 7 (8.0) | ||
| No difference | 23 (26.4) | 19(21.6) | ||
| 1 Level worse | 17(19.5) | 24 (27.3) | ||
| ≥2 Levels worse | 14(16.1) | 28(31.8) | ||
| BSID II psychomotor development index | ||||
| Mean | 63.9(17.3) | 58.9(15.1) | .03 | |
| ≥50 Cutoff | 41 (47.1) | 31 (35.2) | 1.34(0.93–1.92) | .11 |
| ≥70 Cutoff | 38 (43.7) | 28(31.8) | 1.37(0.93–2.02) | .11 |
| ≥85 Cutoff | 13(14.9) | 6 (6.8) | 2.19(0.87–5.50) | .08 |
| Peabody Developmental Motor Scales | ||||
| Stationary | 7.3 ±1.5 | 6.8 ±1.4 | .05 | |
| Locomotion | 3.0 ±1.8 | 2.1 ±1.5 | .001 | |
| Object manipulation | 4.7 ± 2.5 | 3.8 ± 2.2 | .003 | |
| Walking independently at examination | 39 (44.8) | 21 (23.9) | 1.88(1.21–2.92) | .004 |
| Walking status | .01 | |||
| None | 24 (27.6) | 36 (40.9) | ||
| Walk with orthotics/devices | 24 (27.6) | 31 (35.2) | ||
| Walk without orthotics | 39 (44.8) | 21 (23.9) | ||
| WeeFIM instrument | ||||
| Self-care score | 20.8 (4.4) | 19.0(4.3) | .006 | |
| Mobility score | 19.6(6.5) | 16.2(6.2) | <.001 | |
| Cognitive score | 25.0 (5.7) | 24.9 (6.3) | .74 |
Data presented as no. of patients (%) or mean ± SD unless otherwise stated.
BSID II, Bayley Scales of Infant Development, Second Edition; CI, confidence interval.
Includes deaths.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
Children in the prenatal surgery group were more likely to have a level of function ≥2 levels better than expected according to the anatomical level of the defect (26.4% vs 11.4%) and less likely to have a level of function ≥2 levels worse than expected (16.1% vs 31.8%) (P = .02). Children in the prenatal surgery group were more likely to be able to walk independently (44.8% vs 23.9%, P = .004). The prenatal surgery group also performed better on both the BSIDIIPDI and the gross motor Peabody Developmental Motor Scales (Table 2). Parent-reported mobility and self-care, as measured by the WeeFIM instrument, were significantly better in the prenatal surgery group. There were no significant differences between groups in WeeFIM cognitive scores.
Subgroup analyses
There was a nominally significant interaction between gender and surgery group for 2 outcomes: (1) difference between functional and anatomic levels ≥2 levels better), and (2) BSID II PDI ≥70 (Table 3). The proportion of girls with functional level at least 2 better than anatomic level (30.8% in the prenatal surgery group vs 17.9% in the postnatal surgery group, P = .14) was not statistically significant, whereas among boys, 22.9% in the prenatal surgery group had a functional level at least 2 better than their anatomic level vs none in the postnatal surgery group (P = .003). Similarly, there was no significant difference in proportion of girls with PDI of at least 70 (35.9% in the prenatal surgery groups vs 37.5% in the postnatal surgery group, P = .87), whereas among boys, 50% in the prenatal surgery group had a PDI ≥70 in the prenatal surgery group compared with 21.9% in the postnatal surgery group (P = .01). No other interactions were significant.
TABLE 3:
Subgroup analyses for independent walking, functional level ≥2 levels better than anatomic level, Bayley Scales of Infant Development, Second Edition, psychomotor development index ≥70, mental development index ≥70
| Walking independently | Difference, between functional and anatomic, of ≥2 levels better | BSID II PDI ≥70 | BSID II MDI ≥70 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prenatal surgery, N = 91 | Postnatal surgery, N = 92 | P value | Prenatal surgery, N = 91 | Postnatal surgery, N = 92 | P value | Prenatal surgery, N = 91 | Postnatal surgery, N = 92 | P value | Prenatal surgery, N = 91 | Postnatal surgery, N=92 | P value | |
| Lesion level | .27 | .16 | .11 | .46 | ||||||||
| T1-L2 | 5(19.2) | 0 (0.0) | 10(38.5) | 1 (6.3) | 6(23.1) | 5(31.3) | 22 (84.6) | 14(87.5) | ||||
| L3-S1 | 34 (55.7) | 21 (29.2) | 13(21.3) | 9(12.5) | 32 (52.5) | 23(31.9) | 54 (88.5) | 59(81.9) | ||||
| Gender | .08 | .05 | .04 | .07 | ||||||||
| Female | 15(38.5) | 16(28.6) | 12(30.8) | 10(17.9) | .14a | 14(35.9) | 21 (37.5) | .87a | 34 (87.2) | 51 (91.1) | ||
| Male | 24 (50.0) | 5(15.6) | 11 (22.9) | 0 (0.0) | .003a | 24 (50.0) | 7(21.9) | .01a | 42 (87.5) | 22 (68.8) | ||
| Ventricle size, mm | .97 | .64 | .14 | .99 | ||||||||
| <10 | 16(55.2) | 10(29.4) | 9(31.0) | 3 (8.8) | 18(62.1) | 10(29.4) | 26 (89.7) | 29 (85.3) | ||||
| 10–<15 | 17(42.5) | 10(21.7) | 12(30.0) | 7(15.2) | 14(35.0) | 15(32.6) | 35 (87.5) | 38 (82.6) | ||||
| ≥15 | 6 (33.3) | 1 (12.5) | 2(11.1) | 0 (0.0) | 6 (33.3) | 3 (37.5) | 15(83.3) | 6 (75.0) | ||||
| Hindbrain herniation | .57 | .84 | 1.00 | .34 | ||||||||
| Mild/moderate | 31 (49.2) | 16(24.2) | 15(23.8) | 7(10.6) | 30 (47.6) | 23 (34.9) | 58(92.1) | 56 (84.9) | ||||
| Severe | 8 (33.3) | 5 (22.7) | 8 (33.3) | 3(13.6) | 8 (33.3) | 5 (22.7) | 18(75.0) | 17(77.3) | ||||
Data presented as n (%) unless otherwise specified.
P values from Breslow-Day test for homogeneity of odds ratio.
BSID II, Bayley Scales of Infant Development, Second Edition; MDI, mental development index; PDI psychomotor development index.
From χ2 or Fisher exact as appropriate.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
The prenatal group analysis
Factors associated with walking independently
In univariable analyses of the prenatal surgery group only, detectable ankle, knee, and hip movements by ultrasound at baseline were significantly associated with the ability to walk independently at 30 months. All 39 of those who could walk at 30 months showed hip movement at screening ultrasound and 38 showed knee movement. However, only approximately half of those with fetal hip or knee movement could later walk. Nine prenatal surgery patients showed no hip movement at baseline, and none of those 9 could walk independently at 30 months.
Level of the MMC lesion (≤L3), dilation of the third ventricle, and the absence of a sac over the lesion were statistically significantly correlated with the ability to walk independently (P = .002, P = .02, and P = .02, respectively) (Table 4). Size of the lateral ventricles, clubfoot, severe hindbrain herniation, severe cerebellar herniation, and structural abnormalities were not significantly correlated with future ambulatory potential, nor were other measurements taken during the screening ultrasound: amniotic fluid index, biparietal diameter, and head circumference. In multivariable logistic regression of the prenatal surgery group, knee movement, superior lesion level (≤L3), and absence of a sac over the lesion were associated with walking independently (adjusted odds ratio [OR], 14.30; 95% CI, 1.42–144.35; adjusted OR, 9.45; 95% CI, 2.42–36.84; and adjusted OR, 5.12; 95% CI, 1.39–18.83, respectively). Third ventricular dilation was not significant in multivariate analysis when adjusting for the other factors (adjusted OR, 1.94; 95% CI, 0.61–6.15).
TABLE 4:
Walking independently and difference between motor function and anatomic level of ≥2 by risk factor in prenatal surgery group
| Factors | Walking independently | Not walking independently | RR (95% Cl) | Pvalue |
|---|---|---|---|---|
| Ultrasound parameters | ||||
| AFI, cm | 15.2 ±2.98 | 14.9 ±2.72 | .74 | |
| BPD, mm | 51.9 ±5.01 | 52.7 ± 5.72 | .78 | |
| HC, mm | 198.8 ±16.79 | 204.6 ±18.95 | .23 | |
| Ankle movement | 31/58 (53%) | 27/58 (47%) | 1.99 (1.01–3.91) | .02 |
| No ankle movement | 7/26 (27%) | 19/26 (73%) | ||
| Knee movement | 38/74 (51%) | 36/74 (49%) | 6.16 (0.93–40.77) | .006 |
| No knee movement | 1/12(8%) | 11/12(92%) | ||
| Hip movement | 39/77 (51%) | 38/77 (49%) | N/A | .003 |
| No hip movement | 0/9 (0%) | 9/9(100%) | ||
| Any clubfoot | 8/23 (35%) | 15/23 (65%) | 0.72 (0.39–1.33) | .26 |
| No clubfoot | 31/64 (48%) | 33/64 (52%) | ||
| Third ventricle dilation | 15/23 (65%) | 8/23 (35%) | 1.74 (1.11–2.73) | .02 |
| No third ventricle dilation | 21/56 (37.5%) | 35/56 (62.5%) | ||
| L3-S1 lesion level | 34/61 (56%) | 27/61 (44%) | 2.90 (1.28–6.57) | .002 |
| Lesion level >L3 | 5/26 (19%) | 21/26 (81%) | ||
| Ventricle measurement, mm | 11.2 ±3.77 | 12.2 ±4.20 | .19 | |
| Ventricle measurement ≥15 mm | 6/18(33%) | 12/18 (67%) | 0.70 (0.35–1.40) | .27 |
| Ventricle measurement <15 mm | 33/69 (48%) | 36/69 (52%) | ||
| Ventricle measurement <10 mm | 16/29 (55%) | 13/29 (45%) | 1.39 (0.88–2.20) | .17 |
| Ventricle measurement ≥10 mm | 23/58 (40%) | 35/58 (60%) | ||
| Cerebellar measurement below FM, mm | 17.8 ±2.08 | 18.9 ±2.72 | .25 | |
| MRI parameters | ||||
| Severe hindbrain herniation | 8/24 (33%) | 16/24 (67%) | 0.68 (0.36–1.26) | .18 |
| Mild/moderate hindbrain herniation | 31/63 (49%) | 32/63 (51%) | ||
| Any structural abnormalities, brain | 2/5 (40%) | 3/5 (60%) | 0.90 (0.30–2.71) | 1.00 |
| No structural abnormalities, brain | 36/81 (44%) | 45/81 (56%) | ||
| Severe cerebellar herniation | 13/29 (45%) | 16/29 (55%) | 1.02 (0.62–1.68) | .93 |
| Mild/moderate cerebellar herniation | 25/57 (44%) | 32/57 (56%) | ||
| Sac over lesion | 23/62 (37%) | 39/62 (63%) | 0.58 (0.37–0.90) | .02 |
| No sac over lesion | 16/25 (64%) | 9/25 (36%) | ||
| Difference, between functional and anatomic, of ≥2 levels better | Difference, between functional and anatomic, of <2 levels better | |||
| Ultrasound parameters | ||||
| AFI, cm | 14.2 ±2.85 | 15.3 ±2.78 | .13 | |
| BPD, mm | 51.0 ±4.32 | 52.8 ± 5.71 | .24 | |
| HC, mm | 197.9 ± 13.24 | 203.5 ±19.50 | .18 | |
| Ankle movement | 19/58 (33%) | 39/58 (67%) | 2.13 (0.80–5.64) | .10 |
| No ankle movement | 4/26 (15%) | 22/26 (85%) | ||
| Factors | Walking independently | Not walking independently | RR (95% Cl) | Pvalue |
| Knee movement | 22/74 (30%) | 52/74 (70%) | 3.57 (0.54–24.07) | .17 |
| No knee movement | 1/12(8%) | 11/12(92%) | ||
| Hip movement | 22/77 (29%) | 55/77 (71%) | 2.57 (0.39–16.87) | .43 |
| No hip movement | 1/9(11%) | 8/9 (89%) | ||
| Any clubfoot | 2/23 (9%) | 21/23 (91%) | 0.27 (0.07–1.04) | .02 |
| No clubfoot | 21/64 (33%) | 43/64 (67%) | ||
| Third ventricle dilation | 4/23 (17%) | 19/23 (83%) | 0.65(0.24–1.75) | .37 |
| No third ventricle dilation | 15/56 (27%) | 41/56 (73%) | ||
| L3—S1 lesion level | 13/61 (21%) | 48/61 (79%) | 0.55(0.28–1.10) | .10 |
| Lesion level >L3 | 10/26 (38%) | 16/26 (62%) | ||
| Ventricle measurement, mm | 11.0 ± 3.11 | 12.0 ±4.29 | .34 | |
| Ventricle measurement ≥15 mm | 2/18(11%) | 16/18 (89%) | 0.37(0.09–1.41) | .14 |
| Ventricle measurement <15 mm | 21/69 (30%) | 48/69 (70%) | ||
| Ventricle measurement <10 mm | 9/29 (31%) | 20/29 (69%) | 1.29(0.63–2.61) | .49 |
| Ventricle measurement ≥10 mm | 14/58 (24%) | 44/58 (76%) | ||
| Cerebellar measurement below FM, mm | 17.6 ±2.26 | 18.8 ±2.54 | .20 | |
| MRI parameters | ||||
| Severe hindbrain herniation | 8/24(33%) | 16/24 (67%) | 1.40 (0.68–2.87) | .37 |
| Mild/moderate hindbrain herniation | 15/63 (24%) | 48/63 (76%) | ||
| Any structural abnormalities, brain | 1/5 (20%) | 4/5 (80%) | 0.77(0.13–4.63) | 1.00 |
| No structural abnormalities, brain | 21/81 (26%) | 60/81 (74%) | ||
| Severe cerebellar herniation | 10/29 (34%) | 19/29 (66%) | 1.64 (0.81–3.33) | .18 |
| Mild/moderate cerebellar herniation | 12/57 (21%) | 45/57 (79%) | ||
| Sac over lesion | 11/62 (18%) | 51/62 (82%) | 0.37(0.19–0.72) | .004 |
| No sac over lesion | 12/25 (48%) | 13/25 (52%) | ||
Data presented as no. of patients (%) or mean ± SD unless otherwise stated.
AFI, amniotic fluid index; BPD, biparietal diameter; CI, confidence interval; FM, foramen magnum; HC, head circumference; MRI, magnetic resonance imaging; N/A, not applicable; RR, relative risk.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
Factors associated with motor function ≥2 levels better than anatomic lesion level
Within the prenatal surgery group, absence of clubfoot at screening and absence of sac over the lesion were significantly associated with difference in anatomic and motor levels of ≥2 better than expected (P = .02 and P = .004, respectively) (Table 4). In the multivariable analysis, only absence of a sac was significantly associated with the difference in anatomic and motor levels of ≥2 better than expected (adjusted OR, 3.39; 95% CI, 1.19–9.69). Presence of clubfoot did not remain significant.
Factors associated with the BSID II PDI ≥70
Within the prenatal surgery group third ventricle dilation, lesion level (≤L3) and ventricular measurements were significantly associated with the BSID II PDI ≥70 in univariable analysis (Table 5). In multivariable analysis, dilation of the third ventricle (adjusted OR, 16.72; 95% CI, 3.63–77.02), and atrial ventricular measurement (adjusted OR, 0.80; 95% CI, 0.68–0.93) were significant. Superior lesion level (≤L3) did not remain significant.
TABLE 5:
Bayley Scales of Infant Development, Second Edition, psychomotor and mental development indices ≥70 by risk factor in prenatal surgery group
| Factors | BSID II PDI ≥70 | BSID II PDk <70 | RR (95% Cl) | Pvalue | ||||
|---|---|---|---|---|---|---|---|---|
| Ultrasound parameters | ||||||||
| AFI, cm | 15.3 ±2.83 | 14.9 ±2.84 | .38 | |||||
| BPD, mm | 52.0 ± 5.26 | 52.5 ± 5.55 | .85 | |||||
| HC, mm | 199.6 ±18.61 | 203.8 ±17.74 | .32 | |||||
| Ankle movement | 29/58 (50%) | 29/58 (50%) | 1.44(0.80–2.60) | .19 | ||||
| No ankle movement | 9/26 (35%) | 17/26 (65%) | ||||||
| Knee movement | 34/74 (46%) | 40/74 (54%) | 1.38 (0.60–3.18) | .41 | ||||
| No knee movement | 4/12(33%) | 8/12(67%) | ||||||
| Hip movement | 34/77 (44%) | 43/77 (56%) | 0.99 (0.46 –2.15) | 1.00 | ||||
| No hip movement | 4/9 (44%) | 5/9 (56%) | ||||||
| Any clubfoot | 9/23 (39%) | 14/23 (61%) | 0.86 (0.49–1.54) | .61 | ||||
| No clubfoot | 29/64 (45%) | 35/64 (55%) | ||||||
| Third ventricle dilation | 17/23 (74%) | 6/23 (26%) | 2.30 (1.46–3.61) | <.001 | ||||
| No third ventricle dilation | 18/56 (32%) | 38/56 (68%) | ||||||
| L3-S1 lesion level | 32/61 (52%) | 29/61 (48%) | 2.27 (1.08–4.77) | .01 | ||||
| Lesion level >L3 | 6/26 (23%) | 20/26 (77%) | ||||||
| Ventricle measurement, mm | 10.7 ± 3.94 | 12.5 ±3.95 | .02 | |||||
| Ventricle measurement ≥15 mm | 6/18(33%) | 12/18 (67%) | 0.72 (0.36–1.45) | .32 | ||||
| Ventricle measurement <15 mm | 32/69 (46%) | 37/69 (54%) | ||||||
| Ventricle measurement <10 mm | 18/29 (62%) | 11/29 (38%) | 1.80 (1.14–2.84) | .01 | ||||
| Ventricle measurement ≥10 mm | 20/58 (34%) | 38/58 (66%) | ||||||
| Cerebellar measurement below FM, mm | 18.09 ± 2.23 | 18.7 ±2.70 | .38 | |||||
| MRI parameters | ||||||||
| Severe hindbrain herniation | 8/24 (33%) | 16/24 (67%) | 0.70 (0.38–1.30) | .23 | ||||
| Mild/moderate hindbrain herniation | 30/63 (48%) | 33/63 (52%) | ||||||
| Any structural abnormalities, brain | 2/5 (40%) | 3/5 (60%) | 0.90 (0.30–2.71) | 1.00 | ||||
| No structural abnormalities, brain | 36/81 (44%) | 45/81 (56%) | ||||||
| Severe cerebellar herniation | 12/29 (41%) | 17/29 (59%) | 0.91 (0.54–1.52) | .71 | ||||
| Mild/moderate cerebellar herniation | 26/57 (46%) | 31/57 (54%) | ||||||
| Sac over lesion | 24/62 (39%) | 38/62 (61%) | 0.69 (0.43–1.10) | .14 | ||||
| No sac over lesion | 14/25 (56%) | 11/25 (44%) | ||||||
| BSID II MDI ≥70 | BSID II MDI < 70 | |||||||
| Ultrasound parameters | ||||||||
| AFI, cm | 15.0 ± 2.80 | 15.5 ±3.103 | .59 | |||||
| BPD, mm | 51.8 ± 5.03 | 56.2 ± 6.78 | .06 | |||||
| HC, mm | 200.6 ± 17.19 | 212.7 ±22.37 | .15 | |||||
| Ankle movement | 50/58 (86%) | 8/58 (14%) | 0.90 (0.79–1.02) | .26 | ||||
| No ankle movement | 25/26 (96%) | 1/26 (4%) | ||||||
| Knee movement | 64/74 (86%) | 10/74 (14%) | 0.86 (0.79–0.95) | .34 | ||||
| Factors | BSID II PDI ≥70 | BSID II PDk 70 | RR (95% Cl) | Pvalue | ||||
| No knee movement | 12/12(100%) | 0/12(0%) | ||||||
| Hip movement | 68/77 (88%) | 9/77 (12%) | 0.99(0.78–1.27) | 1.00 | ||||
| No hip movement | 8/9 (89%) | 1/9(11%) | ||||||
| Any clubfoot | 23/23 (100%) | 0/23 (0%) | 1.21 (1.08–1.35) | .03 | ||||
| No clubfoot | 53/64 (83%) | 11/64 (17%) | ||||||
| Third ventricle dilation | 21/23(91%) | 2/23 (9%) | 1.02 (0.88–1.19) | 1.00 | ||||
| No third ventricle dilation | 50/56 (89%) | 6/56 (11%) | ||||||
| L3—S1 lesion level | 54/61 (89%) | 7/61 (11%) | 1.05(0.87–1.26) | .73 | ||||
| Lesion level >L3 | 22/26 (85%) | 4/26 (15%) | ||||||
| Ventricle measurement, mm | 11.5 ±3.70 | 13.1 ± 5.85 | .61 | |||||
| Ventricle measurement ≥15 mm | 15/18 (83%) | 3/18(17%) | 0.94(0.75–1.18) | .69 | ||||
| Ventricle measurement <15 mm | 61/69 (88%) | 8/69 (12%) | ||||||
| Ventricle measurement <10 mm | 26/29 (90%) | 3/29 (10%) | 1.04 (0.89–1.22) | .75 | ||||
| Ventricle measurement ≥10 mm | 50/58 (86%) | 8/58 (14%) | ||||||
| Cerebellar measurement below FM, mm | 18.4 ±2.46 | 18.4 ±3.50 | .92 | |||||
| MRI parameters | ||||||||
| Severe hindbrain herniation | 18/24 (75%) | 6/24 (25%) | 0.81 (0.64– 1.04) | .06 | ||||
| Mild/moderate hindbrain herniation | 58/63 (92%) | 5/63 (8%) | ||||||
| Any structural abnormalities, brain | 5/5 (100%) | 0/5 (0%) | 1.16(1.06–1.26) | 1.00 | ||||
| No structural abnormalities, brain | 70/81 (86%) | 11/81 (14%) | ||||||
| Severe cerebellar herniation | 25/29 (86%) | 4/29 (14%) | 0.98(0.83–1.17) | 1.00 | ||||
| Mild/moderate cerebellar herniation | 50/57 (88%) | 7/57 (12%) | ||||||
| Sac over lesion | 55/62 (89%) | 7/62 (11%) | 1.06 (0.87–1.28) | .72 | ||||
| No sac over lesion | 21/25 (84%) | 4/25 (16%) | ||||||
Ventricular measurements were made via ultrasound study using ventricle on downside, or further from transducer.
AFI, amniotic fluid index; BPD, biparietal diameter; BSID II, Bayley Scales of Infant Development, Second Edition; CI, confidence interval; FM, foramen magnum; HC, head circumference; MDI, mental development index; MRI, magnetic resonance imaging; PDI, psychomotor development index; RR, relative risk.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
Factors associated with the BSID II MDI ≥70
No prenatal factor in the prenatal group (other than clubfoot) was significantly associated with the BSID II MDI ≥70 (P = .03).
Association of ventriculoperitoneal shunt with subsequent outcome at 30 months
Presence of a ventriculoperitoneal (VP) shunt at 1 year was not associated with any of the 4 outcomes: walking independently, difference in anatomic and motor levels of ≥2 better than expected, BSID II PDI ≥70, or BSID II MDI ≥70.
Comment
Main findings
The final results for all the children at 30 months of age whose mothers participated in MOMS validate the original published report. As compared with postnatal repair, prenatal repair of MMC <26 weeks of gestation improved scores on a composite of mental and motor function at 30 months despite having worse prognostic indicators in the prenatal surgery group, higher lesion level, and increased premature delivery (34.1 vs 37.3 weeks).8 The full cohort analysis also confirms that prenatal surgery improved several secondary outcomes, including motor function (as measured by the difference between anatomic level and neuromuscular functional level) and the likelihood of being able to walk independently. As was reported previously, babies in the prenatal surgery group still had an increased rate of preterm delivery compared to babies in the postnatal surgery group, with 11% <30 weeks.11 Despite prematurity, neurodevelopmental outcomes were not less favorable for children who underwent prenatal surgery. Notably, motor function was independent of the need for VP shunting at 30 months.
Meaning/clinical implications
These favorable full cohort 30-month motor and developmental outcomes, despite increased prematurity, in addition to the decreased need for shunting at 1 year reported by Tulipan et al10 (68% vs 98%, P < .001), must be weighed against the risk of increased maternal morbidity. Maternal morbidity was unchanged from the initial report12 and it is important to reemphasize that all mothers who undergo prenatal surgery need to understand that subsequent pregnancies must have cesarean delivery before the onset of labor, to prevent uterine rupture and potential fetal and maternal death.
There are several clinically important observations to help guide future counseling. Boys seem to fare slightly better than girls with respect to functional level and PDI. For fetal surgical patients, hip movement appears necessary for ambulation, but not all patients with hip movement were subsequent ambulators and absence of hip movement was associated with a lack of independent ambulation. Absence of a sac over the lesion on prenatal MRI was also a predictor of independent ambulation.
Neither ventricular size nor degree of hindbrain herniation was associated with future ambulatory potential (Table 3). No structural brain abnormality was reported with enough frequency to be associated with better or worse outcomes. Finally, there was no association between shunt status at 1 year and motor function at 30 months (Table 6). Shunt need and distal motor function should be viewed and counseled as independent outcome goals.
TABLE 6:
Cerebrospinal fluid shunting at 12 months: association with 30-month outcomes in prenatal surgery group
| 30-mo Outcome | Shunt by age 1 y N = 39 | No shunt by age 1 y N = 48 | RR (95% Cl) | P value |
|---|---|---|---|---|
| Walking independently | 15(38%) | 24 (50%) | 0.77 (0.47–1.25) | .28 |
| Not walking independently | 24 (62%) | 24 (50%) | ||
| Difference ≥2 levels better than expected | 10(26%) | 13(27%) | 0.95(0.47–1.92) | .88 |
| Difference <2 levels better than expected | 29 (74%) | 35 (73%) | ||
| BSID II PDI ≥70 | 13(33%) | 25 (52%) | 0.64(0.38–1.08) | .08 |
| BSID II PDI <70 | 26 (67%) | 23 (48%) | ||
| BSID II MDI ≥70 | 32 (82%) | 44 (92%) | 0.90 (0.75–1.06) | .21 |
| BSID II MDI <70 | 7(18%) | 4 (8%) |
Data presented as no. of patients (%) or mean ± SD unless otherwise stated.
BSID II, Bayley Scales of Infant Development, Second Edition; CI, confidence interval; MDI, mental development index; PDI, psychomotor development index; RR, relative risk.
Farmer et al. Management of Myelomeningocele Study. Am J Obstet Gynecol 2018.
Strengths and limitations
The strength of the MOMS is in its role as the only prospective randomized controlled trial with blinded outcome evaluation. This intense level of rigor elevates the results from this trial over any other MMC study to date, and sets the bar for maternal and childhood outcome by which all therapies need to be evaluated, including fetoscopic repair methods.
It was important to examine if there were prenatal markers of which fetuses would be most likely to benefit from the prenatal treatment to guide appropriate counseling. For this reason, secondary analyses were performed to examine potential associations between prenatal physiologic markers and good outcomes. However, the sample size within the prenatal surgery group was not large and limits the ability to detect many associations.
Future research
We look forward to the results of the longer-term follow-up study of these children (MOMS2) at school age to determine whether these results remain durable, and we encourage the fetal therapy community to continue data collection that might inform more precise patient selection.
Introduced in the late 1990s,3 fetoscopic methods were put on hold in the United States during MOMS in an effort to standardize MMC repair procedures. A recent meta-analysis suggests that the primary advantage of a fetoscopic approach would be obviating the need for cesarean delivery, but the ability to prevent the need for VP shunting is reduced.12 Once optimized, a randomized clinical trial should be conducted to investigate the maternal and fetal outcomes associated with open hysterotomy vs fetoscopic repair.
Tandem work by many investigators is focusing on how to increase the percentage of independently ambulating patients. Results from applications of placenta-derived mesenchymal stem cells have shown significant increases in motor function.13,14 Other studies have established that transamniotic stem cell therapy and basic fibroblast growth factor sponges can affect coverage of the MMC defect in rodent models.15–18
Future work should focus on the underlying cause of MMC. A recent analysis of the amniotic fluid RNA transcriptome suggests that specific genes and neural signaling pathways may be abnormally regulated in MMC-affected fetuses.19 Understanding what induces the “first hit” of the neural tube malformation could prevent fetal MMC development, and obviate the need for surgical intervention.
Conclusion
The full cohort data for all 183 patients validate in utero surgical repair as an effective treatment for patients diagnosed with fetal MMC and remains the bar by which all other treatments should be compared. On subgroup analysis, boys nominally demonstrated better improvement with functional level and PDI. For patients receiving prenatal surgery, the presence of in utero ankle, knee, and hip movement; absence of a sac over the lesion; and a MMC lesion of ≤L3 were significantly associated with independent ambulation. The outcomes for VP shunting are not linked to the outcome for distal motor function, so counseling for these 2 aspects of the disorder should be distinct.
Acknowledgment
We again wish to thank the mothers, children, and families, and the fetal therapy community, for making this important study possible, and Bailey Deal for her assistance with manuscript preparation.
Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD041666, U01 HD041665, U10 HD041667, and U10 HD041669) and Clinical and Translational Science Awards, National Institutes of Health (UL1-RR-024134, UL1-RR-024131, and UL1-RR-024975).
Appendix Risk factors for prenatal surgery
Following data were not included as predictors in analyses due to either >40% of data missing or ≤3 responses in any category
| Evaluated | Missing | ||
|---|---|---|---|
| Magnetic resonance imaging | |||
| Brainstem kinking (yes/no) | 51 | 40 | 50% Yes |
| Brainstem hypoplasia (none, mild, moderate, severe, present, not assessed) | 23 | 68 | 61 % None |
| Tectal beaking (yes/no) | 83 | 8 | 99% Yes |
| Corpus callosum present (yes/no) | 90 | 1 | 98% Yes |
| Brain hemorrhages (yes/no) | 90 | 1 | 100% No |
| Nonhemorrhagic brain lesions (periventricular leukomalacia, atrophy, infarct) (yes/no) | 91 | 0 | 99% No |
| Supratentorial subarachnoid space (small, normal, large) | 90 | 1 | 99% Small |
| Syringomyelia (none, mild, severe) | 84 | 7 | 100% None |
| Tethered cord (yes/no) | 89 | 2 | 100% Yes |
| Scoliosis/kyphosis (yes/no) | 91 | 0 | 98% No |
Footnotes
The authors report no conflict of interest.
Partial findings were presented as poster 116 at the 37th Annual Pregnancy Meeting of the Society for Maternal-Fetal Medicine, Las Vegas, NV, Jan. 2328, 2017.
References
- 1.Ouyang L, Grosse SD, Armour BS, Waitzman NJ. Health care expenditures of children and adults with spina bifida in a privately insured US population. Birth Defects Res A Clin Mol Teratol 2007;79:552–8. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control. Economic burden of spina bifida–United States, 19801990. MMWR Morb Mortal Wkly Rep 1989;38: 264–7. [PubMed] [Google Scholar]
- 3.Farmer DL, von Koch CS, Peacock WJ, et al. In-utero repair of myelomeningocele: experimental pathophysiology, initial clinical experience, and outcomes. Arch Surg 2003;138: 872–8. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MP, Sutton LN, Rintoul N, et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol 2003;189: 482–7. [DOI] [PubMed] [Google Scholar]
- 5.Johnson MP, Gerdes M, Rintoul N, et al. Maternal-fetal surgery for myelomeningocele: neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol 2006;194:1145–52. [DOI] [PubMed] [Google Scholar]
- 6.Danzer E, Gerdes M, Bebbington MW, et al. Lower extremity neuromotor function and short-term ambulatory potential following in utero myelomeningocele surgery. Fetal Diagn Ther 2009;25:47–53. [DOI] [PubMed] [Google Scholar]
- 7.Danzer E, Finkel RS, Rintoul NE, et al. Reversal of hindbrain herniation after maternal-fetal surgery for myelomeningocele subsequently impacts on brain stem function. Neuropediatrics 2008;39:359–62. [DOI] [PubMed] [Google Scholar]
- 8.Adzick NS, Thom EA, Spong CY, et al. A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 2011;364:993–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson MP, Bennett KA, Rand L, et al. The Management of Myelomeningocele Study: obstetrical outcomes and risk factors for obstetrical complications following prenatal surgery. Am J Obstet Gynecol 2016;215:778 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tulipan N, Wellons JC III, Thom EA, et al. Prenatal surgery for myelomeningocele and the need for cerebrospinal fluid shunt placement. J Neurosurg Pediatr 2015;16:613–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brock JW III, Carr MC, Adzick NS, et al. Bladder function after fetal surgery for myelomeningocele. Pediatrics 2015;136:e906–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabagambe SK, Jensen GW, Chen YJ, Vanover MA, Farmer DL. Fetal surgery for myelomeningocele: a systematic review and meta-analysis of outcomes in fetoscopic vs open repair. Fetal Diagn Ther 2017. Sep 15. 10.1159/000479505 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 13.Wang A, Brown EG, Lankford L, et al. Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Transl Med 2015;4:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EG, Keller BA, Lankford L, et al. Age does matter: a pilot comparison of placenta-derived stromal cells for in utero repair of myelomeningocele using a lamb model. Fetal Diagn Ther 2016;39:179–85. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe M, Li H, Kim AG, et al. Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of myelomeningocele. Biomaterials 2016;76: 133–43. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe M, Li H, Roybal J, et al. A tissue engineering approach for prenatal closure of myelomeningocele: comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Eng Part A 2011;17:1099–110. [DOI] [PubMed] [Google Scholar]
- 17.Dionigi B, Brazzo JA III, Ahmed A, et al. Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. J Pediatr Surg 2015;50: 1037–41. [DOI] [PubMed] [Google Scholar]
- 18.Feng C D Graham C, Connors JP, Brazzo J III, Zurakowski D, Fauza DO. A comparison between placental and amniotic mesenchymal stem cells for trans-amniotic stem cell therapy (TRASCET) in experimental spina bifida. J Pediatr Surg 2016;51:1010–3. [DOI] [PubMed] [Google Scholar]
- 19.Tarui T, Kim A, Flake A, et al. Amniotic fluid transcriptomics reflects novel disease mechanisms in fetuses with myelomeningocele. Am J Obstet Gynecol 2017;217:587.e1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]


