Abstract
BACKGROUND:
Fine-needle aspiration (FNA) cytology is a common approach to evaluating thyroid nodules, although 20% to 30% of FNAs have indeterminate cytology, which hampers the appropriate management of these patients. Follicular (or oncocytic) neoplasm/suspicious for a follicular (or oncocytic) neoplasm (FN/SFN) is a common indeterminate diagnosis with a cancer risk of approximately 15% to 30%. In this study, the authors tested whether the most complete next-generation sequencing (NGS) panel of genetic markers could significantly improve cancer diagnosis in these nodules.
METHODS:
The evaluation of 143 consecutive FNA samples with a cytologic diagnosis of FN/SFN from patients with known surgical outcomes included 91 retrospective samples and 52 prospective samples. Analyses were performed on a proprietary sequencer using the targeted ThyroSeq v2 NGS panel, which simultaneously tests for point mutations in 13 genes and for 42 types of gene fusions that occur in thyroid cancer. The expression of 8 genes was used to assess the cellular composition of FNA samples.
RESULTS:
In the entire cohort, histologic analysis revealed 104 benign nodules and 39 malignant nodules. The most common point mutations involved the neuroblastoma RAS viral oncogene homolog (NRAS), followed by the Kirsten rat sarcoma viral oncogene homolog (KRAS), the telomerase reverse transcriptase (TERT) gene, and the thyroid-stimulating hormone receptor (TSHR) gene. The identified fusions involved the thyroid adenoma associated (THADA) gene; the peroxisome proliferator-activated receptor γ (PPARG) gene; and the neurotrophic tyrosine kinase, receptor, type 3 (NTRK3) gene. Performance characteristics were similar in the retrospective and prospective groups. Among all FN/SFN nodules, preoperative ThyroSeq v2 performed with 90% sensitivity (95% confidence interval [CI], 80%-99%), 93% specificity (95% CI, 88%-98%), a positive predictive value of 83% (95% CI, 72%-95%), a negative predictive value of 96% (95% CI, 92%-100%), and 92% accuracy (95% CI, 88%-97%).
CONCLUSIONS:
The current results indicate that comprehensive genotyping of thyroid nodules using a broad NGS panel provides a highly accurate diagnosis for nodules with FN/SFN cytology and should facilitate the optimal management of these patients.
Keywords: thyroid cancer, thyroid nodules, genetics, cytology, molecular diagnosis
INTRODUCTION
Thyroid cancer is the fastest growing type of cancer in the United States and in many other countries.1 It typically presents as a thyroid nodule identified accidentally by the patient or by physical examination or imaging studies involving the neck region. However, the vast majority of thyroid nodules are benign. Making an accurate distinction between benign nodules and cancer is important so that patients with cancer receive appropriately definitive treatment and unnecessary treatments, like diagnostic surgery, can be avoided for patients with benign nodules. In those thyroid nodules that have suspicious ultrasound or other worrisome clinical features, the appropriate diagnostic procedure is fine-needle aspiration (FNA) followed by cytologic examination of the collected cells. FNA allows a diagnosis of cancer or benign nodule in most patients, although from 20% to 30% of FNA cytology samples yield 1 of 3 types of indeterminate cytologic diagnoses: atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular or oncocytic (Hurthle cell) neoplasm/suspicious for a follicular or oncocytic (Hurthle cell) neoplasm (FN/SFN), and suspicious for malignant cells (SUSP).2–7 In a meta-analysis of 25,445 thyroid FNA samples reported from 8 studies using The Bethesda System for Reporting Thyroid Cytology, 9.6% of all samples were diagnosed as AUS/FLUS, 10.1% were diagnosed as FN/SFN, and 2.7% were diagnosed as SUSP, with an average cancer risk of 15.9%, 26.1%, and 75.2%, respectively.8 Such cancer risks are not low enough to defer surgical management completely. However, with the possible exception of SUSP cytology, the risks also are not high enough to indicate definitive cancer surgery either. Consequently, most of these patients undergo diagnostic surgery, typically lobectomy, which is unnecessary if the nodule proves to be benign or may not be adequate for some nodules that are identified as cancerous after diagnostic surgery.
For nodules with AUS/FLUS cytology, which have the lowest risk of cancer, several recently introduced molecular tests may be helpful in ruling out a substantial risk of cancer, at least for those populations in which the pretest probability of cancer associated with this cytologic diagnosis is comparable to that reported in other studies.9–12 However, for nodules diagnosed as FN/SFN, none of the currently available molecular tests provide both high sensitivity and high specificity for cancer detection.
Remarkable progress in the understanding of molecular genetics of thyroid cancer has occurred over the last decade, and it has accelerated more recently, leading to the identification of driver mutations in the majority of thyroid cancers.13–17 Moreover, new approaches, such as next-generation sequencing (NGS), allow testing for a large variety of genetic alterations simultaneously using a very small amount of nucleic acids extracted from cells obtained during an FNA procedure. In the current study, we applied the most complete NGS panel of thyroid cancer-related genetic markers (ThyroSeq v2) to cancer diagnosis in thyroid nodules and established the performance of this panel in a large series of nodules with the cytologic diagnosis of FN/SFN.
MATERIALS AND METHODS
Study Cohorts
With the approval of the University of Pittsburgh Institutional Review Board, we studied consecutive thyroid FNA samples with the cytologic diagnosis of FN/SFN from 143 patients who underwent surgery with known surgical pathology outcome and for whom nucleic acids isolated from FNA samples were available for molecular testing. The study consisted of 2 cohorts. The first cohort included 91 patients who had an FN/SFN diagnosis established at the Department of Pathology, University of Pittsburgh Medical Center from December 2012 to September 2013, for whom molecular testing was performed retrospectively. The second cohort included 52 consecutive samples from thyroid nodules with an FN/SFN diagnosis established from October 2013 to May 2014, for which molecular testing was performed prospectively. One additional sample diagnosed as FN/SFN during this time interval had an insufficient amount of isolated nucleic acids and was excluded from the analysis. Researchers who performed molecular tests were not aware of the results of surgical excision for patients from either cohorts. Pathologists who evaluated the surgically removed thyroid nodules were aware of the results of molecular testing using a limited 7-gene panel11 in the retrospective cohort and the ThyroSeq v1 panel18 in the prospective cohort, but they were blinded to the results obtained by the ThyroSeq v2 panel. Histologic slides of surgical samples were reviewed to confirm the diagnosis and to evaluate histopathologic features corresponding to the aspirated nodule.
Sample Collection and Molecular Testing Using the ThyroSeq v2 Assay
At the time of ultrasound-guided FNA procedure, small portions of the first and second passes were collected for molecular analysis as previously described.18 Total nucleic acids were extracted using Compact MagNA Pure (Roche, Basel, Switzerland). Samples were tested for ThyroSeq v2 using targeted NGS on an Ion 318-chip and an Ion Torrent Personal Genome Machine (PGM) sequencer (Life Technologies, Carlsbad, Calif). For each sample, 2 libraries were created to test for 1) point mutations and small indels and 2) gene fusions and gene expression controls used to estimate the proportion of thyroid follicular cells and other relevant cell types in the collected FNA samples.
Detection of point mutations and indels
This was performed using 10 ng of DNA as previously described for ThyroSeq v1.18 In addition to the previously validated ThyroSeq v1 panel, which included mutational hotspots in 12 genes (v-akt murine thymoma viral oncogene homolog 1 [AKT1]; B-Raf proto-oncogene, serine/ threonine kinase [BRAF]; neuroblastoma RAS viral (v-ras) oncogene homolog [NRAS]; Harvey rat sarcoma viral oncogene homolog [HRAS]; Kirsten rat sarcoma viral oncogene homolog [KRAS]; phosphatase and tensin homolog [PTEN]; tumor protein 53 [TP53]; thyroid-stimulating hormone receptor [TSHR]; GNAS complex locus [GNAS]; catenin [cadherin-associated protein], β1, 88 kDa [CTNNB1]; ret proto-oncogene [RET]; and phosphatidylinositol 4,5-bisphosphate 3-kinase, catalytic subunit α [PIK3CA]). Primers for detecting mutations at the cytosine-to-thymine 228 and 250 (C228T and C250T, respectively) hotspots of the telomerase reverse transcriptase (TERT) gene promoter, recently identified in thyroid cancer,19,20 were added to the primer pool. Although the analytic sensitivity of the method was approximately 3% for mutant alleles, the clinical sensitivity for all mutations known as early driver mutations in thyroid carcinogenesis (BRAF, NRAS, HRAS, KRAS, PTEN, TSHR, RET) was set at 10%, ie, only those mutations that had an allelic frequency ≥10% were scored as positive test results. For mutations known to typically develop late in thyroid carcinogenesis and during tumor dedifferentiation (TP53, AKT1, CTNNB1, PIK3CA, and TERT), the clinical sensitivity was set at 5%. Finally, the finding of a GNAS mutation at any allelic frequency was considered a marker of benign nodule, and those samples were scored as negative for mutations.
Detection of gene fusions
Ten nanograms of RNA were used to test for all types of gene fusions reported in thyroid cancer using a ThyroSeq-RNA NGS panel. The panel tests for 38 types of RET fusion genes (B-Raf proto-oncogene, serine/threonine kinase [BRAF]; neurotrophic tyrosine kinase, receptor, type 3 [NTRK1]; NTRK3; ALK; peroxisome proliferator-activated receptor γ [PPARG]; and thyroid adenoma associated [THADA]) to different partners15–17,21,22 by sequencing of the fusion transcripts (Supporting Table 1; see online supporting information). The presence of at least 50 high-quality reads crossing the fusion point of the transcript was required to consider the test positive.
Assessment of cell type composition of FNA samples
Gene expression analysis of 8 genes was used as part of the RNA panel to estimate the quantity of all types of cells (PGK1 gene), thyroid follicular cells (TG, TTF1, NIS, KRT7), C-cells and medullary thyroid carcinoma (CALCA), and parathyroid tissues (PTH) and to assess for additional coverage of nonthyroidal epithelial cells (KRT20) present in the specimen. The expression of thyroid follicular cell markers at levels >10% of all sequencing reads was used to determine that samples were adequate for molecular analysis.
Statistical Analysis
The characteristics of the 2 cohorts were compared by testing for similarity in the true-positive fraction (TPF), false-positive fraction (FPF), positive predictive value (PPV), and negative predictive value (NPV). This method involved computing the ratios of the retrospective cohort to the prospective cohort for each of the 4 statistical parameters and then computing the asymptotic 95% confidence interval (CI) for the ratio using the method described by Simel and colleagues.23 The CIs were computed using the R statistical package v3.1 (available at: http://www.R-project.org/; accessed June 20, 2014).
RESULTS
Surgical Pathology of Nodules in the Study Cohorts
In the retrospective cohort, which comprised 91 patients who had thyroid nodules diagnosed as FN/SFN by cytology, surgical excision of nodules sampled by FNA revealed 66 benign nodules (35 follicular adenomas [FAs], including 13 oncocytic FAs, and 31 hyperplastic nodules [HNs]) and 25 malignant nodules. The latter included 22 papillary thyroid carcinomas (PTCs) (19 follicular variants and 3 classic PTCs) and 3 follicular thyroid carcinomas (FTCs) (2 conventional type FTCs and 1 oncocytic variant FTC). In the prospective cohort of 52 patients who had nodules with FN/SFN cytology, surgical excision revealed 38 benign lesions (22 HNs and 16 FAs, including 8 oncocytic FAs) and 14 malignant nodules, including 11 PTCs (8 follicular variant and 3 classic PTCs) and 3 FTCs (2 oncocytic variants and 1 conventional FTC). The prevalence of cancer was similar in both cohorts (27.5% vs 26.9% for the retrospective vs prospective cohorts, respectively).
Molecular Analysis Using ThyroSeq v2
In the retrospective cohort, FNA samples were all collected before the initiation study and underwent molecular analysis retrospectively. In the prospective cohort, the molecular analysis was carried out prospectively in real-time. Molecular analysis using the ThyroSeq v2 NGS panel revealed multiple point mutations and gene fusions in both cohorts. Overall, among point mutations, the most commonly affected gene was NRAS, which was mutated in 16 nodules (a glutamine-to-arginine substitution at position 61 [Q61R] in 11 nodules, a glutamine-to-lysine substitution at position 61 [Q61K] in 4 nodules, and a glutamine-to-arginine substitution at position 13 [Q13R] in 1 nodule), followed by KRAS (a glycine-to-valine substitution at position 12 [G12V] in 3 nodules, a glycine-to-cysteine substitution at position 12 [G12C] in 1 nodule, and a glycine-to-arginine substitution at position 61 [G61R] in 2 nodules), TERT (C228T in 4 nodules), and TSHR (an isoleucine-to-phenylalanine substitution at position 486 [I486F], an isoleucine-to-leucine substitution at position 630 [I630L], and a phenylalanine-to-leucine substitution at position 631 [F631L]) (Table 1). In 13 samples, 1 point mutation was identified, whereas 3 nodules yielded more than 1 mutation. In 2 samples, a combination of NRAS and TERT mutations was identified; whereas 1 nodule revealed NRAS, TP53, and PIK3CA mutations. In addition, several mutations were detected below the cutoff level; therefore, the FNA samples were considered mutation-negative, including 4 KRAS mutations at an allelic frequency of 4% to 6.3%, 2 NRAS mutations at an allelic frequency of 5.3% to 7%, and 1 TSHR mutation at an allelic frequency of 6%. The identified gene fusions involved THADA in 5 samples, PPARG in 4 samples, and NTRK3 in 2 samples. No overlap between gene fusions and point mutations was observed, and no multiple gene fusions were identified in any samples.
TABLE 1.
Molecular Alterations Detected in Fine-Needle Aspiration Samples and Associated Cancer Risk
| No. of Samples |
||||
|---|---|---|---|---|
| Alteration | Positive | Unique Diagnostic Events | Cancer Identified at Surgery (Cancer Risk, %) | Negative Because of Low Level |
| Point mutations | ||||
| NRAS | 16 | 13 | 13 (81) | 2 |
| KRAS | 6 | 6 | 5 (83) | 4 |
| HRAS | 2 | 2 | 2 (100) | 0 |
| TERT | 4 | 2 | 4 (100) | 0 |
| TSHR | 3 | 3 | 1 (33) | 1 |
| BRAF V600E | 1 | 1 | 1 (100) | 0 |
| BRAF K601E | 1 | 1 | 0 (0) | 0 |
| TP53 | 1 | 0 | 1 (100) | 0 |
| PIK3CA | 1 | 0 | 1 (100) | 0 |
| Gene fusions | ||||
| THADA | 5 | 5 | 5 (100) | 0 |
| PPARG | 4 | 4 | 4 (100) | 0 |
| NTRK3 | 2 | 2 | 2 (100) | 0 |
Abbreviations: BRAF, B-Raf proto-oncogene, serine/threonine kinase; FNAs, fine-needle aspiration samples; HRAS, Harvey rat sarcoma viral oncogene homolog; K601E, lysine to glutamic acid substitution at position 601 in BRAF; KRAS, Kirsten rat sarcoma viral oncogene homolog; NRAS, neuroblastoma RAS viral (v-ras) oncogene homolog; NTRK3, neurotrophic tyrosine kinase, receptor, type 3; PIK3CA, phosphatidylinositol4,5-bisphosphate 3-kinase, catalytic subunit α; PPARG, peroxisome proliferator-activated receptor γ; TERT, telomerase reverse transcriptase; THADA, thyroid adenoma associated; TP53, tumor protein 53; TSHR, thyroid-stimulating hormone receptor; V600E, valine to glutamic acid substitution at position 600 in BRAF.
Correlation With Surgical Follow-Up
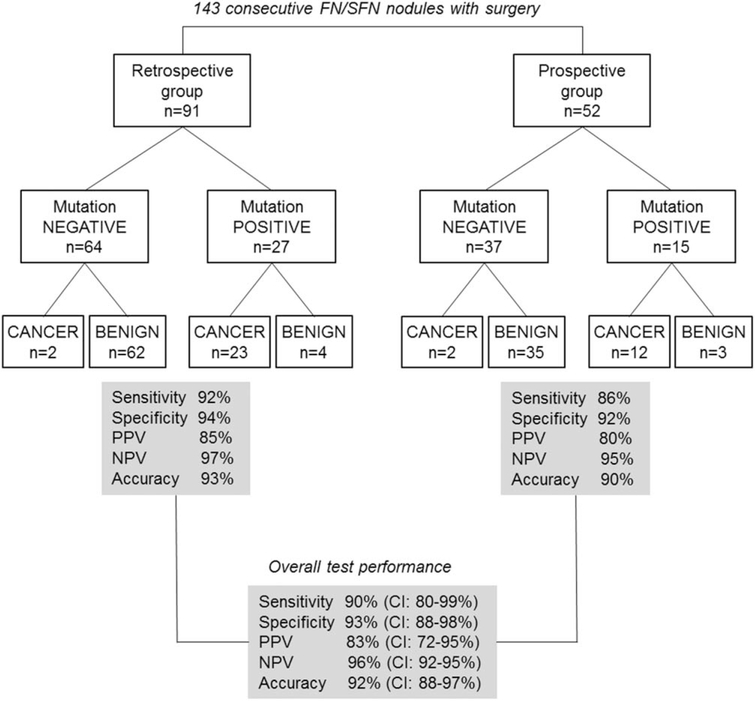
In the retrospective cohort, among 64 aspirates that were negative by molecular analysis, 62 nodules were identified as histologically benign and 2 were identified as malignant after surgery. Two cancers that lacked molecular alterations were identified as a papillary carcinoma, encapsulated follicular variant and a follicular carcinoma, oncocytic variant, minimally invasive. Among 62 benign nodules, there were 30 HNs, 21 conventional FAs, and 11 oncocytic FAs. Among 27 mutation-positive samples, there were 23 cancers, including 20 PTCs (18 follicular variants and 2 classic PTCs) and 3 FTCs (2 oncocytic, 1 conventional type); whereas 4 mutation-positive samples were benign on histology. The latter nodules, which were diagnosed histologically as FA, oncocytic FA, or hyperplastic nodule (n=2), harbored NRAS (n=2), KRAS, and TSHR mutations. The performance of ThyroSeq v2 in the retrospective cohort demonstrated 92% sensitivity, 94% specificity, a 97% NPV, and an 85% PPV (Fig. 1).
Figure 1.
This is a schematic representation of the study cohorts, test results, and overall performance of the targeted next-generation sequencing panel of thyroid cancer-related genetic markers (ThyroSeq v2). FN/FSN indicates follicular (or oncocytic) neoplasm/suspicious for a follicular (or oncocytic) neoplasm; PPV, positive predictive value; NPV, negative predictive value; CI, confidence interval.
In the prospective cohort, among 37 aspirates that were negative by molecular analysis, 35 nodules were identified as benign and 2 were identified as malignant after surgery. Two cancers that lacked molecular alterations in our panel were both papillary carcinomas (follicular variant and classic PTC; both intrathyroidal). Among 35 benign nodules, there were 21 HNs, 7 conventional FAs, and 7 oncocytic FAs. Among 15 mutation-positive FNA samples, there were 12 cancers, including 9 PTCs (8 follicular variants and 1 classic PTC) and 3 FTCs (2 conventional type, 1 oncocytic type). The 3 nodules that were benign on histology (an FA, an oncocytic FA, and a hyperplastic nodule) were positive for the BRAF K601E (lysine-to-glutamic acid substitution at position 601), TSHR, and NRAS mutations, respectively. In the prospective cohort, the performance of the test revealed 86% sensitivity, 92% specificity, a 95% NPV, and an 80% PPV.
The performance characteristics, including TPF, FPF, PPV, and NPV, were tested for homogeneity between the 2 study cohorts and were similar. The analysis indicated that there were no differences in operating characteristics between the 2 cohorts; therefore, they could be combined to assess the test performance. In the combined cohort of 143 patients, the test demonstrated 90% sensitivity (95% CI, 80%-99%), 93% specificity (95% CI, 88%-98%), a PPV of 83% (95% CI, 72%-95%), an NPV of 96% (95% CI, 92%-100%), and 92% accuracy (95% CI, 88%-97%).
Expected ThyroSeq v2 Performance in Populations With Different Disease Prevalence
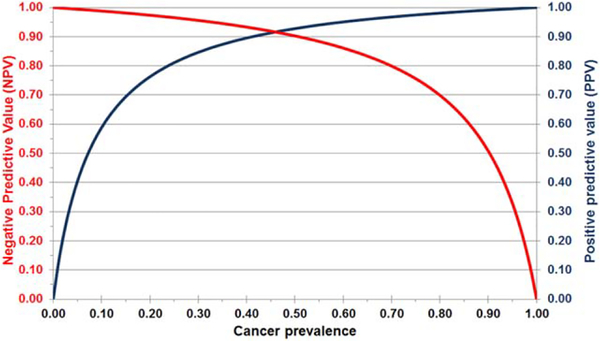
Although the sensitivity and specificity of any diagnostic test depends only on test performance, the NPV and PPV depend on the prevalence of disease in the tested population. Therefore, variation in the cancer rate in thyroid samples with FN/SFN cytology at different institutions may significantly affect the PPV and NPV of any diagnostic test.24 Indeed, the risk of malignancy in nodules with FN/SFN cytology ranged from 14% to 34% in 8 studies summarized by Bongiovanni et al.8 To estimate the performance of ThyroSeq v2 in patients with FN/SFN nodules in populations with different pretest cancer probability, we calculated the expected PPV and NPV of the test using the Bayes theorem based on the sensitivity and specificity determined in the current study (Fig. 2). The analysis demonstrated that, with a cancer probability ranging between 14% and 34%, the NPV of the test would range between 98% and 95%, and the PPV would range between 68% and 87%.
Figure 2.
Expected negative and positive predictive values for the targeted next-generation sequencing panel (ThyroSeq v2) are illustrated in relation to cancer prevalence based on the specificity and sensitivity of the test detected in the current study.
DISCUSSION
In this study, we validated the performance of a novel genetic test based on a comprehensive panel of point mutations and gene fusions occurring in thyroid cancer in a large series of thyroid nodules with FN/SFN cytology and demonstrated that it allows accurate cancer risk assessment in these nodules, opening the possibility for improved management of these patients. On the basis of The Bethesda System, the cytologic diagnosis of FN/SFN is established in those aspirates that: 1) have follicular cells arranged in an architectural pattern characterized by cell crowding and/or microfollicle formation and lacking nuclear features of papillary carcinoma, or 2) are comprised almost exclusively of oncocytic (Hurthle) cells.3 Such cytologic patterns are observed in follicular and oncocytic carcinomas and in the follicular variant of papillary carcinoma, but they also are common in FAs and cellular hyperplastic nodules. Because such benign lesions are common, they determine a high false-positive rate on FN/ SFN cytology, because only approximately 25% of nodules (range, 14%-34% of nodules) with FN/SFN cytology are identified as malignant after surgery.8 Typically, the accepted management of patients who have nodules that fall in this cancer risk category is diagnostic lobectomy, which could be avoided for nodules that are identified as benign after surgery.
Currently available ancillary molecular tests improve either the PPV or the NPV for FN/SFN nodules, but not both at the same time. The gene expression classifier (GEC) test, commercially known as Afirma (Veracyte, South San Francisco, Calif), offers a high NPV, but its PPV is as low as 15% to 37%.12,24 Therefore, the GEC test does not prevent surgery (ie, diagnostic lobectomy) in the majority of patients with this cytologic diagnosis who are classified as GEC suspicious but ultimately have benign histology. In contrast, testing for a 7-oncogene panel offers a high PPV but a low NPV,9–11 which helps to select patients with a higher cancer risk for the appropriate therapeutic surgery (ie, total thyroidectomy) but does not prevent diagnostic surgeries for most patients with benign nodules. Although it is highly specific for thyroid cancer, the limited NPV of the 7-gene panel is expected, because from 30% to 35% of all thyroid cancers do not harbor 1 of these 7 genes.
In the current study, we took advantage of more recent discoveries of point mutations and gene fusions in different types of thyroid cancers and of new technology that allows simultaneous testing for a large variety of mutations in FNA samples, expanding the diagnostic panel to include approximately 60 genetic markers and increasing the sensitivity of cancer detection to 90%. A dramatic improvement in sensitivity led to a similar effect on the NPV, which was 96% in this study. It is noteworthy that our study also was based on the largest series of FN/SFN nodules with molecular testing reported to date, and the cancer prevalence in the series was 27%, which is similar to the average cancer rate reported in many studies of nodules with FN/SFN cytology.8 Moreover, even in a broad range of pretest cancer probability reported in FN/ SFN nodules (ie, 14%-34%), the NPV of this test would not drop below 95%.
Furthermore, the 4 cancers that were missed because of the lack of any mutations included 2 encapsulated follicular variant PTCs, 1 classic PTC, and 1 minimally invasive oncocytic FTC, all of which were intrathyroidal tumors with no histologic features of aggressive behavior at presentation. That no aggressive tumors were missed in this study may be because many of the more aggressive thyroid cancers are expected to be positive for TERT mutations,19,20,25 BRAF mutations,26,27 or to carry multiple mutations18,28 and, thus, can be detected by this broad panel of genetic markers, as we observed in 3 tumors with multiple mutations. On the basis of the high NPV and missing a few low-grade cancers, it is reasonable to propose that patients who have thyroid nodules with FN/SFN cytology and no mutations identified using this broad panel of molecular markers can be followed with active surveillance, ie, without surgery. Possible exceptions would include high-risk ultrasonographic features of the nodule or other significant risk factors, such as prior irradiation, a strong family history of thyroid cancer, or a clinical setting with an unusually high pretest probability of cancer. Indeed, for those practices in which the cancer prevalence of nodules with FN/SFN cytology is very high (ie, approaching 50%), the achieved NPV of this test will drop to 90%. In those situations, the NPV of the test may not be sufficiently robust to avoid diagnostic surgery.
Despite a significant increase in NPV because of adding a large number of additional genetic markers, the PPV of ThyroSeq v2 did not decrease substantially from the 86% reported for the prior 7-oncogene panel.11 The overall PPV of ThyroSeq was 83%, and the risk of cancer was different in specific mutation groups. A finding of TERT, HRAS, BRAF V600E, TP53, and PIK3CA mutations and any gene fusion conferred a 100% risk of cancer in these series. The risk of cancer in the nodules with NRAS or KRAS mutations was 81% to 83%, similar to previously reported rates.9–11,29 Finally, only 1 of 3 TSHR mutation-positive nodules was identified as cancer after surgery; therefore, additional features are needed for more accurate cancer prediction in nodules that are positive for TSHR mutations. The knowledge of cancer risk associated with specific mutations, together with prognostic associations conferred by TERT and other mutations, should help in choosing the appropriate surgical approach for these patients, which, in many cases, would be a therapeutic thyroidectomy.
It is noteworthy that several mutations in these nodules were identified at low levels. NGS provides quantification of mutations and, with sufficient coverage, offers very high analytic sensitivity for mutation detection. In this study, several mutations in the KRAS, NRAS, and TSHR genes were identified at a low levels, ie, <10% of alleles, corresponding to <20% of cells that carried these mutations. This suggests the presence of a subclone within the larger nodule, which may or may not expand if not surgically resected. It is noteworthy that none of the nodules that carried these mutations at low levels were identified as malignant at surgery, which points to the importance of clinical validation of NGS tests and setting up appropriate cutoff levels for clinical sensitivity of mutation detection based on the results of validation. However, it also raises a possibility that patients with low-level mutations may need closer follow-up with repeat FNA and molecular testing to monitor for the expansion of the mutated clone within the nodule. In summary, in this study, we report validation of a molecular test that allows highly accurate stratification of thyroid nodules with FN/ SFN cytology into benign and malignant, effectively resolving the diagnostic uncertainty that has plagued modern thyroid nodule management to date and offering more optimal management for these patients.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work was supported in part by funds from the University of Pittsburgh Cancer Institute and the University of Pittsburgh Medical Center, by the Richard A. and Leslie A. Snow Fund for Thyroid Cancer Research, and by a generous gift from Drs. David and Nancy Brent.
We are grateful to Drs. Daniel Kuriloff (Lenox Hill Hospital) and Madeline Vazquez, CBLPath, for providing follow-up information on their patient, who was included in this study.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Nikiforov reports consulting fees from Quest Diagnostics. Dr. Ferris serves without compensation on the American Thyroid Association Surgical Affairs Subcommittee.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Ali SZ, Cibas ES. The Bethesda System for Reporting Thyroid Cytopathology. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 3.Baloch ZW, LiVolsi VA, Asa SL, et al. Diagnostic terminology and morphologic criteria for cytologic diagnosis of thyroid lesions: a synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference. Diagn Cytopathol. 2008;36: 425–437. [DOI] [PubMed] [Google Scholar]
- 4.Gharib H. Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract. 2004;10:31–39. [DOI] [PubMed] [Google Scholar]
- 5.Sclabas GM, Staerkel GA, Shapiro SE, et al. Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg. 2003;86:702–709; discussion 709–710. [DOI] [PubMed] [Google Scholar]
- 6.Yassa L, Cibas ES, Benson CB, et al. Long-term assessment of a multidisciplinary approach to thyroid nodule diagnostic evaluation. Cancer. 2007;111:508–516. [DOI] [PubMed] [Google Scholar]
- 7.Lewis CM, Chang KP, Pitman M, Faquin WC, Randolph GW. Thyroid fine-needle aspiration biopsy: variability in reporting. Thyroid. 2009;19:717–723. [DOI] [PubMed] [Google Scholar]
- 8.Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: a meta-analysis. Acta Cytol. 2012;56:333–339. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. [DOI] [PubMed] [Google Scholar]
- 10.Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. [DOI] [PubMed] [Google Scholar]
- 11.Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. [DOI] [PubMed] [Google Scholar]
- 13.Nikiforov YE, Nikiforova MN. Molecular genetics and diagnosis of thyroid cancer. Nat Rev Endocrinol. 2011;7:569–580. [DOI] [PubMed] [Google Scholar]
- 14.Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006;6:292–306. [DOI] [PubMed] [Google Scholar]
- 15.Ricarte-Filho JC, Li S, Garcia-Rendueles ME, et al. Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest. 2013;123:4935–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leeman-Neill RJ, Kelly LM, Liu P, et al. ETV6-NTRK3 is a common chromosomal rearrangement in radiation-associated thyroid cancer. Cancer. 2014;120:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelly LM, Barila G, Liu P, et al. Identification of the transforming STRN-ALK fusion as a potential therapeutic target in the aggressive forms of thyroid cancer. Proc Natl Acad Sci U S A. 2014;111:4233–4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE. Targeted next-generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab. 2013;98: E1852–E1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Landa I, Ganly I, Chan TA, et al. Frequent somatic TERT promoter mutations in thyroid cancer: higher prevalence in advanced forms of the disease. J Clin Endocrinol Metab. 2013;98:E1562–E1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Bishop J, Shan Y, et al. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer. 2013; 20:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ciampi R, Knauf JA, Kerler R, et al. Oncogenic AKAP9-BRAF fusion is a novel mechanism of MAPK pathway activation in thyroid cancer. J Clin Invest. 2005;115:94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloth L, Belge G, Burchardt K, et al. Decrease in thyroid adenoma associated (THADA) expression is a marker of dedifferentiation of thyroid tissue [serial online]. BMC Clin Pathol. 2011;11:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simel DL, Samsa GP, Matchar DB. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol. 1991;44:763–770. [DOI] [PubMed] [Google Scholar]
- 24.McIver B, Castro MR, Morris JC, et al. An independent study of a gene expression classifier (Afirma) in the evaluation of cytologically indeterminate thyroid nodules [published online ahead of print April 29, 2014]. J Clin Endocrinol Metab. [DOI] [PubMed] [Google Scholar]
- 25.Melo M, da Rocha AG, Vinagre J, et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J Clin Endocrinol Metab. 2014;99:E754–E765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing M, Alzahrani AS, Carson KA, et al. Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA. 2013;309:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basolo F, Torregrossa L, Giannini R, et al. Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab. 2010;95:4197–4205. [DOI] [PubMed] [Google Scholar]
- 28.Xing M, Liu R, Liu X, et al. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence [published online ahead of print July 14, 2014]. J Clin Oncol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta N, Dasyam AK, Carty SE, et al. RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab. 2013;98:E914–E922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.