Summary
Background:
Relapsed/refractory (R/R) Hodgkin lymphoma (HL) remains a significant clinical challenge; immune microenvironment promotes tumor growth. Recognizing that the immune suppressive microenvironment promotes tumor growth, we hypothesized that activating immunity might augment the efficacy of targeted chemotherapy and evaluated the safety and activity of combinations of brentuximab (BV), nivolumab (Nivo), and ipilimumab (Ipi) in patients with R/R HL.
Methods:
R/R HL patients ≥18 who had relapsed after ≥1 line of therapy, with an ECOG performance (PS) status ≤2, and adequate organ and marrow function, with no pulmonary dysfunction were eligible. Phase I primary objectives were to determine the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of BV combined with Nivo, Ipi, or Nivo and Ipi using 3+3 design, with expansion cohorts. Patients were enrolled sequentially into cohorts of BV 1.8mg/kg with Ipi 1mg/kg and 3mg/kg (cohorts A-C), followed by BV 1.2mg/kg and 1.8mg/kg combined with Nivo 3mg/kg, (cohorts D-F), and BV 1.2mg/kg and 1.8mg/kg with Nivo 3.mg/kg, and Ipi 1mg/kg (cohorts G-I). All drugs were given IV; BV and Nivo were given every 3 weeks, Ipi was given every 6 weeks in A-C and every 12 weeks in G-I. The primary endpoint was toxicity, all eligible and treated patients were included in the analysis. The phase I/II study is registered with ClinicalTrials.gov (NCT01896999). The phase I results are reported here; the Phase 2 randomized portion of the trial is still enrolling.
Findings:
Sixty-four patients were enrolled during 3/7/2014–12/28/2017; 61 were evaluable. Twenty-five patients (40%) had prior hematopoietic cell transplantation (HCT), and 35 (57%) were refractory to most recent therapy. A total of 6 DLTs were reported on 4 patients. There were two (3%) grade 5 adverse events (AEs); there were no additional treatment related deaths. There were 10 (43%), 3 (16%) and 11 (50%) cases reported grade 3–4 treatment related AEs including: rash 13% (8/64), and colitis, gastritis, pancreatitis and arthritis, and diabetic ketoacidosis each occurring in 1 patient (2%). The overall response rate (ORR) was 76% (95% CI: 53–92%), 89% (95% CI: 65–99%) and 82% (95% CI:60–95%), and the complete response (CR) rate was 57% (95% CI: 34–78%), 61% (95% CI: 36–83%) and 73% (95% CI: 50–89%). With a median follow-up of 2.6 (IQR:1.8–2.9), 2.4(IQR:2.2–2.6), and 1.7 (IQR:1.6–1.9) years, the median PFS is 1.2 (95% CI: 1.7-NA) years for BV-Ipi and not reached for BV-Nivo and BV-Nivo-Ipi. The median OS has not been reached.
Interpretation:
While each of the combinations has been safety evaluated and appear highly active there are clear differences in activity and toxicity. The tolerability and activity for the two most active regimens BV-Nivo and BV-Nivo-Ipi are being compared in the ongoing randomized phase 2 component of the study If the durable responses seen here are confirmed, the more effective regimen may be selected for further study either in second line either as a bridge to HCT or compared to HCT for selected patients.
Keywords: Ipilimumab, Nivolumab, Brentuximab in Relapsed Hodgkins
Introduction
Classical Hodgkin lymphoma (cHL) remains the most common lymphoma of adolescents and young adults (7), with an estimated 8,500 new cases in the US per year (8). Although up to 80% of cHL patients are cured with first line therapy, treatment for patients with relapsed and refractory (R/R) cHL remains challenging; approximately 1,200 patients in the US succumb to this disease annually (9).
The cHL tumor microenvironment (TME) is composed of a mixture of immune cells and stroma, (10); the primary lymphoma tumor cells, the Hodgkin Reed Sternberg (HRS) cells comprise less than 1% of the total tumor volume, and are dependent upon pro-survival signals from the TME for growth and survival (11–15). Somatically acquired alterations of chromosome 9p24.1/CD274(PD-L1)/PDCD1LG2(PD-L2), leads to HRS cell overexpression of programmed cell death ligands 1 and 2 (PD-L1 and PD-L2), which facilitates HRS cell evasion of immune surveillance and survival within the TME (16–18). Chromosomal rearrangements of CIITA, the master regulator of MHC class II expression, are found in approximately 15% of cHL, resulting in downregulated MHC class II expression and over-expression of fusion partners such as PD-L1 and PD-L2 (19). Recent characterization of the HL TME using customized time of flight mass cytometry (CYTOF) revealed high numbers of CD4+ cells, with expansion of regulatory T cells (20). Overall, T cell exhaustion and deficient anti-tumor immunity play a key role in propagating a permissive milieu for cHL growth.
For patients with R/R cHL, tumor targeting therapies alone are inadequate to induce a high complete remission (CR) rate. Considering that the TME represents a significant contributor to cHL biology, this study used a novel approach of potentiating the peri-tumoral T cells with the checkpoint inhibitors ipilimumab (Ipi) and nivolumab (Nivo) and targeting the HRS cells with the CD30 specific antibody drug conjugate (ADC) brentuximab vedotin (BV). Both Nivo and BV are FDA approved for R/R cHL and have significant single agent activity but lower CR rates (21–23), and there is clear synergy between Ipi and Nivo in multiple malignancies (24). We hypothesized that a therapeutic strategy, which depleted CD30+ expressing HRS cells and activated T effector cells could target HRS cell killing and overcome therapeutic resistance. The goal for this Phase 1 study was to confirm safety and to obtain a preliminary efficacy signal that would support Phase 2 development.
Methods
Study Design and Participants
This was a CTEP sponsored multicenter, ECOG-ACRIN phase I/II study consisting of 3 treatment regimens: BV-Ipi, BV-Nivo and BV-Nivo-Ipi for patients with R/R classical HL. Thirteen US centers participated in the trial and accrued patients (Appendix page 1).. Patients were enrolled sequentially to the three treatment combinations in Phase I. At the of initial study design in 2013, there was little known use of checkpoint blockade in lymphoma, and the only immune agent available through CTEP was Ipi; Nivo was added to the CTEP portfolio later, and the trial was amended to include the two additional Nivo containing arms.
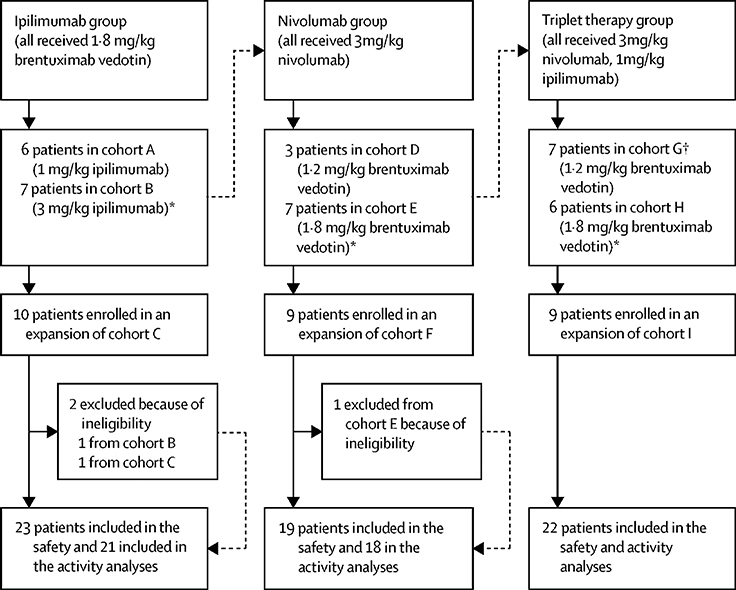
A modified 3 × 3 design (6+6) for BV-Ipi, and a standard 3+3 design for BV-Nivo and BV-Nivo-Ipi were used for dose escalation. Two dose levels plus one de-escalation level were planned (Figure 1). A minimum of 18 patients and a maximum of 36 patients were treated in the escalation portion of the study. Once the MTD was reached, 9 additional patients were planned for an expansion cohort (for a total of 15 patients on the MTD level) to better characterize the safety of the treatment combination. Therefore, a minimum of 18 and a maximum of 63 patients were planned for the Phase I study. Allowing for 10% ineligibility and path exclusion rate, total accrual goal was 70 patients.
Figure 1:
Schema of Phase 1 Trial Design. Cycle Length: 21 days. Treatment Schedule: Day 1 of every cycle BV (for up to 1 year) and Nivo (for up to 2 years), Ipi given on day 1 of cycle 1 and thereafter every 6 weeks for up to 1 year for Arms A-C, and every 12 weeks for up to 2 years for Arms G-I* (*2 patients received Ipi q 6 in Arms G-I). Premedication: Prior to treatment with BV and Ipi patients received prophylactic Pepcid 40mg IV and Benadryl 50mg at every cycle. For patients in cycle 5 and beyond who had had no infusion reactions the Benadryl dose could be lowered to 25mg or omitted per investigator discretion. Acetaminophen 650mg was included as a premedication per investigator discretion. Premedication was not required when patients receive Nivo alone.
DLT was evaluated within first cycle and defined as any grade 3 or 4 hematologic or non-hematological toxicity that was possibly, probably or definitely attributable to therapy, with exceptions including grade 3 nausea, vomiting, fever, diarrhea or mucositis of < 3 days, and cytopenias < 7 days. Response was evaluated after 4 cycles of therapy (12 weeks) and thereafter every 3 months. Responses were investigator assessed in accordance with the International Harmonization Project Group 2007 Revised Response Criteria according to Cheson and Deauville criteria.
Eligible patients were 18 years of age or older with no upper limit for age, had pathologically confirmed R/R HL, ECOG performance status of ≤2, FEV1/FVC ≥ 60%, DLCO >50%, and adequate bone marrow and organ function based on complete blood count and complete metabolic panel. Patients were excluded if they had prior treatment with checkpoint inhibitor, active autoimmune disease, ongoing infections, or significant compromise of organ function. Post Allo HCT patients could not have evidence of active GVHD, or ongoing treatment with immunosuppression. Prior BV was allowed as long as patients had not been treated with BV or progressed within 6 months. The institutional review boards of all participating centers approved the study, which was performed according to the Declaration of Helsinki and International Harmonization Guidelines for Good Practice. All enrolled patients provided written informed consent.
Procedures
All cycles were 21 days, and BV was given on day 1 in all arms. All drugs were given IV. Patients in Arms D-I received Nivo on day 1 of every cycle. Ipi was given on day 1 of cycle 1 and thereafter every 6 weeks for up to 1 year for Arms A-C, and every 12 weeks for up to 2 years for Arms G-I* (*2 patients received Ipi q 6 in Arms G-I). Toxicity and laboratory evaluations (CBC, CMP, ESR) were assessed every week during cycle 1 and at every cycle afterwards, highest grade of each type of toxicity across the entire treatment period was reported. The maximum duration of BV was 1 year (16 doses), and for Nivo 2 years (34 doses), Ipi 1 year in Arms A-C (7 doses) and 2 years (9 doses) in Arms G-I. Patients were removed from study due to patient preference, progression of disease, toxicity, or non-compliance. Dose reductions and interruptions of BV were allowed. Dose interruptions were allowed for Nivo and Ipi, but no dose reductions of either drug were allowed. Imaging assessment (PET/CT and/or CT) was performed at study entry, after cycle 4, and thereafter at 3 month intervals for patients receiving treament. Further details of the study design are provided in the clinical protocol included in Data Supplement (Appendix 1, pages 41–138). Nivo and Ipi were provided by CTEP under a cooperative research and development agreement with Bristol-Myers-Squibb (BMS).
Outcomes
The Phase I primary objectives were to determine the maximum tolerated dose (MTD) and dose limiting toxicities (DLT) of each combination. MTD in 3+3 design is defined as the highest dose level with <2 DLTs observed out of 6 treated patients. The secondary objectives were to evaluate preliminary efficacy including complete response (CR) rate, overall response rate (ORR), duration of response (DOR), progression-free survival (PFS) and overall survival (OS), and to characterize toxicities in patients post HCT. PFS evaluated by imaging (PET/CT and/or CT) and/or clinical assessment was defined as the time from registration to progression or death whichever occurred first and censored at last assessment documenting progression free for patients who continued in remission. OS was defined as the time from registration to death and censored at last contact date for those that remained alive. DOR was defined as the time response first observed to the time of relapse and was measured in responders.
Statistical Analysis
The phase I/II study is registered with ClinicalTrials.gov (NCT01896999). The study was initially designed as a single arm Phase I of BV-Ipi, and was amended to include the 2 Nivo containing combinations in phase I, and later was amended to add a randomized phase 2 comparison of BV-Nivo and BV-Nivo-Ipi. The phase I results are presented here, the phase 2 randomized trial is still enrolling.
A minimum of 18 patients and a maximum of 36 patients were treated in the escalation portion of the study. Once the MTD was reached, 9 additional patients were treated in an expansion cohort (for a total of 15 patients on the MTD level) to better characterize the safety of this treatment combination. Therefore, a minimum of 18 and a maximum of 63 patients were planned for the study. Allowing for 10% ineligibility and path exclusion rate, total accrual goal was 70 patients.
Baseline patient characteristics and toxicity outcomes were summarized by descriptive statistics under each treatment regimens. All study endpoints, primary or secondary, were reported. CR and ORR rates were reported with point estimates with associated 95% confidence intervals (CIs). PFS, OS and DOR were estimated with the Kaplan-Meier method. Patients who came off study to proceed to HCT were followed for PFS and OS. Each treatment regimen was evaluated separately for safety and efficacy. Evaluable (eligible and treated) patients were included in determining MTD and evaluating efficacy, all treated patients were used for safety analysis. All analyses were conducted with SAS (version 9.4) and R (version 3.5.3). There were no planned protocol deviations.
Role of the Funding Source
The role of the sponsors in the study design: The sponsor of this trial is the National Cancer Institute (NCI) of the U.S. Department of Health and Human Services which reviewed and approved the study design. Elad Sharon and Howard Streicher, as co-authors, advised and made substantial contributions to the development of the study.
The role of the sponsors in the collection, analysis, or interpretation of data: The NCI had no role in the collection, analysis, or interpretation of the data. The role of the sponsors in the writing of the report: The NCI had no role in writing of the report, with the exception of Elad Sharon and Howard Streicher, who as co-authors reviewed, revised, and approved the report for important intellectual content. Those who have had access to the raw data by author initials: Only FH the study statistician, had access to the raw data. The corresponding author CD had full access to all of the data and the final responsibility to submit for publication.
Results
A total of 64 patients were enrolled to the phase I study during 3/7/2014–12/28/2017; 61 were evaluable with 21, 18 and 22 on BV-Ipi, BV-Nivo and BV-Nivo-Ipi respectively. Three patients were ineligible due to one each: prior immunotherapy, baseline labs out of window, and ANC of 1450. Patient characteristics of 61 eligible patients are shown in Table 1. The median ages were 33 (range 20–49), 40 (range 21–70), and 35 (range 19–60). Gender was evenly divided across all cohorts. ECOG PS 1–2 was assessed in 23 (38%) patients. There was a median of 2 (range 1–9) prior treatments; HCT was not considered an independent salvage therapy. Twenty-five patients (40%) had prior HCT: 21 (34%) auto-HCT, and 4 (7%) allo-HCT. Eight patients (13%) had prior BV. Thirty-five patients (57%) were refractory to their most recent therapy.
Table 1:
Baseline patient characteristics for all n=61 eligible treated patients
| BV-Ipi (n=21) | BV-Nivo (n=18) | BV-Nivo-Ipi (n=22) | All (N=61) | |
|---|---|---|---|---|
| Age | 33 (20–49) | 40 (21–70) | 35 (19–60) | 34 (19–70) |
| Median (range) | ||||
| Time Since Initial Diagnosis: n (%) | ||||
| <6 mo | 2 (10%) | 2 (11%) | 1 (4%) | 5 (8%) |
| 6–12 mo | 3 (14%) | 3 (17%) | 5 (23%) | 11 (18%) |
| 1–2 yr | 6 (29%) | 3(17%) | 11 (50%) | 20 (33%) |
| >=2 yrs | 10 (48%) | 10 (55%) | 5 (28%) | 25 (41%) |
| Time since most recent treatment: n (%) | ||||
| <6 mo | 7 (33%) | 7 (39%) | 10 (45%) | 24 (39%) |
| 6–12 mo | 4(19%) | 4 (22%) | 6 (27%) | 14 (23%) |
| 1–2 yr | 7 (33%) | 3 (17%) | 2 (9%) | 12 (20%) |
| >=2 yrs | 3 (14%) | 4 (22%) | 4 (18%) | 11 (18%) |
| Female Gender: n (%) | 10 (48%) | 9 (50%) | 11 (50%) | 30 (49%) |
| Stage: n (%) | ||||
| I | 0 | 1 (6%) | 2 (9%) | 3 (5%) |
| II | 10 (48%) | 7(39%) | 12(55% | 29 (48%) |
| III | 6 (29%) | 4(22%) | 3(14%) | 13 (21%) |
| IV | 5 (24%) | 6(33%) | 5 (23%) | 16 (26%) |
| B Symptoms: n (%) | 3 (14%) | 5 (28%) | 5 (23%) | 13 (21%) |
| ECOG PS: n (%) | ||||
| 0 | 16 (76%) | 10 (56%) | 12 (55%) | 38 (62%) |
| 1–2 | 5 (24%) | 8 (44%) | 10 (45%) | 23 (38%) |
| # extra nodal sites: n (%) | ||||
| 0–1 | 18 (86%) | 16(89%) | 20 (91%) | 54 (88%) |
| >=2 | 3 (14%) | 1 (11%) | 2 (9%) | 7 (12%) |
| Bulky Disease (≥7cm) : n (%) | 0 | 2 (11%) | 2 (9%) | 4 (7%) |
| # Prior Chemotherapies: n (%) | ||||
| 1 | 9 (43%) | 9 (50%) | 8 (36%) | 26(43%) |
| 2 | 5 (24%) | 3 (17%) | 9 (41%) | 17 (28%) |
| 3 | 2 (10%) | 4 (22%) | 4 (18%) | 10 (16%) |
| >=4 | 5 (23%) | 2 (11%) | 1 (5%) | 8 (13%) |
| Prior BV: n (%) | 3 (14%) | 4 (22%) | 1 (5%) | 8 (13%) |
| Time since last BV (yr) | 1.20 (1.14–2.94) | 1.27 (0.66–2.05) | 1.34 | 1.27 (0.66–2.94) |
| Median (range) | ||||
| Prior Transplant: n (%) | ||||
| Allogeneic | 1 (5%) | 2 (11%) | 1 (5%) | 4 (7%) |
| Autologous | 7 (33%) | 6 (33%) | 8 (36%) | 21 (34%) |
| Response to last therapy | ||||
| Refractory | 10 (48%) | 9 (50%) | 16 (73%) | 35 (57%) |
| ≥ 6 mos ≤ 1 year | 4 (19%) | 4 (22%) | 2 (9%) | 10 (17%) |
| Response ≥ 1 year | 5 (24%) | 5 (28%) | 3 (14%) | 13 (21%) |
| Unevalauble | 2 (9%) | 0 | 1 (4%) | 3 (5%) |
3 patients ineligible: 1) Received prior immunotherapy; 2) Labs out of window; 3) Baseline ANC < 1500
A total of 6 DLT events were reported, 5 on 3 patients during dose escalation, and 1 during dose expansion. In dose escalation there was one patient with grade 4 pneumonitis and grade 3 typhlitis (BV-Nivo dose level 2), one patient without pre-existing diabetes with grade 4 diabetic ketoacidosis (BV-Nivo-Ipi dose level 1), and one patient with transient grade 3 transaminase (AST, ALT) elevation (BV-Nivo-Ipi dose level 2). Therefore, dose level 2 was declared as the MTD for each of the 3 treatment regimens and patients were subsequently enrolled onto expansion cohorts. One additional DLT event (post allo-HCT grade 4 GVHD / Stevens-Johnson Syndrome) was reported on one patient treated on BV-Nivo-Ipi expansion cohort.
All 64 treated patients are included in the safety analysis. Table 2 lists all common and immune grade 1–2 and all grade 3–5 toxicities. Common toxicities, primarily grade 1–2, included: fatigue 41% (26/64), elevated AST 27% (17/64) ALT 34% (22/64) rash predominantly with BV-Ipi 39% (9/23), compared to BV-Nivo and BV-Nivo-Ipi combined 27% (11/41), fever 25% (16/64), peripheral sensory neuropathy 52% (33/64), and diarrhea 41% (26/64). Uncommon grade 1–2 treatment related immune toxicities included: blurred vision 7% (5/64), hypothyroidism 8% (5/64), and alopecia 6% (4/64). One (2%) grade 1–2 infusion reaction was reported. A total of 3% (2/64) lethal events was reported on study, one each in BV-Nivo and BV-Nivo-Ipi, both were treatment related grade 5 pneumonitis. An additional 43% (10/23), 16% (3/19), and 50% (11/22) cases of grade 3–4 treatment related events including: rash 13% (8/64) and colitis, gastritis, pancreatitis and arthritis, and diabetic ketoacidosis were reported each occurring in 1 patient (2%). Full treatment-related toxicity of all grade are listed in Supplementary Table S1.
Table 2:
Frequencies of all grade 3–5 toxicities, and all grade common and immune toxicity possibly related to treatment for all n=64 treated patients
| Toxicity | Grade | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BV-Ipi (n=23) | BV-Nivo (n=19) | BV-Nivo-Ipi (n=22) | |||||||||
| 1–2 | 3 | 4 | 1–2 | 3 | 4 | 5 | 1–2 | 3 | 4 | 5 | |
| Abdominal pain | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Alanine aminotransferase increased | 11 | 0 | 0 | 9 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Allergic reaction | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 |
| Alopecia | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Anemia | 5 | 1 | 0 | 9 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Anorexia | 6 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Arthritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Aspartate aminotransferase increased | 9 | 0 | 0 | 6 | 0 | 0 | 0 | 2 | 1 | 0 | 0 |
| Blurred vision | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Chills | 3 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Colitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Cough | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Diarrhea | 13 | 1 | 0 | 4 | 0 | 0 | 0 | 9 | 1 | 0 | 0 |
| Dyspnea | 3 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
| Endocrine disorders - Other, specify | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Fatigue | 13 | 0 | 0 | 5 | 0 | 0 | 0 | 8 | 2 | 0 | 0 |
| Fever | 6 | 0 | 0 | 6 | 0 | 0 | 0 | 4 | 0 | 0 | 0 |
| Gastritis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Headache | 6 | 0 | 0 | 6 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Hyperglycemia | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| Hypertension | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| Hyponatremia | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Hypophosphatemia | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Hypoxia | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Immune system disorders - Other, specify | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Lipase increased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 0 |
| Mucositis oral | 3 | 1 | 0 | 1 | 0 | 0 | 0 | 3 | 0 | 0 | 0 |
| Nausea | 16 | 0 | 0 | 8 | 0 | 0 | 0 | 11 | 0 | 0 | 0 |
| Neutrophil count decreased | 7 | 0 | 0 | 4 | 1 | 0 | 0 | 2 | 0 | 1 | 0 |
| Pain | 7 | 0 | 0 | 5 | 0 | 0 | 0 | 7 | 0 | 0 | 0 |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| Peripheral sensory neuropathy | 15 | 1 | 0 | 10 | 0 | 0 | 0 | 8 | 0 | 0 | 0 |
| Platelet count decreased | 1 | 0 | 1 | 4 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Pneumonitis | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| Pruritus | 6 | 1 | 0 | 4 | 1 | 0 | 0 | 3 | 1 | 0 | 0 |
| Rash maculo-papular | 9 | 5 | 0 | 5 | 1 | 0 | 0 | 6 | 2 | 0 | 0 |
| Respiratory failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Serum amylase increased | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Stevens-Johnson syndrome | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| Typhlitis | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vomiting | 5 | 1 | 0 | 5 | 0 | 0 | 0 | 5 | 2 | 0 | 0 |
| White blood cell decreased | 6 | 0 | 0 | 5 | 0 | 0 | 0 | 4 | 2 | 0 | 0 |
| WORST Grade | 13 | 9 | 1 | 15 | 3 | 0 | 1 | 10 | 8 | 3 | 1 |
A median of 7 (range 1–46) cycles of treatment were received by all patients, with a median 7 (2–16), 7 (1–46), and 5.5 (1–39) cycles for BV-Ipi, BV-Nivo and BV-Nivo-Ipi, respectively. The most common reasons for treatment discontinuation included: treatment related adverse events: 3 (BV-Ipi), 2 (BV-Nivo), and 7 (BV-Nivo-Ipi); disease progression during treatment: 6 (BV-Ipi), 2 (BV-Nivo), 1 (BV-Nivo-Ipi); auto HCT: 5 (BV-Ipi), 8 (BV-Nivo), and 8 (BV-Nivo-Ipi). The median number of cycles of therapy for patients who came off for HCT was 4.5, range (1–10). Dose reductions of BV were reported on 7 cases: 3 (BV-Ipi), 1 (BV-Nivo), 3 (BV-Nivo-Ipi). No dose reduction was reported for Nivo and Ipi, A total of 4 cases (3 on BV-Ipi, 1 on BV-Nivo), had BV discontinuation, and 6 cases (3 on BV-Ipi, and 3 on BV-Nivo-Ipi) had Ipi dose discontinuation. Detailed data for dose modification is shown in Supplementary Table S2.
Patient characteristics are similar between patients with/without prior HCT (data not shown). Supplementary Tables S3 and S4 list the frequencies of treatment related grade 3 or higher toxicity for n=27 (2 were not eligible) patients who received prior HCT (allo-HCT (4), auto-HCT (23)). No patients had active GVHD. In BV-Ipi grade >3 toxicities (rash and allergic reaction), occurred in 0/1 allo-HCT and 22% (2/9) of auto-HCT patients () compared with 43% (10/23) of all patients. In BV-Nivo grade ≥3 toxicities were reported in 33% (2/6) auto-HCT, and 50% (1/2) allo-HCT vs. 21% (4/19) all patients. A second pneumonitis, grade 5, occurred in the non HCT population. In BV-Nivo-Ipi there was 100% (1/1) for allo-SCT, 75% (6/8) for auto-SCT and 55% (12/22) for all patients that reported grade >=3 AEs. A grade 5 pneumonitis occurred in one auto-SCT patient. Notable AEs included reactivation of GVHD/Steven’s Johnson’s syndrome in an allo-HCT patient, and and one incidence of grade 3 gastritis in an auto-HCT patient.
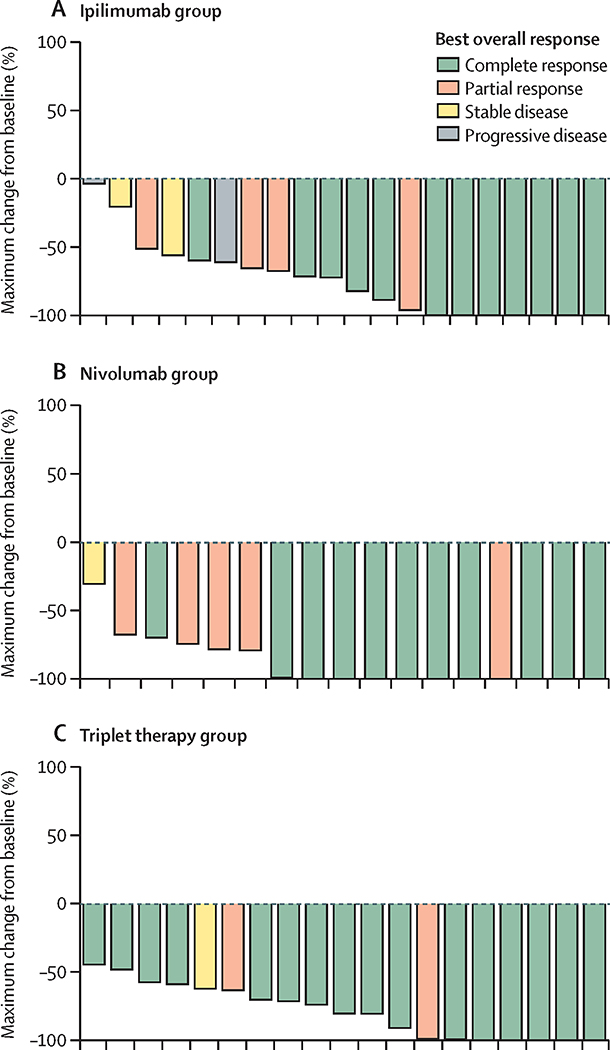
For n=61 evaluable patients (21 BV-Ipi, 18 BV-Nivo, 22 BV-Nivo-Ipi), the ORR is 76% (95% CI: 53–92%), 89% (95% CI: 65–99%) and 82% (95% CI:60–95% ), and the CR rate is 57% (95% CI: 34–78%), 61% (95% CI: 36–83%) and 73% (95% CI: 50–89%) for BV-Ipi, BV-Nivo and BV-Nivo-Ipi, respectively. For evaluable patients with at least two cycles of therapy and one follow-up disease assessment (20 BV-Ipi, 17 BV-Nivo, 19 BV-Nivo-Ipi): the ORR is 80% (95% CI: 56–94%), 94% (95% CI: 71–100 %) and 95% (95% CI: 74–100%). The CR rate is 60% (95%CI: 36–81%), 65% (95%CI: 38–86%) and 84% (95%CI: 60–97%) for BV-Ipi, BV-Nivo, and BV-Nivo-Ipi (Figure 2). The median time to best response was 72 (range 62–245) days for BV-Ipi, 66 (range 55–211) days BV-Nivo, and 66 (range 38–210) days for BV-Nivo-Ipi. Deepening of response from PR to CR were seen across all arms: 5 of 9 PRs in BV-Ipi, 4 of 9 PRs in BV-Nivo, and 1 of 3 PRs in BV-Nivo-Ipi converted to CRs by subsequent assessment. There was no difference in CR rate between patients who were BV naïve 64% (34/53), or BV pretreated 63% (5/8).
Figure 2:
Waterfall plot showing maximum percentage change in tumor size from baseline, (a) BV-Ipi (BV-I), (b) BV-Nivo (BV-N), (c) BV-Nivo-Ipi (BV-N-I)
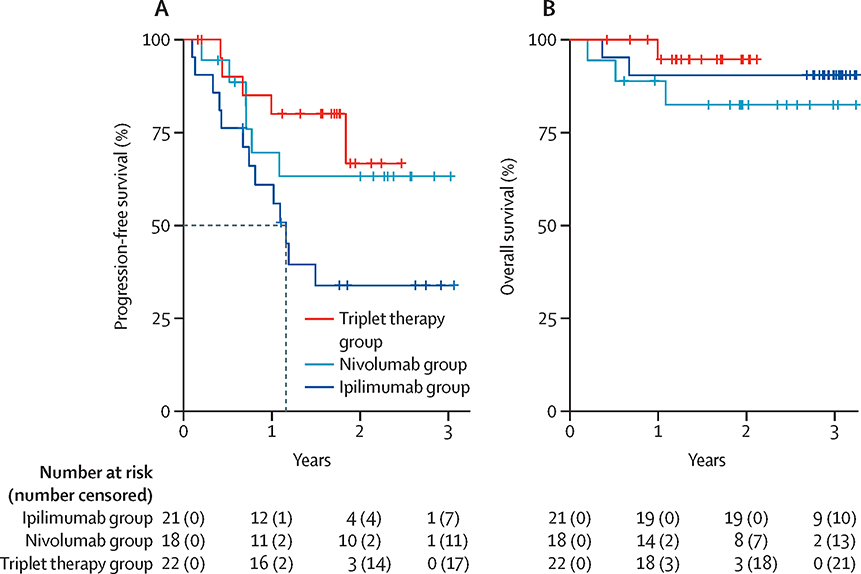
With a median follow-up of 2.6 (IQR:1.8–2.9), 2.4(IQR:2.2–2.6), and 1.7 (IQR:1.6–1.9) the 1-year PFS is 61% (95%CI 43–86%), 70% (95%CI 50–96%), and 80%(95%CI 64–100%) for BV-Ipi, BV-Nivo and BV-Nivo-Ipi, (Figure 3A), and the median PFS is 1.2 (95% CI, 074-NA) years for BV-Ipi and not reached for BV-Nivo and BV-Nivo-Ipi. The median OS has not been reached for any of the arms (Figure 3B). Combining patients treated with all three regimens, 11 of 39 (28%) CR patients progressed compared to 5 of 11 (45%) PR patients, median PFS is 1.84 (95% CI, 0.74-NA) for PR patients and not reached for CR patients (Supplementary Figure S1A). PFS estimates for the 22/61 patients (36%) who discontinued therapy for HCT versus all patients, suggest that consolidation with HCT was associated with longer PFS in both B-Ipi and BV-Nivo but not for BV-Nivo-Ipi (Supplementary Figures S1B, S1C, S1D). DOR is shown in Supplementary Figure S2. The median DOR is 1.32 (95% CI 0.91-NA) years for BV-I responders, not reached for BV-N and BV-N-I responders. One-year relapse free probability is 59% (95%CI 39–91%), 77% (95%CI 57–100%) and 87% (95%CI 71–100%) for BV-I, BV-N, BV-N-I responders.
Figure 3:
Kaplan Meier estimate of progression-free survival (a) and overall survival (b) by treatment combinations for BV-Ipi (B-I), BV-Nivo(B-N), and BV-Nivo-Ipi (B-N-I).
Discussion
The treatment in all 3 treatment arms was generally safe and well tolerated, with primarily grade 1–2 immune toxicities, although two deaths attributed to pneumonitis were noted. One patient was elderly and heavily pre-treated including prior gemcitabine, however a second patient was young and had no risk factors other than prior grade 3 gastritis, and lung involvement of his lymphoma. There were no clear risk factors suggesting which patients were vulnerable to develop these or other unusual immune toxicities. Along with a higher CR rate, the incidence of immune related toxicity, was higher on the triplet regimen raising the concern for potentially higher immune activation related toxicity. Correlative studies are ongoing to evaluate whether distinct biologic risk factors can be identified which may predict and mitigate these unusual toxicities.
While this study was not powered to examine toxicity for patients who use this therapy as a bridge to HCT, we attempted to examine whether there was any increased pattern of immune toxicity in post HCT patients. For the 40% of predominantly post auto HCT patients, no increase in toxicity was seen compared to the general population (Supplementary Table S4). The allo-HCT number is too small, 4 patients, to draw significant conclusions, (Supplementary Table S3).
Relapsed cHL remains a significant clinical challenge. Historically, survival for patients who relapse after HCT has been poor with a median OS of 1–2 years following HCT failure. (25–27). More recent studies indicates that, with the advent of BV and checkpoint inhibitors, this has improved to a median OS of 4–5 years post auto-HCT failure (28, 29). Despite the high ORR for both BV and Nivo, the CR rates are lower. BV has a CR rate of 33% and a PFS of 5.6 months (2); the CR rate to pembrolizumab (Pembro) is 22%, and the CR to Nivo ranges from 12% to 29%. The median DOR is 16.5 months for Pembro, and 16.6 months for Nivo (21–23, 30), and the long-term follow-up data for R/R cHL patients treated with checkpoint blockade agents suggest that relapses continue to occur over time (23, 30). Recent studies suggest that the combination of checkpoint inhibitors and cytotoxic chemotherapy may induce high response rates as a bridge to HCT in small numbers of patients (31), the individual contributions of these agents is not clear, and the question of whether BV-checkpoint or chemotherapy-checkpoint combinations are superior has not been determined in large scale clinical trials.
In this study, the activity of all combination treatment regimens shows a CR rate significantly higher than expected for single agent BV or Nivo. Patients who had been treated previously with BV (13%), did not have an inferior complete response compared to BV naïve patients. In a heavily pretreated R/R HL patient population, 40% of whom had prior SCT and 57% of whom were refractory to their last therapy, all 3 treatment combinations were highly active with ORR/CR rates of 80/60% (BV-Ipi), 94/64% (BV-Nivo), and 95/84% (BV-Nivo-Ipi) for evaluable patients, suggesting a deepening of response compared to expected responses for single agent BV and Nivo. This Phase 1 study was not powered for direct comparisons between the 3 treatment regimens, however, the ORR and CR rate in both Nivo containing cohorts appears superior to the BV-Ipi doublet.
This study contains the longest follow-up to date for patients treated long-term with BV and checkpoint combinations, and is particularly significant in that 40% of the patients in our study had previously received HCT, and consequently did not receive our therapy as a bridge to HCT. This is in contrast to the data presented by Herrera et al. in which patients were HCT naïve received 4 cycles of treatment, and subsequently proceeded to HCT (32). While the CR rates are similar in both studies, the median follow-up time for that study was 7.8 months, while the median follow-up time for our study is 2.63 years, 2.38 years, and 1.74 years for BV-Ipi, BV-Nivo, and BV-Nivo-Ipi. Although 36% of patients used our therapy as a bridge to HCT, 40% of our patients were post HCT. This gives us significantly enhanced view of the impact of our therapy on long-term disease control. The median PFS is 1.16 years for BV-Ipi and not reached for BV-Nivo and BV-Nivo-Ipi. Among CR patients, the regimen received appeared to impact the risk of progression with 6 of 12 (50%) of CRs on BV-Ipi progressing compared to only 2 of 11 (18%) of BV-Nivo and 3 of 16 (18%) of BV-Nivo-Ipi. For the 36% of patients who proceeded auto HCT following E4412, the are too few patients in each arm to make any direct comparisons between the arms but the PFS is uniformly excellent, between 80 and 100%, with a plateau in the PFS curve at 2 years. For patients who received BV-Nivo-Ipi and were not HCT eligible the PFS to date appears equivalent to that of patients who underwent HCT. However, the two populations were not identical, as the post HCT patient were more heavily pre-treated and refractory then the HCT eligible patients.
With long-term follow-up beyond 2 years patients on all arms who did not receive HCT continue to have sustained remissions, and prolonged OS. Longer follow up will be required to determine whether a higher CR rate translates into a higher durable remission rate and survival for patients who do and do not proceed to SCT after BV-Nivo and BV-Nivo-Ipi. Critical questions raised by this study include: which Nivo containing regimen between the doublet or triplet arm, has a superior response rate with manageable toxicity; whether CRs translate into durable responses; and what the role of auto-HCT and allo-HCT used as a consolidation therapy add to OS following BV-immunotherapy combinations. This first question is under investigation in an ongoing randomized phase 2 clinical trial, comparing the doublet of BV-Nivo to the triplet of BV-Nivo-Ipi (clinical trials.gov # NCT01896999), both regimens chosen for comparison because of the higher response rate and improved PFS compared to BV-Ipi. The significant question of whether some second line patients could use this therapy to postpone or forego HCT rather than as a bridge to HCT, will require the selection of an optimal regimen, and should be evaluated in a Phase 3 study, currently in the early planning stages.
Limitations of this study include the heterogeneity of the patient population, with respect to number of prior treatments, however this quite typical of early phase clinical trials, and we are encouraged that the response rates were demonstrated across multiple variables, with no clear increase in benefit for less heavily pre-treated patients. The small sample size makes comparisons across arms impossible, however the ongoing randomized Phase 2 study will address the deficiency.
Conclusion
In this study, which was the first to combine BV and immunotherapy in the R/R HL population, and the first to combine dual checkpoint blockade with BV, toxicity was manageable in all arms, although highest for the triplet combination of BV-Nivo-Ipi. We saw a markedly higher response rate for both doublets and the triplet BV-Nivo-Ipi when compared to historical data for any of the agents used as monotherapy. Durability appears sustained for many CR patients, who received BV-Nivo and BV-Nivo-Ipi. Important questions include whether the doublet or the triplet arm has a superior response rate with manageable toxicity, whether CRs translate into durable responses, and what the role of this therapy is as a bridge to HCT in the second line, or potentially for selected patients, in place of HCT. A randomized phase 2 study, comparing the doublet of BV-Nivo to the triplet of BV-Nivo-Ipi is active through the National Clinical Trials Network (NCTN) (clinical trials.gov # NCT01896999) to better assess the efficacy of the two most promising arms; further randomized studies are in the early planning stages to address these questions.
Supplementary Material
Research in Context.
Evidence before the study:
Preclinical immunologic data suggested that systemic immunologic dysfunction in HL patients reflects the tumor microenvironment (1) Published data demonstrated the activity single agent BV in HL (2), and the high activity of checkpoint inhibitors in solid tumor malignancies such as melanoma (3, 4). Two clinical trials, one of iplimumab in NHL patients (5), and the other of ipilimumab in a post transplant setting which included HL patients (6), suggested that ipiliumumab was safe in lymphoma, and potentially had some activity in HL in a post-transplant setting. However, there was, to our knowledge, no data for the combination of checkpoint inhibitor and antibody drug conjugate in hematologic malignancies or lymphoma at the time this study was developed. Literature searches performed before the study revealed no additional clinical data. There was no existing data on the use of PD-1/PDL1 and brentuximab vedotin (BV) in HL at the time this study was designed.
Added value of the study:
This study contains the only data for HL patients treated with dual checkpoint blockade in combination with an antibody drug conjugate (BV). It demonstrates the safety for both doublet combinations (BV-Ipi and BV-Nivo) and for the triplet combination of (BV-Nivo-Ipi), toxicity was manageable in all arms although highest in the triplet arm. It further demonstrates the longest follow-up to date for patients treated long-term with BV and checkpoint combinations. It is particularly significant in that 40% of the patients in our study had previously received HCT, and consequently did not receive our therapy as a bridge to HCT, in contrast to other published data of BV-Nivo combinations, giving us a significantly enhanced view of the impact of our therapy on long-term disease control.
Implications of all of the available evidence:
This study demonstrates that the combination of BV with Ipi, Nivo, or NIvo-Ipi was safe and active in patients with relapsed HL. Significant future questions raised by this study include: whether the triplet arm is more active then the doublet of BV-Nivo with manageable toxicity, this question is currently under investigation in a randomized phase 2 trial open throughout the CTN. The significant questions of whether this should become standard in second line as a bridge to HCT, or whether some second line patients could use the most effective arm of this study as a therapy in place of HCT, will be evaluated in a Phase 3 study, currently in the early planning stages.
Acknowledgements
Funding: This study was coordinated by the ECOG-ACRIN Cancer Research Group (Peter J. O’Dwyer, MD and Mitchell D. Schnall, MD, PhD, Group Co-Chairs) and supported by the National Cancer Institute of the National Institutes of Health under the following award numbers: U10CA180820, U10CA180794, UG1CA233196, UG1CA233247, UG1CA233341, UG1CA233198, UG1CA233270, UG1CA233320, UG1CA233339, UG1CA233277, and UG1CA232760. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. CSD is supported by American Cancer Society grant: MRSG-14-052-01-LIB
The authors thank Yiji Suh for her creation of the study schema figure.
CSD: supported by American Cancer Society grant: MRSG-14-052-01-LIB
Footnotes
Data Sharing
Data from ECOG-ACRIN clinical trials are available to researchers by directly contacting ECOG-ACRIN, https://ecog-acrin.org/contact-us. De-identified data, including data dictionaries, are available for request per the ECOG-ACRIN Data Sharing Policy after publication of the primary publication. Access to supplemental materials will be considered at time of the request. If the request is approved and if there are no restrictions to ECOG-ACRIN sharing data, a data use agreement between the requestor’s institution and ECOG-ACRIN must be in place before requested data can be released.
References
- 1.Diefenbach CS, Sabado R, Clark-Garvey S, Cruz C, Vengco I, Rojas CN, et al. PD-1 Is Elevated on the Peripheral Blood T Cell Subsets of Patients with Relapsed/Refractory Hodgkin Lymphoma Compared to Normal Volunteers. ASH Annual Meeting Abstracts; November 18, 20112011. p. 4860. [Google Scholar]
- 2.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, et al. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin’s lymphoma. J Clin Oncol. 2012;30(18):2183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. The New England journal of medicine. 2010;363(8):711–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ansell SM, Hurvitz SA, Koenig PA, LaPlant BR, Kabat BF, Fernando D, et al. Phase I study of ipilimumab, an anti-CTLA-4 monoclonal antibody, in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2009;15(20):6446–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bashey A, Medina B, Corringham S, Pasek M, Carrier E, Vrooman L, et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood. 2009;113(7):1581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugieres L, Brice P. Lymphoma in Adolescents and Young Adults. Prog Tumor Res. 2016;43:101–14. [DOI] [PubMed] [Google Scholar]
- 8.Cancer Stat Facts: Hodgkin Lymphoma. NIH, Natl Cancer Institute, SEER Data; [Internet]. Available from: https://seer.cancer.gov/statfacts/html/hodg.html [Google Scholar]
- 9.Ansell SM. Hodgkin Lymphoma: Diagnosis and Treatment. Mayo Clin Proc. 2015;90(11):1574–83. [DOI] [PubMed] [Google Scholar]
- 10.Ansell SM. Targeting immune checkpoints in lymphoma. Curr Opin Hematol. 2015;22(4):337–42. [DOI] [PubMed] [Google Scholar]
- 11.Khan G Epstein-Barr virus, cytokines, and inflammation: a cocktail for the pathogenesis of Hodgkin’s lymphoma? Exp Hematol. 2006;34(4):399–406. [DOI] [PubMed] [Google Scholar]
- 12.Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A. The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumour growth and immune escape. J Pathol. 2010;221(3):248–63. [DOI] [PubMed] [Google Scholar]
- 13.Steidl C, Connors JM, Gascoyne RD. Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(14):1812–26. [DOI] [PubMed] [Google Scholar]
- 14.Hsi ED. Biologic features of Hodgkin lymphoma and the development of biologic prognostic factors in Hodgkin lymphoma: tumor and microenvironment. Leuk Lymphoma. 2008;49(9):1668–80. [DOI] [PubMed] [Google Scholar]
- 15.Kuppers R The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9(1):15–27. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto R, Nishikori M, Kitawaki T, Sakai T, Hishizawa M, Tashima M, et al. PD-1-PD-1 ligand interaction contributes to immunosuppressive microenvironment of Hodgkin lymphoma. Blood. 2008;111(6):3220–4. [DOI] [PubMed] [Google Scholar]
- 17.Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O’Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010;116(17):3268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pericart S, Tosolini M, Gravelle P, Rossi C, Traverse-Glehen A, Amara N, et al. Profiling Immune Escape in Hodgkin’s and Diffuse large B-Cell Lymphomas Using the Transcriptome and Immunostaining. Cancers (Basel). 2018;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steidl C, Shah SP, Woolcock BW, Rui L, Kawahara M, Farinha P, et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nature. 2011;471(7338):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cader FZ, Schackmann RCJ, Hu X, Wienand K, Redd R, Chapuy B, et al. Mass cytometry of Hodgkin lymphoma reveals a CD4(+) exhausted T-effector and T-regulatory cell rich microenvironment. Blood. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen R, Zinzani PL, Fanale MA, Armand P, Johnson NA, Brice P, et al. Phase II Study of the Efficacy and Safety of Pembrolizumab for Relapsed/Refractory Classic Hodgkin Lymphoma. J Clin Oncol. 2017;35(19):2125–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Armand P, Engert A, Younes A, Fanale M, Santoro A, Zinzani PL, et al. Nivolumab for Relapsed/Refractory Classic Hodgkin Lymphoma After Failure of Autologous Hematopoietic Cell Transplantation: Extended Follow-Up of the Multicohort Single-Arm Phase II CheckMate 205 Trial. J Clin Oncol. 2018;36(14):1428–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang Y, Jin G, Pang Y, Huang Y, Wang W, Zhang H, et al. Comparative Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Advanced Cancer: A Systematic Review and Meta-Analysis. Front Pharmacol. 2020;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sureda A, Constans M, Iriondo A, Arranz R, Caballero MD, Vidal MJ, et al. Prognostic factors affecting long-term outcome after stem cell transplantation in Hodgkin’s lymphoma autografted after a first relapse. Ann Oncol. 2005;16(4):625–33. [DOI] [PubMed] [Google Scholar]
- 26.Kewalramani T, Nimer SD, Zelenetz AD, Malhotra S, Qin J, Yahalom J, et al. Progressive disease following autologous transplantation in patients with chemosensitive relapsed or primary refractory Hodgkin’s disease or aggressive non-Hodgkin’s lymphoma. Bone Marrow Transplant. 2003;32(7):673–9. [DOI] [PubMed] [Google Scholar]
- 27.Crump M Management of Hodgkin lymphoma in relapse after autologous stem cell transplant. Hematology Am Soc Hematol Educ Program. 2008:326–33. [DOI] [PubMed] [Google Scholar]
- 28.Badar T, Epperla N, Szabo A, Borson S, Vaughn J, George G, et al. Trends in Post-Relapse Survival in Classical Hodgkin Lymphoma Patients after Experiencing Therapy Failure Following Autologous Hematopoietic Cell Transplantation. Blood. 2018;132(Supplement 1):2918-. [Google Scholar]
- 29.Bair SM, Strelec L, Nagle SJ, Nasta SD, Landsburg DJ, Mato AR, et al. Outcomes of patients with relapsed/refractory Hodgkin lymphoma progressing after autologous stem cell transplant in the current era of novel therapeutics: A retrospective analysis. Am J Hematol. 2017;92(9):879–84. [DOI] [PubMed] [Google Scholar]
- 30.Chen R, Zinzani PL, Lee HJ, Armand P, Johnson NA, Brice P, et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: Two-year follow-up of KEYNOTE-087. Blood. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moskowitz AJ, Shah GL, Schoder H, Hancock H, Davey T, Joong C, et al. High Complete Response Rate Observed with Second-Line Chemo-Immunotherapy with Pembrolizumab and GVD (Gemcitabine, Vinorelbine, and Liposomal Doxorubicin) in Relapsed and Refractory Classical Hodgkin Lymphoma. Blood. 2019;134(Supplement_1):2837-. [Google Scholar]
- 32.Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, et al. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.