Abstract
Bioprinting is emerging as a promising tool to fabricate 3D human cancer models that better recapitulate critical hallmarks of in vivo tissue architecture. In current layer-by-layer extrusion bioprinting, individual cells are extruded in a bioink together with complex spatial and temporal cues to promote hierarchical tissue self-assembly. However, this biofabrication technique relies on complex interactions among cells, bioinks and biochemical and biophysical cues. Thus, self-assembly may take days or even weeks, may require specific bioinks, and may not always occur when there is more than one cell type involved. We therefore developed a technique to directly bioprint pre-formed 3D breast epithelial spheroids in a variety of bioinks. Bioprinted pre-formed 3D breast epithelial spheroids sustained their viability and polarized architecture after printing. We additionally printed the 3D spheroids onto vascular endothelial cell networks to create a co-culture model. Thus, the novel bioprinting technique rapidly creates a more physiologically relevant 3D human breast model at lower cost and with higher flexibility than traditional bioprinting techniques. This versatile bioprinting technique can be extrapolated to create 3D models of other tissues in additional bioinks.
Introduction
3D in vitro vascularized tumor models are essential tools for mechanistic study of cancer growth and metastasis. For breast cancer in particular, breast epithelial cells cultured in Matrigel organize into polarized spheroids that more closely resemble the in vivo mammary acinus architecture1, 2, 3, 4, 5, 6, 7, 8. 3D breast epithelial cell culture also impacts cell function, with 3D cultures showing differences in epidermal growth factor (EGF) receptor modulation8, 9; oncogene function, including ErbB210; growth and apoptosis signaling11, 12; and chemotherapy resistance13, 14. Vascular endothelial cells similarly respond differently to environmental stimuli in 3D vs. traditional 2D culture15, 16, 17, 18. However, much of the understanding of vascular endothelial and breast epithelial interactions comes from 2D culture using conditioned medium or Transwell inserts, or 3D models in which the two cell types are physically separated19, 20, 21, 22, 23. These co-culture models provide limited physiological insight, since both 3D culture and cell-cell contact are critical to vascular endothelial – breast epithelial cell interactions24, 25, 26.
3D cancer models have been fabricated using a variety of techniques, including hanging drop spheroid formation, bioprinting, magnetic assembly, and culture within hydrogels or on engineered scaffolds5, 27, 28, 29. More recently, 3D tumor models were created with multiple cell types arranged in their respective 3D structures. In one example of a tumor-on-a-chip platform, cancer, endothelial, and stromal cells were mixed into a matrix and then injected into the three central tissue chambers in a polydimethylsiloxane (PDMS) device. The tissue chambers were bordered by two outer channels that represented an artery and venule. After 5-7 days of culture, endothelial cells formed a microvascular network and cancer cells proliferated to form small tumors near the vasculature. This platform was then used to screen drugs and drug combinations30. Additional tumor-on-a-chip platforms have been created to study metastasis and cancer types with specific mechanical stimuli (e.g., mechanical strain in the lung)31 , 32. However, these platforms generally do not include both vasculature and cancer in their respective 3D structures.
Biofabrication shows great promise in advancing 3D in vitro vascularized tumor models, since it enables tight spatial control over cell location. Despite growth of bioprinting over the past decade, few studies focus specifically on tumors33 , 34. In one example, 3D printing of HeLa cells in a gelatin/alginate/fibrinogen hydrogel was used to create an in vitro cervical cancer model. Tumor cells were bioprinted as individual cells and then allowed to form spheroids, which showed a higher proliferation rate, increased matrix metalloproteinase expression, and higher chemoresistance than cells in 2D culture35. In these studies, as in many others36, 37, dissociated cell suspensions were bioprinted, and then the cell cultures were provided with the required mechanical and biochemical cues to enable the cells to form a 3D structure. However, cellular self-assembly may take days or weeks, may require complex spatial and temporal environmental cues, or may not occur when two cell types are co-cultured. For example, breast epithelial cells induced cell death in endothelial cells in 2D co-culture, and dissociated breast epithelial cells did not form 3D spheroids when bioprinted in alginate/gelatin hydrogels38. Dissociated breast epithelial or cancer cells formed spheroids in alginate based bioinks only when entrapped in circular PDMS molds. In other cases, spheroids were formed using suspended droplets in ultra-low attachment circular well plates and then mixed into alginate based bioinks39, 40.
We now describe an alternative 3D tissue biomanufacturing method in this protocol. Rather than seed dissociated cells and wait for these cells to form the 3D structures, we describe how to create and bioprint 3D tumor spheroids on a vascular tube network to create a tumor co-culture model that can be used almost immediately. Tumor spheroids can be grown in vitro or derived from human tissues (organoids). Similarly, vascular tubes can be grown or can be derived from adipose tissue microvascular fragments. Bioinks can range from biologically inactive alginate to the highly biologically active Matrigel41. Since this 3D tumor co-culture model can be created with a variety of cell structures and bioinks, it can incorporate multiple cell types, extracellular matrices, and chemokine gradients15, 42. While in its current formulation, the endothelial networks cannot be perfused, future iterations could integrate this method with microfluids or -on-chip systems. Bioprinting 3D breast epithelial spheroids onto endothelial networks enables rapid biofabrication of human breast models for drug testing and personalized precision medicine27.
Protocol
1. Breast Epithelial Cell Growth and Assay Media
-
MCF10A breast epithelial cells
NOTE: The non-tumorigenic immortalized breast epithelial cell line is derived from a patient with fibrocystic disease43. Cells do not express estrogen receptor.- To prepare 20 μg/mL epidermal growth factor (EGF), dissolve 100 μg of lyophilized EGF in 500 μL of sterile dH2O to make 200 μg/mL EGF. Add 500 μL of 200 μg/mL EGF into 4.5 mL of sterile 0.1% BSA in dH2O to make a 20 μg/mL EGF stock solution. Store prepared 20 μg/mL EGF aliquots at −20 °C for up to 12 months.
- To prepare 500 μg/mL hydrocortisone, dilute 1 mg of hydrocortisone in 1 mL of absolute ethanol (200 proof). Add 1 mL of sterile DMEM F:12 to this mixture to make a 500 μg/mL hydrocortisone stock solution. Store hydrocortisone stock aliquots at −80 °C for up to 12 months.
- To prepare 10 μg/mL cholera toxin, dissolve 1 mg of cholera toxin lyophilized powder in 1 mL of sterile dH2O to make a 1 mg/mL cholera toxin stock solution. Store cholera toxin stock aliquots at −80 °C for up to 12 months.
- To prepare Growth Medium, add 500 μL of EGF (20 μg/mL), 500 μL of hydrocortisone (500 μg/mL), 5 μL of cholera toxin (1 mg/mL), 500 μL of bovine insulin (10 mg/mL), 10 mL of penicillin and streptomycin, and 25 mL of horse serum to 500 mL of DMEM F:12 for a final concentration of 20 ng/mL EGF, 500 ng/ mL hydrocortisone, 10 ng/mL cholera toxin, 10 ng/mL bovine insulin, 2% v/v penicillin and streptomycin, and 5% v/v horse serum. Antibiotic concentration was increased to 2% to account for decreased sterility in the bioprinting process; however, the antibiotic concentration can be lowered to 1%.
- To prepare Assay Medium, add 500 μL of hydrocortisone (500 μg/mL), 5 μL of cholera toxin (1 mg/mL), 500 μL of bovine insulin (10 mg/mL), 10 mL of penicillin and streptomycin, and 25 mL of horse serum to 500 mL of DMEM F:12 for a final concentration of 500 ng/mL hydrocortisone, 10 ng/mL cholera toxin, 10 ng/mL bovine insulin, 2% v/v penicillin and streptomycin, and 5% v/v horse serum.
-
MDA-MB-231
NOTE: The triple-negative (lacking estrogen receptor, progesterone receptor, and epidermal growth factor receptor 2) breast cancer epithelial cell line is created from pleural effusion of a female patient with metastatic mammary adenocarcinoma44. Cells represent an aggressive, invasive, and poorly differentiated breast cancer.- To prepare Growth and Assay Medium, add 50 mL of fetal bovine serum, 10 mL of penicillin and streptomycin, and 25 mL of horse serum to 500 mL of DMEM for a final concentration 10% v/v fetal bovine serum, 2% v/v penicillin and streptomycin, and 5% v/v horse serum.
2. Breast Epithelial Cell Culture
Seed 500,000 MCF10A or MDA-MB-231 cells in a 10 cm (P100) tissue culture dish with 10 mL of MCF10A or MDA-MB-231 growth media. MCF10A and MDA-MB-231 cells reach >90% confluence in 48 hours.
To passage breast epithelial cells, first wash cells with 10 mL of warm PBS.
Detach breast epithelial cells by adding 2 mL of 0.05% Trypsin-EDTA to the dish. Place the dish in a 37 °C, 5% CO2 incubator for 20-25 minutes.
Add 5 mL of MCF10A or MDA-MB-231 Growth Medium to the trypsinized cells to neutralize the trypsin. Pipette the cell-media mixture up and down to resuspend cells and break up cell clusters.
Add the cell suspension to a 15 mL conical tube and centrifuge at 1,200 x g for 3 minutes. Carefully aspirate the supernatant and resuspend the cell pellet in 5 mL of MCF10A or MDA-MB-231 Growth Medium.
Plate 1 mL of cell suspension in 5 new 10 cm tissue culture dishes along with MCF10A or MDA-MB-231 Growth Medium. Place the dishes in a 37 °C, 5% CO2 incubator. Cells will be ready to be passaged again in 2-3 days. MCF10A cells can be maintained to passage number 35, after which they show morphological changes. MDA-MB-231 cells can be maintained to passage number 24, after which they show morphological changes.
3. Breast Epithelial Spheroid Formation
Freeze 200 μL pipette tips for 30 minutes prior to starting the assay. Keep growth-factor reduced matrix solution (e.g., Matrigel) (10 mg/mL) on ice for the entire process.
Slowly pipette 30 μL of ice-cold matrix solution using ice-cold 200 μL pipette tips into each well of an 8-well chamber slide. Start from the sides and drag the pipette tip along the corners, adding a final drop to the center of the well to ensure even coating of each well. Avoid air bubbles during this step to ensure a uniform matrix solution layer. Use new pipette tips for each well.
Incubate chamber slides in a 37 °C, 5% CO2 incubator for 15-20 minutes to polymerize the matrix solution.
Resuspend trypsinized MCF10A cells in MCF10A Assay Medium at 200,000 cells/mL or resuspend trypsinized MDA-MB-231 cells in MDA-MB-231 Growth Medium at 200,000 cells/mL.
If using MCF10A cells, prepare fresh MCF10A Spheroid Growth Medium by adding 5 μL of EGF (20 μg/mL) and 100 μL of matrix solution to 5 mL of Assay Medium for a final concentration of 2% matrix solution.
Remove the chamber slide precoated with matrix solution from the incubator. Add 50 μL of MCF10A or MDA-MB-231 cell suspension (10,000 cells) to each well. If using MCF10A cells, add 450 μL of MCF10A Spheroid Growth Medium. If using MDA-MB-231 cells, add 450 μL of MDA-MB-231 Growth Medium premixed with 2% matrix solution (9 μL). Immediate place the chamber slide in a 37 °C, 5% CO2 incubator.
-
Replace the medium every 4 four days. Carefully pipette 200 μL of old media out from one corner of each well using a 200 μL pipette tip. During this process, tilt the chamber slide at 45° to ensure that the spheroids are not disturbed. Add 200 μL of freshly prepared MCF10A Spheroid Growth Medium or MDA-MB-231 Growth Medium previously mixed with 2% matrix solution to each well at the corner in drops to ensure that spheroids remain attached to the matrix layer. Only ~50% of the media is replaced so that all the cytokines produced by the spheroids are not completely depleted.
NOTE: MCF10A breast epithelial spheroids take up to 8 days to polarize and form hollow centers. MDA-MB-231 breast epithelial spheroids take up to 5 days to form and have no hollow centers.
4. Endothelial Cell Network Formation
- Human Umbilical Vein Endothelial Cell (HUVEC) Culture
- Add the contents of a single Endothelial Growth Medium-2 kit containing insulin growth factor, fibroblast growth factor, vascular endothelial growth factor, ascorbic acid, epidermal growth factor, heparin and gentamicin-amphotericin B, along with 50 mL of fetal bovine serum, 5 mL penicillin-streptomycin and 5 mL of 200 mM glutamine to 500 mL of Endothelial Basal Medium-2 (EBM-2) to create complete Endothelial Growth Medium (EGM-2).
- Seed 500,000 endothelial cells in a 10 cm tissue culture dish in 10 mL of EGM-2. Place cells in a 37 °C, 5% CO2 incubator until they reach >80% confluency (around 48 hours).
- To passage cells, wash endothelial cells with 10 mL of warm PBS. Detach HUVEC by adding 2 mL of 0.05% Trypsin-EDTA to the 10 cm tissue culture dish. Closely monitor the cells by phase contrast microscopy. Cells are ready when they are balled up but remain attached to the dish.
- Carefully aspirate the trypsin out of the dish. Add 8 mL of EGM-2 to the dish. Wash cells off the dish by pipetting the EGM-2 up and down over the entire dish surface.
- Alternatively, add 5 mL of EGM-2 to the trypsinized cells to neutralize the trypsin. Pipette the cell-media mixture up and down to resuspend cells and break up cell clusters. Add the cell suspension to a 15 mL conical tube and centrifuge at 1,200 x g for 3 minutes. Carefully aspirate the supernatant and resuspend the cell pellet in 10 mL of EGM-2.
- Add 1 mL of cell suspension to 9 mL of EGM-2 in a 10 cm tissue culture dish. Place the dishes in a 37 °C, 5% CO2 incubator. Exchange 2/3 of the medium with fresh EGM-2 every 2 days. Cells will be ready to passage in 3-5 days. HUVEC can be maintained up to passage 8 after which they fail to form networks.
Endothelial Cell (HUVEC) Network Formation
Freeze 200 μL pipette tips for 30 minutes prior to starting the assay. Keep growth-factor reduced matrix solution (10 mg/mL) on ice for the entire process.
Slowly pipette 30 μL of ice-cold matrix solution using ice-cold 200 μL pipette tips into each well of an 8-well chamber slide. Start from the sides and drag the pipette tip along the corners, adding a final drop to the center of the well to ensure even coating of each well. Avoid air bubbles during this step to ensure a uniform matrix solution layer. Use new pipette tips for each well.
- Incubate chamber slides in a 37 °C, 5% CO2 incubator for 15-20 minutes to polymerize Matrigel.
- Pre-stain a 10 cm dish of HUVEC with 10 μL of red cell tracker (1:1000) for 30 minutes in 37 °C, 5% CO2 incubator.
- Wash endothelial cells with 10 mL of warm PBS. Detach HUVEC by adding 2 mL of 0.05% Trypsin-EDTA to the 10 cm tissue culture dish. Place the dish in a 37 °C, 5% CO2 incubator for 5 minutes to fully detach the cells.
- Add 5 mL of EGM-2 to the dish to neutralize the trypsin. Transfer the cell suspension to a 25 mL conical tube. Centrifuge HUVEC at 1,200 x g for 5 minutes. Aspirate the supernatant and resuspend the cell pellet in 5 mL of serum-free EBM-2.
- Count cells using Trypan blue to determine viable cells. Create a 1 × 106 cell/mL solution.
- Add 100,000 HUVEC to each well with 200 μL of serum free EBM-2. If more cells are added, they will create a surface monolayer rather than a tube network.
- Incubate HUVEC in a 37 °C, 5% CO2 incubator. After 6 hours, image HUVEC networks by phase contrast microscopy. Networks are now ready for coculture with breast spheroids.
5. Bioprinting Breast epithelial spheroids on preformed HUVEC Networks
NOTE: A dual nozzle bio-deposition system should be used for the biofabrication process. In this case, the system had three motion arms to allow micron-scale spatial control of material deposition as well as two screw driven motors to deposit bioink from 10 mL syringes. The system should be functionalized with a high efficiency particulate air filtration system as well as UV-sterilization capabilities to maintain a sterile environment during bioprinting. The bioprinter is UV sterilized for an hour before the printing process.
Create breast epithelial spheroids and HUVEC networks as previously described. Estimate the number of breast epithelial spheroids in each well by counting spheroids in representative phase contrast microscopy images.
-
Cut 0.5 cm off the end of a 1000 μL pipette tip. Use the cut pipette tip to carefully pipette all spheroids out of the 8-well chamber slide and into a 50 mL tube. Resuspend spheroids in the selected bioink at 100 spheroids/100 μL.
NOTE: Higher spheroid concentrations may result in spheroid clustering and prevent visualization of interactions between the spheroids and the endothelial networks.
Pass the spheroid mixture through a 70 μm cell strainer to remove any large or clustered spheroids.
Load the pooled spheroids into a 10 mL sterile syringe and cap it with a 25-gauge sterile needle. Attach the syringe to the bio-deposition system.
Aspirate the medium off the HUVEC networks in the 8-well chamber slide.
Extrude 100 μL of breast epithelial spheroids onto 6 wells of HUVEC networks at a flow rate of 1 mL/min. Maintain 2 wells of HUVEC networks as controls.
Add 400 μL of MCF10A Spheroid Growth Medium if using MCF10A spheroids printed on HUVEC networks or add 400 μL MDA-MB-231 Growth Medium previously mixed with 2% matrix solution if using MDA-MB-231 spheroids printed on HUVEC networks. The respective spheroid growth medium should be used in HUVEC control wells.
Incubate co-cultures in a 37 °C, 5% CO2 incubator for 24 – 96 hours without a media change. Co-cultures will remain viable in the original medium for up to four days.
6. Confocal Microscopy
- Immunofluorescence and PBS-Glycine Wash Buffer Preparation
- Prepare Immunofluorescence (IF) buffer 10x stock solution by adding 2.5 g of sodium azide, 5 g of bovine serum albumin, 10 mL of Triton X-100, and 2.05 mL of Tween-20 in 500 mL of 10x PBS. Adjust pH to 7.4. Store the stock solution for up to a year at 4 °C to avoid sedimentation.
- Create a working IF buffer by diluting 50 mL of 10x IF buffer in 450 mL of sterile deionized water. Store the working solution at room temperature for up to one week.
- Prepare 10x PBS-Glycine buffer stock solution by adding 37.5 g of glycine to 500 mL of 10x PBS. Adjust pH to 7.4. Store the stock solution for up to 6 months at 4 °C to avoid sedimentation.
- Create a working PBS-Glycine solution by diluting 50 mL of 10x PBS-Glycine buffer in 450 mL of sterile deionized water. Store the working solution at 4 °C for up to one week.
- Label Bioprinted Samples and Image by Confocal Microscopy
- Aspirate medium from bioprinted 3D co-cultures and rinse 3 times with warm PBS. Fix bioprinted 3D co-cultures with 4% paraformaldehyde for 1 h at room temperature. Rinse samples 3 times for 20 minutes with 1x PBS-Glycine.
- Block samples with IF buffer mixed with 10% goat serum for 90 minutes (primary block), followed by 40 minutes with IF buffer plus 10% goat serum and Affinipure Fab fragment (1:100, secondary block).
- If using MCF10A spheroids, label samples with a primary antibody for integrin α6 (1:100) in secondary blocking buffer overnight at 4 °C, followed by an Alexa Fluor 488 secondary antibody (1:200) and Hoescht 33342 (1:1000) for 1 h at room temperature protected from light. MCF10A cell lines express high levels of integrin α6, which is essential for showing spheroid polarization and morphology.
- If using MDA-MB-231 spheroids, which express low levels of integrin α6, label samples with Alexa Fluor 488 phalloidin (1:100) and Hoescht 33342 (1:1000) in secondary blocking buffer for 4 hours at room temperature protected from light. Phalloidin enables actin filament visualization so spheroid amorphous and invasive morphology can be assessed.
- Wash samples with 1X PBS-glycine 3 times for 20 minutes.
- Prepare samples for mounting by removing the chamber slide using the manufacturer’s tool. Add a small drop of antifade solution to each well. Place a 22 mm x 60 mm coverslip on each chamber slide and carefully seal the edges with clear nail polish.
- Image samples using a confocal microscope as Z stacks of ~10 slices in 5 μm steps. If desired, compress Z planes into a single plane using the Extended Focus command in cell imaging software.
- Quantify spheroid adhesion to endothelial networks using Image J. The number of adhered spheroids can be quantified using the analyze plugin with the appropriate particle size and circularity. Normalize the number of attached spheroids to the image area.
- To ensure reproducibility, quantify the number of adhered spheroids in 4 x 4 tiled images of co-cultures. If the spheroid number is statistically significantly lower than other experiments, the experiment should be repeated.
Representative Results
Breast epithelial cells should self-organize into 3D spheroids after 5-8 days of culture on matrix solution and in culture medium with 2% matrix solution. Non-tumorigenic MCF10A breast epithelial spheroids should appear round and have a hollow center, with integrin α6 polarized to the outer edge of the spheroid (Figure 1, inset shows hollow centers). Highly invasive MDA-MB-231 breast cancer epithelial cells form irregular spheroids. Spheroids should be used when they are around 100 – 300 μm in diameter. When spheroids become too large and get in close proximity, the spheroids will join together to form megaspheroids. In addition, MDA-MB-231 breast epithelial spheroids may show cells migrating out of the spheroids if maintained in the Matrigel culture for too long.
Figure 1: Representative confocal microscopy images of breast epithelial spheroids.
MCF10A spheroids were labeled for integrin α6 (green) and nuclei (blue). Cell phenotype can be confirmed after bioprinting when spheroids appear round with a hollow center (inset) and have integrin α6 polarized at the outer edges. MDA-MB-231 spheroids were labeled for actin (green) and nuclei (blue). Cell phenotype can be confirmed when spheroids are irregularly shaped without hollow centers and have cell processes invading into the surrounding matrix. Scale bar = 50 μm.
HUVEC should self-organize into tube-like networks after 6-8 hours of sparse, serum-free culture. Samples will have multicellular nodes with connections that are formed of lines of 1-3 cells in parallel. The HUVEC networks can be imaged by phase contrast microscopy or by confocal microscopy if they are labeled with Cell Tracker and Hoescht (Figure 2). The ImageJ angiogenesis analyzer can be used to quantify network junctions, segments, and branches. HUVEC networks will die if left in serum-free medium for longer than 16 hours.
Figure 2: Representative images of HUVEC networks by phase contrast and confocal microscopy.
HUVEC networks appear as small multicellular nodes with lines of cells connecting the nodes. For confocal microscopy, cells were labeled with Cell Tracker Red and Hoescht for nuclei (blue). Scale bar = 100 μm.
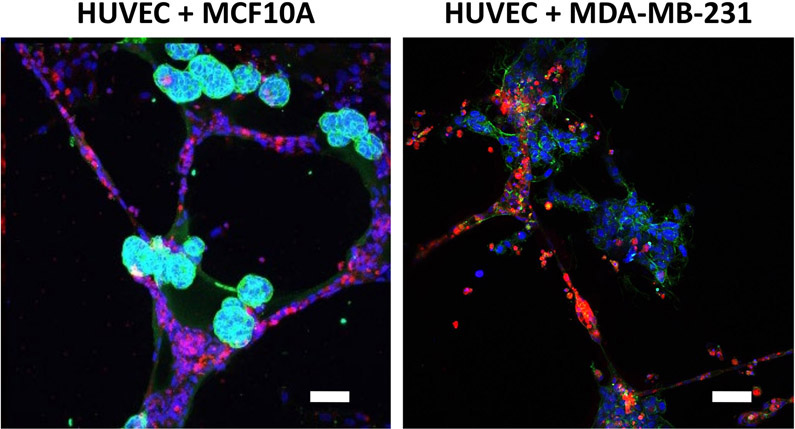
When breast epithelial spheroids are bioprinted onto the HUVEC networks, both spheroids and networks should maintain their original morphology for at least 24 hours. MCF10A breast epithelial spheroids will appear as round objects that adhere directly to the endothelial networks, while MDA-MB-231 breast epithelial spheroids will appear more amorphous yet still attached or in close proximity to the endothelial networks (Figure 3). HUVEC networks will be maintained when co-cultured with breast epithelial spheroids. For co-cultures longer than 24 hours, the breast epithelial cells may migrate out of the spheroids and along the endothelial networks. In our experience, this happens earlier in tumorigenic rather than non-tumorigenic breast epithelial cells38. We previously demonstrated using bioprinted co-cultures that drug testing can be initiated as early as 2 hours after spheroid bioprinting, for example to test spheroid adhesion onto endothelial networks45. We have also shown that 3D breast spheroids are more resistant to anti-cancer drugs like Paclitaxel than when printed as individual cells or in co-culture41 , 45. In the absence of bioprinted spheroids, HUVEC network control wells on their own fail to hold their network morphology and die after 16 h.
Figure 3: Representative images of breast epithelial spheroids co-cultured with HUVEC networks.
MCF10A spheroids, labeled for integrin α6 (green) and nuclei (blue), remain round and appear adhered directly to the endothelial networks. MDA-MB-231 spheroids, labeled for actin (green) and nuclei (blue), appear amorphous yet remain near or on endothelial networks. Co-cultures maintain this morphology for at least 24 hours after bioprinting, after which breast cells may migrate out along the endothelial tubes. Scale bar = 50 μm.
Discussion
This protocol is the first of its kind to bioprint spheroids in their 3D architecture for co-culture with endothelial cells also in their 3D architecture. Critical protocol steps include the initial formation of breast epithelial spheroids and HUVEC networks. Extreme caution must be taken in feeding breast epithelial spheroids, as they are easily disrupted from the matrix solution. Similarly, breast epithelial spheroids must be treated with care when they are pipetted off the matrix solution and mixed into the networks. HUVEC networks should not be plated at too high of a density or left for longer than 16 hours, as they will form a monolayer or die, respectively. Finally, all bioprinting should occur in a sterile environment at 37 °C to maximize cell viability.
Breast epithelial spheroids can be bioprinted in a variety of bioinks besides Matrigel, including alginate and alginate-collagen blends. We demonstrated that spheroids were viable and maintained their morphology when printed in alginate-based bioinks41. Thus while we present bioprinting here in matrix solution-based bioink, other less expensive and easier to use bioinks are also possible. We additionally used other breast cancer cell lines, including MCF-7 and genetically modified MCF10A-NeuN cells with similar success38 , 41 , 45. Alternative means could also be used to create the breast epithelial spheroids. For example, Lee et al. used hydrogel microwell arrays produced using PDMS stamps to create uniformly sized spheroids of controlled size46. Finally, alternative endothelial cells such as tumor-derived endothelial cells could be used, and rather than forming HUVEC networks, adipose-tissue derived microvessels could be directly bioprinted as 3D vascular structures47.
A primary limitation of this method was the challenge in controlling bioprinted spheroid location and number. Spheroids had to be printed with relatively large nozzles and in inviscid fluids to prevent spheroid damage. Rapidly gelling bioinks might better control spheroid location. Spheroids also could not be counted in the bioink, since they were too large for our cell counter. We relied instead on spheroid counts derived from phase contrast images taken prior to pipetting spheroids off the Matrigel surface. Alternative means of forming the spheroids could better control their number and size. We were able to control spheroid number and size with moderate accuracy by using cell strainers and culturing spheroids in the same way each time. A final limitation is that breast epithelial cells migrate out of the spheroids and along the endothelial networks over time. It is possible that alternative bioinks would abrogate this limitation.
Direct bioprinting of breast epithelial spheroids on pre-formed HUVEC networks enables the creation of a 3D in vitro tumor co-culture model in a short time. Researchers can then rapidly examine interactions between spheroids and vasculature with higher throughput. In the future, breast epithelial spheroids could be bioprinted onto perfused vasculatures, which would allow study of flow effects. In addition, tumor-derived organoids and endothelial cells could be bioprinted to enable precision medicine through testing of drug efficacy in a patient-specific model.
Acknowledgments
This research was funded by NIH 1R01HL140239-01 to AMC. We would like to thank the Cell Imaging Center at Drexel University.
Footnotes
Disclosures
The authors have nothing to disclose.
References
- 1.Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development. 105 (2), 223–235 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Debnath J, Muthuswamy SK, Brugge JS Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 30 (3), 256–268 (2003). [DOI] [PubMed] [Google Scholar]
- 3.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 89 (19), 9064–9068 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weaver VM et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. 137 (1), 231–245 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol ES et al. Growth of human breast tissues from patient cells in 3D hydrogel scaffolds. Breast Cancer Research. 18 (1), 19 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howlett AR, Bailey N, Damsky C, Petersen OW, Bissell MJ Cellular growth and survival are mediated by beta 1 integrins in normal human breast epithelium but not in breast carcinoma.Journal of Cell Science. 108 (Pt 5), 1945–1957 (1995). [DOI] [PubMed] [Google Scholar]
- 7.Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 89 (19), 9064–9068 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver VM et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. Journal of Cell Biology. 137 (1), 231–245 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang F et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proceedings of the National Academy of Sciences of the United States of America. 95 (25), 14821–14826 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nature Cell Biology. 3 (9), 785–792 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kirshner J, Chen CJ, Liu P, Huang J, Shively JE CEACAM1-4S, a cell-cell adhesion molecule, mediates apoptosis and reverts mammary carcinoma cells to a normal morphogenic phenotype in a 3D culture. Proceedings of the National Academy of Sciences of the United States of America. 100 (2), 521–526 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debnath J et al. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 111 (1), 29–40 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Weaver VM et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2 (3), 205–216 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada KM, Clark K Cell biology: survival in three dimensions. Nature. 419 (6909), 790–791 (2002). [DOI] [PubMed] [Google Scholar]
- 15.Schmeichel KL, Bissell MJ Modeling tissue-specific signaling and organ function in three dimensions. Journal of Cell Science. 116 (Pt 12), 2377–2388 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weigelt B, Ghajar CM, Bissell MJ The need for complex 3D culture models to unravel novel pathways and identify accurate biomarkers in breast cancer. Advanced Drug Delivery Reviews. 69-70 42–51 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koh W, Stratman AN, Sacharidou A, Davis GE In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods in Enzymology. 443 83–101 (2008). [DOI] [PubMed] [Google Scholar]
- 18.Sacharidou A et al. Endothelial lumen signaling complexes control 3D matrix-specific tubulogenesis through interdependent Cdc42- and MT1-MMP-mediated events. Blood. 115 (25), 5259–5269 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchanan CF et al. Cross-talk between endothelial and breast cancer cells regulates reciprocal expression of angiogenic factors in vitro. Journal of Cellular Biochemistry. 113 (4), 1142–1151 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Szot CS, Buchanan CF, Freeman JW, Rylander MN In vitro angiogenesis induced by tumor-endothelial cell co-culture in bilayered, collagen I hydrogel bioengineered tumors. Tissue Engineering Part C: Methods. 19 (11), 864–874 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franses JW, Baker AB, Chitalia VC, Edelman ER Stromal endothelial cells directly influence cancer progression. Science Translational Medicine. 3 (66), 66ra65 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. International Journal of Cancer. 133 (6), 1334–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phamduy TB et al. Printing cancer cells into intact microvascular networks: a model for investigating cancer cell dynamics during angiogenesis. Integrative Biology (Cambridge). 7 (9), 1068–1078 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Connor Y et al. Physical nanoscale conduit-mediated communication between tumour cells and the endothelium modulates endothelial phenotype. Nature Communications. 6 8671 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ghajar CM et al. The perivascular niche regulates breast tumour dormancy. Nature Cell Biology. 15 (7), 807–817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strilic B et al. Tumour-cell-induced endothelial cell necroptosis via death receptor 6 promotes metastasis. Nature. 536 (7615), 215–218 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Asghar W et al. Engineering cancer microenvironments for in vitro 3-D tumor models. Materials Today (Kidlington). 18 (10), 539–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belgodere JA et al. Engineering Breast Cancer Microenvironments and 3D Bioprinting. Frontiers in Bioengineering and Biotechnology. 6 66 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jang J, Yi HG, Cho DW 3D Printed Tissue Models: Present and Future. ACS Biomaterials Science & Engineering. 2 (10), 1722–1731 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Sobrino A et al. 3D microtumors in vitro supported by perfused vascular networks. Scientific Reports. 6 31589 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen MB, Whisler JA, Jeon JS, Kamm RD Mechanisms of tumor cell extravasation in an in vitro microvascular network platform. Integrative Biology (Cambridge). 5 (10), 1262–1271 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassell BA et al. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In vitro. Cell Reports. 21 (2), 508–516 (2017). [DOI] [PubMed] [Google Scholar]
- 33.Knowlton S, Onal S, Yu CH, Zhao JJ, Tasoglu S Bioprinting for cancer research. Trends in Biotechnology. 33 (9), 504–513 (2015). [DOI] [PubMed] [Google Scholar]
- 34.Asghar W et al. Engineering cancer microenvironments for in vitro 3-D tumor models. Materials Today. 18 (10), 539–553 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y et al. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 6 (3), 035001 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Zhang YS et al. Bioprinting the Cancer Microenvironment. ACS Biomaterials Science & Engineering. 2 (10), 1710–1721 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouyang L et al. Three-dimensional bioprinting of embryonic stem cells directs highly uniform embryoid body formation. Biofabrication. 7 (4), 044101 (2015). [DOI] [PubMed] [Google Scholar]
- 38.Swaminathan S, Ngo O, Basehore S, Clyne AM Vascular Endothelial-Breast Epithelial Cell Coculture Model Created from 3D Cell Structures. ACS Biomaterials Science & Engineering. 3 (11), 2999–3006 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vorwald CE, Ho SS, Whitehead J, Leach JK High-Throughput Formation of Mesenchymal Stem Cell Spheroids and Entrapment in Alginate Hydrogels. Methods in Molecular Biology. 1758 139–149 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaji S, Al-Saleh J, Gomillion CT Bioprinted Three-Dimensional Cell-Laden Hydrogels to Evaluate Adipocyte-Breast Cancer Cell Interactions. Gels. 6 (1) (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swaminathan S, Hamid Q, Sun W, Clyne AM Bioprinting of 3D breast epithelial spheroids for human cancer models. Biofabrication. 11 (2), 025003 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Radisky D, Muschler J, Bissell MJ Order and disorder: the role of extracellular matrix in epithelial cancer. Cancer Investigation. 20 (1), 139–153 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu Y et al. Evaluation of MCF10A as a Reliable Model for Normal Human Mammary Epithelial Cells. PLoS One. 10 (7), e0131285 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chavez KJ, Garimella SV, Lipkowitz S Triple negative breast cancer cell lines: one tool in the search for better treatment of triple negative breast cancer. Breast Disease. 32 (1-2), 35–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swaminathan S, Cranston AN, Clyne AM A Three-Dimensional In vitro Coculture Model to Quantify Breast Epithelial Cell Adhesion to Endothelial Cells. Tissue Engineering Part C: Methods. 25 (10), 609–618 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JM et al. Generation of uniform-sized multicellular tumor spheroids using hydrogel microwells for advanced drug screening. Scientific Reports. 8 (1), 17145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frueh FS, Spater T, Scheuer C, Menger MD, Laschke MW Isolation of Murine Adipose Tissue-derived Microvascular Fragments as Vascularization Units for Tissue Engineering. Journal of Visualized Experiments. (122) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]