Abstract
The current study reports the community succession of different toxin and non-toxin producing cyanobacteria at different stages of cyanobacterial harmful algal blooms (CyanoHABs) and their connectivity with nitrogen and phosphorus cycles in a freshwater lake using an ecogenomics framework. Comprehensive high throughput DNA sequencing, water quality parameter measurements, and functional gene expressions over temporal and spatial scales were employed. Among the cyanobacterial community, the lake was initially dominated by Cyanobium during the months of May, June, and early July, and later primarily by Aphanizomenon and Dolichospermum depicting functional redundancy. Finally, Planktothrix appeared in late August and then the dominance switched to Planktothrix in September. Microcystis aeruginosa and Microcystis panniformis; two species responsible for cyanotoxin production, were also present in August and September, but in significantly smaller relative abundance. MC-LR (0.06–1.32 μg/L) and MC-RR (0.01–0.26 μg/L) were two major types of cyanotoxins detected. The presence of MC-LR and MC-RR were significantly correlated with the Microcystis-related genes (16SMic/mcyA/mcyG) and their expressions (r = 0.33 to 0.8, p < 0.05). The metabolic analyses further linked the presence of different cyanobacterial groups with distinct functions. The nitrogen metabolisms detected a relatively higher abundance of nitrite/nitrate reductase in early summer, indicating significant denitrification activity and the activation of N-fixation in the blooms dominated by Aphanizomenon/Dolichospermum (community richness) during nutrient-limited conditions. The phosphorus and carbohydrate metabolisms detected a trend to initiate a nutrient starvation alert and store nutrients from early summer, while utilizing the stored polyphosphate and carbohydrate (PPX and F6PPK) during the extreme ortho-P scarcity period, mostly in August or September. Specifically, the abundance of Aphanizomenon and Dolichospermum was positively correlated with the nitrogen-fixing nif gene and (p < 0.001) and the PPX enzyme for the stored polyphosphate utilization (r = 0.77, p < 0.001). Interestingly, the lake experienced a longer N-fixing period (2–3 months) before non-fixing cyanobacteria (Planktothrix) dominated the entire lake in late summer. The Provo Bay site, which is known to be nutrient-rich historically, had early episodes of filamentous cyanobacteria blooms compared to the rest of the lake.
Keywords: Harmful algal blooms, Cyanobium, Aphanizomenon, Dolichospermum, Nitrogen fixation, P Scavenging genes, Cyanotoxins
Graphical Abstract
1. Introduction
The input of excess nutrients, primarily nitrogen and phosphorus, causes eutrophication in surface water bodies, leading to harmful algal blooms (HABs) in many freshwater lakes (Heisler et al., 2008; Dodds et al., 2009; Keck and Lepori, 2012; Drobac et al., 2013). Nitrogen (N) and phosphorus (P) are two of the most important nutrients of concern, although their relative contribution to eutrophication is always debatable (Carpenter, 2005; Conley et al., 2009; Kolzau et al., 2014; Paerl et al., 2017). Early studies recognized P as the primary limiting nutrient in most lakes based on the stoichiometry of N and P in phytoplankton (Schindler, 1977; Hecky and Kilham, 1988; Lewis and Wurtsbaugh, 2008). P addition-based bioassays have shown that P addition enhanced the growth of toxin-producing Microcystis (Davis et al., 2009). However, subsequent studies also found that N was often the limiting nutrient in shallow eutrophic lakes, while the oligotrophic deep lake was mostly P limited (Downing and McCauley, 1992; Reynolds, 2006). A switch from spring P to summer N limitation has also been demonstrated in some locations (Conley, 1999). Recent studies also recognized the dominance of cyanobacteria under low N/P ratios (Søndergaard et al., 2017; Isles et al., 2017). Generally, an N-limitation condition could result from nitrate lost to heterotrophs (e.g., denitrifiers) via assimilation, denitrification and other biochemical processes (Allen et al., 2005; Chen et al., 2012; Holmroos et al., 2012), while the levels of P were determined by interactions between sediment and water column of seasonal hydrological processes (Armon and Starosvetsky, 2015; Hogsett et al., 2019; Ma et al., 2019). Nevertheless, none of the past efforts or recent literature have denied the importance of nitrogen and phosphorus in supporting surface water eutrophication (Downing et al., 2001; Håkanson et al., 2007).
With new species of cyanobacteria being identified, the paradigm that surface water is either N limited or P limited is fast changing because nutrient limitations also depend on which cyanobacterial species dominate the bloom (Cottingham et al., 2015). Under N stress conditions, many filamentous cyanobacteria (e.g., Aphanizomenon, Dolichospermum) can conduct both nitrogen fixation and photosynthesis by cell differentiation. It is well-known that vegetative cells conduct primary productivity, whereas the specialized cells, heterocysts, perform nitrogen fixation by utilizing nitrogenases (encoded by nif genes; Schindler et al., 2008; Paerl, 2017). Additionally, N regulatory genes (e.g., ntrA, ntrC) and PII signal transduction proteins are widely spread in bacteria that regulate the N assimilations under N starvations (Hirschman et al., 1985; Herrero et al., 2004; Huergo et al., 2013).
Similar to N systems, one of the commonly recognized strategies for bacteria to enhance phosphate assimilation is inducing the high-affinity inorganic phosphate (Pi) scavenging system-Pho regulon (Adams et al., 2008; Santos-Beneit, 2015), which includes members having the high-affinity Pi transport systems (encoded by pst genes; Makino et al., 1988; Pitt et al., 2010), enzymes polyphosphate kinase (PPK; Brown and Kornberg, 2004), exopolyphosphatase (PPX; Gomez-Garcia et al., 2003), and others. The P correlated metabolisms are even more complex to study, as many P-containing compounds in cells are tightly linked with carbohydrates assimilations (Harke et al., 2012; Harke and Gobler, 2013) or the stringent conditions alert (Abranches et al., 2009; Santos-Beneit, 2015). It is reported that phosphate bioavailability for diazotrophs was one of the constraint factors for nitrogen fixation rates as an interaction between N and P (Ward et al., 2013; Wu et al., 2018).
Recent studies have suggested that cyanobacterial N2-fixation and Pi-scavenging also play important roles in promoting and sustaining cyanobacterial harmful algal blooms (CyanoHABs) (Beversdorf et al., 2013; Harke et al., 2015). A very recent meta-transcriptomic based study by Lu et al. (2019) revealed that expressions of genes involved in N2-fixation (nifDKH) and high-affinity Pi transporter (pstSABC) were significantly upregulated during the bloom compared to pre-bloom in Harsha Lake. In this study, these researchers found that the temporal action of N2-fixation (nifDKH) and high-affinity Pi transporter genes (pstSABC) controlled the ecology of cyanobacterial populations in Harsha Lake. Interestingly, many studies have observed the co-presence or succession of N-fixers (or nif genes) and toxin-producing strains at different stages of blooms (Elser et al., 2000; Beversdorf et al., 2013; Chia et al., 2018; Lu et al., 2019).
Eutrophication is a dynamic process where harmful toxin-producing and nontoxic blooms coexist, although their relative abundance may vary. Additionally, the transition of a lake ecosystem from being N limited to P limited or vice versa would not only depend on the exogenous input of nutrients but the relative expressions of N-fixing and P-affinity genes. Lastly, the presence of toxic cyanobacteria identified taxonomically does not necessarily mean that they are expressing their toxin-producing functional genes. Recent studies successfully linked the dynamics of certain cyanobacterial species with their metabolic activities (Beversdorf et al., 2013; Harke et al., 2015; Lu et al., 2019). However, studies are still scarce for a whole-picture investigation into nutrient utilization pathways and toxin-producing functional genes at the entire bacterial community level during HABs.
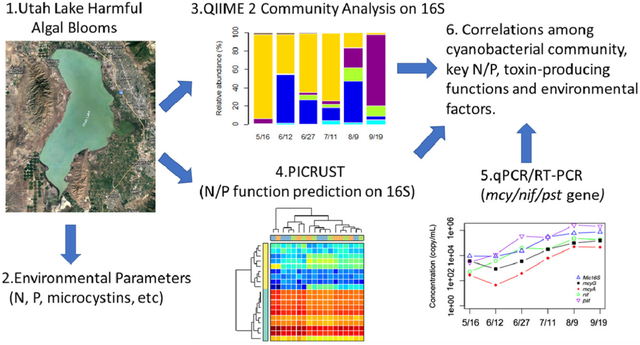
The overall objective of this research was to take a holistic approach to illustrate the interdependency of CyanoHABs with several factors, including water quality parameters and genomic contents in a freshwater peri-urban lake. This study fills an important gap elated to the dynamics of P and N cycles during cyanoHABs. Unlike a previous publication from this group on the ecology of cyanobacteria in Utah Lake (Li et al., 2019), the objective also included studying N-fixing, P-regulating, and toxin-producing functional genes before the onset, during and after cyanoHABs in addition to spatial and temporal variations in the abundances of different cyanobacteria. The general N and P metabolic pathways and functions were predicted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) (Langille et al., 2013); the functional gene/gene expressions for nutrient scavenging (nif/pst) and microcystin-producing (mcy) were precisely targeted by qPCR and Reverse-transcript qPCR (Freeman et al., 1999). The main objectives of the study were to: (1) detect the microbial community shift and predict key functional dynamics related to N and P; (2) study the dynamic behavior of nif genes, pst genes, and mcy genes during the CyanoHABs; (3) investigate the correlations among bacterial community presence, functions, and environmental factors.
2. Materials and methods
2.1. Sampling sites
Freshwater Utah Lake, which is located nearly 50 miles south of Salt Lake City, was considered as the model freshwater lake. Utah Lake is the largest natural freshwater lake in the western United States, with a maximum length of 38.6 km and a maximum width of 20.9 km. As a shallow alkaline lake, it has an average depth of 3.0–3.4 m in open water during standard reservoir operating conditions and has calcium-rich sediments. Utah Lake has experienced frequent CyanoHABs in recent years, with the cyanobacteria cell numbers up to 36 million cells per mL in 2016 and Aphanizomenon flos-aquae as the primary species (UDWQ, 2016a). Utah Lake was also shut down for recreational and irrigation purposes as MCs concentrations were detected to be much higher at some locations than the recreational exposure guideline of 10 μg/L by the World Health Organization (Bartram and Chorus, 1999). Site selections were based on the Utah Division of Water Quality’s (UDWQ’s) regular sampling. All of the sites are in open water and are located near major tributaries with varying depths (Fig. 1 and Table S1). Deepwater sites (depth> 1 m) included Saratoga Springs, Geneva Discharge, Vineyard Buoy, Provo Buoy, and Bird Island Buoy, while Entrance to Provo Bay and Goshen Bay were the shallow sites, with water levels less than 0.5 m during the regular summertime (e.g., early May to September). Sampling was conducted on a monthly basis from early May to late September in 2018, except for sampling twice in June when CyanoHABs typically occurred, based on historical observation updated warnings issued by UDWQ. During sampling, water samples were collected at three depths at each site and combined onsite into one composite sample. For most in-lab analysis, samples were collected in HDPE sampling bottles following the Standard Operating Procedure for the collection of phytoplankton to detect harmful algal blooms (UDWQ, 2016b). Two exceptions were cyanotoxin and chlorophyll a, measurements of which required the use of glass amber bottles to prevent sunlight exposure. The containers were immediately stored in coolers and transferred to the Environmental Engineering and Microbiology Lab at the University of Utah for further physicochemical and biological analysis.
Figure 1:
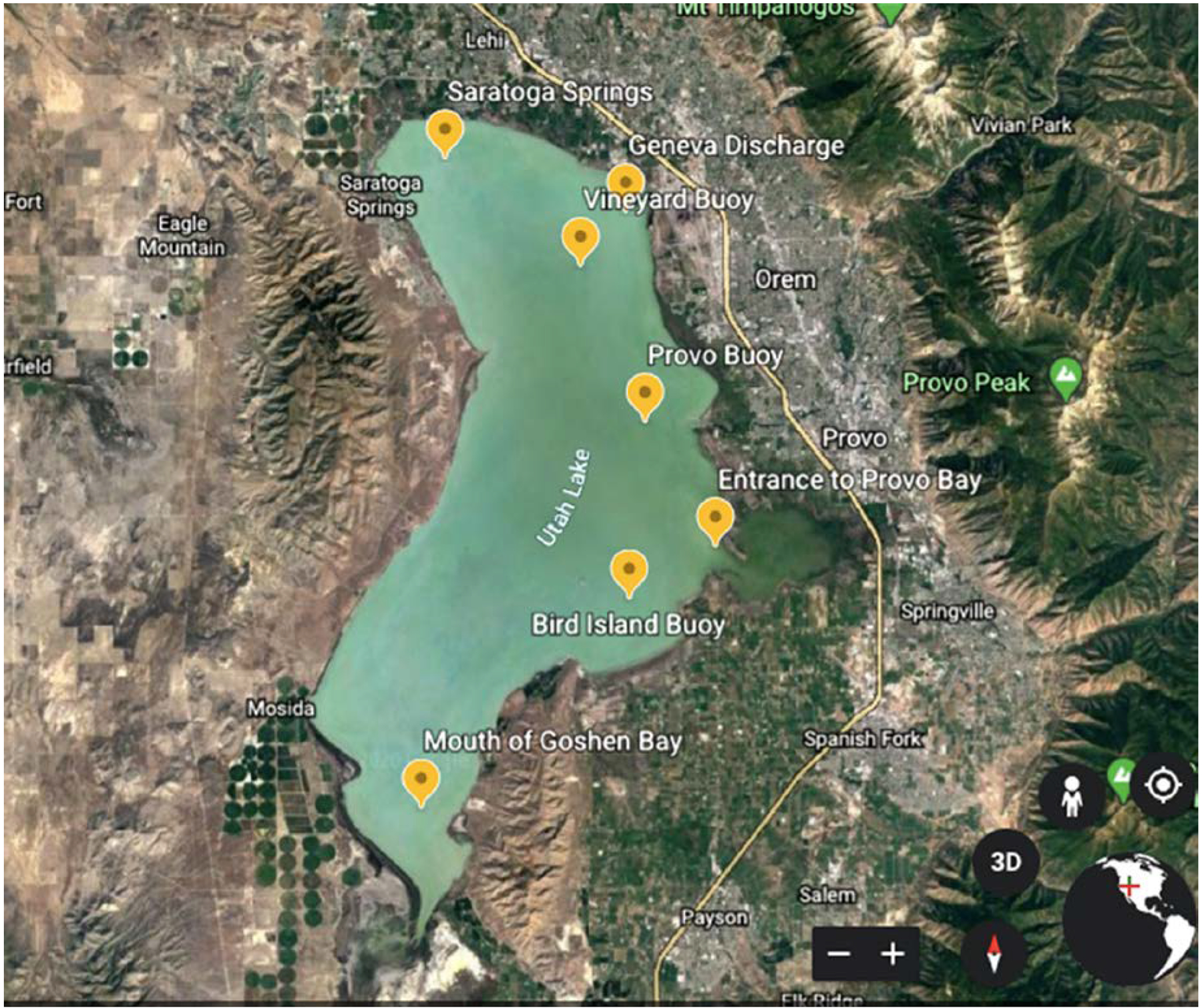
Map of Utah Lake showing site locations and surroundings
2.2. Measurement of physicochemical parameters
Temperature, pH, dissolved oxygen, conductivity, and depth were measured in-situ. Samples were directly filtered through 0.22 μm filters (HPLV 4700, Fisher Scientific) before the measurement of soluble nutrients. The filters with planktonic biomass were kept at −20 °C before genomic (Sections 2.3 and 2.4) analysis and in InvitrogenⓇ RNALater stabilization solution before gene expression (Section 2.4) analysis. Dissolved nutrient anions (nitrate-N, nitrite-N, and orthophosphate-P) in the filtrate were analyzed using Ion Chromatography (IC) (Metrohm 883 Basic IC plus) following EPA method 300 (Pfaff, 1993). The ammonia-N, total dissolved nitrogen (TDN), and total dissolved phosphorus (TDP) were measured using Low Range Ammonia TNTplus Vial Test (TNT830, Hach, USA), Total Nitrogen TNT Reagent Set (LR, Hach), and Total Phosphorus TNT Regent Set (LR, Hach), respectively. Chl a was measured spectrophotometrically and corrected for pheophytin following the standard methods of water and wastewater (APHA, 1999). The dissolved organic carbon was measured by the standard carbonaceous biochemical oxygen demand (cBOD5) bottle test following EPA 450.1. The microcystin concentrations (MCs) were measured starting in June according to EPA method 544. More precisely, microcystins were extracted from both filtrate (lake water) and filter (algal biomass), concentrated by solid-phase extraction, and detected on a WatersⓇ ACQUITY UPLC with TQD mass spectrometry.
2.3. Genomic analysis of blooms
2.3.1. Genomic DNA extraction and high-throughput sequencing
Genomic DNA was extracted from the filtered biomass using a PowerWaterⓇ DNA isolation kit (Qiagen Inc, Valencia, California) according to the manufacturer’s instructions. Concentrations were determined on a ThermoⓇ NanoDrop 2000c, and samples with 260/280 ratios higher than 1.80 were selected for further analysis. Before being sent for IlluminaⓇ MiSeq sequencing, samples were diluted to 10 ng/μL for a total volume of 10 μL. The amplicon library preparation of the bacterial 16S rRNA gene V4 gene region was conducted in the RTSF Genomics Core at Michigan State University using primer set 515F/806R, following the protocol described by Kozich et al., 2013. The final PCR products obtained from the protocol were batch normalized using InvitrogenⓇ SequalPrep DNA Normalization plates and pooled into each well. The pool was cleaned up using Ampure XP beads, quantified using a combination of QubitⓇ dsDNA HS, AgilentⓇ Bioanalyzer DNA 1000, and IlluminaⓇ Kapa Library Quantification qPCR assays. It was then loaded onto an IlluminaⓇ MiSeq Standard v2 flow cell and sequenced in a 2 × 250 bp paired-end format using a v2 Standard 500 cycle MiSeq reagent cartridge. Custom sequencing and index primers were added to appropriate wells of the reagent cartridge as described. The Base calling was done by IlluminaⓇ RealTime Analysis (RTA) v1.18.54, and the output of RTA was demultiplexed and converted to Fastq format with IlluminaⓇ Bcl2fastq v2.19.1.
2.3.2. The analysis of microbial community and the prediction of metabolic pathways and functional groups
The amplicon sequencing results were analyzed according to QIIME 2 “moving picture” tutorials (https://docs.qiime2.org/2018.11/tutorials/moving-pictures/) (Vázquez-Baeza et al., 2013; Bolyen et al., 2018). The overall analysis with read quality filtering demultiplexing, truncating of paired- end reads, operational taxonomic units (OTUs) formation, alpha diversity analysis, and other analyses were conducted similar to our earlier protocol (Li et al., 2019). The predicted metabolic functions of the microbial community were determined by PICRUSt v1.1.3 (Langille et al., 2013). To do so, features (OTUs or sequence variants) were closed-reference picked using “qiime vsearch cluster-features-closed-reference” against the database that was trained on the Greengenes version 13_5 with 99% identity cluster OTUs from the 515F/806R region of sequences (McDonald et al., 2012). The percent identity at which clustering should be performed (–p-perc-identity) was set at 1 to prevent any mismatches. The generated feature table was exported as a biom format file, which was then used as the input for PICRUSt to predict metabolic function counts by referencing the Kyoto Encyclopedia of Genes and Genome (KEGG) Orthology (KO) Database (Kanehisa and Goto, 2000; Kanehisa et al., 2014). The function prediction was achieved by processing through scripts of “normalize_by_copy_number.py” and “predict_metagenomes.py.” The accuracy of metagenomic prediction was estimated by the Nearest Sequenced Taxon Index (NSTI) value, which is considered the standard method for validation of KEGG functional groups (Langille et al., 2013; Koo et al., 2017). A lower NSTI value (< 0.15) implies that samples are phylogenetically close in relationship and suitable for PICRUSt analysis. In our analysis, the weighted NSTI values ranged from 0.105 to 0.169 with a mean value of 0.127 ± 0.014. Thousands of predicted functions were further collapsed into KEGG pathways by “categorize_by_function.py.” The KEGG Orthology (KO) counts of N and P/carbohydrate metabolisms related pathways were plotted using the heatmap option at the log scale in STAMP (Parks et al., 2014).
2.4. The quantitative PCR (qPCR) and functional gene expressions using reverse transcript (RT)-qPCR
Cloning and sequencing was conducted using mcyAcya/mcyEcya primers (Table 1) to identify possible mcy gene clusters within the entire bacterial community. Cloning was conducted from the lake biomass using TOPOⓇ TA cloning kit (Invitrogen, USA), and plasmids were extracted by ZyppyⓇ Plasmid Miniprep Kit (Zymo Research, USA). The cloned plasmids were sent for Sanger Sequencing at the Health Science Center Cores, University of Utah. The species or target genes were identified by blasting against the National Center for Biotechnology Information (NCBI) database.
Table 1.
List of Primers applied in the study
| Oligo name | Sequences (5’−3’) | Tm (°C) | Target | bp | Limit of detection (gn rx−1) | Reference |
|---|---|---|---|---|---|---|
| MICf | GCCGCRAGGTGAAAMCT | 60 | 16S rRNA inMicrocystis | 248 | 1 | Neilan et al., 1997 |
| MICr | AATCCAAARACCTTCCTCCC | |||||
| mcyEcyaf | TTTGGGGTTAACTTTTTTGGGCATAGTC | 56 | mcyE or ndaFin Cyanobacteria | 470 | 10 | Jungblut and Neilan, 2006 |
| mcyEcyar | AATTCTTGAGGCTGTAAATCGGGTTT | |||||
| mcyAcyaf | AAAAGTGTTTTATTAGCGGCTCAT | 56 | mcyA in Cyanobacteria | 302 | 10 | Hisbergues, 2003 |
| mcyAcyar | ATCCAGCAGTTGAGCAAGC | |||||
| mcyAmsf | ATCCAGCAGTTGAGCAA | 60 | mcyA inMicrocystis | 171 | 10 | Furukawa et al., 2006 |
| mcyAmsr | GCCGATGTTTGGCTGTAAAT | |||||
| mcyGmicf | CAACCCAACAGGTTCTTAAAGC | 60 | mcyG inMicrocystis | 244 | 10 | Ngwa, 2012 |
| mcyGmicr | TGAGGCAAGGTTTCCTCTTG | |||||
| nif_nostF3 | ATCGTTCAACACGCAGAATTG | 60 | Ana, Nos, Cyl | 90 | 10 | Lu et al., 2019 |
| nif_nostR3 | TCATCCATTTCGATAGGTGTGG | |||||
| pstSf3 | TGGAATGTTACCAGCAGGAATAA | 60 | A Flos Aq, AFA | 110 | 10 | Lu et al., 2019 |
| pstSr3 | AGTGCTGCTTGACGTAAACT |
Based on the cloning sequencing results, real-time quantitative polymerase chain reaction (qPCR) was further performed to quantify total gene copy numbers and reverse transcript qPCR (RT-qPCR) was conducted to estimate expressions of key functional genes responsible for nitrogen fixation, high-affinity Pi-transporter for Nostocales, and MCs related genes for Microcystis. Primers used to quantify absolute gene copy numbers and genes targeted for expression are listed in Table 1. The genomic DNA templates were diluted to 20 ng/μL to prevent inhibition at high concentrations. Total RNA was extracted using PureLinkⓇ RNA Mini Kit (Thermal Fisher, USA) and immediately stored at −80 °C until used. Following RNA extraction, residual genomic DNA was removed from total RNA using an on-column PureLinkⓇ DNase set (Life Technologies, NY, USA). It was then converted to cDNA by SuperScriptⓇ VILO mastermix (Thermal Fisher,USA). The qPCR and RT-qPCR reaction mixture are 20 μL in total, containing 10 μL 2×Power SYBRⓇ Green Master Mix (Applied Biosystems, Foster City, CA), 0.5 μM of each primer, 2 μL of templates (blank), and 6 μL nuclease-free water. The quantification cycling was conducted with a QuantStudioⓇ 3 Real-Time PCR System (Applied Biosystems) following the process of an initial 2 min at 50 °C and 10 min at 95 °C, followed by 40 cycles of 15 s at 95 °C, 30 s at respective annealing temperature, and 30 s at 72 °C. Before running samples, cloned standards for each gene were serially diluted to a range of 101 to 106 copies per 20 μL reaction using 10% purified plasmid DNA and 90% nuclease-free water. After the qPCR process, the template gene copies were directly calculated based on the linear regressions of standards versus the cycle threshold. The copy numbers were multiplied by the diluting factors to gain results of copies/mL of lake water.
2.5. Statistical analysis
Except for the default graph tools in bioinformatics software, all of the figures were created using R studio 1.1.419 (R Development Core Team, 2013). Specifically, the boxplots for predicted metabolic pathways were generated using the R package “ggpubr.” The associations of ambient water parameters, bacterial communities, metabolism prediction results and real-time gene/gene expression quantifications were estimated by Spearman correlation analysis using the R package “corrplot” at a 95% confidence interval.
3. Results
3.1. Water quality parameters
Water parameters sampled from May to September are listed in Table S2 and cyanotoxin concentrations in supplementary Table S3. Briefly, water temperatures increased from May (16 – 20 °C) to July (25 – 28 °C) and decreased to around 20 °C in September’s. The overall pH increased from May (8.23 – 8.38) to August (8.64 – 8.98) and leveled off in September. The only exception was the Entrance to Provo Bay site when the bloom and pH peaked on June 27th. Chl a and DO results were consistent with pH results. The Entrance to Provo Bay had earlier indicating of bloom in June (Chl a 100 μg/L and DO 10.6 −14.6 mg/L) than the entire lake. The sudden rise in cBOD in the lake was detected in August (5.2 – 22.4 mg/L) and September (6.6 −14.3 mg/L). As for cyanotoxins, MC-LR and MC-RR were two main variants of microcystins detected in the lake water, which peaked on June 12th at the sites Mouth of Goshen Bay and Entrance to Provo Bay and another peak on Sep 19th at all sites. The highest concentrations were 0.69 – 1.16 μg/L for MC-LR and 0.15 – 0.26 μg/L for MC-RR in September (Table S3).
The nutrient concentrations varied at locations and sampling dates. Generally, ammonium-N, nitrate-N, and ortho-P were measured in the range of 0 – 0.97 mg N/L, 0 – 0.09 mg N/L, 0 – 0.07 mg P/L respectively. Nitrite-N was mostly non-detectable. The south part of the lake showed high concentrations of ammonium-N, as high ammonium concentrations were measured in the Mouth of Goshen Bay (0.97 mg N/L) and Bird Island Buoy (0.63 mg N/L). Nutrients observed a decreasing trend overall except for some sudden increases in ammonium-nitrogen concentrations in July and August. Nitrate-N and ortho-P were non-detectable in August and September’s sampling. Because of higher TDN concentrations (0.17 −7.07 mg N/L) compared with TDP (0.23 – 1.04 mg PO43−/L), the atomic N:P ratios were generally higher than 16:1, except for some sites in September. Overall, Provo Bay experienced early blooming period in June followed by blooms in other parts of the lake in July, August and September.
3.2. Sequencing depth and diversity analysis
To identify the microbial community composition, a total of 54 samples were collected during different months, from different locations, and were then sequenced. High-throughput sequencing yielded a total of 8,099,216 sequences. A total 6,482,848 sequences remained after quality control and were clustered into 10,163 features. Table S4 summarizes the sequencing details and number of sequences obtained after quality filtering. The refraction curves tended to approach the saturation plateau, implying adequate sampling depth and coverages (Supplemental Fig, 1 A). Shannon’s diversity index indicated a relatively lower abundance or evenness of species present in August’s sampling (Supplemental Fig, 1 B). The observed OTU counts were detected moderately lower on May 16th than the other months (Supplemental Fig, 1A).
3.3. Microbial community classification at phylum and genus levels
The taxonomy classification and calculations of relative abundance were based on bacterial 16S sequencing. The microbial community at the phylum level is shown in panel A of Fig. 2. Actinobacteria, Bacteroidetes, Proteobacteria, and Cyanobacteria were the most observed phyla (Fig. 2). The relative abundance of cyanobacteria generally increased starting May 16th (7.88–22.7%) onward for subsequent months to 28.2–38.1% in September (depending on the site). However, different sites exhibited varying trends in the relative abundances of Cyanobacteria. For example, the Mouth of Goshen Bay, Bird Island Buoy, and Entrance to Provo Bay sites, which are mostly located toward the south part of the lake, reached the highest relative abundance, varying from 28.6 to 48.3% for cyanobacteria on June 27th when the lake was declared bloom dominated by the UDWQ based on cell counts. On the other hand, sites inside the Provo Buoy, Geneva Discharge, Vineyard Buoy, and Saratoga Springs, which are located toward the north part of the lake, had the highest relative abundance of cyanobacteria varying from 42.6 to 46.3% as observed in the August 9th sampling. Overall, among all sites and sampling occasions, the highest relative abundance for cyanobacteria (48.3%) was detected on June 27th at the Entrance to Provo Bay. Cyanobacteria was the highest phylum in terms of relative abundance during the occurrence of CyanoHABs, while Proteobacteria, Bacteroidetes, and Actinobacteria generally occupied higher relative abundance before or after the blooms (Fig. 2). A trend of decrease in the relative abundance of heterotrophic bacterioplankton was also observed with increases of cyanobacterial relative abundance.
Figure 2.
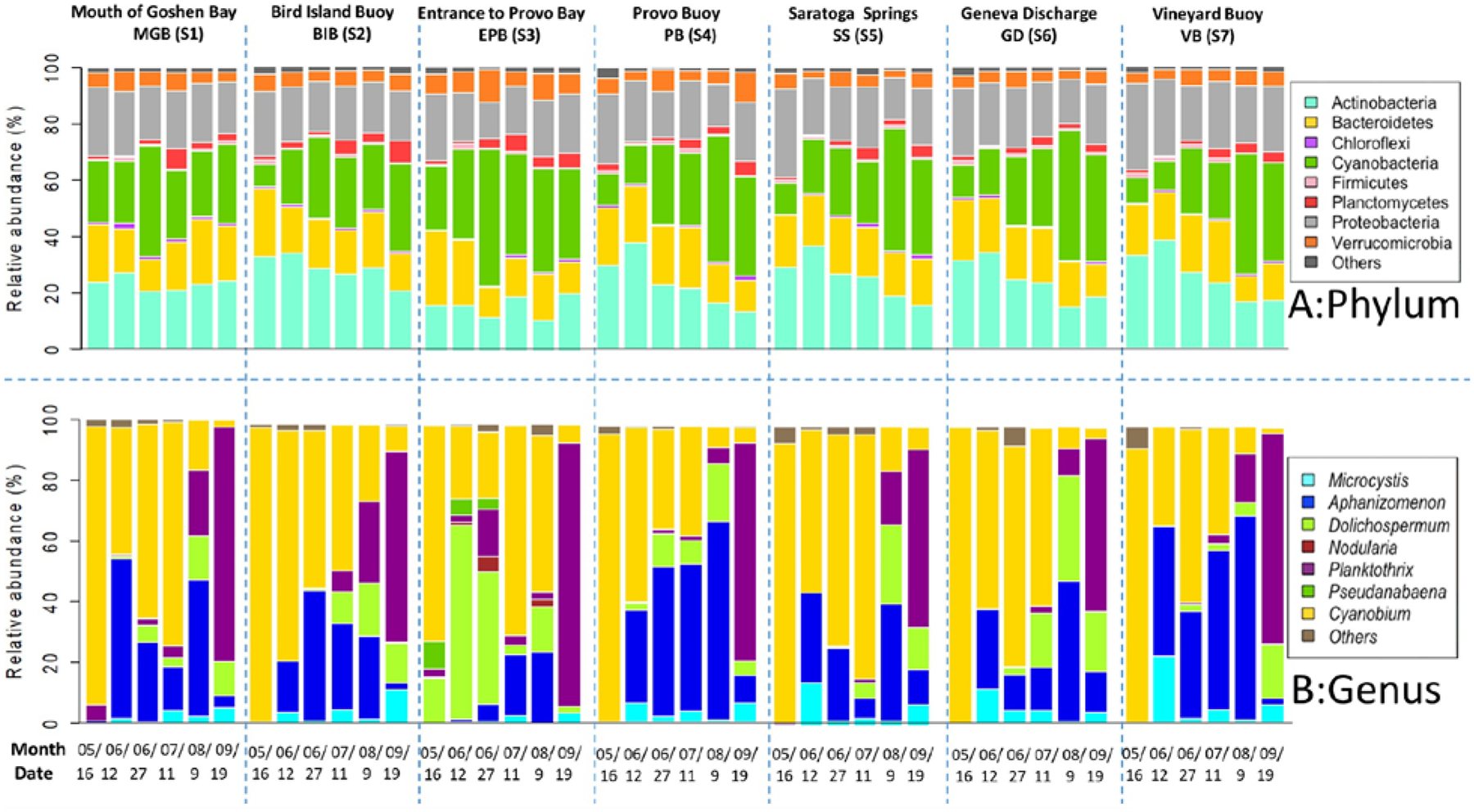
Microbial community composition at the phylum (top panel) and cyanobacterial genus level (bottom panel) from different sampling sites. “Others” contains “unknown,” “unclassified,” and taxa with small relative abundance.
Due to the focus of this work on lake eutrophication and to further classify the taxonomic composition of cyanobacteria, we considered the bacterial assignments at the genus level and compared them within and among sites (Panel B in Fig. 2). Cyanobium, Aphanizomenon, Dolichospermum, Microcystis, and Planktothrix were the dominant genera present at all sites (Fig. 2). Initially, picocyanobacteria Cyanobium (72.2–99.8%) dominated the lake from May until July. The relative abundances of this genera varied from 91.7% at the Mouth of Goshen Bay to a high percentage of 98.2% Bird Island Buoy and Provo Buoy in May, thus representing itself the dominant cyanobacterial genera. In June and July sampling events, the relative abundances of Cyanobium varied between 22.1% and 81.8%, depending upon the site. There is not much information available about the genus Cyanobium. One paper by Komárek et al. (1999) suggests a closer relationship between the picocyanobacteria Synechococcus and Cyanobium with the identical thylakoid arrangement.
As for the bloom-forming genera, Aphanizomenon and Dolichospermum belonging to Nostocales appeared and gradually occupied higher percentages from May end until mid-August. In fact, Aphanizomenon was the dominant genera or equally abundant in late June, July, and August samplings at Bird Island Buoy, Provo Buoy, and Vineyard Buoy sites. Aphanizomenon and Dolichospermum appeared and gradually occupied higher percentages (a maximum of 68.9- for Aphanizomenon and 64.6% for Dolichospermum) until August’s sampling. Microcystis was detected at all sites from June, although its relative abundance varied from 0.27–22.3% depending upon the sampling month (e.g., 22.3% at Vineyard Buoy on June 12th). Except for the first peak detected on June 12th, the relative abundance of this genus was generally high in September, varying from 3.66 to 11.4%. Planktothrix, mostly a non-diazotroph and common toxin producer in temperate lakes, started to appear in August and dominated in September with relative abundances as high as 58.3–87.9% at all sites. The Entrance to Provo Bay was unique as it formed early filamentous cyanobacteria blooms (mostly Dolichospermum) in June, while other sites had a peaked bloom mostly in August.
3.4. Function predictions by PICRUSt
PICRUSt matched 2,961,431 sequences to the Greengenes 13_5 and finally generated 655 features. The weighted NSTI values ranged from 0.105 to 0.169 with a mean value of 0.127 ± 0.014, suggesting the high reliability of predictions (Koo et al., 2017). Zhu et al., 2018 mentioned the NSTI scores of each sample to be varied from 0.11 to 0.17 (mean=0.14), which were mostly lower than the mean NSTI score (0.17) in soil samples reported by Langille et al., 2013, suggesting that the predictions were accurate and reliable. Hence, an average NSTI score of 0.127 ± 0.014 seems reasonably reliable. KEGG pathways of cellular process, environmental information processing, genetic information processing, microbial metabolism, and organismal systems at the highest hierarchy were predicted from the sequencing results. The main metabolisms predicted were amino acid (10.3% – 11.7%), carbohydrate (9.6% – 11.0%), energy (5.9% – 7.5%), membrane transport (10.3% – 11.4%), and replication and repair (7.1% – 7.6%). However, we primarily focused on nutrient-related pathways as these directly reflect the bacterioplankton assimilations and metabolic dynamics. Fig. 3 shows these predictions for nitrogen, phosphatase, phosphotransferase, and photosynthesis-related pathways in different panels. Generally, the “nitrogen metabolism” contains genes that regulate the N-fixation (nif) and inorganic N reductions (Fig. 3A). All of them, as well as some N transporters and regulation genes, regulated N cycles in the lake. As for phosphate and carbohydrate assimilations, two of the important pathways for bacterioplankton are “Phosphonate (phn) and phosphinate metabolism” and “Phosphotransferase system (PTS)” (Fig. 3B, C) (Kotrba et al., 2001; Gomez-Garcia et al., 2011). Both pathways are highly correlated with the transferase and utilization of P and carbohydrate in phytoplankton cells. The PTS system enables bacteria to import sugars by forming P derivatives. However, no pathways were reported for the “Inorganic phosphorus scavenging system (pst)” due to the limitations of the database whereas our functional gene expressions show high abundances of pst between samples (see results later). Photosynthesis was counted relatively higher in August (Fig. 3D). PICRUSt can only predict gene families that are already known and included in the orthology reference used (KEGG KOs) by default. This could be considered as one of the limitations of PICTRUSt. Another limitation of PICRUSt includes any gaps or inaccuracies in pathway annotation or assignments of gene function. For example, many KEGG Orthology groups listed as participating in pathways not found in bacteria or otherwise not reflective of true function. This is simply due to bacteria containing homologs of enzymes showing important roles. Therefore, it is worth carefully checking KEGG pathway annotations to ensure that they are reasonable for any system studied. This result together with higher chl a and cBOD content detected in August, potentially implying higher carbon-fixation activities.
Figure 3.
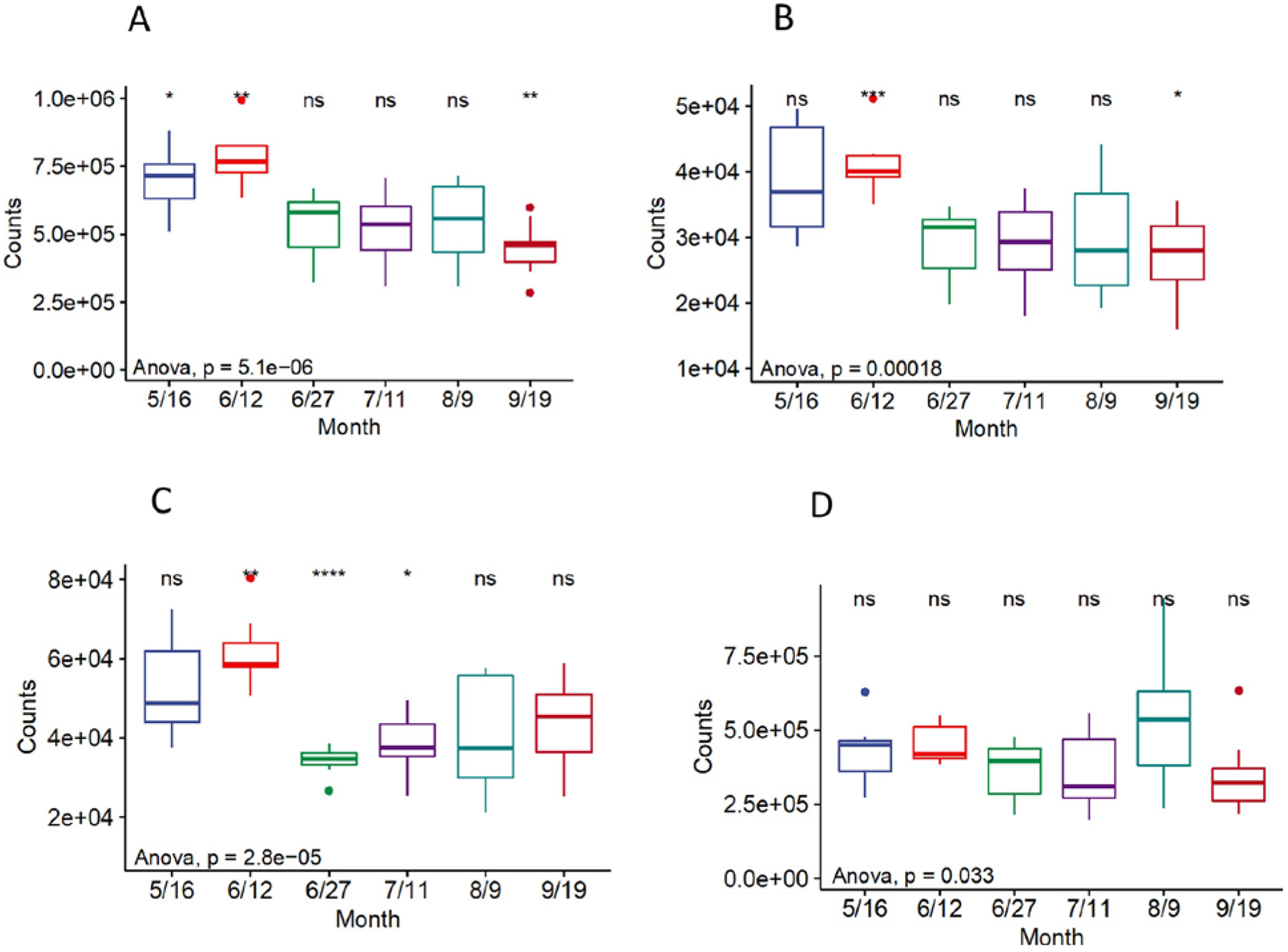
Predicted metabolic pathways at a 99% sequence similarity. (A) Nitrogen Metabolism. (B, C) Carbohydrate/Phosphorus related metabolism. (D) Photosynthesis. Note the differences of y-axis scales. Reference group is “all”. The significance levels were symbolled as: ns: p > 0.05, *: p <= 0.05, **: p <= 0.01, ***: p <= 0.001 ****: p <= 0.0001.
The KOs for N, P, and some carbohydrate-related pathways were further investigated. As for nitrogen cycles, the predicted functions were mainly divided into three groups, namely N-fixation genes (e.g., nif), N reduction-related genes (e.g., nap, nir, nor), and N regulatory proteins (e.g., PII) (Fig. 4). The most abundant counts were nitrate/nitrite reductase subunits, followed by the nif related genes and some specific N transporter or reductase proteins. It is noted that some nif or N-regulatory proteins were elevated at the end of June, early July, and August (e.g., nifX, nifH, nifV), while some are more abundant in May (e.g., nifH, N regulatory protein). By contrast, the main nitrate and nitrite reductase subunits were relatively higher in May and early June and less detectable in August and September, suggesting the availability of inorganic N at the beginning of the summer months.
Figure 4.
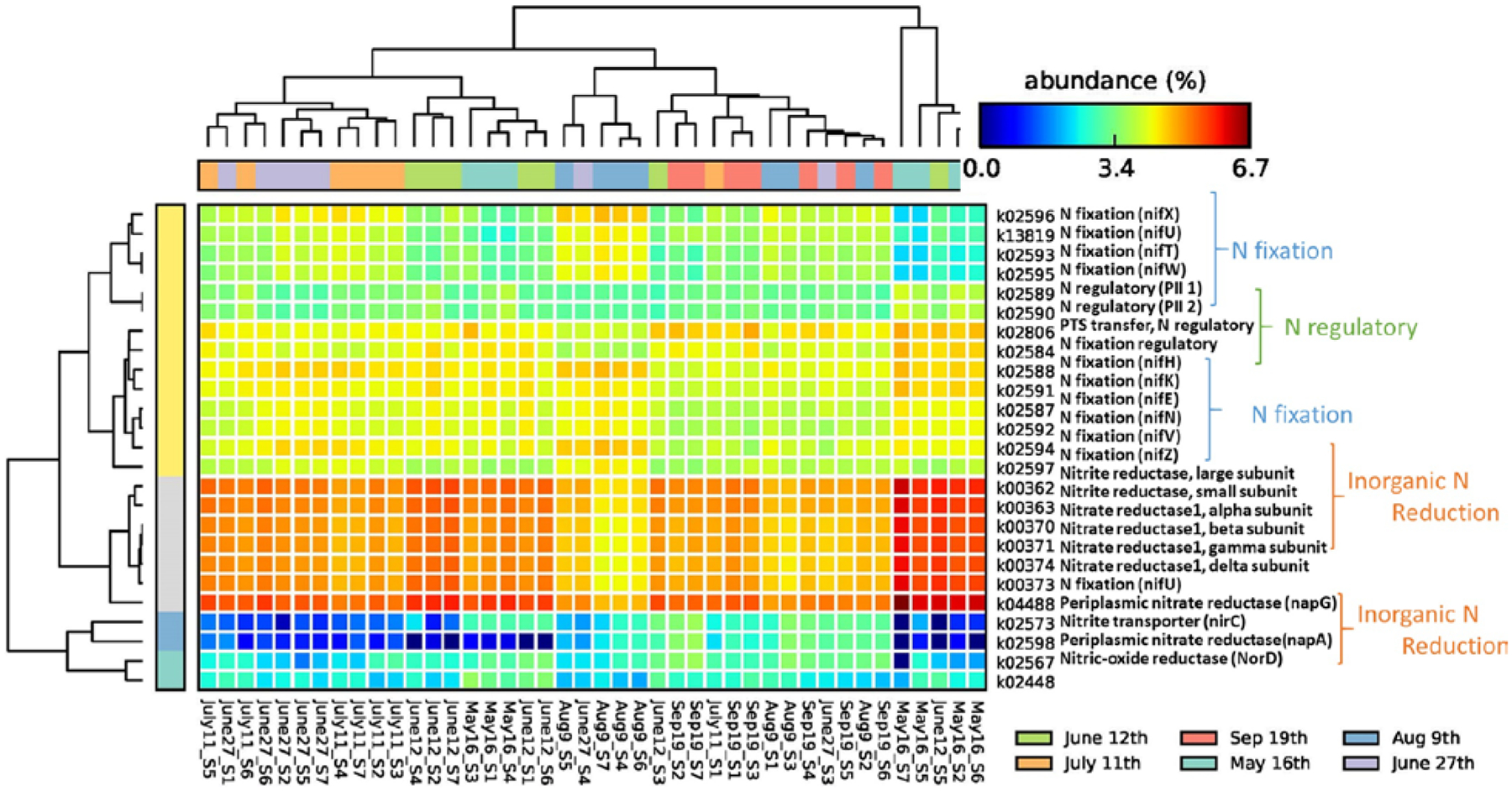
KOs correlated with nitrogen metabolisms at log scale (samples were clustered by date).
As for P and carbohydrate-related functions, the KOs in response to assimilation or coregulated as part of the Pho regulon were mostly studied (Fig. 5). Specifically, polyphosphate kinase (PPK), inorganic pyrophosphatase (PPA), pyrophosphatase (PpaX), guanosine 3′-diphosphate 5′-diphosphate ((p)ppGpp), the inorganic phosphorus transporter (PiT) family, polyphosphate glucokinase (PPGK), and glucose-6-phosphate dehydrogenase (G6PD) were more abundant in May. It is suggested that phytoplankton responded to early summer nutrient stress and started to assimilate P and maybe created storage from the early summer. The exopolyphosphatase (PPX) and fructose-6-phosphate phosphoketolase (F6PPK) counts were elevated in August, indicating the possibility of utilizing stored nutrients during the peak bloom and extreme nutrient limitation conditions. The phosphonate and phosphinate-related proteins were increased in May (phnB, phnP) or August (phnM, phnJ), which could be most active in heterotrophic bacteria and some cyanobacteria for organic P utilization (Dyhrman et al., 2009). Similarly, PTS-related functions were highly abundant, mostly during May or August, for the transportation and phosphorylation of a variety of sugars and sugar derivatives (Deutscher et al., 2014).
Figure 5.
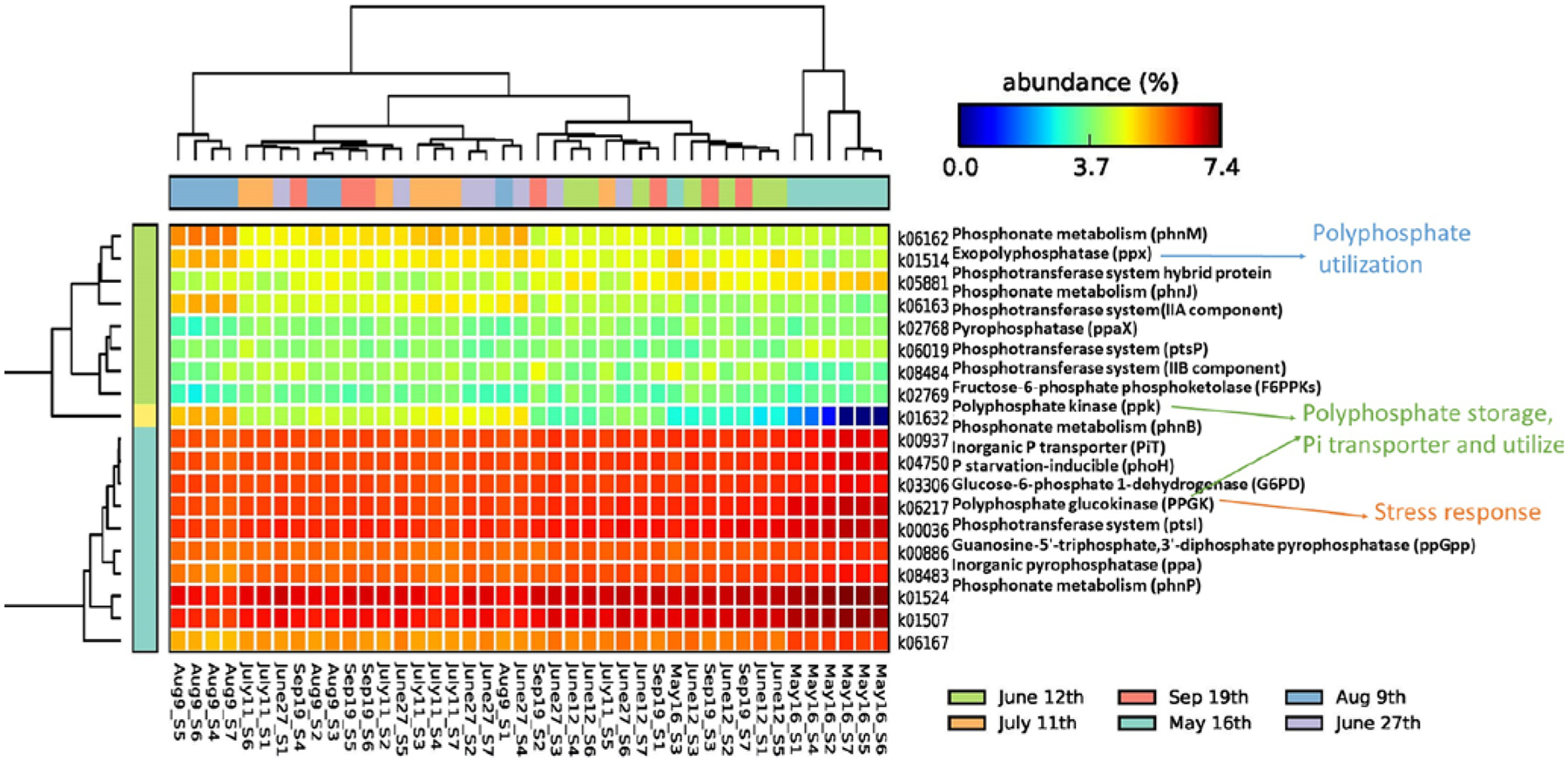
KOs correlated with phosphorus and carbohydrate at log scale (samples were clustered by date).
3.5. Genomic DNA-based gene quantification and mRNA-based gene expressions for nutrient starvation and MC related genes
The Sanger sequencing of cloned plasmids for the mcy gene suggested the presence of Microcystis panniformis FACHB-1757 (14 out of 24 cloned) and Microcystis aeruginosa strain UV027 (7 out of 24 cloned) in the lake. Nevertheless, no direct evidence was found for common mcy gene possessors, such as Planktothrix (Christiansen et al., 2003), Anabaena (Halinen et al., 2007), or Dolichospermum (Teikari et al., 2019). The change of functional genes (e.g., their absolute presence) and their corresponding expressions (based on mRNA) for Microcystis 16S (Mic16S), microcystin-producing (mcyA and mcyG in Microcystis), N-fixing (nif), and high-affinity Pi transport genes (pst) along with time are plotted in Figs. 6 and 7. All standards were amplified at efficiencies of nearly 80%. As for the absolute gene copies based on qPCR of genomic DNA, most sites had increased copies for all five genes with time and peaked in either early June or Aug/Sep (Fig. 6). The trend of the nif gene was consistent with the pst gene, while Mic16S, mcyA, and mcyG could be considered as a group. The copies of nif were mostly close to pst, except for the sites Mouth of Goshen Bay and Bird Island Buoy, where pst gene copies were 1–2 folds more than nif copies. Apart from that, the nif/pst gene copies were significantly higher than Mic16S/mcyA/mcyG at the Entrance to Provo Bay on June 12th (beginning of bloom) and June 27th (peak of bloom). Overall, the copies for nif/pst mostly peaked in the period of June 12th to Aug 9th; however, the highest copy numbers for Mic16S/mcyA/mcyG were found in September.
Figure 6.
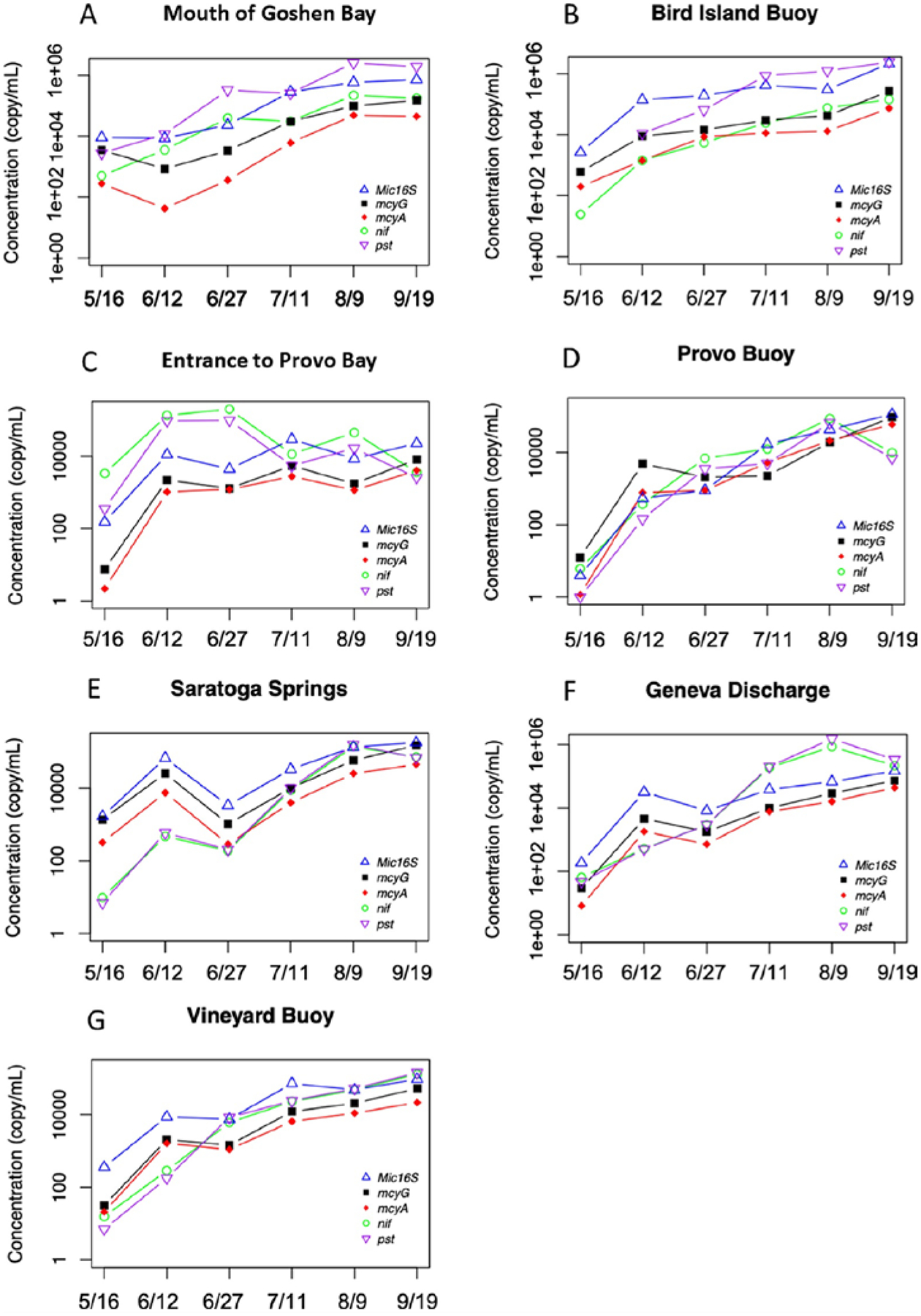
qPCR for the quantification of gene distributions.
Figure 7.
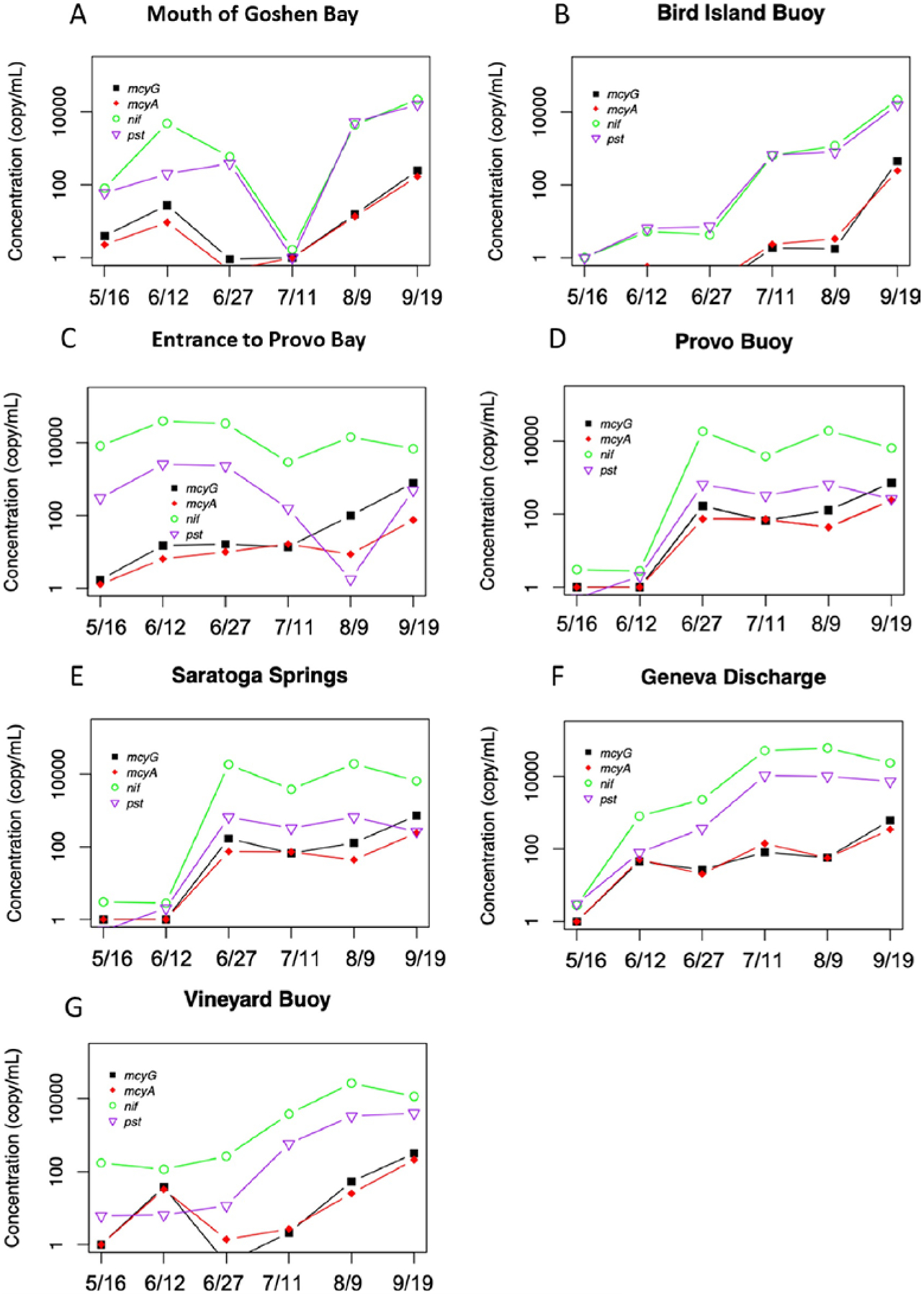
RT-qPCR for the quantification of gene expressions.
Fig. 7 shows the actual expressions of four functional genes based on mRNA and RT-qPCR. The expression of nif/pst genes (0 to 105 copies/mL) were relatively higher than Microcystis’s mcyA/mcyG (0 to 103 copies/mL) groups at all the sites. Apart from the sites Mouth of Goshen Bay and Entrance to Provo Bay that had early significant expressions of nif/pst on May 16th and June 12th, most other sites experienced an increased expression after June 27th. The expressions of toxin-related genes kept increasing until September. Additionally, there was a small peak on June 12th at sites Mouth of Goshen Bay, Entrance to Provo Bay, Geneva Discharge, and Vineyard Buoy. The gene expressions were at the plateau from the end of June to September for the two deeper sites (Provo Buoy and Saratoga Springs). The Mic16S was presented at 4–8 folds and not shown in the graphs.
4. Discussion
4.1. Community successions in the microbial community during CyanoHABs
The analysis of bacterioplankton at the phylum level demonstrated the dominance of Actinobacteria, Bacteroidetes, and Proteobacteria in the summer season (panel A in Fig. 2). The most significant change at the phylum level was the increased relative abundance of Cyanobacteria during the bloom period, which is similar to the findings in 2017 and some other eutrophic lakes (Parulekar et al., 2017; Scherer et al., 2017; Li et al., 2019). Algal blooms generally had a greater effect on community evenness rather than richness, according to the alpha diversity analysis (Berry et al., 2017), which was also detected by the pielou_e test in this study (Figure S1). For the phylum interactions, Cyanobacteria was significantly negatively correlated with Actinobacteria (r = −0.84, p < 0.001), Bacteroidetes (r = −0.67, p < 0.001), and Proteobacteria (r = −0.68, p < 0.001), based on the Spearman correlations.
In terms of the cyanobacterial community, the summer CyanoHABs in the shallow alkaline lake experienced three distinct stages based on cyanobacterial composition (Panel B in Fig. 2). Initially, in May, the lake was mainly composed of the genera Cyanobium belonging to the phylum Cyanobacteria and to the order Synechococcales. Cyanobium morphotypes are among the most abundant cyanobacteria in marine environments. As a consequence, their ability to produce toxins can represent a health risk worldwide (Das and Dash, 2019). Among all cyanobacteria, Cyanobium had a negative correlation with all the other genera, and the most significant correlation was with Planktothrix (r = −0.81, p < 0.001). Compared with larger phytoplanktonic cells, picocyanobacteria have a relatively smaller volume and larger surface-to-volume ratios, which enables faster nutrient uptake and growth rates (Suttle and Harrison, 1986). However, special N-fixation capabilities were not reported in either Cyanobium or Synechococcus (Zehr, 2011), showing them to be outcompeted by other, larger phytoplankton under N starvation.
The second stage was detected in early June when Aphanizomenon and Dolichospermum appeared in Provo Bay and gradually accounted for a higher percentage of the cyanobacterial community (Fig. 2). Toxin-producing Microcystis also appeared at this stage. The active presence Microcystis was further confirmed by absolute mcy gene quantification (Fig. 6) and mcy gene expression (Fig. 7). The bloom of Aphanizomenon and Dolichospermum in Provo Bay continued until the end of June when it gradually spread to the south and north parts of the lake. The early formation of Aphanizomenon and Dolichospermum community in Provo Bay could be attributed to the richer nutrients, which were rapidly consumed by earlier-stage algae and piccocyanobacteria Cyanobium and further triggered nitrogen-limitation conditions promoting the dominance of Aphanizomenon and Dolichospermum. Similar to other eutrophic lakes, this stage could be highly correlated with the N-fixation nature of diazotrophs (Beversdorf et al., 2013; Harke et al., 2015; Lu et al., 2019), which helped dominate the nutrient-limitation conditions. However, different from the lakes that observed cyanobacteria shift to toxic non-diazotrophs soon after the bloom of N-fixers, the relative abundance of Cyanobium again increased at some sites. As a result, the co-occurrence of Cyanobium and Aphanizomenon/Dolichospermum on the entire lake scale lasted for 2–3 months until Aphanizomenon/Dolichospermum became the dominant genus at most sites in August. The success of Aphanizomenon and Dolichospermum could be attributed to their having nif/pst gene clusters (Figs. 6 and 7) when available nitrate and ortho-P were extremely scarce in the lake (Beversdorf et al., 2013; Komárek, 2013; Lu et al., 2019). This was confirmed by our water quality sampling, where we found nitrate-N and ortho-P were non-detectable in August’s and September’s samplings. Further, the coexist of these two genera for a long period of time could be attributed to their similar genomic features, nutrient acquisition systems, and environmental niche (Driscoll et al., 2018).
In the third stage, Planktothrix, a genus typically without heterocyst, appeared in August’s sampling and became the dominant group in September. A significant correlation was found between Planktothrix and Aphanizomenon (r = −0.14, P < 0.01), because Planktothrix dominated after Aphanizomenon in the lake. Along with them, the MCs-producing population was also enlarged and more MCs/MCs-producing genes were detected at this stage (Figs. 6 and 7). The previous sampling generally neglected the late summer Planktothrix blooms (Li et al., 2019) and attributed the presence of MCs mostly to the lysis of cell debris. The successional stages of CyanoHABs were also identified by alpha diversity (Figure S1). The unique nature of Provo Bay and its relatively higher nutrient conditions made it an easy target for blooms.
4.2. Bacterial dynamics in relation to different environmental factors
At the bacterial community level, Proteobacteria, Actinobacteria, and Bacteroidetes were observed to be negatively correlated with the bloom indicators (e.g., pH, DO, Chla, and cBDO), but mostly positively correlated with nutrients (r = 0.03 to 0.55). The varying r values indicated differences in nutrient requirements among organisms (Schauer et al., 2005; Allgaier et al., 2007; Jezbera et al., 2011), but could generally result in N or P deprivation conditions. Cyanobacteria was significantly negatively correlated with Actinobacteria (r = −0.84, p < 0.001), Bacteroidetes (r = −0.67, p < 0.001), and Proteobacteria (r = −0.68, p < 0.001), based on the Spearman correlations. By contrast, Cyanobacteria responded less to the surrounding nutrient conditions. Some cyanobacteria are affected less by the surrounding nutrient conditions and can trap into nutrient pools that are not typically accessible to other phytoplankton (Cottingham et al., 2015). It could have resulted from their possession of high-phosphorus system that activated under low P conditions (Dignum et al., 2005), as well as hydrolysis enzymes (e.g., PPA and PPX) that can release ortho-P from pyro- or poly-phosphates (Gómez-García et al., 2003). The N-fixation strategy is another tool for cyanobacteria to bring new “N” source into the ecosystem and fuel the phytoplankton community (Schindler et al., 2008; Beversdorf et al., 2013; Scott and Grantz, 2013). Among all cyanobacteria, Cyanobium had a negative correlation with all the other genera and the most significant negative correlation was with Planktothrix (r = −0.81, p < 0.001). Cyanobium and Microcystis were positively correlated with nitrate-N (r = 0.23–0.39, p < 0.05) and ortho-P (r = 0.09–0.36, p < 0.05). Compared with larger phytoplanktonic cells, picocyanobacteria have a relatively smaller volume and larger surface-to-volume ratios, which enables faster nutrient uptake and growth rates (Suttle and Harrison, 1986). However, only a few of them have special nutrient management strategies (e.g., N-fixation), causing them to be outcompeted by other, larger phytoplankton under nutrient starving conditions.
Another significant correlation was found between Planktothrix and Aphanizomenon (r = −0.14, P < 0.01), because Planktothrix dominated after Aphanizomenon/Dolichospemum in the lake. As for their response to nutrients, Dolichospemum and Planktothrix were negatively related to all the nutrients measured (r = −0.1 to 0.44). The increased temperature effect was positive for Aphanizomenon (r = 0.53, p < 0.001) but negative for Planktothrix (r = −0.14, p < 0.01). It is reported that Aphanizomenon flos-aquae could grow above 8 °C with an optimum temperature ranging from 23 to 29 °C (Tsujimura et al., 2001). Although Planktothrix favored higher temperatures (> 25 °C) (Lürling et al., 2013; Gomes et al., 2015), our study did observe a dominance of Planktothrix when the temperature fell to around 20 °C.
4.3. The N metabolisms of bacterial community and successions
The N metabolisms mostly correlated the bacterial activities with the N-fixation, nitrite/nitrate reduction, and nitrogen genes regulation during different periods of bloom (Figs. 4, 6, and 7). Cyanobacteria bring new “N” sources into the ecosystem with strategies such as N-fixation under N-limiting conditions (Schindler et al., 2008; Beversdorf et al., 2013; Scott and Grantz, 2013). Although the lake was not N-limited most of the time, except for some occasions in September based on the TDN:TDP ratios (> 16:1, Table S2), potential N-fixers (e.g., Aphanizomenon and Dolichospermum) were some of the most dominant genera found in nitrate and ortho-P depleted conditions in August. Interestingly, Aphanizomenon was positively related to all the nif genes (nifZ, nifW, nifV, nifT, nifK, nifN, nifX, nifE, and nifU) reported in this study (r = 0.60 to 0.79, p < 0.001). Aphanizomenon is long found as a genus dominating summer assemblages and tends to enhance the growth of non-fixing cyanobacteria afterward (Elser et al., 2000; Beversdorf et al., 2013; Lu et al., 2019). Additionally, Dolichospermum is positively linked with nif/nif expressions (r = 0.79 to 0.89, p < 0.001), as it is known for the possession of heterocysts at regular intervals across the filament (Komárek, 2013) and blooms with/without N limitation (Yema et al., 2016; Scherer et al., 2017).
Different from the N-fixation period, nitrate and nitrite reductase subunits were relatively higher in the early summer (Fig. 4). The correlation analysis found that heterotrophic bacterioplankton (Actinobacteria, Bacteroidetes, and Proteobacteria) were positively linked with many nitrate reductase subunits (r = 0.27 to 0.84, p < 0.05) but negatively linked with nif gene and gene expressions (r = −0.21 to −0.73, p < 0.05). Among cyanobacteria, Cyanobium was negatively linked with nif gene/gene expressions (r = −0.65 to −0.70, p < 0.01), but positively correlated with the presence of some nitrate and nitrite reductase subunits (r = 0.34 to 0.49, p < 0.05) (Fig. 4). These cyanobacteria are known for not having heterocysts for N-fixation. This phenomenon could result from the significant composition of nitrate reducers in Proteobacteria, Actinobacteria, Firmicutes, and Cyanobacteria groups (Bru et al., 2007; Palmer and Horn, 2012; Zhao et al., 2015). In fact, except for a few N-fixers, most cyanobacteria rely on ammonia assimilation followed by glutamine synthesis and photosynthetic nitrate assimilations for biosynthesis in CyanoHABs (Andriesse et al., 1990; Flores et al., 2005; Muro-Pastor et al., 2005). Apart from that, cyanobacteria can act as a carbon source for predominant denitrifiers to remove N from the lake (Chen et al., 2012), creating an N-limitation environment. This condition may also favor the long-term stay of N-fixers in the lake.
As for N gene regulation systems, the N regulatory system PII has a higher abundance in May or early June, while the nif-specific regulation protein was also detected to be relatively high during other periods (Fig. 4). The N regulatory system PII did not significantly affect or correlate with any communities, which is surprising as it contains a broad group of signal transduction proteins present in Bacteria, Archaea, chloroplasts in Algae and plants (Herrero et al., 2001; Huergo et al., 2013). By contrast, the nif-specific regulatory protein was negatively correlated with Aphanizomenon and nif gene (r = −0.34, p < 0.05), which is contrary to the finding that detected upregulation of nif- specific proteins under N-fixation conditions (Yan et al., 2010).
4.4. The P and some carbohydrate metabolisms of bacterial community and successions
The P and carbohydrate metabolisms detected a trend to report and respond to nutrient starvation (PPK, (p)ppGpp, PiT, Pho, and G6PD) during early summer, while utilizing the stored polyphosphate (PPX and F6PPK) during the extreme ortho-P scarce period, mostly in August or September (Fig. 5). The dominance of cyanobacteria during blooms could have resulted from their possession of a high-affinity Pi transport system that activated under low Pi conditions (Dignum et al., 2005), as well as hydrolysis enzymes (e.g., PPA and PPX) that can release ortho-P from pyro- or poly-phosphates (Gómez-García et al., 2003). Specifically, the PPX gene is activated at P starvation conditions to degrade poly-P (Adams et al., 2008), which is highly correlated with the presence of Aphanizomenon (r = 0.77, p < 0.001). Similar to PPX, PPA and PPaX are commonly upregulated under Pi-limitation conditions that convert pyrophosphates into two phosphate ions (Gómez-García et al., 2003; Harke and Gobler, 2013). In this study, the PPA and PPaX were activated early in May, when Cyanobium/heterotrophic bacterioplankton were the dominant groups and more pyrophosphate could be present (Fuszard et al., 2013). Apart from that, F6PPK was also highly elevated in August and significantly positively correlated with the presence of Aphanizomenon (r = 0.79, p < 0.001), which was a crucial enzyme involved in the central carbohydrate metabolism in heterofermentative bacteria and recently characterized in Anabaena sp. PCC 7120 (Moriyama et al., 2015). Cyanobacteria (especially Aphanizomenon) was also found positively correlated with “Phosphonate and phosphinate metabolism” related KOs (r = 0.82, p < 0.001), such as PhnM and PhnJ proteins, which are responsible for the hydrolysis of the C-P bond (Metcalf and Wanner, et al., 1993). It is not surprising that cyanobacteria may utilize organic matter (e.g., phosphonates), as it may give them more competition over autotrophs at Pi-limiting conditions (Gilbert et al., 2004; Vahtera et al., 2007; Harke et al., 2012; Harke and Gobler, 2013; Teikari et al., 2018).
By contrast, more functions related to nutrient storage and acquisition into cells were elevated during the early summer, including some stress or starvation-induction functions (Fig. 5). For example, the presence of PPK was slightly correlated with Aphanizomenon (r = 0.08) but highly correlated with heterotrophic bacterioplankton (r = 0.28 to 0.61, p < 0.001), which is a highly conserved region in prokaryotes and responsible for the reversible polymerization of ATP to make polyphosphate for Pi storage in cells (Brown and Kornberg, 2004). PPGK is a polyphosphate-dependent glucokinase found in many organisms, such as diazotrophic cyanobacteria (Klemke et al., 2014; Albi Rodríguez and Serrano, 2015), and was positively correlated with Aphanizomenon (r = 0.33, p < 0.01). Moreover, the G6PD was activated in May, not significantly correlated with Aphanizomenon or Dolichospermum, but it was reported to be reluctant for N-fixation in heterocysts and respiration for vegetative cells under dark conditions (Summers et al., 1995). Furthermore, Pi transporters represented by pst clusters were upregulated starting in May (Dyhrman and Haley, 2006; Pitt et al., 2010). Specifically, the PiT family was only positively correlated with Aphanizomenon (r = 0.33, p < 0.01), while the pst gene/gene expression quantified by qPCR and RT-qPCR were positively correlated with Aphanizomenon, Dolichospermum, and Planktothrix. It is reported that Nostocales (e.g., Aphanizomenon and Dolichospermum) are selectively promoted by high P/low N conditions (Suikkanen et al., 2013; Andersson et al., 2015). Their associations with pst clusters may provide new thoughts into the P assimilation and explain their dominance in the lake’s summer nutrient-limiting conditions (Lu et al., 2019).
The stress and starvation-inducible functions were induced in May (Fig. 5). Specifically, the phosphate starvation-inducible protein PhoH was upregulated during May and faded slightly after (Santos-Beneit, 2015). Although it’s not clear evidence in the study, the activation of pho-like genes would cause an increase in both Pi uptake and polyphosphate accumulation rates (Morohoshi et al., 2002). Moreover, stringent response signaling (p)ppGpp, a stress response alarmone in response to amino acid starvations and mediate polyphosphate accumulation under nutritional stress (Kuroda, 2006; Abranches et al., 2009), was found activated in early May. It is commonly accumulated at the initial stage of heterocyst formation and triggered by darkness in cyanobacteria (Zhang et al., 2013; Hood et al., 2016).
4.5. The potential MCs- producing cyanobacteria and associations with Nostocales
Similar to other studies that observed an interaction of N-fixers and toxin-producing strains (Elser et al., 2000; Beversdorf et al., 2013; Chia et al., 2018; Lu et al., 2019), the lake also experienced a pre-toxic period in June and a post-toxic bloom in September, together with the bloom of Nostocales. The microcystin concentrations (Table S3) increased near the Provo Bay area in June, together with the observation of the first significant evidence of N-fixing genes/gene expressions or N-fixers. It was also correlated with the time when Microcystis initially appeared in the lake. However, the relative abundance of Microcystis decreased during June and again increased in September with enhanced total MCs concentrations at the sites Provo Buoy, Saratoga Springs, Geneva Discharge, and Vineyard Buoy. The post-toxic bloom was observed in September when CyanoHABs were considered to retreat due to temperature effects. The UDWQ also reported the detection of microcystins above 0.1 μg/L in the open water area until November (UDWQ, 2018). Before September, the entire lake experienced 2–3 months’ dominance of Aphanizomenon/Dolichospermum from June to August. Above all, two peaks of Microcystis (up to 106 copies/mL 16S rRNA gene) were observed on June 12th (at some sites) and Sep 9th (the entire lake), which is right after or coexistent with the presence of filamentous cyanobacteria. Since Microcystis coexists or blooms after N-fixing cyanobacteria, it is safe to hypothesize that N-fixation would be one of the main N-providing pathways (Beversdorf et al., 2013).
The correlation analysis showed a significant negative correlation between toxin-producing genes/gene expressions (Figs. 6 and 7) and Cyanobium (r = −0.61 to − 0.78, p < 0.005), while showing positive correlations with Planktothrix (r = 0.62 to 0.73, p < 0.05) and Microcystis (r = 0.39 to 0.62, p < 0.1). Moreover, the presence of MC-LR and MC-RR are significantly correlated with the 16SMic/mcyA/mcyG gene and gene expressions (r = 0.33 to 0.8, p < 0.05). As for the detected MCs producers, Microcystis aeruginosa is commonly presented in many eutrophic lakes, while Microcystis panniformis is a species originated from tropical lakes (Bittencourt-Oliveira et al., 2007). Microcystis panniformis was first reported in Lake Taihu, a temperate lake, implying global warming has driven its distribution from tropical zones to subtropical zones (Zhang et al., 2012). Typically, the proliferation of Microcystis is a phenomenon in many eutrophic lakes and was affected by N availability in the lake (Xu et al., 2010). Under P deficient conditions, the increases of MCs production were observed due to the activation of Pho regulon (Oh et al., 2000; Harke and Gobler, 2013). However, the N-limitation typically decreased the net microcystin-production by decreasing the specific cell division rate (Orr and Jones, 1998; Harke and Gobler, 2013). Microcystis and Planktothrix have distinct morphologies and functions (Guellati et al., 2017), however, they are both bloom-forming, potential MCs-producing cyanobacteria, and their co-habitation was seen in some freshwater lakes (Nixdorf et al., 2003; Paerl et al., 2011; Davis et al., 2014; Francy et al., 2016). Typically, Planktothrix dominated lakes with high TDP and low light conditions (Bonnilla et al., 2012). Their proliferation may be complemented with N sources produced by N-fixers. In this study, lower temperatures in late summer could be one of the main factors favoring Planktothrix (27.5 °C, Lürling et al., 2013) rather than Microcystis or Aphanizomenon, which require relatively higher growth temperatures (Reynolds, 2006).
5. Conclusions
By screening bacterial communities, specific functions, and monitoring water quality changes, we successfully found linkages among these parameters. Our data the suggested long-term dominance of N-fixing cyanobacteria in the eutrophic Utah Lake. The shift of the cyanobacterial community was driven by both environmental factors and metabolism dynamics, especially N and P metabolisms in the lake. The results suggested that the long-term dominance of Aphanizomenon and Dolichospermum in the lake could have been attributed to their activation of the nitrogen fixation (nif) and P-affinity (pst) genes under nutrient stress conditions. Additionally, the cop-presence of Aphanizomenon and Dolichospermum in the lake at different sampling events depicted the functional redundancy of in CyanoHABs. The results suggested how genomic contents can influence cyanobacterial diversity and richness, along with environmental factors such as nutrients. Additionally, cyanobacteria may function in early summer to initiate a starvation alert and store nutrients, while utilizing the stored nutrients or turn on N-fixation during heavy CyanoHABs and nutrient-limiting conditions. Excess N, fixed by diazotrophic filamentous cyanobacteria, could also be the food supplying the succeeding growth of Microcystis and Planktothrix. The detection of Microcystis panniformis, an MCs-producing species originated from the tropical zone, may imply potential climate change in subtropical areas. The correlations with different functions (e.g., organic matter utilization) suggested that cyanobacteria may develop varieties of metabolites to acquire energy. For future suggestions, sampling frequencies could be increased to closely monitor the community successions during CyanoHABs. More attention needs to be paid to late summer community succession and the post-toxic period (August and September). It is suggested that the reduction of nutrient input into the lake alone may not prevent CyanoHABs, but could reduce the toxicity levels by supplying fewer nutrients to non-fixing communities, thus preventing possible community shifting from nontoxic to toxic species in the future. Results acquired from this study could be helpful in identifying mechanisms in freshwater lakes that observed a co-existence or succession of various members of a cyanobacterial community with distinct functions.
Supplementary Material
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The United States Environmental Protection Agency through its Office of Research and Development’s (ORD’s) research programs: Science to Achieve Results (STAR) and Safe and Sustainable Water Resources (SSWR: SSWR 4.01D, 4.3.1 and 4.3.3) funded the research described here. The EPA Grant number to the University of Utah is 83586601 https://finance.apps.utah.edu/uofu/fin/projectlookup?cmd=go&award_num=83586601&status=both. The views expressed in this manuscript are those of authors and not necessarily reflect on the funding agency. It has been subjected to Agency review and approved for publication. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Declaration of Competing Interest
No conflict of interest declared.
References
- Abranches J, Martinez AR, Kajfasz JK, Chávez V, Garsin DA, Lemos JA, 2009. The molecular alarmone (p) ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J. Bacteriol 191 (7), 2248–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams MM, Gómez-García MR, Grossman AR, Bhaya D, 2008. Phosphorus de- privation responses and phosphonate utilization in a thermophilic Synechococcus sp. from microbial mats. J. Bacteriol 190 (24), 8171–8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albi Rodríguez T, Serrano A, 2015. Two strictly polyphosphate-dependent gluco (manno) kinases from diazotrophic Cyanobacteria with potential to phosphory- late hexoses from polyphosphates. Appl. Microbiol. Biotechnol 99, 3887–3900. [DOI] [PubMed] [Google Scholar]
- Allen AE, Booth MG, Verity PG, Frischer ME, 2005. Influence of nitrate avail- ability on the distribution and abundance of heterotrophic bacterial nitrate assimilation genes in the Barents Sea during summer. Aquat. Microb. Ecol 39 (3), 247–255. [Google Scholar]
- Allgaier M, Brückner S, Jaspers E, Grossart HP,2007. Intra-and inter-lake variability of free-living and particle-associated Actinobacteria communities. Environ. Microbiol 9 (11), 2728–2741. [DOI] [PubMed] [Google Scholar]
- Andersson A, Höglander H, Karlsson C, Huseby S, 2015. Key role of phosphorus and nitrogen in regulating cyanobacterial community composition in the northern Baltic Sea. Estuar. Coast. Shelf Sci 164, 161–171. [Google Scholar]
- Andriesse X, Bakker H, Weisbeek P, 1990. Analysis of nitrate reduction genes in cyanobacteria In: Ullrich WR, Rigano C, Fuggi A, Aparicio PJ (Eds.), Inorganic Nitrogen in Plants and Microorganisms Springer, Berlin, Heidelberg. [Google Scholar]
- Apha A, 1999. Standard Methods for the Examination of Water and Wastewater American Public Health Association. Inc., Washington, DC. [Google Scholar]
- Armon RH, Starosvetsky J, 2015. Algal Bloom Indicators In: Armon R, Hänni- nen O (Eds.), Environmental Indicators Springer, Dordrecht. [Google Scholar]
- Bartram J, Chorus I (Eds.), 1999. Toxic Cyanobacteria in Water: a Guide to their Public Health Consequences, Monitoring and Management CRC Press. [Google Scholar]
- Berry MA, Davis TW, Cory RM, Duhaime MB, Johengen TH, Kling GW, …Denef VJ, 2017. Cyanobacterial harmful algal blooms are a biological disturbance to western Lake Erie bacterial communities. Environmental microbiology 19 (3), 1149–1162. [DOI] [PubMed] [Google Scholar]
- Beversdorf LJ, Miller TR, McMahon KD, 2013. The role of nitrogen fixation in cyanobacterial bloom toxicity in a temperate, eutrophic lake. PLoS ONE 8 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittencourt-Oliveira MC, Moura AN, Gouvêa-Barros S, Pinto E, 2007. HIP1 DNA fingerprinting in Microcystis panniformis (Chroococcales, Cyanobacteria). Phycologia 46 (1), 3–9. [Google Scholar]
- Bonilla S, Aubriot L, Soares MCS, Gonzalez-Piana M, Fabre A, Huszar VL, …Kruk C, 2012. What drives the distribution of the bloom-forming cyanobacteria Planktothrix agardhii and Cylindrospermopsis raciborskii? FEMS Microbiology Ecology 79 (3), 594–607. [DOI] [PubMed] [Google Scholar]
- Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet C, Al-Ghalith GA, …Bai Y, 2018. QIIME 2: Reproducible, Interactive, Scalable, and Extensible Microbiome Data Science. PeerJ Preprints (No. e27295v2). [Google Scholar]
- Brown MRW, Kornberg A, 2004. Inorganic polyphosphate in the origin and survival of species. Proc. Natl. Acad. Sci. USA 101, 16085–16087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bru D, Sarr A, Philippot L,2007. Relative abundances of proteobacterial mem- brane-bound and periplasmic nitrate reductases in selected environments. Appl. Environ. Microbiol 73 (18), 5971–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SR, 2005. Eutrophication of aquatic ecosystems: bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 102, 10 0 02–10 0 05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Yang L, Xiao L, Miao A, Xi B, 2012. Nitrogen removal by denitrification during cyanobacterial bloom in Lake Taihu. J. Freshw. Ecol 27 (2), 243–258. [Google Scholar]
- Chia MA, Jankowiak JG, Kramer BJ, Goleski JA, Huang IS, Zimba PV, …Gobler CJ, 2018. Succession and toxicity of Microcystis and Anabaena (Dolichos- permum) blooms are controlled by nutrient-dependent allelopathic interactions. Harmful algae 74, 67–77. [DOI] [PubMed] [Google Scholar]
- Christiansen G, Fastner J, Erhard M, Börner T, Dittmann E, 2003. Microcystin biosynthesis in Planktothrix: genes, evolution, and manipulation. J. Bacteriol 185 (2), 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley DJ, 1999. Biogeochemical nutrient cycles and nutrient management strategies. Hydrobiologia 410, 87–96. [Google Scholar]
- Conley DJ, Paerl HW, Howarth RW, Boesch DF, Seitzinger SP, Havens KE, …& Likens GE (2009). Controlling eutrophication: nitrogen and phosphorus 1014–1015. [DOI] [PubMed] [Google Scholar]
- Cottingham KL, Ewing HA, Greer ML, Carey CC, Weathers KC, 2015. Cyanobacteria as biological drivers of lake nitrogen and phosphorus cycling. Ecosphere 6 (1), 1–19. [Google Scholar]
- Davis TW, Berry DL, Boyer GL, Gobler CJ, 2009. The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8 (5), 715–725. [Google Scholar]
- Davis TW, Watson SB, Rozmarynowycz MJ, Ciborowski JJ, McKay RM, Buller- jahn GS, 2014. Phylogenies of microcystin-producing cyanobacteria in the lower Laurentian Great Lakes suggest extensive genetic connectivity. PLoS ONE 9 (9), e106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S and Dash HR (2019). Microbial diversity in the genomic era 10.1016/C2017-0-01759-7. [DOI]
- Deutscher J, Aké FMD, Derkaoui M, Zébré AC, Cao TN, Bouraoui H, …Joyet P, 2014. The bacterial phosphoenolpyruvate: carbohydrate phosphotransferase system: regulation by protein phosphorylation and phosphorylation-de- pendent protein-protein interactions. Microbiol. Mol. Biol. Rev 78 (2), 231–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignum M, Matthijs HCP, Roel PEL, Laanbroek HJ, Mur LR, 2005. Nutrient limitation of freshwater cyanobacteria In: Huisman J, Matthijs HCP, Visser PM (Eds.), Harmful Cyanobacteria Springer, New York, New York, USA, pp. 65–86. [Google Scholar]
- Dodds WK, Bouska WW, Eitzmann JL, Pilger TJ, Pitts KL, Riley AJ, …& Thornbrugh DJ (2009). Eutrophication of US freshwaters: analysis of potential economic damages 12–19. [DOI] [PubMed] [Google Scholar]
- Downing JA, McCauley E, 1992. The nitrogen: phosphorus relationship in lakes. Limnol. Oceanogr 37 (5), 936–945. [Google Scholar]
- Downing JA, Watson SB, McCauley E, 2001. Predicting cyanobacteria dominance in lakes. Can. J. Fisheries Aquat. Sci 58 (10), 1905–1908. [Google Scholar]
- Driscoll CB, Meyer KA, Šul čius S, Brown NM, Dick GJ, Cao H, …Otten TG, 2018. A closely-related clade of globally distributed bloom-forming cyanobacteria within the Nostocales. Harmful algae 77, 93–107. [DOI] [PubMed] [Google Scholar]
- Drobac D, Tokodi N, Simeunović J, Baltić V, Stani ć D, Svir čev Z, 2013. Human exposure to cyanotoxins and their effects on health. Arch. Ind. Hyg. Toxicol 64 (2), 305–316. [DOI] [PubMed] [Google Scholar]
- Dyhrman ST, Haley ST, 2006. Phosphorus scavenging in the unicellular marine diazotroph Crocosphaera watsonii. Appl. Environ. Microbiol 72 (2), 1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyhrman ST, Benitez-Nelson CR, Orchard ED, Haley ST, Pellechia PJ, 2009. A microbial source of phosphonates in oligotrophic marine systems. Nat. Geosci 2 (10), 696–699. [Google Scholar]
- Elser JJ, Sterner RW, Galford AE, Chrzanowski TH, Findlay DL, Mills KH, …Schindler DW, 2000. Pelagic C: N: P stoichiometry in a eutrophied lake: re- sponses to a whole-lake food-web manipulation. Ecosystems 3 (3), 293–307. [Google Scholar]
- Flores E, Frías JE, Rubio LM, Herrero A, 2005. Photosynthetic nitrate assimilation in cyanobacteria. Photosyn. Res 83 (2), 117–133. [DOI] [PubMed] [Google Scholar]
- Francy DS, Brady AM, Ecker CD, Graham JL, Stelzer EA, Struffolino P, …Loftin KA, 2016. Estimating microcystin levels at recreational sites in western Lake Erie and Ohio. Harmful algae 58, 23–34. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Walker SJ, Vrana KE, 1999. Quantitative RT-PCR: pitfalls and potential. BioTechniques 26 (1), 112–125. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Noda N, Tsuneda S, Saito T, Itayama T, Inamori Y, 2006. Highly sensitive real-time PCR assay for quantification of toxic cyanobacteria based on microcystin synthetase A gene. J. Biosci. Bioeng 102 (2), 90–96. [DOI] [PubMed] [Google Scholar]
- Fuszard MA, Ow SY, Gan CS, Noirel J, Ternan NG, McMullan G, Biggs CA, Reardon KF, …Wright PC, 2013. The quantitative proteomic response of Synechocystis sp. PCC6803 to phosphate acclimation. Aquatic biosystems 9 (1), 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glibert PM, Heil CA, Hollander D, Revilla M, Hoare A, Alexander J, Murasko S, 2004. Evidence for dissolved organic nitrogen and phosphorus up- take during a cyanobacterial bloom in Florida Bay. Mar. Ecol. Prog. Ser 280, 73–83. [Google Scholar]
- Gómez-García MR, Losada M, Serrano A, 2003. Concurrent transcriptional activation of ppa and ppx genes by phosphate deprivation in the cyanobacterium Synechocystis sp. strain PCC 6803. Biochem. Biophys. Res. Commun 302 (3), 601–609. [DOI] [PubMed] [Google Scholar]
- Gomez-Garcia MR, Davison M, Blain-Hartnung M, Grossman AR, Bhaya D, 2011. Alternative pathways for phosphonate metabolism in thermophilic cyanobacteria from microbial mats. ISME J 5 (1), 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AMDA, Azevedo SMFDO, Lürling M, 2015. Temperature effect on ex- ploitation and interference competition among Microcystis aeruginosa, Planktothrix agardhii and, Cyclotella meneghiniana. Sci. World J 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guellati FZ, Touati H, Tambosco K, Quiblier C, Humbert JF, Bensouilah M, 2017. Unusual cohabitation and competition between Planktothrix rubescens and Microcystis sp.(cyanobacteria) in a subtropical reservoir (Hammam Debagh) located in Algeria. PLoS ONE 12 (8), e0183540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkanson L, Bryhn AC, Hytteborn JK, 2007. On the issue of limiting nutrient and predictions of cyanobacteria in aquatic systems. Sci. Total Environ 379 (1), 89–108. [DOI] [PubMed] [Google Scholar]
- Halinen K, Jokela J, Fewer DP, Wahlsten M, Sivonen K, 2007. Direct evidence for production of microcystins by Anabaena strains from the Baltic Sea. Appl. Environ. Microbiol 73 (20), 6543–6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harke MJ, Davis TW, Watson SB, Gobler CJ, 2015. Nutrient-controlled niche differentiation of western Lake Erie cyanobacterial populations revealed via meta- transcriptomic surveys. Environ. Sci. Technol 50 (2), 604–615. [DOI] [PubMed] [Google Scholar]
- Harke MJ, Gobler CJ, 2013. Global transcriptional responses of the toxic cyanobacterium, Microcystis aeruginosa, to nitrogen stress, phosphorus stress, and growth on organic matter. PLoS ONE 8 (7), e69834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harke MJ, Berry DL, Ammerman JW, Gobler CJ, 2012. Molecular response of the bloom-forming cyanobacterium, Microcystis aeruginosa, to phosphorus limitation. Microb. Ecol 63 (1), 188–198. [DOI] [PubMed] [Google Scholar]
- Hecky RE, Kilham P, 1988. Nutrient limitation of phytoplankton in freshwater and marine environments: a review of recent evidence on the effects of enrichment 1. Limnol. Oceanogr 33 (4part2), 796–822. [Google Scholar]
- Heisler J, Glibert PM, Burkholder JM, Anderson DM, Cochlan W, Dennison WC, …Lewitus A, 2008. Eutrophication and harmful algal blooms: a scientific consensus. Harmful algae 8 (1), 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero A, MuroPastor AM, Valladares A, Flores E, 2004. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev 28, 469–487. [DOI] [PubMed] [Google Scholar]
- Herrero A, Muro-Pastor AM, Flores E, 2001. Nitrogen control in cyanobacteria. J. Bacteriol 183 (2), 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschman J, Wong PK, Sei K, Keener J, Kustu S, 1985. Products of nitrogen regulatory genes ntrA and ntrC of enteric bacteria activate glnA transcription in vitro: evidence that the ntrA product is a sigma factor. Proc. Natl. Acad. Sci 82 (22), 7525–7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisbergues M, Christiansen G, Rouhiainen L, Sivonen K, Börner T, 2003. PCR-based identification of microcystin-producing genotypes of different cyanobacterial genera. Arch. Microbiol 180 (6), 402–410. [DOI] [PubMed] [Google Scholar]
- Hogsett M, Li H, Goel R, 2019. The role of internal nutrient cycling in a freshwater shallow alkaline lake. Environ. Eng. Sci 36 (5), 551–563. [Google Scholar]
- Holmroos H, Hietanen S, Niemistö J, Horppila J, 2012. Sediment resuspension and denitrification affect the nitrogen to phosphorus ratio of shallow lake waters. Fundam. Appl. Limnol./Archiv für Hydrobiologie 180 (3), 193–205. [Google Scholar]
- Hood RD, Higgins SA, Flamholz A, Nichols RJ, Savage DF, 2016. The stringent response regulates adaptation to darkness in the cyanobacterium Synechococcus elongatus. Proc. Natl. Acad. Sci 113 (33), E4 867–E4 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huergo LF, Chandra G, Merrick M, 2013. PII signal transduction proteins: nitro- gen regulation and beyond. FEMS Microbiol. Rev 37 (2), 251–283. [DOI] [PubMed] [Google Scholar]
- Isles PD, Xu Y, Stockwell JD, Schroth AW, 2017. Climate-driven changes in energy and mass inputs systematically alter nutrient concentration and stoichiometry in deep and shallow regions of Lake Champlain. Biogeochemistry 133 (2), 201–217. [Google Scholar]
- Jezbera J, Jezberová J, Brandt U, Hahn MW, 2011. Ubiquity of Polynucleobacter subspecies asymbioticus results from ecological diversification. Environ. Micro- biol 13, 922–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungblut AD, Neilan BA, 2006. Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria. Arch. Microbiol 185 (2), 107–114. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, 2000. KEGG: kyoto encyclopedia of genes and genomes. Nucl. Acids Res 28, 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M, Tanabe M, 2014. Data, information, knowledge and principle: back to metabolism in KEGG. Nucl. Acids Res 42, D199–D205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck F, Lepori F, 2012. Can we predict nutrient limitation in streams and rivers? Freshw. Biol 57, 1410–1421. [Google Scholar]
- Klemke F, Beyer G, Sawade L, Saitov A, Korte T, Maldener I, …Volkmer T, 2014. All1371 is a polyphosphate-dependent glucokinase in Anabaena sp. PCC 7120. Microbiology 160 (Pt 12), 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolzau S, Wiedner C, Rücker J, Köhler J, Köhler A, Dolman AM, 2014. Seasonal patterns of nitrogen and phosphorus limitation in four German lakes and the predictability of limitation status from ambient nutrient concentrations. PLoS ONE 9 (4), e96065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komárek J, Kopecký J, Cepák V, 1999. Generic characters of the simplest cyanoprokaryotes Cyanobium, Cyanobacterium and Synechococcus. Cryptogamie Algologie 20 (3), 209–222. [Google Scholar]
- Komárek J, 2013. Süßwasserflora von Mitteleuropa, Bd. 19/3: cyanoprokaryota. 3. Teil/3rd part: heterocytous Genera Süßwasserflora Von Mitteleuropa Spektrum Academischer Verlag, Heidelberg, Germany. [Google Scholar]
- Koo H, Mojib N, Hakim JA, Hawes I, Tanabe Y, Andersen DT, Bej AK, 2017. Microbial communities and their predicted metabolic functions in growth laminae of a unique large conical mat from lake untersee, East Antarctica. Front. Microbiol 8, 1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotrba P, Inui M, Yukawa H, 2001. Bacterial phosphotransferase system (PTS) in carbohydrate uptake and control of carbon metabolism. J. Biosci. Bioeng 92 (6), 502–517. [DOI] [PubMed] [Google Scholar]
- Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD, 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Env- iron. Microbiol 79, 5112–5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda A, 2006. A polyphosphatelon protease complex in the adaptation of Escherichia coli to amino acid starvation. Biosci. Biotechnol. Biochem 70 (2), 325–331. [DOI] [PubMed] [Google Scholar]
- Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, …Beiko RG, 2013. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nature biotechnology 31 (9), 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis WM Jr., Wurtsbaugh WA, 2008. Control of lacustrine phytoplankton by nutrients: erosion of the phosphorus paradigm. Int. Rev. Hydrobiol 93 (4–5), 446–465. [Google Scholar]
- Li H, Alsanea A, Barber M, Goel R, 2019. High-throughput DNA sequencing reveals the dominance of pico-and other filamentous cyanobacteria in an urban freshwater Lake. Sci. Total Environ 661, 465–480. [DOI] [PubMed] [Google Scholar]
- Lu J, Zhu B, Struewing I, Xu N, Duan S, 2019. Nitrogen–phosphorus-associated metabolic activities during the development of a cyanobacterial bloom revealed by metatranscriptomics. Sci. Rep 9 (1), 2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lürling M, Eshetu F, Faassen EJ, Kosten S, Huszar VL,2013. Comparison of cyanobacterial and green algal growth rates at different temperatures. Freshw. Biol 58 (3), 552–559. [Google Scholar]
- Ma J, Wang P, Ren L, Wang X, Paerl HW, 2019. Using alkaline phosphatase activity as a supplemental index to optimize predicting algal blooms in phospho- rus-deficient lakes: a case study of Lake Taihu, China. Ecol. Indic 103, 698–712. [Google Scholar]
- Makino K, Shinagawa H, Amemura M, Kimura S, Nakata A., Ishihama A, 1988. Regulation of the phosphate regulon of Escherichia coli: activation of pstS transcription by PhoB protein in vitro. J. Mol. Biol 203 (1), 85–95. [DOI] [PubMed] [Google Scholar]
- McDonald D, Price MN, Goodrich J, Nawrocki EP, DeSantis TZ, Probst A, …Hugenholtz P, 2012. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. The ISME journal 6 (3), 610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf WW, Wanner BL, 1993. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene 129 (1), 27–32. [DOI] [PubMed] [Google Scholar]
- Moriyama T, Tajima N, Sekine K, Sato N, 2015. Characterization of three putative xylulose 5-phosphate/fructose 6-phosphate phosphoketolases in the cyanobacterium Anabaena sp. PCC 7120. Biosci. Biotechnol. Biochem 79 (5), 767–774. [DOI] [PubMed] [Google Scholar]
- Morohoshi T, Maruo T, Shirai Y, Kato J, Ikeda T, Takiguchi N, …Kuroda A, 2002. Accumulation of inorganic polyphosphate in phoU mutants of Escherichia coli and Synechocystis sp. strain PCC6803. Appl. Environ. Microbiol 68 (8), 4107–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muro-Pastor MI, Reyes JC, Florencio FJ,2005. Ammonium assimilation in cyanobacteria. Photosyn. Res 83 (2), 135–150. [DOI] [PubMed] [Google Scholar]
- Neilan BA, Jacobs D, Blackall LL, Hawkins PR, Cox PT, Goodman AE, 1997. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. Int. J. Syst. Evol. Microbiol 47 (3), 693–697. [DOI] [PubMed] [Google Scholar]
- Ngwa F, Madramootoo C, Jabaji S, 2014. Monitoring toxigenic Microcystis strains in the Missisquoi bay, Quebec, by PCR targeting multiple toxic gene loci. Environ. Toxicol 29 (4), 440–451. [DOI] [PubMed] [Google Scholar]
- Nixdorf B, Mischke U, Rücker J, 2003. Phytoplankton assemblages and steady state in deep and shallow eutrophic lakes—An approach to differentiate the habitat properties of Oscillatoriales In: Phytoplankton and Equilibrium Concept: the Ecology of Steady-State Assemblages. Springer, Dordrecht, pp. 111–121. [Google Scholar]
- Oh HM, Lee SJ, Jang MH, Yoon BD, 2000. Microcystin production by Microcystis aeruginosa in a phosphorus-limited chemostat. Appl. Environ. Microbiol 66 (1), 176–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr PT, Jones GJ, 1998. Relationship between microcystin production and cell division rates in nitrogen-limited Microcystis aeruginosa cultures. Limnol. Oceanogr 43 (7), 1604–1614. [Google Scholar]
- Paerl HW, Hall NS, Calandrino ES, 2011. Controlling harmful cyanobacterial blooms in a world experiencing anthropogenic and climatic-induced change. Sci. Total Environ 409 (10), 1739–1745. [DOI] [PubMed] [Google Scholar]
- Paerl H, 2017. The cyanobacterial nitrogen fixation paradox in natural waters. F10 0 0Research 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer K, Horn MA, 2012. Actinobacterial nitrate reducers and proteobacterial denitrifiers are abundant in N2O-metabolizing palsa peat. Appl. Environ. Micro- biol 78 (16), 5584–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks DH, Tyson GW, Hugenholtz P, Beiko RG,2014. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics 30 (21), 3123–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parulekar NN, Kolekar P, Jenkins A, Kleiven S, Utkilen H, Johansen A, …Sæbø M, 2017. Characterization of bacterial community associated with phytoplankton bloom in a eutrophic lake in South Norway using 16S rRNA gene amplicon sequence analysis. PloS one 12 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaff John. Method 300.0 Determination of Inorganic Anions by Ion Chromatography. USEPA: inorganic Chemistry Branch, Chemistry Research Division; <https://www.epa.gov/sites/production/files/2015-08/documents/method_300-0_rev_21_1993.pdf. [Google Scholar]
- Pitt FD, Mazard S, Humphreys L, Scanlan DJ, 2010. Functional characterization of Synechocystis sp. strain PCC 6803 pst1 and pst2 gene clusters reveals a novel strategy for phosphate uptake in a freshwater cyanobacterium. J. Bacteriol 192 (13), 3512–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core team, R. C. (2013). R: a language and environment for statistical computing
- Reynolds CS, 2006. Ecology of Phytoplankton Cambridge University Press, Cam- bridge. [Google Scholar]
- Santos-Beneit F, 2015. The Pho regulon: a huge regulatory network in bacteria. Front. Microbiol 6, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer M, Kamenik C, Hahn MW, 2005. Ecological differentiation within a cosmopolitan group of planktonic freshwater bacteria (SOL cluster, Saprospiraceae, Bacteroidetes). Appl. Environ. Microbiol 71 (10), 5900–5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PI, Millard AD, Miller A, Schoen R, Raeder U, Geist J, Zwirglmaier K, 2017. Temporal dynamics of the microbial community composition with a focus on toxic cyanobacteria and toxin presence during harmful algal blooms in two South German lakes. Front. Microbiol 8, 2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler DW, 1977. Evolution of phosphorus limitation in lakes. Science 195 (4275), 260–262. [DOI] [PubMed] [Google Scholar]
- Schindler DW, Hecky RE, Findlay DL, Stainton MP, Parker BR, Paterson MJ, …Kasian SEM, 2008. Eutrophication of lakes cannot be controlled by reducing nitrogen input: results of a 37-year whole-ecosystem experiment. Proceedings of the National Academy of Sciences 105 (32), 11254–11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JT, Grantz EM, 2013. N2 fixation exceeds internal nitrogen loading as a phy- toplankton nutrient source in perpetually nitrogen-limited reservoirs. Freshw. Sci 32 (3), 849–861. [Google Scholar]
- Søndergaard M, Lauridsen TL, Johansson LS, Jeppesen E, 2017. Nitrogen or phosphorus limitation in lakes and its impact on phytoplankton biomass and sub- merged macrophyte cover. Hydrobiologia 795 (1), 35–48. [Google Scholar]
- Suikkanen S, Pulina S, Engström-Öst J, Lehtiniemi M, Lehtinen S, Brutemark A, 2013. Climate change and eutrophication induced shifts in northern summer plankton communities. PLoS ONE 8 (6), e66475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers ML, Wallis JG, Campbell EL, Meeks JC, 1995. Genetic evidence of a major role for glucose-6-phosphate dehydrogenase in nitrogen fixation and dark growth of the cyanobacterium Nostoc sp. strain ATCC 29133. J. Bacteriol 177 (21), 6184–6194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suttle CA, Harrison PJ, 1986. Phosphate uptake rates of phytoplankton assemblages grown at different dilution rates in semicontinuous culture. Can. J. Fisheries Aquat. Sci 43 (8), 1474–1481. [Google Scholar]
- Teikari JE, Fewer DP, Shrestha R, Hou S, Leikoski N, Mäkelä M, …Sivonen K, 2018. Strains of the toxic and bloom-forming Nodularia spumigena (cyanobacteria) can degrade methylphosphonate and release methane. The ISME journal 12 (6), 1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teikari JE, Popin RV, Hou S, Wahlsten M, Hess WR, Sivonen K, 2019. Insight into the genome and brackish water adaptation strategies of toxic and bloom–forming Baltic Sea Dolichospermum sp. UHCC 0315. Sci. Rep 9 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura S, Ishikawa K, Tsukada H, 2001. Effect of temperature on growth of the cyanobacterium Aphanizomenon flos-aquae in Lake Biwa and Lake Yogo. Phyco- logical Res 49 (4), 275–280. [Google Scholar]
- UDWQ (2016a). “Utah Lake, Jordan River, Canals Algal Bloom 2016”. deq.utah.gov/water-quality/utah-lake-jordan-river-canals-algal-bloom-2016. Assessed 28 July, 2020.
- UDWQ (2016b). Recommended standard procedures for phytoplankton collection to detect harmful algal blooms, revision 3, effective May 3, 2016
- UDWQ (2018). “Utah Lake Algal Bloom Monitoring 2018”. https://deq.utah.gov/health-advisory-panel/harmful-algal-blooms-habs/utah-lake-jordan-river-canals-algal-bloom-monitoring-2018 Assessed 28 July, 2020.
- Vahtera E, Laamanen M, Rintala JM, 2007. Use of different phosphorus sources by the bloom-forming cyanobacteria Aphanizomenon flos-aquae and Nodularia spumigena. Aquat. Microb. Ecol 46 (3), 225–237. [Google Scholar]
- Vázquez-Baeza Y, Pirrung M, Gonzalez A, Knight R, 2013. EMPeror: a tool for visualizing high-throughput microbial community data. Gigascience 2 (1), 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BA, Dutkiewicz S, Moore CM, Follows MJ, 2013. Iron, phosphorus, and nitrogen supply ratios define the biogeography of nitrogen fixation. Limnol. Oceanogr 58 (6), 2059–2075. [Google Scholar]
- Wu C, Fu FX, Sun J, Thangaraj S, Pujari L, 2018. Nitrogen Fixation by Trichodesmium and unicellular diazotrophs in the northern South China Sea and the Kuroshio in summer. Sci. Rep 8 (1), 2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Wang G, Yang W, Li R, 2010. Dynamics of the water bloom-forming Microcystis and its relationship with physicochemical factors in Lake Xuanwu (China). Environ. Sci. Pollut. Res 17 (9), 1581–1590. [DOI] [PubMed] [Google Scholar]
- Yan Y, Ping S, Peng J, Han Y, Li L, Yang J, …Li D,2010. Global transcriptional analysis of nitrogen fixation and ammonium repression in root-associated Pseudomonas stutzeri A1501. BMC genomics 11 (1), 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yema L, Litchman E, de Tezanos Pinto P, 2016. The role of heterocytes in the physiology and ecology of bloom-forming harmful cyanobacteria. Harmful Algae 60, 131–138. [DOI] [PubMed] [Google Scholar]
- Zehr JP, 2011. Nitrogen fixation by marine cyanobacteria. Trends Microbiol 19 (4), 162–173. [DOI] [PubMed] [Google Scholar]
- Zhao L, Meng Q, Ren L, Liu W, Zhang X, Huo Y, Zhou Z, 2015. Effects of nitrate addition on Rumen fermentation, bacterial biodiversity and abundance. Asian-australas. J. Anim. Sci 28 (10), 1433–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhu B, Wu Z, Xu T, Lu Z,2012. Microcystis panniformis–a newly recorded species of Microcystis in China. J. Lake Sci 24 (4), 647–650. [Google Scholar]
- Zhang SR, Lin GM, Chen WL, Wang L, Zhang CC, 2013. ppGpp metabolism is involved in heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol 195 (19), 4536–4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Wang Y, Shi T, Zhang X, Huang G, Gong J, 2018. Intertidal zonation affects diversity and functional potentials of bacteria in surface sediments: a case study of the Golden Bay mangrove, China. Appl. Soil Ecol 130, 159–168. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


