Abstract
Objectives:
Vaginal estrogen therapy has been shown to decrease the risk of recurrent urinary tract infections in postmenopausal women, but the mechanism of action has not been fully described. Our objectives were to assess whether the postmenopausal urine inflammatory profile changes in response to vaginal estrogen therapy.
Methods:
We prospectively enrolled postmenopausal patients into three groups: 1) currently using vaginal estrogen therapy without a history of recurrent urinary tract infections; 2) history of urinary tract infections, currently using vaginal estrogen therapy; and 3) history of recurrent urinary tract infections, not using vaginal estrogen therapy but willing to start. We followed patients over 6–19 months and collected urine samples at three time points. We performed comprehensive cytopathologic analysis, quantitative urine inflammatory scoring, and enzyme-linked immunosorbent assay for IL-6.
Results:
Seventy patients were recruited (Group 1, n=30; Group 2, n=20; Group 3, n=20). Urine from patients in Groups 2 and 3 demonstrated increased inflammatory cells, debris, and exfoliated urothelial cells. Quantitative urine inflammatory scores and IL-6 were significantly higher in postmenopausal patients with recurrent urinary tract infections not on vaginal estrogen therapy (0.12 vs 0.93, p<0.05) and decreased significantly after initiating vaginal estrogen therapy (0.93 vs 0.38, p<0.05).
Conclusions:
Postmenopausal women with recurrent urinary tract infections on vaginal estrogen therapy demonstrate decreased cell shedding, reduced urine inflammatory scores, and decreased urine IL-6. Modulation of the genitourinary inflammatory profile may represent one mechanism through which vaginal estrogen therapy helps prevent recurrent urinary tract infections in postmenopausal women.
Keywords: recurrent UTI, estrogen therapy, Interleukin 6, aging, bladder, urothelium
Introduction
Urinary tract infections (UTIs) are common, accounting for more than 8 million ambulatory care visits totaling nearly 2 billion dollars per year in the US alone, and are responsible for significant health care utilization in the United States.1,2 Women are disproportionately affected and more likely to experience recurrent UTIs (rUTI), defined as two or more infections in the preceding six months or at least three infections in 12 months4,5. Incidence increases with age, and UTIs account for nearly 25% of all infections in adults over age 65.2–4 More than 50% of UTIs in women over age 55 will recur within one year despite appropriate antibiotic therapy.6 The increased risk in older women has been attributed, in part, to the relative estrogen deficiency that occurs after menopause. Additional risk factors include vaginal prolapse, incomplete bladder emptying, and urinary incontinence.7,8 Age-related immune dysfunction likely further contributes to this population’s increased susceptibility to UTIs.
Interleukin-6, a pro-inflammatory cytokine, is altered in aged mice and humans and appears to play a significant role in immune dysregulation. IL-6 is rapidly induced and recruited to sites of infection or stress. It plays a prominent role in pathogen clearance and tissue repair but also contributes to resolution of the inflammatory response, thus excessive or prolonged IL-6 production can lead to immunopathology and tissue damage.9 In mice following ovariectomy, IL-6 levels are found to be elevated, suggesting that systemic estrogen levels influence IL-6.10 Human studies support a similar relationship. Circulating levels of IL-6 increase with age and menopause and are decreased in the setting of systemic estrogen replacement therapy.9,11,12
To date, cumulative evidence to support the use of estrogen therapy for rUTI prophylaxis has been limited to a heterogenous group of small studies that employ a variety of methods of estrogen administration.13 Vaginal estrogen therapy (VET) has been shown to decrease the risk of rUTIs in postmenopausal women and appears to be more efficacious than systemic estrogen therapy.13 In large part, this is thought to be due to the local effects of vaginal estrogen leading to a reduction in vaginal pH, restoration of the normal lactobacillus population, and overall improvement in tissue integrity, which ultimately results in a shift in the genitourinary environment to resemble a premenopausal state.13 Estrogen may also affect the inflammatory profile in the postmenopausal bladder,14 but no studies have investigated the effect of vaginal estrogen therapy on IL-6 levels. We hypothesize that postmenopausal women with rUTIs demonstrate an exaggerated inflammatory response and increased IL-6 levels compared to postmenopausal women without rUTIs, and this can be modulated with VET to resemble levels seen in postmenopausal women without a history of rUTIs.
Materials and Methods
Recruitment
This prospective cohort study was conducted in the Female Pelvic Medicine and Reconstructive Surgery subspecialty clinic at Washington University in St. Louis from August 2016 to May 2018. Postmenopausal women with and without a history of recurrent UTIs, defined as at least two symptomatic, culture-proven infections (>105 colony-forming units/mL) in the preceding six months or three infections in the past year, were approached for participation. Patients were enrolled into one of three groups based on VET use and history of recurrent UTIs: 1) no history of recurrent UTIs, on VET at enrollment; 2) history of recurrent UTIs, on VET at enrollment; and 3) history of recurrent UTIs, not on VET but willing to start. To be considered postmenopausal, women must have had cessation of menses or a hysterectomy with bilateral oophorectomy at least 12 months preceding enrollment. Exclusion criteria included use of chronic immunosuppressant medications, chronic conditions with associated immune dysfunction including Acquired Immune Deficiency Syndrome (AIDS) and multiple sclerosis, or any contraindication to VET including postmenopausal bleeding, history of estrogen-sensitive malignancy, history of deep vein thrombosis or pulmonary embolus, known liver disease, or history of allergy to sensitivity to vaginal estrogen preparations. Patients were also excluded if they had urinary retention requiring indwelling catheter or clean intermittent straight catheterization. Additionally, patients were excluded from Group 1 if they had a urine culture with >105 colony-forming units/mL in the preceding 12 months, regardless of symptoms. Primary outcomes included a qualitative assessment of inflammation using urothelial cell shedding and quantitative measures of inflammation using urine inflammatory scoring and IL-6 levels. This study was approved by the Institutional Review Board at Washington University in St. Louis (IRB # 201604038).
The electronic medical record was reviewed to identify urine culture results, and records were obtained if performed at an outside lab. Informed consent was obtained from all participants. Patients enrolled into Groups 1 and 2 continued their VET use as prescribed. Patients in Group 3 were started on VET at time of enrollment after the first samples were collected. VET type (conjugated equine estrogen, 0.625mg or estradiol, 1mg) and frequency were at the discretion of the prescribing physicians. Frequency of VET use among the full sample varied from 1–3 times per week with median twice per week. All patients in Group 3 used VET twice per week, and all patients in this study used a cream-based formulation of VET. Patients were instructed to insert the vaginal estrogen cream into the vagina using the applicator supplied with the prescription. Compliance with therapy was assessed by patient report. Demographic information was collected by a questionnaire administered at enrollment and from the electronic medical record.
Sample collection and processing
Urine samples were collected from patients at enrollment and at approximately 3-month intervals for a total of 3 visits and processed by a research assistant according to the following protocol. Urine was collected by straight catheterization, if performed for a clinical indication, or by voided midstream clean catch. A total of 10 mL urine was collected from each patient.
After collection, protease inhibitor (Halt, EDTA-free, Thermo Scientific 78439) was added to the urine sample to achieve 100X dilution. If necessary, the specimen was stored at −4°C at this stage until processing could be completed. Samples were placed on a rotating mixer until uniformly mixed then aliquoted into 2ml aliquots and stored in labeled cryotubes at −80°C.
Cytopathologic analysis and inflammatory scoring
If previously frozen, urine samples were allowed to thaw to room temperature, then 100 μl was cyto-centrifuged onto positively-charged glass slides. Papanicolaou staining was performed and samples were examined using light microscopy. Urine inflammatory scoring was performed according to established protocols.15,16 Briefly, the number of polymorphonuclear leukocytes (PMN) per high power field (hpf) were counted and assigned a semi-quantitative score ranging 0–4 as previously published with higher scores indicating more significant inflammation.15,16 Urothelial cell shedding was subjectively assessed. Samples from patients with an acute UTI, defined as UTI-like symptoms reported by the patient and a corresponding positive urine culture (>105 CFU/mL), at time of sample collection were excluded from the analysis to avoid confounding the inflammatory score results. Samples from these patients were collected after completion of appropriate antibiotic therapy as determined by their treating physician.
Enzyme-Linked Immunosorbent Assay (ELISA)
Human IL-6 Quantikine HS ELISA Kit (Catalog #: HS600B. R&D systems, Minneapolis, MN) and creatinine colorimetric assay kit (Catalog #:500701, Cayman chemical, Michigan, MI) were used according to the manufacturer’s instructions. Samples from patients with an acute UTI at the time of sample collection were excluded from the analysis to avoid confounding the ELISA results.
Statistical analysis
One-way ANOVA with Tukey’s posttest was used to compare continuous variables between the three groups. Categorical variables were compared using Chi-square and Fisher’s exact test as appropriate. Statistical significance was set at a p value of <0.05.
Sample size determination
Sample size was determined a priori based on the following assumptions: 1) IL-6 is log-normally distributed in women; 2) the standard deviation of the log-transformed distribution of IL-6 is no greater than 0.76 ng/ml; and 3) the standard deviation of the log-transformed distribution of IL-6 change is no greater than 0.28 ng/ml. Based on these assumptions and prior work in Dr. Mysorekar’s lab16, we would need 20 patients per group for 80% power to detect: 1) a minimum two-fold change in IL-6 between each group, and 2) at least a 30% minimum difference in IL-6 levels between time points, which we believe is clinically significant.
Results
Patient population
A total of 70 postmenopausal patients were enrolled from 93 potentially eligible patients; 30 without history of rUTI who were already on VET (Group 1), and 40 with rUTI. Of the 40 with rUTI, 20 were already on VET (Group 2) and 20 were not on VET but willing to start (Group 3). Potentially eligible patients were ultimately found to be ineligible for the following reasons: 1) immunosuppressant medication use (n=10); 2) incomplete bladder emptying requiring clean intermittent self-catheterization (n=6), immunocompromised condition (n=2); and 3) presence of a vesicovaginal fistula (n=2). Three eligible patients declined participation. Patients in each group were similar in age and body mass index (BMI, kg/m2, Table 1). Recurrent UTI history and VET use at enrollment were consistent with the groups to which the participants were enrolled. Most patients were using VET twice per week. For patients with history of rUTI, the predominant organism on the most recent culture prior to enrollment was recorded (Table 1). Patients with rUTI and already on a VET regimen (Group 2) showed a predominance of E. coli, while there was not a predominant organism in the group not yet on VET (Group 3). Many patients were on other prophylactic agents in addition to VET (Table 1). There was no significant difference in use of prophylactic agents overall or specific prophylactic agents between groups with rUTI. No patients were using systemic estrogen therapy.
Table 1.
Demographic characteristics of enrolled patients
| Group 1 −rUTI/+VET (n=30) |
Group 2 +rUTI/+VET (n=20) |
Group 3 +rUTI/−VET (n=20) |
|
|---|---|---|---|
| Age [mean (SD)] | 75.4 (7.3) | 74.3 (7.1) | 71.9 (8.5) |
| BMI [mean (SD)] | 27.0 (4.0) | 29.7 (7.0) | 29.8 (5.2) |
| Recurrent UTI (% yes) | 0 | 100 | 100 |
| VET at enrollment (% yes) | 100 | 100 | 0 |
| Frequency of VET use [median (range)] | 2 (1–3) | 2 (2–3) | -- |
| Most recent uropathogen [n(%)] | |||
| E. coli | -- | 11 (55) | 5 (25) |
| K. pneumoniae | -- | 3 (15) | 4 (20) |
| Enterobacter spp | -- | 0 (0) | 2 (10) |
| Other* | -- | 6 (30) | 9 (45) |
| Other rUTI prophylactic agents | 0 | 15 (75) | 11 (55) |
| D-mannose | 0 | 13 (65) | 8 (40) |
| Methenamine hippurate | 0 | 6 (30) | 3 (15) |
| Vitamin C | 0 | 10 (50) | 6 (30) |
| Cranberry supplementation | 0 | 4 (20) | 2 (10) |
Group 1: no history of recurrent urinary tract infection (rUTI), on vaginal estrogen therapy (VET). Group 2: history of rUTI, on VET. Group 3: history of rUTI, not on VET. BMI, body mass index. No difference between groups in age or BMI (p>0.05 for both). No difference between Group 1 and 2 in overall use of other rUTI prophylactic agents or specific agents (D-mannose, methenamine hippurate, vitamin C, cranberry, p>0.05 for all).
includes Enterococcus spp, Group B streptococcus, actinobactim, coagulase negative staphylococcus, Aerococcus, salmonella, pseudomonas aerongenes, streptococcus viridans
95% of patients completed three follow-up visits within 6–8 months. Follow-up extended to 12 months in 22% of patients, and 3 patients completed their follow-up at greater than 12 months. Patient-reported compliance with VET was high for patients in each group at follow-up (Group 1: 97.5%, Group 2: 95%, Group 3: 95%). During the study period, a total of 24 patients experienced 58 symptomatic, culture-proven UTIs: 15 patients in Group 2 (62.5%) and 9 patients in Group 3(37.5%). Frequency of UTI was similar between patients in Group 2 (36 UTIs in 15 patients, 2.4 UTIs/patient; median 2 UTIs/patient, interquartile range (IQR) 1–3) and Group 3 (22 UTIs in 9 patients, 2.4 UTIs/patient; median 3 UTIs/patient, IQR 1–3). No patients in Group 1 experienced a UTI.
Cytopathologic analysis and urine inflammatory scoring
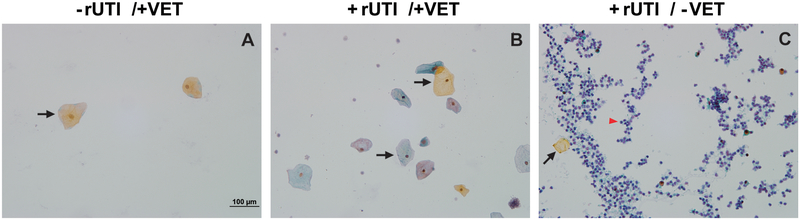
On subjective cytopathologic analysis, urine from postmenopausal patients without a history of rUTI showed scant urothelial cell shedding (Figure 1A). Patients with recurrent UTI, however, demonstrated increased inflammatory cells, debris, and exfoliated urothelial cells (Figure 1B and C). This finding is most pronounced in the patients not on VET (Figure 1C).
Figure 1. Postmenopausal women with a history of recurrent UTI not on vaginal estrogen therapy display increased urothelial cell shedding and inflammatory cell infux in the urine.
(A) Scant urothelial cell shedding and debris is seen in patients without recurrent urinary tract infections (rUTIs) already on vaginal estrogen therapy (VET). (B) Slightly increased urothelial cell shedding, inflammatory cells, and debris are seen in patients with rUTIs. (C) Patients with rUTIs not on VET demonstrate significantly more urothelial shedding, inflammatory cells, and debris. Black arrow, sloughed urothelium; red arrow head, neutrophils.
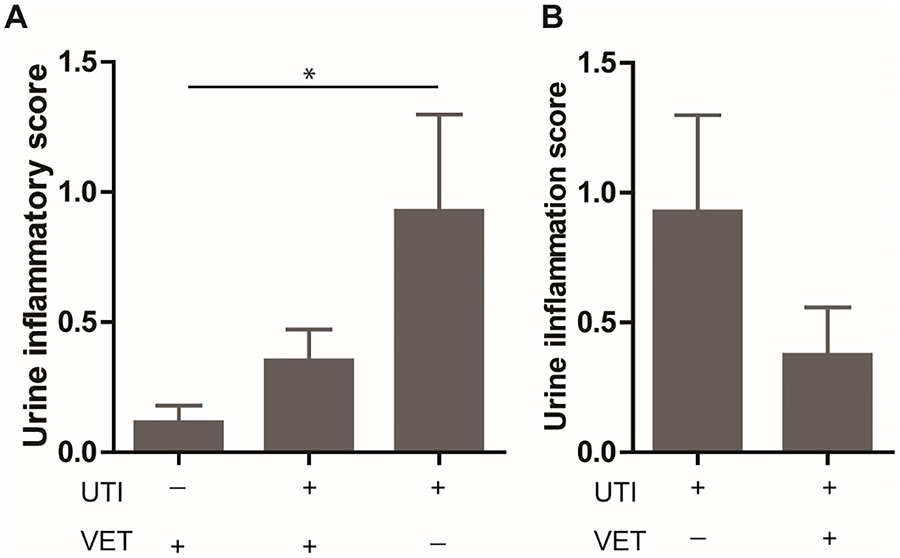
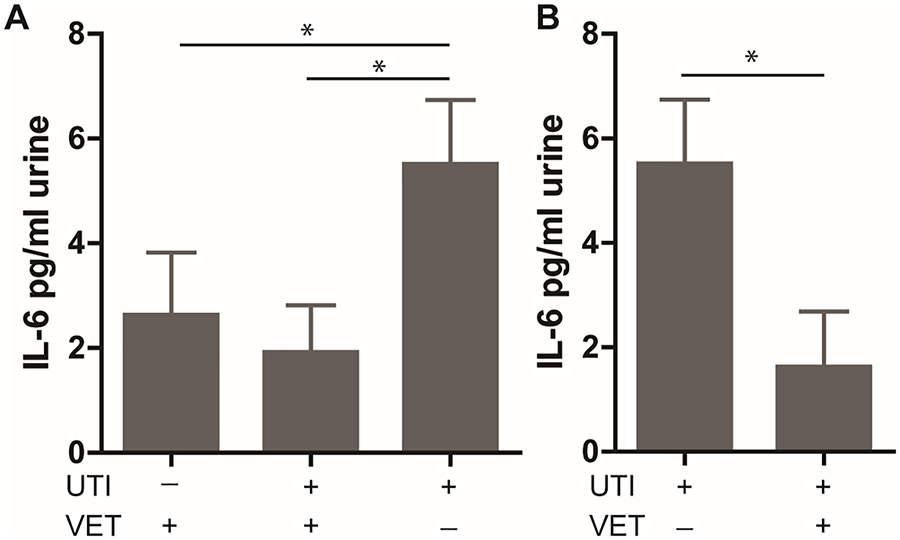
At baseline, quantitative urine inflammation scores were significantly higher in patients with rUTI not on VET (Group 3, Figure 2A) compared to patients without rUTI on VET (Group 1; p<0.05). Urine IL-6 levels were significantly higher in the patients with recurrent UTIs not VET (Group 3, Figure 3A) compared to patients without rUTI on VET (Group 1, p<0.05) and patients with rUTI on VET (Group 2, p<0.05).
Figure 2. Postmenopausal women with a history of recurrent UTI not on vaginal estrogen therapy display increased inflammatory cell influx in the urine.
Representative samples from each group. (A) At baseline, patients with rUTI demonstrate significantly higher urine inflammation scores compared to patients without rUTI (0.93 vs 0.12). (B) Follow-up evaluation demonstrated significant decrease in urine inflammation scores after 3 months of VET in patients with rUTI (0.93 vs 0.38). rUTI, recurrent urinary tract infections; VET, vaginal estrogen therapy. Bars represent mean ± SEM. *p<0.05.
Figure 3. Vaginal estrogen therapy decrease IL-6 production in postmenopausal women with a history of recurrent UTI.
(A) At baseline, patients with rUTI not on VET demonstrate higher IL-6 in the urine than patients already on VET with (Group 1) and without (Group 2) VET. (B) Urine IL-6 levels decreased significantly after 3 months of VET in patients with rUTIs. rUTI, recurrent urinary tract infections; VET, vaginal estrogen therapy. Bars represent mean ± SEM.*p<0.05.
After initiation of VET, urothelial cell shedding, and the presence of debris and inflammatory cells decreased. Quantitative urine inflammation scores also decreased (p<0.05, Figure 2B). Additionally, urine IL-6 levels decreased significantly after initiation of VET (p<0.05, Figure 3B). In order to explore whether urine inflammation scores were related to UTI occurrence, follow up quantitative urine inflammation scores were compared between groups based on whether a UTI was experienced during the study period. There was no significant difference in urine inflammation score between patients that did and did not experience a UTI during the study period (Table 2).
Table 2. Follow up urine inflammatory scores by Group and whether or not a UTI was experienced during the course of the study.
No samples were collected during an acute UTI or during a course of antibiotics for a UTI. Patients who experienced a UTI during the study period submitted samples after completion of a course of appropriate antibiotic therapy. In Group 2, patients with a history of rUTI who were on VET at study initiation, there were 36 UTIs in 15 patients during the study period. There was no difference in urine inflammatory score between those who did and did not experience a UTI during the study period (p=0.35). In Group 3, patients with a history of rUTI who were not on VET at study initiation but started at enrollment, 9 patients experienced a total of 22 UTIs. There was no difference in urine inflammatory score between those who did and did not experience a UTI during the study period (p=0.47). There was no difference between follow-up urine inflammatory score in patients in Group 2 and Group 3 who experienced a UTI during the study period (p=0.75) and those that did not experience a UTI during the study period (p=0.17).
| Group 1 -rUTI/+VET |
Group 2 +rUTI/+VET |
Group 3 +rUTI/-VET |
||||
|---|---|---|---|---|---|---|
| UTI | No UTI | UTI | No UTI | UTI | No UTI | |
|
Inflammatory Score Mean (SD) |
n/a | 0.08 (0.41) | 0.44 (1.33) | 0 (0) | 0.63 (0.92) | 0.33 (0.52) |
Discussion
Vaginal estrogen therapy has been shown to be an effective prophylaxis for rUTI in postmenopausal women, and our data suggests it does so, in part, through modulation of the inflammatory profile as illustrated in our findings with increased IL-6 levels, and reduction in urine inflammation. Recurrent urinary tract infections are common in postmenopausal women, and can be associated with significant morbidity. Treatment usually requires antibiotics, which can be associated with side effects and intolerance. In particular, antibiotic resistance is increasing, and the need for stronger antibiotics with potential for more severe side effects is a concern in this population.17,18 An effective, non-antibiotic prophylactic regimen is thus imperative.
Compared to postmenopausal women without history of rUTI, patients with rUTI demonstrated evidence of an enhanced urine inflammatory response. Excess urothelial cell shedding, which occurs in postmenopausal women with rUTI, appears to be reduced after VET. While urothelial cell exfoliation is an important host defense to clear invading pathogens, excessive exfoliation may impair barrier function and promote increased risk of persistent or recurrent infection and formation of quiescent intracellular reservoirs.14,19 Additionally, levels of the proinflammatory cytokine, IL-6, are associated with vaginal estrogen supplementation. Systemic estrogen administration has been shown to affect serum IL-6 levels,14 but to our knowledge, this is the first data to demonstrate an association between vaginal estrogen therapy and urine IL-6. This finding supports an additional mechanism through which VET prevents rUTI and may support a role for VET in other, noninfectious, inflammatory bladder conditions.
The findings of this study are strengthened by our inclusion of postmenopausal women with symptomatic, culture-proven urinary tract infections with documented culture results. Further, our predefined criteria for postmenopausal status, strict rUTI definition, and exclusion of patients with chronic conditions that could influence their systemic inflammatory profile ensure we have correctly characterized these patients. We allowed VET use to be prescribed and continued according to the clinical recommendations by the patients’ providers. While allowance of both conjugated equine estrogen and estradiol in this study may introduce some heterogeneity in VET type and dose, this likely strengthens the generalizability of our findings.
This study is limited by our lack of a control group of postmenopausal women without rUTIs who are not using VET. As a result, we are unable to evaluate the influence of VET on urine cytopathology or IL-6 levels outside the setting of rUTI. Because we were most interested in understanding the role of VET as an inflammatory mediator in the setting of rUTI, we felt this comparison was sufficient for the purpose of this study. While we have data on the number and frequency of culture-proven infections after starting VET, we do not have complete data on this variable for patients with rUTI prior to starting VET aside from cultures sufficient to diagnose rUTI. As a result, we are unable to report a quantitative decrease in UTI occurrence or frequency with the initiation of VET. Furthermore, follow-up was limited to less than one year in the majority of patients, so we are unable to draw definitive conclusions on the effect of VET on UTI recurrence in this group.
Frequency of UTI was similar among postmenopausal patients with a history of rUTI already on VET and those who initiated VET as part of this study (2.4 UTIs/patient). Since we do not have data on the duration of VET use prior to enrollment for patients in Group 2 we are unable to analyze the effect of duration of VET use on risk of UTI recurrence. Nevertheless, the similar frequency of UTI recurrence was similar between groups and samples were excluded from patients with acute UTIs, thus we would suggest that acute UTI did not significantly contribute to the quantitative inflammatory results we present here. Furthermore, when urine inflammatory scores were analyzed by UTI occurrence within and between groups with a history of rUTI, the urine inflammatory scores did not differ significantly suggesting that the decrease in urine inflammatory score associated with the initiation of VET was not simply due to a decrease in the frequency of UTI. Our sample is likely underpowered to fully investigate the difference in urine inflammatory scores based on UTI occurrence, and further investigation into this preliminary finding is warranted. If found to be true, this would lend further support to modulation of the inflammatory profile as an additional mechanism through which VET helps reduce the risk of rUTI in postmenopausal women.
Finally, many patients in this cohort were on additional prophylactic agents for rUTI including D-mannose, cranberry tablets, and methenamine hippurate. and compliance with VET use was measured using patient report, which may introduce additional error due to recall. The initiation of these agents often coincided with the initiation of VET and was not restricted as part of participation in this study. It is possible that these additional prophylactic agents actually exert a synergistic effect with VET on prevention of rUTIs. Because of our limited sample size, we were unable to further explore these potential interactions. Studies to determine the relative contributions of the additional agents are ongoing as are efforts to increase the number of patients to control for the use of these other agents.
We present compelling evidence to support a role for VET in the inflammatory response as one mechanism through which this agent effectively prevents rUTI. Moving forward, additional studies are needed to determine whether VET influences serum IL-6 levels. Luthje et al and Wang et al were able to show a decrease in serum IL-6 after systemic estrogen supplementation.10,14 Since VET is known to act locally with minimal systemic absorption20 one would expect serum IL-6 to be unaffected by local application, however, this remains to be investigated. Additionally, assessment of urine cytopathology and IL-6 levels in postmenopausal women without rUTI and not using VET would strengthen the findings presented here. This study provides a strong foundation on which to continue to explore the effect of VET on the inflammatory milieu of the postmenopausal bladder, especially in the setting of rUTI. Additional investigation into the effect of VET on other inflammatory mediators will further contribute to our understanding of the mechanism of action of this agent and may elucidate a role for this therapy in other non-infectious bladder conditions. Nevertheless, VET remains an effective, non-antibiotic agent for use in postmenopausal women with rUTI and should be considered early in the treatment algorithm for these patients.
Acknowledgements
We thank Ms. Zoe Jennings for her assistance with patient recruitment, sample processing, and data collection. This work was supported by the Washington University Institute of Clinical and Translational Sciences grant UL1TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) (JLL and IUM); NIH/NIDDK Developmental Center for Interdisciplinary Research in Benign Urology Award P20DK119840 (IUM) and NIH/NIA R01AG052494; and NIH Reproductive Epidemiology Training Grant (T32HD055172–08)(MM).
Footnotes
This work was presented at the International Continence Society Annual Meeting in Florence, Italy, September 12–15, 2017 and the American Urogynecologic Society Annual Meeting in Providence, Rhode Island, October 3–7, 2017.
FINANCIAL DISCLAIMER/CONFLICT OF INTEREST: NONE
Contributor Information
Melanie R. MEISTER, St. Louis, Missouri; Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, Washington University School of Medicine.
Caihong WANG, St. Louis, Missouri; Department of Obstetrics & Gynecology, Division of Basic Research, Center for Reproductive Health Sciences, Washington University School of Medicine.
Jerry L. LOWDER, St. Louis, Missouri; Department of Obstetrics & Gynecology, Division of Female Pelvic Medicine and Reconstructive Surgery, Washington University School of Medicine.
Indira U. MYSOREKAR, St. Louis, Missouri; Center for Reproductive Health Sciences, Department of Obstetrics & Gynecology, Pathology & Immunology, Washington University School of Medicine.
References
- 1.Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat 2011; 169:1–38 [PubMed] [Google Scholar]
- 2.Foxman B Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113:5–13S. [DOI] [PubMed] [Google Scholar]
- 3.Ruben FL, Dearwater SR, Norden CW, et al. Clinical infections in the noninstitutionalized geriatric age group: methods utilized and incidence of infections. The Pittsburgh Good Health Study. Am J Epidemiol 1995; 141(20): 145–157 [DOI] [PubMed] [Google Scholar]
- 4.Foxman B, Barlow R, D’Arcy H, et al. Urinary tract infection: self-reported incidence and associated costs. Ann Epidemiol. 2000; 10:509–515. [DOI] [PubMed] [Google Scholar]
- 5.Caljouw MA, den Elzen WP, Cools HJ, Gussekloo J. Predictive factors of urinary tract infections among the oldest old in the general population. A population-based prospective follow-up study. BMC Med 2011; 9: 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta K, Trautner BW. Diagnosis and management of recurrent urinary tract infections in non-pregnant women. BMJ 2013; 346: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012; 366: 1028–37 [DOI] [PubMed] [Google Scholar]
- 8.Rowe TA, Juthani-Mehta M. Urinary tract infection in older adults. Aging Health 2013; 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maggio M, Guralnik J, Longo DL Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 2006; 61: 575–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Symington JW, Ma E, et al. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect Immun 2013; 81(3): 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim OY, Chae JS, Paik JK, et al. Effects of aging and menopause on serum interleukin-6 levels and peripheral blood mononuclear cell cytokine production in healthy nonobese women. Age 2012; 34: 415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Straub RH, Hense HW, Andus T, et al. Hormone replacement therapy and interrelation between serum interleukin-6 and body mass index in postmenopausal women: a population-based study. J Clin Endocrin Metab 2000; 85(3): 1340–1344 [DOI] [PubMed] [Google Scholar]
- 13.Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database of Systematic Reviews 2008, Issue 2 Art. No.: CD005131. DOI: 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Luthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med. 2013; 5(190):190ra80. [DOI] [PubMed] [Google Scholar]
- 15.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog 2010; 6(8): e1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma E, Vetter J, Bliss L, et al. A multiplexed analysis approach identifies new association of inflammatory proteins in patients with overactive bladder. Am J Physiol Renal Physiol. 2016; 311(1): F28–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhanel GG, Hisanaga TL, Laing NM, et al. , NAUTICA Group. Antibiotic resistance in outpatient urinary isolates: final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA). Int J Antimicrob Agents. 2005; 26(5): 380–388 [DOI] [PubMed] [Google Scholar]
- 18.Swami SK, Liesinger JT, Shah N, et al. Incidence of antibiotic-resistant Escherichia coli bacteriuria according to age and location of onset. Mayo Clin Proc 2012; 87: 753–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hannan TJ, Hooton TM, Hultgren SJ. Estrogen and recurrent UTI: what are the facts? Sci Transl Med. 2013; 5(190):190fs23. [DOI] [PubMed] [Google Scholar]
- 20.Santen RJ. Vaginal administration of estradiol: effects of dose, preparation and timing on plasma estradiol levels. 2015; 18(2): 121–134 [DOI] [PubMed] [Google Scholar]