Abstract
Upregulation of programmed death ligand 1 (PD-L1) allows cancer cells to evade antitumor immunity. Despite tremendous efforts in developing PD-1/PD-L1 immune checkpoint inhibitors (ICIs), clinical trials using such ICIs have shown inconsistent benefits. Here, we hypothesized that the ICI efficacy would be dictated by the binding strength of the inhibitor to the target proteins. To assess this, hyperbranched, multivalent poly(amidoamine) dendrimers were employed to prepare dendrimer-ICI conjugates (G7-aPD-L1). Binding kinetics measurements using SPR, BLI, and AFM revealed that G7-aPD-L1 exhibits significantly enhanced binding strength to PD-L1 proteins, compared to free aPD-L1. The binding avidity of G7-aPD-L1 was translated into in vitro efficiency and in vivo selectivity, as the conjugates improved the PD-L1 blockade effect and enhanced accumulation in tumor sites. Our results demonstrate that the dendrimer-mediated multivalent interaction substantially increases the binding avidity of the ICIs and thereby improves the antagonist effect, providing a novel platform for cancer immunotherapy.
Keywords: immunotherapy, multivalent binding, PD-1/PD-L1 interaction, dendrimer, immune checkpoint inhibitor
Graphical Abstract
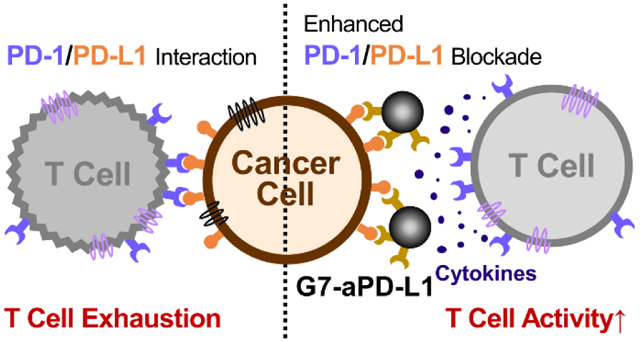
The immune system is responsible for the detection of abnormal cells and suppression of their rapid growth.1 Activation of the innate immune system stimulates T cells to attack malignant tumor cells.2 However, tumor cells frequently adapt to evade immune surveillance and interfere with the T cell response by triggering immune checkpoint regulators. This causes a dysregulation of the antitumor immune response, thereby exhibiting immune-inhibitory behaviors.3–5 Cancer immunotherapy is a burgeoning treatment that restores and/or reactivates the immune system via blockade of the immune checkpoint pathways.6 A number of immune checkpoint inhibitors (ICIs) have been developed to modulate these pathways through the targeting of immunosuppressive molecules, notably the interaction between programmed death ligand 1 (PD-L1) on cancer cells and its counter receptor PD-1 on T cells.7–11
PD-L1 is a bidirectional membrane protein that is widely expressed in many cancer types, including ovarian cancer, renal cell carcinoma, hepatocellular carcinoma, and lung cancer.12–16 Its interaction with PD-1 receptors disrupts the natural immune response mounted against tumors. This has led to the development of several PD-L1 specific antagonists, primarily in a form of monoclonal antibodies, which include three FDA-approved drugs, Atezolizumab, Avelumab, and Durvalumab.17 However, the currently available PD-L1-targeted immunotherapy agents have faced considerable challenges in clinical trials, due to heterogeneity of PD-L1 expressions in tumor, active redistribution of the ligand after the treatment, and low target efficacy or binding strength of the prevalent antibody drugs.18–23
In this study, we hypothesized that the integration of dendrimer nanoparticles with ICI antibodies would enhance the binding avidity of the PD-L1 antagonists, substantially increasing the therapeutic efficacy (Scheme 1). Note that we previously reported that dendrimers effectively facilitate multivalent binding, as evidenced by significant reduction in dissociation rate and enhancement in surface targeting.24–27 This was attributed to the unique capability of dendrimers that accommodate multiple ligands on its nanoscale surface area and that deforms to enable the conformational optimization of the multiple ligands to bind to their counterparts simultaneously.24, 28 Based on these, we assess the following specific hypotheses in this study: i) conjugation of ICIs to dendrimers would result in a significant increase in binding kinetics; ii) the increased binding kinetics would in turn improve in vitro efficiency and in vivo tumor accumulation of the ICI-dendrimer conjugates.
Scheme 1.
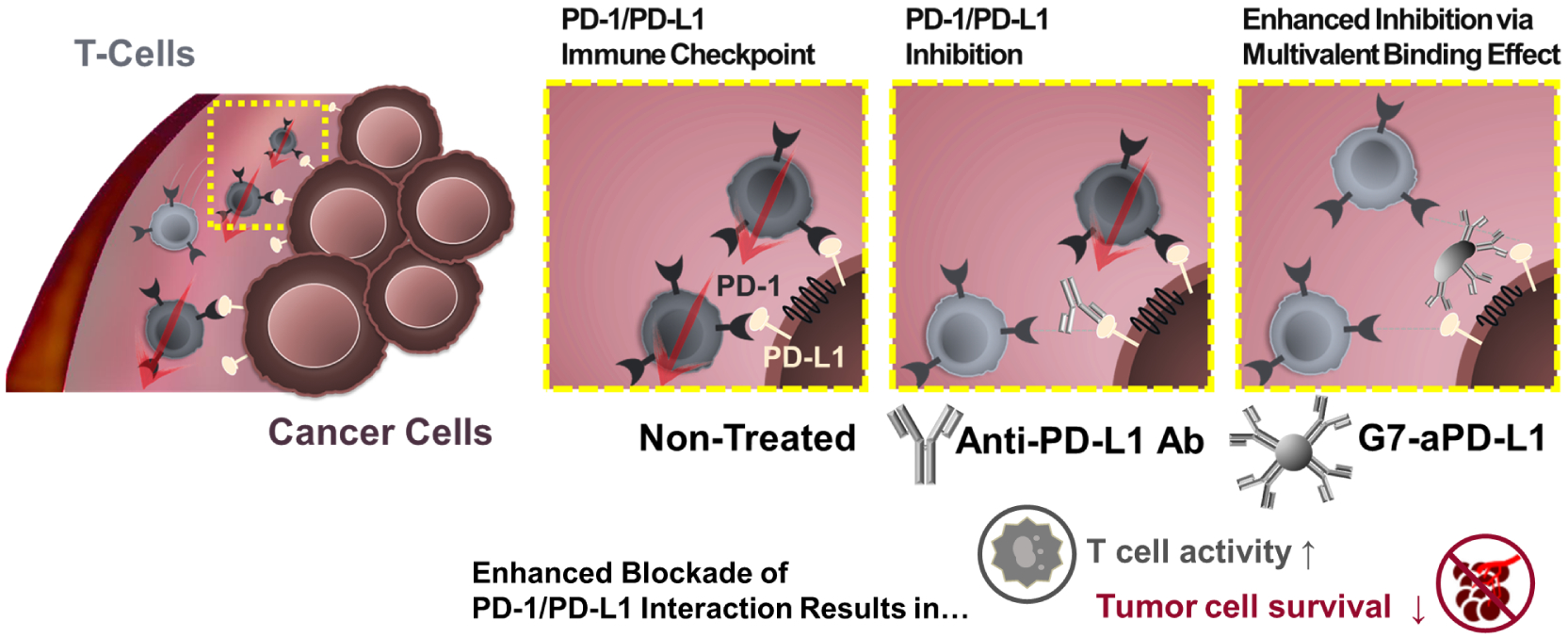
A schematic diagram illustrating the hypothesis that the dendrimer-mediated multivalent interaction would substantially increase the antagonist effect of ICIs as a result of increased binding kinetics.
To test these hypotheses, we designed a nanoparticle drug delivery platform consisting of generation 7 (G7) poly(amidoamine) (PAMAM) dendrimers conjugated with multiple PD-L1-targeting molecules per dendrimer (G7-aPD-L1). The G7-aPD-L1 conjugates were synthesized as described in Figure 1A. G7 PAMAM dendrimers were first labeled with Alexa Fluor 647 (AF647). The dendrimers were then reacted with acetic anhydride to obtain primary amine acetylation. Approximately 75–90% of the peripheral functional groups were acetylated to provide a more neutral surface charge.29 The remaining amine groups on the partially acetylated dendrimers were subsequently carboxylated through the reaction with succinic anhydride. The presence of the different terminal groups after each chemical reaction was confirmed using proton nuclear magnetic resonance (1H NMR), as shown in Figure S1. Following the surface modification, the dendrimers were activated with N-hydroxysuccinimide (NHS) and subsequently reacted with anti-PD-L1 human antibodies (aPD-L1h) at a molar ratio of 1:5. Samples were then purified using centrifugal filters in order to remove unconjugated reactants.
Figure 1.
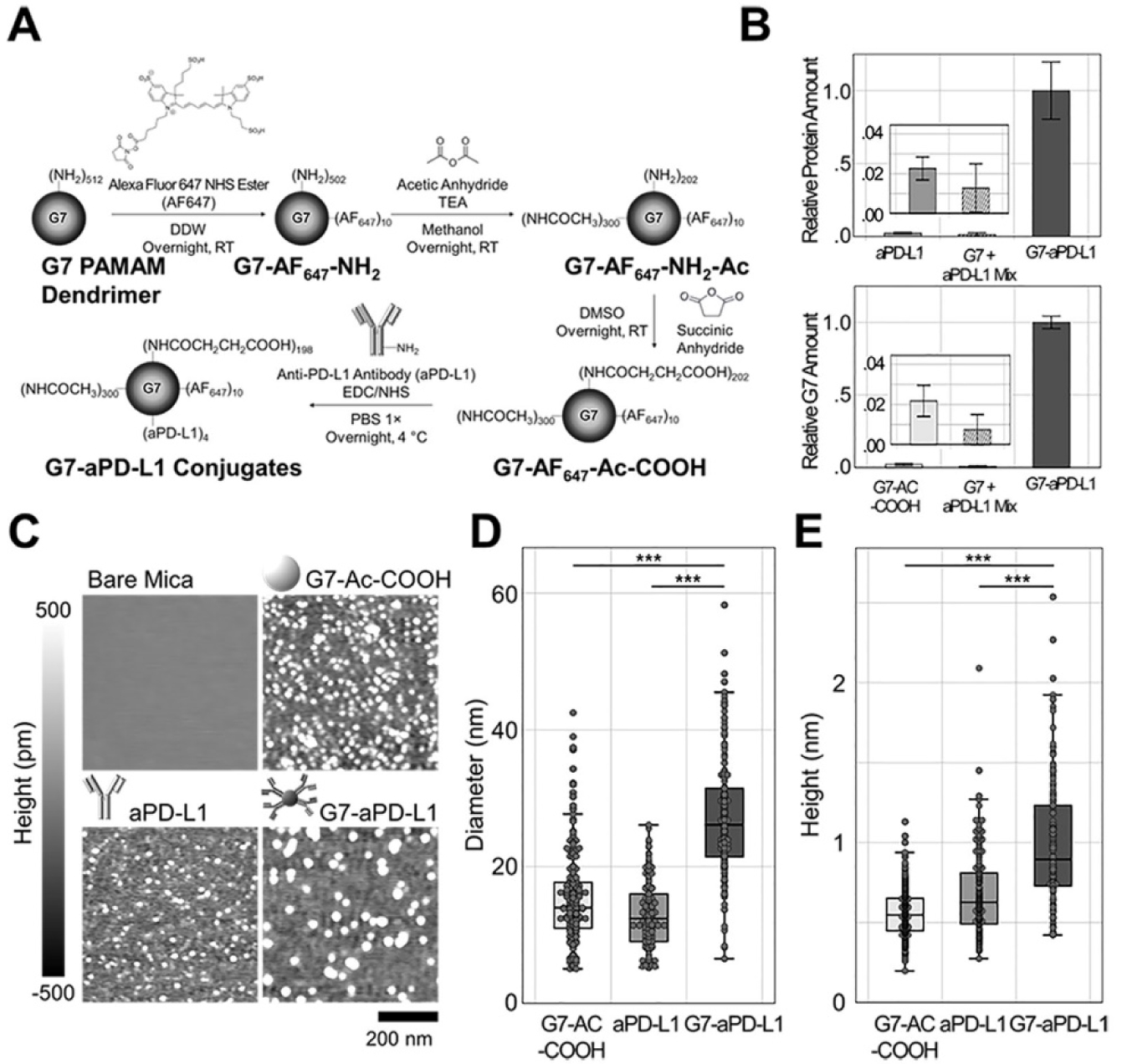
Synthesis and characterization of the G7-aPD-L1 conjugates. (A) Schematics describing the synthetic route of the G7-aPD-L1 conjugates. (B) The molar ratios of impurities, i.e., free antibodies (top) and non-conjugated dendrimers (bottom), after the conjugation reaction between G7-Ac-COOH and aPD-L1. Error bars represent standard deviations (SD). (C-E) The G7-aPD-L1 conjugates characterized using AFM. (C) AFM images of surface-adsorbed G7-Ac-COOH, aPD-L1h, and G7-aPD-L1h conjugates, obtained in air. (D-E) Box plots for the diameters and heights of the nanoparticles obtained using AFM. The differences in height and diameter imply the flattening of the nanoparticles on the mica surface. Note that the center lines in box plots represent the median, boxes represent interquartile ranges (IQR), and error bars range from the first quartile (Q1) − 1.5 × IQR to Q3 + 1.5 × IQR.
The final G7-aPD-L1h conjugates were comprised of 3.7 ± 0.5 antibodies per dendrimer, as assessed using a bicinchoninic acid (BCA) assay and fluorescent intensity measurements (Figure S2). The molar ratio of impurities, including free aPD-L1h or unconjugated dendrimers, to the anticipated conjugates (G7-aPD-L1h) was confirmed to be less than 3% (Figure 1B and S2). The G7-aPD-L1h conjugates were further characterized using atomic force microscopy (AFM) and gel permeation chromatography (GPC). The AFM images revealed that the diameter (D) and height (h) of the G7-aPD-L1h conjugates were significantly larger (D = 27.4 ± 8.9 nm and h = 9.8 ± 3.9 Å) than those of free antibodies (D = 12.7 ± 4.4 nm; p < 0.001 and h = 6.7 ± 2.5 Å; p < 0.001) and unconjugated dendrimers (D = 16.3 ± 7.3 nm; p < 0.001 and h = 5.6 ± 1.6 Å; p < 0.001) (Figure 1C–E). Note that the differences in height and diameter imply the flattening of the nanoparticles on the mica surface, which was also reported elsewhere.30 The GPC chromatograms of the G7-aPD-L1h conjugates, unmodified dendrimers, and aPD-L1h (Figure S3) further supported successful conjugation between antibodies and dendrimers. At the detection wavelength of 280 nm (characteristic to proteins), the G7-aPD-L1h conjugates displayed a faster elution time compared to aPD-L1h (21.9 ± 0.4 min vs. 23.0 ± 0.1 min; p = 0.007), confirming the increased molecular weight of the conjugates. Furthermore, area under the peak from the conjugates was larger than that from the free antibody by ~3.6-fold, indicating that ~3.6 antibodies were conjugated to each of the dendrimers, which is consistent with the results obtained using the BCA assay.
The binding kinetics of the dendrimer-ICI conjugates to PD-L1 was quantitatively analyzed using three direct measurement methods: biolayer interferometry (BLI), surface plasmon resonance spectroscopy (SPR), and AFM. Binding affinities determined using PD-L1-functionalized BLI probes demonstrated that the G7-aPD-L1h conjugates interact more strongly with PD-L1 molecules than aPD-L1h (Figure 2A). The G7-aPD-L1h conjugates showed an average dissociation constant (KD) of (8.5 ± 2.3) × 10−11 M, which was an order of magnitude lower than the KD obtained from aPD-L1h ((9.6 ± 1.7) × 10−10 M; p = 0.016), at inhibitor concentrations between 6.25 and 25.0 μg/mL. Binding affinity was further assessed using SPR, by infusing the inhibitors through the protein-immobilized SPR chip at a flow rate of 10 μL/min (Figure 2B). The G7-aPD-L1h conjugates exhibited 5.8-fold enhanced binding avidity with PD-L1, compared to free aPD-L1h ((6.6 ± 2.7) × 10−11 M vs. (3.8 ± 1.0) × 10−10 M; p = 0.007).
Figure 2.
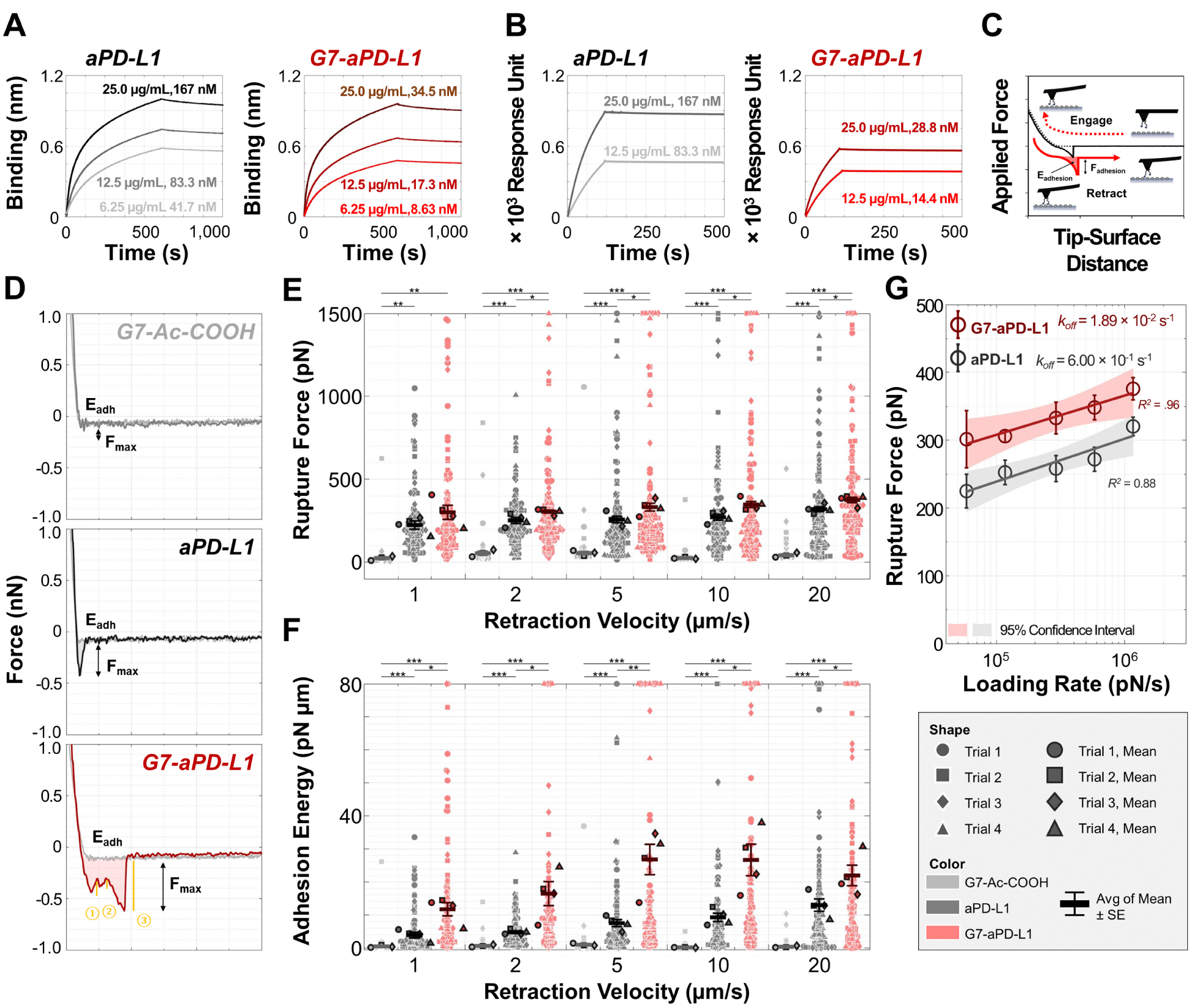
Binding kinetics of the dendrimer-aPD-L1 conjugates, free aPD-L1, and controls to PD-L1 quantitatively analyzed using three direct measurement methods: (A) BLI analysis; (B) SPR analysis; (C-G) AFM force spectroscopy. (C) A schematic diagram of the experimental set up for AFM analysis, representing the working principle of measuring dissociation kinetics between PD-L1 and its binding counterparts employed in this study. (D) Representative FD curves obtained from a PD-L1-immobilized probe, upon interaction with surfaces modified with G7-Ac-COOH (upper), aPD-L1h (middle), and G7-aPD-L1h (bottom). (E, F) Maximum adhesion forces and adhesion energies collected from FD curves for interaction of probe-immobilized PD-L1 with surface-immobilized inhibitors. (G) Bell-Evans model fitting of the FD curves obtained at different pulling velocities. koff values of the G7-aPD-L1h conjugates and free aPD-L1h were calculated as 1.86 × 10−2 s−1 and 6.00 × 10−1 s−1, respectively. For (E-G), error bars represent standard error of mean.
The lower KD of the G7-aPD-L1h conjugates, compared to those of free aPD-L1h were most likely attributed to their faster association (kon) with the target proteins (Table S1 and S2). In contrast, the difference in off-rate kinetics between the two inhibitors was not prominent. Both the G7-aPD-L1h conjugates and aPD-L1h demonstrated significantly slow dissociation (koff) at inhibitor concentrations of 6.25 – 25.0 μg/mL, ((1.4 ± 0.6) × 10−4 s−1 vs. (2.6 ± 0.2) × 10−4 s−1; BLI). The more sensitive AFM force spectroscopy was thus employed to resolve the difference in dissociation kinetics of the two inhibitors, as the multivalent binding typically results in a significant reduction in koff (Figure 2C).24, 31–35 The detailed description for the preparation steps are provided in Supporting Information. Representative force-distance (FD) curves obtained from aPD-L1h- and G7-aPD-L1h-functionalized surfaces at a loading rate of 1,160 nN/s are shown in Figure 2D. Multivalent interaction between the G7-aPD-L1h conjugates and PD-L1 was identified from the FD curves, as represented by two or more discrete unbinding events (rupture force >50 pN) occurring at a retraction phase. These multivalent interactions were more frequently found from the G7-aPD-L1h conjugates than aPD-L1h (Table S3), and for the both inhibitors, the maximum adhesion forces and energies obtained from the curves with multiple unbinding events were significantly larger than those obtained from single unbinding events, regardless of the loading rate (Table S4–S7).
As a result, the average of mean maximum adhesion forces on four G7-aPD-L1h-functionalized surfaces ranged from 301 – 376 pN, depending on the pulling velocity (1 – 20 μm/s) (Figure 2E and S4). These were 1.2- to 1.3-fold stronger than the forces obtained from the aPD-L1h surfaces (225 – 320 pN) at the same pulling velocity. The differences in dissociation kinetics were more pronounced when comparing the adhesion energies (Figure 2F). The energies ranged from 11.7–27.0 pN∙μm and 3.9–13.0 pN∙μm for the G7-aPD-L1h conjugates and aPD-L1h, respectively. The force spectrum of the G7-aPD-L1h conjugates versus aPD-L1h was further analyzed using the Bell-Evans model (Figure 2G).36 The koff of the G7-aPD-L1h conjugates was ~30 times lower than that of aPD-L1h (1.86 × 10−2 s−1 vs. 6.00 × 10−1 s−1), supporting our first hypothesis that conjugation of aPD-L1h to dendrimers would significantly increase the PD-L1 binding. Note that the rupture forces higher than 50 pN were rarely detected from the control G7-Ac-COOH surface (Figure 2E), indicating that the interaction between the dendrimers and PD-L1 is negligible. In addition, BSA-immobilized probe exhibited significantly weaker interaction with the G7-aPD-L1h conjugates, compared to PD-L1-immobilized probe, further demonstrating a high specificity of the conjugates (Figure S5). Furthermore, the inverted configuration did not affect the results, as the G7-aPD-L1h-functionalized probe still showed stronger interactions with PD-L1 immobilized on the surface, compared to aPD-L1h, although the results were statistically less significant (Figure S6). Note that discrepancy in koff measured using BLI, SPR, and AFM have been commonly reported,37 which is attributed to differences in experimental condition and detection sensitivity among such techniques. Particularly for AFM, the results could be affected by the parameters such as the number of molecules on the probe/surface.
The enhanced binding kinetics of the G7-aPD-L1h conjugates compared to aPD-L1h were then tested in vitro using the human renal cell carcinoma cancer cell line 786-O and breast cancer cell line MCF-7, which are known to express high and low levels of PD-L1, respectively.38, 39 The western blot analysis of PD-L1 in these two cell lines confirmed significantly higher PD-L1 expression in 786-O, compared to MCF-7 (Figure S7). The target specificity of the G7-aPD-L1h conjugates was then examined by treating cells with 67 nM of inhibitor for 3 h, followed by staining with 4’,6-Diamidino-2-Phenylindole (DAPI) on a nucleus. The expressions of aPD-L1h and the G7-aPD-L1h conjugates were both significantly higher on 786-O cells than MCF-7 cells (Figure 3A).
Figure 3.
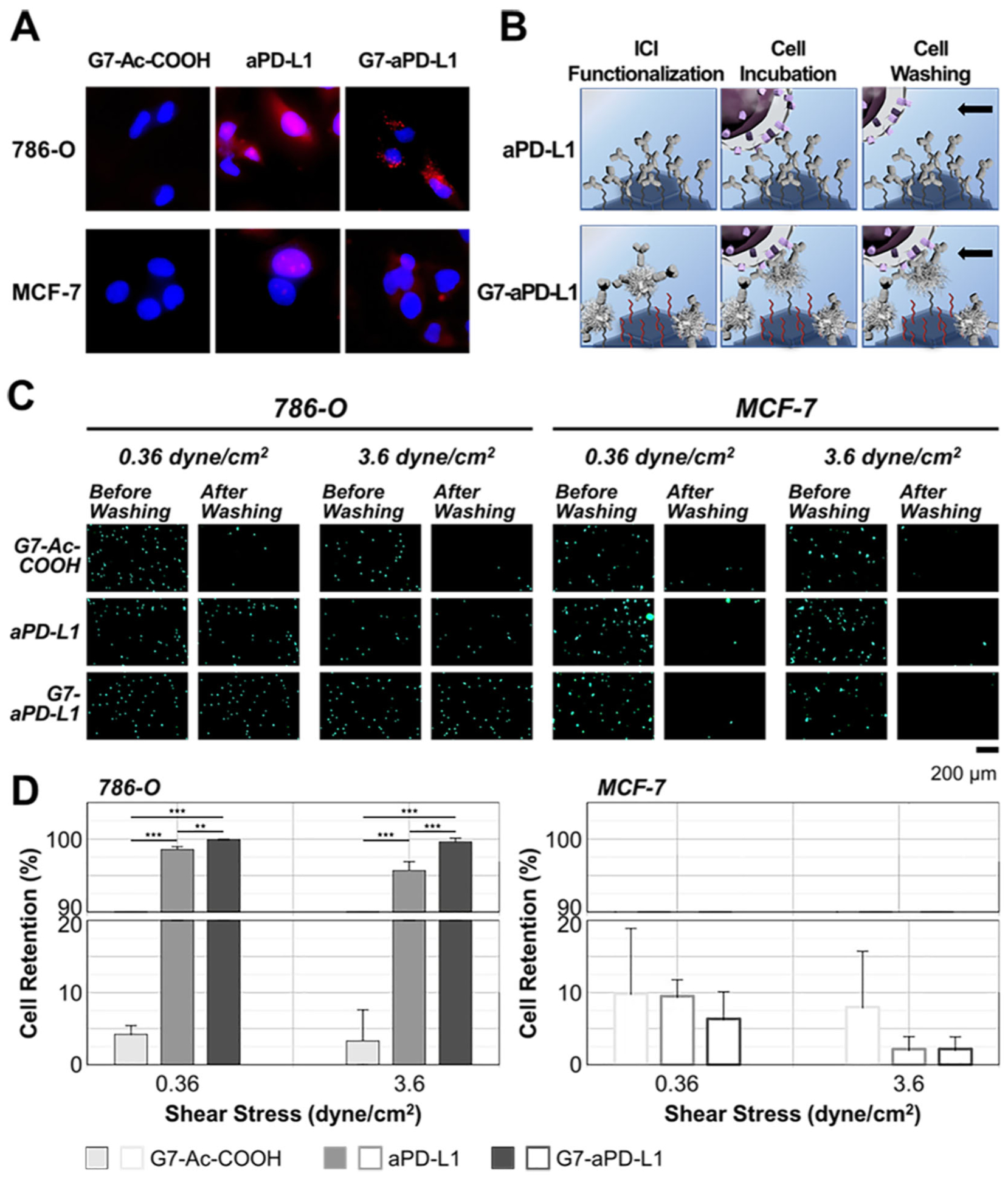
In vitro cell selectivity and enhanced binding avidity of the G7-aPD-L1h conjugates: (A) In vitro specificity of free aPD-L1 and the G7-aPD-L1h conjugates to PD-L1 observed using an inverted fluorescence microscope. (B-D) In vitro cell retention assay demonstrating the enhanced binding of the G7-aPD-L1h conjugates to PD-L1 expressing cells in a selective manner. An equivalent number of antibodies was immobilized on each of the aPD-L1h and G7-aPD-L1h surfaces, whereas the dendrimer-coated surface without aPD-L1 (G7-Ac-COOH) was used as a negative control. The numbers of cells remained attached to the G7-Ac-COOH-, aPD-L1h-, and G7-aPD-L1h-functionalized surfaces were compared after exposure to shear stresses of 0.36 and 3.6 dyne/cm2. All results indicate that the G7-aPD-L1h surfaces exhibit the strongest cell binding as a result of specific aPD-L1/PD-L1 adhesion. Error bars: SD.
Next, we measured the in vitro binding affinity/avidity of the G7-aPD-L1h conjugates, which was compared to that of dendrimers without the antibodies (G7-Ac-COOH) and free aPD-L1h, using a cell retention assay (Figure 3B).40 A flow chamber (Figure S8), consisting of a basal PEGylated slide functionalized with either G7-Ac-COOH, aPD-L1h, or the conjugates was used for the assay. Note that the amount of aPD-L1h immobilized on each surface was controlled to be comparable between the aPD-L1h- and G7-aPD-L1h-functionalized surfaces, by blocking the three fourths of surface reactive groups for immobilization of the G7-aPD-L1h conjugates (Figure S9). The detailed procedures are provided in Supporting Information. The BCA assay confirmed that the amounts of antibodies immobilized on the both surfaces were equivalent (28 ± 7 ng/mm2 vs. 27 ± 4 ng/mm2 for G7-aPD-L1h vs. aPD-L1h; p = .650) (Figure S10).
Cell retention was determined upon washing the cells at shear stresses of 0.36 or 3.6 dyne/cm2 for 20 min, after 15 min incubation inside the chamber. The retention of PD-L1High 786-O cells was significantly higher on the G7-aPD-L1h-functinoalized surface, compared to the surface with free aPD-L1h (Figure 3C and 3D). The difference was more significant at the higher flow rate, as only 0.4 ± 0.5% of 786-O cells were detached from the G7-aPD-L1h-functionalized surface, which is a ~10-fold higher retention than the same surface without dendrimers (4.3 ± 1.2%; p<.001). These findings indicate the successful translation of the improved binding kinetics measured at the nanoscale into selective in vitro cell adhesion.
The higher retention observed on the G7-aPD-L1h surface, compared to aPD-L1h, is likely due to an increase in local antibody density.41, 42 Despite the equivalent number of antibodies presented on the both surfaces, the numerical analysis model (Figure S11) demonstrated a wider distribution in local antibody density on the G7-aPD-L1h surface than the aPD-L1h surface. This implies that the dendrimers cluster antibodies into a small, compacted area, forming an aPD-L1h-concentrated region that effectively mediates strong multivalent binding. For PD-L1Low MCF-7 cells, the cells displayed no noticeable difference in retention among the three surfaces, indicating that the G7-aPD-L1h conjugates have a high selectivity towards target proteins. Furthermore, over 96% of 786-O cells were washed away from the dendrimer-coated surface without antibodies, confirming that the G7-Ac-COOH do not induce cell binding.
In vitro/in vivo functional assays were conducted to confirm our second hypothesis: the increased binding kinetics would in turn improve the blockade of PD-1/PD-L1 interaction. We assessed the T cell interleukin-2 (IL-2) production and cancer cell chemoresistance to doxorubicin (DOX), as described elsewhere.4, 43, 44 The PD-1/PD-L1 interaction has been reported to affect T cell functions, including its cytokine production.4 We quantitatively measured the amount of IL-2 secreted by PD-1 activated T cells via a coculture with cancer cells (Figure 4A). ELISA was utilized to assess IL-2 levels in the supernatants collected from two-day cocultures of cancer cells pre-treated with interferon-γ (IFN-γ, 10 ng/mL) and Jurkat T cells pre-treated with phorbol 12-myristate 13-acetate (PMA, 50 ng/mL)/phytohemagglutinin (PHA, 1 μg/mL). Different inhibitors, including the G7-aPD-L1h conjugates, aPD-L1h, and G7-Ac-COOH, were applied to the IFN-γ-treated cancer cells at 33 nM, prior to the coculture. The IL-2 secretion from Jurkat T cells was observed to be the highest when 786-O cells were treated with the G7-aPD-L1h conjugates (Figure 4B). More specifically, G7-aPD-L1h increased the T cell IL-2 secretion by 1.9-fold (p = 0.036), which was ~35% more effective than aPD-L1h (1.4-fold increased; p = 0.004). Note that the dendrimer without antibodies did not affect the T cell IL-2 secretion (p = 0.861).
Figure 4.
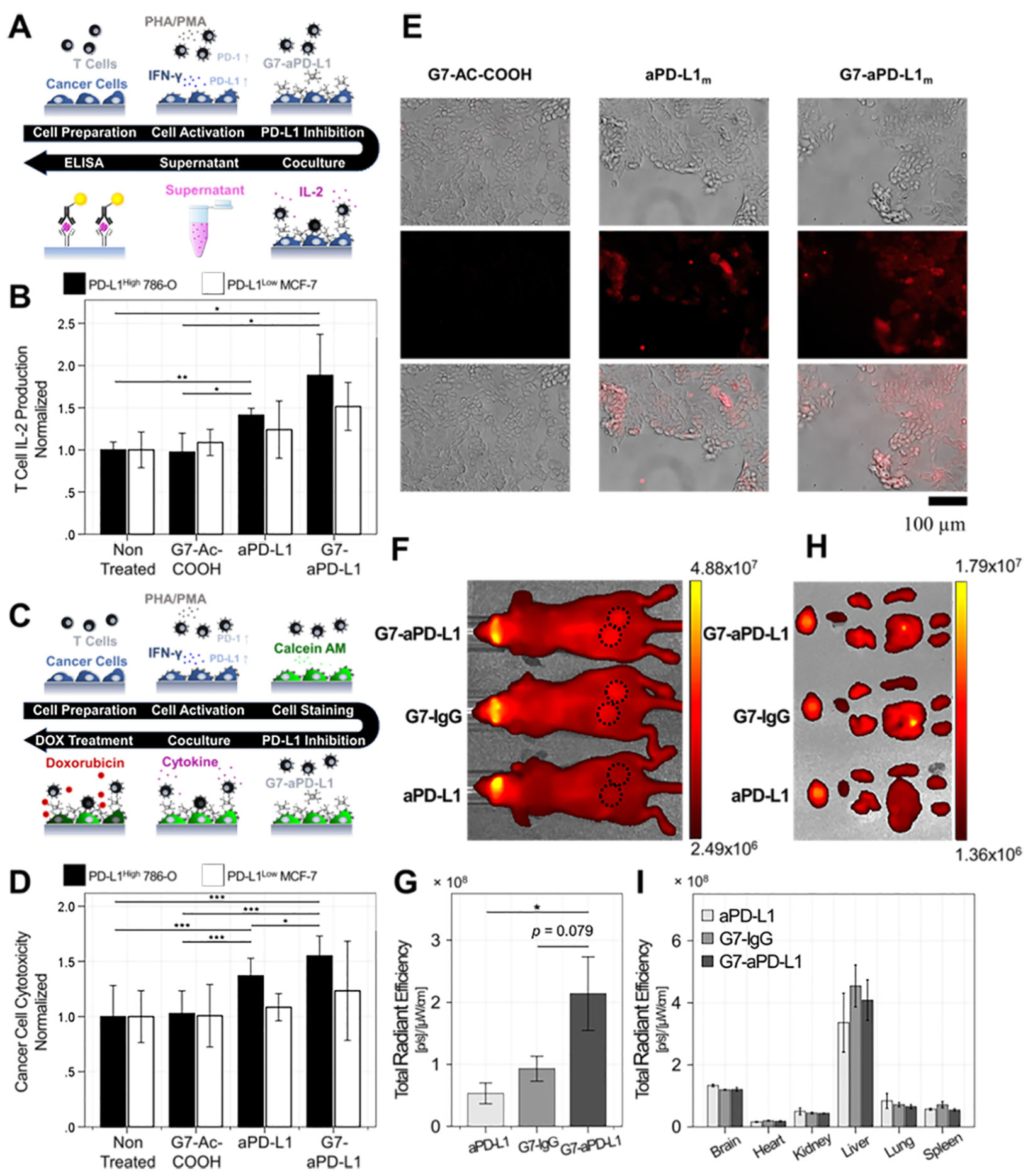
Enhanced in vitro PD-L1 blockade efficacy and in vivo selectivity of the G7-aPD-L1 conjugates compared to aPD-L1: (A, B) T cell IL-2 production assessed following the coculture of T cells and cancer cells (n = 3). Error bars represent standard deviation. (C, D) Cancer cell chemoresistance to DOX measured after coculturing the cells with the Jurkat T cells (n≥8). Note that cancer cells were pre-treated with either the G7-aPD-L1h conjugates, aPD-L1h, or surface-modified dendrimers for A-D. Error bars: SD. (E) The in vitro target binding of the G7-aPD-L1m conjugates confirmed using MOC1 cells. (F, G) In vivo imaging system (IVIS) analysis assessed using MOC1-tumor bearing mice (n = 8–10). Error bars represent standard error of means. (H, I) Biodistribution of the major organs and tumors obtained at 72 h after injection of the G7-aPD-conjugates. Error bars represent standard error of means.
Recent reports suggest that chemotherapy in combination with PD-1/PD-L1 antagonists enhances antitumor effect, compared to chemotherapy alone.43, 45 This is at least partially attributed to the fact that blockade of PD-1/PD-L1 interaction is known to prevent cancer cells from acquiring resistance to chemo-drugs.43 The tumor cell lines were treated with the G7-aPD-L1h conjugates together with DOX, to investigate how enhanced binding kinetics of the conjugates to PD-L1 affects cytotoxicity of DOX (Figure 4C). Briefly, prior to co-culture with PD-1-activated T cells, the IFN-γ-treated cancer cells were incubated with G7-aPD-L1h, aPD-L1h, and G7-Ac-COOH, followed by Calcein-AM staining (see Supporting Information for details). Following 48 h of coculture, the cytotoxicity of DOX was measured by reduction of the Calcein-AM signal. As shown in Figure 4D, G7-aPD-L1h was more cytotoxic than aPD-L1h when used in combination with DOX. For PD-L1High 786-O cells, the cells pre-treated with G7-aPD-L1h and aPD-L1h demonstrated 1.6-fold (p<0.001) and 1.4-fold (p<0.001) greater cell death, respectively, than untreated cells. The dendrimers without antibodies did not display any noticeable cytotoxic effect (p = 0.785). Furthermore, the effect of PD-L1 blockade was not pronounced in MCF-7 cells, demonstrating in vitro selectivity of the dendrimer-ICI conjugates to PD-L1.
The in vivo behaviors of the G7-aPD-L1 conjugates were then tested using a tumor-bearing mouse model. For the in vivo study, mouse aPD-L1 (aPD-L1m) was employed instead of aPD-L1h, and the ratio of antibodies per dendrimer was increased to 9:1 in order to assure their selective tumor accumulation via stronger binding to PD-L1.46 BCA assay demonstrated the molar ratio between dendrimers and antibodies to be 1:10. AFM images further revealed the larger size of the new conjugates, compared to the conjugates having ~3.7 antibodies per dendrimer (Figure S12). All the dendrimers and free antibodies were labeled with AF647, to be fluorescently observed. Prior to the mouse model study, the in vitro selectivity of the G7-aPD-L1m conjugates to PD-L1m was confirmed using a mouse oral squamous cell carcinoma MOC1 cell line that overexpresses PD-L1.47 As shown in Figure 4E, significant interactions of both aPD-L1m and G7-aPD-L1m with MOC1 cells were observed by the red fluorescence, while unconjugated dendrimers did not bind to the cells.
The G7-aPD-L1m conjugates were then applied to a MOC1 tumor-bearing mouse model. To establish the mouse tumor model, ~5 × 105 MOC1 cells were inoculated into nude mice (4- to 6-week-old; female). Once tumor size reached 300–500 mm3, mice were randomized and 50 μL of either the G7-aPD-Llm conjugates or aPD-L1m was injected through the tail vein at a concentration of ~128 nM (Figure S12). In vivo imaging system (IVIS) analysis after 72 hours injection revealed 2.5-fold (p = 0.025) increased targeting of the G7-aPD-L1m conjugates, compared to aPD-L1m (Figure 4F and 4G). Note that accumulation of the aPD-L1m was similar with that of G7-IgGm, due to longer circulation half-life and less renal excretion of G7-IgGm which mediate strong passive targeting.48, 49 The subsequent comparison of biodistribution analysis corroborated the target selectivity of the G7-aPD-L1m conjugates (Figure 4H and 4I). The biodistribution of the three nanoparticles was not significantly different in other major organs, including brain, heart, lung, liver, kidney, and spleen. These findings suggested that the enhanced binding kinetics of the G7-aPD-L1m conjugates were successfully translated into in vivo selectivity. Obviously, our approach needs to be further validated by in vivo efficacy tests to confirm that the enhanced binding avidity through dendrimer-aPD-L1 conjugation is an effective method to improve the therapeutic efficacy of ICIs. An extensive in vivo study using syngeneic, immunocompetent mouse models will be the subject of our future publications.
In this study, we have engineered a nanotherapeutic platform which can effectively block PD-1/PD-L1 immune checkpoints by utilizing the multivalent binding effect mediated by hyperbranched dendrimers. The G7-aPD-L1 conjugates formed multiple binding pairs with PD-L1 proteins, creating significantly stronger interaction with the target receptors than free aPD-L1 did. This was confirmed using three direct measurement methods, BLI, SPR, and AFM, which all revealed that the G7-aPD-L1 conjugates achieved significantly enhanced binding avidity, compared to aPD-L1, by up to an order of magnitude. The enhancement in binding kinetics in turns increased the PD-L1 antagonist effect in vitro, as the dendrimer-ICI conjugates increased T-cell cytokine production while reducing cancer cell chemoresistance to DOX. The increased in vivo tumor accumulation of the G7-aPD-L1 conjugates further confirmed the enhanced target selectivity of the dendrimer-ICI conjugates towards the PD-L1 protein. Our current dendrimer-ICI system still has room for improvement to achieve even stronger targeting efficacy. For example, the orientation of the surface-bound antibodies could be better controlled by utilizing a site-specific conjugation chemistry, such as sulfosuccinimidyl 4-(N-maleimidomethyl)cyclohexane-1-carboxylate (SMCC) and click chemistries. Nonetheless, despite the possibilities of having misoriented antibodies, we have demonstrated throughout the manuscript that the current system exhibits high enough binding avidity toward their target protein (Figure S14). In summary, the results presented in this study demonstrate that the dendrimer-mediated multivalent binding effect improves the blockade of immune checkpoints and has potential as a novel nanoscale platform for advanced cancer immunotherapy.
Supplementary Material
ACKNOWLEDGMENT
The authors would like to thank Yun Hwa Choi (UW-Madison) for her help in collecting the force measurement data.
Funding Sources
This study was partially supported by NSF under grant # DMR-1808251 (SH). Research reported in this publication was also supported, in part, by pilot grants from the UW Carbone Cancer Center (DLW and SH, P30 CA014520) and Wisconsin Head & Neck Cancer SPORE (DLW and SH, P50 DE026787).
ABBREVIATIONS
- PD-L1
programmed death ligand 1
- aPD-L1
immune checkpoint inhibitor, anti-PD-L1 antibody
- G7-aPD-L1 conjugates
G7 PAMAM dendrimer-aPD-L1 conjugates
- BLI
biolayer interferometry
- SPR
surface plasmon resonance spectroscopy
- AFM
atomic force microscopy
- 1H NMR
proton nuclear magnetic resonance
- NHS
N-hydroxysuccinimide
- BCA assay
bicinchoninic acid assay
- GPC
gel permeation chromatography
- IL-2
interleukin-2
- IFN-γ
interferon-γ
- DOX
doxorubicin
Footnotes
Supporting Information Supporting information contains supplementary methods, results, and figures. This material is available free of charge via the Internet at http://pubs.acs.org
REFERENCES
- 1.Whiteside TL Immune Suppression in Cancer: Effects on Immune Cells, Mechanisms and Future Therapeutic Intervention. Semin. Cancer. Biol 2006, 16 (1), 3–15. [DOI] [PubMed] [Google Scholar]
- 2.Konstantinidou M; Zarganes-Tzitzikas T; Magiera-Mularz K; Holak TA; Dömling A Immune Checkpoint PD-1/PD-L1: Is There Life Beyond Antibodies? Angew. Chem. Int. Ed. Engl 2018, 57 (18), 4840–4848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keir ME; Butte MJ; Freeman GJ; Sharpe AH PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol 2008, 26, 677–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang W; Chen PW; Li H; Alizadeh H; Niederkorn JY PD-L1: PD-1 Interaction Contributes to the Functional Suppression of T-Cell Responses to Human Uveal Melanoma Cells in Vitro. Investig. Ophthalmol. Vis. Sci 2008, 49 (6), 2518–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J; Xu K; Wu C; Wang Y; Hu Y; Zhu Y; Chen Y; Shi Q; Yu G; Zhang X PD-L1 Expression Analysis in Gastric Carcinoma Tissue and Blocking of Tumor-Associated PD-L1 Signaling by Two Functional Monoclonal Antibodies. Tissue Antigens 2007, 69 (1), 19–27. [DOI] [PubMed] [Google Scholar]
- 6.Thalya B; Jon E; Fister KR Optimal Control Applied to Immunotherapy. Discrete Contin. Dyn.-B 2003, 4 (1), 135–146. [Google Scholar]
- 7.Rodell CB; Arlauckas SP; Cuccarese MF; Garris CS; Li R; Ahmed MS; Kohler RH; Pittet MJ; Weissleder R TLR7/8-Agonist-Loaded Nanoparticles Promote the Polarization of Tumour-Associated Macrophages to Enhance Cancer Immunotherapy. Nat. Biomed. Eng 2018, 2 (8), 578–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choo YW; Kang M; Kim HY; Han J; Kang S; Lee J-R; Jeong G-J; Kwon SP; Song SY; Go S; Jung M; Hong J; Kim B-S M1 Macrophage-Derived Nanovesicles Potentiate the Anticancer Efficacy of Immune Checkpoint Inhibitors. ACS Nano 2018, 12 (9), 8977–8993. [DOI] [PubMed] [Google Scholar]
- 9.Dai L; Li K; Li M; Zhao X; Luo Z; Lu L; Luo Y; Cai K Size/Charge Changeable Acidity-Responsive Micelleplex for Photodynamic-Improved PD-L1 Immunotherapy with Enhanced Tumor Penetration. Adv. Funct. Mater 2018, 28 (18), 1707249. [Google Scholar]
- 10.Guan X; Chen J; Hu Y; Lin L; Sun P; Tian H; Chen X Highly Enhanced Cancer Immunotherapy by Combining Nanovaccine with Hyaluronidase. Biomaterials 2018, 171, 198–206. [DOI] [PubMed] [Google Scholar]
- 11.Lozano T; Soldevilla MM; Casares N; Villanueva H; Bendandi M; Lasarte JJ; Pastor F Targeting Inhibition of Foxp3 by a CD28 2′-Fluro Oligonucleotide Aptamer Conjugated to P60-Peptide Enhances Active Cancer Immunotherapy. Biomaterials 2016, 91, 73–80. [DOI] [PubMed] [Google Scholar]
- 12.D’Incecco A; Andreozzi M; Ludovini V; Rossi E; Capodanno A; Landi L; Tibaldi C; Minuti G; Salvini J; Coppi E; Chella A; Fontanini G; Filice ME; Tornillo L; Incensati RM; Sani S; Crinò L; Terracciano L; Cappuzzo F PD-1 and PD-L1 Expression in Molecularly Selected Non-Small-Cell Lung Cancer Patients. Br. J. Cancer 2015, 112 (1), 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamanishi J; Mandai M; Iwasaki M; Okazaki T; Tanaka Y; Yamaguchi K; Higuchi T; Yagi H; Takakura K; Minato N; Honjo T; Fujii S Programmed Cell Death 1 Ligand 1 and Tumor-Infiltrating CD8+ T Lymphocytes Are Prognostic Factors of Human Ovarian Cancer. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (9), 3360–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CQ; Xu J; Zhou ZG; Jin LL; Yu XJ; Xiao G; Lin J; Zhuang SM; Zhang YJ; Zheng L Expression Patterns of Programmed Death Ligand 1 Correlate with Different Microenvironments and Patient Prognosis in Hepatocellular Carcinoma. Br. J. Cancer 2018, 119 (1), 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakanishi J; Wada Y; Matsumoto K; Azuma M; Kikuchi K; Ueda S Overexpression of B7-H1 (PD-L1) Significantly Associates with Tumor Grade and Postoperative Prognosis in Human Urothelial Cancers. Cancer Immunol. Immunother 2007, 56 (8), 1173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao Q; Wang XY; Qiu SJ; Yamato I; Sho M; Nakajima Y; Zhou J; Li BZ; Shi YH; Xiao YS; Xu Y; Fan J Overexpression of PD-L1 Significantly Associates with Tumor Aggressiveness and Postoperative Recurrence in Human Hepatocellular Carcinoma. Clin. Cancer Res 2009, 15 (3), 971–9. [DOI] [PubMed] [Google Scholar]
- 17.Hargadon KM; Johnson CE; Williams CJ Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol 2018, 62, 29–39. [DOI] [PubMed] [Google Scholar]
- 18.Brahmer JR; Tykodi SS; Chow LQM; Hwu W-J; Topalian SL; Hwu P; Drake CG; Camacho LH; Kauh J; Odunsi K; Pitot HC; Hamid O; Bhatia S; Martins R; Eaton K; Chen S; Salay TM; Alaparthy S; Grosso JF; Korman AJ; Parker SM; Agrawal S; Goldberg SM; Pardoll DM; Gupta A; Wigginton JM Safety and Activity of Anti-PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med 2012, 366 (26), 2455–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ventola CL Cancer Immunotherapy, Part 3: Challenges and Future Trends. P & T : a peer-reviewed journal for formulary management 2017, 42 (8), 514–521. [PMC free article] [PubMed] [Google Scholar]
- 20.Kosmides AK; Sidhom J-W; Fraser A; Bessell CA; Schneck JP Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth. ACS Nano 2017, 11 (6), 5417–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munari E; Zamboni G; Lunardi G; Marchionni L; Marconi M; Sommaggio M; Brunelli M; Martignoni G; Netto GJ; Hoque MO; Moretta F; Mingari MC; Pegoraro MC; Inno A; Paiano S; Terzi A; Cavazza A; Rossi G; Mariotti FR; Vacca P; Moretta L; Bogina G PD-L1 Expression Heterogeneity in Non-Small Cell Lung Cancer: Defining Criteria for Harmonization between Biopsy Specimens and Whole Sections. J. Thorac. Oncol 2018, 13 (8), 1113–1120. [DOI] [PubMed] [Google Scholar]
- 22.Wang H; Yao H; Li C; Shi H; Lan J; Li Z; Zhang Y; Liang L; Fang J-Y; Xu J HIP1R Targets PD-L1 to Lysosomal Degradation to Alter T cell-Mediated Cytotoxicity. Nat. Chem. Biol 2019, 15 (1), 42–50. [DOI] [PubMed] [Google Scholar]
- 23.Chowdhury S; Veyhl J; Jessa F; Polyakova O; Alenzi A; MacMillan C; Ralhan R; Walfish PG Programmed Death-Ligand 1 Overexpression Is a Prognostic Marker for Aggressive Papillary Thyroid Cancer and Its Variants. Oncotarget 2016; 7(22), 32318–32328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myung JH; Gajjar KA; Saric J; Eddington DT; Hong S Dendrimer-Mediated Multivalent Binding for the Enhanced Capture of Tumor Cells. Angew. Chem. Int. Ed. Engl 2011, 50 (49), 11769–11772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poellmann MJ; Bu J; Hong S Would Antioxidant-Loaded Nanoparticles Present an Effective Treatment for Ischemic Stroke? Nanomedicine (Lond) 2018, 13 (18), 2327–2340. [DOI] [PubMed] [Google Scholar]
- 26.Jeong W-J; Bu J; Kubiatowicz LJ; Chen SS; Kim Y; Hong S Peptide-Nanoparticle Conjugates: A Next Generation of Diagnostic and Therapeutic Platforms? Nano Converg. 2018, 5 (1), 38–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie J; Wang J; Chen H; Shen W; Sinko PJ; Dong H; Zhao R; Lu Y; Zhu Y; Jia L Multivalent Conjugation of Antibody to Dendrimers for the Enhanced Capture and Regulation on Colon Cancer Cells. Scientific Reports 2015, 5, 9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeong W; Bu J; Han Y; Drelich AJ; Nair A; Král P; Hong S Nanoparticle Conjugation Stabilizes and Multimerizes β-Hairpin Peptides To Effectively Target PD-1/PD-L1 β-Sheet-Rich Interfaces. J. Am. Chem. Soc 2020, 142 (4), 1832–1837. [DOI] [PubMed] [Google Scholar]
- 29.Khandare J; Kolhe P; Pillai O; Kannan S; Lieh-Lai M; Kannan RM Synthesis, Cellular Transport, and Activity of Polyamidoamine Dendrimer-Methylprednisolone Conjugates. Bioconjug. Chem 2005, 16 (2), 330–337. [DOI] [PubMed] [Google Scholar]
- 30.Li J; Piehler LT; Qin D; Baker JR; Tomalia DA; Meier DJ Visualization and Characterization of Poly(amidoamine) Dendrimers by Atomic Force Microscopy. Langmuir 2000, 16 (13), 5613–5616. [Google Scholar]
- 31.Gestwicki JE; Cairo CW; Mann DA; Owen RM; Kiessling LL Selective Immobilization of Multivalent Ligands for Surface Plasmon Resonance and Fluorescence Microscopy. Anal. Biochem 2002, 305 (2), 149–55. [DOI] [PubMed] [Google Scholar]
- 32.Poellmann MJ; Nair A; Bu J; Kim JKH; Kimple RJ; Hong S, Immunoavidity-Based Capture of Tumor Exosomes Using Poly(amidoamine) Dendrimer Surfaces. Nano Letters 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratto TV; Rudd RE; Langry KC; Balhorn RL; McElfresh MW, Nonlinearly additive forces in multivalent ligand binding to a single protein revealed with force spectroscopy. Langmuir 2006, 22 (4), 1749–57. [DOI] [PubMed] [Google Scholar]
- 34.Sulchek T; Friddle RW; Noy A Strength of Multiple Parallel Biological Bonds. Biophys. J 2006, 90 (12), 4686–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leistra AN; Han JH; Tang S; Orr BG; Banaszak Holl MM; Choi SK; Sinniah K Force Spectroscopy of Multivalent Binding of Riboflavin-Conjugated Dendrimers to Riboflavin Binding Protein. J. Phys. Chem. B 2015, 119 (18), 5785–92. [DOI] [PubMed] [Google Scholar]
- 36.Evans E; Ritchie K Dynamic Strength of Molecular Adhesion Bonds. Biophys. J 1997, 72 (4), 1541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casillas-Ituarte NN; Cruz CHB; Lins RD; DiBartola AC; Howard J; Liang X; Höök M; Viana IFT; Sierra-Hernández MR; Lower SK, Amino acid polymorphisms in the fibronectin-binding repeats of fibronectin-binding protein A affect bond strength and fibronectin conformation. J. Biol 2017, 292 (21), 8797–8810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mittendorf EA; Philips AV; Meric-Bernstam F; Qiao N; Wu Y; Harrington S; Su X; Wang Y; Gonzalez-Angulo AM; Akcakanat A; Chawla A; Curran M; Hwu P; Sharma P; Litton JK; Molldrem JJ; Alatrash G PD-L1 Expression in Triple-Negative Breast Cancer. Cancer Immunol. Res 2014, 2 (4), 361–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu Q; Cai MY; Weng DS; Zhao JJ; Pan QZ; Wang QJ; Tang Y; He J; Li M; Xia JC PD-L1 Expression Patterns in Tumour Cells and Their Association with CD8. J. Cancer 2019, 10 (5), 1154–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Myung JH; Eblan MJ; Caster JM; Park SJ; Poellmann MJ; Wang K; Tam KA; Miller SM; Shen C; Chen RC; Zhang T; Tepper JE; Chera BS; Wang AZ; Hong S, Multivalent Binding and Biomimetic Cell Rolling Improves the Sensitivity and Specificity of Circulating Tumor Cell Capture. Clin. Cancer Res 2018, 24 (11), 2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myung JH; Roengvoraphoj M; Tam KA; Ma T; Memoli VA; Dmitrovsky E; Freemantle SJ; Hong S Effective Capture of Circulating Tumor Cells from a Transgenic Mouse Lung Cancer Model Using Dendrimer Surfaces Immobilized with Anti-EGFR. Anal. Chem 2015, 87 (19), 10096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bu J; Nair A; Kubiatowicz LJ; Poellmann MJ; Jeong W.-j.; Reyes-Martinez M; Armstrong AJ; George DJ; Wang AZ; Zhang T; Hong S, Surface engineering for efficient capture of circulating tumor cells in renal cell carcinoma: From nanoscale analysis to clinical application. Biosens. Bioelectron 2020, 112250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Black M; Barsoum IB; Truesdell P; Cotechini T; Macdonald-Goodfellow SK; Petroff M; Siemens DR; Koti M; Craig AW; Graham CH, Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 2016, 7 (9), 10557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burr ML; Sparbier CE; Chan YC; Williamson JC; Woods K; Beavis PA; Lam EYN; Henderson MA; Bell CC; Stolzenburg S; Gilan O; Bloor S; Noori T; Morgens DW; Bassik MC; Neeson PJ; Behren A; Darcy PK; Dawson SJ; Voskoboinik I; Trapani JA; Cebon J; Lehner PJ; Dawson MA, CMTM6 Maintains the Expression of PD-L1 and Regulates Anti-Tumour Immunity. Nature 2017, 549 (7670), 101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun D; Ma J; Wang J; Han C; Qian Y; Chen G; Li X; Zhang J; Cui P; Du W; Wu Z; Chen S; Zheng X; Yue Z; Song J; Gao C; Zhao X; Cai S; Hu Y, Anti-PD-1 therapy combined with chemotherapy in patients with advanced biliary tract cancer. Cancer. Immunol. Immunother 2019, 68 (9), 1527–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sykes EA; Chen J; Zheng G; Chan WCW, Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8 (6), 5696–5706. [DOI] [PubMed] [Google Scholar]
- 47.Moore EC; Cash HA; Caruso AM; Uppaluri R; Hodge JW; Van Waes C; Allen CT, Enhanced Tumor Control with Combination mTOR and PD-L1 Inhibition in Syngeneic Oral Cavity Cancers. Cancer Immunol. Res 2016, 4 (7), 611–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sunoqrot S; Bugno J; Lantvit D; Burdette JE; Hong S Prolonged Blood Circulation and Enhanced Tumor Accumulation of Folate-Targeted Dendrimer-Polymer Hybrid Nanoparticles. J. Control Release 2014, 191, 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Attia MF; Anton N; Wallyn J; Omran Z; Vandamme TF, An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol 2019, 71 (8), 1185–1198. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


