Abstract
Purpose
Severe acute respiratory coronavirus 2 (SARS-CoV-2) cases are overgrowing globally and now become a pandemic. A meta-analysis was conducted to evaluate the impact of age, sex, comorbidities, and clinical characteristics on the severity of COVID-19 to help diagnose and evaluate the current outbreak in clinical decision-making.
Methods
PubMed, ScienceDirect, and BMC were searched to collect data about demographic, clinical characteristics, and comorbidities of COVID-19 patients. Meta-analysis was conducted with Review Manager 5.3. Publication bias was assessed using Egger's test and Begg-Mazumdar's rank correlation.
Results
Fifty-five studies were included in this meta-analysis, including 10014 patients with SARS-CoV-2 infection. Male cases and cases with an age of ≥50 years (OR = 2.41, p < 0.00001; RR = 3.36, p = 0.0002, respectively) were severely affected by SARS-CoV-2. Patients having age≥65 years are not associated (p = 0.110) with the severity of COVID-19. Presence of at least one comorbidity or hypertension, diabetes, cerebrovascular disease, cardiovascular diseases, respiratory disease, malignancy, chronic kidney disease and chronic liver diseases individually increased the severity of COVID-19 cases significantly (OR = 3.13, p < 0.00001; OR = 2.35, p < 0.00001; OR = 2.42, p < 0.00001; OR = 3.78, p < 0.00001; OR = 3.33, p < 0.00001; OR = 2.58, p < 0.00001; OR = 2.32, p < 0.00001; OR = 2.27, p = 0.0007; OR = 1.70, p = 0.003, respectively). Clinical manifestation such as fever, cough, fatigue, anorexia, dyspnea, chest tightness, hemoptysis, diarrhea and abdominal pain (OR = 1.68, p = 0.0001; OR = 1.41, p = 0.004; OR = 1.26, p = 0.03; OR = 2.38, p < 0.0001; OR = 4.30, p < 0.00001; OR = 2.11, p = 0.002; OR = 4.93, p < 0.0001; OR = 1.35, p = 0.03; OR = 2.38, p = 0.008, respectively) were significantly associated with the severity of cases. No association of severity was found with myalgia, pharyngalgia, nausea, vomiting, headache, dizziness and sore throat (p > 0.05). No publication bias was found in case of age (≥50 years, age≥65 years), comorbidities and clinical manifestations.
Conclusions
Males patients and elderly or older patients (age ≥50 years) are at higher risk of developing severity, whereas comorbidities and clinical manifestations could significantly affect the prognosis and severity of COVID-19.
Keywords: Microbiology, Virology, Viral disease, Travel medicine, Critical care, Health informatics, Covid-19, Pneumonia, Meta-analysis, Severe, Nonsevere, Risk factor, Comorbidity, Clinical manifestation
Microbiology; Virology; Viral disease; Travel medicine; Critical care; Health informatics; Covid-19; Pneumonia; Meta-analysis; Severe; Nonsevere; Risk factor; Comorbidity; Clinical manifestation
1. Introduction
The evolving coronavirus disease 2019 (COVID-19), caused by the novel coronavirus (2019-nCoV) or severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged from Wuhan, Hubei Province, China in late December 2019, declared global pandemic from the World Health Organization (WHO) on 11th March 2020 due to its worldwide potential and deathly outcomes [1, 2]. This deadly infection is mainly transmitting through large respiratory droplets of affected people during coughing or sneezing, though the virus's presence has also been traced from stool and urine of infected individuals [3]. The most common COVID-19 patients' symptoms are fever, dry cough, fatigue, nasal congestion, myalgia, sore throat, and diarrhea, whereas the comorbidities are diabetes, hypertension, respiratory disease, cardiovascular disease, malignancy and others [4, 5, 6].
Most coronaviruses can infect different animals, including humans. At present, there are seven classes of coronaviruses that have been isolated from humans, including α-coronaviruses (229E and NL63), β-coronaviruses (OC43), Middle East Respiratory Syndrome Coronavirus (MERS-CoV), HKU1, and Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) [7, 8, 9]. SARS-CoV2 was isolated from the lower respiratory tract of patients suffering from pneumonia in Wuhan, and it was named as 2019-nCoV [10]. The International Committee on the Taxonomy of Viruses (ICTV), on the other hand, officially renamed it SARS-CoV-2 [10,11]. It is very similar to the genome sequences of previously identified coronaviruses, most importantly, to the SARS-CoV [12, 13]. So, this novel coronavirus has been classified as a β-coronavirus which can be transmitted into humans.
Currently, more than 213 countries and territories have confirmed the infection of this contagious virus. The infection rate is rising globally, as confirmed by the WHO, according to an exponential trend. As of May 29, 2020, approximately 5,962,944 confirmed cases of COVID-19 were identified with a total death of 363,905 (6.10%) patients worldwide [14]. Accordingly, countries across the world have undertaken rapid regulatory and migratory activities in response to the COVID-19 attack to control major patient outbreaks and to level the demand for increased hospital beds, testing facilities, oxygen and mechanical ventilation supports while protecting the most vulnerable one from infection, including elderly or older people with comorbidities to reduce their mortalities [15]. However, severe patients need more intensive care that is somehow lacking in most of the countries.
The current knowledge about the characteristics of novel coronavirus is still limited, and this is transmitting rapidly. To understand both the situation and the seriousness of the disease, health workers and researchers have made remarkable efforts concerning new coronavirus infected patients. The healthcare providers have proposed numerous recommendations for overcoming both diagnostic and therapeutic challenges as there are no approved drugs for protecting this assailable population from contagious viral exposure. Moreover, as there are no established vaccines, researchers are trying to develop vaccines to tackle this pandemic [16, 17].
The global outbreak of highly contagious coronavirus has led the nations' medical, psychological, and socio-economic conditions to a challenging situation that they never thought before. COVID-19 portrays probably one of the greatest threats in this century that the countries have to tackle. Therefore, scientists are trying to understand the pathogenesis, clinical implications, and develop novel preventive strategies. To date, researches on this pandemic have produced many scientific results on the clinicopathological findings that are not consistent. We analyzed relevant data from published articles to conduct the present meta-analysis to identify epidemiological attributes, clinical features, the frequency of comorbidities, severity of the infection, the correlation of age, sex, comorbidities, clinical manifestations with the severity of COVID-19 cases for more accurate and precise outcomes. We hope this study will help the existing clinical practices on the prevention, treatment, and management of the pandemic.
2. Methods
2.1. Literature search strategy
The present meta-analysis was carried out following the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The relevant studies written in any language were systematically searched on PubMed, ScienceDirect and BMC Journal database from January 1, 2020, to May 23, 2020. EndNote X 7.0 software was employed to exclude duplicate studies. The following keywords are used in search alone or in combination: ‘clinical characteristics of COVID-19’, ‘severity of COVID-19’, ‘clinical outcome’, ‘death or clinical features’, ‘comorbidities of COVID-19’, ‘signs and symptoms of SARS-CoV-2’ There was no country limitation to identify the studies and search was limited to humans, but only online literature was included. We have reviewed reference lists of included articles to identify missing studies.
2.2. Inclusion and exclusion criteria
The criteria need to be satisfied for inclusion studies are below:
1) Only study samples with confirmed COVID-19 infection; 2) Studies with age, sex, clinical signs & symptoms, comorbidities, disease severity, deaths, and survival as primary outcomes; 3) Cohort studies and case-control studies; 4) No language and geographical restriction; 5) Study with human samples; 6) Studies with sufficient data to calculate OR and 95% CI.
The criteria for exclusion are as follows:
1) Expert opinions, reviews, letters, and commentaries; 2) Studies with children and pregnant women case; 3) Overlapping or duplicate publications; 4) Irrelevant information for data extraction; 5) Animal studies.
2.3. Data extraction
Two investigators (MAB and MAA) independently extracted data with the inclusion criteria. They separately performed the literature search, evaluation, and data extraction to an excel database. Regarding the disagreements of the studies that emerged during the process were resolved by another investigator (MSI). Rayyan QCRI, a systematic review web app, was used to select the studies [18]. Data extraction included the author's name, country, age, sex, number of participants, comorbidities, clinical symptoms, and severe and nonsevere cases.
2.4. Methodological quality assessment
‘Newcastle-Ottawa Scale (NOS)’ was utilized for observational cohort studies to determine the methodological quality of the included studies, as described elsewhere [19]. Any disagreement between investigators was settled through discussion.
2.5. Statistical analysis, heterogeneity, and publication bias
The data analyses were performed by Microsoft Excel and Review Manager 5.3 (RevMan 5.3, the Cochrane Collaboration, Oxford, United Kingdom) software. Review Manager 5.3 was utilized to evaluate the heterogeneity (χ2 and I2) between studies, and heterogeneity in the forest plot was evaluated, applying both the Cochran's chi-square Q-test and I2 statistic. p < 0.1 or I2 > 50% indicated the presence of statistically significant heterogeneity. Accordingly, I2 values of 25%, 50%, and 75% represented low, moderate, and high heterogeneity. To determine any significant variations in risk across the studies for each parameter, we conducted a sensitivity analysis by omitting studies one-by-one in a certain order. The random-effect model was selected throughout the analysis. Publication biases were evaluated by the funnel plot along with Egger's regression test and Begg-Mazumdar's rank correlation. The level of significance selected for publication bias was p < 0.05, and the values higher than this were predicted as no publication bias.
3. Results
3.1. Study selection and quality assessment
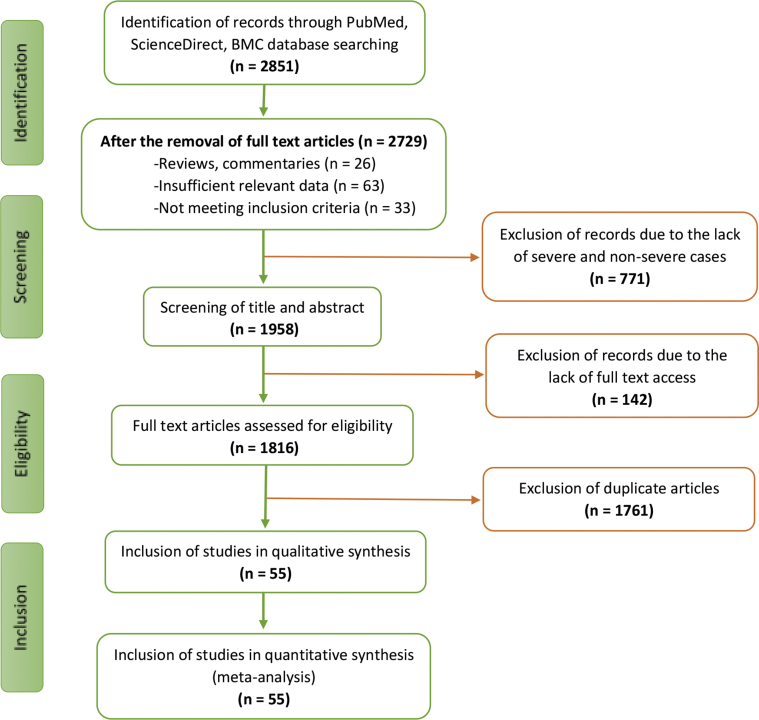
Initially, 2851 articles were identified from three databases (PubMed, ScienceDirect, BMC) during the initial retrieval. A total of 1761 records were excluded because of duplication. Then, 771 articles were removed after reading the title and abstract, and 142 were excluded from the remaining 319 articles for various reasons. In the end, 55 full-text studies involving 10014 COVID-19 patients were included in this meta-analysis based on the detailed assessment and inclusion criteria (Figure 1) [20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30], [31, 32, 33, 34, 35, 36, 37, 38, 39, 40], [41, 42, 43, 44, 45, 46, 47, 48, 49, 50], [51, 52, 53, 54, 55, 56, 57, 58, 59, 60], [61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74]. It was also found that most of these studies (n = 49) were based in China, although three studies were identified from the USA, two studies were from Italy, and one study from South Korea was included. The quality of most included studies was of high quality (score ranges between 6-8) assessed by the Newcastle Ottawa scale. Only two studies being of moderate quality (score 5), as shown in Supplementary Table S1. The baseline characteristics of all studies are presented in Table 1, and other results are presented in Figures 2, 3 and Tables 2, 3.
Figure 1.
Flow chart illustrating the literature search and study selection.
Table 1.
Baseline characteristics of included studies.
| Study ID | Year | Country | Study Design | Sample Size | Male (%) | Median Age/Mean ± SD | Follow-up/Observation period/Data collection period, days |
|---|---|---|---|---|---|---|---|
| Aggarwal S | 2020 | USA | retrospective study | 16 | 12 (75%) | 67/65.5 | 36 |
| Bi X | 2020 | China | retrospective study | 113 | 64 (56.6%) | 46 | 25 |
| Cai Q | 2020 | China | retrospective study | 298 | 145 (48.66%) | 47.5 | 55 |
| Cai Y | 2020 | China | retrospective study | 7 | 5 (71.43%) | 60.29 | ----- |
| Chen G | 2020 | China | retrospective study | 21 | 17 (81%) | 56 | ----- |
| Chen X | 2020 | China | retrospective study | 48 | 37 (77.1%) | 64.6 ± 18.1 | 19 |
| Chen Q | 2020 | China | single center retrospective observational study | 145 | 79 (54.5%) | 47.5 | 71 |
| Chu J | 2020 | China | single center retrospective observational study | 54 | 36 (66.7%) | 39 | 35 |
| Colaneri M | 2020 | Italy | retrospective study | 44 | 28 (63.64%) | 67.5 | 13 |
| Deng Q | 2020 | China | retrospective study | 112 | 57 (50.9%) | 65.0 | 45 |
| Feng Y | 2020 | China | multi-center retrospective study | 476 | 271 (56.9%) | 53.0 | 46 |
| Ferguson J | 2020 | USA | retrospective study | 72 | 38 (52.8%) | 60.4 | 29 |
| Gao Y | 2020 | China | retrospective study | 43 | 26 (60.47%) | 44.08 | 10 |
| Guan WJ | 2020 | China | retrospective study | 1099 | 637 (57.96%) | 47.0 | 49 |
| He R | 2020 | China | retrospective study | 204 | 79 (38.73%) | 49.0 | 34 |
| Hong KS | 2020 | South Korea | Descriptive Study | 98 | 38 (38.8%) | 55.4 ± 17.1 | 90 |
| Huang C | 2020 | China | prospective study | 41 | 30 (73%) | 49.0 | 17 |
| Huang Q | 2020 | China | multi-center retrospective study | 54 | 28 (51.9%) | 41.0 | 24 |
| Huang R | 2020 | China | multi-center retrospective study | 202 | 116 (57.4%) | 44.0 | 19 |
| Jiang Y | 2020 | China | single center retrospective study | 60 | 35 (58.33%) | 41 | 17 |
| Ketcham SW | 2020 | China | retrospective study | 13 | 13 (100%) | 61.0 | 46 |
| Lei S | 2020 | China | retrospective study | 34 | 14 (41.2%) | 55.0 | 36 |
| Li K | 2020 | China | retrospective study | 83 | 44 (53%) | 45.5 | 60 |
| Li S | 2020 | China | retrospective study | 69 | 40 (57.97%) | 48.5 | 60 |
| Li X | 2020 | China | retrospective study | 548 | 279 (50.9%) | 60.0 | 37 |
| Li YK | 2020 | China | retrospective study | 25 | 13 (52%) | 61 | 51 |
| Liang W | 2020 | China | retrospective study | 1590 | 904 (57.4%) | 48.9 | 26 |
| Liu F | 2020 | China | retrospective study | 140 | 49 (35.0%) | 65.5 | 54 |
| Liu J | 2020 | China | retrospective single-center study | 40 | 15 (37.5%) | 48.7 ± 13.9 | 19 |
| Liu Z | 2020 | China | retrospective study | 72 | 39 (54.2%) | 46.2 ± 15.9 | 28 |
| Lodigiani C | 2020 | Italy | retrospective study | 388 | 264 (68%) | 66.0 | 57 |
| Lv Z | 2020 | China | retrospective cohort study | 354 | 175 (49.44%) | 62.0 | 24 |
| Lyu P | 2020 | China | retrospective study | 51 | 29 (56.86%) | 54 ± 17 | 40 |
| Pan L | 2020 | China | descriptive, cross-sectional, multicenter study | 103 | 37 (35.92%) | 48.2 | 60 |
| Peng YD | 2020 | China | retrospective study | 112 | 53 (47.32%) | 62.0 | 26 |
| Pereira MR | 2020 | USA | retrospective study | 90 | 53 (59%) | 57.0 | 20 |
| Shi Y | 2020 | China | retrospective study | 487 | 259 (53.2%) | 46.0 | 15 |
| Sun L | 2020 | China | retrospective study | 55 | 31 (56.4%) | 44.0 | 26 |
| Tian S | 2020 | China | retrospective study | 262 | 127 (48.5%) | 47.5 | 21 |
| Wan S | 2020 | China | retrospective study | 135 | 72 (53.3%) | 47 | 16 |
| Wang D | 2020 | China | retrospective single-center study | 138 | 75 (54.3%) | 56 | 34 |
| Wang R | 2020 | China | single-center, retrospective, descriptive study | 125 | 54 (43.2%) | 38.76 ± 13.80 | 29 |
| Wang F | 2020 | China | retrospective study | 28 | 21 (75.0%) | 68.6 ± 9.0 | 24 |
| Wu J | 2020 | China | retrospective study | 280 | 151 (53.93%) | 43.12 ± 19.02 | 31 |
| Xie H | 2020 | China | retrospective study | 79 | 44 (55.7%) | 60.0 | 21 |
| Xie J | 2020 | China | retrospective study | 56 | 24 (42.86%) | 56.5 | 10 |
| Xiong F | 2020 | China | retrospective study | 131 | 75 (57.3%) | 63.3 | 70 |
| Yang AP | 2020 | China | retrospective study | 93 | 56 (60%) | 46.4 ± 17.6 | 28 |
| Yang P | 2020 | China | retrospective study | 133 | 72 (54.14%) | 50.60 | 90 |
| Yang Y | 2020 | China | retrospective study | 50 | 29 (58%) | 62.0 | 39 |
| Yao Q | 2020 | China | retrospective study | 108 | 43 (39.8%) | 52.0 | 12 |
| Yu X | 2020 | China | descriptive study | 333 | 172 (51.7%) | 56.0 | 26 |
| Zhang JJ | 2020 | China | descriptive study | 140 | 71 (50.7%) | 57.0 | 18 |
| Zheng S | 2020 | China | retrospective study | 96 | 58 (60%) | 55.0 | 61 |
| Zhou Y | 2020 | China | multi-center retrospective study | 366 | 207 (56.6%) | 43.0 | 37 |
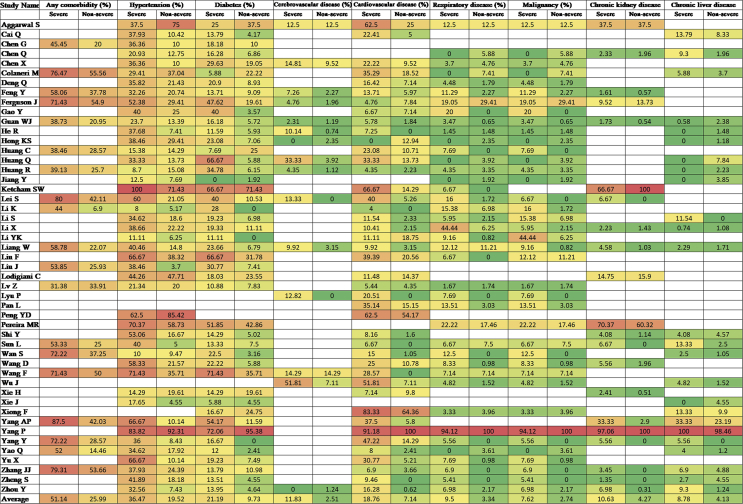
Figure 2.
Comorbidities of COVID-19 cases of the included studies
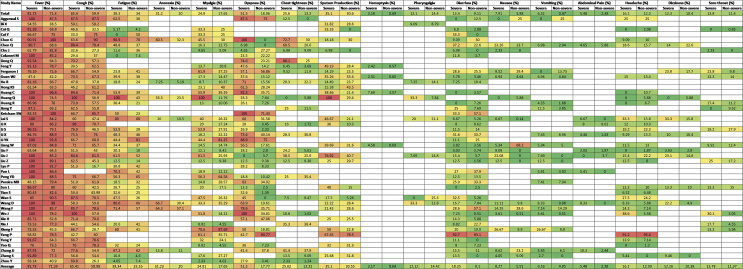
Figure 3.
Clinical symptoms of COVID-19 cases of the included studies
Table 2.
Results of the meta-analysis of the sex, age, comorbidity and clinical manifestation.
| Overall Parameter | Individual Parameter | ∗OR or RR | 95% CI | p value | I2 |
|---|---|---|---|---|---|
| Sex | Sex | 2.41 | 1.93–3.02 | <0.00001 | 67% |
| Age | Age≥50 vs. age<50 | 3.36 | 1.79–6.30 | 0.0002 | 89% |
| Age≥65 vs. age<65 | 0.79 | 0.59–1.06 | 0.11 | 88% | |
| Comorbidity | Any comorbidity | 3.13 | 2.26–4.32 | <0.00001 | 64% |
| Hypertension | 2.35 | 1.83–3.02 | <0.00001 | 66% | |
| Diabetes | 2.42 | 1.84–3.19 | <0.00001 | 58% | |
| Cerebrovascular disease | 3.78 | 2.22–6.43 | <0.00001 | 35% | |
| Cardiovascular disease | 3.33 | 2.47–4.47 | <0.00001 | 47% | |
| Respiratory disease | 2.58 | 1.76–3.77 | <0.00001 | 33% | |
| Malignancy | 2.32 | 1.63–3.32 | <0.00001 | 9% | |
| Chronic kidney disease | 2.27 | 1.41–3.65 | 0.0007 | 32% | |
| Chronic liver disease | 1.70 | 1.19–2.42 | 0.003 | 0% | |
| Overall | 2.59 | 2.31–2.89 | <0.00001 | 49% | |
| Symptoms | Fever | 1.68 | 1.29–2.19 | 0.0001 | 54% |
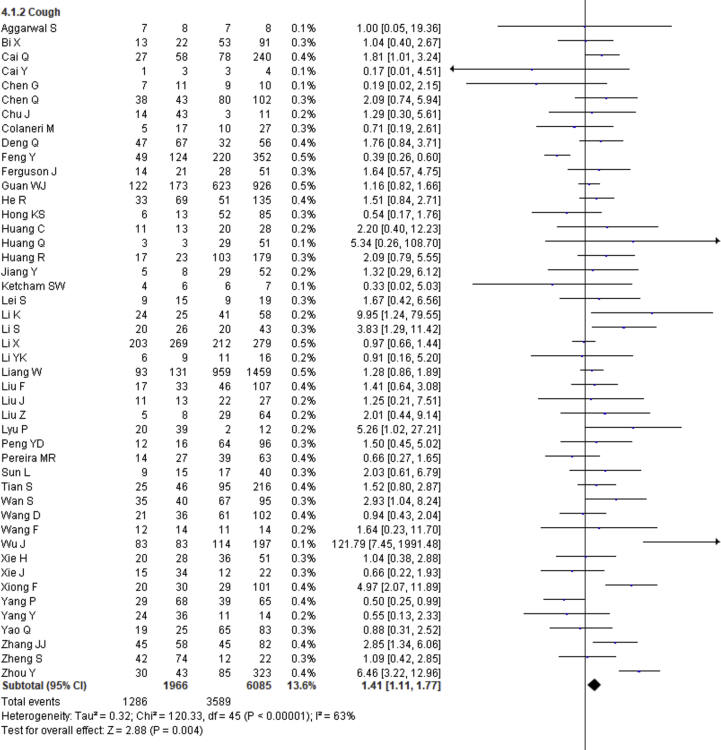
| Cough | 1.41 | 1.11–1.77 | 0.004 | 63% | |
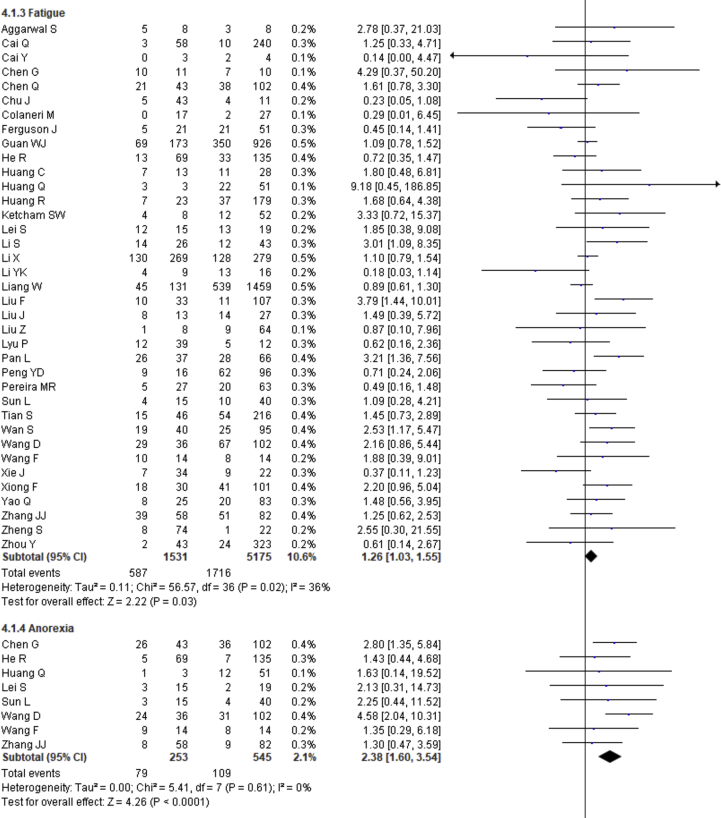
| Fatigue | 1.26 | 1.03–1.55 | 0.03 | 36% | |
| Anorexia | 2.38 | 1.60–3.54 | <0.0001 | 0% | |
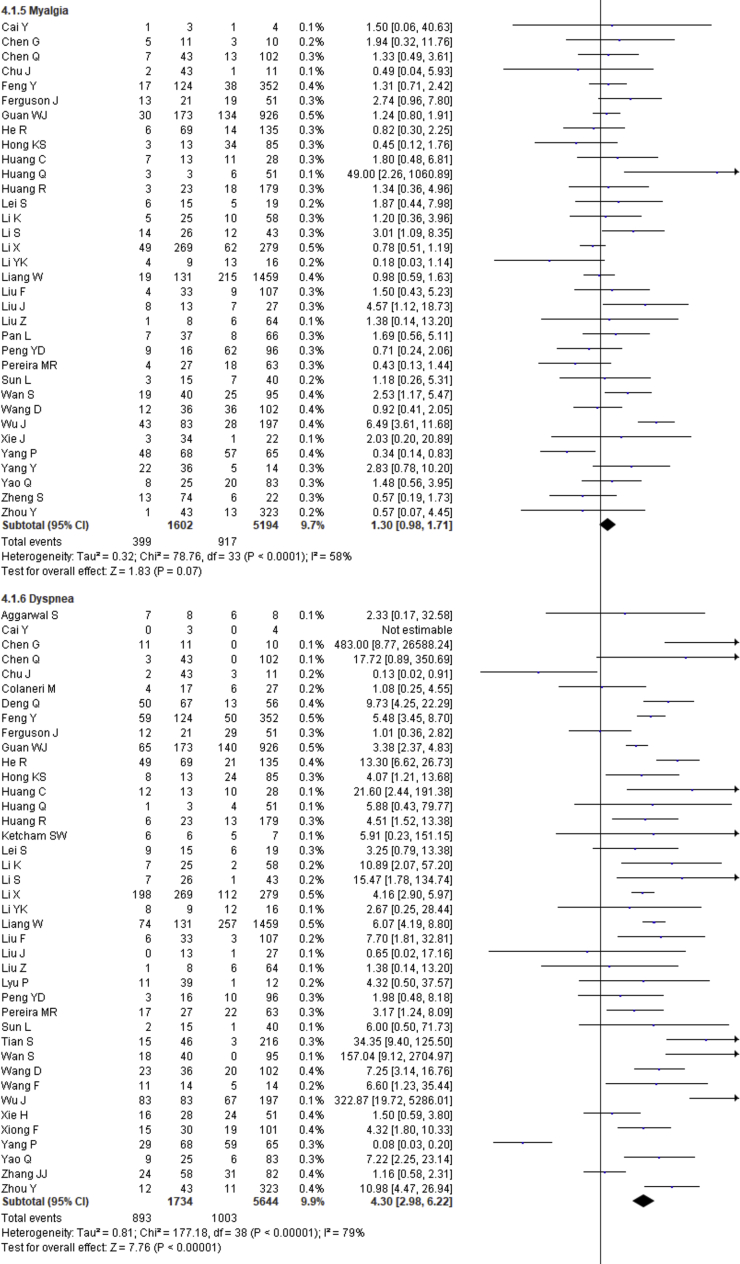
| Myalgia | 1.30 | 0.98–1.71 | 0.07 | 58% | |
| Dyspnea | 4.30 | 2.98–6.22 | <0.00001 | 79% | |
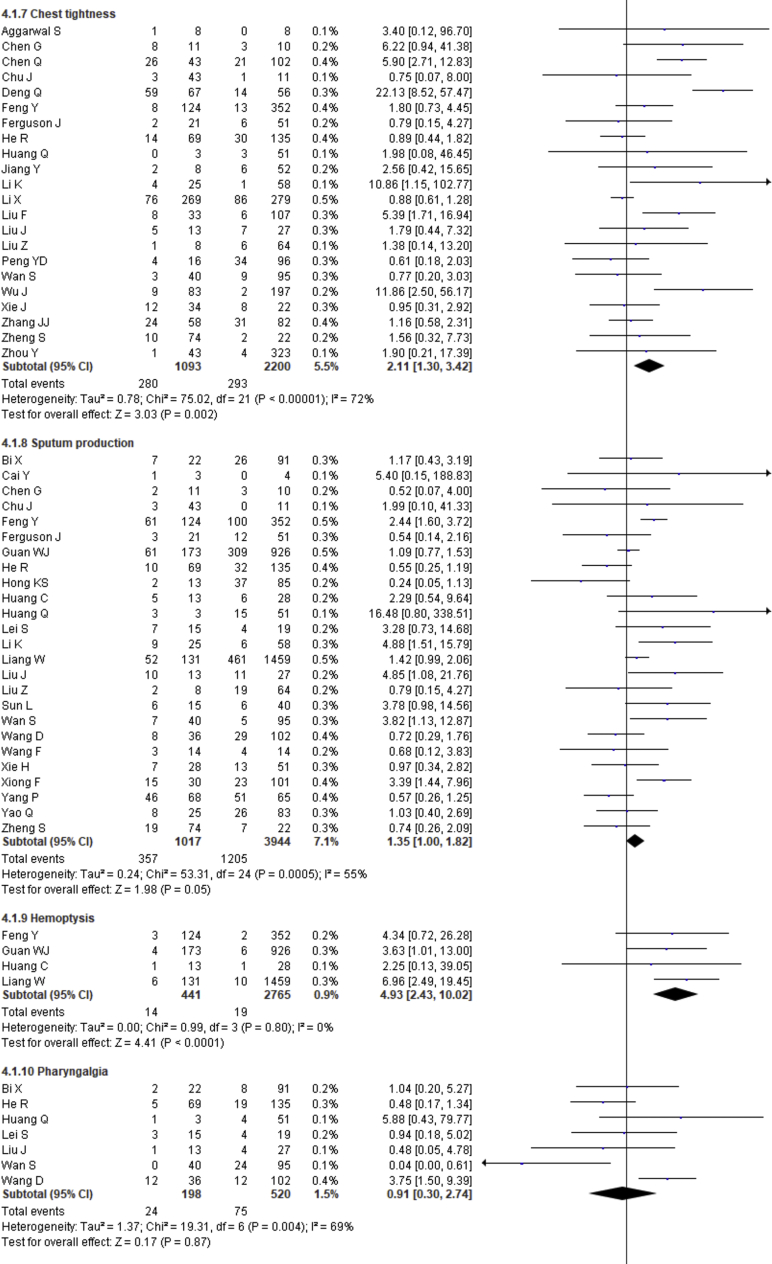
| Chest tightness | 2.11 | 1.30–3.42 | 0.002 | 72% | |
| Sputum production | 1.35 | 1.00–1.82 | 0.05 | 55% | |
| Hemoptysis | 4.93 | 2.43–10.02 | <0.0001 | 0% | |
| Pharyngalgia | 0.91 | 0.30–2.74 | 0.87 | 69% | |
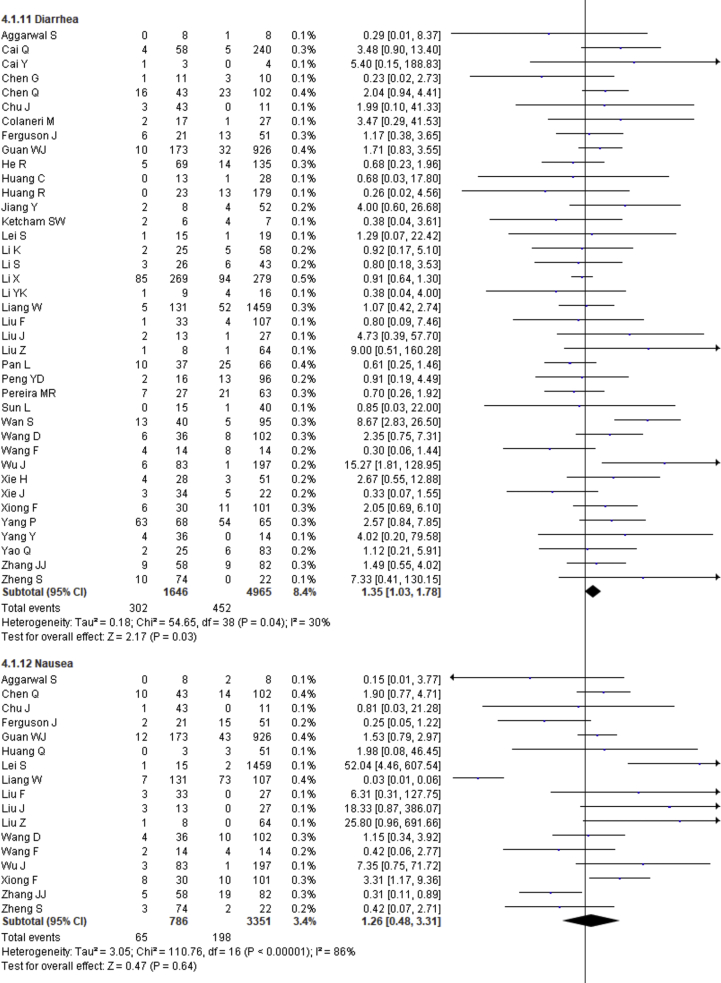
| Diarrhea | 1.35 | 1.03–1.78 | 0.03 | 30% | |
| Nausea | 1.26 | 0.48–3.31 | 0.64 | 86% | |
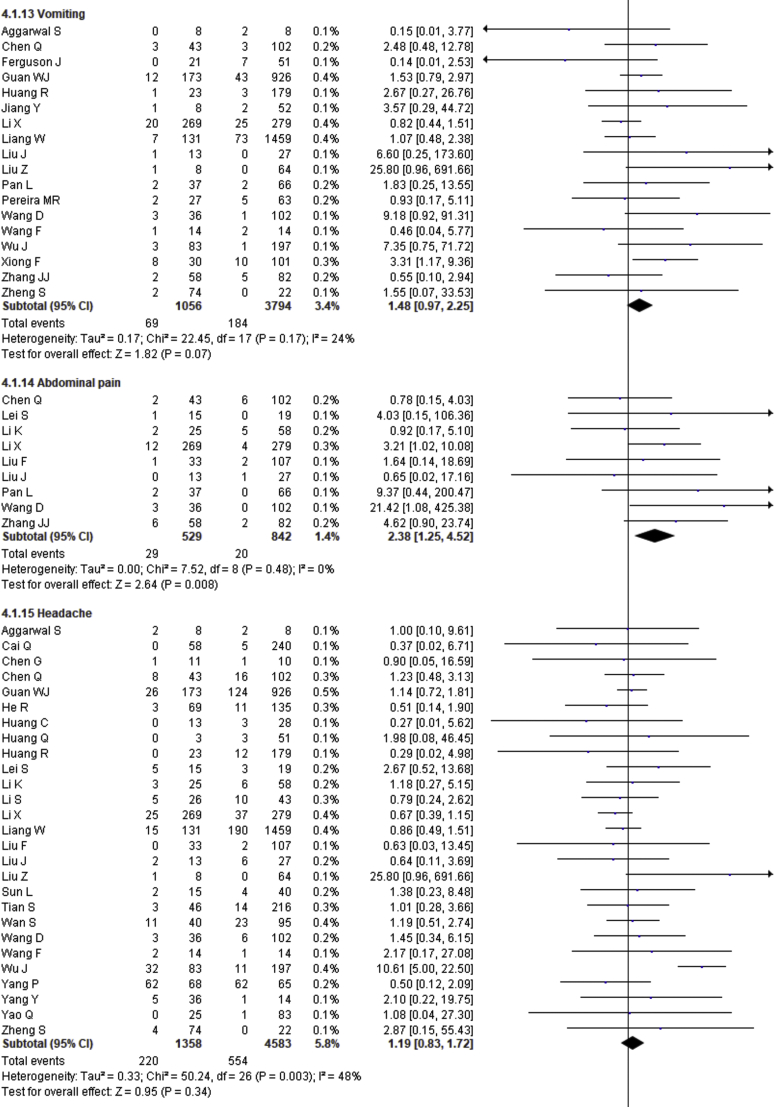
| Vomiting | 1.48 | 0.97–2.25 | 0.07 | 24% | |
| Abdominal pain | 2.38 | 1.25–4.52 | 0.008 | 0% | |
| Headache | 1.19 | 0.83–1.72 | 0.34 | 48% | |
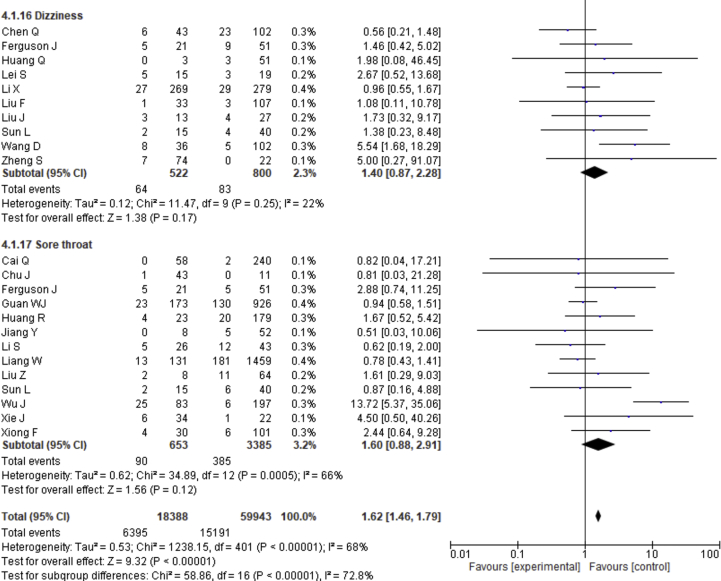
| Dizziness | 1.40 | 0.87–2.28 | 0.17 | 22% | |
| Sore throat | 1.60 | 0.88–2.91 | 0.12 | 66% | |
| Overall | 1.62 | 1.46–1.79 | <0.00001 | 68% |
Risk Ratio (RR) was used only for age.
Table 3.
Publication bias was examined by Egger's linear regression test and Begg and Mazumdar's rank correlation test.
| Parameters | p-value (Egger's test_) | p-value (Begg-Mazumdar's test) |
|---|---|---|
| Sex | 0.063 | 0.126 |
| Age (≥50 vs. <50 years) | 0.116 | 0.835 |
| Age (≥65 vs. <65 years) | 0.926 | 0.891 |
| Any comorbidity | 0.300 | 0.600 |
| Hypertension | 0.953 | 0.992 |
| Diabetes | 0.872 | 0.754 |
| Cerebrovascular disease | 0.207 | 0.656 |
| Cardiovascular disease | 0.731 | 0.683 |
| Respiratory diseases | 0.085 | 0.654 |
| Malignancy | 0.334 | 0.218 |
| Chronic kidney disease | 0.542 | 1.000 |
| Chronic liver disease | 0.751 | 0.779 |
| Fever | 0.035 | 0.819 |
| Cough | 0.125 | 0.977 |
| Fatigue | 0.638 | 0.657 |
| Anorexia | 0.208 | 0.805 |
| Myalgia | 0.793 | 0.563 |
| Dyspnoea | 0.803 | 0.780 |
| Chest tightness | 0.122 | 0.324 |
| Sputum production | 0.813 | 0.513 |
| Haemoptysis | 0.424 | 0.608 |
| Pharyngalgia | 0.675 | 0.543 |
| Diarrhea | 0.305 | 0.762 |
| Nausea | 0.304 | 0.742 |
| Vomiting | 0.287 | 0.472 |
| Abdominal pain | 0.684 | 0.677 |
| Headache | 0.914 | 0.235 |
| Dizziness | 0.164 | 0.531 |
| Sore throat | 0.474 | 0.625 |
3.2. Effect of sex on disease severity
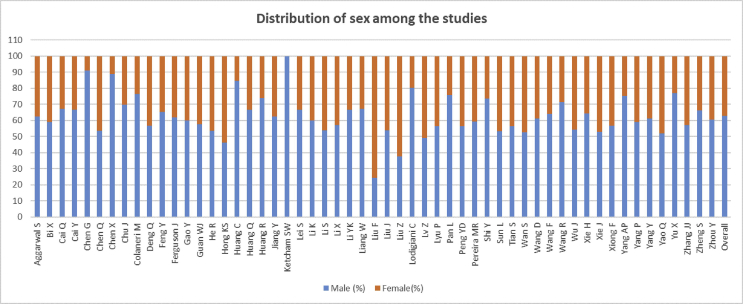
Among the 10014 patients, 2469 were severe or critical cases, 7545 were nonsevere patients. Males (62.83%) were found more than females (37.17%) in severe cases (Figure 4), whereas the males were 53.04%, and 46.6 % were female in nonsevere cases. Significant heterogeneity was found when compared the severity among the male and female COVID-19 patients (I2 = 67%, p < 0.00001). The random-effect model was used in the meta-analysis, and the results showed that the proportion of severe patients in the males was significantly higher than the females and male patients showed 2.41 times more risk of the development severe COVID-19 than female patients (male Vs. female 59.67% vs. 40.33%, OR = 2.41, 95%CI = 1.93–3.02, p < 0.00001) (Table 2, Figure 5.
Figure 4.
Distribution of sex of included studies to analyze the effect of sex on for the severity of COVID-19.
Figure 5.
Meta-analysis for the effect of sex on the severity of COVID-19 cases. Forest plots depict the comparison of the incidences of male and female in severe and nonsevere patients.
3.3. Effect of age on the severity
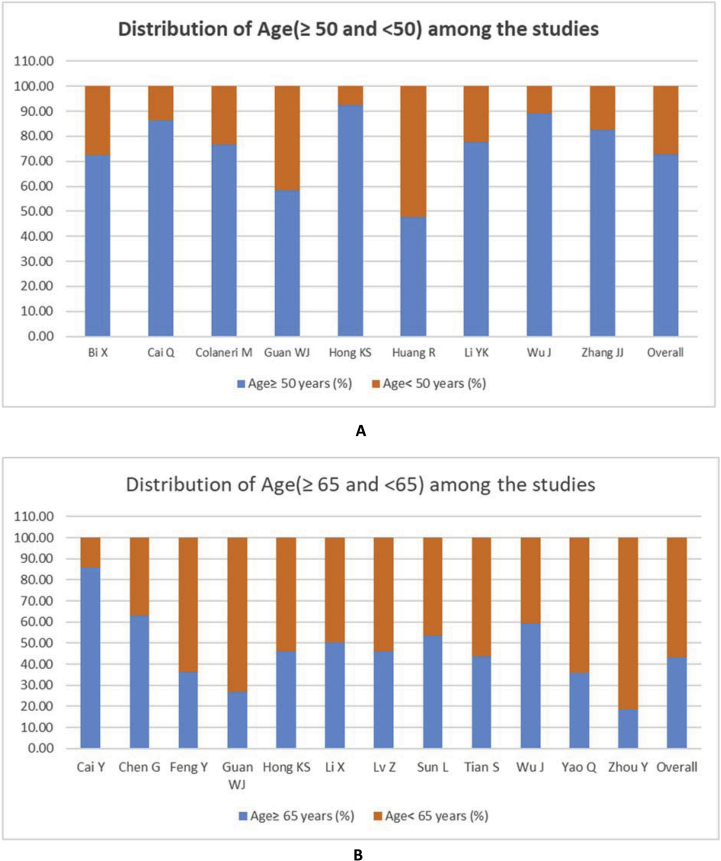
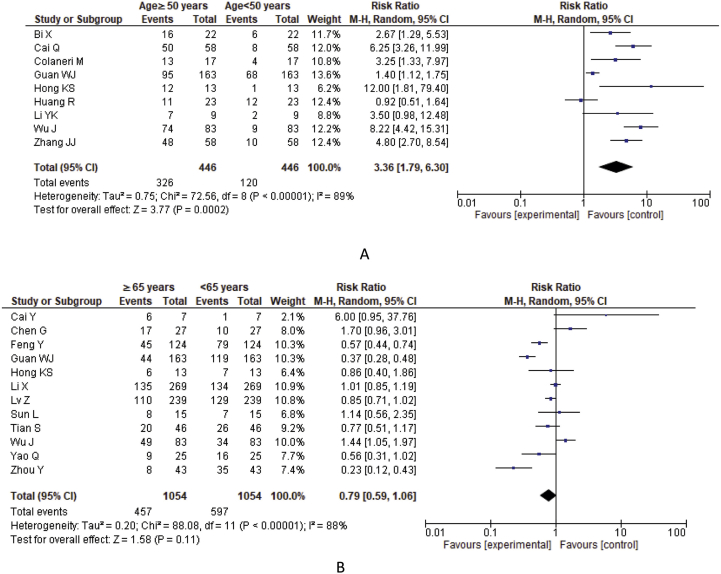
Studies that provided only median or mean age were excluded from the analysis of the association of severity with age. Among the 55 studies, the severe patients of 8 studies were categorized as age ≥50 years (73.09%) and <50 years (26.91%), whereas 12 studies were categorized as age ≥65 years (43.36%) and <65 years (56.64%) (Figure 6). A higher significant heterogeneity also found in both age≥50 vs. age<50 years (I2 = 89%, p < 0.00001) and age≥65 vs. age<65 (I2 = 88%, p < 0.00001) groups. COVID-19 patients with age ≥50 years showed statistically significant 3.36 times more risk of severity in comparison with age below 50 years (age≥50 years Vs. age<50 years, RR = 3.36; 95% CI = 1.79–6.30, p = 0.0002) whereas patients with age ≥65 years showed 0.79 times risk compared to severe patients age below 65 years (age≥65 years Vs. age<65 years, RR = 0.79; 95% CI = 0.59–1.06, p = 0.110) (Table 2, Figure 7).
Figure 6.
Distribution of age A. ≥ 50 Vs. <50 years and B. ≥ 65 and <65 years among the included studies to analyze the effect of age on for the severity of COVID-19.
Figure 7.
Meta-analysis for the effect of age on the severity of COVID-19 cases. Forest plots depict the comparison of the incidences of A) age ≥50 vs. age<50 years B) age ≥65 vs. age<65 years in severe patients.
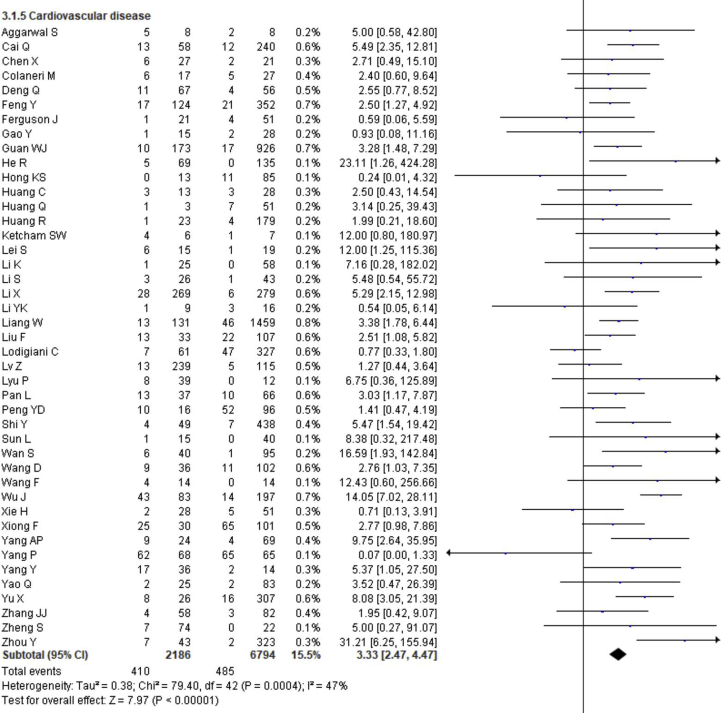
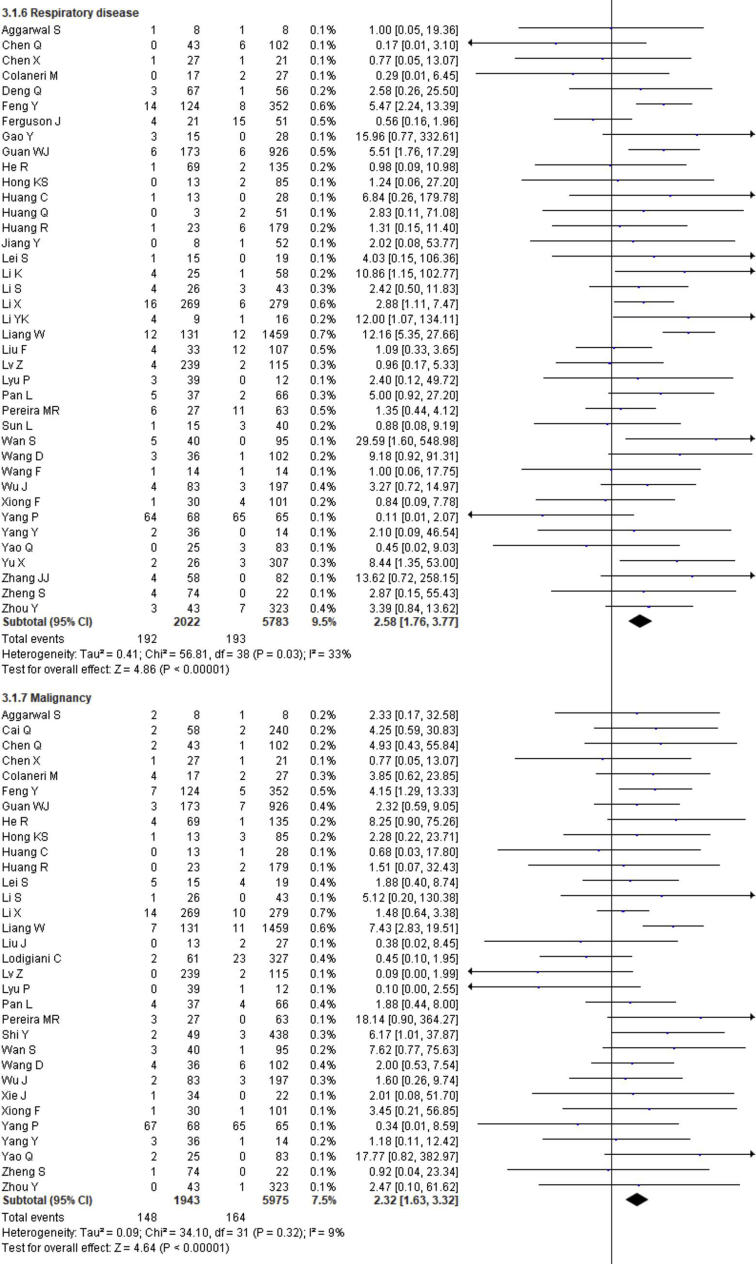
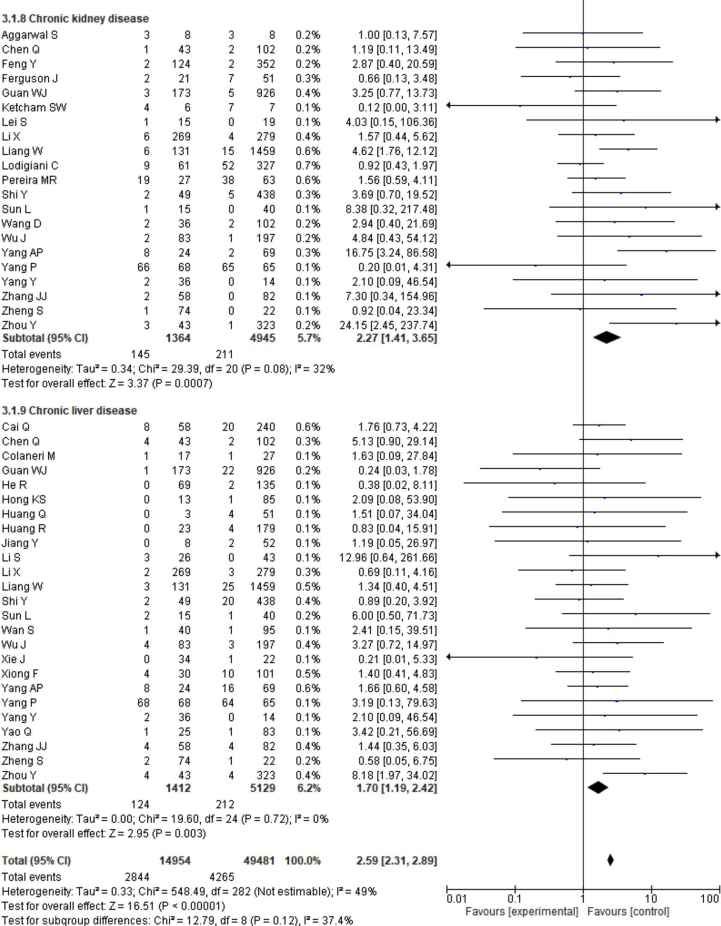
3.4. Effect of comorbidity on the disease severity
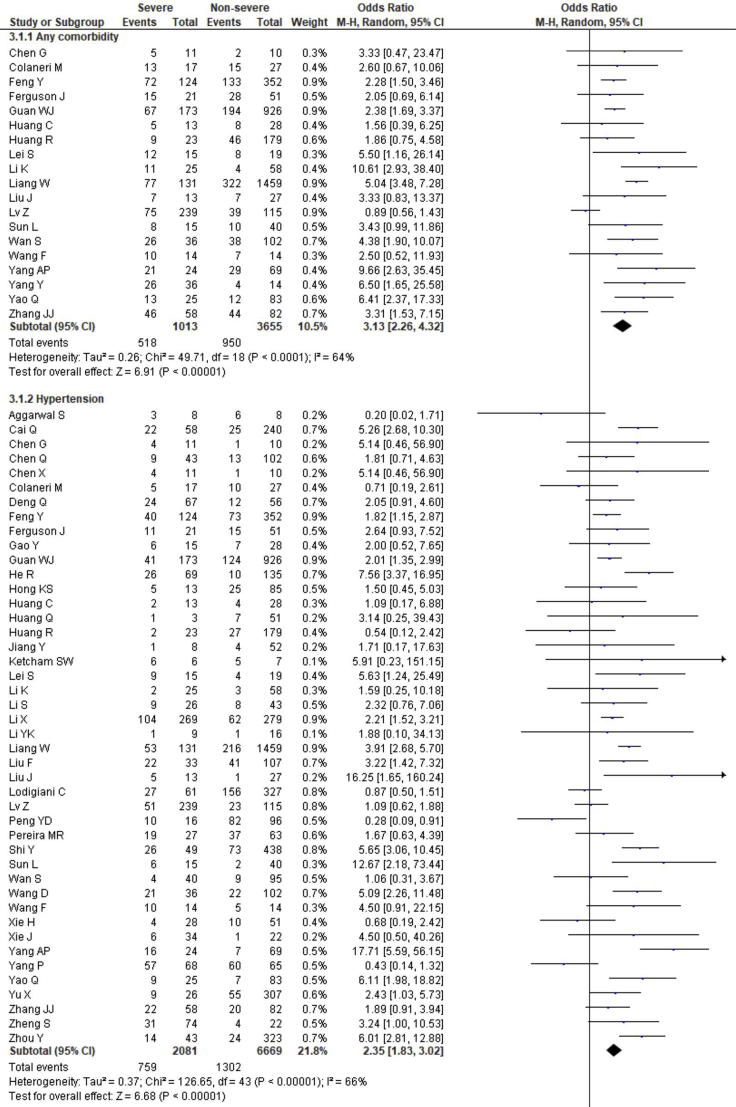
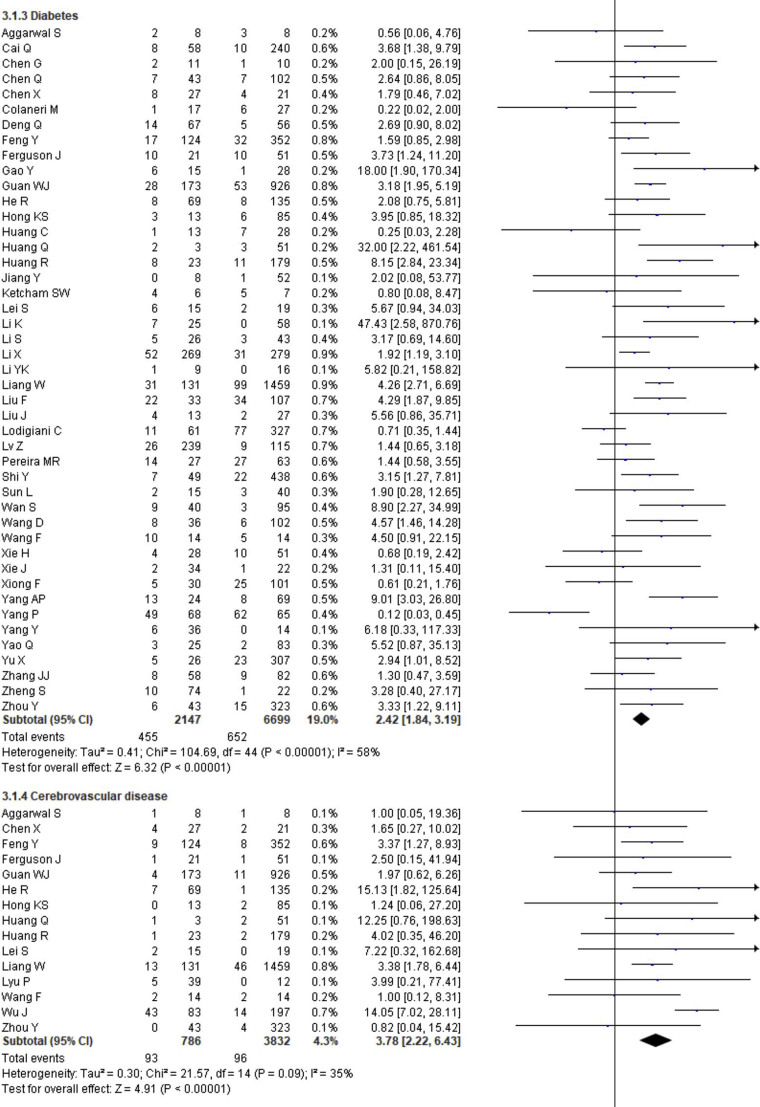
The prevalence of comorbidities including the presence of at least one comorbidity, hypertension, diabetes, cerebrovascular disease, cardiovascular diseases, respiratory disease, malignancy, chronic kidney disease and chronic liver disease in severe and non-severe COVID-19 patients of the included studies is shown in Figures 2, 8 and Table 2. Among the different comorbidities, 51.14% of severe COVID-19 cases had at least one comorbidity and patients having at least one comorbidity had 3.13 times more risk of severe illness than nonsevere patients (severe vs. nonsevere: 51.14 vs. 25.99: OR = 3.13, 95% CI = 2.26–4.32, p < 0.00001, I2 = 64%). A total of 36.47 % of severe patients had hypertension as comorbidity, and the severity of illness was found 2.35 times higher in COVID-19 cases having preexisting hypertension (severe vs. nonsevere: 36.47% vs. 19.52%, OR = 2.35, 95% CI = 1.83–3.02, p < 0.00001, I2 = 66%). The proportion of severe illness in cerebrovascular disease (severe vs. nonsevere: 11.83% vs. 2.51%) and cardiovascular disease (severe vs. nonsevere: 18.76% vs. 7.14%) was also higher than the non-severe patients and the disease severity are strongly associated with preexisting cerebrovascular and cardiovascular diseases (cerebrovascular disease: OR = 3.78, 95% CI = 2.22–6.43, p < 0.00001, I2 = 35%; cardiovascular disease: OR = 3.33, 95% CI = 2.47–4.47, p < 0.00001, I2 = 47%). Preexisting diabetes (severe vs. nonsevere: 21.19% vs. 9.73%), respiratory disease (severe vs. nonsevere: 9.50% vs. 3.34%) and malignancy (severe vs. nonsevere: 7.62% vs. 2.74%) also significantly increased the severity of COVID-19 cases (diabetes: OR = 2.42, 95% CI = 1.84–3.19, p < 0.00001, I2 = 58%; respiratory disease: OR = 2.58, 95% CI = 1.76–3.77, p < 0.00001, I2 = 33%; malignancy: OR = 2.32, 95% CI = 1.63–3.32, p < 0.00001, I2 = 9%). Chronic liver disease (CLD, severe vs. nonsevere: 8.78% vs. 4.13%) and chronic kidney disease (CKD, severe vs. nonsevere: 10.63% vs. 4.27%) were also found as risk factors for increasing the severity of COVID-19 (CLD: OR = 1.70, 95% CI = 1.19–2.42, p = 0.003, I2 = 0; CKD: OR = 2.27, 95% CI = 1.41–3.65, p = 0.0007, I2 = 32%).
Figure 8.
Meta-analysis for the effect of comorbidities on the severity of COVID-19 cases. Random effect model for any comorbidity, hypertension, diabetes, cerebrovascular disease, cardiovascular disease, respiratory disease, malignancy, chronic kidney disease and chronic liver disease.
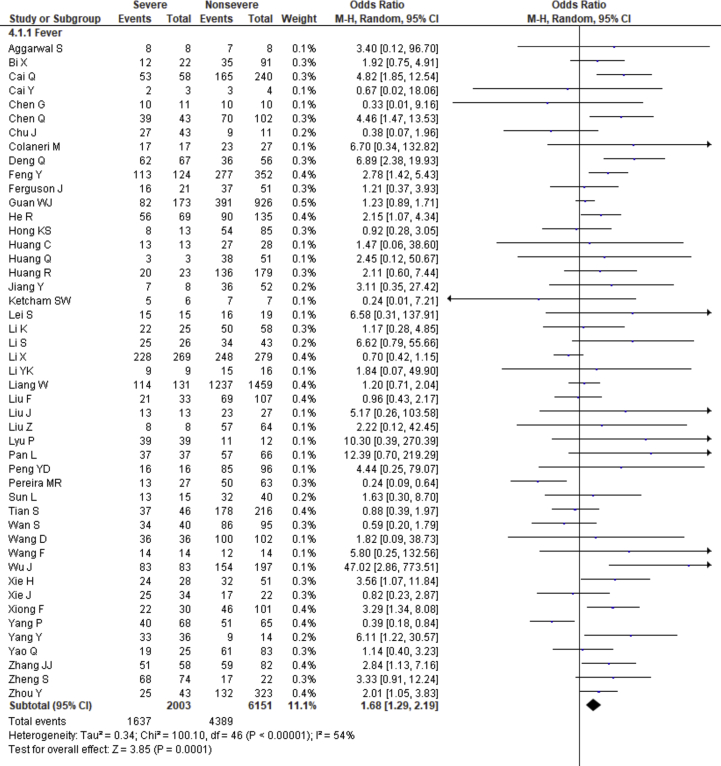
3.5. Effect of clinical symptoms on the disease severity
Fever, cough, fatigue, anorexia, myalgia, dyspnea, chest tightness, sputum production, hemoptysis, pharyngalgia, diarrhea, nausea, vomiting, abdominal pain, headache, dizziness and sore throat were reported in 47, 46, 37, 8, 34, 40, 22, 25, 4, 7, 39, 17, 18, 9, 27, 10 and 13 studies, respectively. The percentages of these symptoms in severe and nonsevere COVID-19 cases are presented in Figure 3 and Table 2, and forest plots are presented in Figure 9. The most prevalent clinical symptoms were fever (81.73%), cough (65.41%) and dyspnea (51.50%) followed by fatigue (38.34%), sputum production (35.10%), anorexia (31.23%), chest tightness (25.62%), myalgia (24.91%), diarrhea (18.35%), headache (16.20%), sore throat (13.78%), dizziness (12.26%), pharyngalgia (12.12%), nausea (8.27%), vomiting (6.53%), abdominal pain (5.48%) and hemoptysis (3.17) in the severe patients.
Figure 9.
Meta-analysis for the effect of clinical symptoms on the severity of COVID-19 cases. Random effect model for fever, cough, fatigue, anorexia, myalgia, dyspnea, chest tightness, sputum production, hemoptysis, pharyngalgia, diarrhea, nausea, vomiting, abdominal pain, headache, dizziness and sore throat.
Regarding the clinical manifestations, fever (OR = 1.68, 95% CI = 1.29–2.19, p = 0.0001, I2 = 54%), cough (OR = 1.41, 95% CI = 1.11–1.77, p = 0.004, I2 = 63%), fatigue (OR = 1.26, 95% CI = 1.03–1.55, p = 0.03, I2 = 36%), anorexia (OR = 2.38, 95% CI = 1.60–3.54, p < 0.0001, I2 = 0%), dyspnea (OR = 4.30, 95% CI = 2.98–6.22, p < 0.00001, I2 = 79%), chest tightness (OR = 2.11, 95% CI = 1.30–3.42, p = 0.002, I2 = 72%), hemoptysis (OR = 4.93, 95% CI = 2.43–10.02, p < 0.0001, I2 = 0), diarrhea (OR = 1.35, 95% CI = 1.03–1.78, p = 0.03, I2 = 30%) and abdominal pain (OR = 2.38, 95% CI = 1.25–4.52, p = 0.008, I2 = 0%) are significantly associated with the severity of COVID-19 cases compared to nonsevere cases whereas myalgia (OR = 1.30, p = 0.07), pharyngalgia (OR = 0.91, p = 0.87), nausea (OR = 1.26, p = 0.64), vomiting (OR = 1.48, p = 0.07), headache (OR = 1.19, p = 0.34), dizziness (OR = 1.40, p = 0.17) and sore throat (OR = 1.60, p = 0.12) are not associated with increased risk of severity of COVID-19 cases and sputum production is in the marginal line (OR = 1.35, 95% CI = 1.00–1.82, p = 0.05, I2 = 55%).
3.6. Sensitivity and publication bias
Publication bias, checked by Egger's regression test and Begg-Mazumdar's rank correlation, are presented in Table 3 and Supplementary Figure S1–S28. No publication bias was found in case of age, sex, comorbidities and clinical symptoms tested by both Egger's and Begg-Mazumdar's tests (p > 0.05). The sensitivity was analyzed for assessing the stability of the results obtained and the influence of each study by omitting each study one by one for age, sex, comorbidities and clinical symptoms (Supplementary Figure S29-56). No significant effect of any single study on the pooled results was found in the case of age, sex and comorbidities and clinical symptoms.
4. Discussion
The new novel coronavirus (SARS-CoV-2) is the seventh human coronavirus, the third type of zoonotic coronavirus, and has genetically similarity with SARS-CoV (79%) and MERS-CoV (50%) [75, 76]. SARS-CoV-2 receptor-binding domain (RBD) is nearly the same as the RBD of SARS-CoV [77]. COVID-19 is highly contagious, and WHO declared it a global pandemic. A total of 5,962,944 confirmed cases and 363,905 (6.10%) deaths were reported as of May 29, 2020, and COVID-19 has spread to 213 countries and territories across all continents [14]. Moreover, China, Europe, and America sufferer more, and now, the severity decreases in China. Although the number of COVID-19 cases continues to grow worldwide, no specific antiviral treatment has been confirmed to be effective against COVID-19. So, clinical demographical characteristics, clinical manifestation, comorbidities of COVID-19 patients are more important to early detection and isolation as well as minimize the spread of the disease, severity, and death rate. In this meta-analysis, we retrospectively analyzed clinical data from patients with COVID-19. So, we completed a systemic meta-analysis. In this meta-analysis, we retrieved 55 independent studies from January 1, 2020, to May 24, 2020, which reported age, sex, severity, comorbidity, clinical symptoms, and different outcomes on 10014 patients with COVID-19 distributed across four countries.
In our study, we observed that males are more likely to be infected by COVID-19 and going to severe conditions (OR = 2.41, p < 0.00001) than females. A similar finding was also reported earlier in some other studies [78, 79]. A study conducted in Spain reported that men are more vulnerable than women because of their irresponsible attitude toward the risk of COVID-19 pandemic [80]. Another Spanish study revealed that the severity and case fatality rate (CFR) are higher in males and old aged people [81]. Moreover, a higher resistance in females is observed, which might be due to female sex hormones, whereas men have lower resistance because of high expression ACE2 receptor to which coronavirus binds easily [82]. Studies also showed that ACE2 expression, decreased B cell and NK cell-specific transcripts, male hormones, and increased NF-κB inhibitor are responsible for the higher viral load in men [83, 84, 85]. According to the data published by Global Health 50/50 presents that men are dying at a more consistent rate than women [86]. Besides, the lifestyle of men, including smoking, leads to high viral load and high severity [87]. A systemic review and meta-analysis also suggested that current smokers are at greater risk than former or non-smokers [88]. One study reported a positive hazard ratio (HR) in the COVID-19 related deaths for current smokers (HR = 1.14, 95%Cl = 1.05–1.23) in a model adjusted for demographic age and sex, whereas a lower HR was found in a fully adjusted model (HR = 0.89, 95%Cl = 0.82–0.97) [89].
Elderly or older people in both sexes (≥50 years) are more susceptible to SARS-CoV-2, which may be associated with a higher frequency of severity (age≥50 years vs. age<50 years, RR = 3.36; 95% CI = 1.79–6.30, p = 0.0002). We did not find any significant association of age ≥65 years with COVID-19 severity. Although we did not find any association of age (≥65 years) with COVID-19 severity, the age ≥50 years group also included some patients with ≥65 years of age. It was thought that elderly or older people are more susceptible to severity for weak immunity and other organ dysfunction. Elderly or older people and a higher frequency of comorbidities patients are more susceptible to SARS-CoV-2 [78,90].
Among 10014 COVID-19 patients, 51.14% had at least one comorbidity in severe groups, and other most common comorbidities in severe cases are hypertension (36.47%), diabetes (21.19%), cardiovascular disease (18.76%), cerebrovascular disease (11.83%) and chronic kidney disease (10.63%). All the preexisting comorbidities are associated with the increased severity in the COVID-19 cases (p < 0.05) in our current meta-analysis. Any comorbidity is a crucial factor in poor prognosis. Diseases such as hypertension, diabetes, respiratory system disease, cardiovascular disease, and their susceptibility conditions are higher risk of severe illness or death [78, 91, 92, 93]. Some articles also reported an association of hypertension and other cardiovascular diseases with COVID-19 [94,95]. Innate immunity response, macrophage, and lymphocyte function are decreased in the presence of comorbidities, which may be more susceptible to the pathogenesis of COVID-19 [96]. A metabolic disorder, inflammation, and infection are induced by diabetes, whereas chronic liver disease was reported to be associated with COVID-19 [97,98]. The presence of respiratory diseases develops acute respiratory distress syndromes (ARDS). Furthermore, a study reported that diabetes, smoking, and heart disease were mainly responsible for MERS-CoV illness [99]. The expression of ACE2 receptors is increased in some comorbid conditions like hypertension and diabetes, and SARS-CoV-2 attacks cells through ACE2 receptors. Therefore, comorbidities increase the severity of COVID-19 cases [90].
We summarized 17 clinical symptoms in our meta-analysis among them we found the significant association of fever (81.73%, OR = 1.68, p = 0.0001), cough (65.41%, OR-1.41, p = 0.004), fatigue (38.34%, OR = 1.26, p = 0.03), anorexia (31.23%, OR = 2.38, p < 0.0001), dyspnea (51.50%, OR = 4.30, p < 0.00001), chest tightness (25.62%, OR = 2.11, p = 0.002), hemoptysis (3.17%, OR = 4.93, p < 0.0001), diarrhea (18.35%, OR = 1.35, p = 0.03), abdominal pain (5.48%, OR = 2.38, p = 0.008) with the severity of COVID-19 cases.
We observed no association of COVID-19 severity with myalgia (24.91%, OR = 1.30, p = 0.07), sputum production (35.10%, OR = 1.35, p = 0.05), pharyngalgia (12.12%, OR = 0.91, p = 0.87), nausea (8.27%, OR = 1.26, p = 0.64), vomiting (6.53%, OR = 1.48, p = 0.07), headache (16.20%, OR = 1.19, p = 0.34), dizziness (12.26%, OR = 1.40, p = 0.17), and sore throat (13.78%, OR = 1.60, p = 0.12). SARS-CoV-2 binds with the ACE-2 receptor, causing diffuse alveolar damage and lymphocytic infiltration in both lungs and may cause several respiratory tract symptoms [100]. Several clinical researchers found that the common clinical manifestations of COVID-19 patients are fever, cough, headache, fatigue, myalgia, nausea, diarrhea, and sputum [101]. Diarrhea has been found in the Middle East respiratory syndrome coronavirus (MERS-COV) patients (up to 30%) [102]. A recent study showed that SARS-CoV-2 was detected in stool samples of patients with abdominal symptoms [103]. There also found expression of SARS-CoV2 receptor in the GI tract that may be related to GI-related symptoms like diarrhea, nausea and vomiting [104]. Shortness of breath or dyspnea indicates an impaired function of the lung and oxygen deficiency. Therefore, while planning to pay great attention to patients with the respiratory system and dyspnea as the primary symptoms, more attention should also be given to patients with cough, fatigue, anorexia, chest tightness, hemoptysis, diarrhea, abdominal pain, headaches, dizziness, nausea, sputum production and vomiting [78, 79, 105, 106].
Nowadays, many articles have been published on epidemiologic and clinical characteristics, but variations in reporting descriptive data may lead to the misunderstanding of the clinical features of COVID-19. Besides, some meta-analysis is also published. However, these meta-analyses pooled a small number of studies (<30). This is the first meta-analysis with the various studies (55 citations) and the most detailed review and clear proof of the clinical characteristics of COVID-19 patients to date. The quality of the publications included in this study is high, the analysis is rigorous, comprehensive, and the conclusions drawn by this meta-analysis are highly credible. Although this is a novel meta-analysis, there were some limitations to our study. First, the studies included were retrospective. Second, the sample size (7–1590) has a considerable variation among the included studies, leading to high heterogeneity. Third, reports being restricted to China and a few other countries, and our goal is to use the findings of this study to predict patients in general, including other countries and races. Without this limitation, this study analyzed the risk factors for progression to critical illness in COVID-19 patients to help to assess patient status and identify critical patients early. Our findings provide valuable information regarding the association of age, sex, comorbidities and clinical symptoms with the severity of COVID-19. We hope this information will support health care professionals and decision-makers in the current global pandemic, and more caution, as well as better early intervention, should be taken to improve the prognosis for older patients with respiratory failure. Effective treatment measures should be taken according to age, sex, comorbidities and clinical symptoms as the severity of COVID-19 is associated with these parameters.
5. Conclusions
Nowadays, COVID-19 is an emerging infectious disease and led to a significant health concern globally. Our study found that male patients and elderly or older patients (age≥50 years) are at a higher risk of developing disease severity. Our study also suggests that the presence of at least one or combined comorbidities like hypertension, diabetes, cerebrovascular disease, cardiovascular diseases, respiratory disease, malignancy, chronic kidney disease and chronic liver disease increases the severity of COVID-19. The prevalence of most common clinical symptoms like fever, cough, fatigue, anorexia, dyspnea, chest tightness, hemoptysis, diarrhea and abdominal pain were significantly higher in severe patients, and these are associated with the disease severity. This meta-analysis will help health care providers make appropriate medical decisions for their patients based on age, sex, comorbidities and clinical symptoms.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supplementary material.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors wish to express their most profound responsibility, indebtedness, appreciation for their generalized assistance, untiring motivation, scholastic supervision, constructive criticism, affectionate feeling and positive advice for the Department of Pharmacy, Noakhali Science and Technology University, Sonapur-3814, Noakhali, Bangladesh.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndrome. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . 2020. WHO Director-General's Opening Remarks at the media Briefing on COVID-19 - March 11 2020.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 [Google Scholar]
- 3.Gupta R., Ghosh A., Singh A.K., Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab. Syndrome. 2020;14(3):211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher D., Heymann D. Q&A: the novel coronavirus outbreak causing COVID-19. BMC Med. 2020;18(1):57. doi: 10.1186/s12916-020-01533-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez-Morales A.J., Cardona-Ospina J.A., Gutiérrez-Ocampo E. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med. Infect. Di. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.-J., Liang W.-H., Zhao Y. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su S., Wong G., Shi W. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490–502. doi: 10.1016/j.tim.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan J.F.-W., Kok K.-H., Zhu Z. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microb. Infect. 2020;9(1):221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang S., Peng W., Zhu Y. Recent progress in understanding 2019 novel coronavirus (SARS-CoV-2) associated with human respiratory disease: detection, mechanisms and treatment. Int. J. Antimicrob. Agents. 2020:105950. doi: 10.1016/j.ijantimicag.2020.105950. 105950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu X., Chen P., Wang J. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci. China Life Sci. 2020;63(3):457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet. Microbiol. 2020;244:108693. doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng J. SARS-CoV-2: an emerging coronavirus that causes a global threat. Int. J. Biol. Sci. 2020;16(10):1678–1685. doi: 10.7150/ijbs.45053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worldometers.info Confirmed cases and deaths by country, territory, or conveyance. https://www.worldometers.info/coronavirus/#countries
- 15.Moujaess E., Kourie H.R., Ghosn M. Cancer patients and research during COVID-19 pandemic: a systematic review of current evidence. Crit. Rev. Oncol. Hematol. 2020;150:102972. doi: 10.1016/j.critrevonc.2020.102972. 102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gulati A., Pomeranz C., Qamar Z. A comprehensive review of manifestations of novel coronaviruses in the context of deadly COVID-19 global pandemic. Am. J. Med. Sci. 2020;360(1):5–34. doi: 10.1016/j.amjms.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst. Rev. 2016;5(1):210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wells G., Shea B., O’Connell D., Peterson J. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 20.Aggarwal S., Garcia-Telles N., Aggarwal G., Lavie C., Lippi G., Henry B.M. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis. 2020;7(2):91–96. doi: 10.1515/dx-2020-0046. [DOI] [PubMed] [Google Scholar]
- 21.Bi X., Su Z., Yan H. Prediction of severe illness due to COVID-19 based on an analysis of initial Fibrinogen to Albumin Ratio and Platelet count. Platelets. 2020;31(5):674–679. doi: 10.1080/09537104.2020.1760230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai Q., Huang D., Ou P. COVID-19 in a designated infectious diseases hospital outside Hubei Province, China. Allergy. 2020;75(7):1742–1752. doi: 10.1111/all.14309. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y., Hao Z., Gao Y. Coronavirus disease 2019 in the perioperative period of lung resection: a brief report from a single thoracic surgery department in wuhan, people's Republic of China. J. Thorac. Oncol. 2020;15(6):1065–1072. doi: 10.1016/j.jtho.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G., Wu D., Guo W. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen X., Zhao B., Qu Y. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. medRxiv. 2020 doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Q., Zheng Z., Zhang C. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection. 2020;48(4):543–551. doi: 10.1007/s15010-020-01432-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu J., Yang N., Wei Y. Clinical characteristics of 54 medical staff with COVID-19: a retrospective study in a single center in Wuhan, China. J. Med. Virol. 2020;92(7):807–813. doi: 10.1002/jmv.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colaneri M., Sacchi P., Zuccaro V. Clinical characteristics of coronavirus disease (COVID-19) early findings from a teaching hospital in Pavia, North Italy, 21 to 28 February 2020. Euro Surveill. 2020;25(16):2000460. doi: 10.2807/1560-7917.ES.2020.25.16.2000460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng Q., Hu B., Zhang Y. Suspected myocardial injury in patients with COVID-19: evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Y., Ling Y., Bai T. COVID-19 with different severities: a multicenter study of clinical features. Am. J. Respir. Crit. Care Med. 2020;201(11):1380–1388. doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jessica F., Joelle I.R., Orlando Q. Characteristics and outcomes of coronavirus disease patients under nonsurge conditions, northern California, USA, march–april 2020. Emerg. Infect. Dis. 2020;26(8) doi: 10.3201/eid2608.201776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao Y., Li T., Han M. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020;92(7):791–796. doi: 10.1002/jmv.25770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He R., Lu Z., Zhang L. The clinical course and its correlated immune status in COVID-19 pneumonia. J. Clin. Virol. 2020;127:104361. doi: 10.1016/j.jcv.2020.104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong K.S., Lee K.H., Chung J.H. Clinical features and outcomes of 98 patients hospitalized with SARS-CoV-2 infection in daegu, South Korea: a brief descriptive study. Yonsei Med. J. 2020;61(5):431–437. doi: 10.3349/ymj.2020.61.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang Q., Deng X., Li Y. Clinical characteristics and drug therapies in patients with the common-type coronavirus disease 2019 in Hunan, China. Int. J. Clin. Pharm. 2020;42(3):837–845. doi: 10.1007/s11096-020-01031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang R., Zhu L., Xue L. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: a retrospective, multi-center study. PLoS Neglected Trop. Dis. 2020;14(5) doi: 10.1371/journal.pntd.0008280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y., He S., Zhang C. Clinical characteristics of 60 discharged cases of 2019 novel coronavirus-infected pneumonia in Taizhou, China. Ann. Transl. Med. 2020;8(8):547. doi: 10.21037/atm.2020.04.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ketcham S.W., Adie S.K., Malliett A. Coronavirus disease-2019 in heart transplant recipients in southeastern Michigan: a case series. J. Card. Fail. 2020;26(6):457–461. doi: 10.1016/j.cardfail.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li K., Wu J., Wu F. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest. Radiol. 2020;55(6):327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li S., Jiang L., Li X. Clinical and pathological investigation of patients with severe COVID-19. JCI Insight. 2020;5(12) doi: 10.1172/jci.insight.138070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X., Xu S., Yu M. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146(1):110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y.K., Peng S., Li L.Q. Clinical and transmission characteristics of covid-19 - a retrospective study of 25 cases from a single thoracic surgery department. Curr. Med. Sci. 2020;40(2):295–300. doi: 10.1007/s11596-020-2176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang W., Liang H., Ou L. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1–9. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu F., Li L., Xu M. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J. Clin. Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J., Li S., Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Jin C., Wu C.C. Association between initial chest CT or clinical features and clinical course in patients with coronavirus disease 2019 pneumonia. Korean J. Radiol. 2020;21(6):736–745. doi: 10.3348/kjr.2020.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lodigiani C., Iapichino G., Carenzo L. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb. Res. 2020;191:9–14. doi: 10.1016/j.thromres.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lv Z., Cheng S., Le J. Clinical characteristics and co-infections of 354 hospitalized patients with COVID-19 in Wuhan, China: a retrospective cohort study. Microb. Infect. 2020;22(4-5):195–199. doi: 10.1016/j.micinf.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lyu P., Liu X., Zhang R., Shi L., Gao J. The performance of chest CT in evaluating the clinical severity of COVID-19 pneumonia: identifying critical cases based on CT characteristics. Invest. Radiol. 2020;55(7):412–421. doi: 10.1097/RLI.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pan L., Mu M., Yang P. Clinical characteristics of COVID-19 patients with digestive symptoms in Hubei, China: a descriptive, cross-sectional, multicenter study. Am. J. Gastroenterol. 2020;115(5):766–773. doi: 10.14309/ajg.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng Y.D., Meng K., Guan H.Q. Clinical characteristics and outcomes of 112 cardiovascular disease patients infected by 2019-nCoV. Zhonghua Xinxueguanbing Zazhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. 0. [DOI] [PubMed] [Google Scholar]
- 55.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am. J. Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shi Y., Yu X., Zhao H., Wang H., Zhao R., Sheng J. Host susceptibility to severe COVID-19 and establishment of a host risk score: findings of 487 cases outside Wuhan. Crit. Care. 2020;24(1):108. doi: 10.1186/s13054-020-2833-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun L., Shen L., Fan J. Clinical features of patients with coronavirus disease 2019 from a designated hospital in Beijing, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian S., Hu N., Lou J. Characteristics of COVID-19 infection in beijing. J. Infect. 2020;80(4):401–406. doi: 10.1016/j.jinf.2020.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wan S., Xiang Y., Fang W. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in wuhan, China. J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang R., Pan M., Zhang X. Epidemiological and clinical features of 125 hospitalized patients with COVID-19 in Fuyang, anhui, China. Int. J. Infect. Dis. 2020;95:421–428. doi: 10.1016/j.ijid.2020.03.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang F., Yang Y., Dong K. Clinical characteristics of 28 patients with diabetes and covid-19 in wuhan, China. Endocr. Pract. 2020;26(6):668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu J., Li W., Shi X. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19) J. Intern. Med. 2020;288(1):128–138. doi: 10.1111/joim.13063. [DOI] [PubMed] [Google Scholar]
- 64.Xie H., Zhao J., Lian N., Lin S., Xie Q., Zhuo H. Clinical characteristics of non-ICU hospitalized patients with coronavirus disease 2019 and liver injury: a retrospective study. Liver Int. 2020;40(6):1321–1326. doi: 10.1111/liv.14449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Xie J., Ding C., Li J. Characteristics of patients with coronavirus disease (COVID-19) confirmed using an IgM-IgG antibody test. J. Med. Virol. 2020 doi: 10.1002/jmv.25930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xiong F., Tang H., Liu L. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in wuhan, China. J. Am. Soc. Nephrol. 2020;31(7):1387–1397. doi: 10.1681/ASN.2020030354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharm. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang P., Wang P., Song Y., Zhang A., Yuan G., Cui Y. A retrospective study on the epidemiological characteristics and establishment of an early warning system of severe COVID-19 patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y., Shen C., Li J. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020;146(1):119–127. doi: 10.1016/j.jaci.2020.04.027. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yao Q., Wang P., Wang X. A retrospective study of risk factors for severe acute respiratory syndrome coronavirus 2 infections in hospitalized adult patients. Pol. Arch. Intern. Med. 2020;130(5):390–399. doi: 10.20452/pamw.15312. [DOI] [PubMed] [Google Scholar]
- 71.Yu X., Sun X., Cui P. Epidemiological and clinical characteristics of 333 confirmed cases with coronavirus disease 2019 in Shanghai, China. Transbound Emerg. Dis. 2020;67(4):1697–1707. doi: 10.1111/tbed.13604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang J.J., Dong X., Cao Y.Y. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 73.Zheng S., Fan J., Yu F. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January-March 2020: retrospective cohort study. BMJ. 2020;369:m1443. doi: 10.1136/bmj.m1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou Y., He Y., Yang H. Development and validation a nomogram for predicting the risk of severe COVID-19: a multi-center study in Sichuan, China. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0233328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lan J., Ge J., Yu J. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature. 2020;581(7807):215–220. doi: 10.1038/s41586-020-2180-5. [DOI] [PubMed] [Google Scholar]
- 78.Zheng Z., Peng F., Xu B. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahmed A., Ali A., Hasan S. Comparison of epidemiological variations in COVID-19 patients inside and outside of China-A meta-analysis. Front Pub. Health. 2020;8:193. doi: 10.3389/fpubh.2020.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de la Vega R., Ruíz-Barquín R., Boros S., Szabo A. Could attitudes toward COVID-19 in Spain render men more vulnerable than women? Global Publ. Health. 2020;15(9):1278–1291. doi: 10.1080/17441692.2020.1791212. [DOI] [PubMed] [Google Scholar]
- 81.Moraga P., Ketcheson D.I., Ombao H.C., Duarte C.M. Assessing the age- and gender-dependence of the severity and case fatality rates of COVID-19 disease in Spain [version 1; peer review: 1 approved] Wellcome Open Res. 2020 doi: 10.12688/wellcomeopenres.15996.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bwire G.M. Coronavirus: why men are more vulnerable to covid-19 than women? SN Compr. Clin. Med. 2020;2:874–876. doi: 10.1007/s42399-020-00341-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lieberman N.A.P., Peddu V., Xie H. In vivo antiviral host transcriptional response to SARS-CoV-2 by viral load, sex, and age. PLoS Biol. 2020;18(9) doi: 10.1371/journal.pbio.3000849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Moeser A. 2020. COVID-19 Affects Men More than Women and This Could Be the Reason Why, According to Scientists.https://www.weforum.org/agenda/2020/06/covid19-mortality-rates-men-women/ Accessed. [Google Scholar]
- 85.Lee J., Yousaf A., Fang W., Kolodney M.S. Male balding is a major risk factor for severe COVID-19. J. Am. Acad. Dermatol. 2020;S0190–9622(20):32262–32263. doi: 10.1016/j.jaad.2020.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Men, sex, gender and COVID-19 . 2020. The Sex, Geneder and Covid-19 Project.https://globalhealth5050.org/the-sex-gender-and-covid-19-project/men-sex-gender-and-covid-19/ Accessed. [Google Scholar]
- 87.Smoking and COVID-19. World Health Organization; 2020. https://www.who.int/news-room/commentaries/detail/smoking-and-covid-19 Accessed. [Google Scholar]
- 88.Alqahtani J.S., Oyelade T., Aldhahir A.M. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Williamson E.J., Walker A.J., Bhaskaran K. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584(7821):430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Landi F., Barillaro C., Bellieni A. The new challenge of geriatrics: saving frail older people from the SARS-COV-2 pandemic infection. J. Nutr. Health Aging. 2020;24(5):466–470. doi: 10.1007/s12603-020-1356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hu Y., Sun J., Dai Z. Prevalence and severity of corona virus disease 2019 (COVID-19): a systematic review and meta-analysis. J. Clin. Virol. 2020;127:104371. doi: 10.1016/j.jcv.2020.104371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Petrie J.R., Guzik T.J., Touyz R.M. Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can. J. Cardiol. 2018;34(5):575–584. doi: 10.1016/j.cjca.2017.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang J., Zheng Y., Gou X. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu J., Zhang X., Zhang X. COVID-19 patients with hypertension have more severity condition, and ACEI/ARB treatment have no influence on the clinical severity and outcome [published online ahead of print, 2020 May 28] J. Infect. 2020 S0163-4453(20)30334-0. [Google Scholar]
- 95.Cappuccio F.P., Siani A. Covid-19 and cardiovascular risk: susceptibility to infection to SARS-CoV-2, severity and prognosis of Covid-19 and blockade of the renin-angiotensin-aldosterone system. An evidence-based viewpoint. Nutr. Metabol. Cardiovasc. Dis. 2020;30(8):1227–1235. doi: 10.1016/j.numecd.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Castle S.C., Uyemura K., Rafi A., Akande O., Makinodan T. Comorbidity is a better predictor of impaired immunity than chronological age in older adults. J. Am. Geriatr. Soc. 2005;53(9):1565–1569. doi: 10.1111/j.1532-5415.2005.53512.x. [DOI] [PubMed] [Google Scholar]
- 97.Odegaard J.I., Chawla A. Connecting type 1 and type 2 diabetes through innate immunity. Cold Spring Harb. Perspect. Med. 2012;2(3) doi: 10.1101/cshperspect.a007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Youssef M., Hussein M., Attia A.S. COVID-19 and Liver Dysfunction: a systematic review and meta-analysis of retrospective studies. J. Med. Virol. 2020 doi: 10.1002/jmv.26055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Alraddadi B.M., Watson J.T., Almarashi A. Risk factors for primary Middle East respiratory syndrome coronavirus illness in humans, Saudi arabia, 2014. Emerg. Infect. Dis. 2016;22(1):49–55. doi: 10.3201/eid2201.151340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Verdecchia P., Cavallini C., Spanevello A., Angeli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur. J. Intern. Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fu L., Wang B., Yuan T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J. Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin. Microbiol. Rev. 2015;28(2):465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poggiali E., Ramos P.M., Bastoni D., Vercelli A., Magnacavallo A. Abdominal pain: a real challenge in novel COVID-19 infection. Eur. J. Case Rep. Intern Med. 2020;7(4) doi: 10.12890/2020_001632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ng S.C., Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. 2020;69(6):973–974. doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li L.Q., Huang T., Wang Y.Q. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92(6):577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sun P., Qie S., Liu Z., Ren J., Li K., Xi J. Clinical characteristics of hospitalized patients with SARS-CoV-2 infection: a single arm meta-analysis. J. Med. Virol. 2020;92(6):612–617. doi: 10.1002/jmv.25735. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material.