Abstract
Background:
Unannounced home-based pill counts conducted in person or on the telephone are reliable and valid for monitoring medication adherence. However, expecting to have one’s pills counted, organizing medications for pill counts, and increased attention from the person conducting the pill counts may have reactive effects and inadvertently improve adherence. The current study determined whether monthly unannounced pill counts conducted by telephone influence adherence over time.
Methods:
Two prospective cohorts, one drawn from a social support condition in a behavioral intervention trial (n=186) and the other an observational study (n=187), were followed for 12 months and 8 months, respectively. Medication adherence was monitored using monthly unannounced pill counts conducted by telephone. In addition, blood plasma viral load was collected at the final pill count for the observational cohort.
Results:
Analyses did not indicate increases in medication adherence over time for antiretroviral or psychiatric medications among men, women, people with detectable and undetectable viral loads, and various medication regimens.
Conclusions:
Unannounced pill counts conducted by telephone do not demonstrate reactivity effects and remain a viable, unobtrusive, objective method of monitoring medication adherence.
Keywords: adherence monitoring, assessment reactivity, HIV treatment adherence, self-monitoring
Antiretroviral therapy (ART) has transformed HIV infection from a life-threatening disease to a chronic condition, and there are now efforts to expand the benefits of ART to prevent HIV transmission.1 Close adherence to ART is necessary to achieve viral suppression, the key to therapeutic and preventive benefits.1–3 Whereas viral load testing ultimately determines the clinical benefits of ART, monitoring adherence is necessary to correct lapses in adherence before HIV viral rebound.4 HIV/AIDS treatment research also depends on reliable and valid methods of monitoring adherence, for which there are few options.
Unannounced pill counts show promise for monitoring ART adherence in research settings, including clinical trials. Unlike office-based pill counts, unannounced pill counts ensure that all medications needed to determine adherence are available at the time that pills are counted. In addition, unannounced pill counts reduce pill dumping, where patients intentionally discard medications to avoid revealing missed doses,5 as well as other serious limitations posed by office-based pill counts.6 Bangsberg et al7 demonstrated that unannounced pill counts conducted in patients’ homes yield adherence data comparable to electronic medication monitoring. Unannounced pill counts also correspond with viral load at a magnitude similar to that observed with electronic medication monitoring. More recently, Bangsberg et al’s home-based pill count procedure was adapted for use over the telephone. Unannounced pill counts conducted by phone have been validated against home-based pill counts with strikingly similar results. The associations between phone-based pill counts and viral suppression also mirror those observed from home-based pill counts.8–10 Unannounced pill counts therefore offer a viable and cost-efficient method of monitoring ART adherence.
One unanswered question about unannounced pill counts is whether counting pills influences medication-taking behavior. Self-monitoring and reactivity effects are a common problem in behavioral assessment, particularly when the assessment method increases awareness, conscientiousness, and self-regulatory processes.11–13 The potential for reactivity to objective adherence assessments has even been capitalized on in medication adherence interventions. For example, feedback from electronic medication monitoring devices has helped patients take corrective action to improve their adherence.14 Monthly contact with someone who counts patients’ pills may also influence adherence behavior through subtle social cues, increased self-conscientiousness, and inadvertent reminders. Assessment reactivity in medication monitoring therefore risks improving and overestimating adherence. Measurement reactivity is of particular concern when interpreting outcomes from adherence intervention trials, where improved adherence from medication monitoring will confound intervention effects. To date, no study has examined the potential adherence reactivity effects of monthly unannounced pill counts.
The current study used data from 2 independent cohorts to examine changes over time in ART adherence monitored by monthly telephone-based unannounced pill counts. The first cohort consisted of participants in support groups that served as a condition in a behavioral intervention trial. Participants in this intervention received a stress reduction and nutrition intervention for people living with HIV/AIDS. Data from the first cohort included unannounced pill counts for ART and psychiatric medication adherence, both monitored for 12 months. Second, reactivity to unannounced pill counts was examined in an 8-month prospective observational cohort. Blood specimens were obtained from participants in the second cohort at the end of the 8-month observation period to examine the association between viral load and changes in adherence over time. We tested whether adherence changed over time in both cohorts and whether changes in adherence differed for ART and psychiatric medications, men and women, and in association with subsequent viral load.
METHODS
Participants
Two cohorts were examined in this study. Cohort 1 consisted of 112 men, 62 women, and 12 transgender persons who were taking ART and were enrolled in a behavioral intervention trial. Participants attended support groups that were focused on stress reduction and nutrition followed by monthly unannounced pill counts conducted by telephone for 1 year. The intervention did not include any adherence-enhancing components. The second cohort included 138 men, 46 women, and 3 transgender persons living with HIV/AIDS and taking ART. Persons in Cohort 2 were enrolled in a prospective observation study that included medication monitoring by unannounced pill counts for 8 months.
Measures
The same adherence and behavioral measures were obtained in both cohorts. In Cohort 1, measures were collected using audio computer-assisted structured interviews (ACASI); in Cohort 2, measures were collected using self-administered procedures. Monthly telephone-based unannounced pill counts were conducted for both cohorts. Blood specimens for viral load testing were collected after the final unannounced pill count in Cohort 2.
Demographic and health characteristics
Participants reported their age, ethnicity, education, income, and related demographic information. We asked participants to report the year that they tested HIV positive and their most recent CD4 cell count.
Unannounced telephone-based pill counts
Using an adaptation of the unannounced home-based pill count,7 we conducted monthly (28 days ± 7 days) unannounced pill counts by telephone. The telephone adaptation of unannounced pill counts has been validated against home-based pill counts and patient viral load.8,9 At an office intake session that included informed consent, participants were trained to count their medications using the following steps after answering the telephone: (a) bring all medications that are in the home to a comfortable flat surface near the telephone, including closed bottles, pocketed doses, and pill boxes; (b) sort medications into clusters; (c) select a medication and tell the pill counter the prescription numbers, refill dates, number of refills remaining, and dispensed quantities; (d) report lost or gained pills since the previous count and whether the drug was taken that day; (e) count pills using pharmacist tray and cup provided by the study (if using a pillbox, open each compartment to count the pills without removing them from containers); (f) repeat procedure to double-count all pills.
Participants were called by a trained pill counter at an unexpected time to count their pills. Participants in Cohort 1 counted ART medications (n = 175) and when applicable they also counted their psychiatric medications prescribed for depression and other mental health conditions (n = 73). Only psychiatric medications taken daily were counted. For Cohort 2, only ART medications were counted. All of the data needed to calculate adherence were tabulated, including prescription numbers, refill dates, and dates that medications may had been stopped and started between pill counts.
Calculating adherence
Adherence was calculated for all points at which there were 2 consecutive pill counts. Participants who missed a pill count were contacted the next month. Adherence was defined by the difference between pills counted at 2 consecutive times divided by the pills prescribed, taking into account the number of pills dispensed, lost, gained, and taken that day. Stopped medications were adjusted for number of days between the previous pill count and the stop date. Refill information, specifically the prescription numbers, filled dates, and remaining number of refills, were used to verify the accuracy of medications dispensed.
Blood specimen collection and laboratory analysis
Participants in Cohort 2 were asked to come to the project office to provide blood specimens to test for viral load within 1 week after their final unannounced pill count. Blood samples were obtained at the project offices using standard phlebotomy and were couriered to the lab for processing. Whole blood specimens in EDTA tube (Becton Dickinson) were centrifuged at 500 g for 10 minutes within 4 hours of collection. The plasma was recovered and aliquoted into 1 mL samples and stored at −70°C. Plasma viral load was determined by the Amplicor HIV-1 Monitor test (Roche Diagnostics, Indianapolis, Indiana, USA) with sensitivity for detecting down to 50 copies/mL.
Data Analyses
In both cohorts, participants were followed using monthly phone assessments regardless of whether they had stopped taking medications or were not reached the previous month. We therefore included participants who started treatment, stopped treatment, and changed regimens. Sample sizes vary at each time point, with some participants initiating medications and stopping medications over time. All analyses used available data at each time point. Generalized estimating equations (GEE) were used to test for changes in adherence over time. We used a repeated measures approach with 12 adherence time points for Cohort 1 and 8 time points for Cohort 2. We also used GEE to test for effects of participant gender and time on adherence in Cohort 2. In these analyses, participant gender and assessment times as well as the Gender x Time interaction were examined in association with monthly adherence. We also examined differences in adherence over time for various ART regimens.
Adherence was also examined in relation to viral load in Cohort 2. A multivariable GEE was performed to test the association between medication adherence over time and subsequent viral load. This analysis tested adherence for persons who later demonstrated detectable and undetectable viral loads. Finally, we examined the correlations between ART adherence at each time point with viral load collected after the final pill count to test for associations between adherence and viral load.
RESULTS
Ninety percent of participants in Cohort 1 were African American, 7% were white, and 3% self-identified as another race/ethnicity. The mean age of Cohort 1 was 45.3 (SD = 6.5) and had been diagnosed with HIV infection a mean of 12.4 years (SD = 5.9) earlier. The average CD4 cell count in Cohort 1 was 429.9 (SD = 286.1), and 54% had an undetectable viral load. Cohort 2 was 89% African American and the average age was 45.8 (SD = 7.0), with participants testing HIV positive an average of 13.4 (SD = 6.8) years earlier. For Cohort 2, the mean CD4 cell count was 465.6 (SD = 306.1), and 61% of participants in Cohort 2 had an undetectable viral load.
For Cohort 1, a total of 175 of the 186 (94%) were taking ART and 73 (39%) participants were taking psychiatric medications. Eighty-five percent of psychotropic medications were anti-depressants and 15% were anti-psychotics. Retention rates for Cohort 1 were 85% at the 6-month phone call and 72% at the 12-month call. For Cohort 2, 87% of participants provided adherence data at the 4-month phone call and 79% at the 8-month phone call. In addition, 161 (86%) participants in Cohort 2 provided blood specimens for viral load testing within 1 week of their final 8-month phone call.
Changes in Adherence Over Time
For Cohort 1, the GEE analysis indicated significant effects of time on ART adherence for Cohort 1, Wald χ211 = 23.6, P < .01; adherence decreased over time (see Figure 1). GEE analysis for psychiatric medications did not indicate significant changes over time, Wald χ211 = 8.0, P = ns. For Cohort 2, GEE analyses showed the main effect for time was significant, Wald χ27 = 14.8, P < .05; adherence decreased. The effects of gender, Wald χ21 = 0.03, P = ns, and the Gender x Time interaction, Wald χ27 = 2.4, P = ns, were not significant (see Figure 2). Once again, final adherence values were lower than the initial adherence values. Examination of adherence to classes of ART medications and number of pills in ART regimens did not indicate any evidence for improved adherence in response to repeated unannounced pill counts (see Table 1). Adherence did not vary more than 5% across time points for standard regimens and regimens with 3 or fewer pills. Adherence for other regimens and regimens with 4 or more medications were somewhat more erratic. However, in no case was there a pattern that suggested adherence improved over time.
Figure 1.
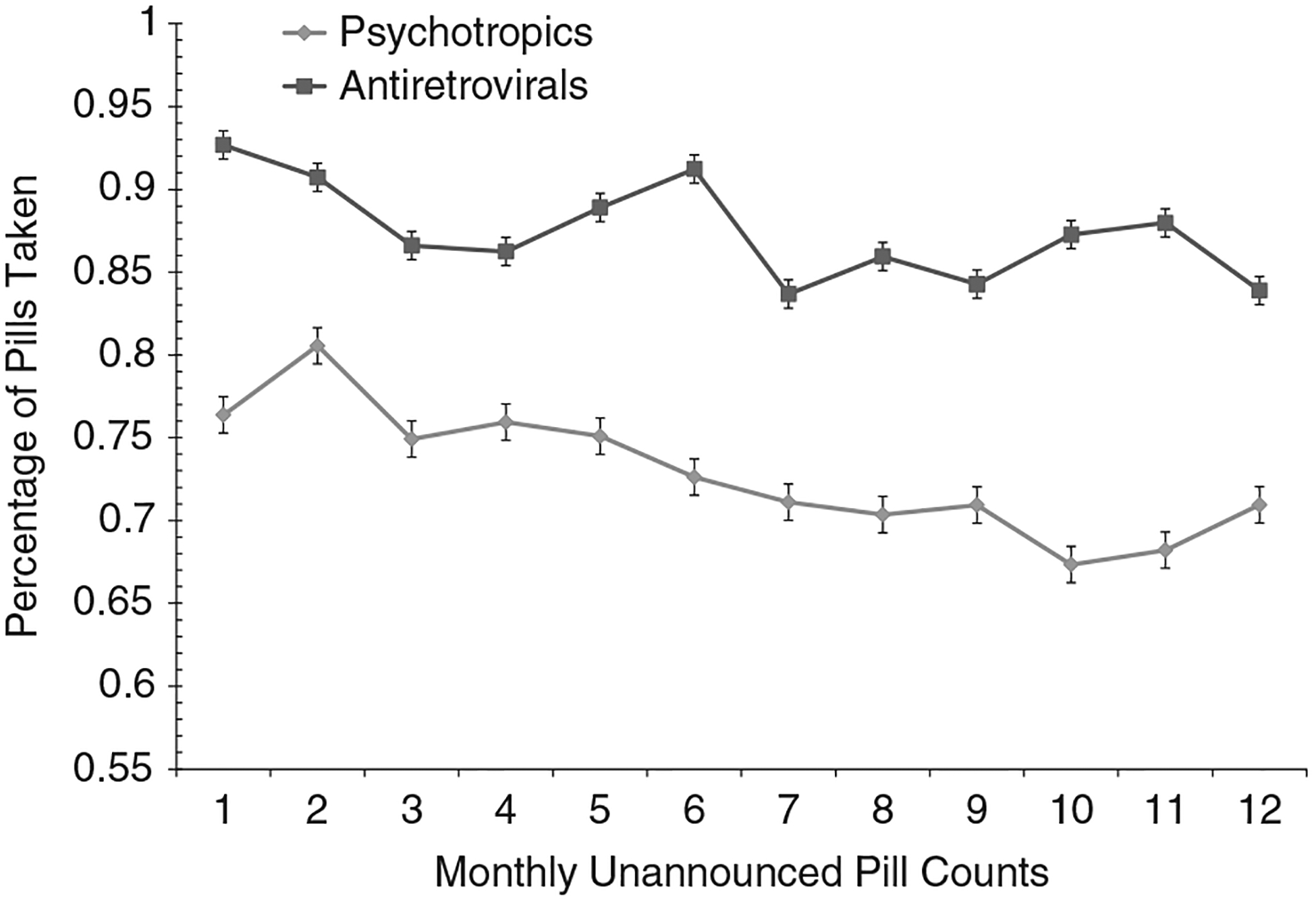
Estimated means for adherence to antiretroviral therapy and psychiatric medications assessed by monthly unannounced pill counts, Cohort 1.
Figure 2.
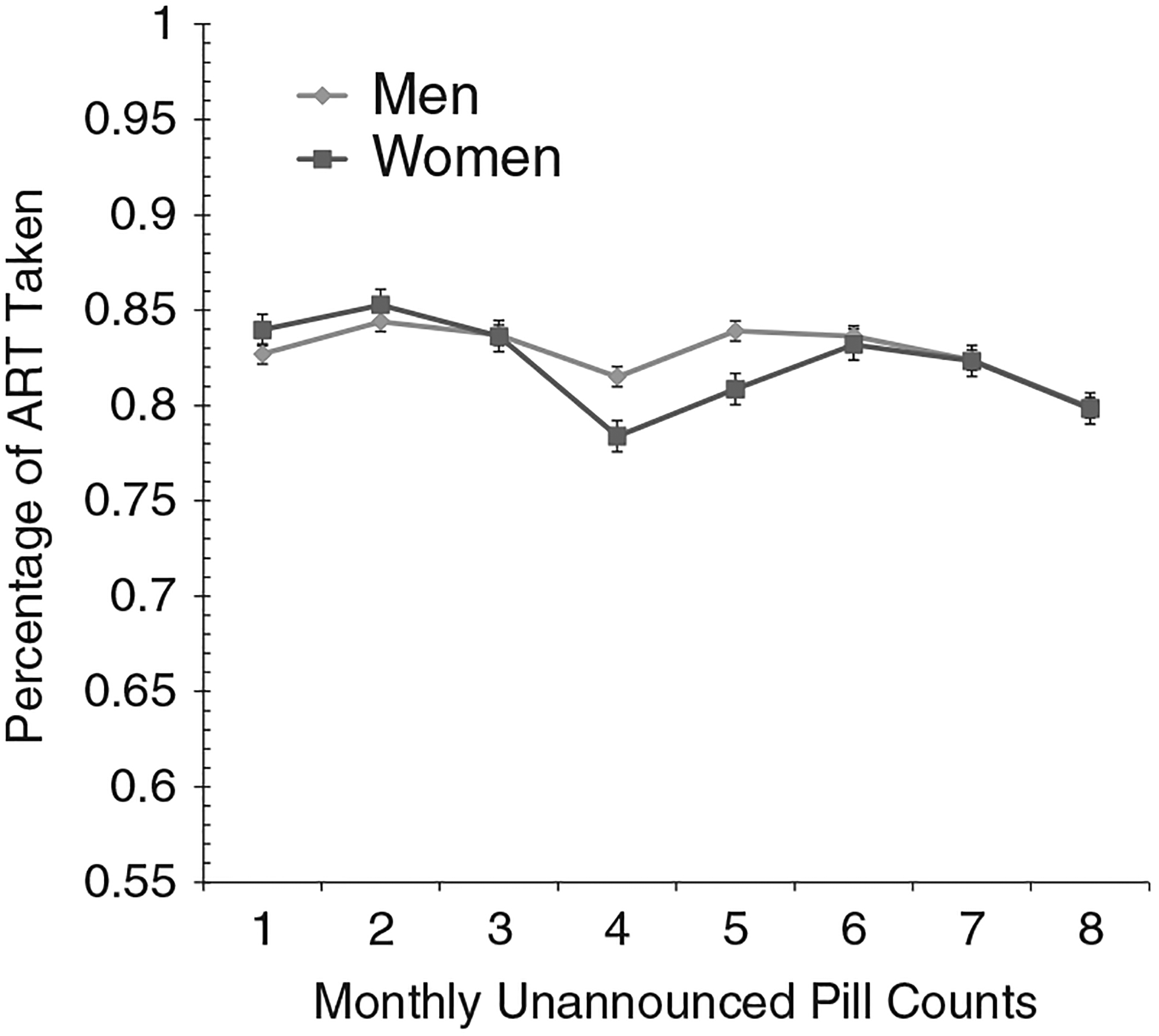
Estimated means for adherence to antiretroviral therapy assessed by monthly unannounced pill counts for men and women, Cohort 2.
Table 1.
Observed mean adherence determined by unannounced pill counts for classes of antiretroviral medications and number of pills in regimen, Cohort 2
| Number of pills in regimen | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time | Total sample | NNRTI | PI boosted | Other | 1 | 2 | 3 | 4+ |
| 1 | 85.2 | 87.8 | 81.0 | 86.8 | 87.8 | 84.5 | 83.6 | 74.3 |
| 2 | 88.1 | 85.4 | 86.0 | 93.1 | 88.8 | 87.5 | 89.4 | 73.6 |
| 3 | 86.4 | 87.4 | 82.7 | 89.2 | 88.4 | 84.8 | 87.3 | 74.7 |
| 4 | 84.6 | 84.8 | 80.2 | 88.9 | 83.7 | 85.5 | 85.5 | 67.8 |
| 5 | 87.3 | 86.8 | 84.3 | 91.0 | 86.9 | 84.1 | 88.5 | 80.4 |
| 6 | 87.1 | 87.3 | 84.5 | 89.6 | 87.5 | 84.4 | 88.5 | 77.8 |
| 7 | 87.6 | 91.0 | 83.9 | 88.1 | 91.9 | 82.5 | 88.2 | 81.7 |
| 8 | 86.2 | 89.9 | 79.9 | 88.3 | 89.9 | 82.7 | 82.4 | 79.2 |
Note: NNRTI = non-nucleoside reverse transcriptase inhibitors; PI boosted = protease inhibitor boosted.
GEE analyses were performed to compare participants who demonstrated undetectable and detectable viral loads on their adherence over time. Results showed a main effect for viral load, Wald χ21 = 20.9, P < .01; persons with detectable viral loads had lower adherence. There was also a main effect for time, Wald χ27 = 17.8, P < .01; adherence diminished over time. There was also a significant Viral Load x Time interaction, Wald χ27 = 19.3, P < .01; participants with undetectable viral loads demonstrated stable adherence, whereas participants with a detectable viral load demonstrated deterioration in adherence (see Figure 3).
Figure 3.
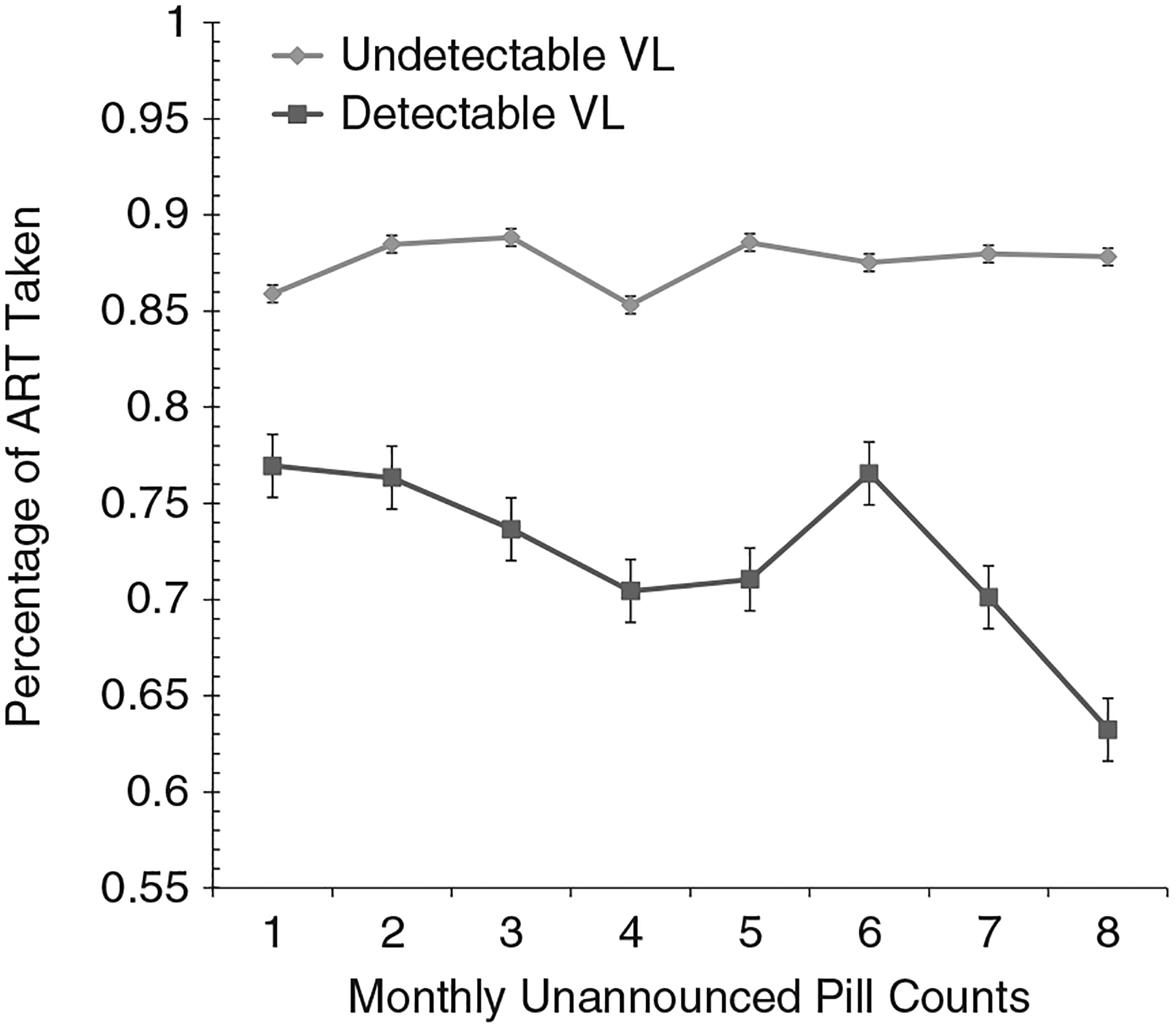
Estimated means for adherence to antiretroviral therapy assessed by monthly unannounced pill counts for participants with undetectable and detectable viral load measured at 8 months, Cohort 2.
With the exception of Time 1, results demonstrated that individuals who had an undetectable viral load at the final follow-up had significantly better ART adherence than those whose viral load was detectable (see Table 2). Adherence was significantly correlated with viral load across time points, with the highest magnitude correlation occurring closest to viral load testing.
Table 2.
Differences in adherence over time for participants with undetectable and detectable viral loads at the final follow-up and the correlation between adherence and final follow-up viral load, Cohort 2
| Undetectable viral load | Detectable viral load | Adherence and viral load correlation | ||||||
|---|---|---|---|---|---|---|---|---|
| Time | n | Mean | SD | n | Mean | SD | t | r |
| 1 | 110 | 85.8 | 20.6 | 51 | 77.8 | 25.5 | 2.1* | −.28** |
| 2 | 109 | 88.8 | 15.4 | 46 | 79.9 | 25.6 | 2.6** | −.33** |
| 3 | 111 | 89.1 | 15.0 | 43 | 75.5 | 24.4 | 4.1** | −.43** |
| 4 | 112 | 85.8 | 19.0 | 46 | 75.3 | 30.0 | 2.6** | −.35** |
| 5 | 114 | 89.1 | 13.9 | 46 | 73.0 | 28.5 | 4.7** | −.45** |
| 6 | 115 | 87.8 | 15.6 | 44 | 79.2 | 23.6 | 2.6** | −.28** |
| 7 | 111 | 88.4 | 16.1 | 39 | 77.5 | 32.6 | 2.7** | −.29** |
| 8 | 107 | 89.1 | 12.6 | 44 | 67.4 | 36.6 | 5.4** | −.57** |
P < .10.
P < .01.
DISCUSSION
There are few methods available to objectively and reliably monitor medication adherence. Unannounced pill counts conducted by telephone offer a new approach to monitoring adherence. The current study replicates previous research to show that phone-based unannounced pill counts correspond with HIV viral load in an expected pattern.8 Results from 2 independent cohorts that measured ART adherence as well as psychiatric medication adherence failed to find evidence that monthly unannounced pill counts improved adherence. In fact, results suggested decreases in adherence over time. This same pattern was observed for men and women, ART and psychiatric medications, people with detectable and undetectable viral loads, various ART regimens, and regimens with a range of numbers of medications. We conclude that monthly unannounced pill counts do not increase medication adherence.
The current study supports the use of unannounced pill counts for monitoring ART. One feature that limits the feasibility of unannounced home-based pill counts is the cost associated with repeatedly sending assessment staff to patients’ homes for pill counts. Conducting unannounced pill counts by telephone substantially reduces staff resources and patient burden. However, the telephone procedure does not allow for visual confirmation of numbers of pills counted. Even though unannounced phone-based pill counts reduce the likelihood of pill dumping and other sources of bias associated with office-based pill counts, mishaps such as miscounting, dropping pills, losing and lending pills, confusing medications, and losing bottles of medication can introduce error in unannounced phone-based pill counts. However, unannounced pill counts may not be feasible for monitoring adherence in all settings. For example, research is needed to determine implementation feasibility in clinical care.
The current findings are consistent with past research on unannounced pill counts.8 The degree to which our findings are able to be generalized to other settings is yet to be determined. Even though it may be possible to monitor medications more often than monthly, such as biweekly, the current reactivity findings may not apply to more frequent pill counts. Our study also monitored participants for 1 year or less, leaving open the question of whether monthly medication monitoring may have reactive effects over the longer term. With these limitations in mind, the current study supports the reliability, stability, and utility of unannounced pill counts conducted by telephone.
The current findings have implications for monitoring medications in clinical trials. Until now, it was unknown whether monthly unannounced pill counts have reactivity effects on medication adherence. The potential to confound intervention outcomes with measurement reactivity could reduce statistical power in clinical trials and would clearly contraindicate using pill counts as an adherence intervention outcome variable. The lack of evidence for assessment reactivity from unannounced pill counts observed in the current study builds confidence that phone-based pill counts can yield reliable and nonconfounding monthly adherence data. Research is now needed to determine the utility of phone-based unannounced pill counts in clinical settings. Adherence nurses and counselors who routinely call patients to check in with how they are doing may benefit from an immediate medication monitoring procedure.
ACKNOWLEDGMENTS
This project was supported by National Institute of Mental Health (NIMH) grants R01-MH71164 and R01-MH82633 to Dr. Kalichman. Drs. Detorio, Caliendo, and Schinazi were supported by the Center for AIDS Research, Emory University School of Medicine, National Institutes of Health (NIH) grant P30 AI050409. Dr. Schinazi was supported by the Department of Veterans Affairs.
REFERENCES
- 1.Dieffenbach CW, Fauci AS. Universal voluntary testing and treatment for prevention of HIV transmission. JAMA. 2009;301(22):2380–2382. [DOI] [PubMed] [Google Scholar]
- 2.Bangsberg D, Kroetz DL, Deeks SG. Adherence-resistance relationships to combination HIV antiretroviral therapy. Curr HIV/AIDS Rep. 2007;4:65–72. [DOI] [PubMed] [Google Scholar]
- 3.Bangsberg DR. Preventing HIV antiretroviral resistance through better monitoring of treatment adherence. J Infect Dis. 2008;197(Suppl 3):S272–278. [DOI] [PubMed] [Google Scholar]
- 4.Bangsberg DR, Deeks SG. Spending more to save more: interventions to promote adherence. Ann Intern Med. 2010;152(1):54–56; W–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd P, Byyny RL, Zachary V, et al. The natural history of medication compliance in a drug trial: limitations of pill counts. Clin Pharmacol Ther. 1989;46(2):169–176. [DOI] [PubMed] [Google Scholar]
- 6.Pullar T, Kumar S, Tindall H, Freely M. Time to stop counting the tablets? Clin Pharmacol Ther. 1989;46(2):163–168. [DOI] [PubMed] [Google Scholar]
- 7.Bangsberg DR, Perry S, Charlebois ED, et al. Comparing objective measures of adherence to HIV antiretroviral therapy: electronic medication monitors and unannounced pill counts. AIDS Behav. 2001;5:275–281. [Google Scholar]
- 8.Kalichman SC, Amaral CM, Cherry C, et al. Monitoring antiretroviral adherence by unannounced pill counts conducted by telephone: reliability and criterion-related validity. HIV Clin Trials. 2008; 9:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kalichman SC, Eaton L, White D, et al. Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. J Gen Intern Med. 2007;22:1003–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalichman SC, Amaral CM, Swetzes C, et al. A simple single-item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Phys AIDS Care. 2009;8(6):367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifford PR, Maisto SA. Subject reactivity effects and alcohol treatment outcome research. J Stud Alcohol. 2000;61(6):787–793. [DOI] [PubMed] [Google Scholar]
- 12.Picciano JF, Roffman RA, Kalichman SC, Walker DD. Lowering obstacles to HIV prevention services: effects of a brief, telephone-based intervention using motivational enhancement therapy. Ann Behav Med. 2007;34(2):177–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalichman SC, Kelly JA, Stevenson Y. Priming effects of HIV risk assessments on related perceptions and behaviors: an experimental field study. AIDS Behav. 1997;1(1): 3–8. [Google Scholar]
- 14.Sabin LL, DeSilva MB, Hamer DH, et al. Using electronic drug monitor feedback to improve adherence to antiretroviral therapy among HIV-positive patients in China. AIDS Behav. 2010;14(3):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]


