Abstract
Severe Combined ImmunoDeficiency (SCID) is defined by the lack of an adaptive immune system. Mutations causing SCID are found naturally in humans, mice, horses, dogs, and recently in pigs, with the serendipitous discovery of the Iowa State University SCID pigs. As research models, SCID animals are naturally tolerant of xenotransplantation and offer valuable insight into research areas such as regenerative medicine, cancer therapy, as well as immune cell signaling mechanisms. Large animal biomedical models, particularly pigs, are increasingly essential to advance the efficacy and safety of novel regenerative therapies on human disease. Thus, there is need to create practical approaches to maintain hygienic severe immunocompromised porcine models for exploratory medical research. Such research often requires stable genetic lines for replication and survival of healthy SCID animals for months post treatment. A further hurdle in the development of the ISU SCID pig as a biomedical model involved the establishment of facilities and protocols necessary to obtain clean SPF piglets from the conventional pig farm on which they were discovered. A colony of homozygous SCID boars and SPF carrier sows has been created and maintained through selective breeding, bone marrow transplants, innovative husbandry techniques and the development of biocontainment facilities.
Keywords: Severe Combined Immunodeficiency (SCID), Snatch farrow, Pig, Colostrum, Specific Pathogen Free (SPF)
INTRODUCTION:
The Iowa State University (ISU) Severe Combined ImmunoDeficiency (SCID) pigs were unexpectedly discovered during a viral challenge study in collaboration with Kansas State University (Cino Ozuna et al. 2012). Animals that died early in the trial revealed a complete lack of antibodies and dysplastic immune tissues (thymus, spleen, and lymph nodes). Genetic analysis using genome wide association study (GWAS) revealed a significant peak on chromosome 10 corresponding to the location of the Artemis gene, which is associated with human SCID (Waide et al. 2015; Cossu 2010). It was determined that two separate point mutations within the Artemis gene segregate in a Mendelian recessive mode of inheritance in which homozygotes or compound heterozygotes produce the SCID phenotype (Waide et al. 2015). Consistent with human Artemis SCID patients, early in life the ISU SCID pig is devoid of B and T cells (Ewen et al. 2014; Waide et al., 2015), but have a population of natural killer (NK) cells that demonstrate normal function in vitro (Powell et al. 2016).
While SCID is naturally occurring in humans, mice, dogs, horses and now pigs (Cossu 2010; Bosma et al. 1983; Perryman et al. 2004; Cino Ozuna et al. 2012) it has also been introduced into rodent and porcine hosts for use as biomedical models through targeted genetic modification (Ito et al. 2012; Huang et al. 2014; Suzuki et al. 2012; Lee et al. 2014 ; reviewed in Powell et al. 2017). Both SCID mice and pigs accept xenotransplants (Basel et al., 2012), and thus make excellent test subjects for cancer research and cell-based therapeutics in regenerative medicine. A related use of SCID animals is ‘humanization’ studies with the engraftment of human hematopoietic stem cells into an immune compromised host creating a human immune system in the SCID animal. This facilitates a non-human research model to explore species-specific pathogens such as HIV and Hepatitis C (Ito R et al. 2012).
The ISU SCID pig permits the growth of two kinds of human cancer cell lines when injected into the ear (Basel et al. 2012), establishing its value for research in cancer drug testing. The ISU SCID pig has also been used to explore the roles of the innate and adaptive immune system during disease challenge, contributing to research focusing on mechanisms of disease for influenza A (Rajao et al. 2017). Although there is widespread interest in developing SCID pigs as a research model, little research is available on the care of large animal SCID models.
Specific Pathogen Free (SPF) SCID mice colonies are typically cared for in a ‘barrier facility’ with group housing boxes under HEPA filtered conditions (Matsumoto et al. 1995). Staff interacting with animals follow strict personal protective equipment (PPE) requirements and animals are maintained with autoclaved feed and bedding (Dietrich et al. 1996). Large animal facilities have many more parameters to balance considering the size and requirements of pigs. We have been unable to keep the immune compromised SCID pig alive for more than 80 days in a conventional pig holding facility or within an isolated standard animal room. Similar viability of SCID pigs including failure to thrive phenotypes were described after engineering defective Recombination Activating Genes (RAG) SCID pigs who had to be terminated before 30 days of age (Lee et al. 2014). To improve the research potential of the SCID pig model by producing longer-lived, healthy SCID pigs, development of biocontainment facilities and appropriate methodologies to raise SPF SCID pigs are needed.
Current approaches for obtaining SPF piglets from non-SPF sows include snatch farrowing (SF) or caesarean derived piglets, of which both may involve colostrum deprivation (CD) (Madson et al. 2016). The risks of CD are well understood, and include mortality, reduced protection from clinical disease, and significantly lower hematocrit and hemoglobin levels compared to naturally sucking piglets (Lecce, 1969; Varley et al. 1985; Meyer et al. 1964; Blanco et al. 2004; Gomez et al. 1998). Colostrum consumption is positively associated with piglet immunity, survival, and growth (Blanco et al. 2004; Devillers et al 2011). Common practices for obtaining SPF piglets from non-SPF sows include supplementing piglets with milk replacer or a bovine colostrum alternative (Huang et al, 2013). The production of dried replacement bovine colostrum is an involved process (Chelack et al. 1993), and does not protect the piglet from porcine-specific pathogens with antibodies they would normally receive from suckling a vaccinated or unvaccinated sow. An interesting study (Gomez et al. 1998) compared circulating IgG levels between naturally suckling and piglets provided with differing formulations of milk replacer. The latter piglets were fed unsupplemented milk replacer, milk replacer supplemented with bovine colostrum, or milk replacer supplemented with pig immunoglobulin (IgG). Naturally suckling piglets achieved the highest levels of total IgG, while supplementation with bovine or pig IgG was sufficient for 100% survival of piglets. However, only 30% of the CD and unsupplemented milk replacer piglets survived (Gomez et al. 1998). While much of the CD piglet rearing literature was developed prior to the availability of SCID pigs, these results indicate that various options may be available to rear SCID pigs isolated from their nursing sow, which is the main source of disease transmission to the piglet.
This manuscript describes the facilities and protocols developed to greatly decrease opportunities for contamination and raise SPF SCID and non-SCID pigs derived from non-SPF sows. This work also provides examples of facilities and protocols to produce disease-naive pigs, which may be important for disease challenge studies. The farrowing protocols herein described provide means for creating founding SPF SCID carrier dams, methods to introduce new genetics or replace animals within an established SPF colony, and provide recommended guidelines for researchers seeking to develop a SCID pig model containment facility.
ANIMALS:
Institutional Animal Care and Use Committee (IACUC) Statement:
All care and animal procedures were approved by the ISU IACUC and adhere to the USDA guide to Large Animals and the Animal Welfare Act. Euthanasia via captive bolt or intravenous overdose of sodium pentobarbital was performed by trained staff.
SCID pigs and non-SCID littermates:
Yorkshire SCID carrier dams were raised at the ISU Lauren Christian Swine Research Center (LCSRC); a non-SPF farm environment (see Table 2). Pregnant SCID carrier dams with potential to produce SCID affected piglets for biomedical use were farrowed in facilities with high sanitation clean rooms at the ISU College of Veterinary Medicine.
Table 2: Testing for Specific Pathogen Free (SPF) status from the Veterinary Diagnostic and Clinical Pathology laboratories.
Animals are tested for specific porcine pathogens and clinical parameters listed. Testing is conducted from serum, EDTA treated whole blood, fecal, and/or nasal swab, and diagnostics are performed by the Veterinary Diagnostic or Clinical Pathology Laboratories(*).
| Test Target | Type of sample (Detection Method) |
|---|---|
| Porcine Reproductive and Respiratory Syndrome | Blood Serum (ELISA) |
| Porcine parvovirus haemagglutination inhibition | Blood Serum (ELISA) |
| Mycoplasma hyorhinis | Nasal Swab (PCR) |
| Mycoplasma hyosynoviae | Nasal Swab (PCR) |
| Lawsonia intracellularis | Fecal, Blood Serum (ELISA, PCR) |
| Fecal Flotation | Fecal (Sugar Float) |
| Swine Influenza Virus NP | Blood Serum (ELISA, PCR) |
| Actinobacillus pleuropneumoniae Antibody 1-2-9-11 | Blood Serum (ELISA) |
| Actinobacillus pleuropneumoniae Antibody 4-5-7 | Blood Serum (ELISA) |
| Actinobacillus pleuropneumoniae Antibody 3-6-8-15 | Blood Serum (ELISA) |
| M. hyopneumoniae | Fecal, Nasal Swab (PCR) |
| M. flocculare | Fecal, Nasal Swab (PCR) |
| Porcine Epidemic Diarrhea virus N gene | Fecal, Blood Serum (ELISA, PCR) |
| Porcine Epidemic Diarrhea Whole Virus | Fecal, Blood Serum (ELISA, PCR) |
| Ingezim Circovirus | Blood Serum (ELISA) |
| Rota Virus A, B, C | Fecal (PCR) |
| Total Blood Chemistry Panel * | Blood Serum |
| Complete Blood Count (CBC)* | Blood EDTA |
Samples Submitted to the Clinical Pathology Laboratory
Bone Marrow Transplantation (BMT) Rescued SCID Sires:
To create litters composed of 50% SCID piglets, we rescued SCID boars via BMT, which resulted in a genetically SCID, phenotypically normal animal that could be used for breeding. Since successful BMT boars have intact immune systems, they can be housed in clean, more traditional pig facilities at lower cost. To accomplish this, SCID piglet containing litters were MHC typed to identify recipient and donor littermate matches (Powell et al. submitted). SCID recipients received unfractionated bone marrow cells (isolated from all long bones of the donor) intravenously delivered via an ear vein. Successful engraftment was verified by CBC, flow cytometry and response to vaccination.
MATERIALS & METHODS:
Maintenance and Construction of facility:
Bubble fabrication:
Custom design of bubbles was completed with expertise from ISU, SCID project leadership, LAR supervisors, and Biobubble, Inc., who created the final design, fabricated and installed the main isolation equipment. Incoming conditioned city water is UV-irradiated and filtered (5 micron before the UV irradiation and 0.5 micron after UV irradiation) (Fig 1 D).
Figure 1. General Bubble Components.

A) Entrance of long term bubble; pigs are housed on either side while middle section is designated for dressing in PPE. B) Staff shown collecting cord blood on newly snatch farrowed piglets in STB. C) HEPA filtration unit filters all incoming air that enters bubble. D) Water is filtered and irradiated before entering bubble. E) All staff don PPE prior to entering either bubble.
Room sterilization:
Prior to animal occupation, rooms are sterilized (Halo’d) using a HaloMist™ Disinfectant Fogging Solution (halosil.com/halo-disinfection-system), which entails a hydrogen peroxide/silver mixture aerosolized treatment of the room and any resident equipment.
Maintenance and Care of animals:
SCID Carrier Dams:
Two sources of pregnant dams were used in this project: the LCSRC and from within the biocontainment facility. Artificial insemination is used at both locations. Before leaving the LCSRC, pregnant carrier sows are tested by blood draw for Porcine Reproductive and Respiratory Syndrome (PRRS), Porcine CircoVirus (PCV2), and Porcine Epidemic Diarrhea Virus (PEDV) through the ISU Veterinary Diagnostic Laboratory (VDL). Test-negative sows are washed at the farm, delivered by pre-sanitized truck and trailer, washed again, and housed in a high sanitation room with other separately penned gestating sows within the LAR facility. A week before farrowing, animals are transferred to a recently cleaned and Halo’d room containing a farrowing crate, and sows are cleaned with chlorhexidine. Once piglets are removed (delivered for snatch farrowing (see below), or weaned for naturally farrowing) sows are transferred back to the sow room. If sows are to be used for artificial insemination within the biocontainment facility where no live boar was available, they are monitored for behavioral estrus (standing heat) with a cloth sprayed with boar pheromones (Part #: 320506, QC Supply state, country). If natural heats are not detected, adjusting the estrous cycle in preparation for insemination of sows was accomplished by administering 15 mg/day altrenogest (Matrix, Merck, NJ, USA) orally for 18 days followed by one dose of PG600 (Merck, Part #: 147448) administered intramuscularly 24 hours after last Matrix dose, and a single dose of Ovugel (JBS United, NDC #: 51233-101-50) administered intravaginally at approximately 80 hours after PG600 injection. Behavioral estrus was detected typically 24-48 hours post Ovugel administration, at which point animals are artificially inseminated using fresh or frozen semen from the desired sire.
Neonatal Piglet Care:
Naturally farrowed piglets are monitored to ensure they are suckling. Snatch farrowed piglets are delivered by personnel wearing PPE (see below) without touching the floor or crate using sterile towels and transferred into sterile rodent boxes with air filtered lids (Innovive, Part #: RS1-H) for transport to the short-term bubble (STB) where they are dried with Mistral micronized clay (Part #: 541005, QC Supply, NE, USA), weighed, and placed in heat lamp-warmed, piglet housing decks (Birthright™, Yellow Sow, Ralco, MN, USA). Within the first 24 hours of life, snatch-farrowed piglets were fed a minimum of 250 mL (Quesnel et al. 2012) pooled pig colostrum collected previously by hand by staff at the LCSRC from farrowing sows, which was then pasteurized at the ISU dairy farm using standard bovine practices. Confirmation of pasteurization was accomplished with a Bulk Tank Analysis/Standard Plate Count at the ISU VDL which includes the detection of Streptococcus agalactiae, Staphylococcus aureus, coliforms, gram negative rods (lactose negative), non- agalactiae Streptococci, coagulase-negative Staphylococci, Corynebacterium, and Bacillus, Mycoplasma, among others. Pasteurization of colostrum dramatically decreases, but does not eliminate, microbial counts. For example, one unpasteurized colostrum sample had colony forming units (CFU) counts of 3.9 x 105 CFU/mL while post pasteurization averages 3.8 x 103 CFU/mL. Piglets were fed with a 60 mL syringe and attached sterile tubing (Part #: 366.78750.4, Midwest Veterinary Supply MN, USA). After 250 mL colostrum consumption, piglets are converted to irradiated milk replacer, and eventually starter feed.
General Piglet Care:
All litters are processed 24-48 hours post birth; piglets were ear notched, their needle teeth clipped and tails docked, and given 1 mL of each iron (Iron Dextran cat# DU3067, Durvet, MO, USA) and EXCEDE (ceftiofur crystalline free acid, used to manufacturer’s specifications, Zoetis, NJ, USA). Piglets are weighed daily until achieving a body weight of 20 kg, and weekly thereafter up to 100 kg. All piglets are weaned from the sow or from milk replacer at day 24-35 days of age and transitioned to an irradiated starter feed. All feed given to pigs other than colostrum (gestation, starter, and milk replacer powder) is gamma-irradiated (5kGy, Iotron, Inc.). Long term carrier animals are vaccinated following standard pig farm practices including Circumvent PCV-M (Intervet), FarrowSure Gold B (Pfizer), LitterGuard LT-C (Pfizer). To aid in the safety and efficiency of animal handling, as well as increase animal enrichment, pigs in our facility are clicker trained with marshmallow rewards for regular care such as weighing, hoof trimming, vaccinations, snaring, and additional sample collection.
IgG ELISA:
A sandwich Enzyme Linked Immunosorbent Assay (ELISA) was used to quantify pig serum IgG concentration. The protocol was adapted from Bethyl laboratories Porcine IgG ELISA protocols. Briefly, plate wells are coated with polyclonal goat antibody (Bethyl labs #A100-104A, 10 μg/mL). Rinsed plates are blocked with 1% Fetal Bovine Serum (FBS) in phosphate buffered saline (PBS) and capture the Fc region of unconjugated pig IgG reference serum (Bethyl labs #RS10-107) or serially diluted serum sample. After washing, secondary horseradish peroxidase (HRP) conjugated antibodies to pig IgG-Fc (Bethyl labs #A100-104P, 1:15,000 dilution) is applied. After incubation with 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) substrate buffer in citric acid, the reaction is stopped with 5% Sodium Dodecyl Sulfate (SDS) and absorbance read at 405 nm (SynergyHI, BioTek, Winooski, VT). Absorbance data is collected and calculated using a four parameter fit of the standard curve (Gen5 Software, BioTek).
Monitoring for disease and SPF Testing:
Biosecurity, staff, and PPE:
Our facility features different levels of biosecurity, and PPE guidelines are dependent on the level of biosecurity required. Staff interacting with animals cannot travel from one level to a higher biosecurity area without 24 hours down time and at least one shower and change of clothing. To enter the highest biosecurity region (either the long term bubble (LTB) or STB), staff must have not been into contact with outside pigs for at least seven days and a minimum of five showers. Staff remove outer clothing, change into freshly laundered scrubs and socks, then don protective disposable outer shoe covers, zip-up full-body long-sleeved plastic suit (Part #: 4902,Valumax, PA, USA), surgical mask, hair net, a pair of long cuffed gloves, and a second pair of short cuffed gloves. Although gloves are non-sterile, gloved hands are then sprayed with a hydrogen peroxide disinfectant and staff immediately enter the high biosecurity area. For staff moving between animal housing areas, innermost scrubs are protected within full-body plastic suits in hallways and new suits are donned before entering a new animal room. Rubber slip-on shoes are worn between animal containing units, and changed for rubber boots that remain inside pig residing areas.
Monitoring Bubble and Research pig health:
All pigs are checked by staff twice per day, with written records updated daily. In accordance with biosecurity management, staff enter the cleanest space first (usually the LTB), and as described above change outer suits and gloves before entering the STB. Only outer gloves are changed for entry into isolated non-SCID sow rooms (or housed naturally farrowed litters). If any animal in any given location is not Bright, Alert, Responsive (BAR) or Quiet, Alert, Responsive (QAR), an on-call veterinarian is called. Depending on the symptoms present and severity, animals are closely monitored with scheduled veterinarian check-ups, and if necessary, veterinarian prescribed treatments.
Monitoring Lauren Christian Swine Research Center pig health:
Animals are routinely tested six times a year unless concerns or symptoms warrant additional testing for the specific pathogens listed (Table 2). The number of animals selected for testing was based on the estimated prevalence and a 95 percent confidence level that the disease will be detected (Chase et al. 2000) or on clinical signs at the time of a disease investigation. Sampling was conducted by the Swine Medicine Education Center. Diagnostics are performed on sera, oral fluid, fecal, and tissue samples. All testing was performed by the ISU Veterinary Diagnostic Laboratory.
Specific Pathogen Free (SPF) Testing:
Piglets snatch farrowed into the STB are evaluated for SPF status at one and two months of age and must be completely negative for all pathogens at both times before they can be moved to the LTB. In the LTB they will continue to be tested for SPF status at 4, 8 and 12 months of age and at six-month intervals thereafter. Litters produced in the LTB are monitored for SPF status at two months of age. For SPF testing serum blood draws are collected from jugular vein bleeds, nasal swabs (BD BBL™ CultureSwab™ Transport Systems: Liquid Amies, Regular Aluminum Wire, Fisher Scientific, Part #: B4320129), and fecal samples are collected into sterile 50 mL conical tubes. Samples are submitted to the ISU VDL or the Clinical Pathology Laboratory (see Table 1).
Table 1: Health testing for the Lauren Christian Swine Research Center from 2015 to 2017.
Carrier dams are sourced from the LCSRC where animals are routinely monitored for herd health by testing for the pathogens listed. All testing was performed by the Veterinary Diagnostic Laboratory.
| Pathogen | Type of sample | Source Farm Records (routine testing 2015-2017 |
|---|---|---|
| Porcine Reproductive and Respiratory Syndrome | Sera, Oral Fluids, tissue (ELISA, PCR) | Negative* |
| Porcine parvovirus haemagglutination inhibition | Sera (PCR) | Negative |
| Mycoplasma hyopneumoniae | Tissue, Oral Fluid (PCR) | Negative |
| Lawsonia intracellularis | Fecal, Tissue (PCR, IHC) | Negative |
| Swine Influenza Virus NP | Sera, Oral Fluid ELISA, PCR) | Positive |
| Actinobacillus pleuropneumoniae | Tissue (Culture) | Positive |
| Culture Staph aureus | Tissue (Culture) | Positive |
| Culture Staph hyicus | Tissue (Culture) | Positive |
| Culture Strep suis | Tissue (Culture) | Positive |
| Porcine Epidemic Diarrhea Whole Virus | Fecal (ELISA, PCR) | Negative |
| Ingezim Circovirus | Tissue (PCR) | Negative |
| Rota Virus A, B, C | Fecal, Tissue (PCR) | Positive |
Single individual ELISA positive out of 30 sera samples in 2015; was not confirmed with PCR. Thought to be a contamination error or a false positive.
RESULTS:
Large Animal Biocontainment bubbles:
The novel SCID pig ‘Bubbles’ create a clean environment where SCID pigs can be raised free of pathogen threat (Fig 1A,B). Air sterility is maintained by a Biobubble containment structure consisting of a positive pressure HEPA-filtered airflow system (Fig 1C) and UV treated water system (Fig 1D). All personnel entering the bubbles or interacting with SCID colony animals wear PPE that minimizes human exposure as described in the materials & methods (Fig 1E). Please see (https://vimeo.com/221432512) or contact the Tuggle lab for more information or detailed standard operating procedures (SOPs).
Snatch Farrow/Cesarean Section:
Protocols for obtaining SPF piglets from non-SPF sows were developed and tested. Piglets obtained by cesarean section are not exposed to vaginal microbiota from their sow. To retain a reproductively sound carrier sow for production of future litters, and produce piglets exposed to maternal microbiota, we developed a snatch farrowing technique based upon prior work (Huang et al. 2013). Farrowing sows were moved into a sterile room, washed, and monitored for signs of labor. As piglets are delivered, they are caught directly from the vaginal canal in sterile towels by gowned staff and immediately have their cords clamped prior to being placed in a sterile rodent box with filter tops for delivery to staff in the bubble.
Upon arrival into the bubble, piglets have cord blood collected and are processed. To provide as safe an exposure to microbiota as possible, piglets are fed pasteurized pooled pig colostrum that was collected by hand from farrowing animals at the farm from which the carrier sows are originally sourced. To date, pooled pasteurized colostrum has been administered to five litters and approximately 27 snatch farrowed piglets. Various pools included samples from 13-45 sows and had an average total IgG concentration of 5.56 (± 0. 13) mg/mL. Since conventional human bottles are inadequate for feeding piglets and difficult to regularly clean in the bubble environment, we feed our piglets with a disposable catheter attached to 60 mL syringe (Fig 2A). We tape the tapered end of the tube to our finger and allow the piglet to suckle while delivering colostrum with the syringe. This feeding apparatus is disposable, and allows us to carefully monitor and record colostrum volumes consumed by each individual (Fig 2B). Piglets are fed pasteurized colostrum every three hours until they have consumed 250 mL at which point they are fed irradiated milk replacer using the same method. Once sufficient-colostrum has been consumed by all piglets in the litter (within the first 24 hours of life), milk replacer is also provided in a separate feed dish inside the farrowing decks. Piglets typically require supplemental tube feeding for the following 48-72 hours as they transition to dish feeding.
Figure 2. Snatch Farrowed Piglet feeding apparatus.
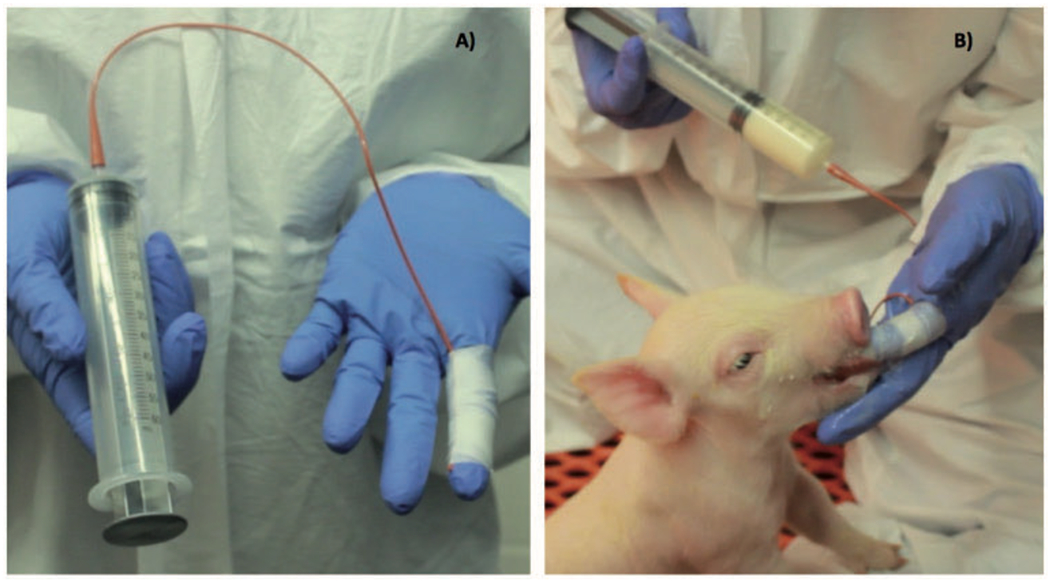
A) A sterile urinary catheter tube is taped to a gloved finger and attached to a 60 ml tube for feeding. B) Porcine colostrum or milk replacer is administered as piglets suckle on the finger with tube attached.
To confirm we were delivering antibody in pasteurized colostrum to the bloodstream before gut closure at approximately 24 hours of age, we collected serum from cord blood (pre-colostrum) and from the jugular, cephalic, or ear vein during the first 1-4 days of life to determine IgG levels by ELISA (Fig3A). Non-SCIDs and SCIDs that were naturally farrowed (NS_NF and S_NF, respectively) had total IgG levels of 13.06 ± 3.63 (mg/mL) and 19.05 ± 4.78 (mg/mL), while non-SCID and SCID snatch farrowed piglets (NS_SF and S_SF respectively) had 7.19 ± 0.51 (mg/mL) and 11.36 ± 4.23 (mg/mL) (Fig3A). To further compare naturally farrowed versus snatch farrowed piglets, we examined growth curves for the first 50 days of life from SCID and non-SCID piglets (Fig 3B). Although tube-fed piglets weighed less for the first 30 days, their growth curves were consistent with the trend of naturally suckling piglets and weight differences are not noticeable after day 35, when piglets were weaned from dam and milk replacer (shown as vertical line in Fig 3B).
Figure 3. ELISA IgG and Growth Curve Data comparing naturally sucking and tube fed piglets.
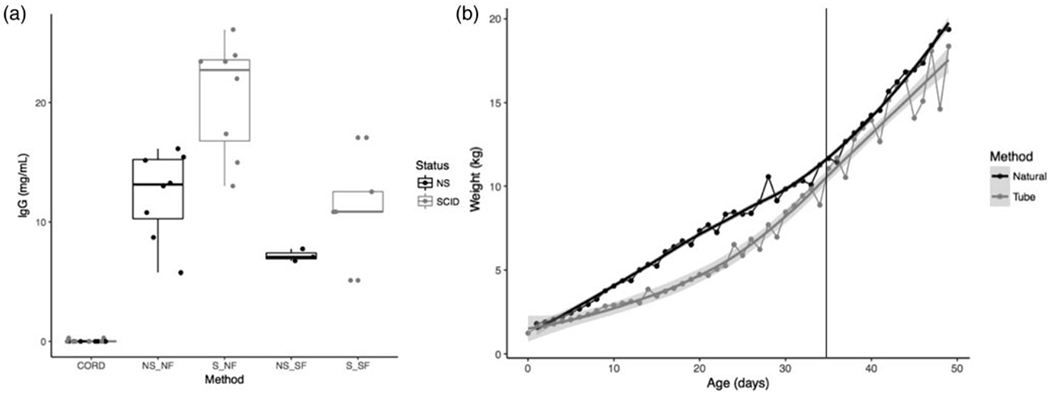
A) The box and whisker plot shows serum levels of IgG (mg/mL) from cord blood of SCID (gray) and non-SCID(black) piglets prior to consumption of colostrum compared to SCID and non-SCID samples post colostrum delivery both from tube fed and naturally suckling piglets. (NS= non-SCID, S= SCID, NF= Naturally Farrowed, SF= Snatch Farrowed respectively). B) Weight (kg) over time for average of combined SCID and non-SCID piglets that were tube fed (gray) or naturally suckled (black) is shown. The gray vertical line denotes typical 35 day wean date (from sow or off milk replacer). Shaded region shows 95% confidence interval.
Animal Flow:
To further reduce the risk of contamination, we established a time- and testing-based quarantine system whereby piglets are tested for SPF status before changing location within the facility. To accomplish this, we utilized two bubbles: the smaller STB that receive snatch farrowed or cesarean derived piglets, and the LTB where SPF females can be raised to sexual maturity. The two bubbles are located in adjacent wings of the same building. Snatch farrowed piglets pass through the vaginal canal during farrowing and are thus exposed to maternal microbiota and possible contaminants before entering the STB. They are tested at one month and two months of age for SPF status (see materials & methods, Table 1). If either test results in the positive identification of pathogen in an individual, litter, or pen mate, the pig cannot progress to the LTB. If SPF status is achieved, female piglets are cleared for transfer to the LTB (Fig 4A).
Figure 4. Animal transfer through facilities.
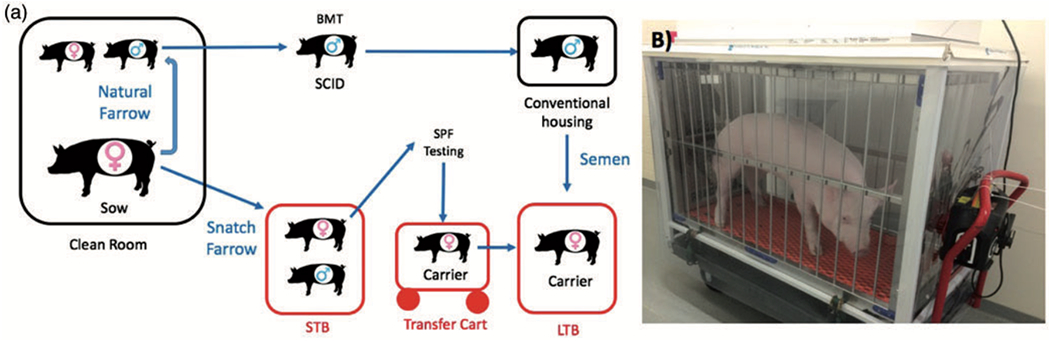
A) The figure shows animal flow from farrowing through short (STB) and long term (LTB) bubbles. Litters are either naturally farrowed where SCID males are BMT rescued and used for subsequent breeding, or naturally farrowed where carrier females are kept SPF clean and moved to the LTB to sexually mature and ultimately be bred to BMT boars to produce naturally farrowed SPF litters in the LTB. B) HEPA filtered transfer cart used to move carrier female pigs from the STB into the LTB.
To decrease the exposure to potential contaminants in the environment outside the positive pressure bubble, we designed and constructed a battery-operated positive pressure, HEPA-filtered transfer cart that allows us to wheel a pig from an internal pen in the STB, to the biocontainment transfer cart, and then into an internal pen in the LTB (Fig 4B).
Disease Prevalence:
Routine testing of animals at the LCSRC where carrier dams are originally from established that LCSRC pigs carry and are exposed to several common pathogens (Table 2). Although the ISU SCID pig is susceptible to virtually all bacterial and viral threats, historically the two most common infections documented in our colony are Streptococcus suis and Staphylococcus hyicus, which are common among commercial pig herds as well as present at the LCSRC (Table 2). Although antibiotic treatment is effective in pigs with a normal immune system, we have not observed any SCID piglets to clear the infection, and infected SCID piglets typically fail to thrive and are euthanized for humane care reasons. The development of the bubble and farrowing system has significantly decreased the prevalence and severity of these infections in snatch farrowed SCIDs. While 71% (5/7 litters) of naturally farrowed litters have presented with diagnosed or suspected incidences of S. hyicus and/or S. suis, we have only identified two individual cases (2/27 or 8% of piglets) of infection in animals snatch farrowed into the STB, and zero events of infectious disease in our LTB to date.
Additional evidence that high levels of biocontainment are necessary for maximum health was provided by two healthy SCID females that were snatch farrowed into the STB and maintained SPF status at one and two months. These gilts were later moved out of biocontainment into non-bubble clean rooms, where they were cared for following strict clean PPE guidelines, irradiated feed, and aforementioned monitoring practices. However, within two months after transfer, both animals had positive confirmation of S. hyicus and one additionally was positive for S. suis.
DISCUSSION:
The potential contributions of a large animal SCID research model to the development of regenerative medicine and cancer therapies, as well as improving our understanding of the mechanisms of immunity, is enormous. The practices described herein focus on the refinement component of the 3R’s (replacement, reduction, and refinement) with an emphasis on improving the overall management, health, and welfare of the ISU SCID pig model.
The described SCID pig bubbles create a clean environment where SCID pigs can be raised free of pathogen threat. To complete future biomedical studies utilizing SCID pigs, we have developed these facilities for maintaining a SPF SCID pig colony and concurrently developed methods using snatch farrowing to produce SPF piglets from non-SPF sows. Our snatch farrowing protocols and feeding of pasteurized colostrum successfully delivered IgG to neonatal piglets, for which growth follows normal rates seen in naturally suckling piglets. Although some exposure to potential contaminants is possible through contact with the vaginal canal of the sow, our high sanitation practices are validated by observed reduced disease incidence and severity.
For the long-term SCID pig colony, our goal is to raise females to reproductive age in the LTB where they can be artificially inseminated with semen from our BMT SCID boars to create SPF litters with 50% SCID and 50% non-SCID piglets for further research. This will decrease labor and the use of resources required by snatch farrowing and raising piglets in the STB. To date, we have successfully raised four SCID carrier gilts to sexual maturity and farrowed a litter within the LTB. The snatch farrowing practices and unique facilities described herein allow us the flexibility of introducing new genetics into our herd to control inbreeding, and provide us with methods to readily replace animals if necessary or re-populate the LTB in the event of contamination. Furthermore, the development of protocols that couple snatch farrowing with biocontainment facilities provides valuable information to collaborators who do not have access to full scale long-term bubbles, but still require SCID piglets for biomedical research.
ACKNOWLEDGEMENTS:
Such a complex project would not be possible without the help of a huge team of highly dedicated individuals. We wish to thank the entire LAR staff (especially Dale Hinderaker and Eldon Whitaker), the LAR veterinarians (especially Dr. Kathleen Mullin, Dr. Amanda Ahrens, and Dr. Giuseppe Dell’Anna), the Swine Medicine Education Center, the SCID pig team of ISU (especially Jackie Jens, Elizabeth Snella, Adrianne Kaiser-Vry, and Austin Putz), and lastly the Iowa State University Farm staff (especially Gary Kuper). This project was made possible with funding from National Institutes of Health 1R24OD019813-1 and the Iowa State University Vice President for Research.
REFERENCES:
- 1.Cino Ozuna AG, Rowland RRR, Nietfeld JC, et al. Preliminary findings of a previously unrecognized porcine primary immunodeficiency disorder. Vet Pathol. 2012; 50(1):144–146. [DOI] [PubMed] [Google Scholar]
- 2.Ewen CL, Cino-Ozuna AG, He H, Kerrigan MA, et al. Analysis of blood leukocytes in a naturally occurring immunodeficiency of pigs shows the defect is localized to T and B cells. Vet Immunol Immunopathol. 2014; 162(3-4): 174–179. [DOI] [PubMed] [Google Scholar]
- 3.Waide EH, Dekkers JCM, Ross JW, et al. Not all SCID pigs are created equally: Two independent mutations in Artemis gene found to cause Severe Combined Immunodeficiency (SCID) in pigs. J Immunol. 2015; 195(7):3171–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cossu F Genetics of SCID. Ital J Pediatr. 2010. 36(76). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell EJ, Cunnick JE, Knetter SM, et al. NK cells are intrinsically functional in pigs with Severe Combined Immunodeficiency (SCID) caused by spontaneous mutations in the Artemis gene. Vet Immunol Immunopathol. 2016; 175 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bosma BC, Custer PR, Bosma M. A severe combined immunodeficiency mutation in the mouse. Nature. 1983; 301(5900): 527–530. [DOI] [PubMed] [Google Scholar]
- 7.Perryman LE. Molecular pathology of severe combined immunodeficiency in mice, horses, and dogs. Vet Pathol. 2004; 41(2): 95–100. [DOI] [PubMed] [Google Scholar]
- 8.Ito R, Takahashi T, Katano I, et al. Current advances in humanized mouse models, Central Institute for Experimental Animals, Kawasaki, Japan. Cell Mol Immunol. 2012; 9(3). 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang J, Guo X, Fan N, et al. RAG1/2 Knockout Pigs with Severe Combined Immunodeficiency. J Immunol. 2014; 193:1496–1503. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki S, Iwamoto M, Saito Y, et al. Il2rg gene-targeted severe combined immunodeficiency pigs. Cell Stem Cell. 2012; 10(6):753–8. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Kwon DN, Ezashi T, et al. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. PNAS. 2014; 111(20):7260–7265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell EJ, Cunnick JE, Tuggle CK. SCID pigs: An Emerging Large Animal Model. J Rare Dis Res Treat. 2017; 2(3):1–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Basel MT, Balivada S, Beck AP, et al. Human xenografts are not rejected in a naturally occurring immunodeficient porcine line: a human tumor model in pigs. Biores Open Access. 2012; 1(2):63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajao DS, Loving CL, Waide EH, Gauger PC, Dekkers JC, Tuggle CK, Vincent AL. Pigs with Severe Combined Immunodeficiency Are Impaired in Controlling Influenza A Virus Infection. J Innate Immun. 2017; 9(2):193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsumoto K, Inukai S, Isaka T, et al. Cell counts in peripheral blood and bone marrow of male C.B-17 scid/scid mice. Lab Anim. 1995; 29,2: 218–222. [DOI] [PubMed] [Google Scholar]
- 16.Dietrich HM, Khaschabi D, Albini B. Isolation of Enterococcus durans and Pseudomonas aeruginosa in a scid mouse colony. Lab Anim. 1996; 30, 2:102–107. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Haines DM, Harding JCS. Snatch-farrowed, porcine-colostrum-deprived (SF-pCD) pigs as a model for swine infectious disease research. Can J Vet Res. 2013; 77(2):81–88. [PMC free article] [PubMed] [Google Scholar]
- 18.Madson DM, Arruda PH, Magstadt DR, et al. Characterization of Porcine Epidemic Diarrhea Virus Isolate US/Iowa/18984/2013 Infection in 1-Day-Old Cesarean-Derived Colostrum-Deprived Piglets. Vet Pathol. 2016; 53(1):44–52. [DOI] [PubMed] [Google Scholar]
- 19.Chelack BJ, Morley PS, Haines DM. Evaluation of methods for dehydration of bovine colostrum for total replacement of normal colostrum in calves. Can Vet J. 1993; 34(7):407–412. [PMC free article] [PubMed] [Google Scholar]
- 20.Lecce JG. Rearing colostrum-free pigs in an automatic feeding device. J Anim Sci 1969. ; 28:27–33. [DOI] [PubMed] [Google Scholar]
- 21.Varley MA, Fowler VR, Maitland A. A rearing system for colostrum-deprived neonatal piglets. Lab Anim. 1985; 19(4):290–296. [DOI] [PubMed] [Google Scholar]
- 22.Meyer RC, Bohl EH, and Kohler EM. Procurement and maintenance of germ-free swine for microbiological investigations. Appl. Microbiol 1964; 12:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blanco I, Galina-Pantoja L, Oliveira S, et al. Comparison between Haemophilus parasuis infection in colostrum-deprived and sow-reared piglets. Vet Microbiol. 2004; 103:21–27. [DOI] [PubMed] [Google Scholar]
- 24.Gomez GG, Phillips O, Goforth RA. Effect of immunoglobulin source on survival, growth, and hematological and immunological variables in pigs. J Anim Sci. 1998; 76:1–7. [DOI] [PubMed] [Google Scholar]
- 25.Devillers N, Dividich JL, Prunier A. Influence of colostrum intake on piglet survival and immunity. Animal. 2011; 5:1605–1612. [DOI] [PubMed] [Google Scholar]
- 26.Powell EJ, Graham J, Ellinwood NM, et al. T-cell lymphoma and leukemia in Severe Combined Immunodeficiency (SCID) pigs following bone marrow transplantation: A Case Report. 8:813. doi: 10.3389/fimmu.2017.00813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniel JA, Carroll JA, Keisler DH, et al. Evaluation of immune system function in neonatal pigs born vaginally or by Cesarean section. Domest Anim Endocrinol. 2008; 35(1):81–87. [DOI] [PubMed] [Google Scholar]
- 28.Quesnel H, Farmer C, Devillers N. Colostrum intake: Influence on piglet performance and factors of variation. Review article. Livestocksci. 2012; 146: 105–114. [Google Scholar]
- 29.Chase C, Polson D. Sampling the Herd: When is enough? Meeting notes. American Association of Swine Veterinarians. 2000; 465. [Google Scholar]


