Abstract
Importance:
Direct-acting antivirals (DAAs) are highly effective in treating hepatitis C virus (HCV). Prior simulations used extended lives as a key health benefit of DAAs. However, real-world evidence on whether DAA treatment reduces mortality is limited.
Objectives:
To examine the association of DAA treatment with mortality among Medicare patients with HCV.
Design, Setting, and Participants:
This retrospective cohort study used data from Medicare patients seeking HCV care between January 1, 2014, and December 31, 2016 after at least a one-year wash-out period. We used Cox proportional hazard regression models with time-varying exposure to compare mortality rates between propensity score-matched cohorts of DAA-treated and untreated patients. Matching and model estimation were done separately for patients with and without cirrhosis. We examined heterogeneity in the association between DAA treatment and mortality by gender and dual eligibility for Medicare and Medicaid. Data were analyzed between September 2019 and March 2020.
Exposure:
Completion of DAA treatment.
Main Outcomes and Measures:
Time to death from the date of newly seeking HCV care after at least a one-year wash-out period.
Results:
Propensity score-matched cohorts of 8,240 patients (36.6% female; mean [SD] age, 62.3 [9.7] years) with cirrhosis and 43,238 patients (41.6% female; mean [SD] age, 58.8 [11.3] years) without cirrhosis were included in the analysis. The adjusted hazard ratio [HR] of dying between DAA-treated and untreated patients with cirrhosis was 0.51 (95% CI, 0.46–0.57). The association of DAA treatment with mortality did not differ by gender (p=0.27) or dual-eligibility status (p=0.80) among cirrhosis patients. The adjusted HR of dying between DAA-treated and untreated patients without cirrhosis was 0.54 (95% CI, 0.50–0.58). The association of DAA treatment with mortality did not differ by gender (p=0.66) among non-cirrhosis patients. However, the survival benefit of DAAs for non-dual eligibles (HR, 0.47; 95% CI, 0.41–0.55) was higher than for dual eligibles (HR, 0.56; 95% CI, 0.52–0.62) among the non-cirrhosis patients, and this difference was significant (p=0.02).
Conclusions and Relevance:
DAA treatment was associated with a decreased mortality in Medicare patients with and without cirrhosis. Increasing access to DAAs for all HCV-infected patients, regardless of disease progression, could improve population health.
Hepatitis C virus infection (HCV) is the most common blood-borne illness in the US.1 About 2.4 million people in the US were estimated to have HCV infection between 2013 and 2016.2 If chronic HCV infection is untreated, serious health problems, such as hepatocellular cancer, cirrhosis, and liver damage, can occur.3 HCV-infected people also experience increased mortality compared with the general population,4–6 and nearly 20,000 Americans die each year from hepatitis C-related causes.7
The availability of second-generation direct-acting antivirals (DAAs) has provided an unprecedented opportunity to address HCV infection and thereby improve population health.8,9 DAAs are highly effective – with cure rates of 90%,10–13 which is much higher than 50% for the earlier interferon-based HCV therapy.14 In addition, DAAs have few adverse effects and improved tolerability, which lead patients to complete the therapy.10–13 Literature on interferon-based therapy indicated that curing HCV was associated with improved clinical outcomes, such as a decrease in the incidence of hepatocellular cancer and decreased mortality rates.15–20 More patients with HCV are expected to have these benefits when treated with DAAs, given the higher cure and completion rates of DAAs.
Several studies of clinical outcomes reported that DAA-treated patients with cirrhosis were less likely to develop hepatocellular cancer than DAA-untreated patients.21,22 Prior simulations used extended lives as a key outcome to indicate that the benefits from DAA treatment can exceed DAA treatment costs.23–25 However, real-world evidence is limited on whether DAA treatment reduces mortality, which is crucial to assess the value of costly DAAs.
Recently, a few studies examined the association of DAA treatment with mortality.26–28 One study focused on patients with a history of hepatocellular carcinoma from 31 health systems across the US and Canada.28 It reported that DAA therapy was associated with a 71% reduction in mortality compared with untreated patients.28 However, the study sample is not representative of the HCV-infected population because only 1%−5% of HCV patients suffer from hepatocellular carcinoma.2 The other two studies used a sample of HCV patients in the Veteran Affairs (VA) health system and reported that DAA treatment was associated with a decrease in mortality.26, 27 However, 97% of the patients in these VA studies were males.26,27 In addition, these VA studies did not match DAA-treated and untreated patients, despite different distributions of health risk in the treated and untreated groups.26,27
We examined whether DAA treatment reduced mortality among Medicare patients with HCV. It is important to assess this issue in Medicare for the following reasons. First, Medicare covers many baby boomers – the group with highest prevalence of HCV.29,30 As this population ages, they experience more HCV complications, which can increase mortality. Medicare is thus expected to play a large role in HCV care. In fact, Medicare paid for half of DAA pills in 2015, making it the largest payer of DAAs in that year.31,32
Second, the Medicare population is 54 percent female,33 and 42 percent of the Medicare patients with HCV in our study are women. HCV progression differs between men and women.34 Women are more likely than men to clear the virus spontaneously after initial HCV infection and have slower rates of liver disease progression after becoming chronically infected.34 Yet, little is known about survival benefits of DAAs among female patients.
Third, Medicare covers non-elderly people who are disabled and have low incomes –demographics that are associated with a higher prevalence of HCV than the general population.35 Most of these patients are dually eligible for both Medicare and Medicaid due to their low-income status, which is associated with poor health outcomes.36 But potential differences in health benefits of DAAs by dual eligibility have not been explored in prior work.
We examined the association between DAA treatment and mortality in a national cohort of Medicare beneficiaries with HCV. We also assessed whether that association varied by patient gender and by dual-eligibility.
METHODS
Data
We used 2013–2016 Medicare claims for inpatient, skilled nursing facility, outpatient, and physician services to identify HCV patients and the presence of cirrhosis. The 2013 files were used only to ensure that patients did not seek HCV care during that year. We used 2014–2017 Medicare Part D files to identify DAA initiation and completion. We required patients to be enrolled in Part D during the entire study period to identify DAA initiation.
Information on death dates was available through December 31, 2017, for all Medicare-enrolled patients from Master Beneficiary Summary Files (MBSF). We also obtained demographic information and indicators of health risks from MBSF.
This study was approved by the Pennsylvania State University’s institutional review board and received a waiver of informed consent. This study follows the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for cohort studies. Data were analyzed between September 2019 and March 2020.
Sample selection
The study population was Medicare Fee-For-Service beneficiaries who newly sought HCV care between January 1, 2014, and December 31, 2016, after at least a one-year wash-out period. We then considered the date of the first HCV claim after the wash-out period as the index date. eAppendix 1 describes details of wash-out periods, index date, and sensitivity checks. We identified patients with HCV following the standard algorithm used by the Centers for Medicare and Medicaid Services (eAppendix 1).37
We excluded patients who died within six months after the index date. DAAs were unlikely to be given to those patients because the course of DAA treatment usually ranges between three and six months.
DAA treated group
DAA initiators were identified as those who were treated with one of the following DAAs: elbasvir/grazoprevir, ledipasvir/sofosbuvir, ombitasvir/paritaprevir/ritonavir plus dasabuvir, sofosbuvir, sofosbuvir/velpatasvir, sofosbuvir/velpatasvir/voxilaprevir, or glecaprevir/pibrentasvir. We defined DAA-treated patients as DAA initiators who completed treatment before December 30, 2017. The completion of a DAA was defined as filling prescriptions for the expected duration of the DAA identified from package inserts or randomized trials. eAppendix A1 describes details of the definition of completion.
DAA untreated group
Patients who did not initiate DAA therapy during the study period were considered DAA- untreated patients. We selected the DAA-untreated group based on one-to-one propensity score matching (within 10% of the standard deviation of propensity scores). Patients were matched on demographics and health risks at the index date. eTable 1 describes the definitions and data sources of all covariates used in matching.
We performed matching separately for patients with and without cirrhosis at the index date. We required patients without cirrhosis at the index date in the DAA-treated group to remain without cirrhosis until treatment. Figure 1 presents a diagram of the study sample selection.
Figure 1:
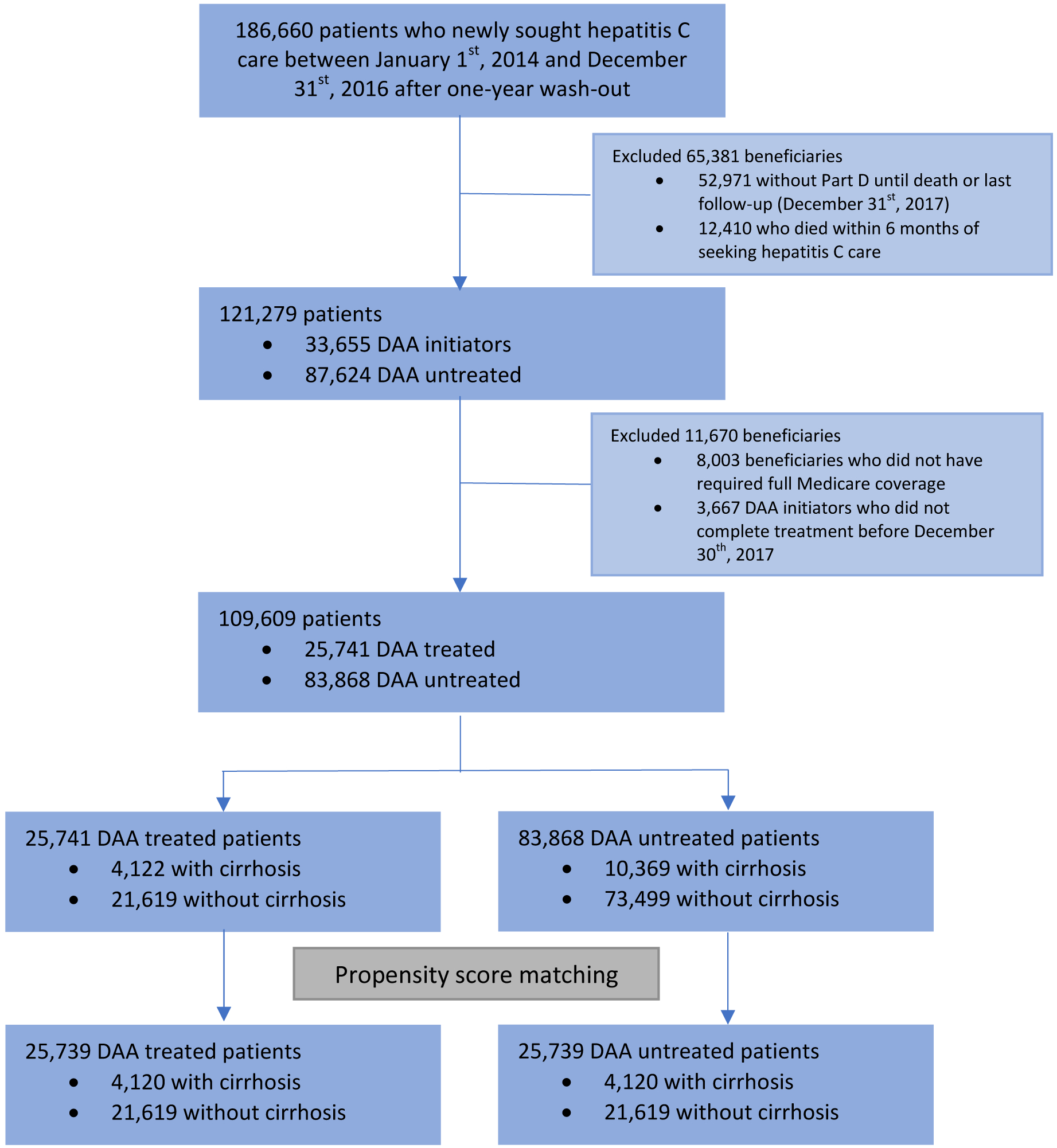
Study sample selection
Abbreviations: DAA, Direct-acting antiviral agent
Outcome and exposure
We followed up all patients from the index date until they died or reached the end of study period [December 31, 2017]. The outcome of analysis was time to death from the index date.
The completion of DAA treatment was examined as a time-varying exposure measure. DAA-treated patients contributed to unexposed person-time until they completed treatment. Following completion of the treatment, they contributed toward exposed person-time. Untreated patients contributed only toward unexposed person-time. Examining DAA treatment as time-varying exposure addresses “immortal bias” – which favors the treatment group because treated individuals survive at least until exposure.38–40
Analysis
We conducted all analyses separately for the cirrhosis and non-cirrhosis cohorts. We first described the patient characteristics at the index date in the full unmatched sample. We then compared patient characteristics at the index date between DAA-treated and untreated patients in the propensity-score matched samples, separately for those with and without cirrhosis. We assessed that the characteristics were “balanced” after matching between the groups when standardized differences were less than 10%.41
We calculated mortality rates as deaths per 100 person-years in the DAA-treated and untreated groups and obtained the crude mortality rate ratios. Kaplan-Meier (KM) survival curves were plotted for DAA-treated and untreated groups. We also estimated crude mortality rate ratios and plotted KM curves separately for females, males, non-dual eligible, and dual eligible patients.
We estimated adjusted hazard ratios of death between DAA-treated and untreated patients using the Cox proportional hazards model with time-varying exposure. We examined heterogeneity in the relation between DAA treatment and mortality rates across different patient groups by estimating separate Cox regression models for the following sub-groups: females, males, non-dual eligibles, and dual eligibles. These separate analyses allow the associations of DAA treatment and all other covariates with mortality to vary in each group. We assessed whether the survival effect of DAAs was significantly different between groups by including interaction terms between DAA treatment and group indicators in a Cox model with all patients.42
We considered p < 0.05 as statistically significant for all comparisons. We used SAS 9.4 (SAS Institute) and Stata15 (StataCorp LLC) for the analyses.
Sensitivity Analysis:
We estimated the model limiting the sample to patients who were alive for at least one year after the index date. This analysis was to consider a recommendation that DAA therapy be given to those with a life expectancy greater than one year.43 We used a propensity-score matched cohort for this analysis.
RESULTS
Patient characteristics
The analysis included a propensity score matched sample of 51,478 beneficiaries (40.8% female; mean [SD] age, 59.4 [11.1] years), consisting of 8,240 patients with cirrhosis (36.6% female; mean [SD] age, 62.3 [9.7] years) and 43,238 patients without cirrhosis (41.6% female; mean [SD] age, 58.8 [11.3] years).
Patient characteristics in the unmatched sample are reported in eTable 2. Compared with DAA-treated patients, untreated patients were likely to be older and have other conditions such as anemia, lung disease, cardiac disease, dementia, diabetes, kidney disorders, and drug and alcohol related disorders.
Table 1 presents the patient characteristics in the matched sample. Baseline patient characteristics after matching were balanced – the estimates of standardized difference scores after matching were all less than 10%. Among patients with cirrhosis, the median time from Hepatitis C index date to treatment completion was 9.1 months (interquartile range {IQR}, 5.8 – 15.5 months), with 28% treated within six months. Among patients without cirrhosis, the median time from Hepatitis C index date to treatment completion was 8.6 months (IQR, 5,2 – 15.7 months), with 33% treated within six months.
Table 1.
Patient characteristics in the propensity-score matched samplea
| Cirrhosis patients (N=8,240) | Non-cirrhosis patients (N=43,238) | |||||
|---|---|---|---|---|---|---|
| DAA Treated (N=4,120) | DAA Untreated (N=4,120) | St.Diff,b % | DAA Treated (N=21,619) | DAA Untreated (N=21,619) | St.Diff,b % | |
| Variable | No. (%) | No. (%) | No. (%) | No. (%) | ||
| Age | ||||||
| Age <65 | 2,355(57.2%) | 2,480(60.2%) | −6.2 | 14,344(66.4%) | 14,544(67.3%) | −2.0 |
| Age 65–70 | 931(22.6%) | 840(20.4%) | 5.4 | 4,144(19.2%) | 4,061(18.7%) | 1.0 |
| Age 70–75 | 458(11.1%) | 421(10.2%) | 2.9 | 1,941(9.0%) | 1,855(8.6%) | 1.4 |
| Age >75 | 376(9.1%) | 379(9.2%) | −0.3 | 1,190(5.5%) | 1,159(5.4%) | 0.6 |
| Female Gender | 1,515(36.8%) | 1,501(36.4%) | 0.7 | 8,994(41.6%) | 8,995(41.6%) | 0.0 |
| Race | ||||||
| White | 2,917(70.8%) | 3,030(73.5%) | −6.1 | 14,358(66.4%) | 14,639(67.7%) | −2.8 |
| African American | 870(21.1%) | 758(18.4%) | 6.8 | 5,823(26.9%) | 5,649(26.1%) | 1.8 |
| Hispanic | 145(3.5%) | 145(3.5%) | 0.0 | 595(2.8%) | 538(2.5%) | 1.7 |
| Other | 188(4.6%) | 187(4.5%) | 0.1 | 843(3.9%) | 793(3.7%) | 1.2 |
| Dual eligibilityc | 2,732(66.3%) | 2,770(67.2%) | −2.0 | 15,182(70.2%) | 15,301(70.8%) | −1.2 |
| Conditions | ||||||
| Decompensated cirrhosis | 1,248(30.3%) | 1,313(31.9%) | −3.4 | - | - | - |
| HIV/AIDS | 109(2.6%) | 98(2.4%) | 1.7 | 1,175(5.5%) | 1,098(5.1%) | 1.6 |
| Hepatocellular cancer | 239(5.8%) | 264(6.4%) | −2.5 | 76(0.4%) | 63(0.3%) | 1.1 |
| Anemia | 1,808(43.9%) | 1,863(45.2%) | −2.7 | 5,572(25.8%) | 5,649(26.1%) | −0.8 |
| Lung Disease | 1,166(28.3%) | 1,229(29.8%) | −3.4 | 5,364(24.8%) | 5,368(24.8%) | 0.0 |
| Cancer | 524(12.7%) | 517(12.6%) | 0.5 | 2,208(10.2%) | 2,089(9.7%) | 1.8 |
| Cardiac disease | 3,140(76.2%) | 3,231(78.4%) | −5.3 | 14,742(68.2%) | 14,975(69.3%) | −2.3 |
| Dementia | 254(6.2%) | 226(5.5%) | 2.9 | 895(4.1%) | 826(3.8%) | 1.6 |
| Psychiatric conditions | 1,810(43.4%) | 1,925(46.7%) | −5.6 | 10,840(50.1%) | 10,809(50.0%) | 0.3 |
| Diabetes | 1,554(37.7%) | 1,579(38.3%) | −1.2 | 6,123(28.3%) | 6,083(28.1%) | 0.4 |
| Eye disease | 673(16.3%) | 630(15.3%) | 2.9 | 3,403(15.7%) | 3,324(15.4%) | 1.0 |
| Kidney disorders | 1,150(27.9%) | 1,206(29.3%) | −3.0 | 4,160(19.2%) | 4,212(19.5%) | −0.6 |
| Drug and alcohol related disorder | 2,052(49.8%) | 2,237(54.3%) | −9.0 | 10,123(46.8%) | 10,061(46.5%) | 0.6 |
| Bone disease | 1,520(36.9%) | 1,570(38.1%) | −2.5 | 8,419(38.9%) | 8,474(39.2%) | −0.5 |
| ESRD | 164(4.0%) | 161(3.9%) | 0.4 | 703(3.2%) | 718(3.3%) | −0.4 |
| Time from index dated to DAA initiation | ||||||
| <6 months | 1,150(27.9%) | NA | 7,161(33.1%) | NA | ||
| 6–12 months | 1,505(36.6%) | 6,907(31.9%) | ||||
| 12–24 months | 1,086(26.4%) | 5,195(24.0%) | ||||
| 24–36 months | 302(7.3%) | 1,866(8.6%) | ||||
| >36 months | 77(1.9%) | 490(2.3%) | ||||
Abbreviations: AIDS, Acquired immunodeficiency syndrome; DAA, Direct-acting antiviral drug; ESRD, End-stage renal disease; HIV, Human immunodeficiency virus; St.Diff, Standardized difference
Patient characteristics are measured at index date
A standardized difference less than 10% is considered to denote balanced patient characteristics
Dual eligibility is an indicator of whether a person is eligible for both Medicare and Medicaid
Index date is the date when the patient first sought HCV care after a one-year wash-out period
Descriptive results
In the cirrhosis group, 480 deaths occurred during 10,531.2 person-years of follow-up among DAA-treated beneficiaries (4.76 deaths/100 person-years; 95% confidence interval [CI], 4.15–4.97). In comparison, 1,310 deaths occurred during 9,234.8 person-years of follow-up (14.19 deaths/100 person-years; 95% CI, 13.42–14.95) in untreated beneficiaries (Table 2). The crude mortality rate ratio between the two groups was 0.32 (95% CI, 0.29–0.35). The 1-year risk of mortality for DAA-treated patients was 2.2%, compared with 10.4% among untreated patients (data not shown). The crude mortality rate ratio between treated and untreated patients did not differ by gender: 0.31 (95% CI, 0.25–0.37) in females versus 0.33 (95% CI, 0.28–0.37) in males. Similarly, no difference was observed by dual-eligibility status: 0.33 (95% CI, 0.27–0.40) in non-duals versus 0.32 (95% CI, 0.28–0.37) in dual eligibles.
Table 2.
Comparison of mortality rates between direct-acting antiviral agent (DAA) treated patients and DAA untreated patients
| DAA untreated versus treated | Patients, n | Deaths, n | Person-years | Mortality-rate per 100 person-years (95% CI) | Crude rate (95% CI) | P value |
|---|---|---|---|---|---|---|
| Cirrhosis patients | ||||||
| Overall - DAA untreated | 4,120 | 1,310 | 9,234.75 | 14.19 (13.42–14.95) | — | |
| Overall - DAA treated | 4,120 | 480 | 10,531.15 | 4.56 (4.15–4.97) | 0.32 (0.29–0.35) | <0.001 |
| Female | ||||||
| DAA untreated | 1,501 | 420 | 3,431.03 | 12.24 (11.07–13.41) | — | |
| DAA treated | 1,515 | 148 | 3,888.59 | 3.81 (3.19–4.42) | 0.31 (0.25–0.37) | <0.001 |
| Male | ||||||
| DAA untreated | 2,619 | 890 | 5,803.72 | 15.33 (14.33–16.34) | — | |
| DAA treated | 2,605 | 332 | 6,642.55 | 5.00 (4.46–5.54) | 0.33 (0.28–0.37) | <0.001 |
| Non dual eligiblesa | ||||||
| DAA untreated | 1,350 | 404 | 2,984.10 | 13.54 (12.22 – 14.86) | — | |
| DAA treated | 1,388 | 159 | 3,517.55 | 4.52 (3.82–5.22) | 0.33 (0.27–0.40) | <0.001 |
| Dual eligiblesb | ||||||
| DAA untreated | 2,770 | 906 | 6,250.65 | 14.49 (13.55–15.44) | — | |
| DAA treated | 2,732 | 321 | 7,013.60 | 4.58 (4.08–5.08) | 0.32 (0.28–0.36) | <0.001 |
| Non-cirrhosis patients | ||||||
| Overall - DAA untreated | 21,619 | 2,955 | 61,587.84 | 4.80 (4.63–4.97) | — | |
| Overall - DAA treated | 21,619 | 912 | 55,792.87 | 1.63 (1.53–1.74) | 0.34 (0.32–0.37) | <0.001 |
| Females | ||||||
| DAA untreated | 8,995 | 978 | 26,253.96 | 3.72 (3.49–3.96) | — | |
| DAA treated | 8,994 | 291 | 23,322.38 | 1.25 (1.10–1.39) | 0.33 (0.29–0.38) | <0.001 |
| Males | ||||||
| DAA untreated | 12,624 | 1,977 | 35,333.88 | 5.59 (5.35–5.84) | — | |
| DAA treated | 12,625 | 621 | 32,466.57 | 1.91 (1.76–2.06) | 0.34 (0.31–0.37) | <0.001 |
| Non dual eligiblesa | ||||||
| DAA untreated | 6,318 | 787 | 17,082.09 | 4.61 (4.28–4.93) | — | |
| DAA treated | 6,437 | 239 | 16,648.38 | 1.44 (1.25–1.62) | 0.31 (0.27–0.36) | <0.001 |
| Dual eligiblesb | ||||||
| DAA untreated | 15,301 | 2,168 | 44,504.75 | 4.87 (4.67–5.08) | — | |
| DAA treated | 15,182 | 673 | 39,140.58 | 1.72 (1.59–1.85) | 0.35 (0.32–0.38) | <0.001 |
Abbreviations: CI, Confidence intervals; DAA, Direct-acting antiviral drug
Non dual eligibles are eligible for Medicare only
Dual eligibles are eligible for Medicare and Medicaid
In the non-cirrhosis group, 912 deaths occurred during 55,794.9 person-years of follow-up among DAA-treated patients (1.63 deaths/100 person-years; 95% CI, 1.53–1.74). In contrast, 2,955 deaths occurred during 61,587.8 person-years of follow-up (4.80 deaths/100 person-years; 95% CI, 4.63–4.97) among the untreated patients (Table 2). The crude mortality rate ratio between the two groups was 0.34 (95% CI, 0.32–0.37). The 1-year risk of mortality for DAA-treated patients was 0.6%, compared with 2.9% among untreated patients (data not shown). We observed little difference in the crude mortality rate ratio by gender: 0.33 (95% CI, 0.29–0.38) in females versus 0.34 (95% CI, 0.31–0.37) in males. No difference in the crude mortality rate ratio was observed by dual eligibility status: 0.31 (95% CI, 0.27–0.36) in non-duals versus 0.35 (95% CI, 0.32–0.38) in dual eligibles.
Figure 2 depicts the Kaplan-Meier (KM) survival curves. DAA treatment was associated with a statistically significant reduction in mortality in both cirrhosis and non-cirrhosis groups. The KM curves for each sub-group – males, females, non-dual eligible, and dual eligible – are provided in the Appendix (eFigure1 and eFigure2). They indicated survival benefits of DAAs in all of those groups, irrespective of cirrhosis at the index date.
Figure 2:
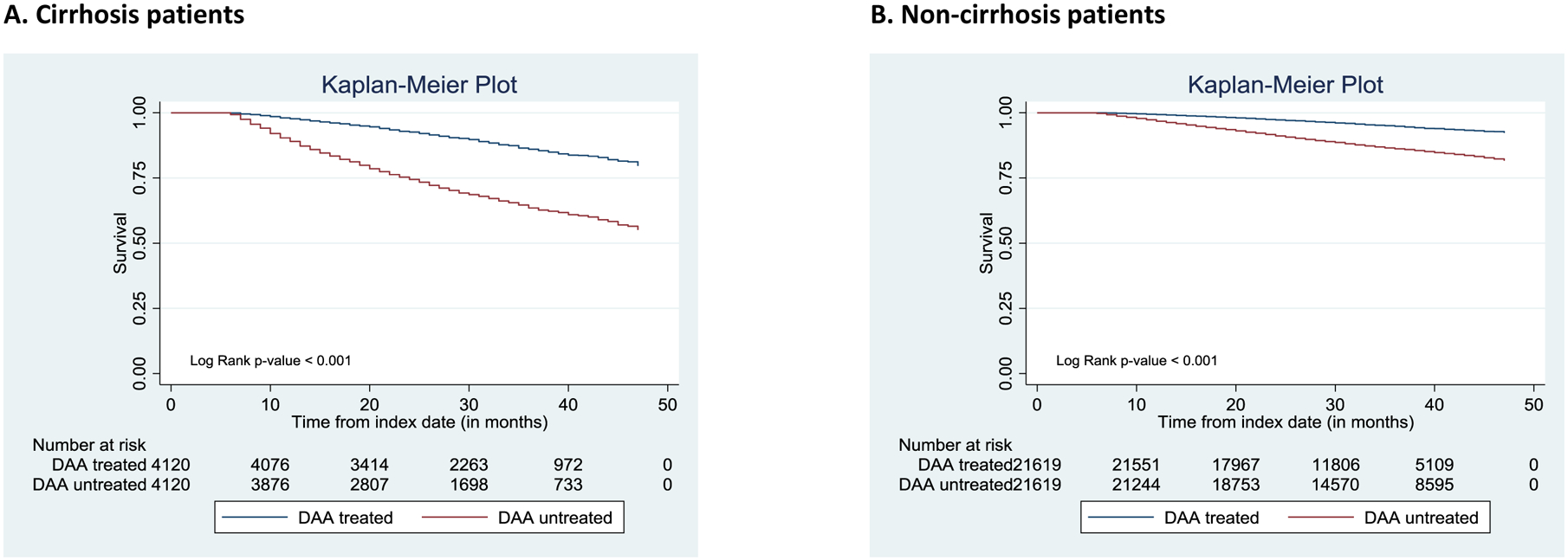
Survival stratified by Direct-Acting Antiviral (DAA) treatment
Abbreviations: DAA, Direct-acting antiviral agent
Cox proportional hazards regression results
DAA treatment was associated with reduced mortality after controlling for patient characteristics (Figure 3). In the cirrhosis group, the adjusted hazard ratio (HR) of dying between DAA-treated and untreated patients was 0.51 (95% CI, 0.46–0.57). Being older, male, having decompensated cirrhosis, and having other health conditions were associated with increased mortality (full regression results are reported in eTable 3). Separate analyses by gender revealed consistent survival benefits of DAAs for both females (HR, 0.46; 95% CI, 0.38–0.56) and males (HR, 0.53; 95% CI, 0.47–0.60). This difference in HR by gender was not statistically significant (p=0.27 on the interaction term, eTable 4). DAA treatment was associated with a slightly smaller reduction in mortality in non-dual eligibles (HR, 0.52; 95% CI, 0.43–0.63) than in dual eligibles (HR, 0.50; 95% CI, 0.44–0.57). This difference was not statistically significant (p=0.80 on the interaction term, eTable 4).
Figure 3:
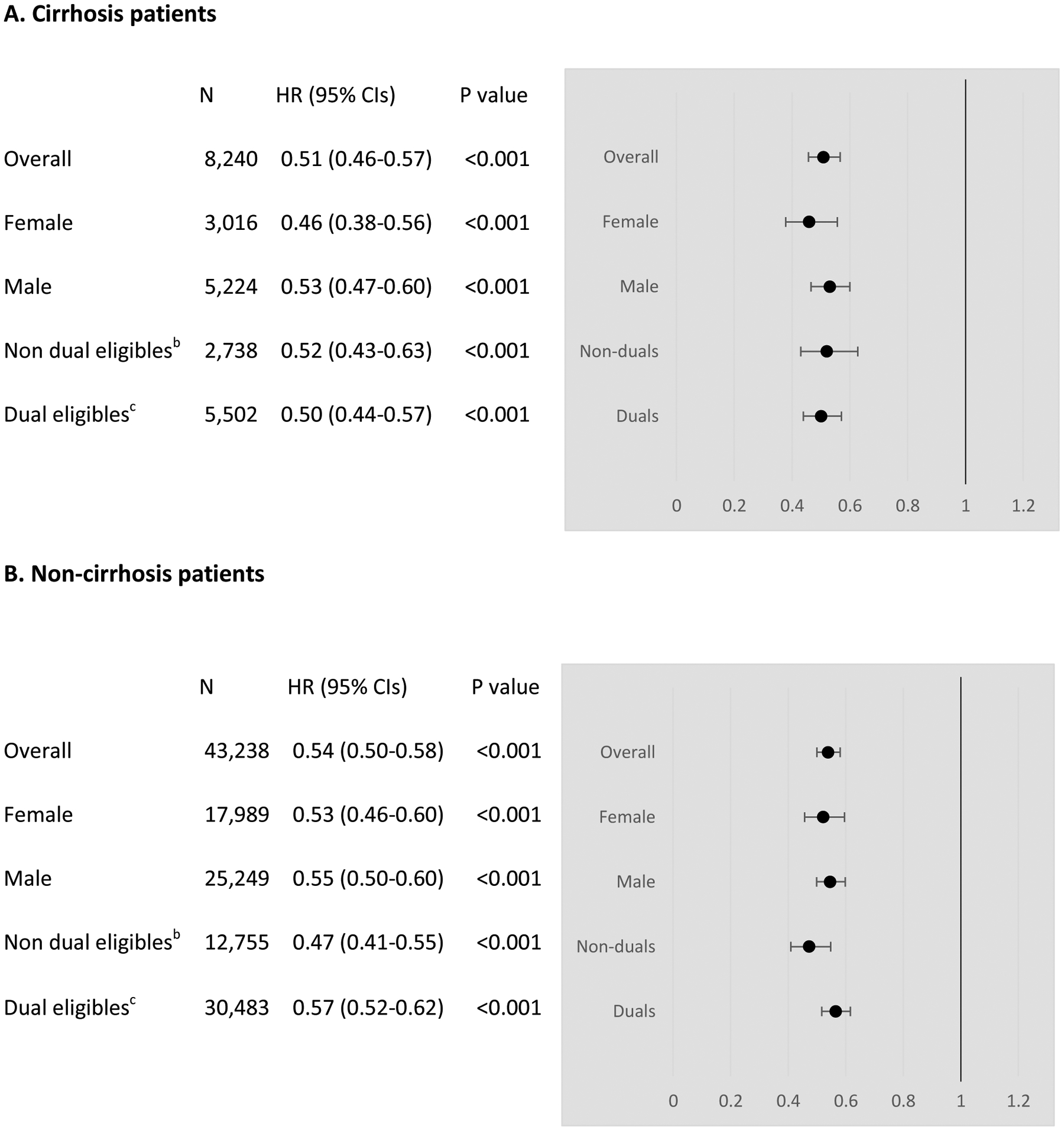
Adjusted hazard ratios for mortality comparing direct-acting antiviral (DAA) treated and DAA untreated patients
Abbreviations: CIs, Confidence intervals; HR, Hazard ratios
a Adjusted for patient characteristics and risk factors summarized in eTable1
b Non dual eligibles are eligible for Medicare only
c Dual eligibles are eligible for Medicare and Medicaid
In the non-cirrhosis group, the adjusted HR of dying between DAA-treated and untreated patients was 0.54 (95% CI, 0.50–0.58). Being older, male, and having other health conditions were associated with increased mortality (eTable 3). Survival benefit of DAAs was observed for both females (HR, 0.53; 95% CI, 0.46–0.60) and males (HR, 0.55; 95% CI, 0.50–0.60). This difference in HR by gender was not statistically significant (p=0.66 on the interaction term, eTable 4). However, DAA treatment was associated with a larger reduction in mortality in non-dual eligibles (HR, 0.47; 95% CI, 0.41–0.55) than in dual eligibles (HR, 0.57; 95% CI, 0.52–0.62). This difference was statistically significant (p=0.02 on the interaction term, eTable 4).
Results from sensitivity analysis
The findings from the analysis with HCV patients who were alive for at least one year after the index date were very similar to those from the main analysis (eFigure 3). The adjusted hazard ratio (HR) of dying between DAA-treated and untreated patients was 0.47 (95% CI, 0.42–0.53) in the cirrhosis group and 0.54 (95% CI, 0.50–0.58) in the non-cirrhosis group.
DISCUSSION
DAA treatment was associated with lower mortality among Medicare patients with and without cirrhosis (adjusted mortality ratio reductions of 49% and 46%, respectively). This finding is important evidence that DAA treatment has a large health benefit, even among patients without advanced liver disease. Because of the high costs of DAAs, payers have restricted coverage for DAAs to patients with advanced fibrosis.44–46 Some payers, such as the VA, have removed this restriction,26 but it still remains in other programs.44–46 Restrictive coverage for DAAs has been based partially on uncertainty about the immediate benefits of treatment in patients without serious HCV progression.26 However, our analysis suggests that expanding coverage for DAAs to all HCV-infected patients regardless of disease progression can avert deaths. Our finding also supports the recent recommendation of HCV testing for all adults between 18–79 years because diagnosis is a precursor to treatment.47
The estimated survival benefit in non-cirrhosis patients (46% reduction in the mortality ratio) is smaller than prior work, which reported a 68% decrease in the mortality ratio.26 This difference may be due to the following. First, it may partially stem from our analytic approach that addressed immortal bias by considering only time after treatment as exposed in the DAA-treated group. Second, we used propensity score matching to improve balance in patient risks between treated and untreated groups. Both of these approaches remove sources of selection bias that would favor the treatment group and thereby result in a smaller effect than otherwise.
The sub-group analyses indicated limited heterogeneity in the health benefit of DAAs across patient groups. First, the mortality ratio reduction after DAA treatment was similar between males and females. Being male is a predictor of HCV prevalence and fast disease progression.34 However, it did not play a role in the association between DAA treatment and mortality, regardless of the presence of cirrhosis. This is an important extension of prior evidence on the survival benefits of DAAs in males or patients with a history of liver cancer.26,27
Second, the association of DAA treatment with mortality was similar for cirrhosis patients who were dually eligible for both Medicare and Medicaid and those who were eligible for Medicare only. However, among non-cirrhosis patients, we found a smaller association of treatment with mortality for dually eligible patients. Dual eligibles are sicker and have lower incomes than Medicare-only beneficiaries.48 They may have encountered barriers to seeking health care and improvement in health outcomes, leading to a smaller association between DAA treatment and mortality. Identifying those barriers was beyond the scope of our study, but it could help explore ways to increase the survival benefit from DAA treatment in this group.
Our study provides real-world evidence that DAA treatment leads to fewer deaths in HCV-infected Medicare patients. This suggests that that improving access to DAAs – perhaps with particular attention to patient groups with low DAA uptake – could have a significant health benefit for the population.
LIMITATIONS
We note several limitations of the study. First, Medicare claims data do not have clinical information, such as genotype or sustained virologic response status. Some DAA treated patients in our analysis may not have cured HCV. However, not accounting for this factor leads to conservative estimates in our analysis. Second, selection bias may remain even after propensity score matching due to differences in unobservable characteristics. However, it is unlikely to change the study conclusions given the large estimates of DAA health impacts. Third, we measured only overall mortality rates – i.e. all-cause deaths. Some deaths in the study sample may not be related to HCV. But condition-specific deaths are not identifiable in claims data. Fourth, we lacked information on the date of initial HCV diagnosis in our five years of claims data. Finally, the study findings may not generalize to patients covered by Medicaid only, commercial insurers, or Medicare Advantage.
CONCLUSIONS
DAA treatment was associated with a decrease in mortality in Medicare patients with HCV regardless of the presence of cirrhosis. This is important real-world evidence on the survival benefit of DAAs and suggests that increasing access to DAAs regardless of disease progression could improve population health.
Supplementary Material
KEY POINTS.
Question:
Is direct-acting antiviral (DAA) therapy to treat hepatitis C virus (HCV) associated with a reduction in mortality among Medicare patients?
Findings:
In this retrospective cohort study of 51,478 propensity-score matched Medicare patients, DAA treatment was significantly associated with a decrease in mortality among patients with and without cirrhosis. The association of DAA treatment with mortality was similar for males and females regardless of the presence of cirrhosis, and it was slightly smaller among non-cirrhosis dual-eligible patients than among Medicare-only patients.
Meaning:
Increasing access to DAAs for all HCV-infected patients, regardless of disease progression, could improve population health.
Acknowledgement
Funding/Support: This study was supported by the National Institute on Aging (NIA) R01 AG055636-01A1 and by the National Institute of Child Health and Human Development (NICHD) R24 HD041025.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The views expressed in this article are those of the authors and do not represent those of the NIA or NICHD.
Conflict of Interest Disclosures: Feldman owns a small amount of stock in Gilead Sciences. No other conflict of interest exists.
Contributor Information
Roger Feldman, Division of Health Policy and Management, School of Public Health, University of Minnesota.
Thomas Riley, III, Department of Medicine, College of Medicine, The Pennsylvania State University.
References:
- 1.Alter MJ. Hepatitis C virus infection in the United States. J Hepatol. 1999;31 Suppl 1:88–91. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Hepatitis C prevalence estimates 2013–2016. https://www.cdc.gov/nchhstp/newsroom/2018/hepatitis-c-prevalence-estimates.html. Accessed February 26, 2020.
- 3.Centers for Disease Control and Prevention. Hepatitis C questions and answers for the public. https://www.cdc.gov/hepatitis/hcv/cfaq.htm. Accessed February 26, 2020.
- 4.Ireland G, Mandal S, Hickman M, Ramsay M, Harris R, Simmons R. Mortality rates among individuals diagnosed with hepatitis C virus (HCV); an observational cohort study, England, 2008 to 2016. Euro Surveill. 2019. July 25; 24(30): 1800695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiltinan AM, Kaidarova Z, Custer B, et al. Increased all-cause, liver, and cardiac mortality among hepatitis C virus seropositive blood donors. Am J Epidemiol. 2008;167:743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Neal KR, Ramsay S, Thomson BJ, Irving WL. Excess mortality rates in a cohort of patients infected with the hepatitis C virus: a prospective study. Gut. 2007;56:1098–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. New hepatitis C infections nearly tripled over five years. https://www.cdc.gov/nchhstp/newsroom/2017/Hepatitis-Surveillance-Press-Release.html. Accessed April 28, 2020.
- 8.Sussman NL, Remien CH, Kanwal F. The end of hepatitis C. Clin Gastroenterol Hepatol. 2014;12(4):533–536. [DOI] [PubMed] [Google Scholar]
- 9.Brennan T, Shrank W. New expensive treatments for hepatitis C infection. JAMA. 2014;312(6):593–594. [DOI] [PubMed] [Google Scholar]
- 10.Afdhal N, Zeuzem S, Kwo P, et al. Ledipasvir and Sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014;370(20):1889–1898. [DOI] [PubMed] [Google Scholar]
- 11.Afdhal N, Reddy KR, Nelson DR, et al. Ledipasvir and Sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014;370(16):1483–1493. [DOI] [PubMed] [Google Scholar]
- 12.Yin S, Barker L, White JZ, Jiles RB. Sofosbuvir-based regimens for chronic hepatitis C in a well-insured U.S. population: patient characteristics, treatment adherence, effectiveness, and health care costs, 2013–2015. J Manag Care Spec Pharm. 2019;25(2):195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andres J, Lott S, Qureshi K. Eight-week outcomes of Ledipasvir/Sofosbuvir in noncirrhotic treatment-naive patients with hepatitis C: analysis of pharmacy-based data. J Manag Care Spec Pharm. 2018;24(1):23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tice AJ, Ollendorf DA, Pearson SD. California Technology Assessment Forum on the comparative clinical effectiveness and value of Simeprevir and Sofosbuvir in the treatment of chronic hepatitis C infection. ©Institute for Clinical & Economic Review. April 2014. http://ctaf.org/sites/default/files/assessments/CTAF_Hep_C_Apr14_final.pdf. Accessed February 26, 2020. [Google Scholar]
- 15.Backus LI, Boothroyd DB, Phillips BR, Belperio P, Halloran J, Mole LA. A sustained virologic response reduces risk of all-cause mortality in patients with hepatitis C. Clin Gastroenterol Hepatol. 2011;9:509–516. [DOI] [PubMed] [Google Scholar]
- 16.van der Meer AJ, Veldt BJ, Feld JJ, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012;308(24):2584–93. [DOI] [PubMed] [Google Scholar]
- 17.Rutter K, Stättermayer AF, Beinhardt S, et al. Successful anti-viral treatment improves survival of patients with advanced liver disease due to chronic hepatitis C. Aliment Pharmacol Ther. 2015;41(6):521–31. [DOI] [PubMed] [Google Scholar]
- 18.Nahon P, Marcellin P, Guyader D, et al. Eradication of hepatitis C virus infection in patients with cirrhosis reduces risk of liver and non-liver complications. Gastroenterology. 2017;152(1):142–156.e2. [DOI] [PubMed] [Google Scholar]
- 19.Tanwar S, Wright M, Foster GR, et al. Randomized clinical trial: a pilot study investigating the safety and effectiveness of an escalating dose of peginterferon α−2a monotherapy for 48 weeks compared with standard clinical care in patients with hepatitis C cirrhosis. Eur J Gastroenterol Hepatol. 2012;24(5):543–50. [DOI] [PubMed] [Google Scholar]
- 20.Francesco A, Esterita A, Giovanni N, et al. Interferon plus ribavirin and interferon alone in preventing hepatocellular carcinoma: A prospective study on patients with HCV related cirrhosis. World J Gastroenterol. 2004; 10(21): 3099–3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park H, Wang W, Henry L, Nelson DR. Impact of all-oral direct-acting antivirals on clinical and economic outcomes in patients with chronic hepatitis C in the United States. Hepatology. 2019;69(3):1032–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology. 2016;64(1):130–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C virus. Ann Intern Med. 2015;162(6):407–419. doi: 10.7326/M14-1152. [DOI] [PubMed] [Google Scholar]
- 24.Linas BP, Barter DM, Morgan JR, et al. The cost-effectiveness of Sofosbuvir-based regimens for treatment of hepatitis C virus genotype 2 or 3 infection. Ann Intern Med. 2015; 162(9): 619–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Younossi ZM, Part H, Dieterich D, Saab S, Ahmed A, Gordon SC. Assessment of cost of innovation versus the value of health gains associated with treatment of chronic hepatitis C in the United States The quality-adjusted cost of care. Medicine (Baltimore). 2016;95(41):e5048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Direct-acting antiviral sustained virologic response: impact on mortality in patients without advanced liver disease. Hepatology. 2018;68(3):827–838. [DOI] [PubMed] [Google Scholar]
- 27.Backus LI, Belperio PS, Shahoumian TA, Mole LA. Impact of sustained virologic response with direct-acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 2019;69(2):487–497. [DOI] [PubMed] [Google Scholar]
- 28.Singal AG, Rich NE, Mehta N, et al. Direct-acting antiviral therapy for hepatitis C virus infection is associated with increased survival in patients with a history of hepatocellular carcinoma. Gastroenterology. 2019;157(5):1253–1263.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis – United States, 2014. https://www.cdc.gov/hepatitis/statistics/2014surveillance/commentary.htm#summary. Accessed February 26, 2020.
- 30.Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144(10):705–14. [DOI] [PubMed] [Google Scholar]
- 31.IMS Institute for Health Informatics. Medicines use and spending in the U.S. – A review of 2015 and outlook to 2020. http://www.imshealth.com/en/thought-leadership/ims-institute/reports/medicines-use-and-spending-in-the-us-a-review-of-2015-and-outlook-to-2020. Accessed April 2016.
- 32.Alonso-Zaldivar R Medicare spending $9B on hepatitis C drugs. Business Insider. November 13, 2015. www.businessinsider.com/ap-apnewsbreak-medicare-spending-9bon-hepatitis-c-drugs-2015-1. Accessed June 22, 2016. [Google Scholar]
- 33.Kaiser Family Foundation. Distribution of Medicare beneficiaries by gender. https://www.kff.org/medicare/state-indicator/medicare-beneficiaries-by-gender/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D. Accessed February 26, 2020.
- 34.Baden R, Rockstroh JK, Buti M. Natural history and management of hepatitis C: does sex play a role? J Infect Dis. 2014;209 Suppl 3:S81–5. [DOI] [PubMed] [Google Scholar]
- 35.Jung JK, Feldman R, Cheong C, et al. Coverage for hepatitis C drugs in Medicare Part D. Am J Manag Care. 2016;22:SP220–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Omland LH, Osler M, Jepsen P, et al. Socioeconomic status in HCV infected patients – risk and prognosis. Clin Epidemiol. 2013; 5: 163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Center for Medicare & Medicaid Services. Chronic conditions data warehouse CCW chronic condition algorithms. https://www2.ccwdata.org/web/guest/condition-categories. Accessed January 22, 2019.
- 38.Cho IS, Chae YR, Kim JH, et al. Statistical methods for elimination of guarantee-time bias in cohort studies: a simulation study. BMC medical research methodology. 2017;17:126–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gleiss A, Oberbauer R, Heinze G. An unjustified benefit: immortal time bias in the analysis of time-dependent events. Transpl Int. 2018;31(2):125–130. [DOI] [PubMed] [Google Scholar]
- 40.Zhou Z, Rahme E, Abrahamowicz M, Pilote L. Survival bias associated with time-to-treatment initiation in drug effectiveness evaluation: a comparison of methods. Am J Epidemiol. 2005;162(10):1016–23. [DOI] [PubMed] [Google Scholar]
- 41.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barraclough H, Govindan R. Biostatistics primer: what a clinician ought to know: subgroup analyses. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2010;5:741. [DOI] [PubMed] [Google Scholar]
- 43.American Association for the Study of Liver Diseases. HCV guidance: recommendations for testing, managing, and treating hepatitis C. https://www.hcvguidelines.org/evaluate/when-whom. Accessed April 29, 2020. [DOI] [PMC free article] [PubMed]
- 44.Canary LA, Klevens RM, Holmberg SD. Limited access to new hepatitis C virus treatment under state Medicaid programs. Ann Intern Med. 2015;163(3), 226–228. [DOI] [PubMed] [Google Scholar]
- 45.Barua S, Greenwald R, Grebely J, Dore GJ, Swan T, Taylor LE. Restrictions for Medicaid reimbursement of Sofosbuvir for the treatment of hepatitis C virus infection in the United States. Ann Intern Med. 2015;163(3), 215–223. [DOI] [PubMed] [Google Scholar]
- 46.Liao JM, Fischer MA. Restrictions of hepatitis C treatment for substance-using Medicaid patients: cost versus ethics. Am J Public Health. 2017; 107(6): 893–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.US Preventive Services Task Force. Hepatitis C virus infection on adolescents and adults: screening. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening. Accessed on April 29, 2020.
- 48.Cubanski J, Swoope C, Boccuti C, et al. A primer on Medicare: Key facts about the Medicare program and the people it covers. Kaiser Family Foundation. https://www.kff.org/report-section/a-primer-on-medicare-what-is-the-role-of-medicare-for-dual-eligible-beneficiaries/view/print/. Accessed February 26, 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


